Abstract
Purpose: To elucidate the effect of ouabain on the regulation of proliferation and apoptosis of HUVECs and involvement of different Na+-K+-ATPase α-subunits and NF-κB. Methods: HUVECs were isolated by collagenase perfusion, and MTT assays and cell cycle analysis were performed to study proliferation. NF-κB expression and function were examined by immunohistochemical staining and western blotting. Na+-K+-ATPase activity was determined by measuring released ouabain inhibitable inorganic phosphate (Pi). The expression of different α-subunits was investigated by real RT-PCR, western blotting and cell immunofluorescence. Results: 0.3 nM ouabain treatment for 0.5 h triggered the proliferation of HUVECs, peaking at 1-2 h. At 1.8 nM for 0.5 h, ouabain induced an increase of cell proliferation for a short time, and then triggered a decrease after 1 h. Cell cycle analysis show that 37% of HUVECs were in G2/M phase of the cell cycle following incubation with 1.8 nM ouabain, compared with 18% with 0.3 nM ouabain. NF-κB activity was assessed by western blot analysis of IκB expression, which was significantly reduced with 0.3 nM ouabain treatment; there was no different between 1.8 nM ouabain treatment and untreated cells. Na+-K+-ATPase activity in HUVECs was markedly reduced after treatment with 0.3 nM and 1.8 nM ouabain. Real RT-PCR and western blotting indicated that Na+-K+-ATPase α1-subunit mRNA expression levels increased after 0.3 nM ouabain treatment and decreased after 1.8 nM ouabain treatment. However, α2- and α3-subunit mRNA decreased after 0.3 nM ouabain treatment and increased after 1.8 nM ouabain treatment. Conclusion: Ouabain at different concentrations caused dual effects on proliferation and apoptosis in HUVECs.
Keywords: Ouabain, endothelium, α subunit, NF-κB
Introduction
Vascular endothelial cells, of which the functional integrity is crucial for the maintenance of blood flow and antithrombotic activity, may be targets for endogenous ouabain (EO) [1]. Following an original report published in 1991 [2], EO has been isolated and detected by different laboratories. EO shares many of the properties of ouabain obtained from exogenous sources. Exogenous ouabain is a cardiac glycoside isolated from the bark and roots of the ouabain tree and the seeds of Strophanthus gratus. It was identified in the 1800s as one of several poisons used by African tribes to make poison arrow tips. As an endogenous regulator of Na+-K+-ATPase activity [3,4], mounting evidence shows that ouabain is involved in the pathogenesis of hypertension. Chronic ouabain treatment results in hypertension [5] and triggers cardiac remodeling [6,7], the onset of which is associated with endothelial dysfunction, which markedly affects the overall integrity and function of the cardiovascular system. Our previous study indicated that ouabain at physiological concentrations (0.3-0.9 nmol/l) stimulated the proliferation of human umbilical vein endothelial cells (HUVECs). However, at pathological concentrations (0.9-1.8 nmol/l), cell death was induced, as characterized by the swelling phenomenon and appearance of apoptotic bodies [8]. The results of mRNA profile analysis indicated that the most up regulated genes were related to signal transduction and metabolism, including nuclear factor-κB (NF-κB) activator TANK (NM_004180). Many experiments have shown that Na+-K+-ATPase activity regulated by ouabain is dependent on NF-κB [9]. However, the relationship between the different Na+-K+-ATPase α-subunits, NF-κB and the dual effects on the endothelium by ouabain remain unclear. Our study focused on the pathway by which ouabain regulates endothelial function. We examined the effect of varying concentrations of ouabain on endothelial function according to the activity of different α-subunits and NF-κB.
Materials and methods
Cell culture and morphological analysis by light microscopy
The research was approved by the ethics committee of Xi’an Jiaotong University. All chemicals were obtained from Sigma Aldrich Co. (St. Louis, M, USA) unless otherwise specified. The collagenase perfusion method was used to isolate HUVECs [10], which were then cultured in RPMI-1640 supplemented with 10% fetal bovine serum (FBS) at 37°C in a humidified atmosphere containing 5% CO2. HUVECs were identified by their typical cobblestone morphology using microscopy (Olympus). After identification, HUVECs were incubated with either 0.3 nM or 1.8 nM ouabain for 2 h to study morphological differences.
MTT cell proliferation assay
HUVECs at passage three were cultured to 80% confluence and then plated at a density of 5×103 cells/well in 96-well multiplates. Complete growth medium was replaced with serum-free medium after the cells had reached confluency to down regulate cell proliferation and block cells in G1 phase of the cell cycle. Serum-free medium was replaced once again 24 h later, and 0.3 nmol/L or 1.8 nmol/L ouabain was added to the cells in quadruplicate; an untreated group was set up as a control. After varying incubation periods (0.5, 1, 2, 4, 8, 16, 24, and 48 h), cells were pulsed with 500 μg/ml methyl thiazolyl tetrazolium (MTT; Sigma) for 4 h, and the resulting MTT formazan was solubilized with 10% sodium dodecyl sulfate (SDS) overnight. Absorbance at 490 nm was measured with a Model 680 microplate reader. Proliferation rates (%) were calculated as (experimental absorbance/control absorbance-1) ×100%.
