Abstract
Background
The public health burden of chronic kidney disease (CKD) and end-stage kidney disease is a national priority and is the subject of recent guidelines. In the UK, ethnic minority groups are over-represented in the renal replacement population (17.8%) compared with the white population (11%).
Aim
Non-steroidal anti-inflammatory drugs (NSAIDs) are a preventable cause of renal damage. Previous studies suggest a prescribing prevalence between 9% and 36% among those with CKD, but have not examined differences by ethnic group.
Design and setting
Cross-sectional survey of 12 011 patients with identified CKD (stages 3–5) in the three PCTs of Tower Hamlets, Hackney, and Newham.
Method
Assessment of NSAID prescribing rates in a multi-ethnic, socially-deprived population, using descriptive and multivariate analysis.
Results
NSAIDs were prescribed for 11.1% of patients with CKD in the year prior to November 2012. Prescribing rates decreased stepwise by stage of renal impairment. Using daily defined dosages this study shows that in comparison with white groups both South Asian and black groups are much less likely to be in the top decile of NSAID prescribing, hence the overall prescribing load will be less: (odds ratio [OR] for South Asians = 0.34, 95% confidence interval [CI] = 0.22 to 0.54, OR for black groups = 0.34, 95% CI = 0.19 to 0.63).
Conclusion
National rates of NSAID prescribing continue to rise, and over-the-counter sales remain unmonitored, despite longstanding concerns about renal outcomes. Prescribing patterns indicate that GPs reduce prescribing as CKD progresses. Differential use of NSAIDs by ethnic group is unlikely to contribute to the high rates of end-stage kidney disease in ethnic minority groups.
Keywords: chronic kidney disease, ethnicity, general practice, NSAID prescribing
INTRODUCTION
The public health burden of chronic kidney disease (CKD) and end-stage kidney disease is a national priority in the UK,1 and is the subject of recently published national CKD guidelines.2 Identifying patient groups at risk of progressive CKD offers the potential to provide targeted health care to groups at higher risk of complications. Identifying reversible causes of CKD progression is a key priority.
In the UK it is of concern that a higher proportion of the prevalent renal replacement population comes from ethnic minority groups compared with the white UK population (17.8% versus 11%).3
Ethnic group, particularly South Asian, has been identified as a risk factor for the prevalence and severity of concomitant CKD in patients with diabetes mellitus and hypertension.4,5 Progressive renal decline in patients with diabetes shows differences by ethnic group, with non-white groups progressing more rapidly, but overall rates of decline are slow.6,7 Currently there is insufficient evidence to determine why ethnic minorities are over-represented on UK renal replacement programmes.
Non-steroidal anti-inflammatory drugs
Analgesics, including non-steroidal anti-inflammatory drugs (NSAIDs), are among the most common prescription and over-the-counter (OTC) medications taken by populations. NSAIDs have long been identified as nephrotoxic, with both acute and chronic effects on renal function. There is mixed evidence on the long-term effects of chronic NSAID use on renal function, with a number of studies showing a risk increase between two- and eight-fold for renal decline or admission for acute kidney injury,8–10 with some studies showing a dose-response effect.11–13 No UK studies were found on NSAID prescribing in ethnic minority groups with CKD.
Although studies of younger, healthy adults have shown little risk from long-term exposure to NSAIDs,14,15 recent studies provide robust evidence of a risk of decline in renal function among older people experiencing high cumulative NSAID exposure.8,16,17
Studies on the effects of drug interactions highlight the additional risk of acute kidney injury when there is concurrent use of diuretics with angiotensin-converting-enzyme inhibitors/angiotensin receptor blockers (ACEIs/ARBs) and NSAIDs.18
In the UK, as in many countries, some oral NSAID preparations are available without prescription, and may additionally form part of OTC combination products marketed for symptoms of febrile illnesses and pain. This provides additional potential for renal damage, which may occur when a combination of prescription and OTC medications are used together. Surveys in the US show high levels of combined prescription and OTC NSAID use.19,20
This analysis uses data from 12 000 adult patients from east London general practice with estimated glomerular filtration rate (eGFR) values of <60 ml/min/1.73m2. With high levels of ethnic group recording in the primary care population (over 95%), it was possible to examine the prevalence and intensity of oral NSAID prescribing by ethnic group. It is hypothesised that ethnic minority groups may be more likely to receive a prescription for NSAIDs, and that this in turn may help to explain the disproportionate prevalence of patients from black and South Asian groups on renal replacement treatment (RRT) in the UK.
