Abstract
To study the changes of interstitial cells of Cajal (ICCs) and expression of c-kt and scf mRNA in terminal ileum tissue during cholesterol gallstone formation in guinea pigs fed on high cholesterol diet, forty guinea pigs were divided into the gallstone group and the control group. The animals in the gallstone group were fed on a high cholesterol diet (HCD), while those in the control group fed on a standard diet (StD). The guinea pigs were sacrificed at the 8th week. The expression of c-kit and scf in terminal ileum were determined by RT-PCR and the morphological characteristics and number of ICCs were observed and calculated by using immunohistochemistry. RT-PCR showed that, compared with the control group, the c-kit and scf mRNA expression levels in the gallstone group were significantly declined. In the animal assay, the decreased number of ICCs was present obviously in the gallstone group. We concluded from the study that decreased number of ICCs, decreased expression of c-kit and scf in terminal ileum are present in guinea pigs fed on high cholesterol diet. The c-kit/scf pathway inhibition might be involved in the decline of intestinal transit function during cholesterol gallstone formation.
Keywords: Cholesterol gallstone, intestinal transit, interstitial cells of Cajal, c-kit, stem cell factor
Introduction
Cholesterol gallstone (CG) formation is a complicated process which may involving many factors such as gallbladder dysmotility, hypersaturation of biliary cholesterol, changes of bile salts pool in bile juice, etc [1,2]. Recently, the role of intestinal transit (IT), which may affect the reabsorption rate of bile salts in terminal ileum and the coordinating motility of small intestine and gallbladder, in the causes of CG has been gradually noticed [3,4]. Our previous study found that in high cholesterol diet (HCD)-induced golden hamster CG model, the intestinal transit (IT) function decreased obviously [5], indicating that the decline of IT might play a role in CG formation. However, the underlying mechanism has not been fully clarified.
Recently our understanding of physiology of the gastrointestinal smooth muscle system has changed due to studies of the population of the interstitial cells of Cajal (ICCs). ICCs are considered to be important in gastrointestinal tract motility by providing electrical impulses for slow wave generation and regulating smooth muscle activity [6-8]. Sufficient evidence suggests that the c-kit/stem cell factor (scf) signal pathway plays a crucial role in the regulation of cellular survival, proliferation and differentiation. So, whether the decline of IT in HCD-induced CG animal models is caused by the abnormalities of ICCs and c-kit/scf pathway? In this experiment, we used immunofluorescence staining technique and reverse transcriptase polymerase chain reaction (RT-PCR) to detect the changes of ICCs number and the expressions of c-kit and scf mRNA in terminal ileum tissue of guinea pig CG models, aiming to explore the underlying mechanisms of the IT decline during CG formation.
Materials and methods
Estabolishment of animal model and grouping
40 adult guinea-pigs (4 weeks, 120-125 g) were obtained from the Animal Research Center in Shengjing Hospital of China Medical University. The animals were randomly divided into two groups: gallstone group (GG, n = 20) which were given a HCD (2% cholesterol, purchased from Shanghai Sinopharm Chemical Reagent Co., Ltd.), and control group (CoG, n = 20) which were given a standard diet. All the animals were fed for 8 weeks before experiment. This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The animal use protocol has been reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of Shengjing Hospital of China Medical University.
Polarizing microscope study and composition analysis of gallstones
8 weeks later, bile juice was obtained from the gallbladder with fine-needle penetration after the animals were killed by cervical dislocation, and a small amount of bile was dropped on the slide to form the bile smear, the situation of cholesterol crystals in each group were observed using DP-71 polarized light microscope (Olympus Co., Ltd, Tokyo, Japan). In GG group, gallstones were collected after rapid cholecystectomy. The stones were dried naturally after washed with distilled water, and then 2 mg stone was mixed with 100 mg KBr, fully milled for 10 min, and baked under an infrared lamp for about 30 min before pressed into a transparent sheet with the tablet machine. FITR-55 infrared spectrometer (Bioon Co., Ltd, Bern, Switzerland) was used to analyze the compositions of the sheet.
