Abstract
Background
Preterm infants often receive mechanical ventilation and oxygen at birth. Exposure to large tidal volumes (VT) at birth causes lung inflammation and oxygen may amplify the injury. We hypothesized that normal VT ventilation at birth causes lung injury that is exacerbated by 95% oxygen.
Methods
The head and chest of anesthetized preterm fetal sheep (129±1d gestation) were surgically exteriorized while maintaining the placental circulation. Fetuses were randomized to four groups with either: 1) VT ventilation to 6 mL/kg or 2) CPAP of 5 cm H2O, and either: a) 95%O2/5%CO2 or b) 95%N2/5%CO2. Age-matched fetuses were controls. After a 15-minute intervention, the fetal lamb was returned to the uterus for 1 h 45 min.
Results
In ventilated lambs, VT was 6.2±0.4 mL/kg at 15 min. Ventilation increased pro-inflammatory cytokines compared to control and CPAP only lambs, with recruitment of primarily monocytes to bronchioalveolar lavage fluid. Early response protein 1 was activated around the bronchioles in VT ventilated animals. The 15-min oxygen exposure did not change inflammatory mediators or other markers of lung and oxidative stress.
Conclusions
A VT of 6–7 mL/kg at birth increased early markers of injury and lung inflammation. Brief exposure to 95% oxygen did not alter lung inflammation.
Introduction
The initiation of ventilation at birth is unique because the fetal lung must rapidly transition from fluid-filled airspaces to gas exchange to sustain life1. Approximately 10% of newborns need some assistance with this transition and the majority of preterm infants less than 1500 grams receive some assisted ventilation2,3. Many preterm infants are exposed to positive pressure ventilation with large tidal volumes (VT) and oxygen, and this can cause airway epithelial injury and lung inflammation4–6. Recent clinical studies of resuscitation demonstrate that clinicians are not able to regulate positive end expiratory pressure (PEEP), peak inspiratory pressures (PIP), or VT given to infants (or in simulation) reliably, and the delivery of high VT is frequent6,7. The initiation of ventilation in preterm sheep with escalating VT to 15 mL/kg stretches the airways and causes airway epithelial injury and diffuse lung inflammation8–10. Large VT ventilation at birth also permits plasma and interstitial proteins to move into airspace, which decreases the response to subsequent surfactant treatment11. The path to bronchopulmonary dysplasia (BPD) may begin with the initiation of ventilation because large VT at birth increases acute phase response genes involved in inflammation, angiogenesis, vascular remodeling, and apoptosis within the lung10. It is assumed but untested that lower VT during resuscitation, similar to the normal VT 5 mL/kg of spontaneously breathing infants, would cause less injury.
The 2010 International liaison committee on resuscitation (ILCOR) guidelines recommend that term infants be resuscitated with room air instead of oxygen2 since resuscitation with 100% oxygen can delay the first spontaneous breath and may increase mortality compared to room air resuscitation12. Unfortunately preterm infants often do not respond to resuscitation with room air, with the majority of preterm infants requiring oxygen concentrations of about 40% to maintain appropriate oxyhemoglobin saturations and heart rates during the first minutes of life13,14. Extremely preterm infants resuscitated with 90% oxygen had increased markers of oxidative stress, inflammation, and a risk of BPD compared to infants resuscitated with 30% oxygen15. Exposure to high oxygen concentrations may also have a direct effect on the gene expression patterns within the epithelial cells16. It is still unclear if a brief exposure to oxygen during resuscitation can contribute to lung inflammation and alter gene expression in preterm infants.
Since it is difficult to isolate the individual components of resuscitation from subsequent ventilation at birth, we have used a premature fetal sheep model to maintain placental support during ventilation to allow time for markers of injury to develop8,10. Although we previously used an escalating VT to 15 mL/kg for 15 minutes to cause lung injury, Brew et al. recently demonstrated modest anatomic injury at 24 hr following 2 hr VT ventilation to 5 mL/Kg with repair by 15 days17. To test the hypothesis that initiation of ventilation with a lower VT would not injure the preterm lung, we have evaluated a VT, below the normal VT of 8 mL/kg of spontaneously breathing preterm lambs on CPAP18. We asked if the lower VT caused activation of acute phase responses and inflammation. In addition, we tested whether exposure to 95% oxygen for 15 minutes changed lung inflammation and markers of oxidative stress.
Results
All lambs (n=5–6/group) survived the fetal interventions and in utero recovery, and there were no differences in the venous blood gas values between ventilation groups before or after the intervention (Table 1). The target VT of 6 to 7 mL/kg was achieved in the ventilated groups by 6 minutes (6.0±0.1 mL/kg), but required the maximal PIP of 40 cm H2O in all animals. The average VT remained stable by 15 minutes at 6.2±0.2 mL/kg. Total protein levels from the BALF were low (19±2 mg/kg) and similar between all ventilation groups and control animals. Injury scoring of H&E stained tissues demonstrated minimal epithelial sloughing, hemorrhage or inflammatory cells for all groups with no difference between groups (Table1). Thus the VT of 6.2 mL/kg or the oxygen did not cause microscopic injury.
Table 1.
