Abstract
The centrosome is the major organelle responsible for the nucleation and organization of microtubules into arrays. Recent studies demonstrate that microtubules can nucleate outside the centrosome. The molecular mechanisms controlling acentrosomal microtubule nucleation are currently poorly defined, and the function of this type of microtubule regulation in tumor cell biology is particularly unclear. Since microtubule nucleation is initiated by the gamma-tubulin protein, we examined the regulation of gamma-tubulin in a panel of human breast tumor cell lines, ranging from non-tumorigenic to highly aggressive. We have identified a more dispersive subcellular localization of gamma-tubulin in aggressive breast cancer cell lines, while gamma-tubulin localization remains largely centrosomal in non-invasive cell lines. Delocalization of gamma-tubulin occurs independently from changes in protein expression and is therefore regulated at the post-translational level. Subcellular fractionation revealed that tumor cell lines show an aberrantly increased release of gamma-tubulin into a soluble cytoplasmic fraction, with the most dramatic changes observed in tumor cell lines of greater metastatic potential. Extraction of soluble gamma-tubulin revealed acentrosomal incorporation of gamma-tubulin in cytoplasmic microtubules and along cell junctions. Moreover, acentrosomal delocalization of gamma-tubulin yielded resistance to colchicine-mediated microtubule collapse. These findings support a model where the solubility of gamma-tubulin can be altered through post-translational modification and provides a new mechanism for microtubule dysregulation in breast cancer. Gamma-tubulin which is delocalized from the centrosome can still clearly be incorporated into filaments, and defines a novel mechanism for tumor cells to develop resistance to microtubule-targeted chemotherapies.
Keywords: γ-tubulin, centrosomes, microtubule nucleation, breast cancer, solubility
Introduction
Microtubules are cytoskeletal filaments involved in fundamental cellular processes such as motility, morphology, and division, and are a common target for cancer therapeutics. Nucleation and organization of microtubules is the responsibility of the centrosome in animal cells, a small organelle also known as the microtubule organizing center (MTOC). Centrosome abnormalities are common hallmarks of breast cancer and thought to cause conditions such as aneuploidy, mitotic defects, and aberrant cellular motility.1-5 Previous work concerning centrosome abnormalities in breast cancer focus mainly on the role centrosome amplification plays during mitosis as a preceding step towards aneuploidy.2, 4, 6 Growing evidence has implicated a larger role for aberrant microtubule nucleation in tumor metastasis beyond mitosis. Recent immunohistochemistry data from breast cancer patients reveals that both pre-invasive lesions and aggressive breast carcinomas show increased expression of gamma-tubulin,7 one of the necessary protein for microtubule nucleation.8
Microtubule nucleation in mammalian cells involves several proteins that make up the gamma-tubulin ring complex (gamma-TuRC), the core functional unit of the centrosome. The centrosome is made up of a pair of orthogonally situated centrioles surrounded by a mass of proteins known as the pericentriolar matrix (PCM). Previous studies on PCM proteins have indicated their role in localization of gamma-tubulin.9-11 The gamma-TuRC is composed of a class of proteins known as the gamma-tubulin complex proteins (GCP). Gamma-tubulin (GCP1) is the best characterized constituent of the centrosome and the essential protein involved in microtubule nucleation. In lower eukaryotes (e.g., plants and fungi) a smaller version of the gamma-TuRC, called the gamma-tubulin small complex (gamma-TuSC), has been found to be sufficient for microtubule nucleation. The gamma-TuSC is composed of only three GCPs: GCP1, GCP2, and GCP3. Many of these lower organisms, such as plants and yeast, possess no centralized MTOC.12, 13 Others possess spindle pole bodies, organelles similar in function to centrosomes but lacking key centrosomal features such as the PCM.14-16 Recent evidence also shows microtubule nucleation occurring acentrosomally in several differentiated mammalian cell types, specifically polarized epithelial cells and neurons.17-19 Dysregulation of gamma-tubulin localization provides a potential mechanism to promote the centrosomal abnormalities in breast cancer and a target for new therapeutic strategies.
Current chemotherapies that target microtubules aim to disrupt cell division in tumor cells which are more rapidly dividing than most somatic cells.20, 21 Taxane-based drugs, such as paclitaxel or docetaxel, are widely used as a breast cancer treatment for their anti-mitotic effects. A major drawback of taxane-based treatments is drug resistance develops over time,22 leaving patients with more aggressive tumors and fewer or no treatment options. Most breast cancer patients eventually acquire multidrug resistance by P-glycoprotein and the multidrug resistance protein family.23, 24 Another major drawback is the side effects of such drugs. Indiscriminate targeting of microtubules affects all rapidly dividing cells, such as gut epithelial cells and hair follicles, leading to the classic chemotherapy side effects of nausea and hair loss. Centrosome amplification and acentrosomal microtubule nucleation may aid in the resistance to these drugs by allowing the cell multiple avenues of cell division. Very little is understood about the role microtubule nucleation plays in interphase cells, although this information could be critical as most dormant cells spend the majority of their time in interphase until signaled for propagation. Understanding the mechanisms that lead to disruption of the microtubule nucleation machinery may assist in further refining many current and developing breast cancer chemotherapeutics that target microtubules.
In this study, we show that localization of gamma-tubulin is radically varied across a panel of 11 human breast tumor cell lines. Cells lines were categorized for metastatic potential based on a collection of previous studies looking at invasiveness and gene profiling.25-31 The alteration in gamma-tubulin localization in more invasive cell lines was independent of the expression level of gamma-tubulin protein, which remained consistent throughout the panel. The greatest difference was observed in the balance between soluble and insoluble gamma-tubulin which was significantly altered in the more aggressive breast tumor cell lines. We further show that gamma-tubulin incorporated into cytoplasmic microtubules confers resistance of these microtubules to colchicine-mediated catastrophe. Given the central role of microtubules in cell motility, cytokinesis, and chromosome segregation, disruption of gamma-tubulin localization and solubility provides a potential mechanism to promote the aberrant microtubule structures observed in breast tumors. This result reveals a potential molecular marker indicating the aggressiveness of malignant breast tumors, as well as a potential molecular target for treatment of breast tumors.
Materials and Methods
Cell culture
The non-tumorigenic, immortalized mammary epithelial cell line, MCF-10A, was used as a negative control for transformation and cultured in DMEM/F12 culture media (Cellgro) supplemented with 5% horse serum, epidermal growth factor (20 ng/mL), hydrocortisone (500 ng/mL), insulin (5 μg/mL), 1% L-Glutamine, and 1% Pen/Strep. BT-20, SKBR3, BT-549, MCF-7, MDA-MB-436, Hs578T, and MDA-MB-231 were cultured in DMEM (Cellgro) with 10% Bovine Calf Serum (BCS), 1% L-Glutamine, and 1% Pen/Strep. BT-474, HCC1428, and HCC1937 cell lines were cultured in RPMI 1640 (Cellgro) supplemented with 10% Fetal Bovine Serum (FBS) and 1% Pen/Strep. Cell lines were categorized for metastatic potential based on previous studies that determined invasion27-29 or used genetic profiling matched to patient prognosis.25, 26, 28-31 MCF-10A were classified as no metastatic potential. BT-20, SKBR3, MCF-7, and BT-474 were classified as low metastatic potential. MDA-MB-436 was classified as medium metastatic potential. BT-549, Hs578T, and MDA-MB-231 were classified as high metastatic potential. No previous data is available for HCC1428 and HCC1937, but were thought classified in this study as having high metastatic potential based on their genetic profiles and their chemoresistant properties.