Cell cycle analysis
Cells were stained with propidium iodide (PI) and DNA content was analyzed using flow cytometry. Briefly, cells were fixed in 70% ethanol, incubated with PI staining solution (containing 10 μg/ml PI and 100 μg/ml RNase) for 15 min at 37°C, and analyzed by flow cytometry.
Immunohistochemical staining of NF-κB p65 subunit
Following pretreatment of glass cover slips with poly-L-lysine in 6-well multiplates, HUVECs were seeded at a density of 5×104 cells/well. Complete growth medium was replaced with serum-free medium once the cells had reached confluency, and plates were incubated for 6 h. Then, different doses of ouabain (0, 0.3 and 1.8 nmol/L) were added to each plate in medium containing 100 ml·L-1 FBS for 4 h. After washing three times with PBS, the cover slips were fixed for 30 min with cold acetone and dried naturally. NF-κB immunohistochemical staining was performed in accordance with the instructions provided by the manufacturer (Wuhan Boster Bio-engineering Co., Ltd); the primary antibody was diluted to 1:200.
NF-κB activity assay
Activation of NF-κB by ouabain was investigated by examining the degradation of its inhibitory subunit IκBα. The expression of IκB was studied by western blot analysis as described above, using 10% polyacrylamide gels and an anti-IκBα monoclonal antibody (1:200) with a goat anti-mouse secondary IgG conjugated to horseradish peroxidase (1:200) (Boster Bio-engineering Co., Ltd Wuhan, China).
Na+-K+-ATPase activity assay
Homogenates of HUVECs were suspended in Tris buffer (1300 mM NaCl, 200 mM KCl, 40 mM MgCl2 and 150 mM histidine; pH 7.4) to a concentration of 2 mg/ml. Saponin (1%) was then added at a ratio of 1:4, and the samples were incubated at room temperature for 30 min in different concentrations of ouabain (0 nM, 0.3 nM, 1.8 nM), a specific inhibitor of the ATPase. Adenosine tri-phosphate (ATP) was then added to a final concentration of 7.5 mM, and samples were incubated for an additional 30 min period at 37°C. At the end of incubation period, an equal volume of trichloroacetic acid (11.5%) was added to stop the reaction, and the samples were spun at 3000×g for 5 min. The amount of inorganic phosphate liberated was measured colorimetrically according to the method of Taussky and Shorr [11]. Enzymatic activity was determined by measuring the ouabain inhibitable inorganic phosphate (Pi) released.
Real RT-PCR
Total RNA was extracted from HUVECs using Sepasol-RNA and reverse-transcribed to cDNA using the Superscript III First-Strand System for RT-PCR (Life Tech, California, U.S.A.) according to the manufacturer’s instructions. cDNA was amplified with a Bio-Rad iCycler (Bio-Rad Laboratories) using KOD DNA polymerase and the following primers (designed and synthesized by Beijing Sunbiotech Co.,Ltd.): α1-subunit (forward): 5’-ACACAGCCTTCTTCGTCAGTATCG-3’, α1-subunit (reverse): 5’-ACATCCTAAGAGCAACACCCATTCC-3’; α2-subunit (forward): 5’-GGTCTCCTTCTTCGTGCTCTCC-3’, α2-subunit (reverse): 5’-GTTCTTCACCAGGCAGTTCTTCC-3’; α3-subunit (forward): 5’-CATCTTCCTCATCGGCATCATCG-3’, α3-subunit (reverse): 5’-GTTCTTCACCAGGCAGTTCTTCC-3’; β-actin (forward): 5’-ATCGTGCGTGACATTAAGGAGAAG-3’, β-actin (reverse): 5’-AGGAAGGAAGGCTGGAAGAGTG-3’. PCR conditions for α-subunits and β-actin were 95°C for 3 min, followed by 40 to 45 cycles of 95°C for 10 s, 60°C for 30 s, 72°C for 30 s, and 55°C for 30 s, followed by 81 s. The PCR products were analyzed by 1.5% agarose gel electrophoresis, and stained with 1 μg/ml ethidium bromide (Life Tech).
