Abstract
Evaluation of sleep apnea-hypopnea syndrome (SAHS) is mainly based on the polysomnogram (PSG), which is considered as the golden diagnostic criteria. As a novel noninvasive and low cost alternative method to detect SAHS patients, the diagnostic power of ECG-derived respiration (EDR) hasn’t been well determined. In light of this, we tested whether EDR can be utilized as a feasible tool to diagnose SAHS in Chinese patients. Overnight sleep investigation was performed in 120 subjects using polysomnogram (PSG) and 24-hour ambulatory electrocardiogram (AECG). The apnea hypopnea index (AHI) was calculated from EDR and PSG respectively. With EDR assessments, 77 subjects were determined as SAHS (+), 43 were diagnosed as SAHS (-). The diagnostic accordance rate was 87.5% and the area under curve was 0.938. The coefficient correlation of AHI between EDR and PSG was 0.879 (P <0.001), while the correlation of maximum apnea and hypopnea time duration were 0.716 (P <0.001) and 0.281 (P <0.005), respectively. All correlations were statistically significant (P <0.01). EDR can be used as a practical tool for the diagnosis of SAHS.
Keywords: Sleep apnea-hypopnea syndrome, ECG-derived respiration, polysomnogram, diagnosis
Introduction
Sleep apnea-hypopnea syndrome (SAHS) is a severe sleep disorder with growing morbidity in Chinese population. When sleep apnea-hypopnea index (AHI) is greater than 5, the diagnosis of SAHS is established. The prevalance of SAHS is about 2%-4% in general Chinese population, with higher morbidity in males than in females. A further increase of morbidity has been reported in males after middle-age and females after menopause. Being an independent risk for cardiovascular and metabolic disorders, SAHS has been proved to raise potential risk of nocturnal sudden death [1,2]. Anatomically, SAHS is usually associated with abnormality of the upper airway. So far, the diagnosis of SAHS mainly depends on polysomnogram monitoring, which is costly and requires sophisticated medical equipments and faculties for data interpretation. All these features substantially limit its’ utilization, especially in developing countries. Subsequently, various screening tests for SAHS have been developed, such as heart rate variability, pulse oximetry, audiotapes and videotaping. Nevertheless, due to inaccuracy or inconvenience, none of these methods are widely used so far.
The EDR (ECG-Derived Respiration) technique is a unique approach which is based on the observation that the positions of ECG electrodes on the chest surface move relative to the heart, and transthoracic impedance varies, as the lungs fill and empty. Thus the lead axes vary at different points in the respiratory cycle, and any sufficiently precise measurement of the mean cardiac electrical axis shows variations that are correlated with respiration. Accordingly, EDR can be utilized to evaluate sleep disturbance owing to apnea or hypopnea episodes. As a very simple and widely used technique, ambulatory ECG can be carried out for either in hospital or outpatients, contributing to better compliance and flexibility for EDR.
In light of this, we designed this study to evaluate the diagnostic utility of detecting SAHS with EDR from AECG monitoring compared with conventional PSG test.
Patients and methods
Patients
The study was designed as a blinded screening test. Patients who individually registered to sleep center in our hospital with snore and sleep disturbance were enrolled into this study. Subjects with coronary heart disease, history of myocardial infarction, diabetes mellitus, permanent or paroxysmal atrial fibrillation or permanent cardiac pacemaker were excluded. As a result, a total of 120 patients including 101 males and 19 females were recruited. The average age and BMI (body mass index) in the 88 SAHS patients are 48.36±10.61 and 29.09±4.97 kg/m2 respectively, while 32 control subjects aged 39.5±17.82 with BMI of 24.37±3.85 kg/m. This study was approved by the institutional ethics committees of People’s Hospital of Peking University and signed informed consent was obtained from each participant.
