Abstract
Exosomes are small membrane vesicles of endocytic origin with a size of 50 – 100 nm. They can contain microRNAs, mRNAs, DNA fragments and proteins, which are shuttled from a donar cell to recipient cells. Many different cell types including immune cells, mesenchymal cells and cancer cells release exosomes. There is emerging evidence that cancer-derived exosomes contribute to the recruitment and reprogramming of constituents associated with tumor environment. Here, we discuss different mechanisms associated with biogenesis, payload and transport of exosomes. We highlight the functional relevance of exosomes in cancer, as related to tumor microenvironment, tumor immunology, angiogenesis and metastasis. Exosomes may exert an immunosuppressive function as well as trigger an anti-tumor response by presenting tumor antigens to dendritic cells. Exosomes may serve as cancer biomarkers and aid in the treatment of cancer.
Keywords: exosomes, cancer, tumor microenvironment, angiogenesis, metastasis, therapy
Introduction
Malignant tumors are complex structures that consist of cancer cells and the surrounding tumor stroma [1]. A continuous cross-talk between cancer cells and local/distant host environment is required for effective tumor growth and systemic dissemination [2]. Emerging evidence suggests that exosomes may play a pivotal role in local and systemic cell-cell communication in cancer. Exosomes were described by Trams and colleagues, who observed that cultures from various normal and neoplastic cell lines produced vesicles with 5′-nucleotidase activity, which reflected the ecto-enzyme activity of the parent monolayer culture. Inside larger vesicles, they observed a second population of vesicles about 40 nm in diameter, which they termed exosomes [3]. Further publications revealed that exosomes are small membrane vesicles of endocytic origin with a size of 40 – 100 nm. They contain microRNAs (miRNAs), messenger RNAs (mRNA), DNA fragments and proteins. Exosomes can be shuttled from a donator cell to recipient cells [4, 5]. There is emerging evidence that cancer-derived exosomes may contribute to the recruitment and reprogramming of the tumor microenvironment to form a pro-tumorigenic soil.
Biogenesis of exosomes
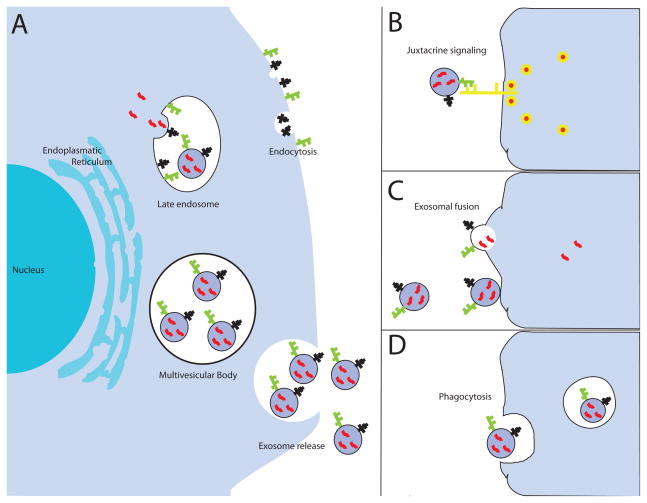
In contrast with larger microvesicles, which are directly shed from the plasma membrane, exosomes derive from the intra-cellular endosomal compartment (Fig. 1a). They are initially formed by a ceramide-triggered process of inward budding from the limiting membrane of late endosomes [6]. This process encapsulates cytoplasmic RNA molecules and functional proteins into exosomes. In a second step, these multivesicular endosomes (also referred to as multivesicular bodies (MVB)) fuse with the cellular membrane to release exosomes into the extracellular space [3, 7]. Multivesicular endosome biogenesis is regulated by the “endosomal sorting complexes required for transport” (ESCRT), although alternative pathways may also exist [8]. The ESCRT complexe recognize ubiquinated membrane proteins and promote their internalization into the multivesicular endosome [9]. The secretion of exosomes is promoted by the Rab GTPases 27a and 27b and can be triggered by hypoxia – often encountered in tumors [10–14]. The mechanism associated with packaging of exosomes content still remains elusive. A comparison of the exosomes composition with their parental cells indicates a selective enrichment process within the exosomes [15–17]. Skog et al. performed a microarray analysis of mRNA in exosomes and their donor glioblastoma cells [17]. They found 3426 transcripts that are present at different in exosomes and their donor cells, with many genes showing a 5-fold difference. Likewise, the expression profile of microRNAs between exosomes and their parent cancer cells displays a different pattern in bladder cancer and gastric cancer [15, 16]. These studies further emphasize the assumption that there is a specific sorting of mRNAs and miRNAs into exosomes and not just a random event. The specific packing of exosomes may help cancer cells to discard tumor suppressive microRNAs, thus increasing the capacity oncogenic potency of cancer cells [16].
Fig. 1. Biogenesis and uptake of exosomes in target cells.
