Abstract
Emotions are not simply concepts that live privately in the mind, but rather affective states that emanate from the individual and may influence others. We explored affect contagion in the context of one of the closest dyadic units, mother and infant. We initially separated mothers and infants; randomly assigned the mothers to experience a stressful positive-evaluation task, a stressful negative-evaluation task, or a nonstressful control task; and then reunited the mothers and infants. Three notable findings were obtained: First, infants’ physiological reactivity mirrored mothers’ reactivity engendered by the stress manipulation. Second, infants whose mothers experienced social evaluation showed more avoidance toward strangers compared with infants whose mothers were in the control condition. Third, the negative-evaluation condition, compared with the other conditions, generated greater physiological covariation in the dyads, and this covariation increased over time. These findings suggest that mothers’ stressful experiences are contagious to their infants and that members of close pairs, like mothers and infants, can reciprocally influence each other’s dynamic physiological reactivity.
Keywords: electrophysiology, emotional development, infant development, social behavior, stress reactions
The human experience of emotion seems personal and internally generated, but people’s feelings can originate from the affective states of others around them. Whether it is the enthusiasm of a team member that ignites excitement or the acute anxiety of a partner that generates a sense of unease, people are highly sensitive to the emotional tenor of their social partners and may unconsciously achieve affective convergence with them. Affect is a neurophysiological state that may vary in valence (positive or negative) as well as arousal (low or high; Barrett, 2006). Affect contagion, then, is the transmission of affect from one person to another and has been suggested to function, in part, to facilitate social connection and coordination (Butler, 2011; Hatfield, Cacioppo, & Rapson, 1994). Consistent with the notion of affect contagion are findings that the regions of the mirror-neuron system that are activated when individuals observe action are similar to the regions that are activated when individuals perform the same action (Iacoboni et al., 1999). In addition, synchrony has been observed in converging voice frequency of dyad members (Gregory & Webster, 1996) and in behavioral mimicry of face and posture (Chartrand & Bargh, 1999; Neumann & Strack, 2000).
Relying on imaging of neural regions or the occurrence of discrete behaviors can pose practical and inferential challenges to measuring affect contagion in the context of dynamic, face-to-face dyadic interactions. In contrast, on-line peripheral physiological responses offer a response channel that reacts quickly to affective changes and allows for temporal precision to examine subtle changes over time in dyad members. Indeed, some of the first psychophysiological studies examining dyadic social interactions found affect contagion in the form of synchronization between autonomic responses of interaction partners (Kaplan, Burch, & Bloom, 1964). More recently, affective scientists have demonstrated that observing or interacting with a stranger experiencing acute stress can engender physiological changes in the observer (Buchanan, Bagley, Stansfield, & Preston, 2012; Butler et al., 2003; Soto & Levenson, 2008), and this ability to “catch” another person’s affect may be related to social sensitivity and emotional accuracy (Guastello, Pincus, & Gunderson, 2006; Hess & Blairy, 2001; Levenson & Ruef, 1992).
Dyadic physiological synchrony is associated with romantic couples’ affective experiences. When romantic partners were instructed to sit face-to-face and “get in sync,” the degree to which women’s physiology synchronized with their partners’ was associated with their responsiveness to their partners’ daily affect (Ferrer & Helm, 2013). In a seminal study, physiological linkage in married couples during a conflict conversation was a significant predictor of self-reported marital dissatisfaction in both partners (Levenson & Gottman, 1983). Further, it has been suggested that couples form a coregulatory unit in which each member provides the feelings of security and support that help the other effectively regulate emotional and neurophysiological arousal during stressful or painful times (Sbarra & Hazan, 2008).
The significance of the romantic pair bond is rivaled only by the significance of the bond between mother and child. The connection that forms between mother and child is an evolutionary adaptation that helps ensure the infant’s nurturance by facilitating the mother’s emotional investment in her child (Bowlby, 1982). In humans, mother-driven behavioral affective attunement fosters children’s developing cognitive and social-emotional skills (Harrist & Waugh, 2002). Several recent studies have found evidence for mother-child cortisol synchrony, especially in the context of negative affect or high anxiety (Hibel, Granger, Blair, & Cox, 2009; Papp, Pendry, & Adam, 2009; Williams et al., 2013). These studies measured naturally occurring variation in physiological synchrony. In the present research, we used an experimental design to induce different affective states in mothers and then examined whether infants caught that affective state. We also examined the extent to which mothers’ and infants’ physiological changes synchronized by measuring the covariation of physiological responses within dyads.
Although behavioral mimicry may be a primary source of affect contagion, within the mother-infant dyad, lower-level actions such as referencing and monitoring are prerequisites for affect contagion. Social referencing refers to how infants nearing the end of their 1st year modify their behavior in accordance with their mothers’ emotional cues (Walden & Ogan, 1988). When mothers exhibit negative emotion, for instance, infants interact with their environments with greater wariness, even if their attention is not deliberately drawn to their mothers’ emotion (de Rosnay, Cooper, Tsigaras, & Murray, 2006). We exposed mothers to negative or positive evaluation during a stressful task in order to examine the contagion of high-arousal negative affect in comparison with high-arousal positive affect. Given that negative affect is typically more salient and impactful than positive affect for both adults (Baumeister, Bratislavsky, Finkenauer, & Vohs, 2001) and infants (Sorce, Emde, Campos, & Klinnert, 1985), we expected to find that infants catch mothers’ negative affect to a greater extent than mothers’ positive affect.
