Summary
A primary cilium is a solitary slender non-motile protuberance of structured microtubules (9+0) enclosed by plasma membrane1. Housing components of the cell division apparatus between cell divisions, they also serve as specialized compartments for calcium signaling2 and Hedgehog (Hh) signaling pathways3. Specialized sensory cilia such as retinal photoreceptors and olfactory cilia employ diverse ion channels4-7. An ion current has been measured from primary cilia of kidney cells8 but the responsible genes have not been identified. The polycystin proteins (PC, PKD), identified in linkage studies of polycystic kidney disease9, are candidate channels divided into two structural classes: 11-transmembrane (TM) proteins (PKD1, PKD1-L1 and PKD1-L2) remarkable for a large extracellular N-terminus of putative cell adhesion domains and a GPCR proteolytic site, and the 6-TM channel proteins (PKD2, PKD2-L1, PKD2-L2; TRPPs). Evidence suggests that the PKD1s associate with the PKD2s via coiled-coil domains10-12. Here, we employ a transgenic mouse in which only cilia express a fluorophore and employ it to directly record from primary cilia and demonstrate that PKD1-L1 and PKD2-L1 form ion channels at high densities in several cell types. In conjunction with the companion manuscript2, we show that the PKD1-L1/PKD2-L1 heteromeric channel establishes the cilia as a unique calcium compartment within cells that modulates established Hedgehog pathways.
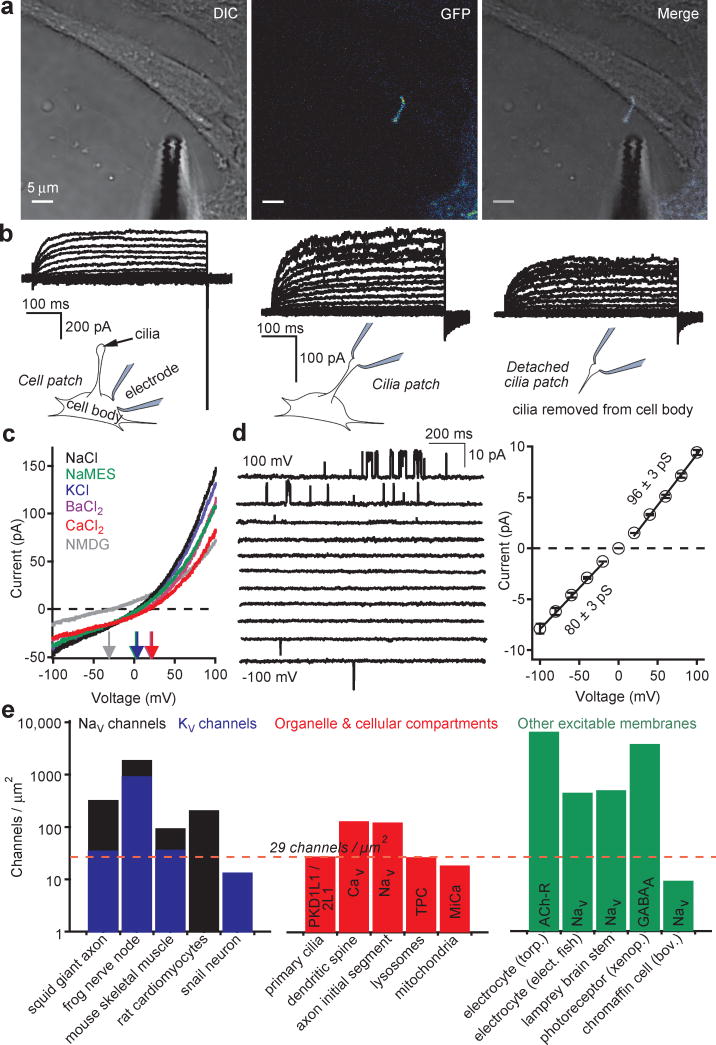
Patch clamp of primary cilia is challenging due to their small dimensions (≈ 0.2-0.5 μm in width, 1-12 μm in length), making them difficult to identify in vivo. Employing a human retina pigmented epithelium cell line stably expressing the cilia-specific EGFP-tagged Smoothened gene (hRPE Smo-EGFP), the cilia could be visualized under confocal fluorescence microscopy and recorded using the method we describe here, whole-cilia patch clamp (Fig. 1a, Extended Data Fig. 1a and Supplementary Video 1 ). After establishing >16 GΩ seals and rupturing the cilia membrane, we recorded a surprisingly large, outwardly-rectifying, non-inactivating current (Icilia). Importantly, Icilia was recorded from cilia attached or detached from the cell body (Fig. 1b, c and Supplementary Video 2). Current density measured in the detached cilia patch was 56-fold higher than that measured from the hRPE cell body (Methods). These measurements indicate that the primary cilium is partly insulated from the cell body by the structures at the cell-cilia junction (Extended Data Fig. 1a). The outwardly rectifying current was cation-nonselective (Fig. 1d) with relative permeabilities of Ca2+ ≈ Ba2+ > Na+ ≈ K+ > NMDG (Extended Data Fig. 1b).
Figure 1. A calcium-selective ion channel is richly expressed in primary cilia.
(a) Confocal image of an hRPE Smo-EGFP cell and patch clamp electrode. (b) Whole-cell leak-subtracted currents elicited by 1 s depolarizing pulses from -100 to 100 mV in +5 mV increments recorded from the cell body, primary cilia and an excised primary cilia (recorded from the same cilium). (c) Whole-cell currents activated by ramp voltage protocols from -100 to +100 mV measured from the primary cilia where extracellular Na+-based saline was replaced by the cation indicated. (d) Single channel currents activated by 1.5 s depolarizations to the indicated potentials (left) and average current amplitudes (right; ± SEM, n = 8 cilia). (e) Estimated endogenous cilia ion channel densities compared to those from other biological preparations17,27-30.
Extended Data Figure 1. Ion selectivity and pharmacology of ciliary hRPE current.