Birth weights, VT, venous blood gases, and injury scores
| Group | n | BW |
VT at 15 min |
Before Fetal Maneuver- Venous | After Fetal Maneuver- Venous | Injury Score | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| pH | pCO2 | pO2 | pH | pCO2 | pO2 | |||||
| kg | mL/kg | mmHg | mmHg | mmHg | mmHg | (out of 6) | ||||
| Controls | 8 | 2.9±0.1 | -- | -- | -- | -- | -- | -- | -- | 1.8±0.3 |
| CPAP - N2 | 5 | 3.0±0.1 | No VT | 7.26±0.05 | 62.4±6.3 | 13.8±2.2 | 7.22±0.08 | 64.2±10.8 | 15.7±2.8 | 2.1±0.2 |
| VT - N2 | 6 | 3.5±0.1 | 6.2±0.4 | 7.22±0.03 | 62.2±3.9 | 13.2±1.6 | 7.21±0.03 | 60.8±3.8 | 14.8±1.8 | 2.0±0.3 |
| CPAP - O2 | 5 | 3.0±0.1 | No VT | 7.30±0.01 | 55.0±0.8 | 15.4±1.2 | 7.32±0.02 | 49.3±2.7 | 15.2±2.3 | 2.1±0.4 |
| VT - O2 | 6 | 2.8±0.1 | 6.2±0.4 | 7.25±0.02 | 63.2±5.2 | 13.4±2.0 | 7.25±0.05 | 60.0±6.4 | 18.1±1.2 | 2.3±0.2 |
Lung Inflammation
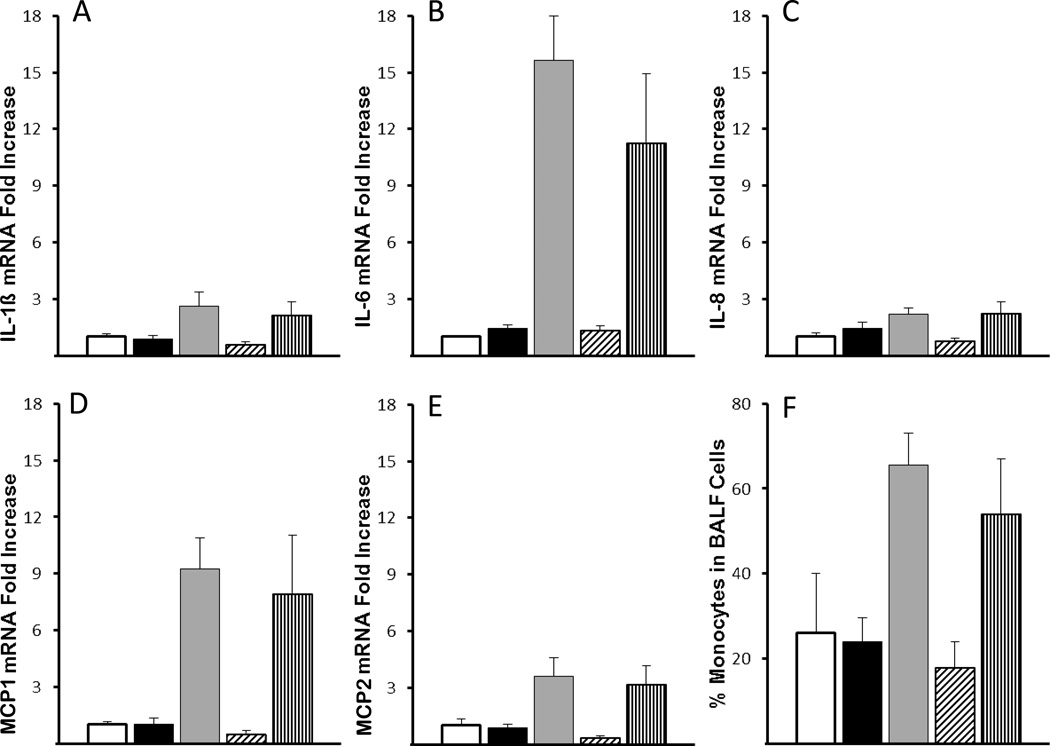
Ventilation for 15 min increased mRNA for the pro-inflammatory cytokines (IL-1β, IL-6, IL-8, MCP-1, MCP-2) in the lung tissue (Figure 1). Oxygen exposure did not increase these cytokines. Exposure to a PEEP of 5 cm H2O with or without 100% oxygen did not increase cytokine mRNA compared to controls. Messenger RNA values for IL-1β, IL-8, and IL-6 in epithelium from the trachea do not change with ventilation or oxygen exposure (data not shown). MCP-1 protein was detected in the cytoplasm of peripheral lung tissue cells in animals receiving VT ventilation (data not shown). Inflammatory cells increased in the BALF of lambs receiving mechanical ventilation, with an overall increase in the percent of monocytes (Figure 1). Compared with the other cytokines, the induction of IL-8 was modest (2 to 3-fold) and the neutrophil recruitment to the BALF was surprisingly low and occurred in lambs with the higher IL-8 mRNA values. Although occasional CD3+ cells were present in the lung tissue, there was no increase in recruitment of these T-cells to the lung with ventilation or oxygen exposure.
Figure 1. VT ventilation increased mRNA for pro-inflammatory cytokines.
(A) IL-1β, (B) IL-6, (C) IL-8, (D) MCP-1 and (E) MCP-2 mRNA increased with mechanical ventilation (VT) (p<0.05 vs Controls and lambs receiving only CPAP). There were no additional effects of oxygen (O2)(Vertical Striped Bar) on the increased mRNA from mechanical ventilation with nitrogen (N2)(Grey bars). Animals receiving only CPAP of 5 cm H2O, with N2 (Black bars) or O2 (Dashed Bars) did not have increased cytokine mRNA. The mRNA was measured from cDNA using RT-PCR and reported as fold increase over the mean of controls. (F) Monocytes in BALF, reported as % of total cells, increased in lambs receiving mechanical ventilation (p<0.05 vs Controls and lambs receiving only CPAP).
white column, controls; black column, CPAP Nitrogen; gray column, VT Nitrogen; diagonal stripes, CPAP Oxygen; vertical stripes, VT Oxygen.