Many of the studies examining physiological synchrony have focused on physiological linkage, in which one individual’s physiological responses influence another person’s physiological responses in a time-lag design, but a second type of physiological synchrony may be more relevant to the current context. Physiological covariation describes the amount of correlation between two individuals’ physiology within a single time period. Conceptually, covariation is believed to result from shared experiences or environments, and positive covariation (i.e., the slopes of responses show the same direction of change) results from the extent to which the individuals’ affective experiences are similar. Given the primacy of affective cues within the mother-infant dyad, we focused on physiological covariation as our model for affect contagion.
We expected mothers’ affective reactivity to vary with condition such that mothers who experienced negative evaluation would have greater physiological reactivity (i.e., sympathetic activation) and more externalizing negative affect than mothers who experienced positive evaluation, whose physiological reactivity would be greater than that of mothers who experienced the low-stress control condition. We expected that infants, who had been separated from their mothers during the manipulation, would catch their mothers’ affective state upon reunion and manifest a pattern of physiological reactivity similar to that induced in the mothers, as well as behavioral responses consistent with environmental wariness if their mothers had been in one of the social evaluation conditions. We also anticipated that affect contagion would be manifested as greater dyadic physiological synchrony in the form of physiological covariation over time. Finally, we expected this covariation over time to be strongest for dyads in which mothers had received negative evaluation.
Method
Participants
Sixty-nine mothers (mean age = 33.6 years, SD = 5.6) and their 12- to 14-month-olds (45% female, 55% male) were recruited from the San Francisco Bay Area and were compensated $75. Mothers were excluded if they were hypertensive, had a pacemaker, took cardiac medications, or were pregnant. (For additional information about the participants, see the Supplemental Material available online.)
Procedure
Figure 1 presents an overview of the procedure (additional details are available in the Supplemental Material). Upon arrival, each mother provided consent for herself and her infant. The infant was taken to a playroom, with a caregiver who came to the experiment with the mother and baby, while the mother moved to a different room. Here, the experimenter attached sensors to measure cardiovascular responses and instructed the mother to relax alone for a 5-min period, during which her baseline cardiovascular responses were obtained. Then, the infant was brought to the mother, and the experimenter attached sensors to measure the infant’s cardiovascular responses. The experimenter instructed the mother to help her infant relax for a 2-min period, during which the infant’s baseline cardiovascular responses were obtained. Afterward, the infant returned to the playroom while the mother remained in the room.
Fig. 1.
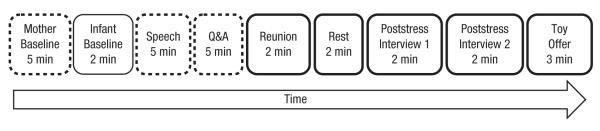
Overview of the procedure. Dashed outlines indicate that the mother was alone; for all other periods, the mother and infant were together. Bold outlines indicate the periods from which the mother’s and infant’s physiological data were taken for covariation analyses. Q&A = question-and-answer session.
The mother completed a questionnaire on her current affect, and then the experimenter introduced the upcoming interview task (modified Trier Social Stress Test; Kirschbaum & Hellhammer, 1994) and obtained verbal consent to continue. The mother was instructed to give a 5-min speech about her strengths and weaknesses to a panel of two evaluators. This speech was followed by a 5-min question and answer (Q&A) session.
Mothers were randomly assigned to one of three conditions: social evaluation with positive feedback, social evaluation with negative feedback, or no evaluation (control). Social evaluation was provided by two trained evaluators (one male, one female), who exhibited non-verbal feedback during the speech and Q&A session. In the positive-evaluation condition, the evaluators became progressively more positive by smiling, nodding, and leaning forward while the participant spoke, whereas in the negative-evaluation condition, the evaluators became progressively more negative, frowning, shaking their heads, crossing their arms, and leaning back. This manipulation of social approval versus social rejection has been used successfully to induce high-arousal positive and negative affective states, respectively (Akinola & Mendes, 2008). In the control condition, mothers were instructed to deliver the speech and verbally answer questions written on cards while alone in the room. Thus, the control condition was similar to the experimental conditions in terms of the physical metabolic demands (i.e., speaking aloud, thinking about the same questions) but did not have the social evaluation component. Immediately following the Q&A session, the mother completed another affect questionnaire.