Western blot
Whole cells lysates were taken from a panel of breast tumor cell lines of increasing aggressiveness and run on 10% SDS-PAGE gels. Protein was semi-dry transferred onto PVDF membranes for 1 hr at 25V or wet transferred for 1.5 hr at 65V at 4°C. Membranes were blocked with 5% milk in TBS-T for 1 hr at room temperature. PVDF membranes were then incubated overnight at 4°C with gamma-tubulin mouse (1:1000; GTU-88, Sigma, St. Louis, MO) or rabbit (1:1000; PRB-433C, Covance, Princeton, NJ) primary antibody in 2.5% milk in TBS-T. Membranes were incubated with HRP-conjugated secondary antibodies to mouse or rabbit (1:2500) for 1 hr at room temperature. Membranes were exposed by chemiluminescence using ECL Plus™ (GE Healthcare, Piscataway, NJ) and imaged using a digital gel imager (UVP, Upland, CA) with CCD camera (Hamamatsu, Bridgewater, NJ).
Immunofluorescence
Indirect immunofluorescence was performed to examine spatial localization and quantify endogenous levels of gamma-tubulin in each cell line. Cells were fixed in ice cold 100% methanol at −20°C for 10 min and permeabilized in 0.25% Triton X-100 for 10 min at room temperature. For prefixation permeabilization experiments, cells were treated with colchicine (100 μM) for 1 hr at room temperature followed by treatment with 0.1% Triton X-100 and microtubule stabilizing buffer (80 mM PIPES, 1 mM MgCl2, 5 mM EGTA) for 1 min at 4°C before ice cold methanol fixation. Cells were blocked for 1 hr at room temperature in 5% BSA with 0.5% NP-40. Primary antibodies against gamma-tubulin (rabbit polyclonal; 1:1000; Covance, Princeton, NJ) and ~180 kDa centrosomal protein (mouse monoclonal; 1:1000; Calbiochem, San Diego, CA) were incubated at 4°C overnight. Secondary antibodies Alexa Fluor 488 and 555 against rabbit and mouse (1:1000; Molecular Probes, Eugene, OR), respectively, were incubated for 1 hr at room temperature. Hoechst 33342 (1:5000; Sigma, St. Louis, MO) was used for nuclei counterstaining.
Ultracentrifugation
Whole cell lysates were taken from 10 cm culture dishes with 1 mL of lysis buffer (50 mM HEPES, pH 7.4, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS) at 4°C for 30 min. Soluble and insoluble fractions of cell lysates were separated by ultracentrifugation (Beckman, Fullerton, CA) at 200,000×g for 1 hr. The soluble fraction (supernatant; s) and insoluble fraction (pellet; p) were collected, and pellet was resuspended in 200 μL Laemelli buffer. Due to the difference in volume collected of supernatant (800 μL) and pellet (200 μL), ¼ volume of the pellet was loaded as compared with supernatant volume to load equal total cellular protein volume. Supernatants and pellets for each cell line were examined by SDS-PAGE to determine protein expression levels in each fraction. Densitometry of bands was used to determine the ratio of soluble to insoluble fraction in each cell line. Statistical significance was measured by Kruskal-Wallis ANOVA.
Image acquisition and analysis
Images were acquired using an Olympus FV1000 laser scanning confocal microscope (Olympus, Center Valley, PA) equipped with a 60×/1.42 NA oil immersion objective lens at the same magnification. Z-stacks at 0.2 μm steps were acquired at 12-bit image depth. Acquisition settings were standardized for all samples and kept consistent throughout image acquisition for correlative image quantification. Signal intensity quantification of gamma-tubulin was analyzed using ImageJ (NIH, Bethesda, MD). Mean intensity of the cytosolic, non-centrosomal portion of gamma-tubulin in each of the panel of cell lines were evaluated by quantifying the total intensity in the field of view using a threshold mask to exclude background and centrosomes, and a mask created from the Hoechst 33342 image to exclude the nuclei. This was then divided by the total number of cells as determined by the Hoechst 33342-stained nuclei to obtain intensity per cell. Three separate fields of view were taken per sample, and data were averaged together. Statistical significance was measured by Student’s T-test.
Radial profiles
Images were analyzed using ImageJ with the radial profile plug-in to determine the gamma-tubulin integrated intensity profile of radial circles centered around the centrosome in individual cells to measure delocalization. The radial profiles were averaged together within each cell line to make composite radial profiles with standard deviations of the full panel of cell lines. Peak vs. average intensity values were calculated amongst the cell lines to quantify differences between cell lines.
Results
Subcellular localization of gamma-tubulin is altered in metastatic cell lines but not due to protein overexpression
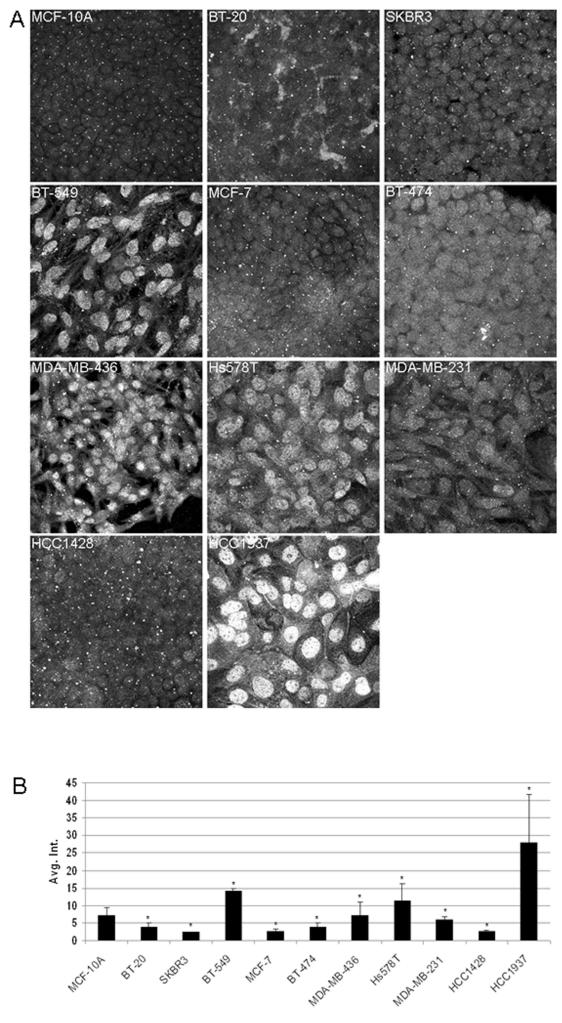
Immunofluorescence revealed that in some aggressive breast cell lines the localization of gamma-tubulin was not contained at the centrosome as in normal interphase cells. In contrast, localization of gamma-tubulin in MCF-10A, an immortalized, non-tumorigenic human mammary epithelial cell line, was restricted to the centrosome (Fig. 1A). The staining pattern for MCF-10As is what is classically reported in the previous literature for centrosome staining.10, 32 Cell lines with low invasiveness, BT-20, SKBR3, MCF-7, and BT-474 showed minimal delocalization, and gamma-tubulin remained predominately at the centrosome (Fig. 1A). Conversely, cell lines with greater metastatic potential (MDA-MB-436, BT-549, Hs578T, MDA-MB-231, HCC1428, and HCC1937) demonstrated greater delocalization from the centrosome (Fig. 1A). Quantitative immunofluorescence intensity analysis of the images show an overall increased cytoplasmic staining per cell in BT-549, MDA-MB-436, Hs578T, and HCC1937 (Fig. 1B). Cytoplasmic staining for gamma-tubulin was strongest in the HCC1937 cell line. Student’s T-test showed a significant difference between all cell lines relative to MCF-10A (p<0.05; n=100 per sample). It is important to note that quantification was performed excluding the centrosomal and nuclear signal, which differs from other centrosome intensity analysis performed by others to determine intensity normalized centrosome amplification.33
Figure 1.