Preparation of cell lysates and western blotting determination of total cellular Na+/K+ ATPase α-subunits
HUVECs were washed once with PBS and lysed in Trion X-100 lysis buffer consisting of 50 mM Tris-HCl (PH 7.4), 1% Triton X-100, 2 mM DTT, 2 Mm sodium orthovanadate, and Complete protease inhibitor mixture (1 mM PSMF, 1 mM benzamidine-HCl, 0.5 μg/ml leupeptin, 0.5 μg/ml aprotinin, 1 mg/ml pepstatin, 50 mM sodium fluoride). Postnuclear lysates were collected as supernatants by centrifugation (800×g for 7 min). Pellets were washed twice with HEPES buffer (20 mM HEPES-NaOH (pH 8.0), 20% glycerol, 100 Mm KCl, 1 mM EDTA, 0.5 Mm DTT, leupeptin (10 μg/ml), and 0.5 Mm PMSF) and then lysed in the HEPES buffer by sonication, followed by centrifugation (9000×g for 2 min). Western blotting was performed on whole cell lysates using procedures similar to those described by Singh et al. [12]. Protein samples (20-30 μg/lane) were separated by SDS-PAGE and transferred to PVDF membranes (Bio-Rad), then blocked with 5% non-fat dehydrated milk in Tris buffered saline containing Tween 20 (TBST; 25 mM Tris (pH 7.4), 137 mM NaCl, 2.7 mM KCl, 0.1% Tween 20) for 1 h at room temperature. The membranes were incubated with primary antibodies (1:150) in TBST (5% BSA) overnight at 4°C, followed by secondary antibody horseradish peroxidase (HRP)-conjugated anti-rabbit IgG (1:200) (Beijing Zhongshan Goldenbridge Biotechnology Co., Ltd.) in TBST (5% non-fat dehydrated milk or 5% BSA) for 1 h at room temperature. Immunoblotting detection was performed using the ECL detection system (Bio-rad) according to the manufacturer’s instructions.
Equal loading of proteins was evaluated by stripping and developing a blot for β-actin (Cell Signaling). The primary antibody used for detection of Na+/K+ ATPase α-subunit was obtained from Cell Signaling (1:1000). Percentage of phosphorylated α-subunit was determined in parallel samples by first developing the membrane to determine the density of phosphorylated signal, and then stripping and exposing the membrane to the Na+/K+ ATPase α-subunit antibody (1:1000; Cell Signaling). Densitometric analyses of α-subunits relative to β-actin were performed using ImageJ, Sigma Stat and Sigma Plot.
Cell immunofluorescence observation of Na+/K+ ATPase α-subunit protein expression
HUVECs were identified as described above and samples were observed under confocal laser microscopy (nIKON). The primary antibody was the same as that used for western blotting, and the secondary antibodies were FITC fluorescence antibody (goat anti-mouse, 1:100; Zhongshan Goldenbridge Biotechnology Co., Ltd.), RBITC fluorescence antibody (rabbit anti-goat, 1:100; Zhongshan Goldenbridge Biotechnology Co., Ltd.) and FITC fluorescence antibody (goat anti-mouse, 1:100; Zhongshan Goldenbridge Biotechnology Co., Ltd.).
Statistical analysis
Values are presented as mean ± SEM. Statistical significance was evaluated using Student’s two-tail t-test and identified as P < 0.05.
Results
Ouabain has dual effects on HUVECs according to morphological evaluation, MTT cell proliferation assay and cell cycle analysis
Fluorescence microscopy showed that HUVECs had elongated spindles, and green fluorescence could be seen in the cell membrane and cytoplasm by staining with vascular-factor III. After incubation with 0.3 nM ouabain for 2 h, HUVECs at passage three grew in a cobblestone-like arrangement. However, cells treated with 1.8 nM ouabain became wider and grew slowly, and the number of dead cells increased. Compared with the control group, different doses of ouabain exhibited time-dependent changes in HUVEC proliferation as measured by MTT assay. Ouabain at 0.3 nM triggered the proliferation of HUVECs from 0.5 h, and the most prominent increases were observed at 1-2 h. This is why all further studies were conducted using 2 h treatment. However, 1.8 nM ouabain treatment for 0.5 h induced an increase in cell proliferation for a short time, and then triggered a decrease from 1 h (Figure 1).
Figure 1.
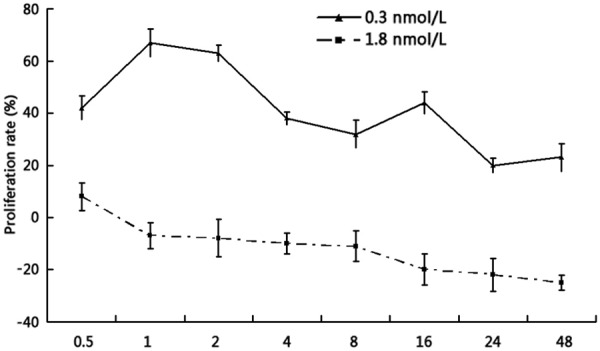
Ouabain affected the proliferation of HUVECs in a time-dependent manner. HUVECS were treated with 0.3 nmol/L or 1.8 nmol/L ouabain over a period of 48 h (n = 4; mean ± SEM). Ouabain at 0.3 nM triggered the proliferation of HUVECs from 0.5 h, and the most prominent increases were observed at 1-2 h. However, 1.8 nM ouabain treatment for 0.5 h induced an increase in cell proliferation for a short time, and then triggered a decrease from 1 h.
To analyze the cell cycle phase in which the HUVECs were localized following stimulation with 0.3 nM and 1.8 nM ouabain, cells were cultured in RPMI-1640 containing 10% FBS and analyzed using PI staining. Figure 2 shows that 37% of HUVECs were in G2/M phase after 1.8 nM ouabain treatment, compared with 18% of those treated with 0.3 nM ouabain (P < 0.01).
Figure 2.