Polysomnography and ambulatory electrocardiography
Overnight sleep investigation (over 7 hours) was administered to all subjects with polysomnogram (PSG) as a golden diagnostic standard. A 16-channel SM2000 PSG (kind courtesy: Mingsi Company, Beijing, China) was used to record electroencephalogram (EEG), electromyogram (EMG), eye movement electrooculogram, nasal airstream, snore index, ambulatory electrocardiogram (ECG), respiratory movement of the chest and abdomen, and oxygen saturation. Time constant was set as 0.3s for EEG and EMG, while noise reduction with a bandpass filter of 25 Hz was used to remove unwanted baseline fluctuation and high-frequency interference in the ECG. Variables including times of sleep and wakefulness, rapid eye movements (REM), non-rapid eye movements (NREM), sleep phases I–IV, the longest and mean durations of apnea, AHI, the minimum and mean blood oxygen saturations were observed. For the EDR assessment, ambulatory electrocardiogram monitoring was carried out simultaneously with the PSG monitoring using a Medilog AR12 12-channel ambulatory electrocardiograph (kind courtesy: Oxford company, UK). Two individual analyzers unaware about the clinical information or PSG results calculated AHI based on AECG using automatic analysis software (Simple View 2.2) and manual protocols, respectively, and gave the diagnosis as SAHS (+) or SAHS (-).
Diagnostic criteria of sleep apnea-hypopnea syndrome
SAHS was diagnosed when AHI was ≥5 or more than 30 episodes of sleep apnea were present during 7 hours of sleep. Apnea is defined as the absence of oral-nasal airflow for >10 seconds. Hypopnea is defined as a ≥50% reduction in the amplitude of respiratory airflow with a ≥4% fall in arterial oxygen saturation (SaO2). The classification of SAHS is based on AHI and arterial oxygen saturation: Mild SAHS (AHI 5~20, SaO2 ≥85%); moderate SAHS (AHI 21~50, SaO2 ≥80%); severe SAHS (AHI >50, SaO2 ≤79%). Same threshold of AHI for SAHS diagnosis is adopted in EDR and PSG monitoring.
Statistical analysis
The primary outcome measurements of the study were the correlation between EDR and PSG derived AHI, as well as receiver operating characteristic (ROC) curve analysis of EDR. Data analysis was carried out using SPSS PC 10.0 software. Values were presented as mean±SD. Comparisons between and among groups were performed with 2-tailed t-test or χ2 test. P values <0.05 were considered statistically significant.
Results
The average age and male gender numbers were significantly greater in the SAHS (+) group compare to the SAHS (-) group (P <0.05), while other parameters such as BMI and the mobidity of hypertension were also higher, though without statistical significance (Table 1).
Table 1.
Comparison of clinical characteristics between SAHS (+) and SAHS (-)
| group | example | Age (year) | male n (%) | BMI (kg/m2) | HBP n (%) |
|---|---|---|---|---|---|
| SAHS (+) | 88 | 48.36±10.61 | 78 (88.6%) | 29.09±4.97 | 52 (59.1%) |
| SAHS (-) | 32 | 39.5±17.82* | 23 (71.9%)* | 24.37±3.85 | 13 (49.6%) |
Comparison between groups
P <0.05.
When diagnosis was performed with EDR technique, we found 77 SAHS (+) results (75 true positive and 2 false positive cases) and 43 SAHS (-) results (30 true negative and 13 false negative cases). Taken together, the sensitivity of diagnosing SAHS with EDR was 85.2%, with a specificity of 93.8%. The positive and negative predictive value were 97.4% and 69.8%, respectively. Moreover, the diagnose accordance rate was 87.5% (Table 2). Using receiver operating characteristic (ROC) method, we confirmed high diagnostic power of EDR for SAHS, with an area under curve (AUC) of 0.938 (Figure 1). The pearson correlation coefficient between EDR and PSG derived AHI was 0.879 (P <0.001), while the correlation coefficient of the maximum apnea and hypopnea duration were 0.716 (P <0.001) and 0.281 (P <0.005), respectively.
Table 2.
Diagnosis accordance rate of EDR and polysomnogram
| SAHS (+) | SAHS (-) | Sensitivity | Specificity | PPV | NPV | AR | |
|---|---|---|---|---|---|---|---|
|
|
|||||||
| Method | (n = 88) | (n = 32) | (%) | (%) | (%) | (%) | (%) |
| EDR | 77 (T75, F2) | 43 (T30, F13) | 85.2 | 93.8 | 97.4 | 69.8 | 87.5 |
PPV- positive predictive value. NPV- negative predictive value. AR- Accordance rate.