Acute phase response genes
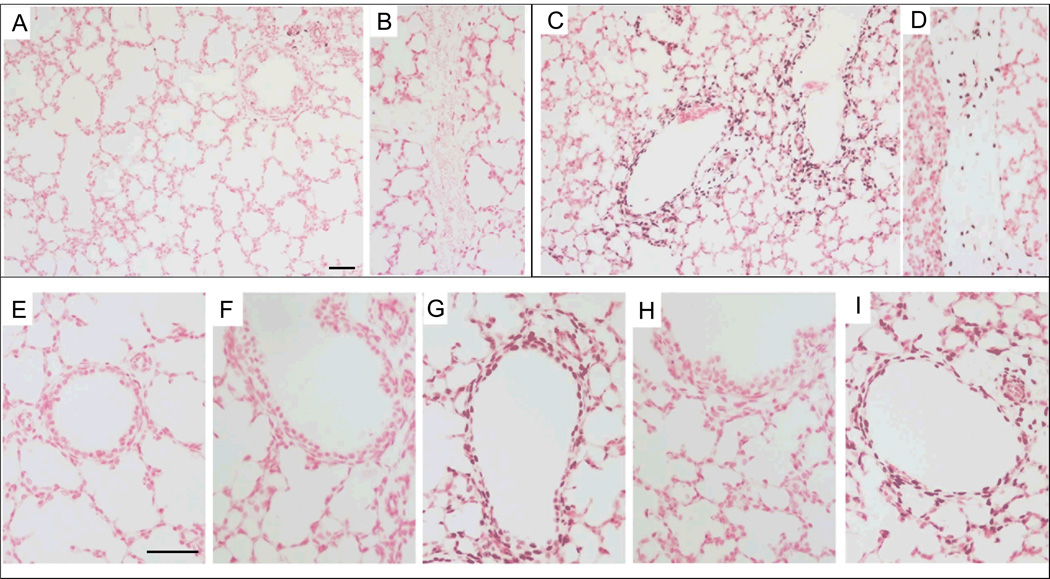
Early growth response protein 1 (Egr-1) activation in control lambs and lambs receiving a PEEP of 5 cm H2O was limited to occasional staining within the blood vessels (Figure 2A, E, F, H). Egr-1 increased in the cells surrounding the smaller airways and the connective tissue with mechanical ventilation (Figure 2C, D) compared to controls (Figure 2A, B), and the pattern of increased expression was not altered by exposure to oxygen (Figure 2E–I). Blinded scoring confirmed increased Egr-1 protein in airways and connective tissue with mechanical ventilation (Table 2). There was little to no Egr-1 activation within the distal lung parenchyma (staining score: 0.13±0.04) (Figure 2C). Although the Egr-1 protein is abundantly present at 2 hours after intervention, the mRNA levels of transcription factors downstream of Egr-1 (PDGFA, FGF2, ALOX5, EGFR) were not altered by ventilation nor was mRNA for the counter regulatory protein NAB2 (Table 2). HSP70 mRNA was localized to the bronchial epithelium of surgical controls and fetal lambs receiving only PEEP, but was lost from the epithelium of lambs receiving mechanical ventilation. HSP70 mRNA was not increased in the airway smooth muscle in lambs receiving ventilation with a VT of 6 mL/kg. HO-1 mRNA from lung tissue did not change compared with the surgical controls (Table 3). HO-1 protein was localized within the cytoplasma of occasional cells within the distal parenchyma, but the distribution or number of cells did not change between intervention groups.
Figure 2. Egr-1 protein increased around bronchioles with mechanical ventilation.
(A–B, E) Control lambs had only occasional Egr-1 staining in the (A) vessels and none was found in (B) intra-lobar connective tissue (20×). (C–D) Egr-1 signal increased with mechanical ventilation with nitrogen (N2) and was localized to cells around bronchioles (C) and in cells within connective tissue (D) (20×). There was minimal staining in the distal epithelium. (E–I) Higher power images of Egr-1 staining. Lambs receiving VT ventilation with N2 (G) and O2 (I) demonstrate increased Egr-1 surrounding small airways. CPAP exposure, with N2 (F) or O2 (H) did not increase staining. Scale bar 50 µm.
Table 2.
Egr-1 protein expression and mRNA for Egr-1 responsive transcription factors
| Egr-1 Protein Expression | ALOX5 | EGFR | FGF2 | PDGFA | NAB2 | |||
|---|---|---|---|---|---|---|---|---|
| +cells/LPF | Bronchioles (Out of 2) |
CT (Out of 2) |
Lung mRNA levels (Fold Increase over Control) |
|||||
| Controls | 8±2 | 0.03±0.03 | 0.4±0.2 | 1.0±0.1 | 1.0±0.1 | 1.0±0.1 | 1.0±0.1 | 1.0±0.1 |
| CPAP - N2 | 13±3 | 0.07±0.03 | 0.5±0.1 | 1.1±0.1 | 0.9±0.1 | 1.3±0.3 | 1.0±0.1 | 1.2±0.1 |
| VT - N2 | 196±19* | 1.86±0.07* | 1.7±0.1* | 0.9±0.1 | 1.0±0.1 | 1.2±0.2 | 1.0±0.1 | 1.4±0.3 |
| CPAP - O2 | 15±2 | 0.08±0.05 | 0.6±0.2 | 0.8±0.1 | 0.9±0.2 | 0.9±0.1 | 0.9±0.1 | 0.7±0.1 |
| VT - O2 | 209±23* | 1.83±0.09* | 1.7±0.1* | 1.0±0.1 | 1.0±0.1 | 1.1±0.1 | 1.0±0.2 | 1.2±0.1 |
p<0.05 vs control and CPAP animals. CT = Connective Tissue
Table 3.