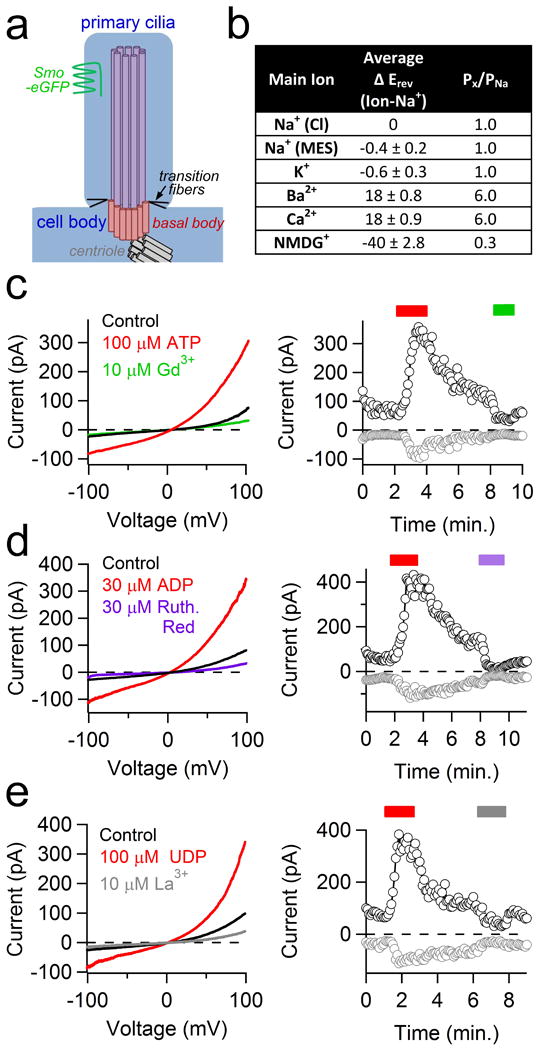
(a) Diagram of the primary cilia depicting the EGFP-labeled Smoothened protein (green), transition fibers (black line), 9+0 axoneme (purple), basal body (pink) and centriole (gray). (b) Table listing the average reversal potential change relative to the standard Na+-based extracellular solution (Average ΔErev) and the estimated relative permeability (Px/PNa; ± SEM, n= 4 cilia). (c-e) left, Representative currents from control (black traces), activation by 100 μM ATP, 30 μM ADP, or 10 μM UDP (red traces) and block by 10 μM Gd3+, 30 μM Ruthenium Red and 10 μM La3+ (green, violet and grey traces respectively). Right, corresponding time course of peak current recorded at -100 mV (gray circles) and +100 mV (black circles).
Consistent with the whole-cilia currents, single channel amplitudes were outwardly rectifying (Fig. 1e) and mean open times substantially longer at more depolarized potentials. Extracellular uridine and adenosine phosphates (UDP, ADP, ATP) activated the ciliary current in perforated-cilia recordings, while the non-selective antagonists Gd3+ and ruthenium red blocked it (Extended Data Fig. 1c-e). Several cell-permeable calmodulin antagonists also activated the conductance. Based on the dimensions of the cilia, we estimate the average membrane surface area to be ∼ 6.3 μm2 (∼0.063 pF). Assuming the entire outward rectifying current is carried by the 96 pS conductance, we estimate the primary cilia channel density to be 29 ± 2 channels/μm2, similar to endogenous channel densities calculated from excitable tissue plasma membranes and larger than those found in intracellular compartments (Fig. 1f). Thus, the primary cilium is richly populated with Ca2+-permeant, relatively nonselective cation channels that enable a much higher dynamic range of ciliary [Ca2+] compared to the cytoplasm.
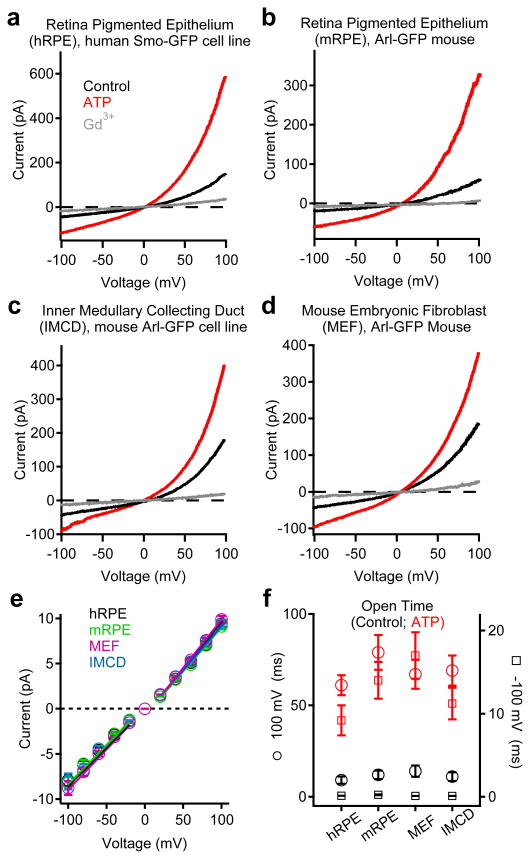
We generated a transgenic mouse expressing the cilia-specific Arl13B gene C-terminally tagged with EGFP (Arl13B-EGFPtg mouse)2 and isolated primary cells from mouse RPE (mRPE) and embryonic fibroblasts (MEFs). Primary cilia currents from these primary cells were outwardly rectifying with the same conductance and pharmacological properties as observed in the cilia from the human RPE cell line (Fig. 2; Extended Data Fig. 2). In addition, ICilia was observed in the cilia of a human kidney-derived inner medullary collecting duct cell line (IMCD stably expressing Arl-EGFP; Fig. 2c, Extended Data Fig. 2d). Ciliary single channel conductances were identical in all 4 cell types (Fig 2e), and activated by extracellular ATP and blocked by Gd3+ in perforated patch recordings. ATP addition to the bath significantly increased the probability of channel opening (Po) and mean open times (5-7 fold, Fig. 2f, Extended Data Fig. 2e). Since these were in the ‘on-cilia’ patch configuration (bath-applied ATP), we reasoned that ATP binds a G-protein coupled purinergic receptor to initiate activation of the channels in the patch. Thus, ICilia is a common feature of many cell types.
Figure 2. Primary cilia currents measured from four different cell types.
Averaged cilia current traces in control and bath-applied 100 μM ATP or 10 μM Gd3+ from: (a) human RPE cell line stably expressing Smoothened-EGFP; (b) Primary mRPE cells from the Arl13B-EGFPtg mouse; (c) Kidney IMCD cell line stably expressing Arl-EGFP and (d) Primary embryonic fibroblasts from the Arl13B-EGFPtg mouse. (e) Average single channel current/voltage relation. The slope is used to estimate conductance (± SEM, n = 4-7 cilia). (f) Average open times in the presence and absence of ATP at -100 and +100 mV potentials measured from the cilia of RPE Smo-EGFP cells (± SEM, n = 6 cilia).
Extended Data Figure 2. ATP indirectly activates the cilia conductance from four different cell types.