Delocalization of gamma-tubulin in metastatic breast cancer cell lines. Immunofluorescence of gamma-tubulin in a panel of breast tumor cell lines with increasing metastatic potential was performed to determine changes in expression of endogenous gamma-tubulin protein. (A) Cell lines which are typically correlated with low invasiveness (BT-20 and SKBR3) displayed classical gamma-tubulin staining at the centrosome. Cell lines with greater metastatic potential showed differential localization of gamma-tubulin with either diffuse cytoplasmic or strong nuclear staining. Fluorescence intensity levels were quantified for each cell line (B), which demonstrated an increased level of gamma-tubulin in cell lines that are considered to be moderately to strongly invasive (BT-549, BT-474, MDA-MB-436, Hs578T, and HCC1937). Values marked with asterisks are statistically significant (p<0.05).
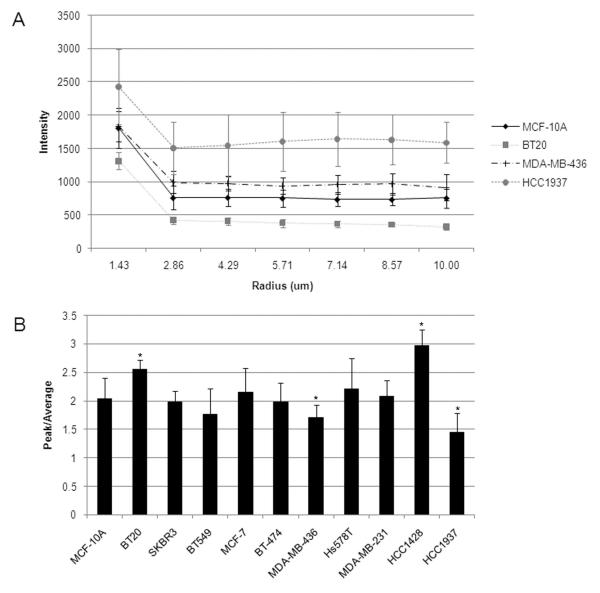
The degree of gamma-tubulin delocalization in each cell line was compiled from a group of individual cells. Intensity line profiles were measured through the centrosomes of cells and averaged from a random sampling of 5-7 cells per cell line. Representative images with averaged line profiles (as detailed in Materials and Methods) are shown (Fig. 2A) of selected cell lines (see Fig. S1 for complete panel of cell lines). The average intensity line profiles demonstrate sharp peaks of gamma-tubulin localization at the centrosomes of most cell lines except for the aggressive MDA-MB-436, Hs578T, and HCC1937 cell lines (Fig. 2B). Analysis of peak vs. average intensity reveals that the BT-549 and HCC1937 cell lines show the lowest differential between centrosomal and cytoplasmic staining of gamma-tubulin.
Figure 2.
(A) Radial profiles of gamma-tubulin staining through the centrosome reveals differential levels of cytosolic vs. centrosomal gamma-tubulin expression. The radial profile for immortalized, non-tumorigenic MCF-10A cell line is shown for comparison. Breast cancer cell lines that are typically associated with low metastatic potential, such as BT-20, produced radial profiles that are consistent with low cytosolic gamma-tubulin dispersive localization and high centrosomal gamma-tubulin expression. The aggressive cell line MDA-MB-436 also produced radial profiles consistent with normal radial profiles of gamma-tubulin. An extreme example of dispersive localization is demonstrated in the radial profile of HCC1937. Figure S1 shows the full panel of cell lines with radial profiles. (B) The ratio of peak to average of the radial profiles was calculated for the panel of cell lines and a ratio higher than MCF-10A signifies less dispersion of gamma-tubulin from the centrosome, while a ratio lower than MCF-10A signifies more dispersion of gamma-tubulin from the centrosome into the cytoplasm.
To determine if gamma-tubulin delocalization occurs through overexpression, the total amount of gamma-tubulin in the cells was measured by Western blot analysis of whole cell lysates. There was no significant upregulation of gamma-tubulin protein (Fig. 3). The lack of change in total protein levels between cell lines indicates that even dramatically altered localization of gamma-tubulin observed in HCC1937 cells does not simply result from overexpression of endogenous gamma-tubulin protein.
Figure 3.
Expression of gamma-tubulin protein in a panel of increasingly metastatic cell lines remained consistent. No significant change in expression level was seen amongst the panel of breast tumor cell lines examined by Western blot.