Next, the infant rejoined the mother for a 2-min reunion period followed by a 2-min resting period in which the mother was instructed to help her infant relax. Mother and infant then experienced two poststress interviews with different female interviewers; these interviews were videotaped for later behavioral coding. In each of these interviews, the interviewer entered the room, sat across from the mother-infant dyad, engaged the mother in a short innocuous conversation about her infant’s development, and then offered the infant a toy for 1 min. In the final phase of the experiment, the two female interviewers entered the room, sat across from the dyad, and offered the infant identical sets of toys (toy offer) for 3 min. Upon completion of the study, the sensors were detached, the mother was debriefed, and payment was given.
Measures
Affect measures
We used the Positive and Negative Affect Schedule (PANAS; Watson, Clark, & Tellegen, 1988) to assess mothers’ affect. Mothers rated the degree to which they were currently experiencing 20 different affect states, using a 5-point scale from 1 (not at all) to 5 (a great deal). We calculated positive- and negative-affect scores for each time point (i.e., before and after the speech task; αs ranged from .85 to .93). Because high-arousal, externalizing negative affect was the expected affective state following negative social evaluation, we further differentiated an externalizing subscale consisting of three of the negative-affect items: “hostile,” “irritable,” and “upset” (αposttask = .83).
As a manipulation check, following the evaluation, we asked mothers assigned to the positive- and negative-evaluation conditions to rate seven statements about their perceptions of the evaluators’ feedback (e.g., “She thought I performed well on the task”). The 7-point rating scale ranged from 1 (strongly disagree) to 7 (strongly agree; Akinola & Mendes, 2008). Perceptions of the male and female evaluators were highly correlated, so the scores were averaged to form a single scale (α = .94).
Autonomic nervous system measures
We measured electrocardiography (Biopac MP150 Data Acquisition System, Biopac Systems, Inc., www.biopac.com) and impedance cardiography (HIC-2000 Impedance Cardiograph, Bio-Impedance Technology, Inc., http://www.microtronics-nc.com/BIT/Home.html) to obtain mothers’ sympathetic nervous system (SNS) reactivity, specifically, preejection period (PEP; the time from contraction of the left ventricle to opening of the aortic valve). PEP is a chronotropic measure, such that greater activation is indicated by a greater decrease in PEP. For ease of interpretation, we multiplied PEP by −1, so that increases in SNS are represented as increases in force of ventricle contractility (VC).
Pilot testing of impedance cardiography collection on infants revealed that application of the adhesive bands was not well tolerated and was quite stressful for them. Unfortunately, it was not feasible to obtain PEP data from the infants. Instead, electrocardiography was collected from two spot sensors on the chest, and we calculated heart rate (HR, in beats per minute) as the measure of infants’ SNS activation.1
The mothers’ physiological measures were collected continuously from the baseline through the toy offer; for infants, physiological responses were obtained at baseline with the mother and then continuously from the 2nd minute of reunion through the toy offer. Thus, we had 10 min of dyadic physiological data following the manipulation. We scored mothers’ PEP and infants’ HR data by first visually inspecting the waveforms for artifacts and then aggregating the data in 30-s segments using Mindware software (Impedance Cardiography Analysis Software 2.6 and Heart Rate Variability Analysis Software 2.6, Mindware Technologies, Ltd., http://www.mindwaretech.com/). As is standard practice, reactivity scores were calculated by subtracting baseline responses (the last 30 s of baseline) from every 30-s segment after the baseline.
Measure of infant behavior
Infants’ behavioral avoidance during the 1st minute of each poststress interview was coded on a 5-point scale from 0 (infant did not hesitate to engage with interviewer) to 4 (infant continuously actively avoided interviewer). Behavioral indicators ranged from passive (e.g., gaze aversion) to active (e.g., twisting bodily away) avoidance of the interviewers (Murray et al., 2008). After achieving reliability with the master coder on 20% of the sample (weighted κ = .78), a female research assistant, naive to mothers’ condition assignment, coded all videotapes. Ten percent of the tapes were uncodable because of equipment malfunction or an inadequate camera angle.
Data analysis
The primary outcome variables were changes in SNS activation (mothers’ VC reactivity and infants’ HR reactivity), mothers’ affective self-reports, and infants’ behavioral avoidance. We first explored physiological reactivity separately for the mothers and babies. To examine effects of evaluation condition, we focused on the time interval of greatest activation, selected a priori on the basis of prior research (e.g., Mendes, Blascovich, Hunter, Lickel, & Jost, 2007). For mothers, this was the 1st minute of the Q&A session (when the task was novel, but feedback had been established), and for infants, this was the 1st minute of each poststress interview (when the situation was novel and before the interviewer attempted to engage directly with the infant). In analyses of mothers’ SNS activation, we controlled for body mass index (Jennings et al., 1981).
