Abstract
MicroRNAs are implicated in an increasing number of diseases and health complications, including asthma. Previous studies have suggested roles for microRNA-21 (miR-21) and microRNA-126 (miR-126) in asthma, although these relationships are incompletely understood. The aim of this study was to further assess the relationship between miR-21, miR-126 and the occurrence and treatment of allergic asthma. Quantitative real-time PCR analyzed expression levels of miR-21 and miR-126 in bronchial epidermal cells from asthma patients treated with (treatment group, n=19) or without (non-treatment group, n=16) inhaled corticosteroids or from non-asthmatic healthy individuals (normal group, n=12). Bronchial epidermal cells were cultured in vitro to determine if there was a relationship between IL-13 and miR-21 and miR-126 expression. Compared to the normal group, miR-21 and miR-126 expression was significantly upregulated in asthma patients regardless of treatment. miR-21 and miR-126 expression was significantly higher in the non-treatment group than the treatment group (P<0.05). In vitro, miR-21 and miR-126 expression increased in bronchial epidermal cells with increasing IL-13 concentration. Because upregulation of miR-126 and miR-21 were associated with occurrence and therapy of allergic asthma, they may be indices of therapeutic effect that are valuable for the evaluation for asthma treatment.
Keywords: Asthma, microRNA-21, microRNA-126, real time PCR
Introduction
Bronchial asthma is one of the most common chronic respiratory tract diseases, with an incidence that rises every year [1]. Asthma is characterized by chronic airway inflammation, airway hyper-responsiveness, and irreversible airway remodeling, but its pathogenesis is unclear. Present asthma treatments mainly rely on long-term inhaled corticosteroids (ICS). While ICS relieve bronchial asthma, they cannot manage refractory asthma involving hormonal resistance and hormone dependence. Further, ICS cause side effects such as growth and development inhibition, obesity, and osteonecrosis. Therefore, it is urgent to further understand the pathogenesis of asthma and identify new methods to treat airway inflammation [2].
MicroRNAs (miRs) were discovered by Lee et al in 1993 [3]. They are a class of small RNAs, 21-25 nucleotides in length that are inherent in eukaryotic organisms. miRs base-pair with target mRNAs to induce silencing complexes that degrade mRNAs or block translation, thus affecting protein synthesis. As shown by miRBase, approximately 1,900 human miR genes are already identified [4]. Over 100 mRNA genes [5] are targeted by each miR, and over 30% of human protein-coding genes are regulated by miRs [6]. Therefore, miRs with abnormal expression are closely associated with the occurrence and development of disease [7], including asthma. Lu et al [8] found that miR-21 is elevated in asthmatic airways and plays a role in asthma. miR-126 expression is correlated with toll-like receptor 4 (TLR4) signal transduction pathways, which are activated by house dust mite allergens to cause asthmatic symptoms [9].
To further understand the roles of miRs in asthma, we used quantitative real-time PCR (qRT-PCR) to measure the expression of miR-21 and miR-126 in cultured bronchial epithelial cells from asthma patients with or without ICS therapy and from non-asthmatic healthy individuals. We further explored the relationship between miR-21 and miR-126 expression in bronchial epithelial cells and interleukin 13 (IL-13) to better inform the occurrence, treatment and prognosis of clinical asthma.
Materials and methods
Subjects
This study included 35 patients (18 males and 17 females) with bronchial asthma admitted to the Respiratory Department, Affiliated Hospital of Hainan Medical University. The inclusion criteria were as follows: 1) patients had previous history of paroxysmal wheezing, dyspnea, chest distress, and/or coughing; 2) according to the Global Initiative for Asthma (GINA), patients had reversible airflow limitation as measured by an increase in forced expiratory volume in one second (FEV1) of at least 15% after inhalation of 200 μg salbutamol, or a decrease in FEV1 of over 20% after inhalation of <8 mg/mL acetylcholine; 3) skin prick tests showed patients were allergic to at least one of the following allergens: house dust mites, mixed grass pollens, mixed tree pollens, dog hair, feathers, cat hair, fine soft hair, cockroaches, or mold; 4) patients had no upper and lower respiratory tract diseases within 2 months and had no chronic heart or lung disease; and 5) within at least 4 weeks, patients had not systemically used corticosteroids, theophylline, long-acting β2-agonists, leukotriene receptor antagonists, or antihistamines. Nineteen cases (10 males and 9 females) presented with less evident asthma symptoms after ICS therapy and were assigned to the treatment group, while the remaining 16 cases (8 males and 8 females) were not given ICS therapy and were assigned to the non-treatment group. For the normal group, 12 healthy non-asthmatic volunteers (5 males and 7 females) were recruited from local college students. Normal volunteers were not allergic to anything or drugs investigated, had no respiratory tract diseases, and had normal lung function. This study met the relevant ethical requirements for human research, approved by the Ethics Committee of the First People’s Hospital of the Affiliated Hospital of Hainan Medical University, and all subjects signed a written consent form.
