Abstract
The transplantation of human pluripotent stem cell-derived cardiomyocytes (hPSC-CMs) is a promising strategy to treat myocardial infarction and reverse heart failure, but to date the contractile benefit in most studies remains modest. We have previously shown that the nucleotide 2-deoxyadenosine triphosphate (dATP) can substitute for ATP as the energy substrate for cardiac myosin, and increasing cellular dATP content by globally overexpressing ribonucleotide reductase (R1R2) can dramatically enhance cardiac contractility. Because dATP is a small molecule, we hypothesized that it would diffuse readily between cells via gap junctions and enhance the contractility of neighboring coupled wild type cells. To test this hypothesis, we performed studies with the goals of (1) validating gap junction-mediated dATP transfer in vitro and (2) investigating the use of R1R2-overexpressing hPSC-CMs in vivo as a novel strategy to increase cardiac function. We first performed intracellular dye transfer studies using dATP conjugated to fluorescein and demonstrated rapid gap junction-mediated transfer between cardiomyocytes. We then cocultured wild type cardiomyocytes with either cardiomyocytes or fibroblasts overexpressing R1R2 and saw more than a twofold increase in the extent and rate of contraction of wild type cardiomyocytes. Finally, we transplanted hPSC-CMs overexpressing R1R2 into healthy uninjured rat hearts and noted an increase in fractional shortening from 41±4% to 53±5% just five days after cell transplantation. These findings demonstrate that dATP is an inotropic factor that spreads between cells via gap junctions. Our data suggest that transplantation of dATP-producing hPSC-CMs could significantly increase the effectiveness of cardiac cell therapy.
Keywords: cardiac cell therapy, ribonucleotide reductase, dATP, cardiac regeneration, gap junction intercellular communication, inotropy, stem cells
Introduction
Cardiovascular disease is the leading cause of death worldwide[1], and current treatments to address the growing problem of heart failure are limited to drugs to slow disease progression or pump replacement. New approaches, including pharmacologic agents and cell- or gene-based strategies, have generated substantial scientific interest for their potential to halt or reverse the deleterious effects of heart failure. However, each of these strategies is also associated with potential challenges to clinical translation. New pharmacologic compounds such as Ca2+ sensitizers provide potent inotropic benefits, but clinical trials thus far have failed to definitively show a decrease in all-cause mortality in patients with acute heart failure[2]. Myosin activators such as omecamtiv mecarbil offer the opportunity to improve cardiac function directly at the myofilament and without changes in [Ca2+]i, but these agents are still in the experimental stage, and concerns of enhancing systolic function at the expense of causing diastolic dysfunction remain to be fully addressed[3]. Similarly, a number of studies have explored the therapeutic potential of cell transplantation therapy using human embryonic stem cell-derived cardiomyocytes (hESC-CMs) to remuscularize the scar with bonafide human myocardium. Unfortunately, this approach requires successful engraftment of large quantities (109) of cells, and most studies to date have demonstrated only a modest benefit in cardiac function[4–6]. Finally, gene therapy approaches have demonstrated efficacy in preclinical and phase I/II clinical trials, but challenges with finding the ideal delivery strategy to maximize efficacy and minimize risk remain[7]. Based upon these challenges, we hypothesized that a number of these limitations could be alleviated by the development of a novel hybrid therapeutic strategy to exploit the strengths of each of the above approaches while minimizing the potential limitations.
We[8–16] and others[17,18] have extensively studied the ability of 2-deoxyadenosine triphosphate (dATP), a novel inotropic agent, to enhance cardiac contractility by acting as an energy substrate for striated muscle. These studies have demonstrated that, compared to ATP, dATP increases both the force and the rate of shortening at all levels of Ca2+ activation in demembranated cardiac trabeculae[11]. The chemomechanical basis for this phenomenon has been well elucidated and is based primarily upon increases in the kinetics of Ca2+-mediated strong actomyosin binding[19], the power stroke[11], and ADP release[10]. Importantly, this occur with cardiac tissue containing either α- and β-cardiac myosin heavy chain isoforms, and the effect persists when dATP accounts for as little as ~1% of the total adenosine nucleotide pool[15,18,19]. More recent work by our group has investigated the effect of overexpressing the enzyme ribonucleotide reductase (R1R2), the enzyme responsible for conversion of ADP to dADP and rate-limiting step in dATP production (Fig. 1A), in intact cardiomyocytes[15]. These studies showed that overexpression of the subunits encoding for R1R2 (R1 and R2) resulted in a 40% increase in shortening magnitude and 80% increase in the shortening rate, as well as an increase in relaxation kinetics, in cultured adult rat cardiomyocytes[15]. Importantly, these effects were noted in the absence of any increase in maximal [Ca2+]i, suggesting that dATP exerts its effects on contractility primarily by acting on the highly cooperative myofilament. Furthermore, we recently reported that transgenic mice overexpressing R1R2 maintain a hypercontractile state without detrimental effects on metabolism or adverse remodeling[16]. Taken together, these findings suggest that dATP may represent a next-generation therapy for the treatment of heart failure. Despite these promising results attained thus far, however, the efficient and effective therapeutic delivery of dATP to the myocardium remains to be demonstrated.
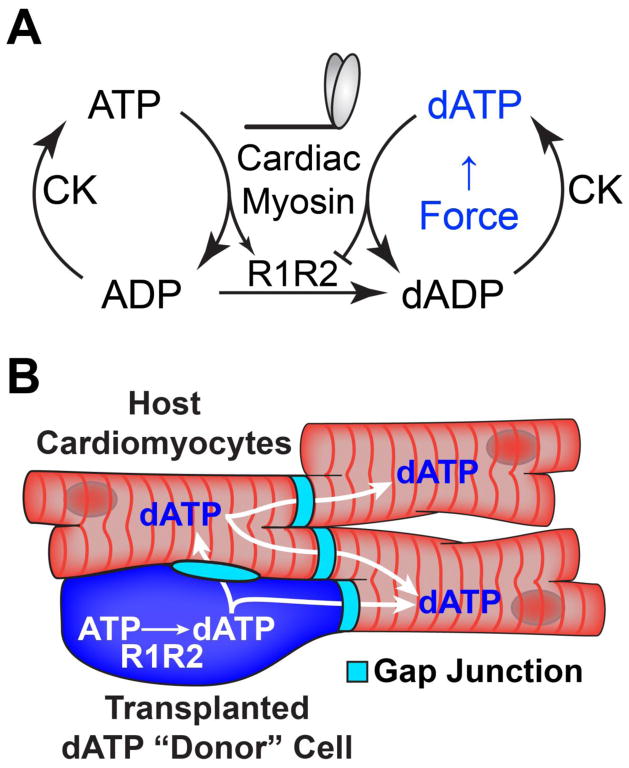
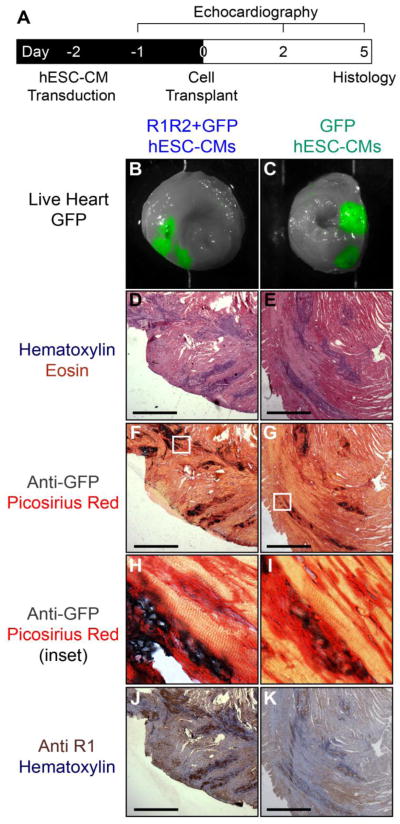
Figure 1. dATP enhances cardiac contractility and may travel between cells via gap junctions.
