Summary
The analyses of protein-protein interactions is crucial for understanding cellular processes including signal transduction, protein trafficking and movement. Protein fragment complementation assays are based on the reconstitution of protein function when non-active protein fragments are brought together by interacting proteins that were genetically fused to these protein fragments. Bimolecular fluorescence complementation (BiFC) relies on the reconstitution of fluorescent proteins and enables both the analysis of protein-protein interactions and the visualization of protein complex formations in vivo. Transient expression of proteins is a convenient approach to study protein functions in planta or in other organisms, and minimizes the need for time-consuming generation of stably expressing transgenic organisms. Here we describe protocols for BiFC analyses in Nicotiana benthamiana and Arabidopsis thaliana leaves transiently transformed by Agrobacterium infiltration. Further we discuss different BiFC applications and provide examples for proper BiFC analyses in planta.
Keywords: Protein fragment complementation, bimolecular fluorescence complementation, protein-protein interactions, Agrobacterium infiltration
1. Introduction
The investigation of protein-protein interactions (PPIs) is essential for the understanding of nearly all cell biological processes. PPI analyses can be performed either in silico, in vitro or in vivo using different methods (summarized in 1, 2). In silico predictions strongly rely on the quality of prediction programs and their algorithms, and provide only an indication for a possible PPI that needs to be further confirmed. Even literature curated datasets are possibly of low quality and error-prone (3). In vitro PPI analyses in particular calorimetric methods (4), surface plasmon resonance (5) or atomic force microscopy (6) enable the measurement of thermodynamic constants of certain PPIs. However, the most dramatic disadvantage of in vitro PPI analyses is that the biochemical and physiological context within a cell cannot be ideally simulated and that the cellular compartmentation is absent. Loss of compartmentation is the main reason for false positive interactions in in vitro PPI analyses (2).
Methods to study PPIs in vivo are based on genetically encoded reporters and rely on fluorescence resonance energy transfer (FRET), bioluminescence resonance energy transfer (BRET), Ca2+/Annexin mediated translocation of protein complexes (7) or protein fragment complementation assays (PCA; 8, 9). For FRET/BRET analyses, proteins of interest are fused to donor and acceptor chromophore pairs with distinct spectral properties (FRET: CFP/YFP variants and GFP/RFP variants [10–12]; BRET: RLuc/YFP variants [13, 14]). In case of a PPI a dipole-dipole resonance mediated energy transfer from the donor- to the acceptor-chromophore occurs leading to an enhanced acceptor emission and to a reduced donor emission and lifetime of its excited state (15). An alternative for FRET/BRET assays is the Ca2+ dependent translocation of protein complexes where one bait protein is fused to Annexin A4 and a fluorescent protein (FP) and its target proteins are fused to FPs with different spectral properties compared to the bait-FP (7). Elevated intracellular Ca2+ levels induce the translocation of Annexin A4 from the cyto- and nucleoplasm to the plasma- and nuclear membrane thereby translocating bait and target proteins. This approach was conducted to visualize the translocation of a four-component protein complex (7).
In PCAs, interaction of protein pairs, which were genetically fused to non active fragments of a reporter gene, reconstitutes the function of this reporter gene. Methods based on protein fragment complementation have either a direct or indirect readout and are reversible or irreversible. PCAs with an indirect readout are the yeast-two-hybrid (Y2H) and split-ubiquitin system (SUS). In Y2H assays PPI pairs are fused to the binding- and activation domain of a transcription factor and positive interaction and translocation of the reconstituted transcription factor to the nucleus induces the activation of a reporter gene (16). The two-hybrid technology has also been applied to Arabidopsis protoplasts (17). SUS is used for PPI analyses of membrane proteins. Here, PPI pairs are respectively fused to the N- and C-terminus of ubiquitin, of which the latter is also fused to a transcription factor. Interaction induces the reconstitution of ubiquitin and subsequent proteolysis by an ubiquitin specific protease enables the transcription factor release from the membrane into the nucleus and the induction of reporter gene expression (18, 19). An alternative method that also can be applied to membrane and soluble proteins is based on split tobacco etch virus protease (split TEV; 20).
PCAs with a direct readout use the reconstitution of an enzyme or a chromophore. Split enzyme assays use fragments of β-galactosidase (21), dihydrofolate reductase (22, 23) or β-lactamase (24–26). Positive interactions induce the catalysis of a specific substrate that can be monitored in a colorimetric assay or by antibiotics resistance. Bioluminiscence complementation (BiLC) relies on the reconstitution and activity of a luciferase as a readout. The splicing based split luciferase system combines split luciferase with split intein (DnaE), where PPI induces the splicing and ligation of luciferase fragments to recover an irreversible luciferase activity (27). Reversible split luciferase assays have also been developed for luciferases with different emission properties and from different species (28–32) and have been successfully applied to plants (33–35). Split luciferase assays have two drawbacks, namely the need for coelenteracin/luciferin as an externally applied substrate and a low sub-cellular resolution, due to the low intensity of this bioluminescence reporter, which usually requires integrated signal detection from whole tissues, or groups of cells.