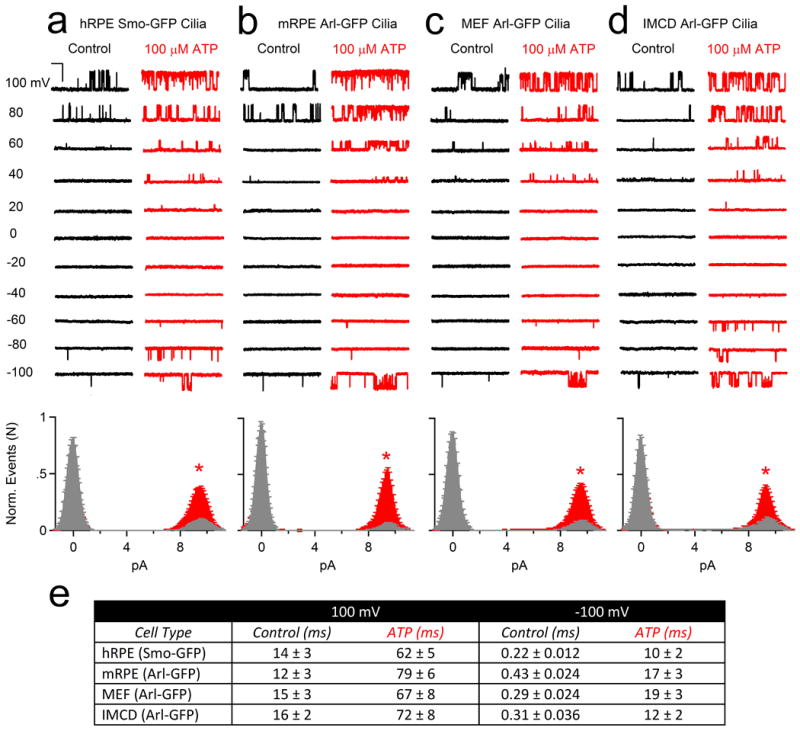
Top, Single channel currents activated by 1.5 s depolarizations to the indicated potentials in control (black traces) and 100 μM extracellular ATP (red traces) recorded from primary cilia derived from (a) human RPE Smo-GFP cell lines, (b) mouse RPE Arl-GFP primary cells, (c) mouse MEF Arl-GFP primary cells, (d) mouse kidney IMCD Arl-EGFP cells (scale = 10 pA and 200 ms). Bottom, corresponding open probability histograms measured in control (grey) and in the presence of 100 μM ATP (red; ± SEM, n = 4-6 cilia, asterisks indicates P < 0.005). (e) Average open dwell times measured from the cilia of these four cell types in control and ATP conditions.
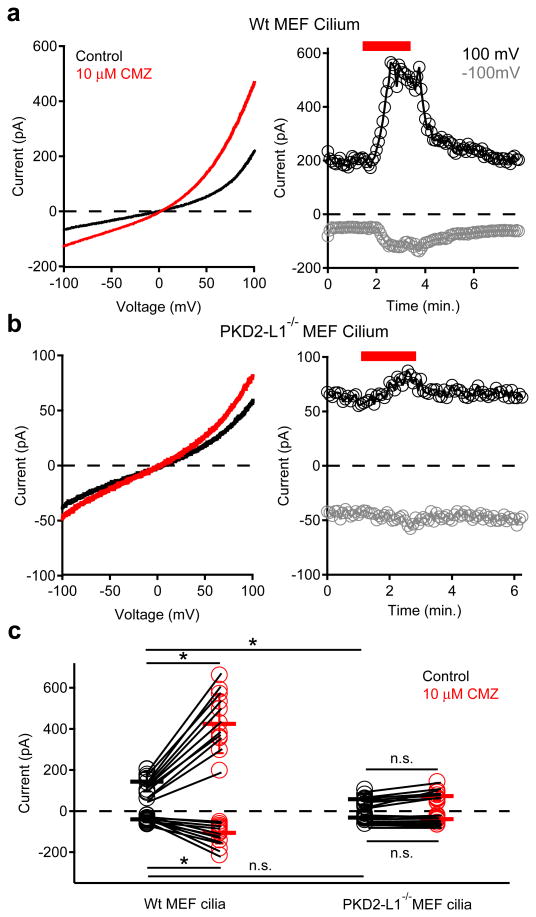
Analysis of hRPE transcripts confirmed expression of several purported ciliary channels13-16 including Tmc7, TRPV4, and PKD1, PKD2, PKD1-L1 and PKD2-L1 (Extended Data Fig. 3a). Only siRNAs specific for PKD1-L1 and PKD2-L1 reduced both inward and outward currents (Extended Data Fig. 3c-d). These results suggest that ICilia is conducted by either PKD1-L1 or PKD2-L1 independently, or together as a heteromeric ion channel. To verify ICilia channel proteins, we patch clamped cilia of homozygous PKD2-L1 knockout (PKD2-L1-/-) crossed with Arl13B-EGFP mice. The much-reduced PKD2-L1-/- MEF ciliary current was linear and failed to activate when stimulated by calmidazolium (Fig. 3b, c). These data establish that PKD2-L1 is a component of ICilia.
Extended Data Figure 3. Anti-PKD1-L1 and -PKD2-L1 siRNA treatment attenuates the RPE ciliary current.
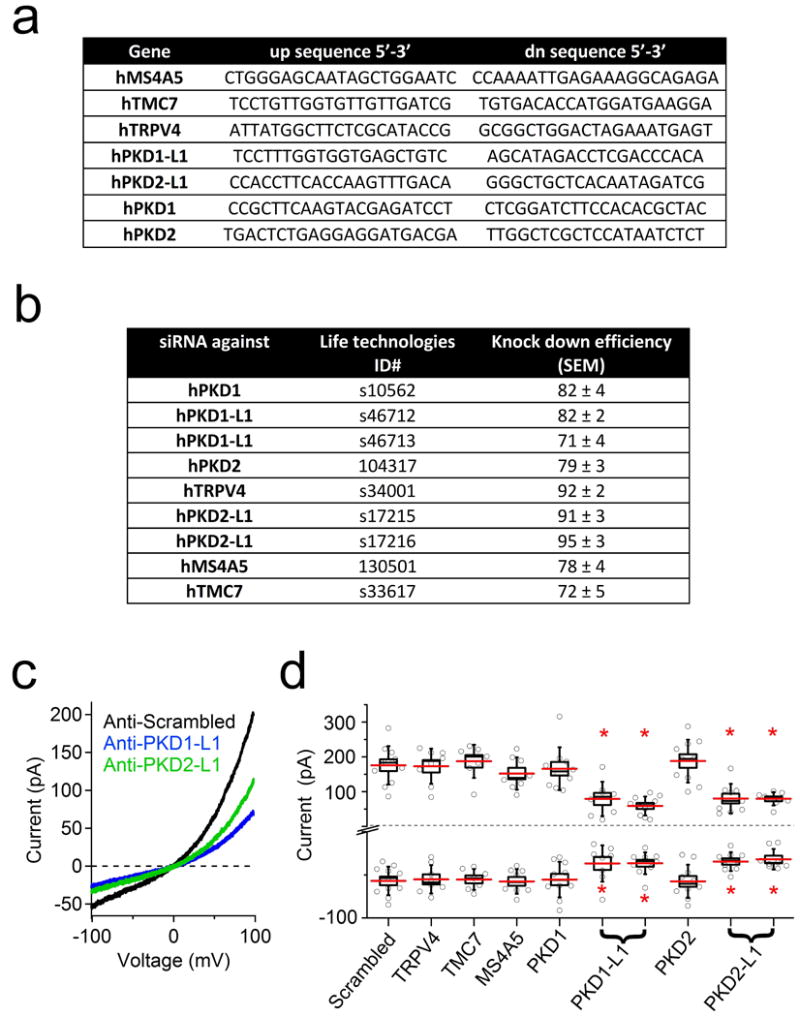
(a) Table of primers used to detect transcript levels present in human RPE cells. (b) Table of siRNAs and their knockdown efficiencies used to identify channel candidates. (c) Example ciliary current measured from cells treated with siRNAs specific for PKD1-L1 or PKD2-L1. (c) Box (± SEM) and whisker (± SD) plots of cilia total outward (+100 mV) and inward (-100 mV) current measured 72 h after double-siRNA treatment. PKD-L mRNAs were targeted by two siRNAs specific for two different regions of the target transcript. Averages are indicated by the red lines. Student's t-test P values comparing treatment groups to scrambled siRNA: * denotes P-value < 0.05; n = 8-12 cilia.