Collection and storage of biological samples
Epithelial cell specimens were collected with a tracheal swab, placed in RNA later (Life technologies, Carlsbad, California, USA) and stored at -80°C. Tracheal lavage fluids were collected in Eppendorf tubes that were disinfected with DEPC water and stored at -80°C.
Total RNA extraction
Total RNA was extracted with kit per manufacturer’s instructions (TaKaRa Biotech, China). Cell specimens were homogenized in ice-cold lysis buffer until all lumps disappeared. Homogenates were transferred to Eppendorf tubes, mixed with 1/10 volume of microRNA homogenate additive, and vortexed or inverted to mix. Solutions were incubated on ice for 10 min, and one volume of chloroform was added to the solution and vortexed for 30-60 s. The mixture was centrifuged at 10,000 rpm for 5 min at room temperature to separate the aqueous and organic phases. Supernatants were transferred to a new tube and 1.25 volumes of 100% ethanol were added. Lysate/ethanol mixtures were transferred to a filter cartridge and centrifuged at 10,000 rpm for 15 s. Filters were washed and RNA was recovered with 95°C eluent. Samples were stored at -80°C.
Determination of RNA concentration and purity
RNA concentration and purity were determined with a DU-640 DNA/RNA analyzer (Beckman, USA). A260/280 ratios of 1.8-2.1 were considered acceptable RNA purity.
qRT-PCR
qRT-PCR was performed with a TaqMan microRNA reverse transcription kit (Ambion), microRNA reverse transcription primers (Applied Biosystems), and U6B internal reference. Reactions included 0.15 μL dNTPs, 1 μL enzyme, 1.5 μL RTbuffer, 0.19 μL RNase inhibitor, 4.16 μL DEPC water, 3 μL primers, and 5 μL total RNA. Reactions were amplified in the PCR Thermal Cycler Dice (TaKaRa Biotech Co. Led, Code No. TP 600) for 30 min at 16°C, 30 min at 42°C, and 5 min at 85°C and stored at 4°C. Relative miR expression was calculated as: RQ=2·ΔΔCT, where ΔΔCT=CTmicroRNA-CTU6B (experimental group)/CTmicroRNA-CTU6B (control group).
Bronchial epithelial cell culture
Bronchial epithelial cell culture was performed according to the previous report [10]. A bronchial mucosa biopsy specimen was taken from one asthma patient and treated by tissue block culture method. The mucosal block was rinsed with L-15 medium (Life technologies, Carlsbad, California, USA), planted in a culture dish pre-coated with collagen (Sigma-Aldrich), and cultured with serum-free media (MCDB-115, Invitrogen Corporation, Carlsbad, California, USA). After primary bronchial epithelial cells proliferated, medium was digested. Analysis of CK-19 antibody showed the purity of bronchial epithelial cells was over 99%. Various concentrations (0, 25, 50, 100, and 200 ng/mL) of IL-13 were added to bronchial epithelial cell cultures.
Statistical methods
All analyses were performed with SPSS 17.0 statistical software (SPSS Inc, Chicago, Illinois, USA) and expressed as mean ± standard deviation (x̅±s). Comparisons between means in multiple groups and paired comparisons between groups (Student-Newman-Keuls method) were made with univariate analysis of variance. Hypothesis tests were two-sided (α=0.05) and P<0.05 was considered significant.
Results
Expression of miR-21 and miR-126 in bronchial epithelial cells
Total RNA extracted from human bronchial and tracheal epithelial cells had A260/280 absorbance ratios of 1.8-2.1 and showed three bands by agarose gel electrophoresis, indicating the RNAs were intact and high-quality. These samples therefore met the quality requirements for qRT-PCR. qRT-PCR analysis showed that miR-21 and miR-126 expression in bronchial epithelial cells was increased in the non-treatment and treatment groups compared to the normal group (Figures 1 and 2). However, expression of both miRs was significantly higher in the non-treatment group than the treatment group (P<0.05).