(a) Early endosomes mature into late endosomes and exosomes are formed by a process of inward budding from the limiting membrane. Via this mechanism, cytoplasmic RNA molecules and functional proteins are encapsulated into exosomes. Moreover, transmembrane proteins maintain the same orientation relative to the cytoplasm and plasma membrane. In a second step, multivesicular endosomes fuse with the cellular membrane to release the exosomes into the extracellular space. Several cellular signals have been suggested: (b) juxtracrine signaling through receptor-ligand interactions, (c) fusion of exosomes with the cellular membrane of the target cell, resulting in a direct release of the cargo into the cytoplasm, (d) phagocytosis in an actin-cytoskeletal and phosphatidylinositol 3-kinase dependent manner.
Exosomes from different cell types contain a core set of identical proteins. These include members of the tetraspanin family (CD9, CD63, CD81 and CD82), members of the ESCRT complex (TSG101, Alix) and heat shock proteins (Hsp 60, Hsp70, Hsp90) [18]. Apart from these protein, exosomes also contain some specific proteins reflective of the parental cell. Epithelial tumor cells secrete exosomes carrying the epithelial cell adhesion molecule (EpCAM) [19, 20]. Melanoma-derived exosomes contain the tumor-associated antigen Mart-1 [21, 22]. Exosomes from gastric cancer, breast cancer or pancreatic cancer express members of the human epidermal receptor (HER) family [23–25].
Exosome uptake in recipient/target cells
The uptake of exosomes occurs in a non-random process and is dependent on transmembrane proteins [26]. Recent studies indicate that the tetraspanin-integrin complex contributes considerably in targeting enabling the binding of exosomes to target cells [27, 28]. Moreover, a pro- inflammatory environment may enhance the expression of receptor molecules such as ICAM-1 on the membrane surface, which increases the exosomes adhesion to the target cells [29]. The subsequent mechanism/s associated with exosomes internalization is still poorly understood. The presence of the T-cell receptor/CD3 complex and the chemokine receptor CXCR4 on exosomes of T-cells suggests a juxtracrine signalling through receptor-ligand interactions (Fig. 1b) [30]. Moreover, exosomes can fuse with the cell membrane of target cells, which results in a direct release of their cargo into the cytoplasm (Fig. 1c) [29, 31]. Cellular internalization of exosomes can occur by phagocytosis in an actin-cytoskeleton and phosphatidylinositol 3-kinase -dependent manner (Fig. 1d) [26]. Additional studies are required to clarify how exosomes are directed to the target cells in cancer and whether the cell specific composition of exosomes results in organotropism associated with metastatic disease.
Functional relevance of exosomes in cancer
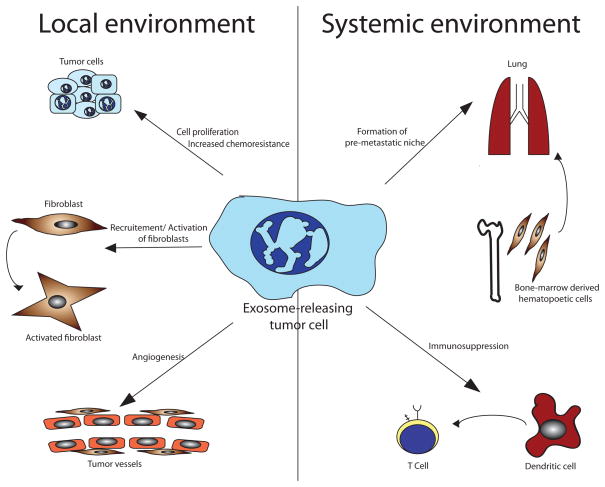
Exosomes can have a bimodal role in cancer. They can manipulate the local and systemic environment to aid in cancer growth and dissemination (Fig. 2). Exosomes may also program the immune system to elicit an anti-tumor response. Exosomes in tumors are heterogenous and secreted by all the cells and this leads to a network of interactions that are likely complex.
Fig. 2. Functional relevance of exosomes in cancer.
Tumor-derived exosomes can alter local and systemic microenvironment. By transferring oncogenic proteins and multidrug transporters, they can facilitate cancer cell proliferation and chemoresistance. Fibroblasts are converted into myofibroblasts, which are a key source of matrix remodelling proteins within the tumor microenvironment and participate in tumor angiogenesis. Endothelial cells are activated to support tumor angiogenesis. In the systemic environment, exosome-mediated signaling results in the recruitment of bone marrow-derived hematopoetic cells in order to form a pre-metastatic niche in distant organs. Tumor-derived exosomes interact with myeloid-derived cells to suppress the anti-tumor immune response.