How this fits in
NSAIDs are widely prescribed and are a preventable cause of nephrotoxicity. High intensity NSAID use is most frequent among people of white ethnicity. Hence differential prescribing is unlikely to contribute to the over representation of ethnic minority groups in renal replacement programmes.
METHOD
This details of the study setting have been described previously.7 In November 2012, demographic, clinical, and prescribing data were extracted for all patients aged ≥18 years, registered with 102 general practices across Tower Hamlets, Newham, and City & Hackney. All data were anonymous and managed according to UK NHS information governance requirements.
Demographic variables included age, sex, ethnic group, and deprivation. Self-reported ethnic group was recorded at the practice during registration or routine consultation. Ethnic categories are based on the UK 2011 census and for this study were condensed into four categories: white (British, Irish, other white), black (black African, black Caribbean, black British, other black, and mixed black), South Asian (Bangladeshi, Pakistani, Indian, Sri Lankan, British Asian, other South Asian, or mixed Asian), and other (Chinese, other, other mixed). Patients of mixed ethnic groups were grouped with their parent ethnic minority. For example, an individual who had classified themselves as mixed South Asian and British was classed as South Asian for the purposes of this study. Patients with an ethnic code of ‘not stated’ or missing ethnic group were excluded from the analysis. The Townsend score, which is derived from census variables and linked to patient place of residence, was used as a measure of social deprivation.21 Patients were included in the study if they had two or more eGFR values below 60 ml/min/1.73m2 recorded in the previous 2 years. eGFR was derived from serum creatinine values using the four-variable modification of diet in renal disease (MDRD) equation, which incorporates an adjustment for black ethnicity.22 Additional clinical variables for analysis included the presence of the following Read Coded comorbidities (ischaemic heart disease, stroke, type 2 diabetes, hypertension, arthritis, and chronic pain) and proteinuria.23 Arthritis was a composite of diagnoses for osteoarthritis, rheumatoid arthritis, ankylosing spondylitis, and psoriatic arthritis. Patients were considered to be proteinuric if they had a protein:creatinine ratio value of ≥15 mg/ mmol, an albumin:creatinine ratio value of ≥2.5 mg/mmol for males, or ≥3.5 mg/mmol for females, or a urine dipstick result for protein of >1.
All prescriptions for oral NSAIDs (excluding low dose aspirin) in the past 12 months were extracted. In addition to deriving an indicator for whether an NSAID had ever been prescribed, the number of defined daily doses, summed for all classes of prescribed NSAIDs, was calculated for each patient.24 Prescribing data were also extracted for paracetamol, as a possible alternative to NSAIDs, and for proton pump inhibitors (PPIs). Dyspepsia is one of the most common side effects of NSAID medication, and there is some evidence that symptomatic dyspepsia is more common among South Asian patients, and may act as a brake on rates of NSAID prescribing.25
Statistical analysis
All statistical analyses were performed using Stata (version 12). Numerical variables with values outside of the valid range were removed from the dataset.
To explore the prevalence and variation in NSAID prescribing, a logistic regression analysis was used to determine whether the odds of being in the top decile of NSAID use differed by ethnic group. This was adjusted for age, sex, deprivation, latest eGFR value, all comorbidities, and clustering by practice. A P-value of <0.05 was considered to represent statistical significance.
RESULTS
Study population
Of the 544 053 adult patients registered in the contributing general practices across east London, 12 011 satisfied the inclusion criteria for the study (two eGFR values <60 ml/min/1.73m2 recorded in the previous 2 years). The characteristics of the study population are shown in Table 1.
Table 1.