(A) Cardiac myosin produces increased force when hydrolyzing dATP as an energy source, and the enzyme R1R2 catalyzes the synthesis of dATP in conjunction with creatine kinase (CK). (B) Schematic depicting the generation of ATP in transplanted R1R2 cells and the subsequent gap junction-mediated transfer of dATP throughout cardiac syncytium.
Our group has previously shown that human embryonic stem cell-derived cardiomyocytes (hESC-CMs) efficiently engraft in both healthy and damaged rodent myocardium[4,20,21]. These cells are capable of electrically coupling and beating synchronously with host myocardium via the formation of host-graft gap junctions[21]. Since gap junctions also allow the efficient intercellular transfer of small metabolites such as cAMP and ATP between cells[22,23], we hypothesized that a small population of engrafted hESC-CMs overexpressing the genes encoding for R1R2 will produce and diffuse the small molecule dATP throughout coupled host myocardium (Fig. 1B), increasing global cardiac function and slowing or reversing the deleterious cardiac remodeling and subsequent heart failure following myocardial infarction. In the present work, we take the first step towards this goal by demonstrating that (1) dATP is capable of efficiently crossing gap junctions between both cardiomyocytes and fibroblasts, (2) cardiomyocytes coupled to cells that overexpress R1R2 show increased contractility, and (3) transplantation of R1R2-overexpressing hESC-CMs can dramatically increase cardiac performance above healthy baseline levels. Taken together, this work suggests that rather than attempting complete scar remuscularization, cells could instead be genetically engineered to exert positive inotropic support via intracellular small molecule delivery in a targeted, cardiac-specific fashion.
Methods
Neonatal rat cardiomyocyte isolation and culture
Neonatal rat ventricular cardiomyocytes (NRVMs) were isolated and cultured as previously described[24]. Briefly, neonatal hearts were hearts rapidly excised and placed into cold buffer (in mmol/L: NaCl 116.4, HEPES 20, NaH2PO4 1, glucose 5.5, KCl 5.4, MgSO4 0.8; pH 7.4). The ventricles were trimmed, minced, and incubated for 5 cycles of 25 min at 37C in collagenase type II (95 U/mL, Worthington) and pancreatin (0.6 mg/mL, Gibco BRL). After each incubation, cells were pelleted and resuspended in DMEM/M199 (4:1) supplemented with 10% horse serum (ICN Flow), 5% fetal bovine serum (HyClone), penicillin G (100 U/mL), and streptomycin (100 μg/mL, Gibco) and preplated for 30 minutes to reduce contaminating nonmyocytes. The cells were then plated in media onto sterile gel-coated 6-well dishes at a concentration of 2x105 cells per well for dye transfer studies.
PSC maintenance and guided cardiac differentiation
All experiments were approved by the University of Washington Embryonic Stem Cell Research Oversight Committee (ESCRO) and conducted using the H7 (NIHhESC-10-0061) hESC line. Human pluripotent stem cells (hPSCs) were maintained and differentiated as previously described[4,25]. Briefly, hESCs were cultured on Matrigel-coated plates (BD Biosciences) with mouse embryonic fibroblast-conditioned medium (MEF-CM) containing 4ng/mL basic fibroblast growth factor (bFGF). Cells were passaged using Versene-EDTA and cardiogenesis was induced with RPMI-B27 (Gibco) containing L-glutamine, Matrigel, and 10μg/mL recombinant human activin A (R&D Systems). After one day, the media was switched to RPMI-B27 containing L-glutamine and 100ng/mL recombinant human bone morphogenetic protein-4 (BMP-4, R&D Systems), and four days later the media was aspirated and subsequently replaced every other day with RPMI-B27 containing L-glutamine. Cells typically began beating spontaneously on approximately day 12–15 post-induction. Prior to experimentation, cells were passaged using 0.05% trypsin-EDTA and replated on PEI-gelatin coated glass bottom petri-dishes as previously described[25].
Dye transfer
Dye transfer experiments were conducted with intracellular microinjection pipettes pulled from 1mm thin-walled capillary glass (Sutter Instruments) pulled to a ~1μm final tip diameter using a Sutter P-97 micropipette puller. Fluorescein-12-dATP in TE buffer was purchased and validated at >99% purity by HPLC (Perkin Elmer). Pipettes were backloaded with ~1μL of either fluorescein-dATP or control fluorescein (1mM in TE buffer) solution and placed in a Eppendorf Celltram microinjector mounted on a Zeiss AxioObserver microscope with AxioCam imaging hardware. Cells were bathed in Tyrode’s buffer at 25C, and a single cell was injected with dye solution. The resulting dye transfer was imaged every 10 seconds for a total of 5 minutes with minimal excitation intensity and length to minimize photobleaching.
Image analysis
Images were manually thresholded and quantitatively analyzed in ImageJ using standard analysis plugins. Images were quantified for both area and maximum distance of dye transfer. To obtain measurements of maximum dye diffusion distance, a minimum of 3 radial measurements to the edges of dye transfer were made, and the largest value was selected. For measurements of dye transfer area, the initial T=0 image was subtracted from the final T=5min image to remove artifacts associated with fluorescence within the injection pipette.
Viral Transduction
The vectors used in this study are identical to those used in a previously publication[15]. Briefly, HEK293 cells were used to generate adenoviral vectors expressing R1 or R2 under the CMV promotor using the AdEasy system. Both vectors contained a green fluorescent protein (GFP) reporter, and as a control we also generated a vector encoding for GFP alone. Cardiomyocytes or fibroblasts were transduced for 2–4hrs at 37C with ~250 particles per cell and rinsed three times with PBS before being placed back in standard media. This transduction protocol typically yielded >95% transduction efficiency with minimal cell death.
fibroblast cardiomyocyte coculture
Human neonatal dermal fibroblasts (NHDFs) were purchased from Lonza and maintained/passaged according to manufacturer recommendations. Confluent monolayers of cells were transduced with experimental (R1 and R2 with GFP) or control (GFP) virus at ~250 particles per cell, and 24 hrs later WT hESC-CMs were sparsely plated on top of the GFP+ confluent monolayers. Cells were allowed to adhere and form gap junctions for 48 hrs, and contractility measurements were performed on spontaneously beating hESC-CMs using the Ionoptix system.
Optical contraction analysis
Optical contraction analysis was performed as previously described[25]. Briefly, cells were rinsed and placed in HEPES-buffered Tyrode’s solution at 25°C and visualized using a Nikon TS100 inverted microscope with a 40x brightfield objective coupled to an Ionoptix videomicroscopy system (Ionoptix). Spontaneous contractions were imaged and cell length data was collected at 1 kHz. There were no differences in spontaneous rate of contraction between any of the control and experimental groups within this study. Transients were quantitatively analyzed using the Ionwizard software package (Ionoptix), and a minimum of five traces were analyzed and averaged for each cell.