Exosomes mediated transfer of oncogenic proteins between cancer cells
Via exosomes mediated transfer, tumor cells exchange proteins with oncogenic activity. The functional purpose for this is till largely undetermined. A mutant epidermal growth factor receptor (known as EGFRvIII), residing on the exosome membrane derived from glioma cells, can be delivered to cells lacking this mutant form [32]. The integration of EGFRvIII into these cells leads to an augmented expression of anti-apoptotic genes and an increase in anchorage independent growth capacity [32]. Colon cancer cells harboring only mutant KRAS alleles are capable of releasing exosomes with mutant KRAS proteins [33]. The mutant isoform is internalized by colon cancer cells expressing the wild-type KRAS. The exosomes mediated exchange of mutant KRAS induces enhanced cell growth and tumorigenicity [33]. Preliminary results suggest that exosomes mediated transfer of proteins between cancer cells can lead to chemoresistance [34]. Exosomes from two docetaxel-resistant prostate cancer cell lines can confer chemoresistance to non-resistant prostate cancer cell lines via exosomes mediated transfer of drug transporter, MDR-1 [34]. Further validation in mouse models is required. However, these observations represent a new paradigm of how malignant cells may increase their tumorigenic potential and develop chemoresistance via exosomes.
Exosome-mediated modulation of the tumor microenviroment and angiogenesis
Exosomes from prostate cancer cells and mesothelioma cell lines contain TGF-β1 protein, which is transferred to recipient cells in a biologically active form [35]. In vitro experiments have shown that TGF-β1 expressing exosomes can trigger the differentiation of fibroblasts to myofibroblasts [35]. Exosomes from breast cancer cells can convert adipose tissue-derived mesenchymal stem cells into myofibroblasts via a SMAD-mediated pathway [36]. Myofibroblasts are a key source of matrix remodelling proteins within the tumor microenvironment and participate in tumor angiogenesis [37]. In this regard, tumor exosome-induced recruitment of fibroblasts could support tumor angiogenesis. Furthermore, cancer cells transfer membrane-bound EGFR to endothelial cells via exosomes [38]. This transfer activates the autocrine VEGF/VEGFR-2 pathway in endothelial cells and likely supports tumor angiogenesis.
Jung et al. show for the first time how cancer-associated exosomes participate in the formation of the pre-metastatic niche in a rodent pancreatic cancer model [39]. Grange et al. have reported that CD105-positive renal carcinoma cells secrete exosomes, which activate endothelial cells to organize capillary-like structures on Matrigel and induce enhanced chemoresistance in vitro [40]. Moreover, this study revealed that CD105-positive exosomes contribute in establishing a pre-metastatic niche in the lung microenvironment of SCID mice by upregulating MMP2, MMP9 and VEGFR1 [40]. Similarly, another study has shown that melanoma-derived exosomes enhance the lung endothelial permeability and increase lung metastases in mice [11]. In the same study, Peinado et al. demonstrated that melanoma cell-derived exosomes are capable of recruiting bone-marrow derived cells to initiate a pre-metastatic niche. Collectively, these studies indicate that tumor-derived exosomes play a crucial role in manipulating the tumor microenvironment for the benefit of cancer cells. Intercellular communication via exosomes is a reciprocal and not unidirectional between cancer cells and cancer-associated stroma. Luga et al. show that fibroblast-derived exosomes, which are positive for the tetraspanin Cd81, activate an autocrine Wnt-signaling pathway in breast cancer cells to facilitate migration [41]. The exosome-induced stimulation of the Wnt-pathway was associated with increased protrusive activity, motility, invasion and lung metastasis in an orthotopic mouse model of breast cancer.
Modulation of immune system by cancer-derived exosomes
Cancer cells recruit immune cells to enhance tumor invasion, tumor angiogenesis and dissemination [42]. Exosome-mediated communication between tumor cells and the immune system is involved in recruiting pro-tumorigenic immune cells. In a murine breast cancer model, 4T1 cancer cells release exosomes in a Rab27a-dependent manner [12]. Blockade of exosome secretion by inhibiting Rab27a is associated with a decreased mobilization of neutrophils. Such impairment results in a decreased primary tumor growth and lung metastasis. MicroRNAs in lung cancer-released exosomes can silence the transcripts associated with Toll-like receptor (TLR) family in macrophages [43]. This mechanism stimulates macrophages to secrete proinflammatory cytokines, which supports enhanced tumor dissemination. Cancer cells are capable of inhibiting anti-tumor functions of the host immune system via an exosome-induced signaling. Chalmin et al. show that tumor-derived exosomes activate myeloid-derived suppressor cells (MDSC) [44]. MDSCs exert immunosuppressive functions in cancer by suppressing the T cell response [45]. Chalmin and colleagues found that tumor-derived exosomes from different cancer cell lines induce interleukin-6 (IL-6) production in MDSCs through the activation of the Toll-like receptor 2 via the membrane-associated heat shock protein 72 (Hsp72) [44]. IL-6 production results in an autocrine phosphorylation of Stat3 in MDSCs, which promotes their immunosuppressive effect. Further studies have revealed that tumor-derived exosomes express Fas ligand [46–48]. Fas containing exosomes can elicit immunosuppressive effect by inducing apoptosis in tumor-reactive CD8+ T lymphocytes [48, 49]. Cancer cells are able to release exosomes that stimulate the expansion of regulatory T (Treg) cells [48, 50]. Tregs cause immunosuppression in the tumor microenvironment by impairing the function of anti-tumorigenic T cells [51]. Cancer-derived exosomes modulate the immune systems by triggering the immunosuppressive response, which in turn favors tumor progression.