Observation and counting of ICCs in terminal ileum
The guinea pigs were killed by cervical dislocation, about 2 cm terminal ileum was then taken and the intestinal contents were cleaned with 0.9% sodium chloride solution and fixed with 100% acetone at 4°C for 30 min. The intestine was removed along the longitudinal axis with microsurgery scissor, and then cut into 3 mm × 3 mm small pieces. Under the operating microscope, the intestinal mucosa and submucosa were peeled with microdissection forceps, while the muscular layer was reserved. After being washed for 4 × 10 min with phosphate buffered saline (PBS), the specimens were treated with PBS containing 0.3% Triton X-100, and then normal goat serum for 30 min at 37°C, respectively, followed by incubation with a rabbit polyclonal anti-c-kit antibody (Beijing Biosynthesis Biotechnology Co., Ltd, Beijing, China) in PBS overnight at 4°C. After this step, the tissues were washed for 4 × 10 min with PBS and incubated with a CY3-conjugated goat anti-rabbit secondary antibody (Beijing Biosynthesis Biotechnology Co., Ltd, Beijing, China) in PBS for 12 h at 37°C. After removing the unbound secondary antibody by washing with PBS, the preparations were coverslipped for confocal microscopic examination. Eclipse IZ laser confocal microscope (Nikon Co., Ltd, Tokyo, Japan) was used to count and photograph the c-kit positive ICCs cells in each group.
Extraction of total RNA
200 mg fresh intestinal tissue of guinea pig was placed in liquid nitrogen, and then repeatedly crushed before adding 1 mL Trizol solution. The total RNA was extracted according to Trizol Reagent kit instructions (Invitrogen, Camarillo, USA).
Detection of c-kit and scf mRNA expression with RT-PCR
cDNA was reverse-transcribed from 1 μg of total RNA and amplified for 35 cycles of denaturation (45 s at 94°C), annealing (45 s at 60°C), and synthesis (45 s at 72°C). The primers of c-kit were 5’-CACAGAGGCTTAGCGG-3’ (forward) and 5’-CGTGAAGGCAACATACC-3’ (reverse), generating an amplified product of 274 bp. The primers of scf were 5’ GCAGCATAATACCACG 3’ (forward), and 5’ AATACCATCATCCGTTC 3’ (reverse), generating an amplified product of 318 bp. The primers of GAPDH were 5’-ACCACAGTCCATGCCATCAC-3’ (forward) and 5’-TCCACCACCCTGTTGGGTA-3’ (reverse), generating an amplified product of 452 bp. Thereafter, electrophoresis was applied to the PCR product with size markers on a 1.5% agarose gel stained with ethidium bromide. GAPDH gene was used as an internal control of the analysis of gene expression. The mRNA values are expressed as relative units calculated according to the following formula: density of the c-kit/scf amplification product/density of the GAPDH amplification product.
Statistical analysis
SPSS 11.5 statistical analysis package was used, and all experimental data were expressed as mean ± standard deviation, inter-group comparison was performed with t test, and the rate comparison used chi-square test, with P < 0.05 considered as statistical significance.
Results
Animal models and stone analysis
3 animals in GG died, with the mortality rate 15%. Autopsy revealed that the causes of death was pneumonia (n = 1), splenic abscess (n = 1) and cervical lymphadenitis (n = 1), respectively; while no death in CoG.
8 weeks later, laparotomy revealed that the gallbladders of GG swelled, with obvious granular, yellow, single or multiple stones; while the gallbladders of CoG were normal with clear and bright bile (Figure 1). There was no stone formation in CoG. In GG, stone formed in the rest 17 guinea pigs, with the stone formation rate 100% (17/17). Polarized light microscopy revealed no cholesterol crystal in the gallbladder bile of CoG, while the typical needle-like crystals of cholesterol could be seen in GG (Figure 2). Infrared spectroscopy revealed obvious cholesterol absorption peaks at the wavelengths of 1418 cm-1 and 1640 cm-1, indicating a successful cholesterol gallstones model.
