Abstract
Purpose
To assess prospectively the dose-response relationship between respiratory disease (ICD10: J1-99), pneumonia (ICD10: J12.0-18.9), and asperation pneumonia mortality (ICD10: J69) vs. baseline walking and running energy expenditure (MET-hours/d, 1 MET = 3.5 ml O2/kg/min).
Methods
Cox proportional hazard analyses of 109,352 runners and 40,798 walkers adjusted for age, sex, smoking, diet, alcohol, and education.
Results
There were 236 deaths with respiratory disease listed as the underlying cause, and 833 deaths that were respiratory disease related (entity axis diagnosis). Included among these were 79 deaths with pneumonia listed as the underlying cause and 316 pneumonia-related deaths, and 77 deaths due to aspiration pneumonia. There was no significant difference in the effect of running compared to walking (per MET-hours/d) on mortality, thus runners and walkers were combined for analysis. Respiratory disease mortality decreased 7.9% per MET-hours/d as the underlying cause (95%CI: 1.6% to 14.0%, P=0.01) and 7.3% for all respiratory disease-related deaths (95%CI: 4.2% to 10.4%, P=10-5). Pneumonia mortality decreased 13.1% per MET-hours/d as the underlying cause (95%CI: 2.6% to 23.2%, P=0.01) and 10.5% per MET-hours/d for all pneumonia-related deaths (95%CI: 5.4% to 15.5%, P=0.0001). The risk for aspiration pneumonia mortality also did not differ between running and walking, and decreased 19.9% per MET-hours/d run or walked (95%CI: 8.9% to 30.2%, P=0.0004). These results remained significant when additionally adjusted for BMI.
Conclusions
Higher doses of running and walking were associated with lower risk of respiratory disease, pneumonia, and aspiration pneumonia mortality in a dose-dependent manner, and the effects of running and walking appear equivalent. These effects appear to be independent of the effects of exercise on cardiovascular disease.
Keywords: physical activity, pneumonia, prevention, cohort
Pneumonia is the leading cause of death from infection in the United States, and in combination with influenza ranks eighth among all underlying causes of death [6,14]. Hospitalization is required for 40% to 50% of those infected, with approximately 10% requiring intensive care [24]. By 2020, the annual number of hospitalizations for community-acquired pneumonia is expected to rise to one million in the United States as the population ages [26]. In the elderly, the risk for community-acquired pneumonia is increased by heart disease, chronic lung disease, immunosuppressive drugs, alcoholism, and increasing age [9]. Long-term survival of community-acquired pneumonia is diminished by age, male sex, low educational achievement, and comorbid illnesses [14], with age and comorbid conditions being stronger predictors of survival than abnormal acute physiologic or laboratory findings [13].
Less common are nosocomical (hospital-acquired) and aspiration pneumonia. Treatment of nosocomical pneumonia is becoming increasingly problematic due to increases in antibiotic resistance of gram-negative bacteria [22], and has greater mortality (38% to over 70% [15]) than community-acquired pneumonia. Aspiration pneumonia, which is an infection of the lung caused by the inhalation of vomit or food, currently ranks as the fifteenth leading cause of death in the United States [6]. Its occurrence may indicate impaired ability to remove food or vomit by coughing due to age, inebriation, a complication of general anesthesia, or debilitation, as, for example, due to stroke [21].
Public health guidelines recommend 150 minutes a week of moderate-intensity, or 75 minutes a week of vigorous-intensity aerobic physical activity [20]. Multiple health benefits have been ascribed to physical activity [20]. However, these benefits do not currently include decreased respiratory disease risk. Two reports from the Nurses' Health Study II suggest that the risk for community-acquired pneumonia decreases with greater leisure-time and recreational physical activity in women, but not when adjusted for body mass index (BMI) [1,17], and, to our knowledge, there have been no significant risk reductions reported for men. However, reductions in pneumonia risk would be consistent with evidence suggesting that regular exercise improves immune function and attenuates its decline with age [3,7,8,11,12,19,35,37].
This report examines the association of running and walking with mortality due to respiratory disease in the National Walkers' and Runners' Health Studies, prospective epidemiological cohorts of over 150,000 walkers and runners whose mortality follow-up has been completed through 2008. The runners and walkers reported usual distance run or walked per week as part of their baseline questionnaire, which has been shown to be a superior to the more traditional time-based physical activity assessments for testing epidemiological hypotheses [32-34]. The large sample size, and the superior quantification of physical activity, provides a unique opportunity to test whether greater exercise energy expenditure predicts lower risk for respiratory diseases in general, and for pneumonia and aspiration pneumonia in particular.
Materials and Methods
The National Walkers' and Runners' Health Studies have been described in detail [27-34]. Walkers were recruited between 1999 and 2001, while runners were recruited in two waves, between 1991 and 1993 (phase I) and between 1998 and 2001 (phase II), through solicitation of subscribers of activity-targeted publications and participants at footrace events. The three cohorts may be more accurately characterized as a single cohort that targeted the runners and walkers, since all three used the same questionnaire (modified slightly for the different activities), the same sampling domain (subscription lists to running and walking publications, running and walking events), the same survey staff, and were funded by the same grants.