Figure 1.
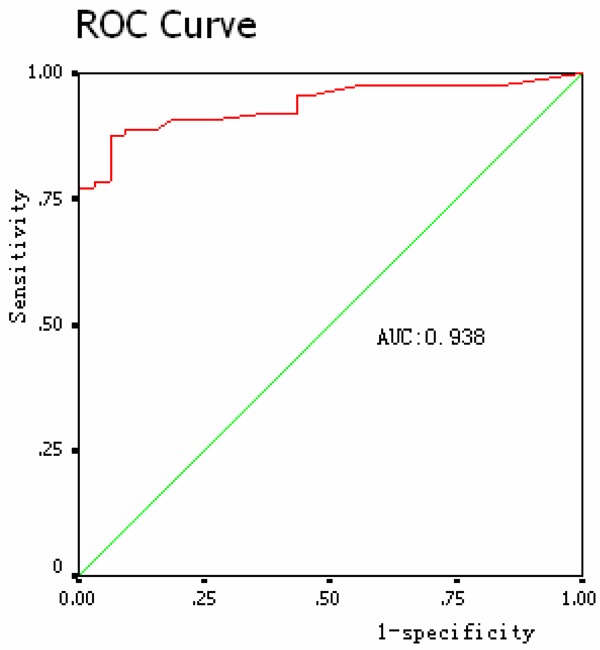
Receiver operating characteristic for EDR compared with PSG.
Discussions
The current study examines the utility of EDR from ambulatory ECG as a potential screening test for the diagnosis of SAHS. The EDR AHI was found to be highly correlated with the PSG-derived AHI, which is considered as the Golden Standard. Using ROC curve analysis, we found that EDR test have optimal screening test performance compared to the PSG gold standard for diagnosis of SAHS.
A growing entity of interest by clinicians has been laid on SAHS, as it’s confirmed as an independent risk factor for cardiovascular disease, such as arrhythmia, hypertension and ischemia [3]. The basic pathophysiological change during SAHS is frequent apnea and hypopnea, which may cause dysfunctions of major organ by eliciting inflammatory response or oxidative stress. Considerable evidence has shown that patients with sleep apnea treated by Continuous Positive Airway Pressure (CPAP), especially at early stage, have improved quality of life, while complications are also reduced, leading to a better overall survival. Thus, early diagnosis of SAHS will have long-term cost-effective benefit. Currently, the Golden diagnostic method for sleep apnea is polysomnogram test, which is somehow under used in developing areas due to its sophistication and high cost. The PSG monitors body functions such as brain (EEG), eye movements (EOG), muscle activity or skeletal muscle activation (EMG) and heart rhythm (ECG) during sleep. Normally, PSG monitoring requires a dedicated sleep-monitoring room with costly medical equipment and well-trained technicians are indispensable for its proper interpretation. The patient will be introduced to the setting and “wired up” so that multiple channels of data can be recorded when he/she falls asleep. So it’s substantially difficult to be widely utilized for the diagnosis of SAHS, especially for outpatients. Moreover, interruption of a natural sleeping scene and indisposition caused by excessive attached probes may also affect the accuracy of the result or even discourage some of the patients from taking the PSG tests.
The easiest approach to predict a patient with SAHS is through clinical history taking and physical examination. The clinical history and physical examination are accurate in ruling in SAHS. Nevertheless, a further objective measurement is required to establish the proper diagnosis with both ideal sensitivity and specificity. Alternatively, screening tests for SAHS including heart rate variability, pulse oximetry, audiotapes and videotaping were developed. However, due to either inaccuracy or inconvenience, these approaches have not been widely used. Previously, Brietzke et al showed PTT as an alternative useful method to screen SAHS in pediatric patients, with a sensitivity of 81% and a specificity of 76%. It requires 2 electrocardiogram chest leads and one coupling pulse oximeter probe. In our study, the EDR only need one Holter device (Ambulatory ECG) while superior diagnostic power is achieved (sensitivity: 85.2%, specificity: 93.8%).