Characteristics of the study population
| Demographic characteristics | White | South Asian | Black | Other | Unknown | Total |
|---|---|---|---|---|---|---|
| Total study population | ||||||
| n | 6315 | 3307 | 1611 | 443 | 334 | 12 011 |
| % | 52.6 | 27.5 | 13.4 | 3.7 | 2.8 | 100.0 |
| % male | 39.1 | 50.4 | 44.8 | 45.4 | 42.5 | 43.3 |
| Mean age, years | 73.7 | 67.0 | 69.6 | 70.8 | 69.0 | 71.1 |
|
| ||||||
| Comorbidities, % | ||||||
| Proteinuria positive | 40.7 | 47.6 | 37.9 | 38.2 | 28.1 | 41.8 |
| Ischaemic heart disease | 24.8 | 28.6 | 14.3 | 20.8 | 14.4 | 24.0 |
| Type 2 diabetes mellitus | 23.9 | 58.1 | 51.6 | 43.5 | 21.9 | 40.5 |
| Stroke/TIA | 7.2 | 4.8 | 7.0 | 5.9 | 6.3 | 6.4 |
| Hypertension | 74.4 | 72.1 | 88.1 | 75.2 | 59.3 | 75.2 |
| Arthritis | 37.8 | 30.7 | 34.8 | 30.5 | 33.8 | 35.0 |
| Chronic pain | 4.6 | 12.7 | 6.7 | 5.0 | 7.2 | 7.2 |
|
| ||||||
| Prescriptions in the previous 12 months, % | ||||||
| NSAID | 11.1 | 11.0 | 10.6 | 12.4 | 11.1 | 11.1 |
| Paracetamol | 39.7 | 51.6 | 51.9 | 37.9 | 33.2 | 44.3 |
| PPI | 37.0 | 52.2 | 34.3 | 39.3 | 29.3 | 40.7 |
NSAID = non-steroidal anti-inflammatory drug. PPI = proton-pump inhibitor. TIA = transient ischaemic attack.
The ethnic minority population with CKD is younger than the white population, but has a higher burden of comorbidities, with rates of diabetes mellitus being more than twice as high.
Prescribing rates for paracetamol are higher for South Asian and black groups compared with the white population, with PPI prescribing rates highest among South Asian groups.
NSAID prescribing
Among the total adult population, 11% had received at least one prescription for NSAID medication in the previous year.
NSAID prescribing rates were then examined among the study population of patients with CKD. Crude prescribing rates by ethnic group, age, CKD stage, and by numbers of comorbidities are shown in Table 2. The proportion of patients receiving a prescription for NSAIDs does not vary by ethnic group. The prescribing rate of NSAIDs falls with increasing severity of CKD from 11.2% of patients with CKD stage 3, to 3% in those with CKD stage 5. Patients with chronic pain or a diagnosis of arthritis receive a higher proportion of prescriptions. However, as the prescribing rate falls, the number of comorbidities rises.
Table 2.
NSAID prescribing rates among 12 011 patients with CKD
| Ethnic group | n | % prescribed NSAIDs in past year | % in 90th decile of NSAID DDD | Latest eGFR |
|---|---|---|---|---|
| Overall | 12 011 | 11.1 | 1.3 | 47.2 |
| Ethnic group | ||||
| White | 6315 | 11.1 | 1.8 | 47.5 |
| South Asian | 3308 | 11.0 | 0.8 | 46.9 |
| Black | 1611 | 10.6 | 0.7 | 46.3 |
| Other | 443 | 12.4 | 1.1 | 48.1 |
| Sex | ||||
| Female | 6809 | 12.3 | 1.7 | 47.3 |
| Male | 5202 | 9.4 | 0.9 | 47.1 |
| Age | ||||
| 18–49 | 861 | 16.6 | 1.4 | 47.5 |
| 50–59 | 1430 | 16.7 | 2.5 | 49.5 |
| 60–69 | 2351 | 14.2 | 1.9 | 49.0 |
| 70–79 | 3954 | 9.6 | 1.1 | 47.1 |
| ≥80 | 3415 | 6.9 | 0.6 | 45.1 |
|
| ||||
| CKD stage (based on latest eGFR) | ||||
| Stage 1 | 22 | 13.6 | 0 | 97.5 |
| Stage 2 | 1083 | 14.2 | 2.4 | 65.8 |
| Stage 3 | 9639 | 11.5 | 1.3 | 48.6 |
| Stage 4 | 897 | 5.5 | 0.5 | 24.0 |
| Stage 5 | 362 | 3.0 | 0.3 | 8.7 |
|
| ||||
| Comorbidity status | ||||
| Proteinuria positive | 5022 | 8.7 | 0.7 | 43.6 |
| Ischaemic heart disease | 2881 | 7.8 | 0.7 | 45.7 |
| Type 2 diabetes mellitus | 4869 | 9.9 | 1.1 | 45.9 |
| Stroke/TIA | 774 | 7.5 | 0.8 | 45 |
| Hypertension | 9032 | 10.3 | 1.3 | 45.1 |
| Arthritis | 4209 | 14.1 | 2.4 | 47.5 |
| Chronic pain | 864 | 16.6 | 2.0 | 47.7 |
| PPI (as proxy for dyspepsia) | 4888 | 14.2 | 1.9 | 46.5 |
|
| ||||
| Comorbidity count, additional conditions | ||||
| None | 1188 | 13.5 | 1.3 | 51.9 |
| 1 | 3307 | 10.9 | 1.1 | 47.8 |
| 2 | 4159 | 11.2 | 1.4 | 46.7 |
| ≥3 | 3357 | 10.2 | 1.5 | 45.5 |
eGFR = estimated glomerular filtration rate. NSAID = non-steroidal anti-inflammatory drug. PPI = proton-pump inhibitor. TIA = transient ischaemic attack.