Figure 3. MEF primary cilium currents compared from wt and PKD2-L1 null animals.
Left; Cilium currents recorded from MEFs isolated from (a) wt and (b) PKD2-L1-/- animals. Currents were elicited by a series of ramps from -100 to +100 mV in control conditions (black traces) or in the presence of 10 μM calmidazolium (CMZ, red trace). Right; Resulting current amplitudes (-100 mV, grey circles; +100 mV, black circles) plotted as a function of time. Red bar indicates application of extracellular 10 μM CMZ. (c) Scatter and whisker (± SD) plots of current magnitudes at +100 mV and -100 mV from MEF cilia. Individual cilia are represented as connected circles in control (black) and after calmidazolium (red). Averages are indicated by the dark horizontal lines. Student's t-test results: * denotes P-value < 0.05; n.s. denotes P-value > 0.05; n = 9-11 cilia.
Immunopreciptation of Flag- or HA-tagged PKD1-L1 and PKD2-L1 demonstrated that PKD1-L1 and PKD2-L1 interact (Extended Data Fig. 4a). In whole-cell patch clamp of HEK-293 cells transiently transfected with members of the PKD family, only PKD2-L1 produced a measurable outward current in the plasma membrane (Extended Data Fig. 4b). Together, these data suggest that PKD2-L1, but not PKD1-L1, can form homomeric channels. Based on an alignment of the PKD2 family of proteins (Extended Data Fig. 4c), a cluster of conserved acidic residues in the putative selectivity filter17 was mutated. Neutralizing two of three extracellular glutamates (E523 and E525, but not E530) to serine or alanine abolished the current (Extended Data Fig. 4b). The homomeric PKD2-L1 channel produced an outwardly rectifying, cationic whole-cell current with a large moderately Ca2+-selective conductance (Fig. 4a; Extended Data Fig. 4d). Our results differ from the presumed PKD2-L1 expressed in oocytes18: PKD2-L1 currents in our HEK293T cells were outwardly rectifying and not activated by extracellular calcium. The unitary conductance (198 ± 3 pS) and rectification ratio of homomeric PKD2-L1 is too large to underlie the primary ciliary conductance (compare Fig. 1e and 4d). However, coexpression of the PKD1-L1 and PKD2-L1 yielded a less rectifying whole-cell current with a single channel outward conductance of 103 ± 3 pS (Fig. 4b-d), consistent with that found in RPE primary cilia. In addition, heterologously expressed PKD2-L1 and PKD1-L1 channels were activated by calmodulin antagonists and blocked by Gd3+ and ruthenium red. Unlike the ciliary current, the heterologously expressed channels were not activated by extracellular uridine and purine analogues (UDP, ADP, ATP; data not shown) suggesting that the receptor/signal transduction pathway in cilia is absent in the whole-cell HEK-293 conditions. These data establish that ICilia is conducted by a heteromer of PKD1-L1 and PKD2-L1 subunits.
Extended Data Figure 4. Heterologous PKD1-L1 and PKD2-L1 form an ion channel.
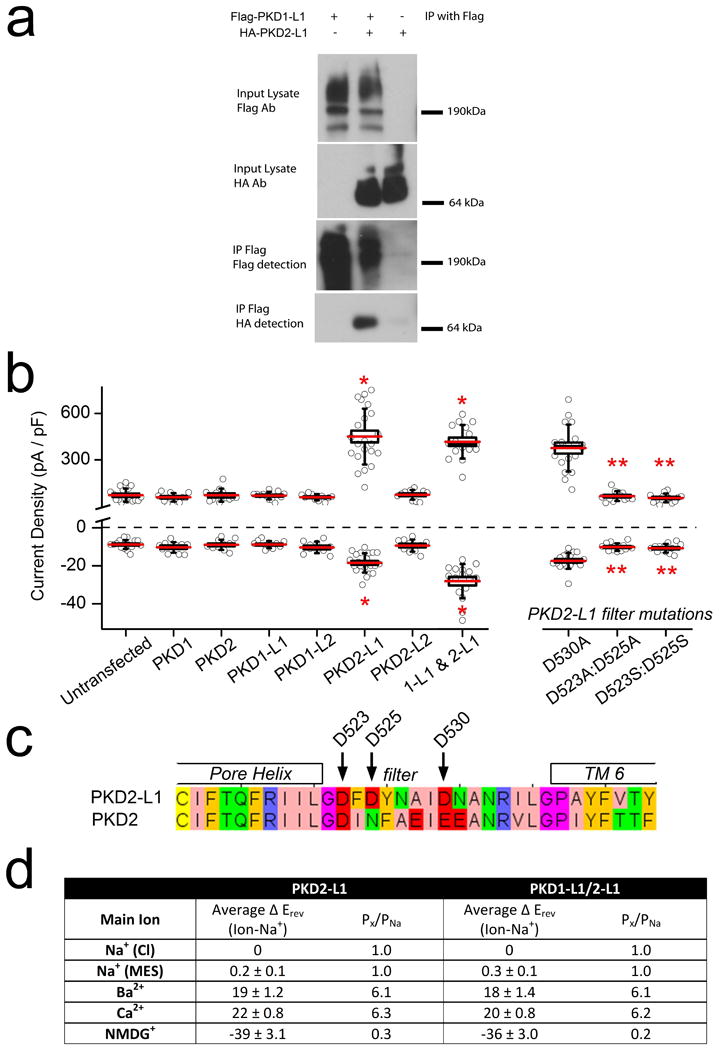
(a) Immunoprecipitation of Flag– and HA–tagged PKD1-L1 and PKD2-L1 heterologously expressed in HEK293T cells. (b) Box (± SEM) and whisker (± SD) plots of the current densities measured from PKDx-L1 family-transfected HEK cells at -100 mV (bottom) and +100 mV (top). Averages are indicated by the red lines. Statistical significance from Student's t-test comparing transfected to untransfected cells are indicated by asterisks (P-value < 0.005; n = 10-23 cells) and those comparing PKD2-L1 to the pore mutants are indicated by double asterisks (* denotes P-value < 0.005 compared to untransfected cells; ** denotes P-value < 0.005 compared to PKD 1-L1/2-L1 transfected cells; n= 9-11 cells). (c) An alignment of the PKD2-L1 and PKD2 (polycystin 2) pore helix and selectivity filter with glutamate residues D523, D525, and D530 indicated. (d) Table listing the average reversal potential change relative to the standard Na+-based extracellular solution (average खErev) and the estimated relative permeability (Px/PNa) for HEK cells transfected with PKD2-L1 alone or with PKD2-L1 and PKD1-L1 (± SEM, n= 4-6 cells).