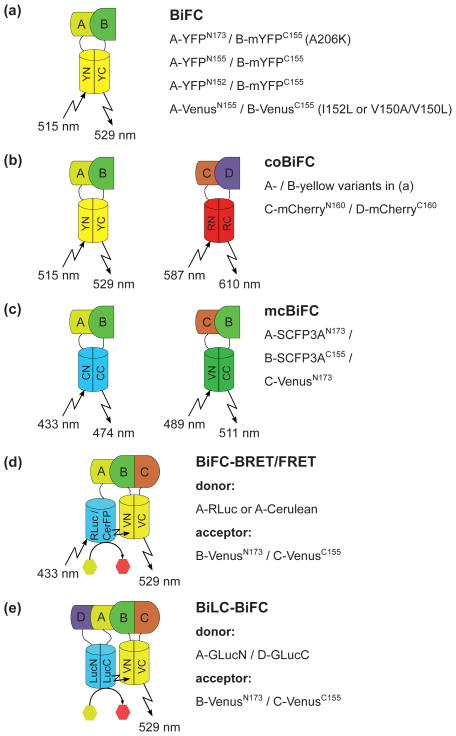
Bimolecular fluorescence complementation (BiFC) is based on the reconstitution of N- and C-terminal fragments of green fluorescent protein (GFP, cut between amino acids Gln157 and Lys158; 36) or of enhanced yellow fluorescent protein (eYFP, cut between amino acids Ala154 and Asp155 or between Glu172 and Asp173; 37, 38, Figure 1a) as a reporter for PPI. Interaction of protein pairs that are fused to the FP fragments results in fluorescence complementation thereby enabling the visualization of complex formation with subcellular resolution. One characteristic of BiFC is its irreversibility (9, 39), which is in some cases considered as drawback because of possible unspecific FP assembly that results in higher background signals. By introducing point mutations (I152L or V150A/V150L) into Venus (40) or by using a YFP N-terminal fragment cut after Ile152, BiFC signal/noise-ratio could be improved (41–44). However, the impossibility of FP fragment dissociation stabilizes weak or transient interactions which are not detectable when reversible methods for PPI analyses are used (9, 39). BiFC has been extensively applied to many living systems (summarized in http://sitemaker.umich.edu/kerppola.lab/kerppola.bifc/bifc_results; 45) including plants (summarized in 15, 46, 47). A whole spectrum of FPs can be used for BiFC analyses. These are CFP and its improved variants Cerulean and SCFP3A (cyan; 38, 48, 49), GFP, super folder GFP (sfGFP) and mKG2 (green; 36, 50–55), YFP, super folder YFP and the YFP variant Venus (37, 38, 48, 49, 56–58), mDsRed, mRFP, mCherry and mLumi (red; 59–63) and finally the reversible photo-switching GFP-like FP Dronpa (64). The simultaneous use of FPs with different spectral properties, e.g. CFP-, GFP- or YFP-BiFC combined with mDsRed-BiFC, enables the visualization of different protein complexes at the same time and in the same cell (62). We call this application coBiFC for co-localization analyses of protein-BiFC complexes (Figure 1b). Besides coBiFC, multicolor BiFC (mcBiFC) visualizes the complex formations of alternative interaction partners (9). In mcBiFC assays protein A is fused to the C-terminal fragment of CFP-(CFPC) or brighter variants and alternative interaction partners B and C are fused to the N-terminal fragment of CFP-(CFPN) and YFP-(YFPN) variants, respectively (38, 48, 49). Interaction of A–B reconstitutes CFP fluorescence (cyan, 474 nm), whereas A–C interaction leads to CFPC-YFPN assembly with intermediate (green, 511 nm) emission maximum (Figure 1c). mcBiFC was more efficient when FPs were separated between amino acids Glu172 and Asp173 compared to Asp155 (38). Co-expression of interacting proteins tagged to YFP- or Venus fragments with one interacting protein tagged to Renilla reniformis luciferase or the FP Cerulean enables BiFC based BRET or FRET analyses for the analyses of three component protein complexes (65–68, Figure 1d). Even further, combination of BiLC and BiFC based BRET, in which reconstituted Gaussia princeps luciferase was used for BRET on reconstituted YFP enabled analyses of four component protein complexes (69, Figure 1e). FRET between two complemented FPs (e.g. Venus and mCherry) may also allow the visualization of such four component protein complexes (70). Other applications of BiFC are ubiquitin-mediated fluorescence complementation (UbFC; 71, 72), the visualization of nucleic acid-protein interactions (73–76), the analysis of protein architecture or folding (77, 78), protein topology (79), protein co-expression (80) and the isolation of BiFC stabilized complexes (iBiSC; 74). Finally the combination of BiFC with fluorescence-activated cell sorting (BiFC-FC) could provide a tool for high throughput screening of weak PPIs (42, 81).
Figure 1.
Different examples for BiFC applications. BiFC can be applied to investigate (a) interactions of single PPI pairs (BiFC), (b) co-localization of different PPI pairs (coBiFC), (c) competition of two protein complex formations (mcBiFC), (d) analyses of ternary protein complex formations (BiFC based FRET or BRET) or (e) the analysis of quaternary protein complexes (by combining BiLC and BiFC). The protein fragment and/or donor/acceptor combinations recommended for each application and the resulting excitation and emission maxima or substrate catalysis of the respective (complemented) proteins are indicated. The proteins to be investigated for PPI are labeled from A – D. For more information refer to main text.
The most common methods to study BiFC in planta employ protoplast transfection of tobacco or Arabidopsis, particle bombardment of onion or tobacco epidermal cells or Agrobacterium infiltration of tobacco, mostly Nicotiana benthamiana (summarized in 15, 46, 47). Protocols for such assays are available (82–89). However, to our knowledge, there are only two reports describing BiFC in Arabidopsis plants that were transiently transformed by Agrobacterium infiltration (56, 90). There is a whole palette of vectors with different FP fragments available to perform BiFC analyses in plants (49, 53, 56, 57, 59, 62, 63, 90–93). However, different BiFC applications may require the use of different FPs and their N- and C-terminal fragments (see Figure 1). Here we present protocols for BiFC assays in Nicotiana benthamiana and Arabidopsis thaliana using the Agrobacterium infiltration method (adapted from 82, 94, 95).
2. Materials
2.1 BiFC vectors and constructs
PPI pairs of interest should be cloned into appropriate vectors that enable the intended BiFC application (see Figure 1; Note 1). The use of BiFC vectors that harbor an epitope tag between FP fragment and cloning site, which enables western blot analyses as expression control in case of negative BiFC results is recommended (49, 56, 57, 59, 63, 93). However GFP antibodies are also suitable for detecting FP N-terminal fragments (56, Figure 2).
Vectors with reference FP (e.g. CFP or RFP variants) with the same vector backbone than the BiFC constructs (Note 2).
Reagents, primers and DNA-Polymerase for PCR-based analyses of BiFC constructs.
Figure 2.