Heme-oxidase 1 and superoxide dismutase 1 in lung
| Lung Tissue | BALF | |||
|---|---|---|---|---|
| HO-1 mRNA | HO-1 protein | SOD1 mRNA | SOD activity | |
| fold increase | (cells / LPF) | fold increase | U/mL | |
| Controls | 1.0±0.12 | 28±6 | 1.0±0.19 | 0.19±0.05 |
| CPAP - N2 | 1.0±0.06 | 50±11 | 1.0±0.08 | 0.31±0.07 |
| VT - N2 | 0.8±0.10 | 59±15 | 0.9±0.04 | 0.22±0.01 |
| CPAP - O2 | 0.9±0.02 | 55±10 | 0.8±0.04 | 0.10±0.04 |
| VT - O2 | 1.0±0.03 | 42±11 | 0.9±0.05 | 0.39±0.08 |
Oxidative response
There were no differences in the inflammatory and acute phase responses when lambs were exposed to 95% oxygen for the resuscitation. SOD1 mRNA from peripheral lung tissue did not change in any of the groups compared with the surgical controls (Table 3). Total SOD activity also did not change in the BALF. Hif1α and Hif2α proteins are expressed in the fetal lung at 129 days in control animals, with staining in the epithelial cells surrounding the bronchioles and in cells with a distribution similar to type II cells. There was no difference in the expression pattern for Hif1α and Hif2α between intervention groups, with or without oxygen, and control animals. Arginase 1 protein also did not change in the epithelium or lung parenchyma with either ventilation or oxygen exposure.
Discussion
We found that, similar to large VT ventilation, ventilation with a VT of 6.2mL/kg for 15 minutes caused increased acute phase response genes and lung inflammation that are localized primarily around the bronchioles. The low overall release of total protein into the BALF and lack of airway epithelial sloughing indicated less damage than previously reported for higher VT of 15 mL/kg5,8. We did not find additional inflammation or markers of oxidative stress when lambs were ventilated for 15 min with 95% oxygen instead of nitrogen.
Ventilation of the preterm lungs with normal VT at birth caused lung inflammation and monocyte recruitment to the bronchoalveolar space, which may have been a response to the cytokines MCP-1, MCP-2, and IL-6. Our previous fetal sheep experiments with larger VT demonstrated increased mRNA for IL-1β, MCP-1, IL-8, and IL-6 at 45 minutes after injury, whereas by 3 hours after injury the IL-6 and IL-8 mRNA levels had begun to fall and returned to baseline by 24 hr8–10. In the current study, we did not find an additive effect of oxygen on pro-inflammatory cytokines. We previously found a small, 2-fold increase in lung IL-1β mRNA with 100% oxygen use in near-term lambs (140d GA) ventilated with VT of 9 mL/kg for 3 hours compared to lambs ventilated with 21% oxygen, but IL-6 and IL-8 did not change with oxygen in those lambs4. The addition of 100% oxygen for 30 minutes to newborn rats ventilated with large VT of 25 or 40 mL/kg caused moderate and inconsistent changes in the mRNA expression of pro-inflammatory cytokines19. Newborn rats exposed to low VT ventilation (3.5 mL/kg) for 8 hours had increased mRNA for IL-6 and CXCL2 (homolog to IL-8), but IL-1β did not change20. The addition of 50% oxygen to low VT ventilation caused a modest increase in the IL-6 and CXCL-2 mRNA levels20. Due to their consistent increases with ventilation, IL-6 or MCP-1 may be appropriate targets for pharmacologic interventions. It should be noted though that our previous attempts to inhibit inflammation at birth with corticosteroids or specific cytokine inhibitors have been unsuccessful21,22. Our data demonstrate that inflammation is activated by low VT ventilation at birth and may be inevitable in the setting of positive pressure ventilation.
Ventilation at birth with a low VT caused the activation of Egr-1, an acute phase gene responsive to mechanical stretch23. Egr-1 is multifunctional and can activate genes ranging from growth factors to coagulation cascades24. Egr-1 plays an important role in the initiation of inflammation and Egr-1 deficient mice are more resistant to ventilator-induced lung injury25. Egr-1 signal was intense surrounding the bronchioles and in the connective tissue, suggesting airway stretch. Unlike with larger VT ventilation or ventilation of newborn lambs after birth, there was little Egr-1 activation in the peripheral lung tissue suggesting lung injury was limited to the airways5,21. Egr-1 can be activated by hyperoxia, but we did not find activation in lambs receiving only oxygen without VT ventilation26. Although the Egr-1 protein showed nuclear staining, there were surprisingly no changes in the mRNA for downstream targets of Egr-1 protein (PDGFA, FGF2, ALOX, and EGFR). We also did not find changes in the mRNA for NAB2, the major counter-regulatory protein for Egr-124. The lack of these responses could be due to the timing of the intervention or it could also result from the overall immaturity of the fetal lung. We also did not find differences in HO-1, which responds like many acute phase response elements and is inducible by a variety of stimuli from LPS to hyperoxia27. We did find a loss of HSP70 mRNA in the epithelium, similar to our previous findings with larger VT ventilation, giving additional support to airway stretch during initiation with normal VT5.