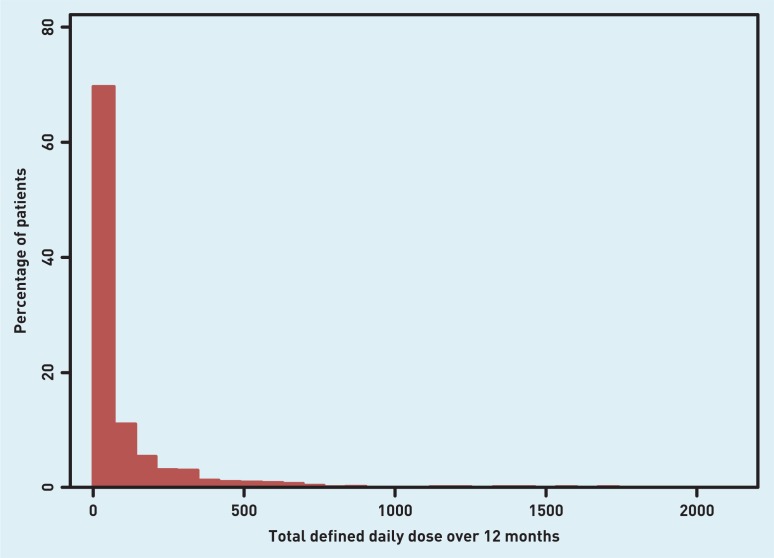
Using the combined daily defined dose (DDD) of all classes of NSAIDs for each patient, the odds ratios, by ethnic group, of being in the top decile of NSAID usage (≥252 DDD per year) were then calculated (Table 3). The model additionally adjusted for age, sex, Townsend deprivation score, latest eGFR, presence of CVD, type 2 diabetes, arthritis, chronic pain, and prescription of PPIs. Errors are adjusted for clustering by practice (Appendix 1). In contrast with the finding of no difference in the crude proportions receiving a prescription, it was found that there are highly significant differences in prescribing intensity by ethnic group, with both South Asian and black groups being much less likely to receive the highest NSAID load. The distribution of DDDs among the study population is illustrated in Appendix 2.
Table 3.
The odds of being in the top decile of NSAID prescribinga
| Explanatory variable | Odds ratio | 95% CI | P-value |
|---|---|---|---|
| White (reference) | 1 | — | — |
| South Asian | 0.34 | 0.21 to 0.54 | <0.001 |
| Black | 0.34 | 0.19 to 0.61 | <0.001 |
Adjusted for age, sex, Townsend deprivation score, latest eGFR, presence of cardiovascular disease, type 2 diabetes mellitus, arthritis, chronic pain, and prescription of PPIs; errors are adjusted for clustering by practice.
Sensitivity analyses
To explore these findings further, some supplementary analyses were undertaken.
Ibuprofen is the most common NSAID available OTC. It was considered plausible that differential purchase of NSAIDs OTC, by ethnic group, may introduce bias to the study findings. The multivariate analysis was therefore repeated excluding ibuprofen, this exclusion did not alter the study findings.