Delocalization of large centrosomal protein
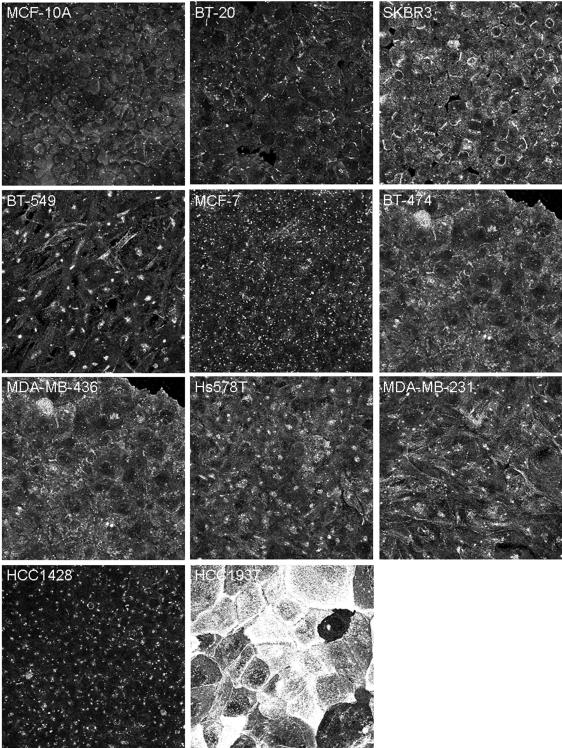
An antibody to the large centrosome protein of ~180 kDa34 was used to counterstain the centrosome. This protein is known primarily for its specific localization to the centrosome, but its function is unknown. Surprisingly, delocalization of this protein was also seen in metastatic cell lines (Fig. 4). This effect was most dramatic in HCC1937, although staining that is atypical of the centrosome localization can be seen in several of the more aggressive cell lines, such as SKBR3, BT-549, BT-474, MDA-MB-436, Hs578T, and MDA-MB-231 (Fig. 4). Due to this concurrent delocalization, this protein could not serve as a central marker to quantify gamma-tubulin delocalization. Future investigation and characterization of the delocalization of this protein is warranted to determine whether disrupted centrosomal organization is coincidental to or in conjunction with the delocalization of gamma-tubulin.
Figure 4.
Concurrent delocalization of the large centrosomal protein of ~180 kDa in human breast tumor cells. Differences include staining at the membrane (SKBR3, BT-549, BT-474, MDA-MB-436, Hs578T, and MDA-MB-231), enlarged centrosome staining (BT-549, MCF-7, Hs578T, MDA-MB-231, and HCC1428), and, most strikingly, punctate undefined staining (HCC1937).
Increased solubility of gamma-tubulin in breast tumor cell lines
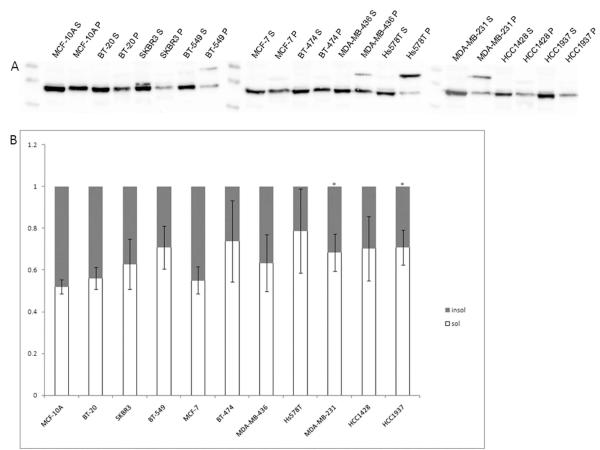
Early experiments using cell fractionation and centrosome isolation found that soluble, cytosolic gamma-tubulin makes up at least 80% of the total gamma-tubulin in KE37 human lymphoid cells.35 Changes in this ratio may indicate a dysfunction or dysregulation of the protein. To examine this, ultracentrifugation was performed to separate the soluble and insoluble fractions in whole cell lysates to determine if this ratio was altered in metastatic breast tumor cell lines. The results revealed that the soluble fraction of gamma-tubulin was increased in metastatic breast tumor cell lines. Western blots showed a significant level of soluble vs. insoluble variation in the panel of cell lines (Fig. 5A). Densitometry measurements showed a significant change in the soluble to insoluble ratio of a number of cell lines (Fig. 5B). The non-tumorigenic cell line, MCF-10A, showed a 52/48 ratio of soluble/insoluble gamma-tubulin. Earlier studies determined a 80/20 ratio of soluble/insoluble gamma-tubulin in KE37 human lymphoblastic cells.35 Unfortuantely, because of the difference in methods used, we cannot directly compare our ratios to this previous work. The ratios of the soluble vs. insoluble fractions in SKBR3 (63/37), BT-549 (71/29), BT-474 (74/26), MDA-MB-436 (63/37), Hs578T (79/21), MDA-MB-231 (68/32), HCC1428 (70/30), and HCC1937 (71/29) all showed increased soluble gamma-tubulin compared to the nontumorigenic MCF-10A cell line, which maintains the morphology of normal epithelial cells (Fig. 5B, see Table 1). This increase in gamma-tubulin solubility was statistically-significant in BT-549, Hs578T, MDA-MB-231, HCC1428, and HCC1937 when compared against nontumorigenic MCF-10A cells (p<0.05; n=3). Statistical significance was determined by Kruskal-Wallis ANOVA.
Figure 5.
Imbalance of soluble and insoluble gamma-tubulin in a panel of human breast tumor cell lines with increasing metastatic potential. Western blot analysis was performed on whole cell lysates after ultracentrifugation to separate soluble and insoluble fractions (A). Bands were analyzed by densitometry measurements using ImageJ and graphed as ratios (B). Soluble/insoluble ratios of MDA-MB231 and HCC1937 are significantly different (denoted by *) from MCF-10A (p<0.05).
Table 1.
BRCA1/ER/PR/HER2 Status of Breast Cancer Cell Lines
| ER | PR | HER2/neu† | BRCA1 Mutation Status |
BRCA1* | gamma-tubulin level/cell‡ |
% soluble gamma-tubulin |
|
|---|---|---|---|---|---|---|---|
| MCF-10A | − | − | − | WT | NA | ++ | 51.9 |
| BT-20 | − | − | + | WT | + | + | 56.1 |
| SKBR3 | − | − | ++ | WT | ++ | + | 62.8 |
| BT-549 | − | − | − | WT | ++ | +++ | 70.9 |
| MCF-7 | + | + | +/− | WT | +/− | + | 55.1 |
| BT-474 | + | + | ++ | WT | ++ | + | 73.8 |
| MDA-MB-436 | − | − | − | 5396 + 1G > A | ++** | ++ | 63.4 |
| Hs578T | − | − | +/− | WT | ++ | +++ | 78.9 |
| MDA-MB-231 | − | − | +/− | WT | ++ | +++ | 68.4 |
| HCC1428 | + | + | − | NA | − | + | 70.3 |
| HCC1937 | − | − | − | 5382insC | ++ | ++++ | 70.9 |
++ normal transcript levels, + low transcript levels, +/− barely detectable levels
different length transcripts
NA data not available
− negative, +/− low, + normal, ++ overexpression
+ low, ++ medium, +++ high, ++++ very high
Delocalization of gamma-tubulin yields colchicine resistant microtubules
In order to determine if the altered solubility of gamma-tubulin yielded differences in the incorporation of gamma-tubulin into cytoplasmic microtubules, we extracted the soluble cytoplasmic gamma-tubulin prior to fixation. By removing the masking effect of the increased soluble protein, we were able to identify that several of the more invasive breast tumor cell lines show incorporation of gamma-tubulin into cytoplasmic microtubules (Fig. 6B red arrow). When MCF10A cells were treated with colchicine to disrupt polymers of α/β-tubulin, cytoplasmic microtubules collapsed but gamma-tubulin remained localized to the centrosome, showing that centrosomal gamma-tubulin is anchored in a manner that does not depend on α/β-tubulin polymers. Cytoplasmic microtubules were more resistant to colchicine in cell lines with delocalized gamma-tubulin, and fragments of α/β-tubulin remained following treatment. This colchicine resistance was most striking in the HCC1937 cells, which showed significant retention of cytoplasmic α/β-tubulin fragments after colchicine treatment (Fig. 6P, white arrow). Most intriguingly, the HCC1937 cell line also showed a distinct pattern of gamma-tubulin localization at the plasma membrane (Fig. 6D, white arrowhead). Treatment with colchicine removed this surface staining (Fig. 6D vs. L), demonstrating that this membrane localization in HCC1937 cells depends on intact polymers of α/β-tubulin, while centrosomal gamma-tubulin remained unaffected. These results indicate that gamma-tubulin can be incorporated into cytoplasmic microtubules in breast tumor cell lines and that this aberrant localization yields a greater resistance to microtubule depolymerization with colchicine.