Figure 1.
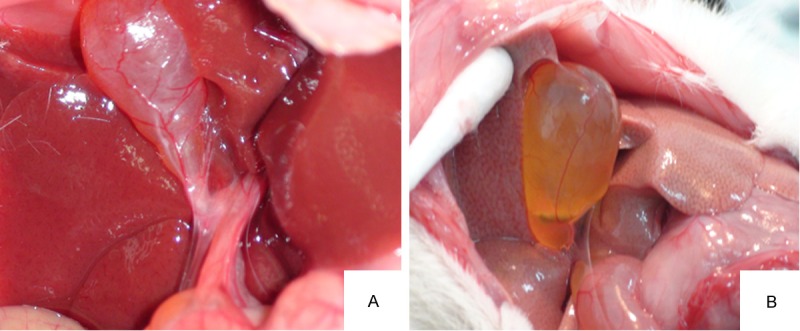
In vivo gallbladders in both groups at the end of lithogenic period. A: The gallbladder of CoG was normal with clear and bright bile juice. B: The gallbladder of GG guinea pig swelled, with obvious granular, yellow, single or multiple stones.
Figure 2.
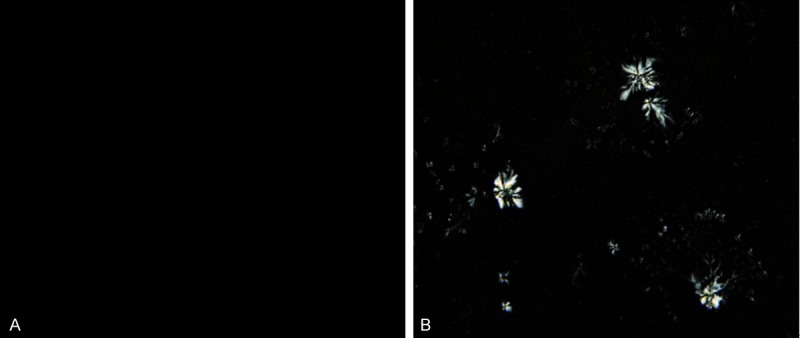
Polarized light microscopy revealed no cholesterol crystal in the gallbladder bile of CoG (A), while the typical needle-like crystals of cholesterol could be seen in GG (B).
Observation and counting of ICCs in terminal ileum
The c-kit-positive ICCs existed in the muscular layer of terminal ileum in guinea pig. Under the confocal microscope, ICCs were fusiform or satellite-shaped, the oval DAPI-blue-stained nuclei were enormous, with less perinuclear cytoplasm and 2~5 long-branched cell processes, making it appeared as spindle-like or satellite-like cell. ICCs were connected to each other, forming a network-like structure (Figure 3). The terminal ileum slide of GG also exhibited c-kit immune-positive ICCs, showing no significant difference with CoG under the light microscope, while the number of ICCs observed within the vision field significantly reduced, and the network structure disappeared (Figure 4).
Figure 3.
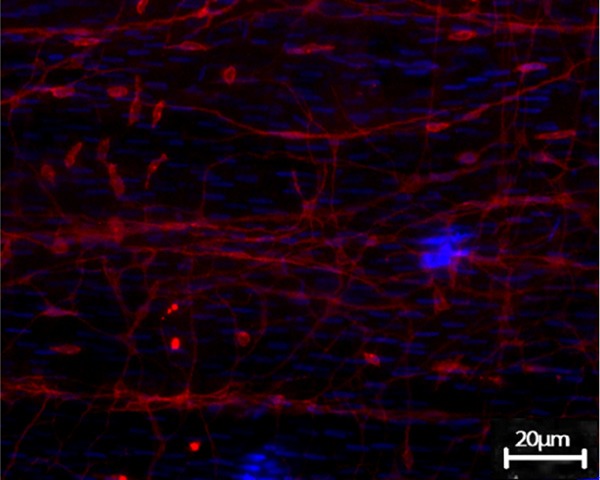
ICCs in terminal ileum in CoG. ICCs were fusiform or satellite-shaped, the oval DAPI-blue-stained nuclei were enormous, with less perinuclear cytoplasm and 2-5 long-branched cell processes, making it appeared as spindle-like or satellite-like cell. ICCs were connected to each other, forming a network-like structure c-kit positive ICCs of the small intestine in CoG (Confocal microscopy, DPAI counterstain, × 200).