Western blot analysis
Cells were harvested using 0.05% trypsin and placed in Laemmli sample buffer at −80°C, and SDS-PAGE separated proteins were transferred to nitrocellulose membrane, blocked using 5% milk (w/v in Tris-buffered saline) and probed with anti-R1 or anti-R2 antibodies (Santa Cruz Biotechnology). Protein bands were quantified using open access software (ImageJ, NIH) and expressed relative to the housekeeping protein GAPDH.
Cell transplantation
All studies were approved by the University of Washington Animal Care and Use Committee and were conducted in accordance with federal guidelines. All surgeries were performed according to protocols previously described in detail by our group[4,6,26]. Briefly, animals were anesthetized using ketamine/xylazine, mechanically ventilated, and subjected to a thoracotomy. 10 million GFP or R1R2-GFP transduced hESC-derived cardiomyocytes were directly injected into the uninjured left ventricular walls of 200–300g male nude athymic rats (Charles River) in 3–4 spatially distinct injections. The chest wall was closed, and the animals were allowed to recover and monitored for signs of discomfort or weight loss.
Histology
After euthanasia, the hearts were rapidly excised, grossly sectioned into 1mm short-axis sections, and imaged using a fluorescent dissecting microscope (Zeiss) with a GFP filter and a Nikon digital camera. Hearts were then fixed overnight in formalin, dehydrated, and embedded in paraffin. 5 μm thin sections were cut, rehydrated, and stained according to protocols previously published by our group[4,26]. The antibodies used for this study included rabbit polyclonal anti-R1 (Proteintech) and goat polyclonal anti-GFP (Novus).
Echocardiography
Transthoractic echocardiography was performed as previously described[4] using a GE Vivid7 (GE, Piscataway, NJ) with a 10 MHz pediatric transducer. Briefly, we lightly anesthetized the animals using isoflurane to minimize movement artifact and stress response, confirmed satisfactory heart rate >300 bpm while under anesthesia, and imaged the short axis of the heart using M-mode to measure left ventricular (LV) end systolic dimension (LVESD) and left ventricular end diastolic dimension (LVEDD) to calculate fractional shortening (FS=100*(LVEDD-LVESD)/LVEDD). All echocardiography measurements were performed in a blinded fashion by two independent reviewers.
Statistical Analysis
All data are presented as mean ± S.E.M. All data were initially compiled in Microsoft Excel, and normal parameter distributions were confirmed using the Shapiro-Wilk test for normality. Statistical tests were performed using the SPSS statistics package (IBM). In experiments comparing two means, a 2-tailed Student’s t-Test with unequal sample variance was performed. In experiments with multiple comparisons, ANOVA was performed with Games-Howell post-hoc analysis for unequal variance to determine significance. In all cases, α was set a priori at 0.05.
Results
hESC-CM contractility is increased with R1R2 overexpression
Our previous work has shown that R1R2 overexpression results in increased contractility in neonatal and mature adult rat cardiomyocytes[15,16]. We first verified that overexpression of R1R2 results in similar increases in contraction magnitude and velocity in hESC-CMs. Consistent with previous findings we indeed saw a doubling in contraction magnitude (Fig. 2A) and a tripling in maximum contraction velocity (Fig. 2B) above baseline values typical for hESC-CMs[25]. Despite these increases in contraction, there were no changes in maximum relaxation velocity (Fig. 2B).
Figure 2. R1R2 overexpression increases hESC-CM contractility. (A).
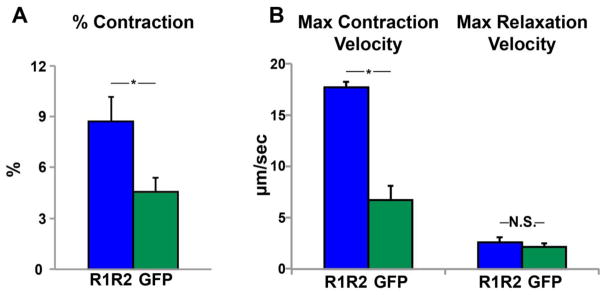
Upregulation of R1R2-GFP in hESC-CMs increases contraction magnitude compared to GFP alone. (B) hESC-CMs containing R1R2-GFP demonstrated significantly increased maximum contraction velocity without altering relaxation velocity. n=3–5 per condition. *p<0.05, N.S. not significant
Neonatal rat ventricular myocytes (NRVMs) rapidly conduct dATP-fluorescein via gap junctions
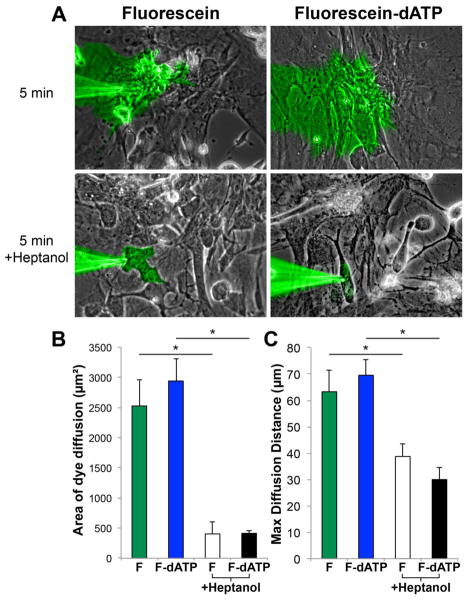
We next tested the hypothesis that like ATP[27], dATP is capable of rapidly crossing between coupled cells via gap junctions. To accomplish this, a single NRVM within a confluent monolayer was microinjected with either a highly purified commercially available dATP-fluorescein conjugate or fluorescein alone using a glass micropipette with a sub-μm tip (Fig. 3A, Supplemental video 1). We restricted our analysis to the 5-minute time point because fluorescein has been shown to compartmentalize and/or leak slowly from certain cell lines with a half-life of 30 mins[28]. We quantified this transfer and found that after 5 minutes, the fluorescein signal covered 2527 ± 432 μm2, whereas the dATP-fluorescein signal similarly occupied 2942 ± 36 μm2 (Fig. 3B, p=0.47). Furthermore, the maximum distance of dye transfer for fluorescein and dATP-fluorescein was 63 ± 8 μm and 70 ± 6 μm, respectively (Fig. 3C, p=0.54). Pretreatment with 2mM of the gap junction blocker heptanol resulted in a ~5-fold lower fluorescence transfer in both experimental groups (p<0.001) and a ~2-fold less maximum distance of dye transfer (p<0.05), suggesting that the dye transfer we observed is indeed gap junction-mediated.
Figure 3. NRVMs rapidly conduct dATP-fluorescein between cells with kinetics similar to fluorescein alone.
(A) NRVM cultures were microinjected with dATP-fluorescein or fluorescein and serially imaged for 5 minutes. Images were thresholded and quantified for (B) maximum area of dye diffusion and (C) maximum diffusion distance from the pipette tip. To assess gap junction specificity, we also added 2mM heptanol and observed a significant decrease in transfer efficiency. n=3–9 per condition. *p<0.05
hESC-CMs support gap junction-mediated dATP-fluorescein diffusion
To confirm that these results were applicable to human cardiomyocytes derived from hESCs, we performed a similar experiment in hESC-CM cultures (Fig. 4). As expected, dATP-fluorescein again rapidly transferred to neighboring cells with kinetics similar to NRVMs, and in many cases second and third order transfer readily occurred.