Figure 4. Plasma membrane expressed PKD1-L1/PKD2-L1 channels match those of Icilia.
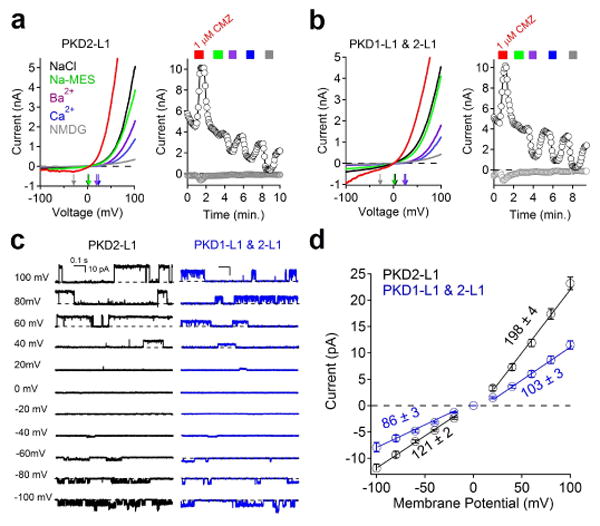
(a, b) Whole-cell currents recorded from cells transfected with (a) PKD2-L1 alone or (b) PKD1-L1 and PKD2-L1, where extracellular 1 μM calmidazolium (CMZ, red trace) was applied and Na+-based saline was exchanged by the cations indicated. (c) Single channel currents measured from the cell membrane of cells transfected by PKD2-L1 (black traces), or PKD1-L1 and PKD2-L1 (blue traces). (d) Resulting average single channel current amplitudes plotted against voltage measured from transfected cells (± SEM, n = 4-5 cells).
Primary cilia have been proposed to sense flow by mechanical activation of the putative PKD1/PKD2 channel16,19. Using pressure clamp20, we observed no difference in single channel activity at pressures of 0-60 mm Hg (8 kPa) for PKD1-L1/PKD2-L1 in mRPE primary cilia or in heterologous expression (Extended Data Fig. 5). Although channel activity measurably increased at high pressure (80-100 mm Hg; 11-13 kPa), the PKD1-L1/PKD2-L1 channel lacks the sensitivity found in designated mechanosensitive channels21,22.
Extended Data Figure 5. The PKD1-L1/PKD2-L1 channel is mechanosensitive only at high pressures.
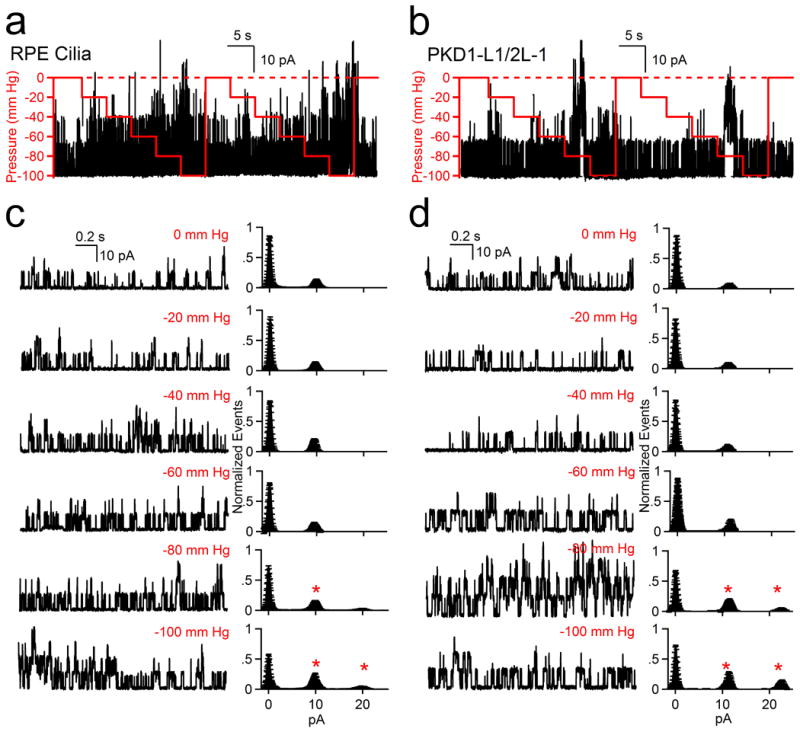
(a, b) Results of pressure clamp (0-100 mm Hg, red line) on PKD1-L1/PKD2-L1 single channel events recorded from (a) RPE primary cilia and (b) HEK-293T cells transfected with PKD1-L1 and PKD2-L1. (c, d) Left, Expanded times scales from (a) and (b). Right, corresponding averaged normalized amplitude histograms are plotted for the indicated applied pipette (± SEM, n = 5 cilia and 6 cells). Cilia or cells were held at +100 mV and pressure changes were applied at 5 s intervals. Asterisks indicate a significant (P > 0.05) increase in channel opening events relative to the zero pressure condition.
Many members of the TRP superfamily are highly temperature-sensitive7,23-25 and primary cilia are proposed to be temperature sensors via activation of a thermosensitive Ca2+ influx26. We tested the effect of increasing temperature (22-37°C) on primary cilia and PKD1-L1/PKD2-L1-transfected cells and observed an increase in the Gd3+-sensitive current (Extended Data Fig. 6 a, b). When rapidly increasing the bath temperature (2.1 ± 0.2°C/s), we observed biphasic current activation in the current from RPE primary cilia (from 24-32°C, Q10 = 6) and heterologously expressed PKD1-L1/PKD2-L1 cilia (from 24-32°C, Q10 = 8; Extended Data Fig. 6 c). This sensitivity is moderate in comparison to highly temperature-sensitive channels, such as TRPV1-TRPV4 (Q10>20). In any case, temperature gradients are likely inconsequential over the length of cilia and cilia to cytoplasm.