Tumor-derived exosomes as a source of antigens associated with tumor rejection
Several studies have demonstrated that exosomes can transport antigens from tumor cells to antigen presenting dendritic cells [22, 52, 53]. Via MHC-I molecules, dendritic cells prime cytotoxic T lymphocytes evoke an anti-tumor response and suppress tumor growth in vivo [52]. Moreover, exosomes from pancreatic cancer cells and hepatocellular carcinoma cell lines contain heat shock protein 70 (Hsp70) which can directly activate natural killer (NK) cells [54, 55]. As result, NK cells initiate apoptosis in tumors through granzyme B. In accordance with these findings, Elsner et al. have reported that exosomal Hsp70 activates mouse NK cells which is associated with decrease in primary tumor size and metastases in a murine melanoma model [56]. However, there is still an ongoing debate whether tumor-derived exosomes are adequate mode of vaccination against cancer cells.
Exosomes in clinical diagnosis and cancer therapy
The use of exosomes in tumor diagnosis
Exosomes contain a wide range of RNA molecules and proteins. Therefore, cancer-derived exosomes may provide information about tumor signature. Exosomes are very stable under different storage conditions including short time storage at 4°C for 96 hours or long time storage at −70°C [57]. Such characteristics qualify circulating serum exosomes as potential biomarkers to predict cancer burden at an early stage and impact personalized cancer care. For example, a clinical subtype of glioblastoma contains EGFRvIII mutant/variant [17]. The expression of this EGFRvIII mutant/variant is decisive in determining the therapeutic approach for such patients [58]. In this regard, Skog et al. have demonstrated that circulating serum exosomes are positive for this mutant/variant EGFRvIII when the parental glioblastoma cells also expressed this mutant/variant [17]. Therefore, determining EGFR status from a small serum sample derived exosomes instead of a need for primary tumor biopsy that involved invasive brain surgery, is of immense benefit for patients. Such application for exosomes in tumor diagnosis have been described for colorectal cancer [19], ovarian cancer [57] and melanoma [11]. Garcia et al. have reported that high levels of exosomes in plasma of patients with colorectal cancer was significantly associated with poorly differentiated tumors and with decreased overall survival [19]. Patients with ovarian cancer exhibit significantly increased levels of serum exosomes compared to benign disease or healthy controls [57]. A profile between exosome-derived miRNA and corresponding tumor-derived miRNA revealed a dysregulated expression of 43 out of 218 detectable miRNAs [57]. These data point toward a specific selection of miRNAs in tumor-derived exosomes. Exosomes derived miRNA and mRNA could serve as a useful tool to classify patients into different risk categories to enable a more tailored cancer therapy.
Exosomes as potential therapeutical target
By releasing exosomes, cancer cells modify the local and systemic environment to support tumor progression and metastasis. Therefore, impairing the secretion of exosomes by cancer cells might constitute a potential target for cancer therapy. Exosome secretion is triggered by an intracellular increase of calcium (Ca2+) [59]. H+/Na+ and Na+/Ca2+ channels regulate the intracellular Ca2+ concentration and likely control the release of exosomes. Preclinical results have suggested that blocking these channels using dimethyl amiloride (DMA) reduces the secretion of exosomes in CT26 tumor bearing mice [44]. Additionally, DMA blunts Stat3 phosphorylation in MDSCs and their T cell-suppressive functions, so specific effects are hard to assign at this point [44]. Exosome-mediated tumor growth can be inhibited by targeting sphingomyelinase 2 with the chemical inhibitor GW4869 [60]. Sphingomyelinase 2 regulates the biosynthesis of ceramide, which triggers the inward budding of exosomes from the limiting membrane of multivesicular endosomes [6]. When lung cancer bearing mice are treated with GW4869, Fabbri et al. describe significantly lower number of lung metastases [43]. Andre et al. incubate activated dendritic cells (ex vivo) with exosomes from autologous ascites of patients. They observed that in seven out of nine patients with cancer, lymphocytes specific to the tumor could be expanded from peripheral blood cells by pulsing autologous activated dendritic cells with autologous ascites exosomes [22]. Based on these results, Andre et al. conclude that exosomes could be used as a new source of tumor-rejection antigens in order to immunize patients against cancer. At the present time, two Phase I trials have evaluated the feasibility of autologous dendritic cell-derived exosomes as immunotherapuetics in patients with lung cancer and melanoma [61, 62]. In most cases, the specificity of any such approaches remains to be established. Nevertheless, exciting work on exosomes is ahead of us.