Participants completed a four-page survey on running and walking history, height, weight, diet, smoking, and history of diseases. Intakes of meat and fruit were based on the questions “During an average week, how many servings of beef, lamb, or pork do you eat”, and “…pieces of fruit do you eat”. Alcoholic beverage consumption was ascertained from the corresponding questions for 4-oz (112 mL) glasses of wine, 12-oz (336 mL) bottles of beer, and mixed drinks and liqueurs, and alcohol intake estimated from 10.8 g/4-oz glass of wine, 13.2 g/12-oz bottle of beer, and 15.1 g/mixed drink. Running energy expenditure was expressed in units of metabolic equivalents (METs), in which one MET is the energy expended sitting at rest (3.5 ml O2•kg-1•min-1) [4]. Running MET values were calculated as 1.02 MET•hours per km [34]. Previously, we reported strong correlations between repeated questionnaires for self-reported running distance (r=0.89) [29]. Walking energy expenditure (MET-hours/d) was computed by converting the reported usual weekly distance into duration (i.e., distance divided by mph for their usual walking pace) and then calculating the product of the average hours walked per day and the MET value corresponding to their reported pace [32,33]. The study protocol was approved by the University of California Berkeley committee for the protection of human subjects, and all subjects provided a signed statement of informed consent.
Mortality surveillance was completed through 2008 using the National Death Index plus [16]. International Classification of Disease codes versions 9 (ICD9) and 10 (ICD10) [36] were used to identify deaths due to respiratory disease (ICD9460-519, 799.1, ICD10J1-99), pneumonia (ICD9480-489, ICD10J12.0-18.9), and aspiration pneumonia (ICD9507, ICD10J69). The underlying cause of death is “the disease or injury that initiated the chain of morbid events that led directly and inevitably to death” [25]. Other contributing causes of death are “all other significant diseases, conditions, or injuries that contributed to death but which did not result in the underlying cause of death” [25]. “All pneumonia-related mortality” and “all respiratory disease-related mortality” refer to pneumonia or respiratory disease listed as either underlying or other contributing causes of death. Aspiration pneumonia was seldom listed as a contributing cause of death (nine deaths) and therefore only underlying cause of death was analyzed.
Cox proportional hazard analyses (JMP version 5.1, SAS institute, Cary SC) were used to test whether total and cause-specific deaths were significantly related to MET-hours/d run or walked when adjusted for baseline age (age and age2), sex, years of education, baseline smoking status (current smoker vs. non-smoker), cohort, and intakes of meat, fruit, and alcohol. Results are presented as hazard ratios (HR) and their percent risk reduction (calculated as 100*(HR-1)) per MET-hours/d run or walked, and for five categories of exercise energy expenditure: 1) falling short of the current exercise recommendations for health (450 MET minutes per week =1.07 MET-hours/d [4]), 2) achieving the exercise recommendations (450 to 750 MET minutes per week =1.07 to 1.8 MET-hours/d [4]), 3) exceeding the recommendations by 1- to 2-fold (1.8 to 3.6 MET-hours/d), 2- to 3-fold (3.6 –5.4 MET-hours/d), or ≥3-fold the recommended levels (≥5.4 MET-hours/d). Deaths occurring within one year of the baseline survey were excluded.
The primary hypothesis was that MET-hours/d of exercise would be inversely related to all pneumonia-relate mortality, with a significance level of P≤0.05. Secondary hypotheses were that MET-hours/d of exercise was significantly related to pneumonia as an underlying cause of death, all respiratory disease-related mortality, respiratory disease as an underlying cause of death, and aspiration pneumonia as an underlying cause of death. Analyses were repeated excluding all cardiovascular disease-related mortality (CVD, ICD9390-448, ICD10I00-78) to ensure the observed associations with respiratory diseases were not due to their associations with CVD. Shoenfeld residuals were checked for serious departures from proportionality. The significance for the decreased mortality with MET-hours/d of running or walking was verified in every case by logistic regression analyses that included follow-up duration as a covariate (one exception, P=0.06 for pneumonia as an underlying cause in females, results not displayed).
Results
The baseline characteristics of the cohorts, and the numbers of death by respiratory disease endpoints during the 11.4-year average follow-up are displayed in Tables 1 and 2, respectively.
Table 1.
Baseline characteristics of the cohorts.
| Males | Females | |||
|---|---|---|---|---|
| Runners | Walkers | Runners | Walkers | |
| Sample (N) | 63,267 | 8103 | 46,085 | 32,695 |
| Age (years)* | 40.40±10.85 | 61.16±13.07 | 38.18±10.10 | 50.39±10.85 |
| Education (years)* | 16.42±2.50 | 15.92±2.80 | 16.04±2.55 | 15.02±2.52 |
| Current smokers (%) | 1.58 | 4.76 | 2.30 | 5.52 |
| Alcohol (g/d)* | 11.52±16.21 | 10.40±16.77 | 7.10±10.43 | 5.50±10.46 |
| Meat (servings/d)* | 0.42±0.40 | 0.46±0.41 | 0.26±0.31 | 0.37±0.49 |
| Fruit (pieces/d)* | 1.51±1.25 | 1.52±1.19 | 1.50±1.11 | 1.57±1.30 |
| BMI (kg/m2)* | 24.16±2.82 | 27.20±4.72 | 21.65±2.74 | 25.83±5.55 |
| Running and walking (MET-hours/d)* | 4.43±3.31 | 2.23±1.66 | 4.89±3.08 | 2.20±1.60 |
means±SD
Table 2.