Paracetamol prescribing was then examined by ethnic group, as there may possibly be selective substitution of paracetamol for NSAIDs by some groups. There are higher rates of paracetamol prescribing for black and South Asian groups, but PPI rates are high only among South Asian groups (Table 1). This suggests that if there is substitution of NSAIDs by paracetamol, it is not driven only by dyspepsia and PPI prescribing among the South Asian community.
DISCUSSION
Summary
This is the largest UK primary care study to examine NSAID prescribing by ethnic group in patients with chronic kidney disease.
The results demonstrate that black and South Asian groups are less than half as likely to be in the top decile of NSAID prescribing in comparison with white population groups hence they will have a lower cumulative prescribing load of NSAID medication. This suggests that NSAID prescribing with the attendant risks of nephrotoxicity is unlikely to be causally related to the more rapid progression of CKD in ethnic minorities, or linked to the higher rates of ethnic minority patients on the renal replacement register.
There is a stepwise decline in NSAID prescribing by CKD staging and by age, indicating that GPs are following current prescription guidance. Prescription of NSAIDs in stage 3 CKD is still high, however, with rates equivalent to those in the general population.
Patients with higher numbers of comorbidities have lower rates of prescriptions than those with only one condition, which may be consequent to increasing age and more frequent review of prescribing.
Strengths and limitations
The strengths of this study include the size of the study population, and the setting within a multi-ethnic area of London where over 50% of the population are from ethnic minority groups. The setting, combined with the exceptionally high rates of self-reported ethnic group, make the results relevant to other areas in the UK with similar populations. In the UK health system access to primary care is free, and hence reduces the confounding caused by differential access to prescription medications by ethnic group noted in some studies based in other countries.
Weaknesses relate to the pragmatic use of this database, designed primarily for clinical contact. There will be variation in the quality of data recording between practitioners. Electronic prescribing is universally used in the locality, however, and effectively abolishes the effect of recall bias on NSAID use.
The major weakness in this study is the lack of data about OTC medication, hence exposure bias to NSAID may still exist. Attempts were made to reduce this bias by running a sensitivity analysis, and considering the rate of prescribing of alternative analgesics to NSAIDs. Despite these efforts, the authors remain uncertain about the extent of OTC oral NSAID use, and any variation by ethnic group.
Comparison with existing literature
Although topical NSAID preparations are widely used as prescribed medication and OTC, they were not included in this study. Sporadic case reports that describe renal effects following topical use are rare,26 and a large UK database study did not find independent risks for acute kidney injury or hospitalisation relating to prior use of topical NSAIDs.27
Implications for research and practice
No evidence was found to suggest that differences in NSAID prescribing rates by ethnic group are associated with the excess of more severe renal disease, and the over-representation on renal replacement programmes, by black and South Asian patients in east London.
Although rates of NSAID prescribing are low among patients with severe CKD, these could be reduced further as this is a group who are most vulnerable to the effects of further renal damage. Improved patient understanding of CKD, and provision of written and web-based information on the potential hazards of NSAIDs, particularly during periods of acute intercurrent illness, is needed.
Further investigation of the reasons for excess progressive renal disease among these groups is needed, with the aim of reducing the costly burden of end-stage kidney disease for patients and health services.
Acknowledgments
We thank the staff at the clinical effectiveness group at QMUL for collating the practice data.