The antioxidant enzymes normally increase 2 to 3-fold in the final 10 to 15% of gestation in many species and parallels the development of the surfactant system28. Lambs born at 129 days GA are just beginning lung maturation and surfactant production. Fetal sheep at 125 days GA can generate a modest increase in some markers of oxidative stress (protein carbonyls and myeloperoxidase activity) following exposure to 7 days of intra-amniotic (IA) LPS29. Since the inflammatory system is immature in preterm lambs, the lambs may not have generated a large response to the oxidative stress30. Although we only measured jugular PvO2 levels at the end of resuscitation, the lack of any increase would suggest maintenance of fetal circulation and shunting across the PDA, thus limiting any systemic increase in PaO2.
The majority of animal models showing oxidative stress responses from resuscitation use acute hypoxia prior to ventilation with oxygen or room-air31. The lack of increased oxidative responses in our study may result from the lack of preceding hypoxia/hypoperfusion. Resuscitation of newborn piglets with 100% oxygen after acute hypoxia increased MMP-2 activity in the brain compared to 21% resuscitation, but animals exposed to 30 min of 100% oxygen without previous hypoxia did not demonstrate these changes32. Similar to our findings on SOD, there were no differences found in anti-oxidant enzymes (SOD, catalase, or glutathione peroxidase) between term lambs ventilated with oxygen or air33.
We did not find changes in oxygen sensitive proteins (Hif1α, Hif2α, or Arginase 1) with a brief oxygen exposure. Hif1α and Hif2α are expressed in fetal pulmonary epithelial, smooth muscle, and endothelial cells34, and help regulate the expression of vascular endothelial growth factor (VEGF). Prolonged ventilation of premature baboons leads to decreased Hif-1α expression35. Ventilation of surfactant deficient very preterm lambs (GA 115d) for 4 hours with high oxygen concentrations decreased Hif1α and Hif2α protein and decreased VEGF mRNA36. Although in our study Hif1α and Hif2α were present in the fetal lung, we did not find consistent decreases with this brief period of normal ventilation or with the addition of oxygen. We previously have not seen differences in VEGF mRNA or VEGFR protein in lambs exposed to short periods of ventilation5. Arginase 1 competes with nitric oxide synthase for L-arginine and activation impairs airway muscle relaxation. In newborn rat pups exposed to 50% oxygen for 7 days, Arginase 1 is increased in the airway epithelium37. Short-term exposure to 95% oxygen during fetal ventilation did not affect Arginase 1 expression. The lack of changes in markers of oxidative stress and oxygen sensitive proteins suggests that short-term exposure of oxygen to preterm infants may not increase injury caused by ventilation.
There are several limitations of the study that should be noted. As with many large animal studies, the number of animals per group was only 5 to 6, so small differences between groups will not be detected. Although we focused are evaluations on inflammation, acute phase genes and some oxidative markers, other genes may have changed within the model. This fetal sheep model, which maintains placental blood flow and ductal shunting, may have limited the systemic effects of oxygen on the lungs, as the PvO2 did not change. We used 5% CO2 in these animals to maintain mild hypercapnia, and our PvCO2 at end of 15 min ventilation (56±4) was similar to the initial PvCO2 (58±3). The exposure time of 15 minutes may also not have been long enough to cause direct oxidant injury to the epithelial cells. We used high peak inspiratory pressures of 40 cm H2O and no PEEP to generate VT of only 6 to 7 mL/kg. We recently demonstrated that PEEP use during resuscitation with high VT decreases pro-inflammatory cytokines 2-fold9. PEEP use with normal VT at birth might lessen, but not abolished, the negative effects of ventilation.
Conclusions
Ventilation of the preterm lung at birth with low VT leads to airway stretch and lung inflammation. The degree of lung injury is less than what is seen with higher VT ventilation (15 mL/kg), and there is minimal protein leak and airway epithelial disruption. In these lambs, oxygen exposure did not alter the inflammatory response to ventilation. Our results support the avoidance of positive-pressure ventilation, even low VT, when possible in preterm infants at birth. Although clinicians should attempt to limit oxygen exposure when possible, the use of supplemental oxygen as needed in preterm infants at birth2 is supported by a lack of short-term effects of oxygen.
Methods
The investigations were approved by the Animal Ethics Committees of the Western Australian Department of Agriculture and Cincinnati Children's Hospital Medical Center.
Maternal Anesthesia and Surgery
Date-mated Merino ewes with singletons or twins at 129±1 days gestation (early alveolarization stage, term is 150 days in sheep) received ketamine (5 mg/kg) IM and xylazine (0.5 mg/kg) IM for induction of general anesthesia prior to halothane (1–2%) anesthesia. Using aseptic techniques, a midline hysterotomy was performed. The head and chest of the preterm lamb were exposed and the lamb was given 10 mg/kg ketamine IM.