Hazard ratios from Cox proportional hazard analyses (95% confidence interval) for respiratory disease, pneumonia, and aspiration pneumonia mortality vs. other risk factors.
| Respiratory disease ICD9460-519, 799.1, ICD10J1-99 | Pneumonia ICD9480-489, ICD10J12.0-18.9 | Aspiration pneumonia ICD9507, ICD10J69 | |||
|---|---|---|---|---|---|
| Underlying | All related mortality | Underlying | All related mortality | Underlying | |
| Deaths | |||||
| Runners | 81 | 349 | 33 | 141 | 28 |
| Walkers | 155 | 484 | 46 | 175 | 49 |
| Female | 0.420 (0.308, 0.569) P=10-8 |
0.561 (0.476, 0.660) P=10-11 |
0.403 (0.229, 0.693) P=0.0009 |
0.458 (0.347, 0.601) P=10-8 |
0.222 (0.118, 0.398) P=10-7 |
| Education, per year | 0.948 (0.904, 0.995) P=0.03 |
0.967 (0.942, 0.992) P=0.01 |
0.990 (0.911, 1.050) P=0.82 |
0.996 (0.956, 1.034) P=0.86 |
0.975 (0.898, 1.049) P=0.54 |
| Smokers | 1.728 (0.818, 3.188) P=0.14 |
3.362 (2.518, 4.397) P=10-12 |
3.367 (1.166, 7.697) P=0.03 |
2.723 (1.542, 4.442) P=0.001 |
0.740 (0.042, 3.365) P=0.75 |
| Meat, per servings/d | 1.105 (0.923, 1.157) P=0.19 |
1.003 (0.824, 1.121) P=0.97 |
1.006 (0.509, 1.187) P=0.99 |
0.782 (0.549, 1.085) P=0.15 |
1.139 (0.727, 1.222) P=0.42 |
| Fruit, per pieces/d | 0.849 (0.749, 0.955) P=0.006 |
0.956 (0.903, 1.012) P=0.12 |
0.845 (0.678, 1.027) P=0.10 |
0.957 (0.870, 1.039) P=0.34 |
0.964 (0.791, 1.072) P=0.69 |
| Alcohol, per g/d | 1.000 (0.991, 1.008) P=0.95 |
1.000 (0.995, 1.004) P=0.88 |
0.984 (0.962, 1.002) P=0.08 |
0.994 (0.985, 1.001) P=0.11 |
0.987 (0.967, 1.004) P=0.14 |
| Runner | 0.364 (0.206, 0.622) P=0.0002 |
0.769 (0.599, 0.987) P=0.04 |
0.940 (0.390, 2.149) P=0.89 |
1.049 (0.705, 1.547) P=0.81 |
0.544 (0.205, 1.313) P=0.18 |
| BMI, per kg/m2 | 0.994 (0.960, 1.028) P=0.73 |
1.018 (1.000, 1.036) P=0.05 |
0.915 (0.848, 0.982) P=0.01 |
0.990 (0.959, 1.022) P=0.55 |
1.023 (0.961, 1.081) P=0.46 |
Hazard ratios when all variables are included simultaneously in the model with age (age, age2) and cohort effect.
Table 2 presents the hazard ratios for the covariates used in the analyses when all were included simultaneously in the model and adjusted for age and exercise level. Compared to men, women had significantly lower risk for all respiratory disease endpoints studied. Runners, as a group, were also at lower risk for respiratory disease mortality than walkers. Other variables showing significant associations with mortality were: 1) years of education with lower risk of respiratory disease as an underlying cause of death and all respiratory disease related deaths; 2) smoking with all respiratory disease- and pneumonia-related deaths, and deaths with pneumonia as an underlying cause; 3) greater fruit intake with lower risk of respiratory deaths as an underlying cause; and 4) greater BMI with increased risk for all respiratory disease related deaths and decreased risk for pneumonia as an underlying cause.
Respiratory disease deaths as the underlying cause
There was no significant difference in the decrease in respiratory disease deaths per MET-hours/d run vs. MET-hours/d walked (P=0.58), so their energy expenditures were combined and the analyses adjusted for cohort effects. The risk for respiratory disease mortality decreased 7.9% per MET-hours/d, which remained essentially unaffected by adjustment for BMI (Table 3). Figure 1 shows that compared to falling short of the recommended exercise level (<1.07 MET-hours/d), there was no significant reduction in risk for merely satisfying the exercise recommendations (10% decrease), whereas the risk for respiratory disease mortality decreased 34.5% for exceeding the recommendations by 1- to 2-fold, and 45.3% for exceeding the recommendations by ≥2-fold (HR: 0.55; 95%CI: 0.36 to 0.83, P=0.005). Very similar risk reductions were experienced in men and women (Table 3), with their difference in statistical significance reflecting more their differences in the number of deaths (163 men, 73 women) rather than their effect size.