Figure 6.
Immunofluorescence with prefixation permeabilization reveals gamma-tubulin is incorporated in microtubules. Cells treated with colchicine provide evidence of gamma-tubulin (A-D and I-L) along microtubules in breast cancer cell lines (panel B, red arrow), while a normal centrosome staining for non-tumorigenic MCF-10A. N-P vs. F-H show a stabilization effect of alpha-tubulin (green stain) in breast cancer cell lines when treated with colchicine. HCC1937 shows short pieces of microtubules when treated with colchicine (panel P, white arrow), which indicates stabilization of fragments of alpha-tubulin by gamma-tubulin. HCC1937 also shows membrane staining of gamma-tubulin without colchicine treatment (panel D, white arrowhead), but this staining pattern is lost with colchicine treatment (panel P).
Discussion
Implications of delocalized microtubule nucleation on breast cancer progression and metastasis
Until recently, it was thought that all microtubules in mammalian cells were organized as an aster focused at the MTOC; however, recent evidence contradicts this dogma. Reilein et al. have shown that microtubules are able to self-organize into a network at the basolateral surface in order to form a cortical microtubule array in MDCK and Caco-2 epithelial cells,19 but the function of this microtubule cortex remains undetermined. Bartolini et al. proposed a three-step model to explain the initiation and formation of non-centrosomal microtubule arrays in mammalian cells,36 but there is still a lack of understanding how cells use non-centrosomal microtubule arrays. Delocalization of microtubule nucleation potentially explains many of the centrosomal abnormalities seen in breast cancer. Dysregulation of microtubule nucleation has been associated with tumorigenesis,3, 37 but these studies provided little data as to why tumors with centrosome abnormalities correlate with aggressiveness and metastasis aside from genomic instability due to mitotic spindle abnormalities.1, 38
Localization modulation of centrosomal and cytoskeletal associated proteins in tumor cells
Initial investigation with immunofluorescence (IF) of gamma-tubulin showed markedly differential staining in cell lines with increased metastatic potential, and what appeared to be an increase in overall staining in more metastatic cell lines (Fig. 1A). Further analysis of the immunofluorescence data revealed some increase in the cytosolic levels of gamma-tubulin in some cell lines (Fig. 1B). We tested whether the increase in cytosolic gamma-tubulin resulted from increased protein expression, but Western blotting revealed that the total amount of gamma-tubulin remains relatively equal among the panel of breast tumor cell lines (Fig. 3). Possible imbalances of soluble/insoluble levels of gamma-tubulin were examined to try to reconcile the IF and Western blot data. Ultracentrifugation confirmed a consistent increase in the ratio of soluble/insoluble gamma-tubulin in breast tumor cells lines, when compared to MCF-10A, with the most significant differences in the aggressive MDA-MB-231 and HCC1937 cell lines (Fig. 5A&B). These data indicate that changes in the soluble/insoluble ratio of gamma-tubulin occur in both non-invasive and invasive cell lines and that dysregulation of gamma-tubulin may be an early step in carcinogenesis. A previous clinical study showing an increase in IHC staining of gamma-tubulin protein in both preinvasive lesions and breast carcinomas7 lends further support for this early role of gamma-tubulin dysregulation. While this study showed that gamma-tubulin transcription also increased via RT-PCR,7 it remains possible that the increased immunohistochemical staining resulted from a change in gamma-tubulin protein localization rather than a significant increase in total protein expression. Certainly our initial immunochemical studies would have predicted a general increase in gamma-tubulin protein in HCC1937 cells (Fig. 1) until more detailed analysis of total protein expression (Fig. 3) and gamma-tubulin partitioning (Fig. 5) showed this dysregulation largely results from aberrant localization to a soluble cellular fraction. Originally, an antibody to the large centrosomal protein of ~180 kDa was used to counterstain the centrosome to examine delocalization of gamma-tubulin. This also produced intriguing localization data, as the centrosome protein also showed differential localization in many of the breast tumor cell lines, most significantly in HCC1937 (Fig. 4). Many of the other cell lines showed either increased staining near or around the centrosome, denoting an increase in size of the centrosome (most notable in BT-549 cells), or membrane staining by this antibody. Despite its widespread use as a marker of centrosomal amplification, little information is available on this centrosomal protein.34 However, the delocalization of this protein implies that the mechanism of delocalization of gamma-tubulin could entail a mis-targeting of multiple components of the microtubule nucleation machinery. The presence of gamma-tubulin in detergent-resistant membrane (DRM) fractions in mammalian epithelial cells has previously been reported, but the basis of this localization remains unclear.35, 39 There is precedent for tumorigenic cells to redistribute cytoskeleton-associated proteins without changes in protein expression. For example, ezrin concentrates at the apical surface in non-tumorigenic cells, but reorganizes diffusely in the cytoplasm and in structures involved in cell motility in invasive cell lines.40 Earlier studies have also examined the role of proteins that make up the PCM in breast cancer.1, 2, 4, 6 Those previous findings suggest that delocalization of PCM proteins, pericentrin and ninein, play a role in centrosome amplification but did not address the localization of gamma-tubulin. These PCM proteins have been shown to play a large role in the localization and anchoring of gamma-tubulin in breast tumors and have a positive correlation with centrosomal abnormalities clinically.41 Determining the role of delocalization of the proteins involved in microtubule nucleation could clarify the mechanisms that lead to centrosome amplification.