Appendix 1. The odds of being in the top decile of NSAID prescribing load
| Explanatory variable | Odds ratio | 95% CI | P-value |
|---|---|---|---|
| Ethnic group | |||
| White (reference) | 1 | — | — |
| South Asian | 0.34 | 0.21 to 0.54 | 0.000 |
| Black | 0.34 | 0.19 to 0.61 | 0.000 |
| Other | 0.61 | 0.26 to 1.47 | 0.272 |
| Unknown | 0.69 | 0.28 to 1.72 | 0.428 |
| Sex | |||
| Female (reference) | 1 | — | — |
| Male | 0.72 | 0.51 to 1.01 | 0.054 |
| Age, years | |||
| 18–49 (reference) | 1 | — | — |
| 50–59 | 1.10 | 0.54 to 2.21 | 0.794 |
| 60–69 | 0.60 | 0.27 to 1.32 | 0.201 |
| 70–79 | 0.35 | 0.16 to 0.80 | 0.013 |
| ≥80 | 0.16 | 0.06 to 0.41 | 0.000 |
| Townsend deprivation score | 1.02 | 0.93 to 1.11 | 0.719 |
| Latest eGFR | 1.02 | 1.00 to 1.04 | 0.018 |
| Comorbidity | |||
| Proteinuria positive | 0.46 | 0.32 to 0.67 | 0.000 |
| Ischaemic heart disease | 0.50 | 0.29 to 0.85 | 0.010 |
| Type 2 diabetes mellitus | 1.04 | 0.76 to 1.41 | 0.815 |
| Stroke/TIA | 0.75 | 0.32 to 1.75 | 0.510 |
| Hypertension | 1.37 | 0.98 to 1.93 | 0.069 |
| Arthritis | 3.98 | 2.58 to 6.12 | 0.000 |
| Chronic pain | 1.22 | 0.65 to 2.28 | 0.536 |
| PPI in last 12 months | 2.21 | 1.51 to 3.25 | 0.000 |
| Constant | 0.01 | 0.00 to 0.02 | 0.000 |
NSAID = non-steroidal anti-inflammatory drug. PPI = proton-pump inhibitor. TIA = transischaemic attack. Errors are adjusted for clustering by practice.
Appendix 2.
The distribution of daily defined doses (DDDs) for all NSAIDs among the study population.
Funding
The study received no external funding.
Ethical approval
Not required.
Provenance
Freely submitted; externally peer reviewed.
Competing interests
The authors have declared no competing interests.
Discuss this article
Contribute and read comments about this article: bjgp.org.uk/letters
REFERENCES
- 1.Department of Health The National Service Framework for Renal Services – Part Two: chronic kidney disease, acute renal failure and end of life care. 2005. http://www.kidney.org.uk/documentlibrary/nsf-pt2.pdf (accessed 27 May 2014)
- 2.National Institute for Health and Care Excellence cg73 chronic kidney disease: nice guideline. early identification and management of chronic kidney disease in adults in primary and secondary care. http://www.nice.org.uk/nicemedia/live/12069/42117/42117.pdf (accessed 3 Jun 2014)
- 3.Ansell DFJ, Feest TG, Ford D, et al. UK Renal Registry. 2007. http://www.renalreg.com/Reports/2007.html (accessed 13 May 2014)
- 4.Dreyer G, Hull S, Aitken Z, et al. The effect of ethnicity on the prevalence of diabetes and associated chronic kidney disease. QJM. 2009;102(4):261–269. doi: 10.1093/qjmed/hcn177. [DOI] [PubMed] [Google Scholar]
- 5.Hull S, Dreyer G, Badrick E, et al. The relationship of ethnicity to the prevalence and management of hypertension and associated chronic kidney disease. BMC Nephrol. 2011;12:41. doi: 10.1186/1471-2369-12-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ali O, Mohiuddin A, Mathur R, et al. A cohort study on the rate of progression of diabetic chronic kidney disease in different ethnic groups. BMJ Open. 2013;3(2) doi: 10.1136/bmjopen-2012-001855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dreyer G, Hull S, Mathur R, et al. Progression of chronic kidney disease in a multi-ethnic community cohort of patients with diabetes mellitus. Diabet Med. 2013;30(8):956–963. doi: 10.1111/dme.12197. [DOI] [PubMed] [Google Scholar]
- 8.Perneger TV, Whelton PK, Klag MJ. Risk of kidney failure associated with the use of acetaminophen, aspirin, and nonsteroidal antiinflammatory drugs. N Engl J Med. 1994;331(25):1675–1679. doi: 10.1056/NEJM199412223312502. [DOI] [PubMed] [Google Scholar]
- 9.Sandler DP, Burr FR, Weinberg CR. Nonsteroidal anti-inflammatory drugs and the risk for chronic renal disease. Ann Intern Med. 1991;115(3):165–172. doi: 10.7326/0003-4819-115-3-165. [DOI] [PubMed] [Google Scholar]