References
- 1.Sund M, Kalluri R. Tumor stroma derived biomarkers in cancer. Cancer metastasis reviews. 2009;28:177–183. doi: 10.1007/s10555-008-9175-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kalluri R, Zeisberg M. Fibroblasts in cancer. Nature reviews Cancer. 2006;6:392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 3.Trams EG, Lauter CJ, Salem N, Jr, Heine U. Exfoliation of membrane ecto-enzymes in the form of micro-vesicles. Biochimica et biophysica acta. 1981;645:63–70. doi: 10.1016/0005-2736(81)90512-5. [DOI] [PubMed] [Google Scholar]
- 4.Balaj L, Lessard R, Dai L, Cho YJ, Pomeroy SL, Breakefield XO, Skog J. Tumour microvesicles contain retrotransposon elements and amplified oncogene sequences. Nature communications. 2011;2:180. doi: 10.1038/ncomms1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nature cell biology. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 6.Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F, Schwille P, Brugger B, Simons M. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science. 2008;319:1244–1247. doi: 10.1126/science.1153124. [DOI] [PubMed] [Google Scholar]
- 7.Fevrier B, Raposo G. Exosomes: endosomal-derived vesicles shipping extracellular messages. Current opinion in cell biology. 2004;16:415–421. doi: 10.1016/j.ceb.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 8.Hurley JH, Odorizzi G. Get on the exosome bus with ALIX. Nature cell biology. 2012;14:654–655. doi: 10.1038/ncb2530. [DOI] [PubMed] [Google Scholar]
- 9.Wollert T, Hurley JH. Molecular mechanism of multivesicular body biogenesis by ESCRT complexes. Nature. 2010;464:864–869. doi: 10.1038/nature08849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ostrowski M, Carmo NB, Krumeich S, Fanget I, Raposo G, Savina A, Moita CF, Schauer K, Hume AN, Freitas RP, et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nature cell biology. 2010;12:19–30sup. 11–13. doi: 10.1038/ncb2000. [DOI] [PubMed] [Google Scholar]
- 11.Peinado H, Aleckovic M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G, Hergueta-Redondo M, Williams C, Garcia-Santos G, Ghajar C, et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nature medicine. 2012;18:883–891. doi: 10.1038/nm.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bobrie A, Krumeich S, Reyal F, Recchi C, Moita LF, Seabra MC, Ostrowski M, Thery C. Rab27a supports exosome-dependent and -independent mechanisms that modify the tumor microenvironment and can promote tumor progression. Cancer research. 2012;72:4920–4930. doi: 10.1158/0008-5472.CAN-12-0925. [DOI] [PubMed] [Google Scholar]
- 13.King HW, Michael MZ, Gleadle JM. Hypoxic enhancement of exosome release by breast cancer cells. BMC cancer. 2012;12:421. doi: 10.1186/1471-2407-12-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Borges FT, Melo SA, Ozdemir BC, Kato N, Revuelta I, Miller CA, Gattone VH, 2nd, Lebleu VS, Kalluri R. TGF-beta1-Containing Exosomes from Injured Epithelial Cells Activate Fibroblasts to Initiate Tissue Regenerative Responses and Fibrosis. Journal of the American Society of Nephrology: JASN. 2012 doi: 10.1681/ASN.2012101031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hessvik NP, Phuyal S, Brech A, Sandvig K, Llorente A. Profiling of microRNAs in exosomes released from PC-3 prostate cancer cells. Biochimica et biophysica acta. 2012;1819:1154–1163. doi: 10.1016/j.bbagrm.2012.08.016. [DOI] [PubMed] [Google Scholar]
- 16.Ohshima K, Inoue K, Fujiwara A, Hatakeyama K, Kanto K, Watanabe Y, Muramatsu K, Fukuda Y, Ogura S, Yamaguchi K, et al. Let-7 microRNA family is selectively secreted into the extracellular environment via exosomes in a metastatic gastric cancer cell line. PloS one. 2010;5:e13247. doi: 10.1371/journal.pone.0013247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Skog J, Wurdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M, Curry WT, Jr, Carter BS, Krichevsky AM, Breakefield XO. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nature cell biology. 2008;10:1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taylor DD, Gercel-Taylor C. Exosomes/microvesicles: mediators of cancer-associated immunosuppressive microenvironments. Seminars in immunopathology. 2011;33:441–454. doi: 10.1007/s00281-010-0234-8. [DOI] [PubMed] [Google Scholar]