We then examined whether physiological covariation varied as a function of evaluation condition and whether it strengthened or weakened over the course of the interaction between mother and infant. To measure covariation, we estimated the relationship between mothers’ VC reactivity and infants’ HR reactivity, using a nomothetic approach in which mothers’ VC reactivity was treated as the criterion variable and infants’ HR reactivity as the predictor; covariation was estimated as a path coefficient. (We note that this analysis does not imply causation, but rather, captures the relationship between variables measured simultaneously; for a similar strategy to estimate dyadic similarity, see West & Kenny, 2011).2 We modeled a linear growth curve in which time was centered at the study midpoint (see the Supplemental Material for random effects of intercept and slope, as well as intercept-slope covariance). This model allowed us to estimate the overall strength of covariation (i.e., the effect of infants’ HR reactivity on mothers’ VC reactivity), the effect of condition on covariation (i.e., the interactive effect of infants’ HR reactivity and condition on mothers’ VC reactivity), whether covariation strengthened over time (i.e., the interactive effect of infants’ HR reactivity and time on mothers’ VC reactivity), and whether it did so differently as a function of condition (i.e., the interactive effect of infants’ HR reactivity, time, and condition on mothers’ VC reactivity). (Note that the main effect of time was also included in the model.)
Data were analyzed using the MIXED procedure in SPSS to account for nonindependence across the 20 time segments of data when mother and baby were together. We note that this procedure uses the Satterthwaite (1946) method to calculate degrees of freedom, which can be fractional; it also allows for missing data.
Results 3
We first examined whether mothers experienced the evaluation conditions as intended by analyzing mothers’ perceptions of the evaluators’ feedback, as well as their self-reported positive and negative affect. Mothers who received negative evaluation perceived the evaluators as less supportive (M = 3.31, SD = 0.98) than did those who received positive evaluation (M = 5.10, SD = 1.05), t(40) = 5.68, p < .001. In addition, mothers experienced greater decreases in positive affect and greater increases in negative affect in the negative-evaluation condition, compared with the positive-evaluation and control conditions (see the Supplemental Material). When we examined externalizing negative affect specifically, we found that it differed significantly by condition, F(2, 64) = 7.32, p = .001. Externalizing negative affect increased significantly after negative evaluation (M = 0.54, SD = 0.98), compared with positive evaluation (M = −0.23, SD = 0.71) and the control task (M = −0.09, SD = 0.23), t(64) = −3.56, p = .001, and t(64) = −2.96, p = .004. The control and positive-evaluation conditions were not significantly different from each other, p = .51. In sum, negative evaluation engendered primarily externalizing (i.e., anger) responses.
Maternal physiological reactivity
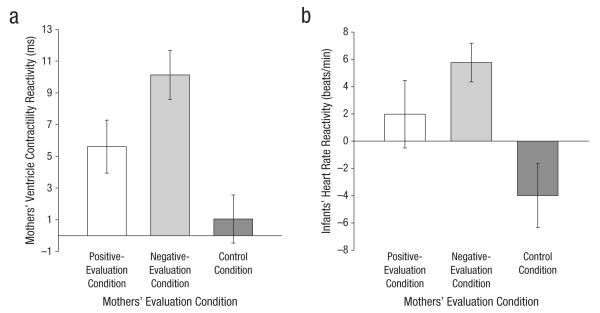
Analysis of covariance revealed a significant main effect of condition on mothers’ VC reactivity, F(2, 62) = 9.53, p < .001 (Fig. 2a). As expected, the positive-evaluation condition (ΔVC = 6.0, SD = 6.49) and negative-evaluation condition (ΔVC = 10.75, SD = 8.81) engendered significant increases in sympathetic activation relative to the control condition (ΔVC = 0.74, SD = 8.29), t(62) = 2.29, p = .03, and t(62) = 4.21, p < .001, respectively. Negative evaluation was associated with greater SNS activation than was positive evaluation, t(62) = 1.80, p = .08. (See the Supplemental Material for information on the covariate body mass index.)
Fig. 2.
Mothers’ and infants’ physiological reactivity during the poststress interviews: (a) mothers’ mean increase in ventricle contractility and (b) infants’ mean heart rate reactivity as a function of mothers’ evaluation condition. Error bars represent ±1 SE.
We then examined the correlation between SNS responses and externalizing negative affect, finding that the magnitude of this relationship varied across conditions—negative evaluation: r(22) = .41, p = .058; positive evaluation: r(20) = .33, p = .16; control: r(23) = .04, p = .85. These data suggest that we successfully engendered greater SNS reactivity in the two evaluation conditions, which nonetheless showed differentiation in both affective quality and the magnitude of physiological responses.
Infants’ physiological reactivity
We examined infants’ HR reactivity during the poststress interviews as a function of mothers’ evaluation condition. Infants’ HR reactivity during the two poststress interviews was significantly correlated, r(54) = .68, p < .001, so responses were averaged. Analysis of variance revealed a significant main effect of condition, F(2, 58) = 5.35, p = .007 (Fig. 2b). Infants whose mothers received negative evaluation exhibited significantly higher HR reactivity during the interviews (ΔHR = 5.76, SD = 6.35) than did infants whose mothers were in the control condition (ΔHR = −3.95, SD = 10.72), t(58) = −3.24, p = .002. The HR reactivity of infants of mothers assigned to the positive-evaluation condition (ΔHR = 1.95, SD = 10.94) fell in between the HR reactivity of infants of mothers in the negative-evaluation condition, t(58) = −1.26, p = .21, and control condition, t(58) = −1.97, p = .05. (Analyses examining the influences of infants’ sex and alternate caregivers’ identity on infants’ outcomes are in the Supplemental Material.)