Fetal Ventilation Procedure
The fetal lamb was intubated with a cuffed tube and fetal lung fluid was removed with a catheter using gentle suction8. Each lamb was randomly assigned to four groups (n=5–6/group) based on the type of ventilation and the inspired gas mixture. Lambs received either: 1) Mechanical ventilation for 15 min with a rate 30 breaths/min, inspiratory time 1 s, PEEP 0 cm H2O (Bourns BP200 time-cycled, pressure-limited infant ventilator with 12L/min flow, Bear Medical Systems, Riverside, CA USA) or 2) CPAP of 5 cm H2O with no VT (under these conditions the fetal lambs do not breathe spontaneously). Our target VT was 6 to 7 mL/kg with a maximal PIP limited to 40 cm H2O. An in-line flow sensor (Florian Infant Graphics Monitor, Acutronic Medical Systems, Hirzel, Switzerland) was used to continuously measure VT and pressures. Lambs were further randomized to one of two heated, humidified gases: a) oxygen – 95%O2/5%CO2 or b) nitrogen – 95%N2/5%CO2. After the 15 minute intervention, the fetus was returned to the uterus, the uterus and maternal abdomen were closed, and the ewe recovered from general anesthesia. Two hours after the intervention, the ewe was humanely killed, followed by immediate surgical delivery of the fetus, which was euthanized with pentobarbital (100 mg/kg IV). Jugular venous blood gases were measured before the ventilation intervention and after the intervention. Cord blood gases were measured at delivery.
Lung Processing and BAL Analysis
At autopsy, a deflation pressure-volume curve was measured after air inflation to 40 cm H2O pressure38. Bronchoalveolar lavage fluid (BALF) of the left lung was collected by repetitive saline lavage. BALF was used for measurement of total protein and differential cell counts39. Total superoxide dismutase (SOD) activity was measured in BALF with a colorimetric activity assay (Arbor Assays, Ann Arbor, Michigan, USA). Tissues from the right lower lung and tracheal epithelial scrapings were snap frozen. The right upper lobe was inflation fixed with 10% formalin at 30 cm H2O and then paraffin embedded40.
Quantitative RT-PCR
Messenger RNA was extracted from tissue from the right lung and trachea5. Complimentary DNA was made using Verso cDNA kit (Thermoscientific, Walthram, MA, USA). Custom Taqman gene primer (Applied Biosystems, Carlsbad, CA USA) were designed from ovine sequences for arachidonate 5-lipoxygenase (ALOX5), early growth response protein 1(Egr-1), Epithelial growth factor receptor (EGFR), Fibroblast growth factor 2(FGF2), heme oxygenase 1(HO-1), interleukin (IL)-1β, IL-6, IL-8, Monocyte chemotactic protein (MCP)-1, MCP-2, NGFI-A binding protein 2(NAB2), platelet derived growth factor A (PDGFA), and SOD1. Quantitative RT-PCR was performed on a 7300 RT-PCR machine and software (Applied Biosystems). 18S primers were used for internal loading control, and results are reported relative to the mean for control animals.
Immunohistochemistry/In situ Hybridization
Immunostaining protocols used 5 µm paraffin sections of formalin-fixed tissues8. Primary antibodies included anti-human Early response protein-1 (Egr-1) (Santa Cruz Biotechnology, Santa Cruz, CA USA, 1:250), Hifα (internal, 1:500), Hif2α (internal, 1:1000), CD3 (Dakocytomation, Glostrup, Denmark, 1:100) or Argniase 1 (Abcam, Cambridge, MA USA, 1:150). In situ localization of mRNA was performed with digoxigenin-labeled anti-sense and sense sheep riboprobes for HSP70 (Roche, Indianapolis, IN USA)5. Random, blinded H&E stained sections (×10/slide) on were scored (0 to 2) based on hemorrhage, inflammatory cells, and epithelial sloughing (total 6 pionts)5. Random sections (×10/slide) of blinded Egr-1 slides were scored (0 to 2 for staining around the airways, connective tissue, and peripheral lung tissue). Egr-1 positive and HO-1 positive cells were counted per low power (10×) field.
Data Analysis and Statistics
Results are shown as mean (SEM). Statistics were analyzed using InStat (GraphPad, USA) with Student’s t-test and Mann-Whitney non-parametric tests as appropriate. Significance was accepted as p<0.05.
Acknowledgements
We thank Megan McAuliffe for her assistance in the lab.
Grant Support: This work was supported by grant HD-12714 from the National Institute of Child Health and Development, K08 HL097085 (NHH), and the Women’s and Infants Research Foundation.