Figure 4. dATP-fluorescein transfers readily in hESC-CMs.
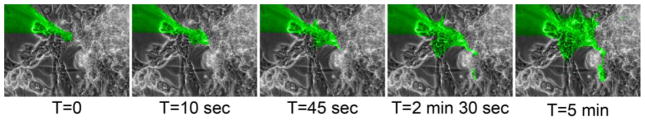
Cultures of hESC-CMs were microninjected with dATP-fluorescein, which rapidly transferred to neighboring cells over the course of 5 mins.
R1R2-overexpressing hESC-CMs enhance the contractility of neighboring cardiomyocytes
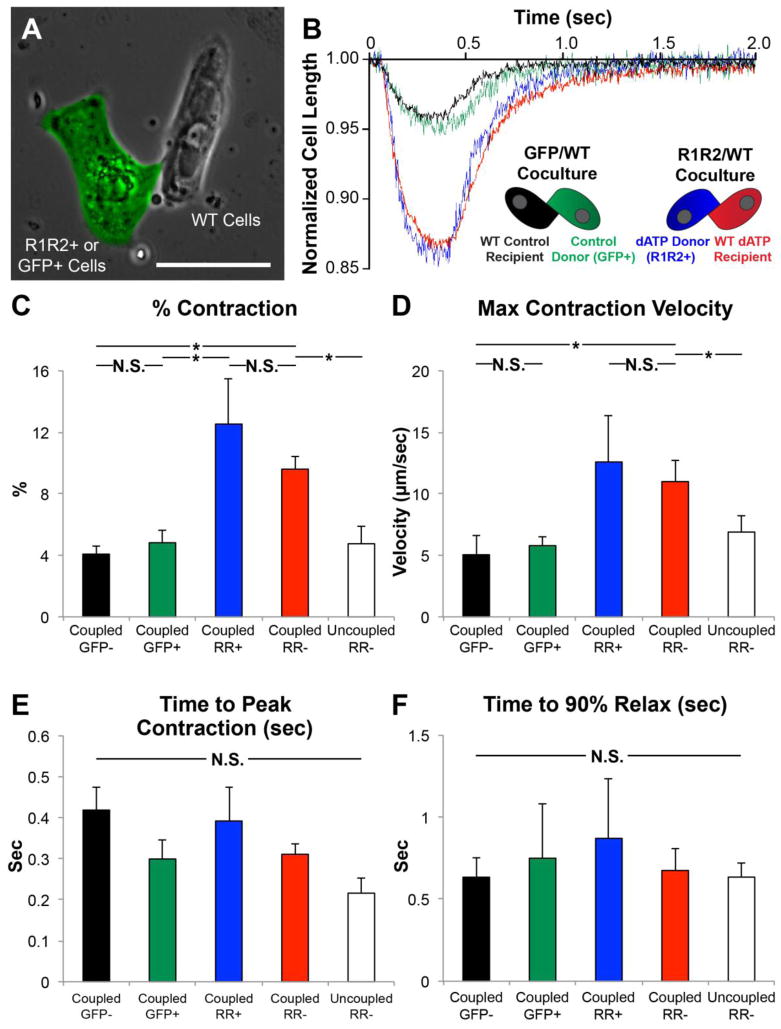
We next sought to demonstrate the functional consequences of dATP overproduction and transfer to neighboring wild type (WT) cardiomyocytes. To accomplish this, hESC-CMs were transduced with R1R2+GFP or GFP adenovirus and subsequently replated into sparse cultures of WT hESC-CMs. Within these cocultures, heterogeneous doublets with one GFP+ cell and GFP-null cell often formed (Fig. 5A). Using videomicroscopy, we measured the contractility of each of these cells, as well as singlet R1R2+GFP or GFP+ cells and GFP− cells in the same cultures as controls. As expected, GFP+ control hESC-CMs (green trace) and adjacent WT hESC-CMs coupled (black trace) exhibited similar contraction magnitudes to each other (4.8 ± 0.8% and 4.1 ± 0.6%, respectively, p=0.48) and to values previously-published by our group[25] (Fig. 5B). In stark contrast, the R1R2+GFP cells (blue trace) exhibited a substantially larger contraction magnitude (12.5 ± 2.9%). Interestingly, the GFP-negative WT hESC-CM in direct contact with R1R2+GFP cells (red trace) exhibited a statistically similar contraction magnitude (9.6 ± 0.85%, p=0.42) despite the lack of R1R2 overexpression in these cells (Fig. 5C). Similarly, both R1R2-GFP hESC-CMs and coupled WT hESC-CMs exhibited increased contraction velocity over respective controls (Fig. 5D, p<0.05), in agreement with the results in Fig. 2. There were no statistically significant differences in the time to peak contraction (Fig. 5E, p=0.11) or the time to 90% relaxation (Fig. 5F, p=0.95) among these groups. Finally, as a control, we also measured the contractile performance of uncoupled singlet WT hESC-CMs in the R1R2-WT coculture wells exhibited similar contractile performance to control GFP+ cells across all parameters measured (Fig. 5, white bars), suggesting that direct physical contact between cells is necessary for improvements in contractility.
Figure 5. R1R2-hESC-CMs enhance contractility of neighboring WT cardiomyocytes.
HESC-CMs were transduced with R1R2-GFP or control GFP adenovirus and cocultured with WT hESC-CMs. (A) The contractile parameters of each cell in a heterogeneous GFP+/GFP− doublet were measured. As a control, measurements were also taken from a WT uncoupled cell in the R1R2 cultures (white bar). (B) Representative traces show that R1R2-GFP+ cells and coupled WT hESC-CMs exhibit increased (C) magnitudes and (D) velocities of contraction but no change in the kinetics of (E) contraction or (F) relaxation. n=3–12 per condition. *p<0.05 N.S. not significant
R1R2-overexpressing fibroblasts modulate the contractility of coupled cardiomyocytes
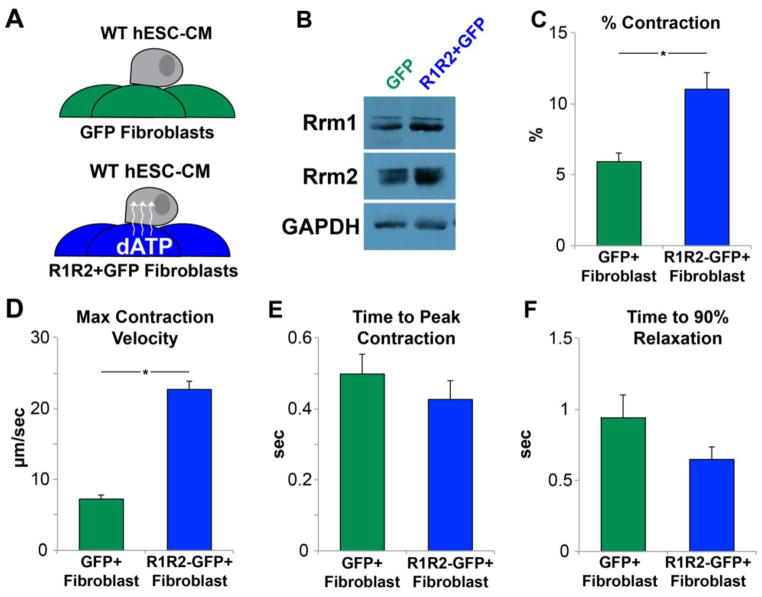
Based upon literature reports of functional coupling between cardiomyocytes and fibroblasts[29], we next sought to examine whether fibroblasts modified to overexpress R1R2 might modulate the contractility of adjacent hESC-CMs connected via gap junctions. Human neonatal dermal fibroblasts (HNDFs) were transduced with R1R2-GFP or GFP control virus and allowed to form a confluent monolayer. hESC-CMs were subsequently plated on top of this monolayer and allowed to adhere for 48hrs (Fig. 6A). Western blot analysis of the fibroblast feeders showed a >3-fold induction of both R1 and R2 at the protein level (Fig. 6B). Contractile measurement of the cardiomyocytes on R1R2-transduced fibroblast feeders had roughly a doubling in contractile magnitude compared to those in GFP-control fibroblast cultures (Fig. 6C, 11.0 ± 1.1% vs 5.9 ± 0.6%, respectively, p<0.001). Similarly, maximum contraction velocities were also increased (Fig. 6D, 22.7 ± 4.0 vs 7.2 ± 1.0 μm/sec, p<0.005). There was no change in the time to peak contraction (Fig. 6E, 498 ± 6 vs 427 ± 5 ms, p=0.36), and though the time to 90% relaxation appeared to be somewhat faster (Fig. 6F) this difference was not significant (941 ± 16 vs 648 ± 90 ms, p=0.13).