Infants’ behavioral avoidance
As was the case for infants’ HR reactivity, behavioral-avoidance scores from the two poststress interviews were significantly correlated, r(58) = .53, p < .001, and thus averaged. A significant main effect of condition was observed, F(2, 54) = 6.89, p = .002. Mothers who had experienced social evaluation had infants who were more avoidant toward the interviewers (positive-evaluation condition: M = 1.55, SD = 1.28; negative-evaluation condition: M = 1.76, SD = 1.1) compared with mothers assigned to the control condition (M = 0.67, SD = 1.0), t(60) = −3.36, p = .001, and t(60) = −4.15, p < .001, respectively (Fig. 3). Behavioral avoidance differed descriptively but not significantly between infants of mothers in the positive-evaluation condition and infants of mothers in the negative-evaluation condition (p = .47). (Recall that a small percentage of behavioral-avoidance data were missing. Analyses of maternal and infants’ physiological reactivity in the subsample with intact behavioral data are in the Supplemental Material.)
Fig. 3.
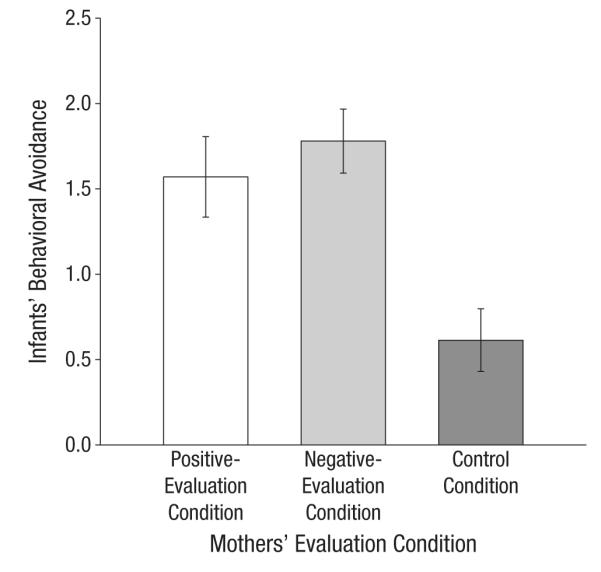
Infants’ mean behavioral avoidance of the interviewers as a function of mothers’ evaluation condition. Error bars represent ±1 SE.
Mother-infant physiological covariation over time
Finally, we tested covariation from the reunion through the toy offer. Recall that covariation was estimated as a path coefficient. The overall relationship between infants’ HR reactivity and mothers’ VC reactivity was positive and significantly different from zero, F(1, 908.64) = 17.21, p = .003, which indicated that, overall, there was covariation.4 The interaction between infants’ HR reactivity and evaluation condition was not significant, p = .54; however, the interaction of infants’ HR reactivity, evaluation condition, and time was significant, F(2, 282.00) = 3.78, p = .02; change in covariation over time varied as a function of evaluation condition (Fig. 4). Specifically, in the negative-evaluation condition, the interaction between infants’ HR reactivity and time was positive and significant, t(368.43) = 2.56, p = .01; the greater the mothers’ SNS activation, the greater their infants’ HR responses, and this effect strengthened over the course of the study. We note that at the end of the study, covariation was positive and significant in the negative-evaluation condition, t(436.41) = 3.49, p = .001. In the positive-evaluation and control conditions, the interaction between infants’ HR reactivity and time was not significant (ps = .92 and .21, respectively); covariation did not change significantly over time in these conditions. We also note that overall covariation was not significantly different from zero in the control condition, p = .12, but was different from zero in the positive-evaluation condition, p = .01.
Fig. 4.
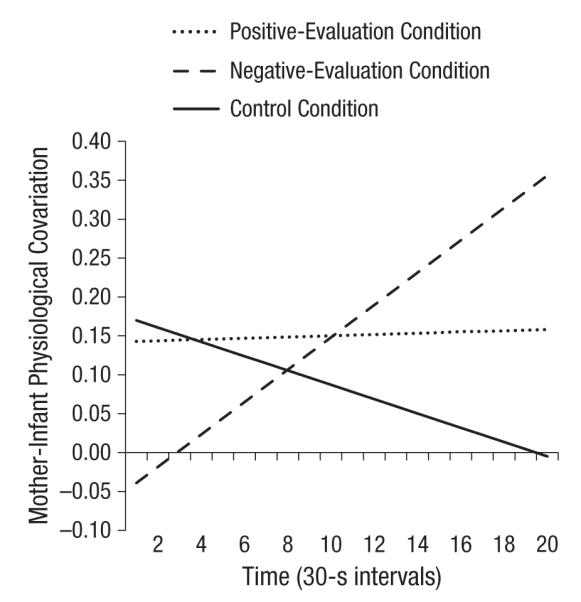
Covariation of infants’ heart rate (HR) reactivity and mothers’ ventricle contractility (VC) reactivity over time, from reunion through toy play, in the three evaluation conditions. Covariation is indexed as the effect of (standardized) infants’ HR reactivity on (standardized) mothers’ VC reactivity.