Footnotes
Author contribution: Conception and design: TMJ, NH, AJ; Animal procedures:TJM, IN; Analysis: NH, AJ: Manuscript preparation: NH, AJ
References
- 1.Jobe AH, Hillman N, Polglase G, Kramer BW, Kallapur S, Pillow J. Injury and inflammation from resuscitation of the preterm infant. Neonatology. 2008;94:190–196. doi: 10.1159/000143721. [DOI] [PubMed] [Google Scholar]
- 2.Kattwinkel J, Perlman JM, Aziz K, et al. Neonatal resuscitation: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Pediatrics. 2010;126:e1400–e1413. doi: 10.1542/peds.2010-2972E. [DOI] [PubMed] [Google Scholar]
- 3.Fanaroff AA, Stoll BJ, Wright LL, et al. Trends in neonatal morbidity and mortality for very low birthweight infants. Am J Obstet Gynecol. 2007;196:147e1–147e8. doi: 10.1016/j.ajog.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 4.Pillow JJ, Hillman NH, Polglase GR, et al. Oxygen, temperature and humidity of inspired gases and their influences on airway and lung tissue in near-term lambs. Intensive Care Med. 2009;35:2157–2163. doi: 10.1007/s00134-009-1624-z. [DOI] [PubMed] [Google Scholar]
- 5.Hillman NH, Kallapur SG, Pillow JJ, et al. Airway injury from initiating ventilation in preterm sheep. Pediatr Res. 2010;67:60–65. doi: 10.1203/PDR.0b013e3181c1b09e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schmolzer GM, Kamlin OC, O'Donnell CP, Dawson JA, Morley CJ, Davis PG. Assessment of tidal volume and gas leak during mask ventilation of preterm infants in the delivery room. Arch Dis Child Fetal Neonatal Ed. 2010;95:F393–F397. doi: 10.1136/adc.2009.174003. [DOI] [PubMed] [Google Scholar]
- 7.Resende JG, Menezes CG, Paula AM, et al. Evaluation of peak inspiratory pressure and respiratory rate during ventilation of an infant lung model with a self-inflating bag. J Pediatr (Rio J) 2006;82:359–364. doi: 10.2223/JPED.1524. [DOI] [PubMed] [Google Scholar]
- 8.Hillman NH, Moss TJ, Kallapur SG, et al. Brief, large tidal volume ventilation initiates lung injury and a systemic response in fetal sheep. Am J Respir Crit Care Med. 2007;176:575–581. doi: 10.1164/rccm.200701-051OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hillman NH, Nitsos I, Berry C, Pillow JJ, Kallapur SG, Jobe AH. Positive end-expiratory pressure and surfactant decrease lung injury during initiation of ventilation in fetal sheep. Am J Physiol Lung Cell Mol Physiol. 2011;301:L712–L720. doi: 10.1152/ajplung.00157.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hillman NH, Polglase GR, Jane Pillow J, Saito M, Kallapur SG, Jobe AH. Inflammation and lung maturation from stretch injury in preterm fetal sheep. Am J Physiol Lung Cell Mol Physiol. 2011;300:L232–L241. doi: 10.1152/ajplung.00294.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wada K, Jobe AH, Ikegami M. Tidal volume effects on surfactant treatment responses with the initiation of ventilation in preterm lambs. J Appl Physiol. 1997;83:1054–1061. doi: 10.1152/jappl.1997.83.4.1054. [DOI] [PubMed] [Google Scholar]
- 12.Saugstad OD, Ramji S, Soll RF, Vento M. Resuscitation of newborn infants with 21% or 100% oxygen: an updated systematic review and meta-analysis. Neonatology. 2008;94:176–182. doi: 10.1159/000143397. [DOI] [PubMed] [Google Scholar]
- 13.Dawson JA, Kamlin CO, Wong C, et al. Oxygen saturation and heart rate during delivery room resuscitation of infants<30 weeks' gestation with air or 100% oxygen. Arch Dis Child Fetal Neonatal Ed. 2009;94:F87–F91. doi: 10.1136/adc.2008.141341. [DOI] [PubMed] [Google Scholar]
- 14.Escrig R, Arruza L, Izquierdo I, et al. Achievement of targeted saturation values in extremely low gestational age neonates resuscitated with low or high oxygen concentrations: a prospective, randomized trial. Pediatrics. 2008;121:875–881. doi: 10.1542/peds.2007-1984. [DOI] [PubMed] [Google Scholar]
- 15.Vento M, Moro M, Escrig R, et al. Preterm resuscitation with low oxygen causes less oxidative stress, inflammation, and chronic lung disease. Pediatrics. 2009;124:e439–e449. doi: 10.1542/peds.2009-0434. [DOI] [PubMed] [Google Scholar]
- 16.Chambellan A, Cruickshank PJ, McKenzie P, et al. Gene expression profile of human airway epithelium induced by hyperoxia in vivo. Am J Respir Cell Mol Biol. 2006;35:424–435. doi: 10.1165/rcmb.2005-0251OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brew N, Hooper SB, Allison BJ, Wallace MJ, Harding R. Injury and repair in the very immature lung following brief mechanical ventilation. Am J Physiol Lung Cell Mol Physiol. 2011;301:L917–L926. doi: 10.1152/ajplung.00207.2011. [DOI] [PubMed] [Google Scholar]
- 18.Mulrooney N, Champion Z, Moss TJ, Nitsos I, Ikegami M, Jobe AH. Surfactant and Physiological Responses of Preterm Lambs to Continuous Positive Airway Pressure. Am J Respir Crit Care Med. 2005;171:1–6. doi: 10.1164/rccm.200406-774OC. [DOI] [PubMed] [Google Scholar]
- 19.Copland IB, Martinez F, Kavanagh BP, et al. High tidal volume ventilation causes different inflammatory responses in newborn versus adult lung. Am J Respir Crit Care Med. 2004;169:739–748. doi: 10.1164/rccm.200310-1417OC. [DOI] [PubMed] [Google Scholar]
