Standfirst
Supramolecular structures composed of inorganic nanoparticles and DNA strands can efficiently target tumours and then be disassembled in order to ease elimination from the body.
The ideal targeted contrast agent should vanish after lighting up at the specific target site in the body1. Similarly, the ideal cargo vehicle, be it nanoparticles or molecular assemblies, should deliver biomedical imaging, diagnostic, or theranostic agents to the target site and then be eliminated from the body2. Writing in Nature Nanotechnology, Warren Chan and colleagues at the University of Toronto now report Lego-like nanoassemblies made from single-stranded DNA and gold nanoparticles that are large enough to be delivered to tumour sites via the enhanced permeation and retention (EPR) effect3. Once the assemblies have reached their target, they degrade into the original building blocks, which are then small enough to be eliminated through the kidneys.
The use of DNA in the nanoassemblies provides several useful features: it allows robust and modular architectures to be built through the programmability of its sequences; it allows different diagnostic probes to be loaded, which can be used for imaging techniques such as positron emission tomography, single photon emission computed tomography, magnetic resonance imaging, or optical imaging; and it allows the delivery of theranostic agents into different sites to be controlled through the attachment of targeting moieties.
Inorganic nanoparticles have been widely used in biomedical applications for bioimaging, diagnostic and theranostic purposes2. Although the US Food and Drug Administration treats (or regulates) nanoparticles like any other diagnostic or therapeutic agents (for example, small molecules)2 the clinical translation of such nanoparticles is fundamentally limited because of the (potential) differences in their uptake and clearance from the body. In particular, most nanoparticles exhibit high uptake in the liver and gastrointestinal tract once they are administered, resulting in their slow complete elimination (through hepatobiliary clearance). For this reason, direct elimination from the kidneys to the bladder (renal clearance) is the preferred route for removal of the unbound targeted nanoparticles in order to reduce nonspecific background uptake in the major organs4. Larger nanoparticles are more effective when taking advantage of the EPR effect but their persistence could be toxic. Smaller nanoparticles are less likely to be taken up by passive targeting, but are eliminated quickly from the body. Chan and colleagues manipulate both of these properties to create degradable nanoassemblies.
To improve biodegradation and renal clearance, the researchers assembled gold nanoparticles using DNA building blocks and decorated their surface using polyethylene glycol (PEG). The structures have a gold core nanoparticle, which is surrounded by one or more layers of gold satellite nanoparticles. Each nanoparticle was modified with single-stranded DNA and linked together using DNA strands with complementary sequences. This design strategy improved tumour accumulation of the nanoassembly via passive targeting and the EPR effect, while significantly reducing uptake in the reticuloendothelial system. Finally, the supramolecular structure decomposed into the original building blocks, which promoted efficient elimination of each component into urine (3–5-nm satellite nanoparticles). This strategy improves the biodistribution and renal clearance of injected inorganic nanoparticles in living animals, while maintaining tumour-targeting efficiency. Thus, the DNA-mediated core–satellite supramolecular nanoassemblies have the potential to replace the toxic and hydrophobic inorganic nanoparticles, such as quantum dots and carbon nanotubes, that are typically used in drug-delivery systems (Fig. 1).
Figure 1. The delivery and clearance of nanoassemblies.
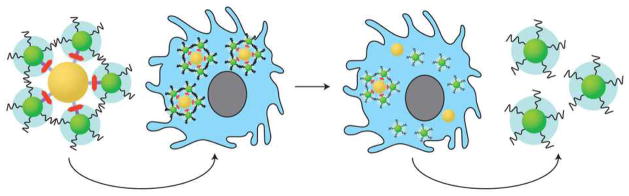
The nanoassemblies comprising core (gold) and satellite (green) nanoparticles are constructed using complementary DNA sequences as linkers (red) and coated with ligands such as PEG (black strands, blue clouds) to control their interactions with cells. When injected intravenously, they are uptaken via the EPR effect to the target site. Once inside the cell (blue), the assemblies exert their therapeutic effect and breakdown into their constituents for elimination.
During the past decade, it has been shown that a number of important factors mediate the ultimate fate of nanoparticles in vivo including solubility, stability, shape and flexibility, size and size distribution, and formulation2, 5, 6. The design of precise nanostructures is typically focused on developing site-specific delivery and targeting capabilities but biodistribution and clearance of the nanostructures from the neighbouring tissue and local environment are also critical. Thus, it is important to increase specificity, selectivity and safety1, which are the fundamental principles that govern the clinical translation of nanoparticles7. In particular, these factors are crucial for determining physiological behaviours and pharmacokinetic parameters of nanoparticles in the body, as well as to ascertain their potential cytotoxicity and in vivo toxicity. The use of biocompatible or biodegradable supramolecular structures is essential to enhance renal clearance, and eventually reduce potential in vivo toxicity (Fig. 1)3.
While taking steps towards overcoming the fundamental limitations of nanoparticles, namely efficient targeting and renal clearance, the DNA nanoassembly developed by Chan and colleagues3 still needs improvement as a targeted probe. First, the biodistribution and targeting process is mediated by macrophage uptake and phagosomal accumulation, which increases nonspecific uptake and retention in the liver and spleen. To avoid this immune response, longer PEG chains were used; however, this eventually will increase the overall hydrodynamic diameter of the nanoassembly, preventing renal clearance of decomposed building blocks. Second, passive targeting via the EPR effect requires the long circulation time of nanoparticles in the body and the EPR effect is only exploited when the size of the nanoparticle is larger than 10 nm in hydrodynamic diameter. Therefore, there is a balance to strike between PEGylation, which increases the size of the nanoparticle, and keeping the diameter of the nanoparticles within limits to allow renal clearance and prevent undesired nonspecific background retentions. These important trade-offs should be explored in future studies.
References
- 1.Lee JH, Park G, Hong GH, Choi J, Choi HS. Quant Imaging Med Surg. 2012;2:266–273. doi: 10.3978/j.issn.2223-4292.2012.12.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Choi HS, Frangioni JV. Mol Imaging. 2010;9:291–310. [PMC free article] [PubMed] [Google Scholar]
- 3.Chou LY, et al. Nature Nanotech. 2014;9:148–155. doi: 10.1038/nnano.2013.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choi HS, et al. Nature Biotechnol. 2007;25:1165–1170. doi: 10.1038/nbt1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu J, Yu M, Zhou C, Zheng J. Materials Today. 2013;16:477–486. [Google Scholar]
- 6.Albanese A, Lam AK, Sykes EA, Rocheleau JV, Chan WCW. Nature Commun. 2013;4:2718. doi: 10.1038/ncomms3718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi HS, et al. Nature Nanotech. 2010;5:42–47. doi: 10.1038/nnano.2009.314. [DOI] [PMC free article] [PubMed] [Google Scholar]