We created two contrast codes to compare the over-time change in covariation in the negative-evaluation condition with the over-time change in covariation in the control condition (Contrast 1) and in the positive-evaluation condition (Contrast 2). The Contrast 1 × Infants’ HR Reactivity × Time interaction was significant, t(278.63) = −2.76, p = .01, which indicated that the slope for the negative-evaluation condition differed significantly from the slope for the control condition. The Contrast 2 × Infants’ HR Reactivity × Time interaction was marginally significant, t(317.37) = −1.65, p = .10, which indicated that the slope for the negative-evaluation condition differed marginally from the slope for the positive-evaluation condition. The slopes for the positive-evaluation and control conditions were not significantly different from each other, p = .35.
Discussion
Employing an experimental design to induce positive or negative social evaluative stress in mothers while they were separated from their infants, we found that a mother’s stress is embodied by her infant upon reunion. Moreover, the mother-infant dyads showed greater physiological covariation after mothers experienced a negative stressor than after they experienced a positive stressor or low-stress task, and this covariation increased over time. We feel confident that infants’ responses were not driven by a combination of their mothers’ reactivity coupled with environmental triggers because the infants were never exposed directly to the mothers’ stressors. To our knowledge, this is the only study in which autonomic nervous system reactivity has been measured simultaneously in mothers and infants following different stress manipulations and in which the resulting physiological attunement has been analyzed.
Mother-infant attunement is likely highly adaptive and presumably evolved for a variety of reasons, such as detecting and communicating danger from imminent threats from conspecifics and other nonhuman animals, and other environmental hazards. In animals, relational processes between mothers and infants have long-lasting modulatory effects on social and health outcomes. Foundational studies of rat pups and their mothers demonstrated that pups who receive higher levels of licking and grooming behavior from their mothers have lower responses to subsequent stressors (Meaney, 2001), and that olfactory and auditory stimuli emitted from rat and mouse pups allow for maternal monitoring of location (Nagasawa, Okabe, Mogi, & Kikusio, 2012). Thus, mother-infant attunement is likely to serve both adaptational and survival purposes. In humans, its function is not fully understood. In the current study, we initially induced stress in only one member of each dyad, which allowed us to test whether such attunement serves to communicate affective information from one member to the other. We found that maternal stress transmission had the greatest impact on infants’ physiology when mothers had experienced a negative-evaluative stressor. This suggests that infants may be predisposed to attend to their mothers’ heightened-arousal states, such as reactions to negative, threatening, or angering events.
Our study has several limitations and suggests several interesting avenues of further inquiry. We suspect that there are a variety of channels through which affect is communicated between mother and infant. Stressed mothers may exhibit changes in facial expression, odor, posture, vocal tone, prosody, and touch, all of which may contribute to the effects we observed. Although a mother’s soothing physical touch helps a distressed infant better regulate himor herself (Feldman, Singer, & Zagoory, 2010; Field, 1998), the touch of an acutely stressed mother has not been well examined. Infants in our study sat on their mothers’ lap during the poststress exchanges, and it is possible that the mothers’ touch was a proximal cause for changes in physiological reactivity. Given the amount of time many young infants spend in physical contact with their parents, understanding of early biobehavioral synchrony would be strengthened by isolating the impact of physical touch following a paradigm like the one we used here.
We designed the study so that we could compare responses to a negative stressor with responses to a milder, positive stressor. However, it is fair to note that the negative-evaluation condition was associated with larger maternal SNS activation than the positive-evaluation condition, so we are unable to completely rule out intensity of reactivity (rather than negative affect) as the causal factor influencing physiological covariation.
In sum, our findings demonstrate that infants catch their mothers’ physiological stress reactivity entirely through interactions with their mothers, without exposure to the stressor itself. These effects have implications for understanding transgenerational health and well-being. By showing that maternal stress immediately influences infants’ stress reactivity, we have demonstrated how stress “gets under the skin” of children whose parents are exposed to psychological stressors and have extended understanding of how the social world influences infants indirectly through exchanges with their close caregivers.
Supplementary Material
Acknowledgments
We thank Kate Hawley, Helena Karnilowicz, and members of the Emotion, Health, and Psychophysiology Lab for their many contributions to this study.
Funding This research was supported by the Sarlo/Ekman endowment awarded to W. B. Mendes.
Footnotes
The heart is dually innervated by sympathetic and parasym-pathetic branches of the autonomic nervous system, so HR is not considered a pure measure of sympathetic activation, in contrast to PEP. That stated, correlations between PEP and HR in active tasks tend to be medium to large; for example, in this study, mothers’ PEP and HR during the stress task were strongly correlated, r(65) = .52, p < .001.