Table 3.
Hazard ratios from Cox proportional hazard analyses (95% confidence interval) for respiratory disease, pneumonia, and aspiration pneumonia mortality vs. MET-hour/d run or walked.
| Sexes combined | Sex specific | |||
|---|---|---|---|---|
| No BMI adjustment | Adjusted for BMI | Males only | Females only | |
| Respiratory disease-underlying cause | ||||
| All cases | 0.921 (0.860, 0.984) P=0.01 |
0.915 (0.853, 0.979) P=0.009 |
0.926 (0.859, 0.996) P=0.04 |
0.918 (0.776, 1.067) P=0.27 |
| All respiratory disease related deaths | ||||
| All cases | 0.927 (0.896, 0.958) P=10-5 |
0.932 (0.901, 0.965) P=0.0001 |
0.938 (0.902, 0.974) P=0.0007 |
0.894 (0.832, 0.958) P=0.001 |
| Excluding CVD related deaths | 0.941 (0.900, 0.983) P=0.005 |
0.936 (0.895, 0.978) P=0.003 |
0.953 (0.905, 1.001) P=0.06 |
0.920 (0.840, 1.002) P=0.06 |
| Pneumonia-underlying cause | ||||
| All cases | 0.869 (0.768, 0.974) P=0.01 |
0.833 (0.848, 0.915) P=0.002 |
0.896 (0.786, 1.009) P=0.07 |
0.623 (0.456, 0.974) P=0.03 |
| All pneumonia related deaths | ||||
| All cases | 0.895 (0.845, 0.946) P=0.0001 |
0.891 (0.840, 0.943) P=0.0001 |
0.918 (0.863, 0.975) P=0.005 |
0.796 (0.685, 0.914) P=0.0008 |
| Excluding CVD related deaths | 0.896 (0.831, 0.961) P=0.002 |
0.890 (0.824, 0.956) P=0.001 |
0.934 (0.863, 1.006) P=0.07 |
0.721 (0.581, 0.875) P=0.0006 |
| Aspiration pneumonia underlying cause | ||||
| All cases | 0.801 (0.698, 0.911) P=0.0004 |
0.809 (0.703, 0.920) P=0.0009 |
0.779 (0.670, 0.894) P=0.0002 |
|
| Excluding CVD related deaths | 0.795 (0.661, 0.939) P=0.006 |
0.798 (0.661, 0.945) P=0.008 |
0.782 (0.639, 0.937) P=0.006 |
|
Hazard ratios adjusted for baseline age (age and age2), years of education, baseline smoking status (current smoker vs. non-smoker), cohort, and intakes of meat, fruit, and alcohol. Sex was also included as a covariate in the analyses of the sexes combined.
There were only 15 deaths from aspiration pneumonia in women, and therefore those results are not included.
Figure 1.
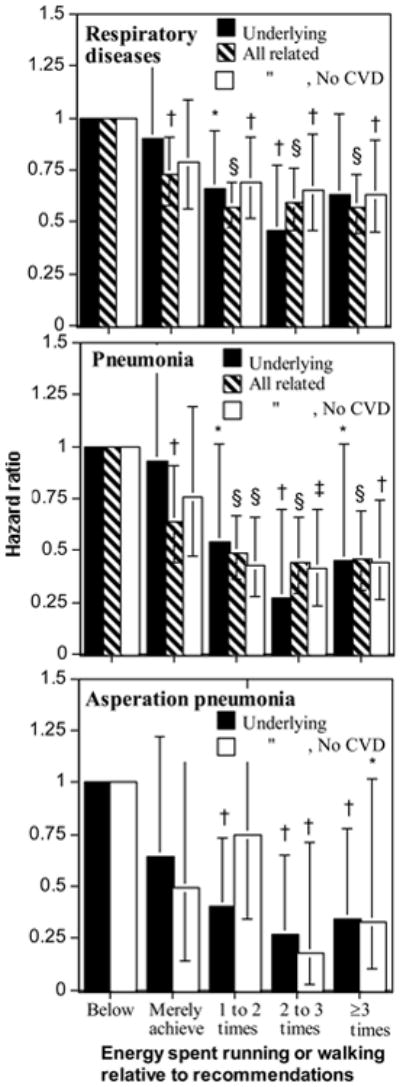
Decrease in the risk for respiratory disease mortality as underlying cause and for all related mortality (underlying and contributing combined) relative to falling below (<1.07 MET-hours/d) or merely meeting the recommendations (1.07 to 1.8 MET-hours/d), and running or walking at 1 to 2-times (1.8 to 3.6 MET-hours/d), 2- to 3-times (1.8 to 3.6 MET-hours/d), or ≥ 3-times (≥3.6 MET-hours/d) the recommended levels. Brackets represent 95% confidence intervals. Significance levels relative to not meeting the current exercise recommendations (<1.07 MET-hours/d) are coded: * P≤0.05; † P≤0.01; ‡ P≤0.001; § P≤0.0001.