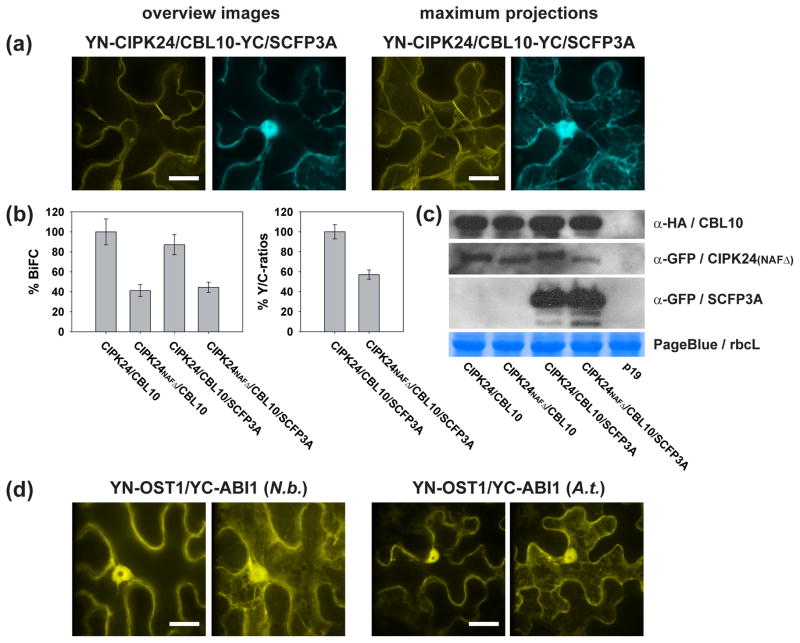
BiFC analyses in Nicotiana benthamiana and Arabidopsis. (a) Representative images of YN-CIPK24/CBL10-YC BiFC (yellow, tonoplast) co-expressed with SCFP3A (cyan, cytoplasm and nucleus) in N. benthamiana. Left panel, overview images and right panel, maximum projections of a z-stack including 32 focal planes of the same cell (60× objective, scale = 20 μm). (b) Normalized YFP fluorescence (BiFC) or BiFC/SCFP3A (Y/C)-ratios from randomly taken images of epidermal cells infiltrated with the indicated BiFC construct combinations using a 20× objective 3 days after infiltration into N. benthamiana. Given are averages ± standard errors (n = 10). Compared to CIPK24/CBL10, expression of CIPK24NAFΔ/CBL10 exhibited only 41 – 57 % BiFC. (c) Western blot analyses of BiFC construct/SCFP3A combinations from the same experiments as in (a and b). From top to bottom, CBL10-YC detected with α-HA antibody, YN-CIPK24(NAFΔ) detected with α-GFP antibody, SCFP3A detected with α-GFP antibody and PageBlue staining of the large RuBisCO subunit (rbcL) in representative SDS-gel as loading control. Loaded were 10 μg total protein for CBL10 and rbcL and 15 μg for CIPK24(NAFΔ) and SCFP3A blots. In the p19 lane protein extracts of only with p19 infiltrated leaves were loaded. Western blots confirm expression of BiFC and SCFP3A constructs in indicated construct combinations. (d) BiFC analyses of YN-OST1/YC-ABI1 in N. benthamiana (N.b.; left panel) and Arabidopsis (A.t.; right panel). Depicted are overview images (left) and maximum projections of 32 focal plane z-stacks (right). Images were taken with a 60× objective 4 (N.b.) and 7 days (A.t.) after infiltration. Scalebars = 20 μm. BiFC analyses indicate OST1/ABI1 complex formations in the cytoplasm and the nucleus.
2.2 Agrobacterium tumefaciens strain and media for Agrobacterium culture and infiltration
Electro-competent Agrobacterium tumefaciens GV3101 (pMP90) (96) or other Agrobacterium strains.
p19 silencing suppressor strain (97).
2 M NaOH.
1 M KOH and 5 M KOH.
1 M MgSO4 (autoclave and store a 4 °C).
1 M MgCl2 (autoclave and store a 4 °C).
1 M 2-(N-morpholino) methanesulfonic acid (MES). Adjust to pH 5.6 with 5 M KOH and filter sterilize through a 0.45 μm filter.
3,5-dimethoxy-4-hydroxyacetophenone (Acetosyringone) 200 mM in dimethyl sulfoxide (DMSO). Store 500 μL aliquots at − 20 °C.
YEB medium: 5 g/L sucrose, 5 g/L beef extract, 1 g/L yeast extract, 1 g/L peptone, 2 mM MgSO4 (add from sterile 1 M stock after autoclaving). Adjust to pH 7.4 with 2 M NaOH and autoclave. For solid medium add 20 g/L agar, autoclave and pour into petri dishes.
Infiltration medium for N. benthamiana infiltration: 10 mM MES pH 5.6, 10 mM MgCl2, 150 μM Acetosyringone (dilute from stocks).
Induction medium for Arabidopsis infiltration: 10.5 g/L K2HPO4, 4.5 g/L KH2PO4, 1 g/L (NH4)2SO4, 0.5 g/L NaCitrate, 1 g/L glucose, 1 g/L fructose, 4 g/L glycerol, 1 mM MgSO4, 10 mM MES. Adjust pH to 5.6 with 1M KOH and autoclave. Before use add antibiotics (see 2.3) and 100 μM Acetosyringone (dilute from stocks).
Infiltration medium for Arabidopsis infiltration: 10 mM MES pH 5.6, 10 mM MgSO4, 200 μM Acetosyringone (dilute from stocks).
2.3 Antibiotics
Rifampicin: 50 mg/mL stock in DMSO. Store 1 mL aliquots at − 20 °C, working concentration 100 μg/mL.
Kanamycin: 100 mg/mL stock in H2O. Store 1 mL aliquots at − 20 °C, working concentration 25 μg/mL.
Gentamycin: 50 mg/mL stock in H2O. Store 1 mL aliquots at − 20 °C, working concentration 25 μg/mL.
2.4 Plants and materials for plant culture
Nicotiana benthamiana seeds.
Arabidopsis thaliana seeds (Note 3).
Plant pots with 5 – 6 cm diameter for Arabidopsis and with 8 – 9 cm diameter for N. benthamiana, trays and plastic covers.
Soil.
Fertilizer.
Fungicide (optional).
Insecticide (optional).
Ultra pure H2O (e.g. Milli Q) autoclaved.
100 % ethanol.
10 % sodium dodecyl sulfate (SDS, sterilized through a 0.45 μm syringe filter).