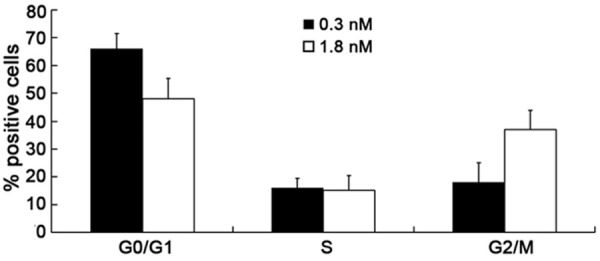
Effect of ouabain on cell cycle distribution. The results show that 37% of HUVECs were in G2/M phase after 1.8 nM ouabain treatment, compared with 18% of those treated with 0.3 nM ouabain (P < 0.01) after 2 h incubation. The data are expressed as a percentage of positive cells in the respective cell cycle phase. Bars correspond to the mean ± SEM. Data are representative of three samples.
Evaluation of the involvement of NF-κB on the dual effects of ouabain in HUVECs
After incubation with 0.3 nM ouabain for 2 h, the nuclei and cytoplasm of HUVECs exhibited NF-κB p65 staining, indicating that NF-κB heterodimers had become free and trans-located to the nucleus. Hence, the nuclei and cytoplasm were all stained with NF-κB p65. However, only the cytoplasm of untreated HUVECs and those treated with 1.8 nM ouabain were stained. This phenomenon indicated that the combination of NF-κB with the IκB inhibitory proteins was confined to the cytoplasm, and NF-κB was not activated. Then western blot analysis was used to determine NF-κB activity after 2 h of incubation with two different concentrations of ouabain. IκB expression was significantly reduced after 0.3 nM ouabain treatment (0.82 ± 0.13 versus 0.21 ± 0.19, P < 0.01). There was no different between 1.8 nM ouabain treatment and the untreated control (0.82 ± 0.13 versus 0.76 ± 0.21, P > 0.05) (Figure 3).
Figure 3.
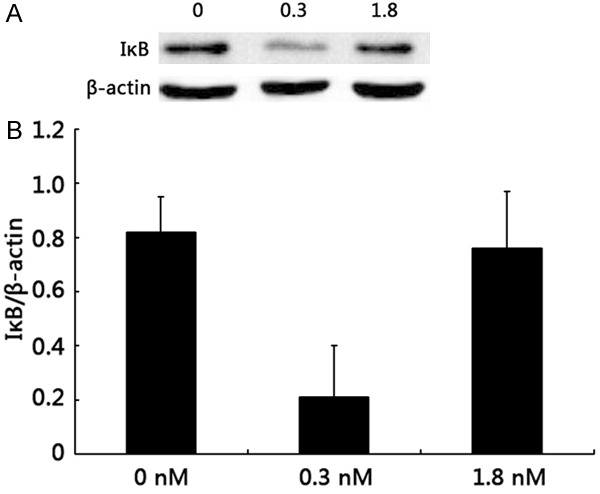
NF-κB activity determination by western blot analysis of IκB expression. (A) Western blot and (B) densitometric analysis of IκB expression. The expression of IκB was significantly reduced after 0.3 nM ouabain treatment (0.82 ± 0.13 versus 0.21 ± 0.19, P < 0.01). No effects were observed for 1.8 nM ouabain treatment or the control group (0.82 ± 0.13 versus 0.76 ± 0.21, p > 0.05). Bars not sharing a common letter are significantly different from each other (P < 0.01).
Examination of the involvement of different Na+-K+-ATPase α-subunits on the dual effects of ouabain in HUVECs by Na+-K+-ATPase activity assay, RT-PCR western blot and immunofluorescence
The activity of Na+-K+-ATPase in HUVECs was significantly reduced after treatment with either 0.3 nM or 1.8 nM ouabain (46 ± 8%, 51 ± 9% versus 100%, P < 0.01), and there was no difference between the two groups (46 ± 8% versus 51 ± 9%, P > 0.05) (Figure 4). To detect the expression level of α-subunits mRNA by RT-PCR, we found that the expression level of α1-subunit mRNA increased after 0.3 nM ouabain treatment (1.78 ± 0.61 versus 1, P < 0.01) and decreased after 1.8 nM ouabain treatment (0.62 ± 0.41 versus 1, P < 0.01). However, α2- and α3-subunits mRNA expression decreased after 0.3 nM ouabain treatment (0.73 ± 0.31 and 0.61 ± 0.42 versus 1, respectively; P < 0.01) and increased after 1.8 nM ouabain treatment (1.27 ± 0.39 and 1.87 ± 0.50 versus 1, respectively; P < 0.01) (Figure 5). Examination of α-subunit protein expression by western blot revealed that α1-subunit protein expression levels were increased after 0.3 nM ouabain treatment (1.3 ± 0.11 versus 0.95 ± 0.08, P < 0.01) and decreased after 1.8 nM ouabain treatment (0.67 ± 0.23 versus 0.95 ± 0.08, < 0.01). But α2 and α3-subunit protein decreased after 0.3 nM ouabain treatment (0.21 ± 0.12 versus 0.38 ± 0.10, and 0.25 ± 0.10 versus 0.37 ± 0.06, respectively; P < 0.01) and increased after 1.8 nM ouabain treatment (0.08 ± 0.19 versus 0.38 ± 0.10, and 0.57 ± 0.13 versus 0.37 ± 0.06, respectively; P < 0.01) (Figure 6). The results of further study to detect protein expression by immunofluorescence were similar to those obtained by RT-PCR and western blotting.