Figure 6. R1R2-Fibroblasts modulate contractility of adjacent cardiomyocytes.
(A) Fibroblasts were transduced with R1R2-GFP or GFP, and WT hESC-CMs were allowed to adhere to the monolayer. (B) Western blot analysis of the fibroblast cultures showed significantly increased quantities of R1 and R2. Measurement of hESC-CM contractility showed a significant increase in the (C) magnitude and (D) velocity of contraction in cells on R1R2-GFP feeders. (E) No changes were noted in the time to peak contraction or (F) the time to 90% relaxation. n=13–17 per condition. *p<0.05
R1R2-overexpressing hESC-CMs efficiently engraft and survive in healthy rat myocardium
Motivated by the in vitro data supporting the hypothesis of gap junction-mediated dATP transfer, we sought to test the ability of R1R2-overproducing cardiomyocytes to elevate global healthy cardiac function. To accomplish this, we transduced hESC-CMs with R1R2+GFP or control GFP virus and two days later transplanted 10 million of these cells into the anterior wall of nude rats in a series of 3–4 (Fig. 7A). Upon excision of the heart and imaging of cross-sectional slices, 100% of both control and experimental hearts displayed positive GFP signal (Figs. 7B–C). Importantly, the GFP+ signal in this experiment remained confined to engrafted cells, as the GFP protein is far too large to diffuse across gap junctions. H&E staining demonstrated multiple areas with morphology consistent with surviving engrafted cells (Figs. 7D–E). Further staining for anti-GFP confirmed the presence of graft cells in both groups (Figs. 7F–G), and high resolution imaging of these graft regions demonstrated clear regions of direct contact with healthy host myocardium (Figs. 7H–I), suggesting potential host-graft gap junction formation. Staining for the R1 subunit identified large regions of positive staining in hearts from the R1R2+GFP cohort (Fig. 7J), while hearts receiving control GFP-hESC-CMs showed diffuse low levels of positive staining without clear foci (Fig. 7K).
Figure 7. In vivo transplantation of R1R2-hESC-CMs.
(A) hESC-CMs were transduced with R1R2-GFP or GFP on D-2 and 15e6 cells transplanted into healthy nude rat hearts on D0. Animals were monitored echocardiographically before and after cell transplantation, and on D5 the hearts were explanted and histology performed. (B,C) 100% of animals receiving grafts had positive GFP signal upon gross sectioning and imaging. (D,E) H&E demonstrated areas of dense nuclei staining consistent with engrafted cells. (F,G) Anti-GFP staining colocalized to the same graft regions identified by H&E. (H,I) High magnification inset of these stains show clear regions of GFP+ signal directly adjacent to host myocardium. (J,K) Staining for the R1 subunit in serial sections revealed strongly positive clusters of cells in R1R2-GFP animals, but only weak signal in GFP-hESC-CM control hearts. Scale bar 500 μm
R1R2-overexpressing hESC-CM grafts improve global cardiac contractility above healthy baseline levels
Finally, using echocardiography, we measured the cardiac performance of hearts receiving R1R2-GFP or GFP cells. Both groups exhibited similar fractional shortening (FS) at baseline (~45%), and the control animals receiving GFP-hESC-CMs had no statistical change in this value over the 5 day course of the experiment. In stark contrast, animals receiving R1R2-hESC-CMs had a fractional shortening of 53.2 ± 2.5% at day 5 following transplantation (Fig. 8A). Not only was this value significantly higher than GFP-hESC-CM recipients at day 5 (p<0.01), but also it was significantly higher than baseline values obtained before transplantation (p<0.05). This increase in FS was not due to changes in diastolic performance or global hypertrophy/dilation, as both groups had indistinguishable weight, heart rate (Supplemental table 1), and left ventricular end diastolic dimensions (LVEDD) at both baseline and day 5 (Fig. 8B, p=0.45). The R1R2-hESC-CM group, however, had a significantly smaller Left Ventricular End Systolic Dimension (LVESD) at day 5 compared GFP-hESC-CM recipient controls (Fig. 8C, p<0.05).
Figure 8. Transplanted R1R2-hESC-CMs increase contractility of healthy hearts to above baseline physiological levels.