Extended Data Figure 6. The PKD1-L1/PKD2-L1 channel is highly temperature sensitive.
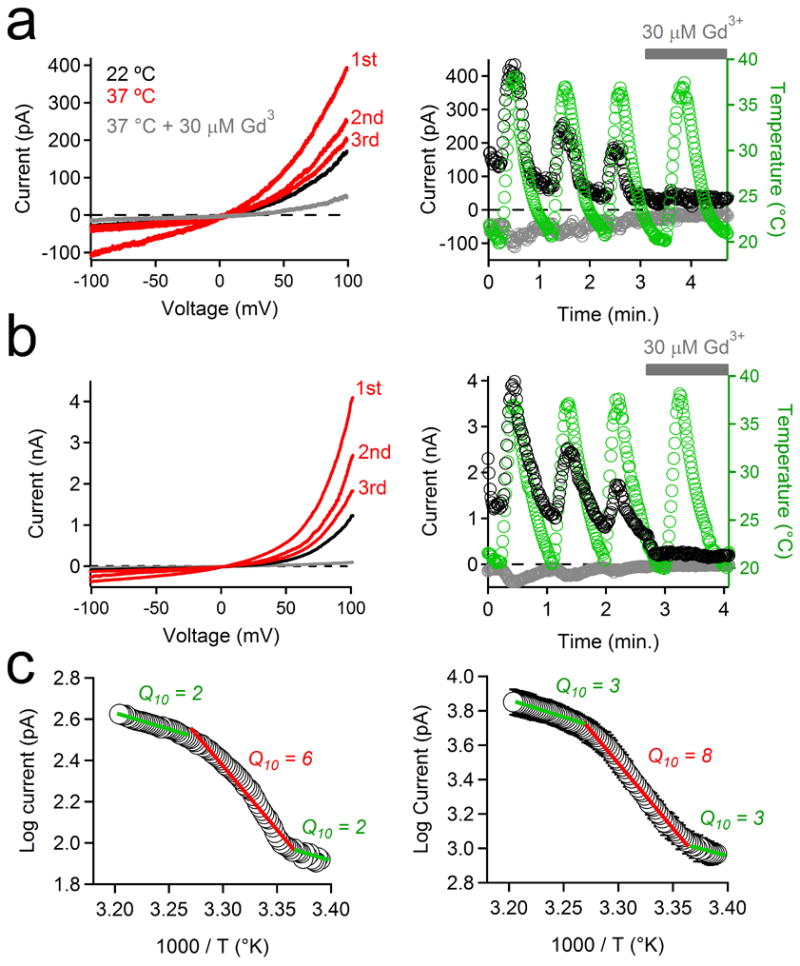
(a, b) The effects of repeated temperature stimulations from 22 to 37°C on the PKD1-L1/PKD2-L1 current recorded from (a) RPE primary cilia and (b) HEK-293T cells (plasma membrane) transfected with PKD1-L1 and PKD2-L1. Left, Currents elicited by a series of 1 Hz voltage ramps from -100 to +100 mV from 21 to 38°C in control conditions (red traces) or in the presence of 30 μM Gd3+ (grey trace). Right, resulting current amplitudes (-100 mV, grey circles; +100 mV, black circles) and cilia temperature (green circles) are plotted as a function of time. Grey bar indicates the duration of extracellular 30 μM Gd3+ application. (c) Arrhenius plots of the PKD1-L1/PKD2-L1 currents recorded from (left) RPE primary cilia and (right) when heterologously expressed in HEK-293T cells. Q10 values were derived from 3 linear fits of the average normalized current magnitude from 3 phases of the thermal response (21-24°C; 24-32°C; 32-38°C; ± SEM; n = 4 cilia or 4 cells).
Delling and DeCaen et al2. demonstrated that cilia are a specialized calcium compartment, regulated by PKD1-L1 and PKD2-L1 channels, and that they in turn regulate ciliary Smoothened signaling and Gli transcription. Here we have demonstrated that PKD1-L1 and PKD2-L1, heteromultermerize to form a calcium-permeant ciliary channel which can be indirectly activated by purines. Future experiments will determine the purine receptor and downstream effectors activating ICilia. Since the PKD1-L1/PKD2-L1 complex is calcium-permeant, but is also inactivated at high internal [Ca2+], we propose that the channel regulates the high resting ciliary calcium level2. We hypothesize that these signals modify regulation of Smoothened target genes, in particular Gli transcription of genes regulating cell division and growth.
Methods
Electrophysiology
hRPE1 Smo-EGFP cells were serum starved 24-72 h prior to electrophysiological recording in order to slow cell growth and induce ciliogenesis. Primary cells cultured from the Arl13B-EGFPtg mouse tissues (MEF and mRPE) were cultured for less than two passages before they were patch clamped. Data were collected using an Axopatch 200B patch clamp amplifier, Digidata 1440A, and pClamp 10 software. For temperature-controlled experiments, the perfusate was heated using a Warner TC-344B heater controller and in-line heater/cooler while bath temperature was monitored using a thermistor placed in close proximity to the recording electrode. For pressure clamp experiments, membrane pressure was applied using a HSPC-1 high-speed pressure system (ALA Scientific) controlled by pClamp software. Whole-cell and excised inside-out patch currents were digitized at 25 kHz and low pass filtered at 10 kHz. The permeability of monovalent cations relative to that of Na+ was estimated from the shift in reversal potential on replacing external Na+ bath solution (150 mM X, 10 mM HEPES, 0.5 mM CaCl2, pH 7.4), where X was NaCl, NaMES, KCl, BaCl2, CaCl2, or NMDG. Permeability ratios were calculated as:
where [X]out is defined as the extracellular concentration of the given ion, P is defined as the permeability of the ion indicated by the subscript, F is Faraday's constant, R is the gas constant, T is absolute temperature and Vrev is the reversal potential for the relevant ion.
Estimates of cilia channels per membrane surface area
The number of channels per cilia was calculated as N = I / Po(i), where N = number of channels; I is the whole-cilium current at +100 mV; Po is the open probability (calculated at +100 mV from ∼1 s-long traces), and i is the single channel amplitude at +100 mV. Based in the slope of the single channel records from RPE cilia, the channel conductance, γ = 80 ± 3 pS inward and γ = 96 ± 3 pS outward. RPE cilia channel mean open time at -100 mV and 100 mV were 0.22 ± 0.02 and 14 ± 3 ms, respectively. For simplification we represent the cilia as a cylinder and thus its surface area is given by A = 2πr(r+h) where h is the height (5 μm on average) and radius (r) varies from 0.15 to 0.25 μm as measured in electron micrographs. Assuming r = 0.2 μm, then the surface area is ∼ 6.3 μm2 and capacitance (Cm, assuming 1 μF/cm2) = 0.063 pF (for comparison, surface areas of typical cells are ∼ 2000 μm2 with Cm = 20 pF). Current density measured in the detached cilia patch and the hRPE cell body was 1730 ± 98 pA/pF and 31 ± 4 pA/pF respectively. The equivalent circuit of the whole-cilia recording assumes that the resistance between cilia and cell is sufficiently high to neglect the contributions from the cell, since cilia ripped from the cell and sealed over show essentially the same result as cilia attached to the cell.