- 19.Silva J, Garcia V, Rodriguez M, Compte M, Cisneros E, Veguillas P, Garcia JM, Dominguez G, Campos-Martin Y, Cuevas J, et al. Analysis of exosome release and its prognostic value in human colorectal cancer. Genes, chromosomes & cancer. 2012;51:409–418. doi: 10.1002/gcc.21926. [DOI] [PubMed] [Google Scholar]
- 20.Runz S, Keller S, Rupp C, Stoeck A, Issa Y, Koensgen D, Mustea A, Sehouli J, Kristiansen G, Altevogt P. Malignant ascites-derived exosomes of ovarian carcinoma patients contain CD24 and EpCAM. Gynecologic oncology. 2007;107:563–571. doi: 10.1016/j.ygyno.2007.08.064. [DOI] [PubMed] [Google Scholar]
- 21.Mears R, Craven RA, Hanrahan S, Totty N, Upton C, Young SL, Patel P, Selby PJ, Banks RE. Proteomic analysis of melanoma-derived exosomes by two-dimensional polyacrylamide gel electrophoresis and mass spectrometry. Proteomics. 2004;4:4019–4031. doi: 10.1002/pmic.200400876. [DOI] [PubMed] [Google Scholar]
- 22.Andre F, Schartz NE, Movassagh M, Flament C, Pautier P, Morice P, Pomel C, Lhomme C, Escudier B, Le Chevalier T, et al. Malignant effusions and immunogenic tumour-derived exosomes. Lancet. 2002;360:295–305. doi: 10.1016/S0140-6736(02)09552-1. [DOI] [PubMed] [Google Scholar]
- 23.Ciravolo V, Huber V, Ghedini GC, Venturelli E, Bianchi F, Campiglio M, Morelli D, Villa A, Della Mina P, Menard S, et al. Potential role of HER2-overexpressing exosomes in countering trastuzumab-based therapy. Journal of cellular physiology. 2012;227:658–667. doi: 10.1002/jcp.22773. [DOI] [PubMed] [Google Scholar]
- 24.Adamczyk KA, Klein-Scory S, Tehrani MM, Warnken U, Schmiegel W, Schnolzer M, Schwarte-Waldhoff I. Characterization of soluble and exosomal forms of the EGFR released from pancreatic cancer cells. Life sciences. 2011;89:304–312. doi: 10.1016/j.lfs.2011.06.020. [DOI] [PubMed] [Google Scholar]
- 25.Baran J, Baj-Krzyworzeka M, Weglarczyk K, Szatanek R, Zembala M, Barbasz J, Czupryna A, Szczepanik A, Zembala M. Circulating tumour-derived microvesicles in plasma of gastric cancer patients. Cancer immunology, immunotherapy: CII. 2010;59:841–850. doi: 10.1007/s00262-009-0808-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feng D, Zhao WL, Ye YY, Bai XC, Liu RQ, Chang LF, Zhou Q, Sui SF. Cellular internalization of exosomes occurs through phagocytosis. Traffic. 2010;11:675–687. doi: 10.1111/j.1600-0854.2010.01041.x. [DOI] [PubMed] [Google Scholar]
- 27.Nazarenko I, Rana S, Baumann A, McAlear J, Hellwig A, Trendelenburg M, Lochnit G, Preissner KT, Zoller M. Cell surface tetraspanin Tspan8 contributes to molecular pathways of exosome-induced endothelial cell activation. Cancer research. 2010;70:1668–1678. doi: 10.1158/0008-5472.CAN-09-2470. [DOI] [PubMed] [Google Scholar]
- 28.Rana S, Yue S, Stadel D, Zoller M. Toward tailored exosomes: the exosomal tetraspanin web contributes to target cell selection. The international journal of biochemistry & cell biology. 2012;44:1574–1584. doi: 10.1016/j.biocel.2012.06.018. [DOI] [PubMed] [Google Scholar]
- 29.Clayton A, Turkes A, Dewitt S, Steadman R, Mason MD, Hallett MB. Adhesion and signaling by B cell-derived exosomes: the role of integrins. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2004;18:977–979. doi: 10.1096/fj.03-1094fje. [DOI] [PubMed] [Google Scholar]
- 30.Blanchard N, Lankar D, Faure F, Regnault A, Dumont C, Raposo G, Hivroz C. TCR activation of human T cells induces the production of exosomes bearing the TCR/CD3/zeta complex. J Immunol. 2002;168:3235–3241. doi: 10.4049/jimmunol.168.7.3235. [DOI] [PubMed] [Google Scholar]
- 31.Montecalvo A, Larregina AT, Shufesky WJ, Stolz DB, Sullivan ML, Karlsson JM, Baty CJ, Gibson GA, Erdos G, Wang Z, et al. Mechanism of transfer of functional microRNAs between mouse dendritic cells via exosomes. Blood. 2012;119:756–766. doi: 10.1182/blood-2011-02-338004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Al-Nedawi K, Meehan B, Micallef J, Lhotak V, May L, Guha A, Rak J. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nature cell biology. 2008;10:619–624. doi: 10.1038/ncb1725. [DOI] [PubMed] [Google Scholar]