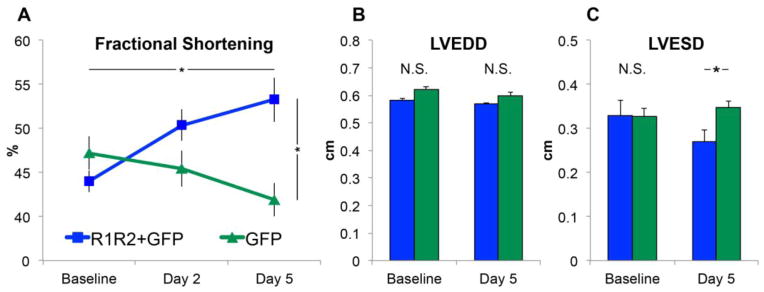
(A) Fractional shortening (FS) of animals receiving R1R2-hESC-CMs and GFP-hESC-CMs were similar at baseline, but at R1R2-hESC-CM recipient hearts had a significant increase in FS when compared to both GFP-hESC-CM recipients and to baseline pre-transplantation levels. (B) No differences were noted in the left ventricular end diastolic dimension (LVEDD), but the (C) R1R2-hESC-CM cohort at day 5 exhibited a smaller left ventricular end systolic dimension (LVESD) than other groups. n=5 per condition. *p<0.05
Discussion
Current strategies to restore lost cardiac function using hPSC-CMs as a cell therapy require substantial remuscularization of the infarct with new force-producing units. Most studies to date, however, have suggested that these cells are immature and produce contractile force two orders of magnitude below adult cardiomyocytes[30–33]. Furthermore, in vivo studies have shown that the vast majority of cells fail to survive the initial transplantation[26]. Rather than fully remuscularizing the infarct, it is possible that cells could be engineered to take advantage of the efficient gap junction intercellular communication (GJIC) network of the heart to distribute the nucleotide dATP, which acts as an energy substrate for cardiac myosin and improves cardiac function specifically at the myofilament (Fig. 1). Importantly, due to the highly cooperative nature of cardiac muscle contractile activation, the similar kD for dATP and ATP with myosin[15], and the rapid rephosphorylation of dADP by intracellular kinases, even small quantities (<1% of the total adenosine content) of dATP appear to be sufficient to accomplish this goal[15]. If successful, this approach would eliminate a number of challenges associated with traditional cell therapy, including the necessity to achieve large-scale engraftment in the hostile infarct zone. Furthermore, this approach is compatible with small quantities of engrafted cells within the healthy viable remaining myocardium, where engraftment has been shown to be more robust[26]. While this strategy could also be accomplished using traditional gene therapy to upregulate R1R2, the enzyme responsible for dATP production, using cardiomyocytes as the delivery vehicle offers three primary advantages. First, unlike viral transduction of native host myocardium, exogenous R1R2-overexpressing cells can be generated using site-specific next-generation genome engineering techniques (e.g. zinc finger nuclease or CRISPR), and individual hPSC clones can be sequenced and exhaustively characterized prior to transplantation. Second, engraftment of new cardiomyocytes, however immature, may provide additional force-producing units and electrically stabilize the myocardium, especially if long-term maturation does indeed occur as suggested by the literature[34,35]. Finally, while R1R2 overexpression has been implicated as potentially oncogenic, these effects have only been appreciated in lung tissue[36], and the choice of human cardiomyocytes, which rapidly senesce[37], further minimizes this risk.
In order for the proposed strategy to be successful, the ideal candidate cell type must (1) be capable of overexpressing R1R2, (2) express sufficient quantities of connexin protein to facilitate transfer (as well as adhesive proteins such as N-cadherin to mechanically stabilize the junction), and (3) survive transplantation into myocardium. Cells such as skeletal myoblasts robustly engraft, but they fail to express the appropriate connexins necessary for coupling[38]. Human mesenchymal stem cells (hMSCs) fulfill some of these requirements[39], but their ability to permanently survive in an infarcted heart remains to be conclusively demonstrated[40]. We chose to pursue the use of hESC-CMs as a cell delivery platform because they are uniquely amenable to targeted genetic engineering, they express high levels of connexins including Cx-43[25,41,42], they efficiently engraft and survive in healthy and diseased rodent hearts[4,26], and importantly they readily form functional gap junctions with host myocardium[21].
Using dye transfer studies with fluorescein dye or dATP-fluorescein conjugates (Figs. 3–4), we showed that cell-to-cell gap junction transfer occurs robustly in both NRVMs and hESC-CMs. Interestingly, there were little differences in the kinetics of transfer between the dye alone and the larger dye-nucleotide, which is likely due to the complex interplay between size and charge that dictates the biophysics of GJIC[43]. The addition of heptanol resulted in a precipitous decline in dye transfer efficiency, suggesting that the effect is indeed gap junction-mediated. Indeed, the positive results supporting gap junction dATP transfer is not entirely surprising, given the literature precedent for highly efficient transfer of ATP through cardiac gap junctions[23,27] and the fact that dATP is highly similar in size and charge profile. Coculture of dATP-producing cardiomyocytes with WT cardiomyocytes caused robust increases in contractile performance in cells directly coupled to R1R2-hESC-CMs (Fig. 5). Critically, uncoupled single WT cardiomyocytes in cultures containing R1R2-hESC-CMs were unaffected and demonstrated contraction characteristics identical to the GFP-hESC-CMs and WT hESC-CMs coupled to them. This demonstrates that direct physical coupling rather than paracrine signaling such as purinergic receptor activation[44] is required for any functional benefit.
The second coculture experiment involving R1R2-overexpressing donor fibroblasts provided two important pieces of information (Fig. 6). First, the use of a non-contractile donor cell population (fibroblasts) rules out passive mechanical effects between two contractile cardiomyocytes as the explanation for increased contractility shown in Fig. 5. Second, this experiment also suggests that fibroblasts actively participate in GJIC in vitro and may play a critical role in facilitating dATP diffusion in vivo as well. This notion is well supported by a number of prior studies showing functional gap junction coupling with fibroblasts[45–48]. In agreement with our previous reports[15,16], this experiment further supports the absence of deleterious effects of dATP on the relaxation kinetics of cardiomyocytes and supports the idea that dATP may represent a way to increase systolic function without impairing diastolic performance.
Finally, as a proof of principle we tested the hypothesis that R1R2-hESC-CM transplantation and dATP transfer will improve the global function of healthy rodent hearts. We chose this model system first because any supraphysiological elevation of function is unlikely to occur from transplantation of the mechanically immature hESC-CMs alone and is instead likely due to the presence of dATP. Importantly, the ability of graft cells to electrically couple 1:1 to host rodent myocardium is unnecessary for GJIC; merely the presence of functional host-graft gap junction communication is sufficient. Previous studies have conclusively shown functional gap junction-mediated coupling and dye transfer between immature (neonatal) cardiomyocytes and adult cardiomyocytes[24], suggesting that transplanted immature hESC-CMs have the capability to electrically couple with and diffuse small molecules to neighboring adult cardiomyocytes. Excitingly, R1R2-hESC-CM transplantation significantly improved fractional shortening not only above GFP-hESC-CMs, but also above baseline echo function in the same animals just 5 days earlier (Fig. 8). This was directly attributable to increases in systolic function, as no changes in heart rate or end diastolic dimensions were noted. To our knowledge this is the first demonstration of hPSC-CM transplantation successfully elevating cardiac function above healthy baseline levels.
Taken together, these findings suggest that transplantation of R1R2-overexpressing hESC-CMs improves global cardiac function to a significant degree and suggests that a hybrid dATP/cell therapy may provide a novel synergistic approach for treating heart disease. While promising, it will be important to demonstrate stable long-term functional benefits in healthy animals beyond the acute time-point shown in the current work, as well as demonstrating rescue of lost cardiac function in diseased models. Additionally, while it still remains unclear if the presence of dATP-producing hESC-CMs might alter Ca2+-handling, evidence from our lab suggests that the presence of small quantities of dATP has minimal effect on Ca2+ homeostasis[15,16], and hESC-CM transplantation may in fact have the opposite effect of suppressing arrhythmias[21]. Nevertheless it is certainly possible that arrhythmogenesis may occur, especially at high heart rates and/or in damaged myocardium, and further work will be necessary to more thoroughly address this question.
In summary, this study provides the first direct evidence that gap junctions and diffusion may be exploited as a strategy to deliver a biological small molecule throughout the cardiac syncytium for therapeutic purposes. This study also suggests that, in addition to regenerating new myocardium, cell therapy itself may be capable of acting as biological drug delivery platform, producing any number of compounds with beneficial effects in a wide variety of different cardiac pathologies.
Supplementary Material
Highlights.