Molecular Biology and Biochemistry
Plasmids and heterologous expression of PKD channels
HEK 293T cells were transfected using Lipofectamine 2000 (Invitrogen) reagent. hPKD2, hPKD2-L1 and hPKD2-L2 cDNAs were obtained from Open Biosystems and a Hemagglutinin (HA) Tag was added to the N-terminus using PCR. The resulting cDNAs were subcloned into an IRES enhanced green fluorescence protein (EGFP)-containing vector. hPKD1-L1 cDNA was synthesized (Bio Basic, Amherst NY) with a Flag-Tag at the C-terminus. hPKD1L1, hPKD1 and hPKD1-L2 were subcloned into an IRES red fluorescence protein (RFP)-containing vector. Transfected cells cultured at 37°C and plated onto glass coverslips and were recorded 24-48 h later. For the co-transfected cells in Figure 4, the cDNA was transfected at a 5:1 ratio for PKD1-L1 and 2L-1. Whole-cell patches for recordings from the cell body were obtained using electrodes of 1.5-2.5 MΩ and excised inside-out patches were obtained using pipettes of 5-7 MΩ resistances. Saline conditions are the same as those used for primary cilia.
Inhibition and Detection of Transcripts
Smo-EGFP expressing hRPE1 cells were transfected with 50 pM of siRNA and 5 μl RNAiMAX (Life Technologies) in a 3.5 cm dish. Cells were seeded at 230,000 cells/plate. 60 h after transfection, cells were serum-starved and cilia recordings performed 48 h after serum starvation. hRPE1 cells in a 3.5 cm dish were washed ×1 with PBS and lysed in 1 ml TRIZOL for RNA extraction according to the manufacturer's instructions. RNA was reverse transcribed using the QuantiTect reverse transcription kit (Qiagen). Gene specific primers were designed using Primerbank (http://pga.mgh.harvard.edu/primerbank/). Gene specific products were amplified by PCR and gene expression was visualized by agarose gel electrophoresis.
Immunoprecipitation
HEK 293T cells were transfected in a 10 cm dish with the indicated combinations of PKD1-L1 Flag and PKD2-L1-HA using Lipofectamine according to the manufacturer's instructions (Life Technologies). 24 h after transfection cells were lysed in 2 ml RIPA buffer (20 mM Tris-HCl; pH 7.5, 150 mM NaCl, 1 mM EDTA, 1% NP-40 and 1% sodium deoxycholate and protease inhibitors added (Complete, Roche). After centrifugation at 15,000 g for 10 min at 40°C, 1.5 ml of the supernatant was added to 30 μl Anti-Flag M2 Agarose (Sigma-Aldrich) and incubated overnight at 40°C. Agarose beads were spun down at 1000 g for 5 min and washed ×5 with RIPA buffer. The agarose beads were resuspended in LDS samples buffer containing 2% β-mercaptoethanol, heated at 75°C for 5 min, and proteins were separated on a 4%-12% BisTris gel. After transfer to PVDF membrane, blots were probed with anti-Flag (Sigma Aldrich) or anti-HA (Roche) antibodies.
Supplementary Material
Supplemental Video 1: Obtaining a ‘whole-cilium’ patch using confocal microscopy An hRPE cell cilia expressing Smo-GCaMP3 is patch clamped by applying the glass pipette to the tip of the cilia and establishing a giga-seal by gentle suction (frames 4-13). Intentional movement of the pipette pulls the attached cilium (frames 14-18). The ciliary membrane was held at -60 mV and ruptured, establishing a ‘whole-cilium’ patch configuration in which the entire cilia membrane is voltage-clamped and the inside of the cilia perfused by the patch pipette. DIC (left), fluorescence (right), and merged (center) images were acquired in an inverted confocal microscope using a 60x objective . Break-in to the whole-cilium is accompanied by an increase in GCaMP fluorescence (frames 22-29). The video playback speed is 7 frames/s.
Supplemental Video 2: Obtaining an ‘excised-cilia’ patch from an RPE Smo-EGFP cell. RPE cells expressing Smo-GFP enable visualization of the primary cilium under confocal microscopy. Once the cilium was patched in the ‘whole-cilium’ configuration, the cilia-pipette connection is demonstrated by intentional movement of the pipette from side to side (frames 1-2). Part of the cilia was then detached from the cell by lifting the electrode (frames 3-4). The focus plane was raised and lowered (gap in fluorescence), demonstrating that the cilium has separated from the cell body (frames 4-9). The video playback speed is 7 frames/s.
Acknowledgments
Paul DeCaen was supported by NIH T32 HL007572. Animal work was, in part, supported by NIH P30 HD18655 to the IDDRC of Boston Children's Hospital. We thank Betsy Navarro, Nat Blair, Julia Doerner, Sebastien Febvay, and the members of the Clapham laboratory for valuable advice and assistance.
Footnotes
Author Information. Reprints and permissions information is available at www.nature.com/reprints.
Online Content. Any additional Methods, Extended Data display items and Source Data are available in the online version of the paper; references unique to these sections appear only in the online paper.
Author Contributions. All authors designed and conducted experiments and wrote the manuscript.
The authors declare no competing financial interests.
Readers are welcome to comment on the online version of the paper.