Seed sterilization solution: 70 % ethanol, 0.04 % SDS.
1 M KOH
0.5 MS agar plates: half concentrated Murashige and Skoog basal medium (98) with Gamborg B5 vitamins adjusted to pH 5.8 with 1 M KOH and supplemented with 0.8 % agar. Autoclave, pour into petri dishes and store at 4 °C.
2.5 Media for protein extraction, SDS-PAGE and western blotting (after 99)
Ultra pure H2O.
Protein extraction buffer: 100 mM Tris (hydroxymethyl) aminomethane (Tris)/HCl pH 8 (1 M stock), 100 mM NaCl (1 M stock), 5 mM EDTA (0.5 M stock, adjust to pH 8 with NaOH tablets), 5 mM EGTA (0.5 M stock, adjust to pH 8 with NaOH tablets), 20 mM DTT (1–2 M stock, − 20 °C), 1× protease inhibitor mix (Roche 11873580001, 25× stock, − 20 °C), 0.5 % Triton X-100 (10 % stock). Prepare fresh before use.
1× SDS sample buffer: 62.5 mM Tris/HCl pH 6.8, 2 % SDS, 5 % β-mercaptoethanol, 10 % glycerol, 0.002 % bromophenol blue. Store 1 mL aliquots at − 20 °C.
4× SDS sample buffer: 250 mM Tris/HCl pH 6.8, 8 % SDS, 20 % β-mercaptoethanol, 40 % glycerol, 0.008 % bromophenol blue. Store 1 mL aliquots at − 20 °C.
Amidoblack staining solution: 10 % acetic acid, 90 % methanol, 0.1 % Amidoblack filtered through a flute filter and stored at 4 °C.
Amidoblack de-staining solution: 10 % acetic acid, 90 % methanol (store at 4 °C).
0.2 M NaOH.
Bovine serum albumin.
1.5 M Tris/HCl pH 8.8 (degas and store at 4 °C).
0.5 M Tris/HCl pH 6.8 (degas and store at 4 °C).
20 % SDS.
40 % Acrylamide/Bisacrylamide solution (37.5:1, store at 4 °C).
N,N,N′,N′-tetramethyl-ethane-1,2-diamine (TEMED, store at 4 °C).
10 % ammonium persulfate (store 1 mL aliquots at − 20 °C).
70 % ethanol.
Acetone.
Isopropanol.
Methanol.
SDS running buffer: 5g/L Tris, 14.4 g/L glycine, 1g/L SDS. Prepare 10× solution and dilute before use.
Blotting buffer: 24 mM Tris, 192 mM glycine, 20 % ethanol. Prepare 5× solution without ethanol and filter sterilize through a 0.45 μm filter. Dilute before use with ultra pure H2O and 100 % ethanol to final concentration.
10× Tris buffered saline (TBS): 100 mM Tris/HCl pH 7.4, 1.5 M NaCl. Filter sterilize through a 0.45 μm filter.
Western blot blocking buffer: 1× TBS, 0.05 % Tween 20, 5 % non fat dry milk powder.
Western blot washing buffer (TBS-T): 1× TBS, 0.05 % Tween 20.
Coomassie brilliant blue (CBB) staining solution: 50 % methanol, 10 % acetic acid, 0.25 % Coomassie brilliant blue R-250. Filtrate through a flute filter and store at 4 °C. Alternatively to CBB staining PageBlue (http://www.fishersci.com) could be used for SDS-gel staining.
Coomassie de-staining solution: 50 % methanol, 10 % acetic acid (store at 4 °C).
Enhanced chemo luminescence (ECL) solution A: 0.1 M Tris/HCl pH 8.6, 0.25 mg/mL luminol (store at 4 °C).
ECL solution B: 1.1 mg/mL para-hydroxycoumaric acid in DMSO.
30 % (8.8 M) H2O2 (store at 4 °C).
ECL developing solution: use for one 7 cm × 9 cm PVDF membrane 2 mL ECL solution A, 200 μL ECL solution B and 0.6 μL 30 % H2O2 (prepare fresh before use).
Precission plus dual-color protein standard (http://www.bio-rad.com/).
Monoclonal HA antibody (http://www.covance.com/). Prepare 15 mL of 1:4000 dilution in TBS-T, 0.05 % NaAcide (reusable, store at 4 °C).
Monoclonal c-myc antibody (http://www.cellsignal.com/; http://www.invitrogen.com/). Prepare 15 mL of 1:4000 dilution in TBS-T, 0.05 % NaAcide (reusable, store at 4 °C).
Monoclonal FLAG M2 antibody (http://www.sigmaaldrich.com/). Prepare 15 mL of 1:4000 dilution in TBS-T, 0.05 % NaAcide (reusable, store at 4 °C).
Monoclonal GFP antibody (Zymed; http://www.invitrogen.com/). Prepare 15 mL of 1:4000 dilution in TBS-T, 0.05 % NaAcide (reusable, store at 4 °C).
Secondary goat anti mouse IgG (H + L) horseradish peroxidase-conjugate antibody (http://www.bio-rad.com/). Prepare 15 mL of 1:10000 dilution and discard after use.
2.6 Consumables
10 μL, 200 μL, 1 mL and 5 mL pipette tips.
Plastic transfer pipettes.
PCR tubes.
1.5 mL and 2 mL reaction tubes.
Sterile 15 mL and 50 mL Falcon tubes.
24 well plates.
1 mL plastic cuvettes.
Electroporation cuvettes.
Petri dishes.
Razor blades.
Microscope slides and cover-glass.
Kimwipes.
Sterile paper covered in aluminum-foil and autoclaved.
Sterile toothpicks stored in 100 mL glass beaker covered with aluminum-foil and autoclaved.
Micropore surgical tape.
Examination gloves.
Plastic syringes without needle (1 and 60 mL).
Fluted filters.
Sterile 0.45 μm syringe filters.
Sterile 0.45 μm filters with bottles (0.5 or 1 L).
Polyvinylidene fluoride (PVDF) Immobilon-Transfer Membrane, 0.2 μm pore size.
Whatman chromatography paper.
X-ray Films.
Parafilm.
Tape.
Cling film.