Ribonucleotide reductase (R1R2) synthesizes dATP
R1R2 increases contractility in human stem cell-derived cardiomyocytes
dATP diffuses via gap junctions and increases coupled cardiomyocyte contractility
Transplantation of R1R2 stem cell-derived cardiomyocytes increases cardiac function
First demonstration of cell-cell diffusive drug delivery to the heart
Proof-of-principle for future cell-based biological drug delivery approaches
Acknowledgments
We thank Benjamin Van Biber for assistance with cell culture, Elina Minami for technical assistance with echocardiography, Marta Scatena for use of the Zeiss fluorescence microscope, and Steve Korte for insightful scientific discussions.
Sources of Funding
This work was supported by NIH grants T32-GM 7266-37 S1 (SDL), R01-HL11197 (MR), R01-HL064387 (MAL, CEM and MR), R01-HL084642 (CEM), P01-HL094374 (CEM and MAL), P01 GM81619 (CEM), U01-HL100405 (CEM and MAL), and R01-HL117991 (MAL). MR is an Established Investigator of the American Heart Association.
Abbreviations
- CM
Cardiomyocyte
- dATP
2-deoxyadenosine triphosphate
- GJIC
Gap junction intercellular communication
- hESC
Human embryonic stem cell
- hPSC
Human pluripotent stem cell
- R1R2
Ribonucleotide reductase
Footnotes
Disclosures
SDL, SAM, and JD declare no financial conflicts. MR, MAL, and CEM are founders and equity holders in BEAT BioTherapeutics Corporation, which has licensed the gene- and cell-based dATP technology discussed in this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.World Health Organization, editor. Global Status Report on Noncommunicable Diseases 2010–2011. pp. 1–176. [Google Scholar]
- 2.Mebazaa A, Nieminen MS, Packer M, Cohen-Solal A, Kleber FX, Pocock SJ, et al. Levosimendan vs dobutamine for patients with acute decompensated heart failure: the SURVIVE Randomized Trial. Jama. 2007;297:1883–91. doi: 10.1001/jama.297.17.1883. [DOI] [PubMed] [Google Scholar]
- 3.Malik FI, Hartman JJ, Elias KA, Morgan BP, Rodriguez H, Brejc K, et al. Cardiac Myosin Activation: A Potential Therapeutic Approach for Systolic Heart Failure. Science. 2011;331:1439–43. doi: 10.1126/science.1200113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laflamme MA, Chen KY, Naumova AV, Muskheli V, Fugate JA, Dupras SK, et al. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol. 2007;25:1015–24. doi: 10.1038/nbt1327. [DOI] [PubMed] [Google Scholar]
- 5.Caspi O, Huber I, Kehat I, Habib M, Arbel G, Gepstein A, et al. Transplantation of human embryonic stem cell-derived cardiomyocytes improves myocardial performance in infarcted rat hearts. J Am Coll Cardiol. 2007;50:1884–93. doi: 10.1016/j.jacc.2007.07.054. [DOI] [PubMed] [Google Scholar]
- 6.Fernandes S, Naumova AV, Zhu WZ, Laflamme MA, Gold J, Murry CE. Human embryonic stem cell-derived cardiomyocytes engraft but do not alter cardiac remodeling after chronic infarction in rats. J Mol Cell Cardiol. 2010;49:941–9. doi: 10.1016/j.yjmcc.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sesti C, Kloner RA. Gene therapy in congestive heart failure. Circulation. 2004;110:242–3. doi: 10.1161/01.CIR.0000137593.62669.67. [DOI] [PubMed] [Google Scholar]
- 8.Regnier M, Homsher E. The effect of ATP analogs on posthydrolytic and force development steps in skinned skeletal muscle fibers. Biophysj. 1998;74:3059–71. doi: 10.1016/S0006-3495(98)78013-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Regnier M, Lee DM, Homsher E. ATP analogs and muscle contraction: mechanics and kinetics of nucleoside triphosphate binding and hydrolysis. Biophysj. 1998;74:3044–58. doi: 10.1016/S0006-3495(98)78012-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Regnier M, Martyn DA, Chase PB. Calcium regulation of tension redevelopment kinetics with 2-deoxy-ATP or low [ATP] in rabbit skeletal muscle. Biophysj. 1998;74:2005–15. doi: 10.1016/S0006-3495(98)77907-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Regnier M, Rivera AJ, Chen Y, Chase PB. 2-deoxy-ATP enhances contractility of rat cardiac muscle. Circulation Research. 2000;86:1211–7. doi: 10.1161/01.res.86.12.1211. [DOI] [PubMed] [Google Scholar]
- 12.Regnier M, Martin H, Barsotti RJ, Rivera AJ, Martyn DA, Clemmens E. Cross-bridge versus thin filament contributions to the level and rate of force development in cardiac muscle. Biophysj. 2004;87:1815–24. doi: 10.1529/biophysj.103.039123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adhikari BB, Regnier M, Rivera AJ, Kreutziger KL, Martyn DA. Cardiac length dependence of force and force redevelopment kinetics with altered cross-bridge cycling. Biophysj. 2004;87:1784–94. doi: 10.1529/biophysj.103.039131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moreno-Gonzalez A, Gillis TE, Rivera AJ, Chase PB, Martyn DA, Regnier M. Thin-filament regulation of force redevelopment kinetics in rabbit skeletal muscle fibres. J Physiol (Lond) 2007;579:313–26. doi: 10.1113/jphysiol.2006.124164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Steven Korte F, Dai J, Buckley K, Feest ER, Adamek N, Geeves MA, et al. Upregulation of cardiomyocyte ribonucleotide reductase increases intracellular 2 deoxy-ATP, contractility, and relaxation. J Mol Cell Cardiol. 2011 doi: 10.1016/j.yjmcc.2011.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nowakowski SG, Kolwicz SC, Korte FS, Luo Z, Robinson-Hamm JN, Page JL, et al. Transgenic overexpression of ribonucleotide reductase improves cardiac performance. Proc Natl Acad Sci USA. 2013;110:6187–92. doi: 10.1073/pnas.1220693110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schoffstall B, Clark A, Chase PB. Positive inotropic effects of low dATP/ATP ratios on mechanics and kinetics of porcine cardiac muscle. Biophysj. 2006;91:2216–26. doi: 10.1529/biophysj.105.079061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schoffstall B, Chase PB. Increased intracellular [dATP] enhances cardiac contraction in embryonic chick cardiomyocytes. J Cell Biochem. 2008;104:2217–27. doi: 10.1002/jcb.21780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caspi O, Huber I, Kehat I, Habib M, Arbel G, Gepstein A, et al. Transplantation of human embryonic stem cell-derived cardiomyocytes improves myocardial performance in infarcted rat hearts. J Am Coll Cardiol. 2007;50:1884–93. doi: 10.1016/j.jacc.2007.07.054. [DOI] [PubMed] [Google Scholar]