All respiratory disease-related deaths
The estimated effects of exercise energy expenditure on all respiratory disease related deaths were also not significantly different between walking and running (P=0.10). Table 3 shows that for running and walking combined, the risk for all respiratory disease-related deaths decreased 7.3% per MET-hours/d, which persisted when adjusted for BMI, and was significant in both males and females separately (Table 3). Forty-nine percent of the respiratory disease deaths had CVD listed as a contributing cause, a mortality endpoint known to decrease with running [30,31]. However, the reduction in the risk for all respiratory disease-related deaths persisted when all CVD-related deaths were excluded (Table 3). Only two-thirds of the potential risk reduction was achieved by meeting the current exercise guidelines (Figure 1). Exceeding the current exercise guidelines was associated with a significantly greater decrease in all respiratory disease-related deaths than merely satisfying the guidelines (i.e., >1.8 vs. 1.07 to 1.8 MET-hours/d, HR: 0.79, 95%CI: 0.63 to 0.98, P=0.04 for all respiratory disease-related deaths).
Pneumonia as the underlying cause
The risk for pneumonia decreased 13.1% per MET-hours/d run or walked, and was significant both with and without adjustment for BMI, and when the analyses were restricted to women (Table 3, P=0.07 for men). Compared to not achieving the current exercise recommendation, the risk for pneumonia as an underlying cause of death decreased 21% for meeting the guidelines, 31% by exceeding the guidelines by 1- to 2-fold, 35% by exceeding the guidelines by 2- to 3-fold, and 37% by exceeding the guidelines by ≥3-fold when all CVD-related deaths were excluded (Figure 1, middle panel).
All pneumonia-related deaths
The risk for all pneumonia-related deaths decreased 10.5% per MET-hours/d run or walked, and was significant both with and without adjustment for BMI, and when the analyses were restricted to men and women. Compared to not achieving the current exercise recommendation, the risk for all pneumonia-related deaths decreased 36.2% for meeting the guidelines, 50.6% by exceeding the guidelines by 1- to 2-fold, 55.9% by exceeding the guidelines by 2- to 3-fold, and 54.0% by exceeding the guidelines by ≥3-fold (Figure 1, middle). The reductions in risk for all pneumonia-related mortality were somewhat weakened by excluding CVD-related deaths, but remained significant except for merely meeting the guidelines.
Asperation pneumonia as the underlying cause
There was no difference in the per MET-hours/d reduction in asperation pneumonia mortality between running and walking (P=0.89). Its risk decreased 19.9% per MET-hours/d run or walked before adjustment for BMI, and 19.1% after adjustment. Figure 1 (bottom) shows that aspiration pneumonia was less likely to occur in those that exceeded 1.8 MET-hours/d run or walked (i.e., exceeded the current recommendations).
Other respiratory disease related deaths
The remaining 441 deaths included 180 respiratory failures of an unspecified nature and 261 deaths involving other respiratory causes. Neither the respiratory failures (HR: 0.985, 95%CI: 0.924 to 1.045, P=0.62) nor the other respiratory causes (HR: 0.943, 95%CI: 0.885 to 1.002, P=0.06) were significantly related to MET-hours/d run or walked.
Discussion
These analyses show that the risks for respiratory diseases and pneumonia as underlying and contributing causes of mortality decreased significantly in association with greater baseline exercise energy expenditure (MET-hours/d), and did not differ significantly between running (a vigorously intense exercise) and walking (a moderately intense exercise). Exceeding physical activity recommendations beyond that currently recommended appeared to produce substantial reductions in risk. Aspiration pneumonia mortality also decreased significantly with greater METs run or walked, with those who exceeded the recommendations having ≥60% lower risk for aspiration pneumonia as those who fell short of the recommendations.
The current results substantially strengthen the evidence linking pneumonia risk to physical activity in women. Specifically, the women's risk for pneumonia as an underlying cause decreased significantly per MET-hour/d both before (Table 3) and after adjustment for BMI (HR: 0.644, 95%CI: 0.420 to 0.916, P=0.01). At best, prior reports suggest a weak association that loses its statistical significance when adjusted for BMI. In particular, a two year follow-up of 78,062 women who participated in the Nurses' Health Study showed that the risk for community acquired pneumonia decreased 6% for the 2nd quintile, 21% for the 3rd, 26% for the 4th, and 34% in the 5th quintile of recreational or leisure-time physical activity relative to the least active quintile [1]. This trend was significant when adjusted for age, smoking, and alcohol intake (P=0.02), but not when further adjusted for BMI. Subsequent 12-year follow-up of this cohort (N=83,185) showed that nurses in the highest activity quintile had 28% lower risk of non-fatal community-acquired pneumonia compared to those in the lowest quintile when adjusted for age, smoking, and alcohol use (P<0.0001 for trend), but again, not when adjusted for BMI [17]. The highest quintile of walking had 18% lower risk of community-acquired pneumonia compared to the lowest quintile, but there was no significant trend across quintiles. Running or jogging >2 hour per week had 54% lower risk than woman who neither ran or jogged [17]. The effects of BMI adjustment on the walking-, jogging-, or running-associations were not reported.