Cellophane.
Paper towels.
2.7 Equipment
Pipettes (2 μL, 20 μL, 100 μL, 200 μL, 1000 μL and 5000 μL).
Stirring bars.
Forceps.
Scalpel.
Scissors.
Cork borer.
Dewar flask.
Styropore box.
Grinding balls for Retsch Mill.
Autoradiography cassette.
Systems for protein electrophoresis, blotting and SDS-gel drying.
Electrophoresis power supply.
Electroporator.
Heat block for 1.5 and 2 mL tubes adjustable to 95 °C.
28 °C incubator with shaking device.
Platform shaker.
Vortex.
Stirrer/Heater combination.
Microwave.
Sonicator.
PCR machine.
Centrifuge for 1.5 and 2 mL tubes ± cooling device.
Centrifuge with swinging bucket rotor and adaptors for 15 mL tubes.
Retsch Mill with buckets resistant to liquid N2 (http://www.retsch.com/).
Spectrophotometer.
X-ray film developing machine.
Scanner.
Ice machine.
Inverted fluorescence microscope or spinning disc confocal microscope equipped with appropriate excitation and emission filters or laser units to image CFP, YFP and RFP, with 20×, 40× and 60× objectives and a CCD camera.
Sterile hood.
Greenhouse and/or plant growth device to maintain stable growth conditions (e.g. growth room or growth chamber) to enable light intensities of 50 – 120 μE m−2 s−1 under long day (16 h light/8 h dark) or short day (8 h light/16 h dark) conditions and 22 – 27 °C.
Freezer (− 20 °C and − 80 °C).
Fridge and/or cold room (4 °C).
Dark room.
2.8 Software
ImageJ (http://rsbweb.nih.gov/ij/) or Fiji (http://fiji.sc/wiki/index.php/Fiji).
Microsoft Excel or other software for data analyses and calculations.
3. Methods
3.1 Transformation and culture of Agrobacteria
Prepare for each transformation one 1.5 mL tube with 100 ng plasmid DNA (BiFC-constructs etc.), one electroporation cuvette and one 2 mL tube containing 1 mL YEB medium without antibiotics and place on ice.
Thaw for each transformation one 30–50 μL aliquot of electro-competent Agrobacteria, stored at − 80 °C, on ice.
Mix plasmid DNA and electro-competent Agrobacteria and transfer into electroporation cuvette.
Transfer cuvette into electroporator, apply a 5 ms electric pulse of 1.8 – 2.5 kV, immediately resuspend Agrobacteria in 1 mL YEB medium without antibiotics and transfer into 2 mL tube.
Shake Agrobacteria for 2 h at 28 °C and 180 – 220 rpm.
Plate 30 – 200 μL of Agrobacteria transformation on YEB agar plates containing appropriate antibiotics and incubate 2 days at 28 °C.
Re-strike four colonies of each transformation to fresh YEB agar plate containing appropriate antibiotics and incubate one additional day at 28 °C.
Re-confirm positive transformation of Agrobacteria by colony-PCR.
Inoculate positively transformed Agrobacteria in 3 mL YEB liquid medium containing appropriate antibiotics and shake at 28 °C and 180 – 220 rpm over night.
Mix 930 μL Agrobacteria culture with 70 μL DMSO in 2 mL tube, incubate on ice for 30 min, flash freeze in liquid N2 and store at − 80 °C. Alternatively, Agrobacteria could be stored on YEB agar plates with appropriate antibiotics at 4 °C if transferred monthly to a fresh plate.
3.2 Growing of Nicotiana benthamiana
Prepare six pots with watered soil containing fertilizer, fungicide and insecticide. Pots should be prepared one day before plant sowing.
Sow nine N. benthamiana seeds per pot using a toothpick and dip seeds gently into the soil. Cover pots with plastic cover to ensure high humidity and transfer to greenhouse or growth device (Note 4).
After seed germination, usually four days, remove plastic cover.
As soon as the first true leaves emerge (12 – 14 days), transfer seedlings to single pots and cover again for four days with plastic cover.
Grow N. benthamiana plants for 5 – 6 weeks and water every day or every second day. Plants should not flower at time point of infiltration.
3.3 Infiltration of Nicotiana benthamiana
Inoculate Agrobacteria harboring BiFC-constructs, a reference FP control (e.g. CFP or RFP variant) and the p19 strain in sterile 50 mL Falcon tubes containing 15 mL YEB medium and appropriate antibiotics and shake over night at 28 °C and 180 – 220 rpm. Do not close Falcon tube lid but prevent lid falling off by taping (Note 5).
Prepare for each Agrobacteria culture one 1 mL plastic cuvette with 900 μL YEB medium. Add 100 μL Agrobacteria culture to the respective cuvette and measure the optical density at 600 nm (OD600) with the spectrophotometer using a cuvette containing 1 mL YEB medium as blank.
Calculate the amount of Agrobacteria needed for a final 4 mL suspension per leaf using the formula Vculture= Vfinal × OD600 final/OD600. For Agrobacteria harboring BiFC and reference FP constructs standard OD600 final is 0.5 (Note 6). Standard OD600 final for the p19 strain is 0.3.
Using the calculated culture volumes (Vculture) and according to the intended BiFC application (see Figure 1) mix the respective Agrobacteria suspensions (except of the p19 strain) in a 15 mL Falcon tube. Transfer the total Vculture of the p19 strain, calculated for all samples, to additional 15 mL Falcon tubes. At this stage do not mix p19 culture with BiFC and FP cultures.
Spin cultures in swinging bucket rotor for 15 min at 4000 g.
Discard supernatant, resuspend BiFC and FP Agrobacteria culture mixes in 2 mL infiltration medium by gentle pipetting and add additional infiltration medium to reach 0.5 Vfinal. Resuspend p19 cultures by gentle pipetting in 2 mL infiltration medium and add additional infiltration medium to reach 0.5 Vfinal.
Mix BiFC and FP culture mix 1:1 (v/v) together with the p19 culture and incubate for 2 h at room temperature.
Select N. benthamiana plants of the same age and size and label for each construct combination the same leaf of individual plants for infiltration (Note 7).
Carefully cut infiltration points on abaxial leaf side by touching with a razor blade at different leaf regions.