- 20.Kroon AA, Wang J, Huang Z, Cao L, Kuliszewski M, Post M. Inflammatory response to oxygen and endotoxin in newborn rat lung ventilated with low tidal volume. Pediatr Res. 2010;68:63–69. doi: 10.1203/PDR.0b013e3181e17caa. [DOI] [PubMed] [Google Scholar]
- 21.Hillman N, Kallapur SG, Pillow JJ, Polglase GR, Nitsos I, Ikegami M, Jobe AH. Inhibitors of inflammation and endogenous surfactant pool size as modulators of lung injury with initiation of ventilation in preterm sheep. Resp Research. 2010;11:1–8. doi: 10.1186/1465-9921-11-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hillman NH, Pillow JJ, Ball MK, Polglase GR, Kallapur SG, Jobe AH. Antenatal and postnatal corticosteroid and resuscitation induced lung injury in preterm sheep. Respir Res. 2009;10:124. doi: 10.1186/1465-9921-10-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Copland IB, Post M. Stretch-activated signaling pathways responsible for early response gene expression in fetal lung epithelial cells. J Cell Physiol. 2007;210:133–143. doi: 10.1002/jcp.20840. [DOI] [PubMed] [Google Scholar]
- 24.Silverman ES, Collins T. Pathways of Egr-1-mediated gene transcription in vascular biology. Am J Pathol. 1999;154:665–670. doi: 10.1016/S0002-9440(10)65312-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoetzel A, Dolinay T, Vallbracht S, et al. Carbon monoxide protects against ventilator-induced lung injury via PPAR-gamma and inhibition of Egr-1. Am J Respir Crit Care Med. 2008;177:1223–1232. doi: 10.1164/rccm.200708-1265OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones N, Agani FH. Hyperoxia induces Egr-1 expression through activation of extracellular signal-regulated kinase 1/2 pathway. J Cell Physiol. 2003;196:326–333. doi: 10.1002/jcp.10308. [DOI] [PubMed] [Google Scholar]
- 27.Choi AM, Alam J. Heme oxygenase-1: function, regulation, and implication of a novel stress-inducible protein in oxidant-induced lung injury. Am J Respir Cell Mol Biol. 1996;15:9–19. doi: 10.1165/ajrcmb.15.1.8679227. [DOI] [PubMed] [Google Scholar]
- 28.Frank L, Sosenko IR. Prenatal development of lung antioxidant enzymes in four species. J Pediatr. 1987;110:106–110. doi: 10.1016/s0022-3476(87)80300-1. [DOI] [PubMed] [Google Scholar]
- 29.Cheah FC, Jobe AH, Moss TJ, Newnham JP, Kallapur SG. Oxidative stress in fetal lambs exposed to intra-amniotic endotoxin in a chorioamnionitis model. Pediatr Res. 2008;63:274–279. doi: 10.1203/PDR.0b013e31815f653b. [DOI] [PubMed] [Google Scholar]
- 30.Kramer BW, Jobe AH, Ikegami M. Monocyte function in preterm, term, and adult sheep. Pediatr Res. 2003;54:52–57. doi: 10.1203/01.PDR.0000066621.11877.33. [DOI] [PubMed] [Google Scholar]
- 31.Gitto E, Pellegrino S, D'Arrigo S, Barberi I, Reiter RJ. Oxidative stress in resuscitation and in ventilation of newborns. Eur Respir J. 2009;34:1461–1469. doi: 10.1183/09031936.00032809. [DOI] [PubMed] [Google Scholar]
- 32.Solberg R, Loberg EM, Andresen JH, et al. Resuscitation of newborn piglets. short-term influence of FiO2 on matrix metalloproteinases, caspase-3 and BDNF. PLoS One. 2010;5:e14261. doi: 10.1371/journal.pone.0014261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kumar VH, Patel A, Swartz DD, et al. Exposure to supplemental oxygen and its effects on oxidative stress and antioxidant enzyme activity in term newborn lambs. Pediatr Res. 2010;67:66–71. doi: 10.1203/PDR.0b013e3181bf587f. [DOI] [PubMed] [Google Scholar]
- 34.Yu AY, Frid MG, Shimoda LA, Wiener CM, Stenmark K, Semenza GL. Temporal, spatial, and oxygen-regulated expression of hypoxia-inducible factor-1 in the lung. Am J Physiol. 1998;275:L818–L826. doi: 10.1152/ajplung.1998.275.4.L818. [DOI] [PubMed] [Google Scholar]
- 35.Asikainen TM, Ahmad A, Schneider BK, White CW. Effect of preterm birth on hypoxia-inducible factors and vascular endothelial growth factor in primate lungs. Pediatr Pulmonol. 2005;40:538–546. doi: 10.1002/ppul.20321. [DOI] [PubMed] [Google Scholar]
- 36.Grover TR, Asikainen TM, Kinsella JP, Abman SH, White CW. Hypoxia-inducible factors HIF-1alpha and HIF-2alpha are decreased in an experimental model of severe respiratory distress syndrome in preterm lambs. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1345–L1351. doi: 10.1152/ajplung.00372.2006. [DOI] [PubMed] [Google Scholar]
- 37.Ali NK, Jafri A, Sopi RB, Prakash YS, Martin RJ, Zaidi SI. Role of Arginase in Impairing Relaxation of Lung Parenchyma of Hyperoxia-Exposed Neonatal Rats. Neonatology. 2011;101:106–115. doi: 10.1159/000329540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jobe A, Kramer BW, Moss TJ, Newnham J, Ikegami M. Decreased indicators of lung injury with continuous positive expiratory pressure in preterm lambs. Pediatric Research. 2002;52:387–392. doi: 10.1203/00006450-200209000-00014. [DOI] [PubMed] [Google Scholar]
- 39.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 40.Kramer BW, Moss TJ, Willet KE, et al. Dose and time response after intraamniotic endotoxin in preterm lambs. Am J Respir Crit Care Med. 2001;164:982–988. doi: 10.1164/ajrccm.164.6.2103061. [DOI] [PubMed] [Google Scholar]