Figure 4.
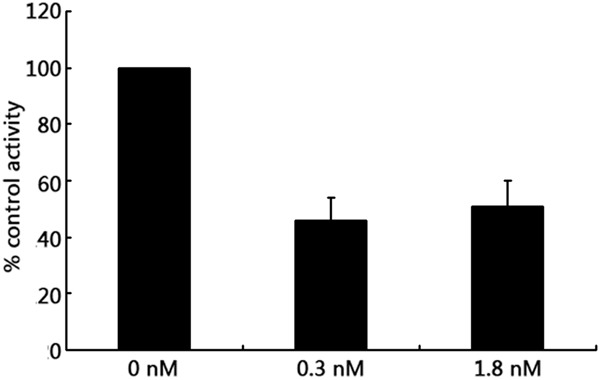
Effects of different concentrations of ouabain on Na+-K+-ATPase activity in HUVECs. The activity of Na+-K+-ATPase in HUVECs was significantly reduced after treatment with either 0.3 nM or 1.8 nM ouabain (46 ± 8%, 51 ± 9% versus 100%, P < 0.01), and there was no difference between the two groups (46 ± 8% versus 51 ± 9%, P > 0.05). Values shown are means ± SEM of three observations assayed in triplicate. Bars not sharing a common letter are significantly different from each other (P < 0.01).
Figure 5.
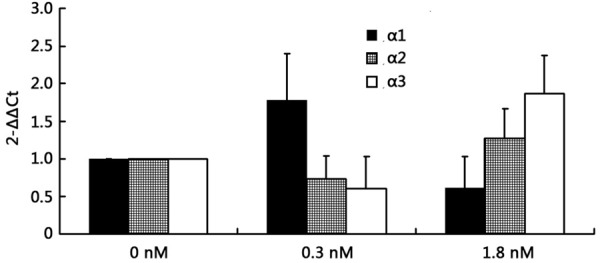
Effect of different concentrations of ouabain on Na+-K+-ATPase α-subunit mRNA expression in HUVECs. The expression level of α1-subunit mRNA increased after 0.3 nM ouabain treatment (1.78 ± 0.61 versus 1, P < 0.01) and decreased after 1.8 nM ouabain treatment (0.62 ± 0.41 versus 1, P < 0.01). However, α2- and α3-subunit mRNA expression decreased after 0.3 nM ouabain treatment (0.73 ± 0.31 and 0.61 ± 0.42 versus 1, respectively; P < 0.01) and increased after 1.8 nM ouabain treatment (1.27 ± 0.39 and 1.87 ± 0.50 versus 1, respectively; P < 0.01). Values shown indicate means ± SEM of three observations assayed in triplicate. In each α-subunit group, bars not sharing a common letter are significantly different from each other (P < 0.01).
Figure 6.
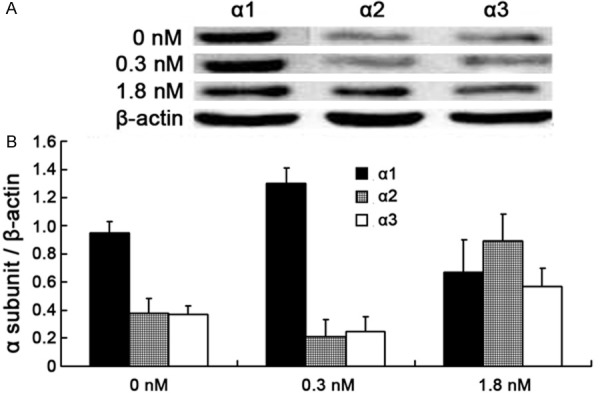
Analysis of α1-, α2- and α3-subunits expression. (A) Western blot and (B) densitometric analysis of α1-, α2- and α3-subunits expression. α1-subunit protein expression levels were increased after 0.3 nM ouabain treatment (1.3 ± 0.11 versus 0.95 ± 0.08, P < 0.01) and decreased after 1.8 nM ouabain treatment (0.67 ± 0.23 versus 0.95 ± 0.08, P < 0.01). But α2 and α3-subunit protein decreased after 0.3 nM ouabain treatment (0.21 ± 0.12 versus 0.38 ± 0.10, and 0.25 ± 0.10 versus 0.37 ± 0.06, respectively; P < 0.01) and increased after 1.8 nM ouabain treatment (0.89 ± 0.19 versus 0.38 ± 0.10, and 0.57 ± 0.13 versus 0.37 ± 0.06, respectively; P < 0.01) Values shown are means ± SEM of three observations assayed in triplicate. In each α-subunit group, bars not sharing a common letter are significantly different from each other (P < 0.01).