Centrosome aberrations are regulated by multiple pathways and gamma-tubulin delocalization is increased in BRCA1 mutant cell lines
Although centrosome amplification is a common feature found in many breast tumors, the regulation of this phenomenon is not yet clear. Previous studies have shown that clinically aggressive tumors have a greater capacity for the creation of microtubule asters than normal or non-aggressive tumors and can drive chromosomal instability.2 The list of post-translational modifications that affect centrosome duplication are plentiful and well reviewed.5 Briefly, this review implicates three main classes of enzymes that effect centrosome abnormalities: kinases, ubiquitin ligases, and poly-ADP-ribosyl-polymerase (PARP). The regulation of gamma-tubulin by several other kinases has been recently proposed. Spleen tyrosine kinase (Syk) has been shown to form complexes with gamma-tubulin and localize at the centrosome to negatively regulate mitotic progression in immune cells and breast epithelial cells.42-45 A loss of Syk expression has also been correlated with increased risk of metastasis in clinical studies (reviewed in reference 43). New evidence has shown that the Src family kinase, Fyn, and phosphoinositide 3-kinase (PI3K) can regulate non-centrosomal microtubule nucleation by forming complexes with gamma-tubulin located in DRMs.39
BRCA1 mutant breast cancers are highly aggressive forms of breast cancer that frequently metastasize. BRCA1 has been demonstrated to directly regulate gamma-tubulin through its ubiquitin ligase activity and act as a negative regulator of centrosome duplication.5 Many of these pathways have been found to be dysfunctional in tumorigenesis, invasion, and metastasis. Interestingly, HCC1937s have a known mutation in BRCA1 which manifests pathogenicly46 and these cells also have the highest levels of delocalization as well as significant changes in the soluble gamma-tubulin fraction (Fig. 1B and 5B). MDA-MB-436 also possess a pathogenic BRCA1 mutation46 and produce significantly different gamma-tubulin expression by IF(Fig. 1B). The soluble fraction of MDA-MB-436s also shows a significant difference as compared with MCF-10As (Fig. 5B). The mutation in MDA-MB-436 (5396 + 1G > A) produce two transcript products that differ from WT BRCA1. One of the predicted protein mutations includes an in-frame deletion of 28 amino acids resulting in the disappearance of exon 20. The other mutant transcript produces a frameshift that causes an early termination sequence. The mutation in HCC1937 (5382insC) also produces a frameshift resulting in early termination. Significant evidence exists suggesting that BRCA1 mutations may lead to centrosomal abnormalities because of BRCA1’s role in inhibiting microtubule nucleation.47 Thus, these data indicate that BRCA1 is a regulator of gamma-tubulin and is supported by previous work that has shown BRCA1 regulates localization of gamma-tubulin by post-translational modifications.5, 47-49 Our findings indicate possible connections between increases in gamma-tubulin delocalization and BRCA1 mutation status and warrant further investigation.
Microtubule nucleation provides a therapeutic target beyond cell division
Many current cancer therapies rely on reducing cell division by targeting the mitotic machinery and microtubules in rapidly dividing cells.20, 21 Many of these drugs have been highly successful for anti-cancer therapies, such as taxanes, Vinca alkaloids, and colchicine. These drugs focus mainly on blocking mitosis to manipulate the rapidly dividing characteristics of tumor cells by binding to their respective domains on tubulin. A major drawback of many of these therapies is the development of multidrug resistance.22 These therapies largely ignore other cellular processes that are associated with centrosomal abnormalities. Increasing evidence suggests that the centrosome plays an important role in interphase cells and not solely during mitosis, and that these abnormalities may have roles clinically in tumor aggressiveness.1, 41, 50 A previous clinical study revealed an increase in gamma-tubulin protein due to amplification of the gene in patient samples of invasive carcinomas, atypical hyperplasias, and in situ carcinomas, with the highest expression seen in high-grade infiltrating ductal carcinomas.7 Recent work has detailed the intricacies of the microtubule nucleation machinery in roles not associated with cell division, but the function of many of these have yet to be determined.5, 17, 19, 39, 51 Our findings provide a molecular mechanism of gamma-tubulin delocalization in breast cancer through increased solubility which could potentially be the precursor to centrosomal abnormalities often seen in breast cancer which promotes the progression of aggressive or metastatic tumors. Specifically, we show the potential for gamma-tubulin delocalization to confer colchicine resistance in cases such as HCC1937, a cell line derived from a very aggressive tumor, through stabilization of small microtubule fragments (Fig. 6P).
Supplementary Material
Figure S1. Line profiles of gamma-tubulin staining through the centrosome reveals differential levels of cytosolic vs. centrosomal gamma-tubulin expression. Breast cancer cell lines that are typically associated with low metastatic potential (BT-20 and SKBR3) produced line profiles that are consistent with low cytosolic gamma-tubulin expression and high centrosomal gamma-tubulin expression. One moderately aggressive (MCF-7) and two highly aggressive cell lines (HCC1428 and MDA-MB-231) also produced line profiles consistent with normal ratios of gamma-tubulin. Several moderate to highly invasive breast cancer cell lines revealed a significant change in ratio (BT-549, MDA-MB-436, Hs578T, and HCC1937).
Acknowledgements
This work is supported by a predoctoral fellowship (BC083126) from the USA Medical Research and Materiel Command (to E.H.C.), 1R01CA124704-01 from the National Cancer Institute (to S.S.M.), a Breast Cancer Idea Award (BC061047) from the USA Medical Research and Materiel Command (to S.S.M.) and a Cigarette Restitution Fund Cancer Research Grant (CH 649 CRF) from the State of Maryland Department of Health and Mental Hygiene.
Abbreviations
- ER
estrogen receptor
- DRM
Detergent-resistant membranes
- gamma-TuRC
gamma-tubulin ring complex
- gamma-TuSC
gamma-tubulin small complex
- GCP
gamma-tubulin ring complex protein
- MTOC
Microtubule organizing center
- PARP
poly-ADP-ribosyl-polymerase
- PCM
pericentriolar material
- PI3K
phosphoinositide 3-kinase
- PR
progesterone receptor
- Syk
spleen tyrosine kinase
Footnotes
Competing interests The authors declare that they have no competing interests.
Author’s contributions EHC and SSM designed the study and wrote the manuscript. RAW, MAM, EMB contributed to data interpretation, provided material and technical support, and critically reviewed the manuscript.