Treating mothers’ VC reactivity as the predictor and infants’ HR reactivity as the criterion yielded the same pattern of results. Although we emphasize that we captured a correlation between mothers’ VC reactivity and infants’ HR reactivity, so the choice of criterion and predictor was arbitrary, we treated mothers’ VC reactivity as the criterion because it allowed us to adjust for the effect of mother’s body mass index on mother’s VC reactivity.
All data and programming code associated with these results can be obtained online at http://mendes.socialpsychology.org/ publications.
We reran all analyses reported here replacing mothers’ VC reactivity with their HR reactivity, and the results were essentially the same (though the covariation between mothers and infants was weaker).
Author Contributions W. B. Mendes and S. F. Waters designed the experiment. S. F. Waters conducted the experiment. S. F. Waters and W. B. Mendes edited and completed diagnostics of the physiological data. S. F. Waters and T. V. West conducted the analyses. All authors contributed to writing the manuscript.
Declaration of Conflicting Interests The authors declared that they had no conflicts of interest with respect to their authorship or the publication of this article.
Supplemental Material Additional supporting information may be found at http://pss.sagepub.com/content/by/supplemental-data
References
- Akinola M, Mendes WB. The dark side of creativity: Biological vulnerability and negative emotions lead to greater artistic creativity. Personality and Social Psychology Bulletin. 2008;34:1677–1686. doi: 10.1177/0146167208323933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF. Are emotions natural kinds? Perspectives on Psychological Science. 2006;1:28–58. doi: 10.1111/j.1745-6916.2006.00003.x. [DOI] [PubMed] [Google Scholar]
- Baumeister RF, Bratislavsky E, Finkenauer C, Vohs KD. Bad is stronger than good. Review of General Psychology. 2001;5:323–370. [Google Scholar]
- Bowlby J. Attachment and loss: Vol. I. Attachment. 2nd ed. Basic Books; New York, NY: 1982. [Google Scholar]
- Buchanan TW, Bagley SL, Stansfield B, Preston SD. The empathic, physiological resonance of stress. Social Neuroscience. 2012;7:191–201. doi: 10.1080/17470919.2011.588723. [DOI] [PubMed] [Google Scholar]
- Butler EA. Temporal interpersonal emotion systems: The “TIES” that form relationships. Personality and Social Psychology Review. 2011;15:367–393. doi: 10.1177/1088868311411164. [DOI] [PubMed] [Google Scholar]
- Butler EA, Egloff B, Wilhelm FH, Smith NC, Erickson EA, Gross JJ. The social consequences of expressive suppression. Emotion. 2003;3:48–67. doi: 10.1037/1528-3542.3.1.48. [DOI] [PubMed] [Google Scholar]
- Chartrand TL, Bargh JA. The chameleon effect: The perception–behavior link and social interaction. Journal of Personality and Social Psychology. 1999;76:893–910. doi: 10.1037//0022-3514.76.6.893. [DOI] [PubMed] [Google Scholar]
- de Rosnay M, Cooper PJ, Tsigaras N, Murray L. Transmission of social anxiety from mother to infant: An experimental study using a social referencing paradigm. Behavior Research and Therapy. 2006;44:1165–1175. doi: 10.1016/j.brat.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Feldman R, Singer M, Zagoory O. Touch attenuates infants’ physiological reactivity to stress. Developmental Science. 2010;13:271–278. doi: 10.1111/j.1467-7687.2009.00890.x. [DOI] [PubMed] [Google Scholar]
- Ferrer E, Helm JL. Dynamical systems modeling of physiological coregulation in dyadic interactions. International Journal of Psychophysiology. 2013;88:296–308. doi: 10.1016/j.ijpsycho.2012.10.013. [DOI] [PubMed] [Google Scholar]
- Field TM. Touch therapy effects on development. International Journal of Behavioral Development. 1998;22:779–797. [Google Scholar]
- Gregory SW, Webster S. A nonverbal signal in voices in interview partners effectively predicts communication accommodation and social status perceptions. Journal of Personality and Social Psychology. 1996;70:1231–1240. doi: 10.1037//0022-3514.70.6.1231. [DOI] [PubMed] [Google Scholar]
- Guastello SJ, Pincus D, Gunderson PR. Electrodermal arousal between participants in a conversation: Nonlinear dynamics and linkage effects. Nonlinear Dynamics, Psychology, and Life Sciences. 2006;10:365–399. [PubMed] [Google Scholar]
- Harrist AW, Waugh RM. Dyadic synchrony: Its structure and function in children’s development. Developmental Review. 2002;22:555–592. [Google Scholar]
- Hatfield E, Cacioppo JT, Rapson RL. Emotional contagion. Current Directions in Psychological Science. 1994;2:96–99. [Google Scholar]
- Hess U, Blairy S. Facial mimicry and emotional contagion to dynamic emotional facial expressions and their influence on decoding accuracy. International Journal of Psychophysiology. 2001;40:129–141. doi: 10.1016/s0167-8760(00)00161-6. [DOI] [PubMed] [Google Scholar]