- 33.Demory Beckler M, Higginbotham JN, Franklin JL, Ham AJ, Halvey PJ, Imasuen IE, Whitwell C, Li M, Liebler DC, Coffey RJ. Proteomic analysis of exosomes from mutant KRAS colon cancer cells identifies intercellular transfer of mutant KRAS. Molecular & cellular proteomics: MCP. 2012 doi: 10.1074/mcp.M112.022806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Corcoran C, Rani S, O’Brien K, O’Neill A, Prencipe M, Sheikh R, Webb G, McDermott R, Watson W, Crown J, et al. Docetaxel-Resistance in Prostate Cancer: Evaluating Associated Phenotypic Changes and Potential for Resistance Transfer via Exosomes. PloS one. 2012;7:e50999. doi: 10.1371/journal.pone.0050999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Webber J, Steadman R, Mason MD, Tabi Z, Clayton A. Cancer exosomes trigger fibroblast to myofibroblast differentiation. Cancer research. 2010;70:9621–9630. doi: 10.1158/0008-5472.CAN-10-1722. [DOI] [PubMed] [Google Scholar]
- 36.Cho JA, Park H, Lim EH, Lee KW. Exosomes from breast cancer cells can convert adipose tissue-derived mesenchymal stem cells into myofibroblast-like cells. International journal of oncology. 2012;40:130–138. doi: 10.3892/ijo.2011.1193. [DOI] [PubMed] [Google Scholar]
- 37.Vong S, Kalluri R. The role of stromal myofibroblast and extracellular matrix in tumor angiogenesis. Genes & cancer. 2011;2:1139–1145. doi: 10.1177/1947601911423940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Al-Nedawi K, Meehan B, Kerbel RS, Allison AC, Rak J. Endothelial expression of autocrine VEGF upon the uptake of tumor-derived microvesicles containing oncogenic EGFR. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:3794–3799. doi: 10.1073/pnas.0804543106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jung T, Castellana D, Klingbeil P, Cuesta Hernandez I, Vitacolonna M, Orlicky DJ, Roffler SR, Brodt P, Zoller M. CD44v6 dependence of premetastatic niche preparation by exosomes. Neoplasia. 2009;11:1093–1105. doi: 10.1593/neo.09822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grange C, Tapparo M, Collino F, Vitillo L, Damasco C, Deregibus MC, Tetta C, Bussolati B, Camussi G. Microvesicles released from human renal cancer stem cells stimulate angiogenesis and formation of lung premetastatic niche. Cancer research. 2011;71:5346–5356. doi: 10.1158/0008-5472.CAN-11-0241. [DOI] [PubMed] [Google Scholar]
- 41.Luga V, Zhang L, Viloria-Petit AM, Ogunjimi AA, Inanlou MR, Chiu E, Buchanan M, Hosein AN, Basik M, Wrana JL. Exosomes Mediate Stromal Mobilization of Autocrine Wnt-PCP Signaling in Breast Cancer Cell Migration. Cell. 2012;151:1542–1556. doi: 10.1016/j.cell.2012.11.024. [DOI] [PubMed] [Google Scholar]
- 42.Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nature reviews Cancer. 2009;9:239–252. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fabbri M, Paone A, Calore F, Galli R, Gaudio E, Santhanam R, Lovat F, Fadda P, Mao C, Nuovo GJ, et al. MicroRNAs bind to Toll-like receptors to induce prometastatic inflammatory response. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:E2110–2116. doi: 10.1073/pnas.1209414109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chalmin F, Ladoire S, Mignot G, Vincent J, Bruchard M, Remy-Martin JP, Boireau W, Rouleau A, Simon B, Lanneau D, et al. Membrane-associated Hsp72 from tumor-derived exosomes mediates STAT3-dependent immunosuppressive function of mouse and human myeloid-derived suppressor cells. The Journal of clinical investigation. 2010;120:457–471. doi: 10.1172/JCI40483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nagaraj S, Gabrilovich DI. Regulation of suppressive function of myeloid-derived suppressor cells by CD4+ T cells. Seminars in cancer biology. 2012;22:282–288. doi: 10.1016/j.semcancer.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Taylor DD, Gercel-Taylor C, Lyons KS, Stanson J, Whiteside TL. T-cell apoptosis and suppression of T-cell receptor/CD3-zeta by Fas ligand-containing membrane vesicles shed from ovarian tumors. Clinical cancer research: an official journal of the American Association for Cancer Research. 2003;9:5113–5119. [PubMed] [Google Scholar]
- 47.Martinez-Lorenzo MJ, Anel A, Alava MA, Pineiro A, Naval J, Lasierra P, Larrad L. The human melanoma cell line MelJuSo secretes bioactive FasL and APO2L/TRAIL on the surface of microvesicles. Possible contribution to tumor counterattack. Experimental cell research. 2004;295:315–329. doi: 10.1016/j.yexcr.2003.12.024. [DOI] [PubMed] [Google Scholar]