The current results provide the only evidence linking pneumonia risk to physical activity in men. A six-year follow-up of 26,429 men aged 44 to 79 in the Health Professionals Follow-up Study showed no significant risk reduction for community acquired pneumonia from the lowest quintile of recreational or leisure-time physical activity to the 2nd (7% increase), 3rd (31% decrease), 4th (20% decrease), and 5th quintiles of activity (4% decrease) [1]. Although Paffenbarger et al. reported that lower physical activity predicted a 3.9-fold increased risk of deaths due to pneumonia in their 22-year follow-up of 3686 San Francisco longshoremen, the increased risk was not statistically significant [18]. In addition, moderate and heavy physical activity were not found to decrease the incidence of hospital-treated pneumonia in 50- to 69-year old male smokers participating in the Alpha-Tocopherol, Beta-Carotene Cancer Prevention (ATBC) Study [5].
The effects of running and walking we observed on pneumonia risk are consistent with other research suggesting that exercise may improve immune function [3,11,35], and attenuate its decline with age [8]. Regular exercise in adults has been associated with higher amounts of anti-influenza IgG and IgM following immunization [7], and to attenuate the decline in natural killer cell activity with age [37]. Chronic resistance training is reported to improve natural killer cell activity in older women [12].
The reduction respiratory disease and pneumonia risk per MET-hours/d run or walked remained significant when CVD-related deaths were excluded. Over one-half of older community acquired pneumonia patients have preexisting chronic cardiac conditions and acute infections such as pneumonia can precipitate acute cardiac events [2]. Although running reduces the risk for coronary heart disease [31] and stroke [30], our results suggest that the reductions respiratory disease and pneumonia risk were not simply the consequence of lowered cardiovascular disease risk due to running or walking.
Whether obesity increases the risk for infectious pneumonia is controversial [10]. Contrary to the Nurses Health Study [1], we did not find BMI was related to increased risk for pneumonia, but this might be explained by the study differences, e.g., our focus on fatal rather than nonfatal pneumonia, and our lack of specificity on the origin of the infections (community vs. hospital acquired). Our cohort was also leaner than either the men of the Health Professionals' Follow-up Study (mean BMI: 24.2 for our males vs. 25.5 kg/m2) or the women of the Nurses' Health Study (mean BMI: 21.7 for our females vs. 24.45 kg/m2), and included fewer obese subjects (3.5% obese males vs. 8.2% for the Health Professionals Follow-up Study; 1.5% obese females vs. 12.1% in the Nurses' Health Study), and therefore many have included too few subjects at risk for their obesity.
We considered all respiratory-related and all pneumonia-related deaths in addition to deaths due to respiratory disease and pneumonia as underlying causes to increase statistical power. Concurrent infections, and the contraction of hospital-acquired pneumonia while being treated for other conditions, are examples of pneumonia's contribution to mortality without necessarily being listed as the underlying cause. Moreover, the mechanism by which physical activity reduces mortality may not simply involve its effect on pneumonia as the underlying cause, but also by affecting whether pneumonia contributes to the fatal consequence of other underlying causes.
Finally, we hypothesize that the observed reduction of aspiration pneumonia with increased exercise may represent a lower risk for stroke or conditions requiring general anesthesia, less loss of vitality with aging, or the sustained ability to cough or otherwise prevent the aspiration of food or vomit. We have previously demonstrated that the risk for stroke decreases with greater running mileage [30]. Greater running may also be associated with reduced susceptibility to lung inflammation when food or vomit is aspirated. The association was unexpected and requires confirmation.
There are important limitations to these analyses. Running, walking, and other baseline variables were self-reported from the participants' baseline questionnaires. Exercise levels, and other subject characteristics could have changed prior to infection. Our use of mortality is both a strength and weakness–a strength in its ease of ascertainment, lack of subjectivity, and broad importance to patients and the critical care community, a weakness in that it is not known whether the risk reduction is due a lower risk of infection, a lower risk of mortality among those infected, or both. Another important limitation of these analyses is the lack of information on the circumstances of the infections. Death certificate diagnoses do not distinguish pneumonias acquired in the community, nursing homes, and health care facilities. Almost all of the diagnoses were pneumonia, unspecified ((ICD9486, ICD10J18.9). Differential mortality could represent differences in susceptibility to infection and well as the adequacy, timeliness, and response to antibiotic treatment [23]. Additional studies are required that track incidence, circumstances of infection, and treatment to determine whether the relationships observed are affected by patient physiology, behavior, or access to quality treatment in addition to exercise. Vital status is known only from the National Death Index and therefore some subjects who have died are likely to be misclassified as alive. Finally, we caution with regards to implying cause and effect in that individuals with greater susceptibility to respiratory disease may choose to exercise less.