Figure 4.
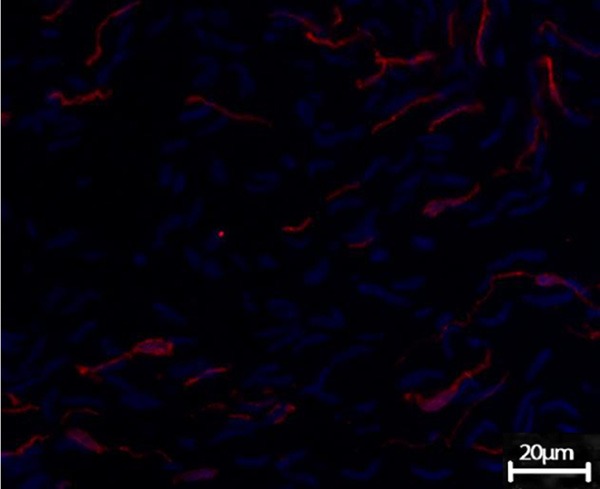
ICCs in terminal ileum in GG. Terminal ileum slide of GG also exhibited c-kit immune-positive ICCs, showing no significant difference with CoG in morphology under the light microscope, while the number of ICCs observed within the vision field significantly reduced, and the network structure disappeared (Confocal microscopy, DPAI counterstain, × 200).
Through the comparative observation of the slides of CoG and GG, each slide was randomly scanned for 3 fields, and the immunofluorescence image analysis software was used to count the number of ICCs. The results showed that the number of ICCs in GG significantly reduced when compared with that in CoG (75 ± 15 vs 20 ± 6.5, t = 19.256, P < 0.01).
mRNA expression of c-kit and scf in terminal ileum
RT-PCR analysis showed the presence of c-kit and scf mRNA in the terminal ileum from the CoG and GG. Compared with the controls, the mRNA expression of c-kit and scf was decreased in terminal ileum tissues in GG (0.416 ± 0.056 vs. 0.923 ± 0.103, t = 6.493, P < 0.01 and 0.487 ± 0.011 vs. 0.879 ± 0.113, t = 6.062, P < 0.01) (Figure 5).
Figure 5.
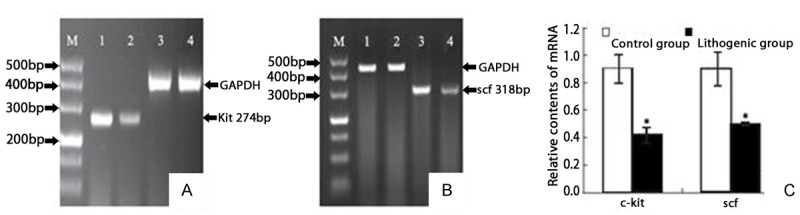
The mRNA expression of c-kit and scf in Guinea pig terminal ileum. Compared with control group (CoG), the mRNA expression of C-kit and scf were decreased in gallstone/lithogenic group (GG). A: The mRNA expression of C-kit markedly decreased in specimens of GG. B: The mRNA expression of scf markedly decreased in specimens of GG group. M: marker; lanes 1 and 3, control group; lane 2 and 4, gallstone group. C: The mean optical density of c-kit and scf mRNA/GAPDH. Each bar represents the mean ± SD (vertical line) with P = 0.001 and P = 0.002, respectively.