Infiltrate Agrobacteria suspensions at infiltration points into the abaxial leaf side using a 1 mL syringe without needle and ensure that the complete leaf is infiltrated.
Return infiltrated N. benthamiana plants to growth device, water them and incubate for 2 – 5 days. Semiquantitative BiFC analyses should be performed on day 3 or 4 post infiltration.
3.4 Growing and preparation of Arabidopsis plants
Prepare 2 mL tubes with 20 – 50 μL Arabidopsis seeds, add 1 mL sterilization solution and shake or rotate gently for 10 min.
Wash seeds three times with 100 % ethanol and discard final ethanol.
Dry seeds in sterile hood.
Transfer sterile seeds in sterile hood to 0.5 MS agar plates using sterile toothpicks, close plates with micropore surgical tape and incubate for 3 – 4 days at 4 °C for stratification. Alternatively, seeds can be directly sowed on soil, covered with plastic cover and stratified for 3 – 4 days at 4 °C.
Transfer sown seeds to growth device and incubate for 5 – 7 days (Note 4).
Prepare pots with watered soil containing fertilizer, fungicide and insecticide. Pots should be prepared one day before plant transfer.
Transfer Arabidopsis seedlings to single pots and cover the plants for 4 days with plastic cover to maintain high humidity. Water plants if needed.
Grow Arabidopsis plants for a total of 3 – 4 weeks. Arabidopsis infiltration should be performed before flowering.
Stop watering plants 2 – 3 days prior to infiltration.
Keep plants under constant dark and high humidity conditions the evening before and until the actual infiltration procedure by covering them with a light impermeable tray.
3.5 Arabidopsis infiltration
Depending on the amount of Agrobacteria culture needed, inoculate Agrobacteria harboring BiFC-constructs and a reference FP control (e.g. CFP or RFP variant) either in sterile 50 mL Falcon tubes containing 15 mL YEB medium and appropriate antibiotics or in sterile 15 mL Falcon tubes containing 3 – 5 mL YEB medium and appropriate antibiotics and shake over night at 28 °C and 180 – 220 rpm. Do not close Falcon tube lid but prevent lid falling off by taping (Note 5).
Transfer 2 – 3 mL of Agrobacteria culture to sterile 15 mL Falcon tube and spin in swinging bucket rotor for 15 min at 4000 g.
Discard supernatant and resuspend Agrobacteria by gentle pipetting in 2 mL induction medium. Add additional 3 mL of induction medium and shake at 28 °C and 180 – 220 rpm for 5 – 6 h.
Prepare for each Agrobacteria culture one 1 mL plastic cuvette with 900 μL H2O. Add 100 μL Agrobacteria culture to the respective cuvette and measure OD600 with the spectrophotometer using a cuvette containing 1 mL H2O as blank.
Calculate the amount of Agrobacteria needed for a final 2 mL suspension using the formula Vculture= Vfinal × OD600 final/OD600. A 2 mL Agrobacteria suspension is sufficient for infiltration of 16 – 20 Arabidopsis leaves. OD600 final of 0.4 should be used as standard but could be adjusted (Note 6).
Using the calculated culture volumes (Vculture) and according to the intended BiFC application (see Figure 1) mix the respective Agrobacteria suspensions by transferring them into a 15 mL Falcon tube and spin in swinging bucket rotor for 15 min at 4000 g.
Discard supernatant, resuspend Agrobacteria by gentle pipetting in 2 mL infiltration medium and transfer to 2 mL tube.
Infiltrate Agrobacteria into the abaxial side of Arabidopsis leaves (4th – 6th leaf) using a 1 mL syringe without needle. Use the leaf tip as infiltration point as this enables the infiltration of the entire leaf at once. Infiltrate each construct combination into a total of eight Arabidopsis plants.
Transfer infiltrated Arabidopsis plants to growth device, water plants, additionally spray with water and use a plastic cover to maintain high humidity. Incubate plants for seven days and remove plastic cover after 24 h.
3.6 Microscopic imaging and semiquantitative BiFC analyses
Prepare 24 well plate for sample harvesting. Label wells and add 1 mL tap water to each well.
Punch out N. benthamiana leaf discs (without veins) of 14 mm diameter or cut off Arabidopsis leaves and transfer to 24 well plate. Harvest maximum four samples simultaneously. For sample comparison punch out leaf discs from the same leaf area of N. benthamiana leaves or harvest always the same leaf (e.g. leaf 5) from different Arabidopsis plants.
Place a drop of water on a microscope slide and transfer a leaf disc or leaf (sample) with the abaxial side up on water drop. If needed, cut off the mid vein of Arabidopsis leaves with a scalpel and place a second drop of water on the sample. Cover the sample with a cover-glass and ensure that there are no air-bubbles between sample and cover-glass by gently pushing with a forceps.
Mount the microscope slide onto an inverted fluorescence microscope or a spinning disc confocal microscope with the abaxial side of the sample towards the objective.
For semiquantitative fluorescence intensity measurements analyze samples with respective FP imaging settings using a 20× objective. Adjust exposure time, gain, etc. with a reference sample to obtain optimal imaging conditions (e.g. sample (a) in Note 5). Image all samples using identical settings for each FP channel.
Take minimum 10 images from different areas of each sample using both FP reference and BiFC settings. For background subtraction take also images of N. benthamiana samples infiltrated with p19 alone or from non-infiltrated Arabidopsis leaves. For N. benthamiana samples use bright field settings to select different leaf regions with a flat focal plane. In Arabidopsis select regions with flat focal plane and high reference FP expression.
Measure mean fluorescence intensities of entire images for each fluorescence channel using the microscope software or Fiji.
Calculate for every sample and FP channel the average mean intensities and standard errors. After background fluorescence subtraction, normalize BiFC fluorescence relative to reference sample (e.g. sample (a) in Note 5). In addition and after background subtraction calculate BiFC/reference FP-ratios for each image individually. Calculate average and standard error of resulting BiFC/reference FP-ratios and normalize values relative to reference sample (Note 8; Figure 2).
For qualitative high resolution BiFC analyses use a 60× objective and adjust exposure time, gain, etc. for each sample individually. As standard procedure record overview images and z-stacks of representative cells. Also convert z-stacks into .avi file and re-constitute 3D maximum projections (Note 9; Figure 2).