Figure 1.
qRT-PCR measurement of miR-21 and miR-126 expression in bronchial epidermal cells from non-treatment (A), treatment (B), and normal (C) patient groups.
Figure 2.
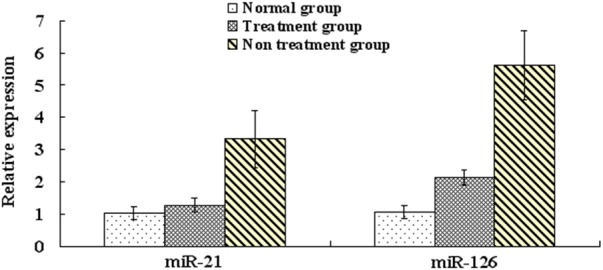
qRT-PCR measurement of miR-21 and miR-126 expression in bronchial epidermal cells from non-treatment, treatment, and normal patient groups.
Expression of miR-21 and miR-126 in bronchial epithelial cells treated with IL-13 in vitro
When bronchial epithelial cells were cultured in vitro with IL-13, qRT-PCR revealed a positive correlation between miR-21 and miR-126 expression and IL-13 concentration (Figure 3).
Figure 3.
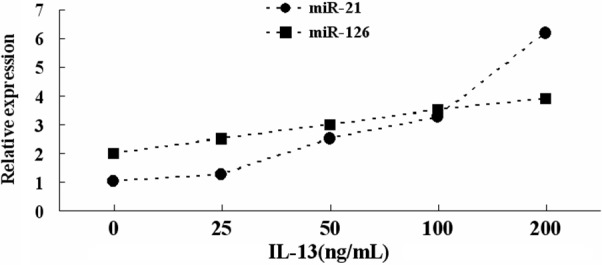
qRT-PCR measurement of miR-21 and miR-126 expression in bronchial epidermal cells treated with the indicated concentration of IL-13.
Discussion
As the innate immune defense of the airways, bronchial epithelial cells bear the full brunt of various asthma-inducing stimuli and respond by releasing inflammatory mediators and factors to contribute to asthma occurrence [11,12]. Normal non-asthmatic bronchial epithelial cells are also exposed to various asthma-inducing stimuli, but do not induce asthma. This may be because normal and asthmatic bronchial epithelial cells respond differently to the same environmental stimuli and release different inflammatory mediators and factors [13].
In an IL-13 transgenic mouse model of asthma, IL-13 induces increased miR-21 expression. miR-21 achieves its proinflammatory role by negatively regulating IL-12 [14]. IL-12 helps regulate Th1/Th2 balance in asthma, so decreased IL-12 expression may induce an excessive Th2 response, or conversely, increased IL-12 expression may induce a Th1 response. In a miR-21-null mouse model of asthma, allergen stimulation with ovalbumin (OVA) increases IL-12 expression, decreases pulmonary eosinophil accumulation, and increases Th1 response in comparison to a wild-type mouse model of asthma [14].
Collison et al. [15] showed that miR-126 expression is significantly increased in the airways of a house dust mite (HDM) mouse model of asthma. However, miR-126 expression does not increase after HDM stimulation in TLR4-null or MyD88-null mice. This suggests that TLR activation may be necessary for increased miR-126 expression. These results support a relationship between miR-126 and asthma, as both airway hyper-reactivity and immune cell migration are decreased in a miR-126-deficient HDM asthma model compared to wild-type asthmatic mice. Similar results are also observed for wild-type asthmatic mice treated with intranasal anti-miR-126. If these mice are challenged with HDM stimulation 24 h after intranasal anti-miR treatment, they exhibit decreases in airway hyper-reactivity, secretion amount, eosinophil/neutrophil accumulation, and secretion of Th2 cytokines IL-5 and IL-13. Similar results for the effect of anti-miR-126 on Th2 responses are also seen in OVA-induced asthma mouse models.
Compared to healthy non-asthmatic individuals, both miR-21 and miR-126 expression are increased in the bronchial epithelia of asthmatic patients with or without ICS treatment. In the non-ICS group, miR-21 and miR-126 expression is significantly higher than in the ICS group, and further, miR-21 and miR-126 expression in the bronchial epithelia increases with IL-13 concentration. These results suggest that miR-21 and miR-126 overexpression in the bronchial epithelia of asthma patients positively correlates with IL-13 as a major inflammatory factor inducing an asthma attack. Further, because ICS therapy can down-regulate miR-21 and miR-126 expression, miR expression can be a judgment index for the occurrence of asthma and its therapeutic efficacy. With increasing studies on the relationship between miRs and asthma, miR-21 and miR-126 could be considered therapeutic targets to provide ideas and strategies for asthma therapy.