It could be noticed with AECG monitoring that heart rate reduces during apnea and increases when the apnea event ends. The heart rate could reach a climax through several breath episodes. This phenomenon is called cyclic variations in heart rate (CVHR). In light of this, analysis of the change in heart rate and HRV among the cycles using ambulatory electrocardiogram can predict the presence of sleep apnea and may present as a potential diagnostic tool for the disease [4-10].
Recently, Sun et al [11] reported that the time-domain and frequency-domain analyses of HRV can be used as two useful analytical approaches for screening of individuals with OSAS. According to previous studies, the sensitivity and specificity of this technique were around 70~90%, when almost of the data were produced from qualitative analysis rather than quantitative tests.
Moody first proposed EDR technique [12] in 1985. He showed that ECGs recorded from the surface of the chest are influenced by motion of the electrodes with respect to the heart and the changes in electrical impedance of the thoracic cavity. The expansion and contraction of the chest which accompany respiration result in motion of chest electrodes. These physical influences of respiration result in amplitude variations in the recorded ECG. Respiratory signals may be derived from body surface ECG by measuring fluctuations in the mean cardiac electrical axis accompanying respiration. This technique costs lower while remaining noninvasive. In addition, it is applicable to any type of automated ECG analysis without the need for additional transducers or hardware update. Clinicians can use EDR technique to detect SAHS patients through both qualitative and quantitative analysis.
In 1986, Moody [13] completed two studies by applying the EDR technique to Holter recordings. The first study demonstrated the feasibility of using Holter recordings as a screening test for sleep apnea. In a sample size of 9 patients, diagnosis of SAHS based on the EDR was confirmed by simultaneous polysomnography in all but one case. In the other study, Cheyne-Stokes respiration was observed in a group of patients with severe congestive heart failure. EDR analysis showed that the phenomenon occurred in 8 patients out of 10 who were studied, and its incidence decreased in 7 of these 8 after chronic oral administration of a positive inotropic agent. Subsequently, Travaglini A tried to derive respiratory signal from eight-lead ECG [14], the results obtained presented to be strongly correlated with conventional measurements of respiration, and the use of multi-lead ECG can provide further noise reduction. Later, the method was extended to ambulatory multiple lead electrocardiogram gradually.
A number of studies showed a significant correlation between the EDR signal and measurements of respiration. However, few evidence showing the diagnostic utility of EDR on SAHS compared with conventional PSG method is available.
Our study indicates that EDR curve correlates well with respiration (Figures 2, 3 and 4). Its sensitivity and rate of accordance for screening SAHS is acceptable with either computer software automatic analysis or manual analysis of EDR.
Figure 2.
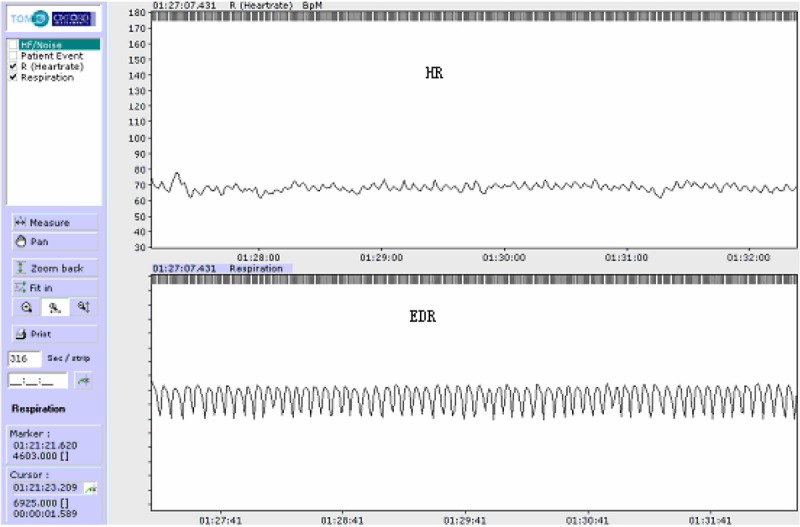
Upper trace: heart rate. Lower trace: EDR curve with normal regular respiration.
Figure 3.