Discussion
The time of bile transit in the intestine or the whole gut is prolonged in patients with gallstones. Therefore, there is a possible relationship between dysfunction of IT and CG formation. The prolongation of IT can cause stasis of bile salts in the gut, which leads to the formation of more deoxycholic acid (DCA). Studies show that patients with gallstones have a longer IT time, higher serum DCA concentration, a higher pH in the colon cavity, more anaerobic bacteria in the cecum, and higher 7α-dehydroxylase activity than healthy controls [9-11]. Theoretically, increased DCA can promote the formation of gallstones in many ways: (1) DCA itself can slow down IT thereby allowing more time for intestinal cholesterol absorption and exerting a positive feedback on its own formation [12]; (2) DCA can enhance biliary cholesterol secretion by an effect on the hepatocyte canalicular membrane [13]; (3) in vitro studies have revealed that DCA enhances biliary cholesterol crystallization by destabilizing cholesterol-rich vesicles [14]; and (4) DCA can affect gallbladder motility through neural reflexes or other regulatory factors. Moreover, gallbladder contraction and intestinal transportation are related to the serum motilin level during digestion. The decline of IT would induce the disorder towards the gallbladder emptying, and the bile concentration would increase [15]; and the IT period extended would also prolong the enterohepatic circulation of bile acids, and bile salt reabsorption rate would slow down. Therefore, the bile salt content would reduce, and then promote the cholesterol supersaturation and crystallization [16]. However, the mechanism of the decline of IT has not been reported yet.
ICC was first reported in 1893 [17]. Recent studies have confirmed its crucial role in the pacing and dissemination of the smooth muscle activity in the gastrointestinal tract, and also in the nerve signal transduction pathway [18-20].
Our results show that the number of terminal ileum ICCs decreased significantly and the network-like structure between cells disappeared in HCD-induced guinea pig CG models, indicating that the number and functional changes of ICCs may contribute to the declined IT function.
One of the most important breakthroughs in ICCs study has been the discovery that the tyrosine kinase receptor c-kit and its ligand-stem cell factor (scf) are critical in the normal development, maturation, and phenotype maintenance of ICCs [21,22]. The animals with spontaneous c-kit mutant could not have normal occurrence and development of ICCs [23]. When ACK II, the c-kit neutralizing antibody, was intraperitoneally injected into the new-born mouse, it was found that the normal contraction phase of mouse intestine was disordered, the electric slow wave activity disappeared and ICCs also missed [24]. When the non-lethal mutation of scf happens, the heterozygous mice (Sl/Sld) would be severely damaged in the synthesis of scf, only capable of synthesizing and secreting a small amount of scf and the intestinal ICCs would be in poor development, forming a loose, and very untypical network structure [25]. Our results showed that in the terminal ileum of HCD-induced CG guinea pigs, the expression of c-kit and scf mRNA significantly decreased, cholesterol could inhibit the expression of c-kit and scf mRNA in terminal ileum tissues, participating the regulation of the number and function of ICCs and then affect IT function.
In summary, decreased number of ICCs, decreased expression of c-kit and scf in terminal ileum are present in guinea pigs fed on Figure 5. The mRNA expression of c-kit and scf in Guinea pig terminal ileum. Compared with control group (CoG), the mRNA expression of C-kit and scf were decreased in gallstone/lithogenic group (GG). A: The mRNA expression of C-kit markedly decreased in specimens of GG. B: The mRNA expression of scf markedly decreased in specimens of GG group. M: marker; lanes 1 and 3, control group; lane 2 and 4, gallstone group. C: The mean optical density of c-kit and scf mRNA/GAPDH. Each bar represents the mean ± SD (vertical line) with P = 0.001 and P = 0.002, respectively. HCD. The c-kit/scf pathway inhibition leading to the decreased number and function of ICCs might be involved in the decline of IT function during CG formation. ICCs may be a therapeutic target in CG prevention.
Acknowledgements
This study was supported by National Natural Science Foundation of China (NSFC) (NO. 81000183), Shenyang Scientific and Technological Funding (NO. F11-264-1-24) and Natural Science Foundation of Liaoning (NO. 2013021060).
Disclosure of conflict of interest
None.