Discussion
EO is associated with blood pressure and cardiovascular complications both in rats infused with ouabain and in humans suffering from early and mild to long-lasting and more severe hypertension [13-16], thus causing cardiac damage. EO increases blood pressure in Sprague Dawley rats when infused at very low doses (15 μg/kg/day) for 4-8 weeks [17,18].
Chronic ouabain treatment produces hypertension by acting on the central nervous system and at the vascular level. On the other hand, EO also triggers cardiac remodeling. Endothelial dysfunction is a very important factor in cardiac remodeling. The vascular endothelium is a monolayer of endothelial cells that lines in the inner surface of all blood vessels, including arteries, veins, and lymphatic vessels. The endothelial cells are continually exposed to circulating blood and must function to regulate and meet the oxygen and nutritional needs of underlying tissue. Therefore, they are not only a physical barrier between the blood and the underlying smooth muscle cells, but also an active player in the regulation of vasodilation, coagulation, inflammation, and immune response. Additionally, they are essential for cardiovascular homeostasis and maintaining vascular tone. Endothelial cells play a pivotal role in the development of atherosclerosis.
As an endogenous hormone, ouabain has different roles in endothelial cells. A previous study found that ouabain can initiate cell proliferation or apoptotic cell death in a dose-dependent manner; ouabain stimulated the proliferation of HUVEC at physiological concentrations (0.3-0.9 nmol/l) and induced cell death at pathological concentrations (0.9-1.8 nmol/l) [19]. The loss of Na+-K+-ATPase activity in the evolution of cellular pathology is well recognized. Thus, a graded reduction of Na+-K+- ATPase activity or a modulation of Na+-K+-ATPase function independent of pump activity [20,21] may activate signals that promote cellular proliferation and/or block programmed cell death. However, the relationship between ouabain and different Na+-K+-ATPase α-subunits and NF-κB in the regulation of proliferation and apoptosis of HUVECs remained unclear.
As a target for ouabain, Na+-K+-ATPase is a ubiquitous cell membrane enzyme involved in regulation of intracellular ion gradients. There are four isoforms of the α-subunit (α1, α2, α3 and α4) and three of the β-subunit (β1, β2 and β3) in vertebrates [22,23]. The Na+-K+-ATPase α-subunit ouabain binding site has been highly conserved during the evolution of higher animals, which may indicate a fundamental role in the regulation of physiological processes [24]. Nevertheless, not all α-subunit isoforms, nor the isoforms in all species, have the same high affinity for ouabain. In rats, ouabain exhibits high affinity for the α2/α3 isoforms of Na+-K+-ATPase. We found that the mRNA and protein expression levels of α1-subunit increased after 0.3 nM ouabain treatment and decreased after 1.8 nM ouabain treatment; in contrasts, that of the α2- and α3-subunits decreased after 0.3 nM ouabain treatment and increased after 1.8 nM ouabain treatment, indicating that signaling pathway of ouabain binding to α1-subunit participates in the proliferation of HUVECs and that of the α2/3-subunits participates in the death of HUVECs in dose-dependent manner.
The transcription factor NF-κB participates in the expression of a wide variety of genes that are involved in the regulation of many cellular mediators of immune and inflammatory responses [25].
I-κB exists as a complex with NF-κB heterodimer in the cytosol [26]. The NF-κB heterodimer becomes free and trans-locates to the nucleus where it activates a variety of genes responsible for inflammation as well as cancer development and progression [27]. Activation of NF-κB results from the phosphorylation of its IκB protein. This phosphorylation is mediated via IκB protein kinases (IKKs), which causes proteolytic degradation of IκB by 26S proteasomes. Subsequently, degraded IκB allows NF-κB to translocate to the nucleus, where it regulates the expression of many genes [28]. Moreover, it has been reported that ouabain is able to activate the NF-κB pathway accordingly to affect the early NF-κB signaling pathway, triggering slow calcium oscillations and preventing NF-κB- inducible protein expression by blocking Na+-dependent amino acid transport [29-32].
The present study was designed as a follow up to our previous study to determine whether NF-κB plays a role in ouabain-mediated proliferation and apoptosis of HUVECs, and to investigate its relationship with different α-subunits. IκB expression was significantly reduced after 0.3 nM ouabain treatment. NF-κB p65 staining was observed in the nuclei and cytosol of HUVECs incubated with 0.3 nM ouabain for 2 h, but in the cytosol alone of untreated HUVECs or those treated with 1.8 nM ouabin. This phenomenon indicated that the binding of NF-κB with the IκB inhibitory proteins was confined to the cytoplasm. NF-κB was activated after ouabain binding to α1-subunit, which induced HUVEC proliferation.
EO, an important endogenous hormone, is associated with many cardiovascular diseases such as hypertension, by its mode of action is unclear. Our results indicated that EO has dual effects on the regulation of proliferation and apoptosis in HUVECs at different concentrations. At 0.3 nM, ouabain promoted cell proliferation via activation of Na+-K+-ATPase α1-subunit and the NF-κB pathway, whereas it induced cell apoptosis via activation of α2- and α3-subunits without participation of NF-κB at 1.8 nM. In conclusion, any mechanism altering Na+-K+-ATPase function and/or expression may be a candidate for hypertension pathogenesis and thus represents a potential new therapeutic target [33].