References
- 1.D’Assoro AB, Barrett SL, Folk C, Negron VC, Boeneman K, Busby R, et al. Amplified centrosomes in breast cancer: a potential indicator of tumor aggressiveness. Breast Cancer Res Treat. 2002;75:25–34. doi: 10.1023/a:1016550619925. [DOI] [PubMed] [Google Scholar]
- 2.Lingle WL, Barrett SL, Negron VC, D’Assoro AB, Boeneman K, Liu W, et al. Centrosome amplification drives chromosomal instability in breast tumor development. Proc Natl Acad Sci U S A. 2002;99:1978–83. doi: 10.1073/pnas.032479999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pihan GA, Wallace J, Zhou Y, Doxsey SJ. Centrosome abnormalities and chromosome instability occur together in pre-invasive carcinomas. Cancer Res. 2003;63:1398–404. [PubMed] [Google Scholar]
- 4.Salisbury JL, D’Assoro AB, Lingle WL. Centrosome amplification and the origin of chromosomal instability in breast cancer. J Mammary Gland Biol Neoplasia. 2004;9:275–83. doi: 10.1023/B:JOMG.0000048774.27697.30. [DOI] [PubMed] [Google Scholar]
- 5.Sankaran S, Parvin JD. Centrosome function in normal and tumor cells. J Cell Biochem. 2006;99:1240–50. doi: 10.1002/jcb.21003. [DOI] [PubMed] [Google Scholar]
- 6.D’Assoro AB, Lingle WL, Salisbury JL. Centrosome amplification and the development of cancer. Oncogene. 2002;21:6146–53. doi: 10.1038/sj.onc.1205772. [DOI] [PubMed] [Google Scholar]
- 7.Liu T, Niu Y, Yu Y, Liu Y, Zhang F. Increased {gamma}-tubulin expression and P16INK4A promoter methylation occur together in preinvasive lesions and carcinomas of the breast. Ann Oncol. 2009 doi: 10.1093/annonc/mdn651. [DOI] [PubMed] [Google Scholar]
- 8.Oakley BR, Akkari YN. Gamma-tubulin at ten: progress and prospects. Cell Struct Funct. 1999;24:365–72. doi: 10.1247/csf.24.365. [DOI] [PubMed] [Google Scholar]
- 9.Mogensen MM, Malik A, Piel M, Bouckson-Castaing V, Bornens M. Microtubule minus-end anchorage at centrosomal and non-centrosomal sites: the role of ninein. J Cell Sci. 2000;113(Pt 17):3013–23. doi: 10.1242/jcs.113.17.3013. [DOI] [PubMed] [Google Scholar]
- 10.Delgehyr N, Sillibourne J, Bornens M. Microtubule nucleation and anchoring at the centrosome are independent processes linked by ninein function. J Cell Sci. 2005;118:1565–75. doi: 10.1242/jcs.02302. [DOI] [PubMed] [Google Scholar]
- 11.Sillibourne JE, Delaval B, Redick S, Sinha M, Doxsey SJ. Chromatin remodeling proteins interact with pericentrin to regulate centrosome integrity. Mol Biol Cell. 2007;18:3667–80. doi: 10.1091/mbc.E06-07-0604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murata T, Hasebe M. Microtubule-dependent microtubule nucleation in plant cells. J Plant Res. 2007;120:73–8. doi: 10.1007/s10265-006-0054-z. [DOI] [PubMed] [Google Scholar]
- 13.Murata T, Sonobe S, Baskin TI, Hyodo S, Hasezawa S, Nagata T, et al. Microtubule-dependent microtubule nucleation based on recruitment of gamma-tubulin in higher plants. Nat Cell Biol. 2005;7:961–8. doi: 10.1038/ncb1306. [DOI] [PubMed] [Google Scholar]
- 14.Keating TJ, Borisy GG. Centrosomal and non-centrosomal microtubules. Biol Cell. 1999;91:321–9. [PubMed] [Google Scholar]
- 15.Luders J, Stearns T. Microtubule-organizing centres: a re-evaluation. Nat Rev Mol Cell Biol. 2007;8:161–7. doi: 10.1038/nrm2100. [DOI] [PubMed] [Google Scholar]
- 16.Wiese C, Zheng Y. Microtubule nucleation: gamma-tubulin and beyond. J Cell Sci. 2006;119:4143–53. doi: 10.1242/jcs.03226. [DOI] [PubMed] [Google Scholar]
- 17.Abal M, Piel M, Bouckson-Castaing V, Mogensen M, Sibarita JB, Bornens M. Microtubule release from the centrosome in migrating cells. J Cell Biol. 2002;159:731–7. doi: 10.1083/jcb.200207076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khodjakov A, Cole RW, Oakley BR, Rieder CL. Centrosome-independent mitotic spindle formation in vertebrates. Curr Biol. 2000;10:59–67. doi: 10.1016/s0960-9822(99)00276-6. [DOI] [PubMed] [Google Scholar]
- 19.Reilein A, Yamada S, Nelson WJ. Self-organization of an acentrosomal microtubule network at the basal cortex of polarized epithelial cells. J Cell Biol. 2005;171:845–55. doi: 10.1083/jcb.200505071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jackson JR, Patrick DR, Dar MM, Huang PS. Targeted anti-mitotic therapies: can we improve on tubulin agents? Nat Rev Cancer. 2007;7:107–17. doi: 10.1038/nrc2049. [DOI] [PubMed] [Google Scholar]
- 21.Jordan MA, Wilson L. Microtubules as a target for anticancer drugs. Nat Rev Cancer. 2004;4:253–65. doi: 10.1038/nrc1317. [DOI] [PubMed] [Google Scholar]
- 22.Fojo T, Menefee M. Mechanisms of multidrug resistance: the potential role of microtubule-stabilizing agents. Ann Oncol. 2007;18(Suppl 5):v3–8. doi: 10.1093/annonc/mdm172. [DOI] [PubMed] [Google Scholar]
- 23.Gonzalez-Angulo AM, Morales-Vasquez F, Hortobagyi GN. Overview of resistance to systemic therapy in patients with breast cancer. Adv Exp Med Biol. 2007;608:1–22. doi: 10.1007/978-0-387-74039-3_1. [DOI] [PubMed] [Google Scholar]
- 24.Gottesman MM, Ling V. The molecular basis of multidrug resistance in cancer: the early years of P-glycoprotein research. FEBS Lett. 2006;580:998–1009. doi: 10.1016/j.febslet.2005.12.060. [DOI] [PubMed] [Google Scholar]
- 25.Blick T, Widodo E, Hugo H, Waltham M, Lenburg ME, Neve RM, et al. Epithelial mesenchymal transition traits in human breast cancer cell lines. Clin Exp Metastasis. 2008;25:629–42. doi: 10.1007/s10585-008-9170-6. [DOI] [PubMed] [Google Scholar]
- 26.Promkan M, Liu G, Patmasiriwat P, Chakrabarty S. BRCA1 modulates malignant cell behavior, the expression of survivin and chemosensitivity in human breast cancer cells. Int J Cancer. 2009 doi: 10.1002/ijc.24684. [DOI] [PubMed] [Google Scholar]
- 27.Sommers CL, Byers SW, Thompson EW, Torri JA, Gelmann EP. Differentiation state and invasiveness of human breast cancer cell lines. Breast Cancer Res Treat. 1994;31:325–35. doi: 10.1007/BF00666165. [DOI] [PubMed] [Google Scholar]
- 28.Thompson EW, Paik S, Brunner N, Sommers CL, Zugmaier G, Clarke R, et al. Association of increased basement membrane invasiveness with absence of estrogen receptor and expression of vimentin in human breast cancer cell lines. J Cell Physiol. 1992;150:534–44. doi: 10.1002/jcp.1041500314. [DOI] [PubMed] [Google Scholar]