References
- 1.Pasternak A, Gil K, Matyja A, Gajda M, Sztefko K, Walocha JA, Kulig J, Thor P. Loss of gallbladder interstitial Cajal-like cells in patients with cholelithiasis. Neurogastroenterol Motil. 2013;25:e17–24. doi: 10.1111/nmo.12037. [DOI] [PubMed] [Google Scholar]
- 2.Portincasa P, Di Ciaula A, Vendemiale G, Palmieri V, Moschetta A, Vanberge-Henegouwen GP, Palasciano G. Gallbladder motility and cholesterol crystallization in bile from patients with pigment and cholesterol gallstones. Eur J Clin Invest. 2000;30:317–324. doi: 10.1046/j.1365-2362.2000.00639.x. [DOI] [PubMed] [Google Scholar]
- 3.Kaur J, Rana SV, Gupta R, Gupta V, Sharma SK, Dhawan DK. Prolonged orocecal transit time enhances serum bile acids through bacterial overgrowth, contributing factor to gallstone disease. J Clin Gastroenterol. 2014;48:365–369. doi: 10.1097/MCG.0b013e3182a14fba. [DOI] [PubMed] [Google Scholar]
- 4.Xie M, Kotecha VR, Andrade JD, Fox JG, Carey MC. Augmented cholesterol absorption and sarcolemmal sterol enrichment slow small intestinal transit in mice, contributing to cholesterol cholelithogenesis. J Physiol. 2012;590:1811–1824. doi: 10.1113/jphysiol.2011.224717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fan Y, Wu SD, Fu BB. Effect of intestinal transit on the formation of cholesterol gallstones in hamsters. Hepatobiliary Pancreat Dis Int. 2007;6:513–515. [PubMed] [Google Scholar]
- 6.Mostafa RM, Moustafa YM, Hamdy H. Interstitial cells of Cajal, the Maestro in health and disease. World J Gastroenterol. 2010;16:3239–3248. doi: 10.3748/wjg.v16.i26.3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huizinga JD, Zarate N, Farrugia G. Physiology, injury, and recovery of interstitial cells of Cajal: basic and clinical science. Gastroenterology. 2009;137:1548–1556. doi: 10.1053/j.gastro.2009.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lammie A, Drobnjak M, Gerald W, Saad A, Cote R, Cordon-Cardo C. Expression of c-kit and kit ligand proteins in normal human tissues. J Histochem Cytochem. 1994;42:1417–1425. doi: 10.1177/42.11.7523489. [DOI] [PubMed] [Google Scholar]
- 9.Shoda J, He BF, Tanaka N, Matsuzaki Y, Osuga T, Yamamori S, Miyazaki H, Sjövall J. Increase of deoxycholate in supersaturated bile of patients with cholesterol gallstone disease and its correlation with de novo syntheses of cholesterol and bile acids in liver, gallbladder emptying, and small intestinal transit. Hepatology. 1995;21:1291–1302. [PubMed] [Google Scholar]
- 10.Colecchia A, Mazzella G, Sandri L, Azzaroli F, Magliuolo M, Simoni P, Bacchi-Reggiani ML, Roda E, Festi D. Ursodeoxycholic acid improves gastrointestinal motility defects in gallstone patients. World J Gastroenterol. 2006;12:5336–5343. doi: 10.3748/wjg.v12.i33.5336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Erpecum KJ, van Berge Henegouwen GP. Intestinal aspects of cholesterol gallstone formation. Dig Liver Dis. 2003;35:S8–11. doi: 10.1016/s1590-8658(03)00086-0. [DOI] [PubMed] [Google Scholar]
- 12.Brown NJ, Read NW, Richardson A, Rumsey RD, Bogentoft C. Characteristics of lipid substances activating the ileal brake in the rat. Gut. 1990;31:1126–1129. doi: 10.1136/gut.31.10.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Erpecum KJ, Carey MC. Influence of bile salts on molecular interactions between sphingomyelin and cholesterol: relevance to bile formation and stability. Biochim Biophys Acta. 1997;1345:269–282. doi: 10.1016/s0005-2760(97)00002-7. [DOI] [PubMed] [Google Scholar]