- 10.Morlans M, Laporte JR, Vidal X, et al. End-stage renal disease and non-narcotic analgesics: a case-control study. Br J Clin Pharmacol. 1990;30(5):717–723. doi: 10.1111/j.1365-2125.1990.tb03841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huerta C, Castellsague J, Varas-Lorenzo C, Garcia Rodriguez LA. Nonsteroidal anti-inflammatory drugs and risk of ARF in the general population. Am J Kidney Dis. 2005;45(3):531–539. doi: 10.1053/j.ajkd.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 12.Griffin MR, Yared A, Ray WA. Nonsteroidal anti-inflammatory drugs and acute renal failure in elderly persons. Am J Epidemiol. 2000;151(5):488–496. doi: 10.1093/oxfordjournals.aje.a010234. [DOI] [PubMed] [Google Scholar]
- 13.Perez Gutthann S, Garcia Rodriguez LA, Raiford DS, et al. Nonsteroidal anti-inflammatory drugs and the risk of hospitalization for acute renal failure. Arch Intern Med. 1996;156(21):2433–2439. doi: 10.1001/archinte.1996.00440200041005. [DOI] [PubMed] [Google Scholar]
- 14.Kurth T, Glynn RJ, Walker AM, et al. Analgesic use and change in kidney function in apparently healthy men. Am J Kidney Dis. 2003;42(2):234–244. doi: 10.1016/s0272-6386(03)00647-4. [DOI] [PubMed] [Google Scholar]
- 15.Curhan GC, Knight EL, Rosner B, et al. Lifetime nonnarcotic analgesic use and decline in renal function in women. Arch Intern Med. 2004;164(14):1519–1524. doi: 10.1001/archinte.164.14.1519. [DOI] [PubMed] [Google Scholar]
- 16.Gooch K, Culleton BF, Manns BJ, et al. NSAID use and progression of chronic kidney disease. Am J Med. 2007;120(3):280, e1–7. doi: 10.1016/j.amjmed.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 17.Dieppe P, Bartlett C, Davey P, et al. Balancing benefits and harms: the example of non-steroidal anti-inflammatory drugs. BMJ. 2004;329(7456):31–34. doi: 10.1136/bmj.329.7456.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lapi F, Azoulay L, Yin H, et al. Concurrent use of diuretics, angiotensin converting enzyme inhibitors, and angiotensin receptor blockers with non-steroidal anti-inflammatory drugs and risk of acute kidney injury: nested case-control study. BMJ. 2013;346:e8525. doi: 10.1136/bmj.e8525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilcox CM, Cryer B, Triadafilopoulos G. Patterns of use and public perception of over-the-counter pain relievers: focus on nonsteroidal antiinflammatory drugs. J Rheumatol. 2005;32(11):2218–2224. [PubMed] [Google Scholar]
- 20.Paulose-Ram R, Hirsch R, Dillon C, et al. Prescription and non-prescription analgesic use among the US adult population: results from the third National Health and Nutrition Examination Survey (NHANES III) Pharmacoepidemiol Drug Saf. 2003;12(4):315–326. doi: 10.1002/pds.755. [DOI] [PubMed] [Google Scholar]
- 21.Townsend P. Deprivation and ill health. Nursing (Lond) 1991;4(43):11–15. [PubMed] [Google Scholar]
- 22.Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130(6):461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 23.O’Neil M, Payne C, Read J. Read Codes Version 3: a user led terminology. Meth Inform Med. 1995;34(1–2):187–192. [PubMed] [Google Scholar]
- 24.Wertheimer AI. The defined daily dose system (DDD) for drug utilization review. Hospital Pharm. 1986;21(3):233–234. 9–41, 58. [PubMed] [Google Scholar]
- 25.Malcolm PN, Chan TY, Li PL, et al. Management of dyspepsia among Asians by general practitioners in east London. BMJ. 1995;310(6984):910–911. doi: 10.1136/bmj.310.6984.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O’Callaghan CA, Andrews PA, Ogg CS. Renal disease and use of topical non-steroidal anti-inflammatory drugs. BMJ. 1994;308(6921):110–111. doi: 10.1136/bmj.308.6921.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Evans JM, McGregor E, McMahon AD, et al. Non-steroidal anti-inflammatory drugs and hospitalization for acute renal failure. QJM. 1995;88(8):551–557. [PubMed] [Google Scholar]