In conclusion, the current results provide strong evidence that greater levels of physical activity may reduce the risk for respiratory disease-related deaths in a dose-dependent manner, including pneumonia and aspiration pneumonia. These effects appear to be independent of the effects of exercise on CVD risk.
Acknowledgments
This research was supported by grant HL094717 from the National Heart, Lung, and Blood Institute and was conducted at the Ernest Orlando Lawrence Berkeley National Laboratory (Department of Energy DE-AC03-76SF00098 to the University of California). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The results of the present study do not constitute endorsement by the American College of Sports Medicine.
Footnotes
The authors have declared that no competing interests exist.
Disclosures: There are no financial conflicts of interests to report.
References
- 1.Baik I, Curhan GC, Rimm EB, Bendich A, Willett WC, Fawzi WW. A prospective study of age and lifestyle factors in relation to community-acquired pneumonia in US men and women. Arch Intern Med. 2000;160:3082–8. doi: 10.1001/archinte.160.20.3082. [DOI] [PubMed] [Google Scholar]
- 2.Corrales-Medina VF, Suh KN, Rose G, et al. Cardiac complications in patients with community-acquired pneumonia: a systematic review and meta-analysis of observational studies. PloS Med. 2011;8:e1001048. doi: 10.1371/journal.pmed.1001048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gleeson M. Immune function in sport and exercise. J Appl Physiol. 2007;103:693–9. doi: 10.1152/japplphysiol.00008.2007. [DOI] [PubMed] [Google Scholar]
- 4.Haskell WL, Lee IM, Pate RR, et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc. 2007;39:1423–34. doi: 10.1249/mss.0b013e3180616b27. [DOI] [PubMed] [Google Scholar]
- 5.Hemila H, Kaprio J, Albanes D, Virtamo J. Physical activity and the risk of pneumonia in male smokers administered vitamin E and beta-carotene. Int J Sports Med. 2006;27:336–41. doi: 10.1055/s-2005-865670. [DOI] [PubMed] [Google Scholar]
- 6.Hoyert DL, Xu J. Deaths: Preliminary data for 2011. National Vital Statistics Reports. 2012;61:1–64. [PubMed] [Google Scholar]
- 7.Kohut ML, Cooper MM, Nickolaus MS, Russell DR, Cunnick JE. Exercise and psychosocial factors modulate immunity to influenza vaccine in elderly individuals. J Gerontol A Biol Sci Med Sci. 2002;57:M557–M562. doi: 10.1093/gerona/57.9.m557. [DOI] [PubMed] [Google Scholar]
- 8.Kohut ML, Senchina DS. Reversing age-associated immunosenescence via exercise. Exerc Immunol Rev. 2004;10:6–41. [PubMed] [Google Scholar]
- 9.Koivula I, Sten M, Mäkelä PH. Risk factors for pneumonia in the elderly. Am J Med. 1994;96:313–20. doi: 10.1016/0002-9343(94)90060-4. [DOI] [PubMed] [Google Scholar]
- 10.Mancuso P. Obesity and lung inflammation. J Appl Physiol. 2010;108:722–8. doi: 10.1152/japplphysiol.00781.2009. M. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mazzeo RS. Altitude, exercise and immune function. Exerc Immunol Rev. 2005;11:6–16. [PubMed] [Google Scholar]
- 12.McFarlin BK, Flynn MG, Phillips MD, Stewart LK, Timmerman KL. Chronic resistance exercise training improves natural killer cell activity in older women. J Gerontol A Biol Sci Med Sci. 2005;60:1315–8. doi: 10.1093/gerona/60.10.1315. [DOI] [PubMed] [Google Scholar]
- 13.Mortensen EM, Coley CM, Singer DE, et al. Causes of death for patients with community-acquired pneumonia: results from the Pneumonia Patient Outcomes Research Team cohort study. Arch Intern Med. 2002;162:1059–64. doi: 10.1001/archinte.162.9.1059. [DOI] [PubMed] [Google Scholar]
- 14.Mortensen EM, Kapoor WN, Chang CC, Fine MJ. Assessment of mortality after long-term follow-up of patients with community-acquired pneumonia. Clin Infect Dis. 2003;37:1617–1624. doi: 10.1086/379712. [DOI] [PubMed] [Google Scholar]
- 15.Muscedere JG, Day A, Heyland DK. Mortality, attributable mortality, and clinical events as end points for clinical trials of ventilator-associated pneumonia and hospital-acquired pneumonia. Clin Infect Dis. 2010;51(Suppl1):S120–5. doi: 10.1086/653060. [DOI] [PubMed] [Google Scholar]
- 16.National Death Index User's Manual. U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control, National Center for Health Statistics; Hyattsville, Md: Sep, 1990. [accessed 6/23/2013]. pp. 1–61. http://www.cdc.gov/nchs/data/ndi/NDI_Users_Guide.pdf. [Google Scholar]
- 17.Neuman MI, Willett WC, Curhan GC. Physical activity and the risk of community-acquired pneumonia in US women. Am J Med. 2010;123:281. doi: 10.1016/j.amjmed.2009.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paffenbarger RS, Jr, Brand RJ, Sholtz RI, Jung DL. Energy expenditure, cigarette smoking, and blood pressure level as related to death from specific diseases. Am J Epidemiol. 1978;108:12–8. [PubMed] [Google Scholar]