3.7 Protein extraction and protein quantification
Harvest two N. benthamiana leaf discs of 14 mm diameter or one Arabidopsis leaf from infiltrated plants and background controls into 2 mL tubes containing one grinding ball (sample) and freeze in liquid N2. Samples could be stored at − 80 °C for few days but immediate protein extraction is recommended.
Prepare protein extraction buffer and keep on ice.
Pre-chill Retsch Mill buckets in liquid N2, place liquid N2 frozen samples inside buckets and grind three times for 30 sec with a frequency of 30 s−1. Re-chill buckets with samples in liquid N2 between grinding steps.
Keep samples in cold buckets, until 100 μL protein extraction buffer was added for N. benthamiana or 75 μL for Arabidopsis samples and rotate samples for 10 min at 4 °C. (Note 10).
Sonicate twice for 10 sec (0.5 sec on/off) on ice.
Spin samples in a microfuge for 10 min at 14000 rpm and 4 °C.
Transfer supernatant to 1.5 mL tube and spin samples for 10 min at 14000 rpm and 4 °C.
Transfer supernatant to 1.5 mL tube, flash freeze in liquid N2 and store at − 80 °C.
Prepare one 1.5 mL tube with 195 μL H2O for each sample, add 5 μL of protein extract and 800 μL Amidoblack staining solution. Vortex vigorously and spin for 20 min at 14000 rpm.
Discard supernatant, add 1 mL Amidoblack de-staining solution, vortex and spin for 10 min at 14000 rpm. Repeat de-staining step.
Discard supernatant and air dry samples.
Resuspend by vortexing in 1 mL 0.2 M NaOH, transfer to 1 mL plastic cuvette and measure OD615 nm in spectrophotometer.
Calculate protein concentrations of each sample according to a BSA standard calibration.
3.8 SDS-gel preparation (prepare one day before use)
Rinse glass plates of gel-pouring system with water and acetone and dry with kimwipes. Clean spacers and combs with water and 70 % ethanol and dry with kimwipes.
Mount gel-pouring system.
Boil 0.375 M Tris/HCl pH 8.8 supplemented with 1 % Agarose in microwave and pour on large glass plate. Immediately place gel-pouring system on polymerizing solution to seal lower part of the gel-pouring system.
Prepare separating-gel solution in 50 mL Falcon tube. For one 8 mL SDS-gel with 10 % Acrylamide/Bisacrylamide use: 3.920 mL ultra pure H2O, 2 mL 1.5 M Tris/HCl pH 8.8, 2 mL 40 % Acrylamide/Bisacrylamide, 40 μL 20 % SDS.
To induce polymerization, add 4 μL TEMED and 40 μL 10 % APS, mix gently and pour into gel-pouring system. Leave space for the stacking-gel and comb. Carefully add 1 mL isopropanol to poured gel and incubate until the SDS separating-gel is polymerized, usually 45 – 60 min.
Prepare stacking-gel solution. For 2.75 mL stacking-gel use: 1.6 mL ultra pure H2O, 625 μL 0.5 M Tris/HCl pH 6.8, 500 μL 40 % Acrylamide/Bisacrylamide, 25 μL 20 % SDS.
Before pouring the stacking-gel, decant isopropanol from separating-gel and wipe off residual isopropanol with kimwipes.
Add 5 μL TEMED and 50 μL 10 % APS to stacking-gel solution, mix by gentle pipetting and pour over separating-gel. Immediately insert comb into gel-pouring system. Stacking-gel will polymerize within 2 min.
Wrap polymerized SDS-gels into wet paper towels and cling film and store at 4 °C.
3.9 SDS-PAGE and western-blotting
Calculate the volume of extracted protein for each sample to reach 15 μg total protein.
Thaw protein extracts on ice and transfer calculated protein amount to 1.5 mL tube. Add 4× SDS sample buffer to reach a final 1× concentration.
Label wells of SDS-gel(s) with a waterproof pen, mount gel(s) into electrophoresis system, add 1× SDS running buffer, remove combs of SDS-gels and wash wells with 1× SDS running buffer using a 200 μL pipette.
Boil protein samples 3 – 5 min in 95 °C heat-block, spin briefly and place on ice.
Load protein samples together with dual-color protein marker on SDS-gel(s), and run at maximum voltage and 20 mA/SDS-gel constant current until the bromophenol blue band reaches the separating gel, then run at constant current of 30 mA/SDS-gel until the bromophenol blue band reaches the bottom end of the SDS-gel(s).
While SDS-PAGE is running, prepare an appropriate volume of blotting buffer, PVDF-membranes and Whatman papers with appropriate SDS-gel size.
After run unmount SDS-gel(s), remove stacking-gel(s) and incubate separating-gel(s) 10 min in blotting buffer.
Activate PVDF-membranes 30 sec in methanol, wash briefly in ultra pure H2O and incubate in blotting buffer.
Mount western-blot(s) according to manufacturer’s instructions, usually in a sandwich of Whatman paper(s), SDS-gel, PVDF-membrane and Whatman paper(s). SDS-gel should be placed on the cathode side and PVDF-membrane on the anode side.
Run western blot(s) 2 h at constant current of 50 mA.
Prepare blocking-buffer and heat up while stirring to solubilize the fat free milk powder. Allow cooling down before use. Up to three PVDF-membranes can be blocked in 100 mL blocking buffer.
After run unmount western blot(s) and check successful blotting by visualization of the dual-color protein marker on the PVDF-membrane(s).
Block PVDF-membrane(s) with gentle shaking in blocking buffer for 1 h at room temperature or over night at 4 °C.
Wash SDS-gel(s) with gentle shaking for 10 min in ultra pure H2O, discard H2O and stain SDS-gel(s) in Coomassie staining-solution or PageBlue for 3 – 4 h or overnight (for Coomassie staining move to 25.).
After blocking, wash PVDF-membrane(s) with gentle shaking three times for 5 min in TBS-T.