- Hibel LC, Granger DA, Blair C, Cox MJ. Intimate partner violence moderates the association between mother–infant adrenocortical activity across an emotional challenge. Journal of Family Psychology. 2009;23:615–625. doi: 10.1037/a0016323. [DOI] [PubMed] [Google Scholar]
- Iacoboni M, Woods RP, Brass M, Bekkering H, Mazziotta JC, Rizzolatti G. Cortical mechanisms of human imitation. Science. 1999;286:2526–2528. doi: 10.1126/science.286.5449.2526. [DOI] [PubMed] [Google Scholar]
- Jennings JR, Bberg WK, Hutcheson JS, Obrits P, Porges S, Turpin G. Publication guidelines for heart rate studies in man. Psychophysiology. 1981;18:226–231. doi: 10.1111/j.1469-8986.1981.tb03023.x. [DOI] [PubMed] [Google Scholar]
- Kaplan HB, Burch NR, Bloom SW. Physiological covariation and sociometric relationships in small peer groups. In: Leiderman PH, Shapiro D, editors. Psychobiological approaches to social behavior. Stanford University Press; Stanford, CA: 1964. pp. 92–109. [Google Scholar]
- Kirschbaum C, Hellhammer DH. Salivary cortisol in psychoneuroendocrine research: Recent developments and applications. Psychoneuroendocrinology. 1994;19:313–333. doi: 10.1016/0306-4530(94)90013-2. [DOI] [PubMed] [Google Scholar]
- Levenson RW, Gottman JM. Marital interaction: Physiological linkage and affective exchange. Journal of Personality and Social Psychology. 1983;45:587–597. doi: 10.1037//0022-3514.45.3.587. [DOI] [PubMed] [Google Scholar]
- Levenson RW, Ruef AM. Empathy: A physiological substrate. Journal of Personality and Social Psychology. 1992;63:234–246. [PubMed] [Google Scholar]
- Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annual Review of Neuroscience. 2001;24:1161–1192. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- Mendes WB, Blascovich J, Hunter S, Lickel B, Jost J. Threatened by the unexpected: Physiological responses during social interactions with expectancy-violating partners. Journal of Personality and Social Psychology. 2007;92:698–716. doi: 10.1037/0022-3514.92.4.698. [DOI] [PubMed] [Google Scholar]
- Murray L, De Rosnay M, Pearson J, Bergeron C, Schofield E, Royal-Lawson M, Cooper PJ. Intergenerational transmission of social anxiety: The role of social referencing processes in infancy. Child Development. 2008;79:1049–1064. doi: 10.1111/j.1467-8624.2008.01175.x. [DOI] [PubMed] [Google Scholar]
- Nagasawa M, Okabe S, Mogi K, Kikusio T. Oxytocin and mutual communication in mother-infant bonding. Frontiers in Human Neuroscience. 2012;6 doi: 10.3389/fnhum.2012.00031. Article 31. Retrieved from http://www.frontiersin.org/Journal/10.3389/fnhum.2012.00031/full#h2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann R, Strack F. Mood contagion”: The automatic transfer of mood between persons. Journal of Personality and Social Psychology. 2000;79:211–223. doi: 10.1037//0022-3514.79.2.211. [DOI] [PubMed] [Google Scholar]
- Papp LM, Pendry P, Adam EK. Mother-adolescent physiological synchrony in naturalistic settings: Within-family cortisol associations and moderators. Journal of Family Psychology. 2009;23:882–894. doi: 10.1037/a0017147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite FE. An approximate distribution of estimates of variance components. Biometrics Bulletin. 1946;2:110–114. [PubMed] [Google Scholar]
- Sbarra DA, Hazan C. Coregulation, dysregulation, self-regulation: An integrative analysis and empirical agenda for understanding adult attachment, separation, loss, and recovery. Personality and Social Psychology Review. 2008;12:141–167. doi: 10.1177/1088868308315702. [DOI] [PubMed] [Google Scholar]
- Sorce JF, Emde RN, Campos J, Klinnert MD. Maternal emotional signaling: Its effect on the visual cliff behavior of 1-year-olds. Developmental Psychology. 1985;21:195–200. [Google Scholar]
- Soto JA, Levenson RW. Emotion recognition across cultures: The influence of ethnicity on empathic accuracy and physiological linkage. Emotion. 2008;9:874–884. doi: 10.1037/a0017399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walden TA, Ogan TA. The development of social referencing. Child Development. 1988;59:1230–1240. doi: 10.1111/j.1467-8624.1988.tb01492.x. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- West TV, Kenny DA. The truth and bias model of judgment (T&B) Psychological Review. 2011;118:357–378. doi: 10.1037/a0022936. [DOI] [PubMed] [Google Scholar]
- Williams SR, Cash E, Daup M, Geronimi EMC, Sephton SE, Woodruff-Borden J. Exploring patterns in cortisol synchrony among anxious and nonanxious mother and child dyads: A preliminary study. Biological Psychology. 2013;93:287–295. doi: 10.1016/j.biopsycho.2013.02.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.