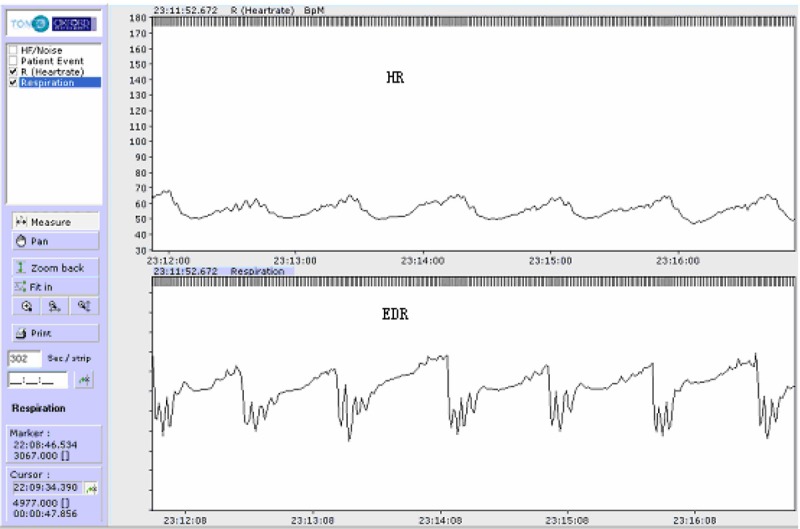
Upper trace: heart rate. Lower trace: EDR curve with sleep apnea.
Figure 4.
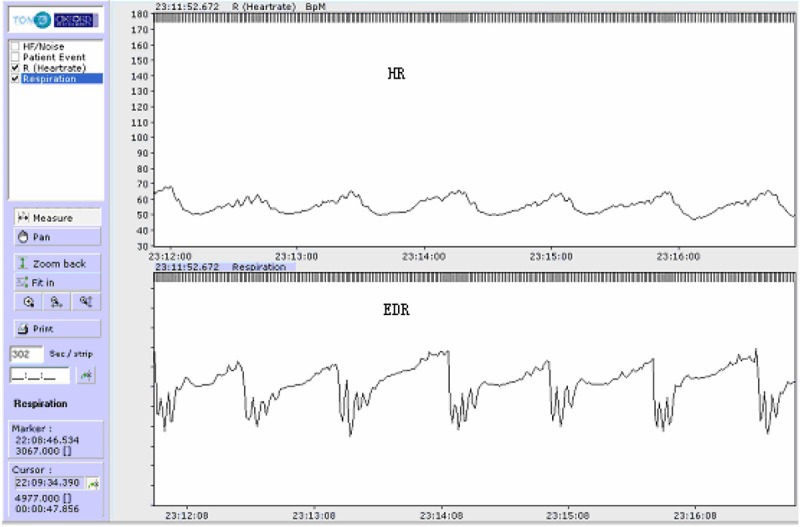
Upper trace: heart rate. Lower trace: EDR curve with sleep Hypopnea.
Limitations of the current study include a relatively small patient population, as well as confounding factors which may interfere with the EDR recording. HoIn certain cases, the calculation of respiratory frequency and apnea was interrupted, with major causes listed as following. First, fluctuation of ECG tracing can be caused by extra noise. Then, detach of an electrode or lead during the monitoring of the AECG may affect the result. Additionally, our current study may be more practical in patients with sinus rhythm, as when the number of ectopic heartbeats exceeds twenty percent of the total, the result can be inaccurate according to calculation errors. Also, the effect of temperature, humidity, and thoracic impedance may also be considered.
Conclusions
In summery, EDR technique is a simple method of lower cost compared with PSG for the diagnosis of SAHS. It can be performed in a setting more close to natural sleeping condition. Automatic analysis with software in combination with manual interpretation is simple and accurate, making it an easier approach for widespread adoption. In summary, EDR technique of AECG is useful to screen subjects suspicious for SAHS, and it can be an ancillary tool to diagnose SAHS. Further multicenter, randomized, double-blinded studies, as well as system upgrade will be required to determine the sensitivity and specificity of diagnosing SAHS with EDR.
Disclosure of conflict of interest
None.