- 48.Wieckowski EU, Visus C, Szajnik M, Szczepanski MJ, Storkus WJ, Whiteside TL. Tumor-derived microvesicles promote regulatory T cell expansion and induce apoptosis in tumor-reactive activated CD8+ T lymphocytes. J Immunol. 2009;183:3720–3730. doi: 10.4049/jimmunol.0900970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Abusamra AJ, Zhong Z, Zheng X, Li M, Ichim TE, Chin JL, Min WP. Tumor exosomes expressing Fas ligand mediate CD8+ T-cell apoptosis. Blood cells, molecules & diseases. 2005;35:169–173. doi: 10.1016/j.bcmd.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 50.Szajnik M, Czystowska M, Szczepanski MJ, Mandapathil M, Whiteside TL. Tumor-derived microvesicles induce, expand and up-regulate biological activities of human regulatory T cells (Treg) PloS one. 2010;5:e11469. doi: 10.1371/journal.pone.0011469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lindau D, Gielen P, Kroesen M, Wesseling P, Adema GJ. The immunosuppressive tumor network: MDSCs, Tregs and NKT cells. Immunology. 2012 doi: 10.1111/imm.12036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wolfers J, Lozier A, Raposo G, Regnault A, Thery C, Masurier C, Flament C, Pouzieux S, Faure F, Tursz T, et al. Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nature medicine. 2001;7:297–303. doi: 10.1038/85438. [DOI] [PubMed] [Google Scholar]
- 53.Dai S, Zhou X, Wang B, Wang Q, Fu Y, Chen T, Wan T, Yu Y, Cao X. Enhanced induction of dendritic cell maturation and HLA-A*0201-restricted CEA-specific CD8(+) CTL response by exosomes derived from IL-18 gene-modified CEA-positive tumor cells. J Mol Med (Berl) 2006;84:1067–1076. doi: 10.1007/s00109-006-0102-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gastpar R, Gehrmann M, Bausero MA, Asea A, Gross C, Schroeder JA, Multhoff G. Heat shock protein 70 surface-positive tumor exosomes stimulate migratory and cytolytic activity of natural killer cells. Cancer research. 2005;65:5238–5247. doi: 10.1158/0008-5472.CAN-04-3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lv LH, Wan YL, Lin Y, Zhang W, Yang M, Li GL, Lin HM, Shang CZ, Chen YJ, Min J. Anticancer drugs cause release of exosomes with heat shock proteins from human hepatocellular carcinoma cells that elicit effective natural killer cell antitumor responses in vitro. The Journal of biological chemistry. 2012;287:15874–15885. doi: 10.1074/jbc.M112.340588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Elsner L, Muppala V, Gehrmann M, Lozano J, Malzahn D, Bickeboller H, Brunner E, Zientkowska M, Herrmann T, Walter L, et al. The heat shock protein HSP70 promotes mouse NK cell activity against tumors that express inducible NKG2D ligands. J Immunol. 2007;179:5523–5533. doi: 10.4049/jimmunol.179.8.5523. [DOI] [PubMed] [Google Scholar]
- 57.Taylor DD, Gercel-Taylor C. MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecologic oncology. 2008;110:13–21. doi: 10.1016/j.ygyno.2008.04.033. [DOI] [PubMed] [Google Scholar]
- 58.Mellinghoff IK, Wang MY, Vivanco I, Haas-Kogan DA, Zhu S, Dia EQ, Lu KV, Yoshimoto K, Huang JH, Chute DJ, et al. Molecular determinants of the response of glioblastomas to EGFR kinase inhibitors. The New England journal of medicine. 2005;353:2012–2024. doi: 10.1056/NEJMoa051918. [DOI] [PubMed] [Google Scholar]
- 59.Savina A, Furlan M, Vidal M, Colombo MI. Exosome release is regulated by a calcium-dependent mechanism in K562 cells. The Journal of biological chemistry. 2003;278:20083–20090. doi: 10.1074/jbc.M301642200. [DOI] [PubMed] [Google Scholar]
- 60.Kosaka N, Iguchi H, Yoshioka Y, Takeshita F, Matsuki Y, Ochiya T. Secretory mechanisms and intercellular transfer of microRNAs in living cells. The Journal of biological chemistry. 2010;285:17442–17452. doi: 10.1074/jbc.M110.107821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Escudier B, Dorval T, Chaput N, Andre F, Caby MP, Novault S, Flament C, Leboulaire C, Borg C, Amigorena S, et al. Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: results of the first phase I clinical trial. Journal of translational medicine. 2005;3:10. doi: 10.1186/1479-5876-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Morse MA, Garst J, Osada T, Khan S, Hobeika A, Clay TM, Valente N, Shreeniwas R, Sutton MA, Delcayre A, et al. A phase I study of dexosome immunotherapy in patients with advanced non-small cell lung cancer. Journal of translational medicine. 2005;3:9. doi: 10.1186/1479-5876-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]