- 19.Phillips B, Marshall ME, Brown S, Thompson JS. Effect of smoking on human natural killer cell activity. Cancer. 1985;56:2789–92. doi: 10.1002/1097-0142(19851215)56:12<2789::aid-cncr2820561213>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 20.Physical Activity Guidelines Advisory Committee. Physical Activity Guidelines Advisory Committee Report, 2008. Washington, DC: U.S. Department of Health and Human Services; 2008. pp. A1–H14. [DOI] [PubMed] [Google Scholar]
- 21.Raghavendran K, Nemzek J, Napolitano LM, Knight PR. Aspiration-induced lung injury. Crit Care Med. 2011;39:818–26. doi: 10.1097/CCM.0b013e31820a856b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raineri E, Crema L, Dal ZS, et al. Rotation of antimicrobial therapy in the intensive care unit: impact on incidence of ventilator-associated pneumonia caused by antibiotic-resistant Gram-negative bacteria. Eur J Clin Microbiol Infect Dis. 2010;29:1015–24. doi: 10.1007/s10096-010-0964-5. [DOI] [PubMed] [Google Scholar]
- 23.Torres A, Ferrer M, Badia JR. Treatment guidelines and outcomes of hospital-acquired and ventilator-associated pneumonia. Clin Infect Dis. 2010;51(suppl 1):S48–53. doi: 10.1086/653049. [DOI] [PubMed] [Google Scholar]
- 24.Torres A, Rello J. Update in community-acquired and nosocomial pneumonia 2009. Am J Respir Crit Care Med. 2010 Apr;181:782–7. doi: 10.1164/rccm.201001-0030UP. [DOI] [PubMed] [Google Scholar]
- 25.U.S. Department of Health and Human Services. Instructions for Completing the Cause-of-Death Section of the Death Certificate. [accessed July 31, 2013];2004 http://www.cdc.gov/nchs/data/dvs/blue_form.pdf.
- 26.Venditti M, Falcone M, Corrao S, Licata G, Serra P Study Group of the Italian Society of Internal Medicine. Outcomes of patients hospitalized with community-acquired, health care–associated, and hospital-acquired pneumonia. Ann Intern Med. 2009;150:19–26. doi: 10.7326/0003-4819-150-1-200901060-00005. [DOI] [PubMed] [Google Scholar]
- 27.Williams PT. High-density lipoprotein cholesterol and other risk factors for coronary heart disease in female runners. N Engl J Med. 1996;334:1298–303. doi: 10.1056/NEJM199605163342004. [DOI] [PubMed] [Google Scholar]
- 28.Williams PT. Relationship of distance run per week to coronary heart disease risk factors in 8283 male runners. The National Runners' Health Study. Arch Intern Med. 1997;157:191–8. [PMC free article] [PubMed] [Google Scholar]
- 29.Williams PT. Vigorous exercise and the population distribution of body weight. Int J Obes Relat Metab Disord. 2004;28:120–8. doi: 10.1038/sj.ijo.0802480. [DOI] [PubMed] [Google Scholar]
- 30.Williams PT. Reduction in incident stroke risk with vigorous physical activity: evidence from 7.7-year follow-up of the national runners' health study. Stroke. 2009;40:1921–3. doi: 10.1161/STROKEAHA.108.535427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Williams PT. Reductions in incident coronary heart disease risk above guideline physical activity levels in men. Atherosclerosis. 2010;209:524–7. doi: 10.1016/j.atherosclerosis.2009.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Williams PT. Distance walked and run as improved metrics over time-based energy estimation in epidemiological studies and prevention; evidence from medication use. PLoS One. 2012;7:e41906. doi: 10.1371/journal.pone.0041906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Williams PT. Advantage of distance- versus time-based estimates of walking in predicting adiposity. Med Sci Sports Exerc. 2012;44:1728–37. doi: 10.1249/MSS.0b013e318258af3f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Williams PT. Non-exchangeability of running vs. other exercise in their association with adiposity, and its implications for public health recommendations. PLoSOne. 2012;7:e36360. doi: 10.1371/journal.pone.0036360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Woods JA, Lowder TW, Keylock KT. Can exercise training improve immune function in the aged? Ann N Y Acad Sci. 2002;959:117–27. doi: 10.1111/j.1749-6632.2002.tb02088.x. [DOI] [PubMed] [Google Scholar]
- 36.World Health Organization. Tenth Revision. Geneva: World Health Organization; 1992. [accessed Oct 5, 2012]. International Statistical Classification of Diseases and Related Health Problems. http://www.cdc.gov/nchs/data/dvs/Volume-1-2005.pdf. [Google Scholar]
- 37.Yan H, Kuroiwa A, Tanaka H, Shindo M, Kiyonaga A, Nagayama A. Effect of moderate exercise on immune senescence in men. Eur J Appl Physiol. 2001;86:105–11. doi: 10.1007/s004210100521. [DOI] [PubMed] [Google Scholar]