- 29.Zajchowski DA, Bartholdi MF, Gong Y, Webster L, Liu HL, Munishkin A, et al. Identification of gene expression profiles that predict the aggressive behavior of breast cancer cells. Cancer Res. 2001;61:5168–78. [PubMed] [Google Scholar]
- 30.Sakai W, Swisher EM, Karlan BY, Agarwal MK, Higgins J, Friedman C, et al. Secondary mutations as a mechanism of cisplatin resistance in BRCA2-mutated cancers. Nature. 2008;451:1116–20. doi: 10.1038/nature06633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neve RM, Chin K, Fridlyand J, Yeh J, Baehner FL, Fevr T, et al. A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell. 2006;10:515–27. doi: 10.1016/j.ccr.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deng CX. Roles of BRCA1 in centrosome duplication. Oncogene. 2002;21:6222–7. doi: 10.1038/sj.onc.1205713. [DOI] [PubMed] [Google Scholar]
- 33.Fleisch MC, Maxwell CA, Kuper CK, Brown ET, Barcellos-Hoff MH, Costes SV. Intensity-based signal separation algorithm for accurate quantification of clustered centrosomes in tissue sections. Microsc Res Tech. 2006;69:964–72. doi: 10.1002/jemt.20372. [DOI] [PubMed] [Google Scholar]
- 34.Chevrier V, Komesli S, Schmit AC, Vantard M, Lambert AM, Job D. A monoclonal antibody, raised against mammalian centrosomes and screened by recognition of plant microtubule organizing centers, identifies a pericentriolar component in different cell types. J Cell Sci. 1992;101(Pt 4):823–35. doi: 10.1242/jcs.101.4.823. [DOI] [PubMed] [Google Scholar]
- 35.Moudjou M, Bordes N, Paintrand M, Bornens M. gamma-Tubulin in mammalian cells: the centrosomal and the cytosolic forms. J Cell Sci. 1996;109(Pt 4):875–87. doi: 10.1242/jcs.109.4.875. [DOI] [PubMed] [Google Scholar]
- 36.Bartolini F, Gundersen GG. Generation of noncentrosomal microtubule arrays. J Cell Sci. 2006;119:4155–63. doi: 10.1242/jcs.03227. [DOI] [PubMed] [Google Scholar]
- 37.Salisbury JL, Lingle WL, White RA, Cordes LE, Barrett S. Microtubule nucleating capacity of centrosomes in tissue sections. J Histochem Cytochem. 1999;47:1265–74. doi: 10.1177/002215549904701006. [DOI] [PubMed] [Google Scholar]
- 38.Godinho SA, Kwon M, Pellman D. Centrosomes and cancer: how cancer cells divide with too many centrosomes. Cancer Metastasis Rev. 2009;28:85–98. doi: 10.1007/s10555-008-9163-6. [DOI] [PubMed] [Google Scholar]
- 39.Macurek L, Draberova E, Richterova V, Sulimenko V, Sulimenko T, Draberova L, et al. Regulation of microtubule nucleation from membranes by complexes of membrane-bound gamma-tubulin with Fyn kinase and phosphoinositide 3-kinase. Biochem J. 2008 doi: 10.1042/BJ20080909. [DOI] [PubMed] [Google Scholar]
- 40.Sarrio D, Rodriguez-Pinilla SM, Dotor A, Calero F, Hardisson D, Palacios J. Abnormal ezrin localization is associated with clinicopathological features in invasive breast carcinomas. Breast Cancer Res Treat. 2006;98:71–9. doi: 10.1007/s10549-005-9133-4. [DOI] [PubMed] [Google Scholar]
- 41.Schneeweiss A, Sinn HP, Ehemann V, Khbeis T, Neben K, Krause U, et al. Centrosomal aberrations in primary invasive breast cancer are associated with nodal status and hormone receptor expression. Int J Cancer. 2003;107:346–52. doi: 10.1002/ijc.11408. [DOI] [PubMed] [Google Scholar]
- 42.Coopman PJ, Do MT, Barth M, Bowden ET, Hayes AJ, Basyuk E, et al. The Syk tyrosine kinase suppresses malignant growth of human breast cancer cells. Nature. 2000;406:742–7. doi: 10.1038/35021086. [DOI] [PubMed] [Google Scholar]
- 43.Coopman PJ, Mueller SC. The Syk tyrosine kinase: a new negative regulator in tumor growth and progression. Cancer Lett. 2006;241:159–73. doi: 10.1016/j.canlet.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 44.Stewart ZA, Pietenpol JA. Syk: a new player in the field of breast cancer. Breast Cancer Res. 2001;3:5–7. doi: 10.1186/bcr261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zyss D, Montcourrier P, Vidal B, Anguille C, Merezegue F, Sahuquet A, et al. The Syk tyrosine kinase localizes to the centrosomes and negatively affects mitotic progression. Cancer Res. 2005;65:10872–80. doi: 10.1158/0008-5472.CAN-05-1270. [DOI] [PubMed] [Google Scholar]
- 46.Elstrodt F, Hollestelle A, Nagel JH, Gorin M, Wasielewski M, van den Ouweland A, et al. BRCA1 mutation analysis of 41 human breast cancer cell lines reveals three new deleterious mutants. Cancer Res. 2006;66:41–5. doi: 10.1158/0008-5472.CAN-05-2853. [DOI] [PubMed] [Google Scholar]
- 47.Sankaran S, Starita LM, Groen AC, Ko MJ, Parvin JD. Centrosomal microtubule nucleation activity is inhibited by BRCA1-dependent ubiquitination. Mol Cell Biol. 2005;25:8656–68. doi: 10.1128/MCB.25.19.8656-8668.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Parvin JD, Sankaran S. The BRCA1 E3 ubiquitin ligase controls centrosome dynamics. Cell Cycle. 2006;5:1946–50. doi: 10.4161/cc.5.17.3208. [DOI] [PubMed] [Google Scholar]
- 49.Sankaran S, Crone DE, Palazzo RE, Parvin JD. BRCA1 Regulates g-Tubulin Binding to Centrosomes. Cancer Biol Ther. 2007;6 doi: 10.4161/cbt.6.12.5164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pujana MA, Han JD, Starita LM, Stevens KN, Tewari M, Ahn JS, et al. Network modeling links breast cancer susceptibility and centrosome dysfunction. Nat Genet. 2007;39:1338–49. doi: 10.1038/ng.2007.2. [DOI] [PubMed] [Google Scholar]
- 51.Hinchcliffe EH, Miller FJ, Cham M, Khodjakov A, Sluder G. Requirement of a centrosomal activity for cell cycle progression through G1 into S phase. Science. 2001;291:1547–50. doi: 10.1126/science.1056866. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Line profiles of gamma-tubulin staining through the centrosome reveals differential levels of cytosolic vs. centrosomal gamma-tubulin expression. Breast cancer cell lines that are typically associated with low metastatic potential (BT-20 and SKBR3) produced line profiles that are consistent with low cytosolic gamma-tubulin expression and high centrosomal gamma-tubulin expression. One moderately aggressive (MCF-7) and two highly aggressive cell lines (HCC1428 and MDA-MB-231) also produced line profiles consistent with normal ratios of gamma-tubulin. Several moderate to highly invasive breast cancer cell lines revealed a significant change in ratio (BT-549, MDA-MB-436, Hs578T, and HCC1937).