Acknowledgements
This work was supported by the National Natural Science Foundation of China (30971222).
Disclosure of conflict of interest
None.
References
- 1.Trevisi L, Pighin I, Luciani S. Vascular endothelium as a target for endogenous ouabain: studies on the effect of ouabain on human endothelial cells. Cell Mol Biol (Noisy-le-grand) 2006;52:64–70. [PubMed] [Google Scholar]
- 2.Hamlyn JM, Blaustein MP, Bova S, DuCharme DW, Harris DW, Mandel F, Mathews WR, Ludens JH. Identification and characterization of a ouabain-like compound from human plasma. Proc Natl Acad Sci U S A. 1991;88:6259–6263. doi: 10.1073/pnas.88.14.6259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hamlyn JM, Hamilton BP, Manunta P. Endogenous ouabain, sodium balance and blood pressure: a review and a hypothesis. J Hypertens. 1996;14:151–167. doi: 10.1097/00004872-199602000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Schoner W, Scheiner-Bobis G. Endogenous cardiac glycosides: hormones using the sodium pump as signal transducer. Semin Nephrol. 2005;25:343–351. doi: 10.1016/j.semnephrol.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 5.Hamlyn JM, Blaustein MP. Salt sensitivity, endogenous ouabain and hypertension. Curr Opin Nephrol Hypertens. 2013;22:51–58. doi: 10.1097/MNH.0b013e32835b36ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferrari P, Ferrandi M, Valentini G, Manunta P, Bianchi G. Targeting Ouabain- and Adducin- dependent mechanisms of hypertension and cardiovascular remodeling as a novel pharmacological approach. Med Hypotheses. 2007;68:1307–1314. doi: 10.1016/j.mehy.2006.07.058. [DOI] [PubMed] [Google Scholar]
- 7.Jiang X, Ren YP, Lv ZR. Ouabain induces cardiac remodeling in rats independent of blood pressure. Acta Pharmacol Sin. 2007;28:344–352. doi: 10.1111/j.1745-7254.2007.00496.x. [DOI] [PubMed] [Google Scholar]
- 8.Ren YP, Huang RW, Lu ZR. Ouabain at pathological concentrations might induce damage in human vascular endothelial cells. Acta Pharmacol Sin. 2006;27:165–172. doi: 10.1111/j.1745-7254.2006.00244.x. [DOI] [PubMed] [Google Scholar]
- 9.Miles MT, Cottey E, Cottey A, Stefanski C, Carlson CG. Reduced resting potentials in dystrophic (mdx) muscle fibers are secondary to NF-kappaB-dependent negative modulation of ouabain sensitive Na+-K+ pump activity. J Neurol Sci. 2011;303:53–60. doi: 10.1016/j.jns.2011.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jaffe EA, Nachman RL, Becker CG, Minick CR. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973;52:2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taussky HH, Shorr E. A microcolorimetric method for the determination of inorganic phosphorus. J Biol Chem. 1953;202:675–685. [PubMed] [Google Scholar]
- 12.Singh R, Millman G, Turin E, Polisiakeiwicz L, Lee B, Gatti F, Berge J, Smith E, Rutter J, Sumski C, Winders WT, Samadi A, Carlson CG. Increases in nuclear p65 activation in dystrophic skeletal muscle are secondary to increases in the cellular expression of p65 and are not solely produced by increases in IkappaB-alpha kinase activity. J Neurol Sci. 2009;285:159–171. doi: 10.1016/j.jns.2009.06.030. [DOI] [PubMed] [Google Scholar]
- 13.Manunta P, Messaggio E, Ballabeni C, Sciarrone MT, Lanzani C, Ferrandi M, Hamlyn JM, Cusi D, Galletti F, Bianchi G. Plasma ouabain-like factor during acute and chronic changes in sodium balance in essential hypertension. Hypertension. 2001;38:198–203. doi: 10.1161/01.hyp.38.2.198. [DOI] [PubMed] [Google Scholar]
- 14.Manunta P, Stella P, Rivera R, Ciurlino D, Cusi D, Ferrandi M, Hamlyn JM, Bianchi G. Left ventricular mass, stroke volume, and ouabain-like factor in essential hypertension. Hypertension. 1999;34:450–456. doi: 10.1161/01.hyp.34.3.450. [DOI] [PubMed] [Google Scholar]
- 15.Pierdomenico SD, Bucci A, Manunta P, Rivera R, Ferrandi M, Hamlyn JM, Lapenna D, Cuccurullo F, Mezzetti A. Endogenous ouabain and hemodynamic and left ventricular geometric patterns in essential hypertension. Am J Hypertens. 2001;14:44–50. doi: 10.1016/s0895-7061(00)01225-5. [DOI] [PubMed] [Google Scholar]