- 14.Wang DQ, Carey MC. Complete mapping of crystallization pathways during cholesterol precipitation from model bile: influence of physical-chemical variables of pathophysiologic relevance and identification of a stable liquid crystalline state in cold, dilute and hydrophilic bile salt-containing systems. J Lipid Res. 1996;37:606–630. [PubMed] [Google Scholar]
- 15.Zhang ZH, Wu SD, Su Y, Jin JZ, Fan Y, Yu H, Zhang LK. Differences and significance of motilin, vasoactive intestinal peptide and gastrin in blood and gallbladder tissues of patients with gallstones. Hepatobiliary Pancreat Dis Int. 2008;7:58–64. [PubMed] [Google Scholar]
- 16.Xie M, Kotecha VR, Andrade JD, Fox JG, Carey MC. Augmented cholesterol absorption and sarcolemmal sterol enrichment slow small intestinal transit in mice, contributing to cholesterol cholelithogenesis. J Physiol. 2012;590:1811–1824. doi: 10.1113/jphysiol.2011.224717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abramovic M, Radenkovic G, Velickov A. Appearance of interstitial cells of Cajal in the human midgut. Cell Tissue Res. 2014;356:9–14. doi: 10.1007/s00441-013-1772-x. [DOI] [PubMed] [Google Scholar]
- 18.McCann CJ, Hwang SJ, Bayguinov Y, Colletti EJ, Sanders KM, Ward SM. Establishment of pacemaker activity in tissues allotransplanted with interstitial cells of Cajal. Neurogastroenterol Moti. 2013;25:e418–428. doi: 10.1111/nmo.12140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huizinga JD, Chen JH. Interstitial cells of Cajal: update on basic and clinical science. Curr Gastroenterol Rep. 2014;16:363. doi: 10.1007/s11894-013-0363-z. [DOI] [PubMed] [Google Scholar]
- 20.Kim SO, Jeong HS, Jang S, Wu MJ, Park JK, Jiao HY, Jun JY, Park JS. Spontaneous electrical activity of cultured interstitial cells of cajal from mouse urinary bladder. Korean J Physiol Pharmacol. 2013;17:531–6. doi: 10.4196/kjpp.2013.17.6.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hirota S, Isozaki K, Nishida T, Kitamura Y. Effects of loss-of-function and gain-of-function mutations of c-kit on the gastrointestinal tract. J Gastroenterol. 2000;35:75–79. [PubMed] [Google Scholar]
- 22.Rich A, Miller SM, Gibbons SJ, Malysz J, Szurszewski JH, Farrugia G. Local presentation of steel factor increases expression of c-kit immunoreactive interstitial cells of Cajal in culture. Am J Physiol Gastrointest Liver Physiol. 2003;284:G313–320. doi: 10.1152/ajpgi.00093.2002. [DOI] [PubMed] [Google Scholar]
- 23.Albertí E, Mikkelsen HB, Wang XY, Díaz M, Larsen JO, Huizinga JD, Jiménez M. Pacemaker activity and inhibitory neurotrans-mission in the colon of Ws/Ws mutant rats. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1499–1510. doi: 10.1152/ajpgi.00136.2006. [DOI] [PubMed] [Google Scholar]
- 24.O’Grady G, Du P, Paskaranandavadivel N, Angeli TR, Lammers WJ, Asirvatham SJ, Windsor JA, Farrugia G, Pullan AJ, Cheng LK. Rapid high-amplitude circumferential slow wave propagation during normal gastric pacemaking and dysrhythmias. Neurogastroenterol Motil. 2012;24:e299–e312. doi: 10.1111/j.1365-2982.2012.01932.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mikkelsen HB, Malysz J, Huizinga JD, Thuneberg L. Action potential generation, Kit receptor immunohistochemistry and morphology of steel-Dickie (Sl/Sld) mutant mouse small intestine. Neurogastroenterol Motil. 1998;10:11–26. doi: 10.1046/j.1365-2982.1998.00082.x. [DOI] [PubMed] [Google Scholar]