Disclosure of conflict of interest
None.
References
- 1.Subbarao P, Mandhane PJ, Sears MR. Asthma: epidemiology, etiology and risk factors. CMAJ. 2009;181:E181–190. doi: 10.1503/cmaj.080612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Qi C, Yunxiao S. Mechanism of action of microRNA and its prospect for use in airway inflammation in asthma. Chinese Journal of Cellular and Molecular Immunology. 2013;29:1117–1119. [Google Scholar]
- 3.Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4encodes small RNAs with antisense complementary to lin-14. Cell. 1993;75:843–54. doi: 10.1016/0092-8674(93)90529-y. [DOI] [PubMed] [Google Scholar]
- 4.Kozomara A, Griffiths-Jones S. miRBase: integrating microRNA annotation and deep-sequencing data. Nucleic Acids Res. 2011;39:D152–157. doi: 10.1093/nar/gkq1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gurtan AM, Sharp PA. The role of miRNAs in regulating gene expression networks. J Mol Biol. 2013;425:3582–3600. doi: 10.1016/j.jmb.2013.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bandiera S, Hatem E, Lyonnet S, Henrion-Caude A. microRNA in disease: from candidate to modifier genes. Clin Genet. 2010;77:306–313. doi: 10.1111/j.1399-0004.2010.01370.x. [DOI] [PubMed] [Google Scholar]
- 7.Sayed D, Abdellatif M. MicroRNAs in development and disease. Physiol Rev. 2011;91:827–887. doi: 10.1152/physrev.00006.2010. [DOI] [PubMed] [Google Scholar]
- 8.Lu TX, Munitz A, Rothenberg ME. MicroRNA-21 is up-regulated in allergic airway inflammation and regulates IL-12p35 expression. J Immunol. 2009;182:4994–5002. doi: 10.4049/jimmunol.0803560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hansbro PM, Scott GV, Essilfie AT, Kim RY, Starkey MR, Nguyen DH, Allen PD, Kaiko GE, Yang M, Horvat JC, Foster PS. Th2 cytokine antagonists: potential treatments for severe asthma. Expert Opin Investig Drugs. 2013;22:49–69. doi: 10.1517/13543784.2013.732997. [DOI] [PubMed] [Google Scholar]
- 10.Genquan Y, Shunying Z, Suping G. The role of Galectin-7 in apoptosis of bronchial epithelial cells in children with asthma. Chinese Journal of Pediatrics. 2006;44:523–525. [Google Scholar]
- 11.Holgate ST, Holloway J, Wilson S, Bucchieri F, Puddicombe S, Davies DE. Epithelial-mesenchymal communication in the pathogenesis of chronic asthma. Proc Am Thorac Soc. 2004;1:93–98. doi: 10.1513/pats.2306034. [DOI] [PubMed] [Google Scholar]
- 12.Holtzman MJ, Morton JD, Shornick LP, Tyner JW, O’Sullivan MP, Antao A, Lo M, Castro M, Walter MJ. Immunity, inflammation, and remodeling in the airway epithelial barrier: epithelial-viral-allergic paradigm. Physiol Rev. 2002;82:19–46. doi: 10.1152/physrev.00020.2001. [DOI] [PubMed] [Google Scholar]
- 13.Davies DE. The bronchial epithelium: translating gene and environment interactions in asthma. Curr Opin Allergy Clin Immunol. 2001;1:67–71. doi: 10.1097/01.all.0000010987.09561.65. [DOI] [PubMed] [Google Scholar]
- 14.Lu TX, Hartner J, Lim EJ, Fabry V, Mingler MK, Cole ET, Orkin SH, Aronow BJ, Rothenberg ME. MicroRNA-21 limits in vivo immune response-mediated activation of the IL-12/IFN-gamma pathway, Th1 polarization, and the severity of delayed-type hypersensitivity. J Immunol. 2011;187:3362–3373. doi: 10.4049/jimmunol.1101235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Collison A, Herbert C, Siegle JS, Mattes J, Foster PS, Kumar RK. Altered expression of microRNA in the airway wall in chronic asthma: miR-126 as a potential therapeutic target. BMC Pulm Med. 2011;11:29. doi: 10.1186/1471-2466-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]