References
- 1.Bornens M. The centrosome in cells and organisms. Science. 2012;335:422–426. doi: 10.1126/science.1209037. [DOI] [PubMed] [Google Scholar]
- 2.Delling M, DeCaen PG, et al. Primary cilia are specialized calcium signaling organelles. Nature. doi: 10.1038/nature12833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Corbit KC, et al. Vertebrate Smoothened functions at the primary cilium. Nature. 2005;437:1018–1021. doi: 10.1038/nature04117. [DOI] [PubMed] [Google Scholar]
- 4.Hardie RC, Minke B. The trp gene is essential for a light-activated Ca2+ channel in Drosophila photoreceptors. Neuron. 1992;8:643–651. doi: 10.1016/0896-6273(92)90086-s. [DOI] [PubMed] [Google Scholar]
- 5.Shin JB, et al. Xenopus TRPN1 (NOMPC) localizes to microtubule-based cilia in epithelial cells, including inner-ear hair cells. Proc Natl Acad Sci U S A. 2005;102:12572–12577. doi: 10.1073/pnas.0502403102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stortkuhl KF, Hovemann BT, Carlson JR. Olfactory adaptation depends on the Trp Ca2+ channel in Drosophila. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1999;19:4839–4846. doi: 10.1523/JNEUROSCI.19-12-04839.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Story GM, et al. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell. 2003;112:819–829. doi: 10.1016/s0092-8674(03)00158-2. [DOI] [PubMed] [Google Scholar]
- 8.Kleene NK, Kleene SJ. A method for measuring electrical signals in a primary cilium. Cilia. 2012;1 doi: 10.1186/2046-2530-1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arnaout MA. Molecular genetics and pathogenesis of autosomal dominant polycystic kidney disease. Annu Rev Med. 2001;52:93–123. doi: 10.1146/annurev.med.52.1.93. [DOI] [PubMed] [Google Scholar]
- 10.Celic A, Petri ET, Demeler B, Ehrlich BE, Boggon TJ. Domain mapping of the polycystin-2 C-terminal tail using de novo molecular modeling and biophysical analysis. J Biol Chem. 2008;283:28305–28312. doi: 10.1074/jbc.M802743200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu Y, et al. Structural and molecular basis of the assembly of the TRPP2/PKD1 complex. Proc Natl Acad Sci U S A. 2009;106:11558–11563. doi: 10.1073/pnas.0903684106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu J, et al. Structural model of the TRPP2/PKD1 C-terminal coiled-coil complex produced by a combined computational and experimental approach. Proc Natl Acad Sci U S A. 2011;108:10133–10138. doi: 10.1073/pnas.1017669108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gherman A, Davis EE, Katsanis N. The ciliary proteome database: an integrated community resource for the genetic and functional dissection of cilia. Nat Genet. 2006;38:961–962. doi: 10.1038/ng0906-961. [DOI] [PubMed] [Google Scholar]
- 14.Andrade YN, et al. TRPV4 channel is involved in the coupling of fluid viscosity changes to epithelial ciliary activity. J Cell Biol. 2005;168:869–874. doi: 10.1083/jcb.200409070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raychowdhury MK, et al. Characterization of single channel currents from primary cilia of renal epithelial cells. J Biol Chem. 2005;280:34718–34722. doi: 10.1074/jbc.M507793200. [DOI] [PubMed] [Google Scholar]
- 16.Yoshiba S, et al. Cilia at the node of mouse embryos sense fluid flow for left-right determination via Pkd2. Science. 2012;338:226–231. doi: 10.1126/science.1222538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hille B. Ion Channels of Excitable Membranes. 3rd. Sinauer Associates Inc; 2001. [Google Scholar]
- 18.Chen XZ, et al. Polycystin-L is a calcium-regulated cation channel permeable to calcium ions. Nature. 1999;401:383–386. doi: 10.1038/43907. [DOI] [PubMed] [Google Scholar]
- 19.Praetorius HA, Spring KR. A physiological view of the primary cilium. Annu Rev Physiol. 2005;67:515–529. doi: 10.1146/annurev.physiol.67.040403.101353. [DOI] [PubMed] [Google Scholar]
- 20.Besch SR, Suchyna T, Sachs F. High-speed pressure clamp. Pflugers Archiv : European journal of physiology. 2002;445:161–166. doi: 10.1007/s00424-002-0903-0. [DOI] [PubMed] [Google Scholar]
- 21.Coste B, et al. Piezo proteins are pore-forming subunits of mechanically activated channels. Nature. 2012;483:176–181. doi: 10.1038/nature10812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sukharev SI, Blount P, Martinac B, Blattner FR, Kung C. A large-conductance mechanosensitive channel in E. coli encoded by mscL alone. Nature. 1994;368:265–268. doi: 10.1038/368265a0. [DOI] [PubMed] [Google Scholar]
- 23.Xu H, et al. TRPV3 is a calcium-permeable temperature-sensitive cation channel. Nature. 2002;418:181–186. doi: 10.1038/nature00882. [DOI] [PubMed] [Google Scholar]
- 24.Benham CD, Gunthorpe MJ, Davis JB. TRPV channels as temperature sensors. Cell calcium. 2003;33:479–487. doi: 10.1016/s0143-4160(03)00063-0. [DOI] [PubMed] [Google Scholar]
- 25.Voets T, et al. The principle of temperature-dependent gating in cold- and heat-sensitive TRP channels. Nature. 2004;430:748–754. doi: 10.1038/nature02732. [DOI] [PubMed] [Google Scholar]
- 26.Kottgen M, et al. TRPP2 and TRPV4 form a polymodal sensory channel complex. J Cell Biol. 2008;182:437–447. doi: 10.1083/jcb.200805124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cang C, et al. mTOR Regulates Lysosomal ATP-Sensitive Two-Pore Na(+) Channels to Adapt to Metabolic State. Cell. 2013 doi: 10.1016/j.cell.2013.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fieni F, Lee SB, Jan YN, Kirichok Y. Activity of the mitochondrial calcium uniporter varies greatly between tissues. Nature communications. 2012;3:1317. doi: 10.1038/ncomms2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shenkel S, Sigworth FJ. Patch recordings from the electrocytes of Electrophorus electricus. Na currents and PNa/PK variability. The Journal of general physiology. 1991;97:1013–1041. doi: 10.1085/jgp.97.5.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brisson A, Unwin PN. Quaternary structure of the acetylcholine receptor. Nature. 1985;315:474–477. doi: 10.1038/315474a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Video 1: Obtaining a ‘whole-cilium’ patch using confocal microscopy An hRPE cell cilia expressing Smo-GCaMP3 is patch clamped by applying the glass pipette to the tip of the cilia and establishing a giga-seal by gentle suction (frames 4-13). Intentional movement of the pipette pulls the attached cilium (frames 14-18). The ciliary membrane was held at -60 mV and ruptured, establishing a ‘whole-cilium’ patch configuration in which the entire cilia membrane is voltage-clamped and the inside of the cilia perfused by the patch pipette. DIC (left), fluorescence (right), and merged (center) images were acquired in an inverted confocal microscope using a 60x objective . Break-in to the whole-cilium is accompanied by an increase in GCaMP fluorescence (frames 22-29). The video playback speed is 7 frames/s.
Supplemental Video 2: Obtaining an ‘excised-cilia’ patch from an RPE Smo-EGFP cell. RPE cells expressing Smo-GFP enable visualization of the primary cilium under confocal microscopy. Once the cilium was patched in the ‘whole-cilium’ configuration, the cilia-pipette connection is demonstrated by intentional movement of the pipette from side to side (frames 1-2). Part of the cilia was then detached from the cell by lifting the electrode (frames 3-4). The focus plane was raised and lowered (gap in fluorescence), demonstrating that the cilium has separated from the cell body (frames 4-9). The video playback speed is 7 frames/s.