References
- 1.Ludka O, Konecny T, Somers V. Sleep apnea, cardiac arrhythmias, and sudden death. Tex Heart Inst J. 2011;38:340–343. [PMC free article] [PubMed] [Google Scholar]
- 2.Gottlieb DJ, Yenokyan G, Newman AB, O’Connor GT, Punjabi NM, Quan SF, Redline S, Resnick HE, Tong EK, Diener-West M, Shahar E. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the sleep heart health study. Circulation. 2010;122:352–360. doi: 10.1161/CIRCULATIONAHA.109.901801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alonso-Fernández A, García-Río F, Racionero MA, Pino JM, Ortuño F, Martínez I, Villamor J. Cardiac rhythm disturbances and ST-segment depression episodes in patients with obstructive sleep apnea-hypopnea syndrome and its mechanisms. Chest. 2005;127:15–22. doi: 10.1378/chest.127.1.15. [DOI] [PubMed] [Google Scholar]
- 4.Roche F, Gaspoz JM, Court-Fortune I, Minini P, Pichot V, Duverney D, Costes F, Lacour JR, Barthélémy JC. Screening of obstructive sleep apnea syndrome by heart rate variability analysis. Circulation. 1999;100:1411–1415. doi: 10.1161/01.cir.100.13.1411. [DOI] [PubMed] [Google Scholar]
- 5.Penzel T, McNames J, Murray A, de Chazal P, Moody G, Raymond B. Systematic comparison of different algorithms for apnoea detection based on electrocardiogram recordings. Med Biol Eng Comput. 2002;40:402–407. doi: 10.1007/BF02345072. [DOI] [PubMed] [Google Scholar]
- 6.Roche F, Pichot V, Sforza E, Court-Fortune I, Duverney D, Costes F, Garet M, Barthélémy JC. Predicting sleep apnoea syndrome from heart period: a time-frequency wavelet analysis. Eur Respir J. 2003;22:937–942. doi: 10.1183/09031936.03.00104902. [DOI] [PubMed] [Google Scholar]
- 7.Roche F, Sforza E, Duverney D, Borderies JR, Pichot V, Bigaignon O, Ascher G, Barthélémy JC. Heart rate increment: an electrocardiological approach for the early detection of obstructive sleep apnoea/hypopnoea syndrome. Clin Sci (Lond) 2004;107:105–110. doi: 10.1042/CS20040036. [DOI] [PubMed] [Google Scholar]
- 8.Stein PK, Duntley SP, Domitrovich PP, Nishith P, Carney RM. A simple method to identify sleep apnea using Holter recordings. J Cardiovasc Electrophysiol. 2003;14:467–473. doi: 10.1046/j.1540-8167.2003.02441.x. [DOI] [PubMed] [Google Scholar]
- 9.Babaeizadeh S, White DP, Pittman SD, Zhou SH. Automatic detection and quantification of sleep apnea using heart rate variability. J Electrocardiol. 2010;43:535–541. doi: 10.1016/j.jelectrocard.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 10.Hayano J, Watanabe E, Saito Y, Sasaki F, Fujimoto K, Nomiyama T, Kodama I, Sakakibara H. Screening for obstructive sleep apnea by cyclic variation of heart rate. Circ Arrhythm Electrophysiol. 2011;4:64–72. doi: 10.1161/CIRCEP.110.958009. [DOI] [PubMed] [Google Scholar]
- 11.Sun JL, Li XY, Guo JH, Zhang HC. Identification of Obstructive Sleep Apnea by Ambulatory Electrocardiogram of Time-domain and Frequency Variability in Chinese Patients. Cell Biochem Biophys. 2011;59:165–170. doi: 10.1007/s12013-010-9128-6. [DOI] [PubMed] [Google Scholar]
- 12.Moody G, Mark R, Zoccola A, Mantero S. Derivation of respiratory signals from multi-lead ECGs. Computers in Cardiology. 1985;12:113–116. [Google Scholar]
- 13.Moody G, Mark R, Bump M, Mantero S. Clinical validation of the ECG-Derived respiration (EDR) technique. Computers in Cardiology. 1986;13:507–510. [Google Scholar]
- 14.Travaglini A, Lamberti C, Debie J, Ferri M. Respiratory of signal derived from eight-lead ecg. IEEE Xplore. 1998;25:65–68. [Google Scholar]