- 20.Laflamme MA, Gold J, Xu C, Hassanipour M, Rosler E, Police S, et al. Formation of human myocardium in the rat heart from human embryonic stem cells. Am J Pathol. 2005;167:663–71. doi: 10.1016/S0002-9440(10)62041-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shiba Y, Fernandes S, Zhu W-Z, Filice D, Muskheli V, Kim J, et al. Human ES-cell-derived cardiomyocytes electrically couple and suppress arrhythmias in injured hearts. Nature. 2012 doi: 10.1038/nature11317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anselmi F, Hernandez VH, Crispino G, Seydel A, Ortolano S, Roper SD, et al. ATP release through connexin hemichannels and gap junction transfer of second messengers propagate Ca2+ signals across the inner ear. Proc Natl Acad Sci USA. 2008;105:18770–5. doi: 10.1073/pnas.0800793105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goldberg GS, Lampe PD, Nicholson BJ. Selective transfer of endogenous metabolites through gap junctions composed of different connexins. Nat Cell Biol. 1999;1:457–9. doi: 10.1038/15693. [DOI] [PubMed] [Google Scholar]
- 24.Reinecke H, Zhang M, Bartosek T, Murry CE. Survival, integration, and differentiation of cardiomyocyte grafts: a study in normal and injured rat hearts. Circulation. 1999;100:193–202. doi: 10.1161/01.cir.100.2.193. [DOI] [PubMed] [Google Scholar]
- 25.Lundy SD, Zhu W-Z, Regnier M, Laflamme MA. Structural and functional maturation of cardiomyocytes derived from human pluripotent stem cells. Stem Cells Dev. 2013;22:1991–2002. doi: 10.1089/scd.2012.0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goldberg GS, Moreno AP, Lampe PD. Gap junctions between cells expressing connexin 43 or 32 show inverse permselectivity to adenosine and ATP. J Biol Chem. 2002;277:36725–30. doi: 10.1074/jbc.M109797200. [DOI] [PubMed] [Google Scholar]
- 27.Stewart WW. Functional connections between cells as revealed by dye-coupling with a highly fluorescent naphthalimide tracer. Cell. 1978;14:741–59. doi: 10.1016/0092-8674(78)90256-8. [DOI] [PubMed] [Google Scholar]
- 28.Pedrotty DM, Klinger RY, Badie N, Hinds S, Kardashian A, Bursac N. Structural coupling of cardiomyocytes and noncardiomyocytes: quantitative comparisons using a novel micropatterned cell pair assay. Am J Physiol Heart Circ Physiol. 2008;295:H390–400. doi: 10.1152/ajpheart.91531.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu J, Sun N, Bruce MA, Wu JC, Butte MJ. Atomic force mechanobiology of pluripotent stem cell-derived cardiomyocytes. PLoS ONE. 2012;7:e37559. doi: 10.1371/journal.pone.0037559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun N, Yazawa M, Liu J, Han L, Sanchez-Freire V, Abilez OJ, et al. Patient-specific induced pluripotent stem cells as a model for familial dilated cardiomyopathy. Sci Transl Med. 2012;4:130ra47. doi: 10.1126/scitranslmed.3003552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pillekamp F, Reppel M, Rubenchyk O, Pfannkuche K, Matzkies M, Bloch W, et al. Force measurements of human embryonic stem cell-derived cardiomyocytes in an in vitro transplantation model. Stem Cells. 2007;25:174–80. doi: 10.1634/stemcells.2006-0094. [DOI] [PubMed] [Google Scholar]
- 32.Hazeltine LB, Simmons CS, Salick MR, Lian X, Badur MG, Han W, et al. Effects of substrate mechanics on contractility of cardiomyocytes generated from human pluripotent stem cells. Int J Cell Biol. 2012;2012:508294. doi: 10.1155/2012/508294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moreno-Gonzalez A, Korte FS, Dai J, Chen KY, Ho B, Reinecke H, et al. Cell therapy enhances function of remote non-infarcted myocardium. J Mol Cell Cardiol. 2009;47:603–13. doi: 10.1016/j.yjmcc.2009.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dai W, Field LJ, Rubart M, Reuter S, Hale SL, Zweigerdt R, et al. Survival and maturation of human embryonic stem cell-derived cardiomyocytes in rat hearts. J Mol Cell Cardiol. 2007;43:504–16. doi: 10.1016/j.yjmcc.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu X, Page JL, Surtees JA, Liu H, Lagedrost S, Lu Y, et al. Broad overexpression of ribonucleotide reductase genes in mice specifically induces lung neoplasms. Cancer Res. 2008;68:2652–60. doi: 10.1158/0008-5472.CAN-07-5873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Snir M, Kehat I, Gepstein A, Coleman R, Itskovitz-Eldor J, Livne E, et al. Assessment of the ultrastructural and proliferative properties of human embryonic stem cell-derived cardiomyocytes. Am J Physiol Heart Circ Physiol. 2003;285:H2355–63. doi: 10.1152/ajpheart.00020.2003. [DOI] [PubMed] [Google Scholar]
- 37.Koh GY, Klug MG, Soonpaa MH, Field LJ. Differentiation and long-term survival of C2C12 myoblast grafts in heart. J Clin Invest. 1993;92:1548–54. doi: 10.1172/JCI116734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Valiunas V, Doronin S, Valiuniene L, Potapova I, Zuckerman J, Walcott B, et al. Human mesenchymal stem cells make cardiac connexins and form functional gap junctions. J Physiol (Lond) 2004;555:617–26. doi: 10.1113/jphysiol.2003.058719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Laflamme MA, Murry CE. Heart regeneration. Nature. 2011;473:326–35. doi: 10.1038/nature10147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kehat I, Gepstein A, Spira A, Itskovitz-Eldor J, Gepstein L. High-resolution electrophysiological assessment of human embryonic stem cell-derived cardiomyocytes: a novel in vitro model for the study of conduction. Circulation Research. 2002;91:659–61. doi: 10.1161/01.res.0000039084.30342.9b. [DOI] [PubMed] [Google Scholar]
- 41.Goh G, Self T, Barbadillo Muñoz MD, Hall IP, Young L, Denning C. Molecular and phenotypic analyses of human embryonic stem cell-derived cardiomyocytes: opportunities and challenges for clinical translation. Thromb Haemost. 2005;94:728–37. doi: 10.1160/TH05-04-0268. [DOI] [PubMed] [Google Scholar]
- 42.Goldberg GS, Valiunas V, Brink PR. Selective permeability of gap junction channels. Biochim Biophys Acta. 2004;1662:96–101. doi: 10.1016/j.bbamem.2003.11.022. [DOI] [PubMed] [Google Scholar]
- 43.Balogh J, Wihlborg A-K, Isackson H, Joshi BV, Jacobson KA, Arner A, et al. Phospholipase C and cAMP-dependent positive inotropic effects of ATP in mouse cardiomyocytes via P2Y11-like receptors. J Mol Cell Cardiol. 2005;39:223–30. doi: 10.1016/j.yjmcc.2005.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tomasetto C, Neveu MJ, Daley J, Horan PK, Sager R. Specificity of gap junction communication among human mammary cells and connexin transfectants in culture. J Cell Biol. 1993;122:157–67. doi: 10.1083/jcb.122.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Spanakis SG, Petridou S, Masur SK. Functional gap junctions in corneal fibroblasts and myofibroblasts. Invest Ophthalmol Vis Sci. 1998;39:1320–8. [PubMed] [Google Scholar]
- 46.Nagira T, Matthew SB, Yamakoshi Y, Tsuchiya T. Enhancement of gap junctional intercellular communication of normal human dermal fibroblasts cultured on polystyrene dishes grafted with poly-N-isopropylacrylamide. Tissue Eng. 2005;11:1392–7. doi: 10.1089/ten.2005.11.1392. [DOI] [PubMed] [Google Scholar]
- 47.Zhang Y, Kanter EM, Yamada KA. Remodeling of cardiac fibroblasts following myocardial infarction results in increased gap junction intercellular communication. Cardiovasc Pathol. 2010;19:e233–40. doi: 10.1016/j.carpath.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.