- 16.Pitzalis MV, Hamlyn JM, Messaggio E, Iacoviello M, Forleo C, Romito R, de Tommasi E, Rizzon P, Bianchi G, Manunta P. Independent and incremental prognostic value of endogenous ouabain in idiopathic dilated cardiomyopathy. Eur J Heart Fail. 2006;8:179–186. doi: 10.1016/j.ejheart.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 17.Winnicka K, Tomasiak M. PST 2238 as an antihypertensive compound that antagonizes the effect of endogenous cardiac glycosides. Acta Pol Pharm. 2005;62:75–79. [PubMed] [Google Scholar]
- 18.Ferrari P, Torielli L, Ferrandi M, Padoani G, Duzzi L, Florio M, Conti F, Melloni P, Vesci L, Corsico N, Bianchi G. PST2238: a new antihypertensive compound that antagonizes the long-term pressor effect of ouabain. J Pharmacol Exp Ther. 1998;285:83–94. [PubMed] [Google Scholar]
- 19.Chueh SC, Guh JH, Chen J, Lai MK, Teng CM. Dual effects of ouabain on the regulation of proliferation and apoptosis in human prostatic smooth muscle cells. J Urol. 2001;166:347–353. [PubMed] [Google Scholar]
- 20.Saini-Chohan HK, Hryshko L, Xu YJ, Dhalla NS. Modification of Ca(2+)-handling in cardiomyocytes by redox sensitive mechanisms in response to ouabain. Can J Physiol Pharmacol. 2013;91:45–55. doi: 10.1139/cjpp-2012-0215. [DOI] [PubMed] [Google Scholar]
- 21.Zulian A, Linde CI, Pulina MV, Baryshnikov SG, Papparella I, Hamlyn JM, Golovina VA. Activation of c-SRC underlies the differential effects of ouabain and digoxin on Ca(2+) signaling in arterial smooth muscle cells. Am J Physiol Cell Physiol. 2013;304:C324–333. doi: 10.1152/ajpcell.00337.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nguyen AN, Jansson K, Sanchez G, Sharma M, Reif GA, Wallace DP, Blanco G. Ouabain activates the Na-K-ATPase signalosome to induce autosomal dominant polycystic kidney disease cell proliferation. Am J Physiol Renal Physiol. 2011;301:F897–906. doi: 10.1152/ajprenal.00095.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pierre SV, Sottejeau Y, Gourbeau JM, Sanchez G, Shidyak A, Blanco G. Isoform specificity of Na-K-ATPase-mediated ouabain signaling. Am J Physiol Renal Physiol. 2008;294:F859–866. doi: 10.1152/ajprenal.00089.2007. [DOI] [PubMed] [Google Scholar]
- 24.Lingrel JB. The physiological significance of the cardiotonic steroid/ouabain-binding site of the Na,K-ATPase. Annu Rev Physiol. 2010;72:395–412. doi: 10.1146/annurev-physiol-021909-135725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zingarelli B. Nuclear factor-kappaB. Crit Care Med. 2005;33:S414–416. doi: 10.1097/01.ccm.0000186079.88909.94. [DOI] [PubMed] [Google Scholar]
- 26.Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132:344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 27.Karin M, Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5:749–759. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- 28.Zingarelli B, Sheehan M, Wong HR. Nuclear factor-kappaB as a therapeutic target in critical care medicine. Crit Care Med. 2003;31:S105–111. doi: 10.1097/00003246-200301001-00015. [DOI] [PubMed] [Google Scholar]
- 29.Takada Y, Matsuo K, Ogura H, Bai L, Toki A, Wang L, Ando M, Kataoka T. Odoroside A and ouabain inhibit Na+/K+-ATPase and prevent NF-kappaB-inducible protein expression by blocking Na+-dependent amino acid transport. Biochem Pharmacol. 2009;78:1157–1166. doi: 10.1016/j.bcp.2009.06.027. [DOI] [PubMed] [Google Scholar]
- 30.Liu X, Spicarova Z, Rydholm S, Li J, Brismar H, Aperia A. Ankyrin B modulates the function of Na,K-ATPase/inositol 1,4,5-trisphosphate receptor signaling microdomain. J Biol Chem. 2008;283:11461–11468. doi: 10.1074/jbc.M706942200. [DOI] [PubMed] [Google Scholar]
- 31.Aizman O, Aperia A. Na,K-ATPase as a signal transducer. Ann N Y Acad Sci. 2003;986:489–496. doi: 10.1111/j.1749-6632.2003.tb07233.x. [DOI] [PubMed] [Google Scholar]
- 32.Kawamoto EM, Lima LS, Munhoz CD, Yshii LM, Kinoshita PF, Amara FG, Pestana RR, Orellana AM, Cipolla-Neto J, Britto LR, Avellar MC, Rossoni LV, Scavone C. Influence of N-methyl-D-aspartate receptors on ouabain activation of nuclear factor-kappaB in the rat hippocampus. J Neurosci Res. 2012;90:213–228. doi: 10.1002/jnr.22745. [DOI] [PubMed] [Google Scholar]
- 33.Rindler TN, Lasko VM, Nieman ML, Okada M, Lorenz JN, Lingrel JB. Knockout of the Na,K-ATPase alpha2-isoform in cardiac myocytes delays pressure overload-induced cardiac dysfunction. Am J Physiol Heart Circ Physiol. 2013;304:H1147–1158. doi: 10.1152/ajpheart.00594.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]