Discard washing solution and bind primary antibodies (monoclonal HA-, c-myc-, FLAG- or GFP-antibody diluted 1:4000) to membrane(s) by gentle shaking for 2 h at room temperature. Binding of HA-, c-myc- and GFP-antibodies can be extended over night at 4 °C.
Recycle primary antibodies and wash PVDF-membrane(s) three times for 5 min in TBS-T with gentle shaking.
Discard washing solution and bind secondary antibody (goat anti mouse horseradish peroxidase-conjugate diluted 1:10000) by gentle shaking for 45 min at room temperature.
Discard secondary antibody and wash PVDF-membrane(s) three times for 5 min in TBS-T with gentle shaking.
While washing the membranes prepare ECL developing-solution and keep in 15 mL Falcon tube wrapped in aluminum foil.
Move to the dark room and briefly dry PVDF-membrane(s) from washing buffer by placing them on kimwipes. Place membrane(s) on cling film and add 2 mL ECL developing solution to each membrane. Embed membrane(s) in cling film to ensure even distribution of ECL developing solution and incubate 2 min in the dark.
Briefly dry PVDF-membrane(s) from ECL developing solution by placing them on kimwipes. Transfer membrane(s) to autoradiography cassette and in the dark place one x-ray film into cassette, close cassette tightly and incubate for 5 min. Incubation time can be adjusted depending on the strength of the detection signal.
Develop x-ray film in the dark using the developing machine.
After developing, label protein marker bands with waterproof pen on developed film. Scan developed x-ray film and save as .tiff file.
Recycle Coomassie staining or PageBlue solution and wash SDS-gel(s) twice with respective de-staining solution until desired contrast is reached. Dry SDS-gel(s) with gel drying system.
Scan SDS-gel(s) and save scans as .tiff file. Dependent on the manufacturer, PVDF-membrane(s) can be Coomassie stained after western blot development by shaking for 1 – 2 h in Coomassie staining-solution. After staining wash membranes two times in de-staining solution, dry on kimwipes, scan and save scan as .tiff file.
Acknowledgments
We thank Alex Costa for critical suggestions with the Arabidopsis infiltration protocol, Katrin Held for providing the YC-ABI1 construct and Jan Niklas Offenborn for providing the OST1 construct. This work was supported by a Feodor Lynen-fellowship from the Alexander von Humboldt-Foundation to RW, a National Institutes of Health grant R01GM060396 to J.I.S. and grants from the DFG (SFB629 and FOR964) to JK.
Abbreviations
- BiFC
bimolecular fluorescence complementation
- BiLC
bimolecular luminescence complementation
- BRET
bioluminescence resonance energy transfer
- CBB
Coomassie brilliant blue
- CFP
cyan FP
- coBiFC
co-localization of BiFC complexes
- ECL
enhanced chemo luminescence
- FP
fluorescent protein
- FPC
FP C-terminal fragment
- FPN
FP N-terminal fragment
- FRET
fluorescence resonance energy transfer
- GFP
green FP
- iBiSC
isolation of BiFC stabilized complexes
- mcBiFC
multicolor BiFC
- OD600
optical density at 600 nm
- PCA
protein fragment complementation assay
- PPI
protein-protein interaction
- PVDF
polyvinylidene fluoride
- RFP
red FP
- RLuc
Renilla reniformis luciferase
- SUS
split-ubiquitin system
- TEV
tobacco etch virus protease
- UbFC
ubiquitin-mediated fluorescence complementation
- Y2H
yeast-two-hybrid
- YFP
yellow FP
Footnotes
Before generating BiFC-fusion constructs, use web resources to analyze topology, sub-cellular localization and targeting signals of proteins of interest (e.g. http://aramemnon.botanik.uni-koeln.de/ [100]; http://www.expasy.ch/ [101] and links therein). Fusion of BiFC-tags to the wrong terminal end could mask targeting signals and affect protein localization. On the other hand, expression levels of some proteins are drastically reduced when a certain tag orientation is used. It is recommended to generate full-length FP fusion constructs in a vector backbone similar to the BiFC-vectors used and to analyze proper localization and expression of fusion proteins before generating the BiFC constructs. If expression and proper localization could be confirmed by microscopic analyses the use of a similar BiFC-tag orientation is recommended. If no information about the protein localization is available, generate FP fusion constructs with respective N- and C-terminal FP-tag orientations (e.g. CFP-query and query-YFP) and perform co-localization analyses. If both tag-orientations are possible, use C-terminal BiFC-tag fusions (query-BiFC) as N-terminal BiFC-tag fusions may result in higher background signals (Waadt and Kudla, unpublished results).
Proper controls are the key for adequate semiquantitative BiFC analyses as BiFC is irreversible and may stabilize randomly formed protein complexes (9, 39). The best negative control is the use of a different family member of one of the investigated proteins. Point mutations that disrupt interaction or deletion constructs of the interacting domain may also be suitable. It is important that the negative control is localized in the same cellular compartment and exhibits a similar expression level compared to the reference protein. The use of empty-vector controls is not recommended as the expression of FP-fragments alone does not fulfill the requirements mentioned above.
In order to suppress plant immune responses one might also consider co-expression of the Pseudomonas syringae type III effector AvrPto (102).
Additional to the infiltration of wild type plants, BiFC experiments could also be conducted in mutant plants, in GVG-AvrPto plants (102) that enable dexametasone inducible immune response suppression or in the rdr6-11 mutant (103) that is deficient in post translational gene silencing.
N. benthamiana plants can be either grown in the greenhouse or in a growth room with long day conditions, 90 – 120 μE m−2 s−1 light intensity and 22 – 27 °C.
Optimal growth conditions for Arabidopsis plants are short day conditions (8 h light/16 h dark) with 90 μE m−2 s−1 light intensity at 23 °C (94, 95). However, transient expression is also achieved in long day conditions (16 h light/8 h dark) with 50 – 80 μE m−2 s−1 light intensity at 27 °C. Most important for achieving high protein expression levels, is the use of young leaves of plants in the pre-bolting stage. Two to three days prior to infiltration Arabidopsis plants should be stopped watering and the evening prior infiltration plants should be kept under constant darkness until the infiltration procedure. This minimizes the syringe pressure needed for the infiltration procedure. High syringe pressure during infiltration could damage the leaves. After infiltration Arabidopsis plants need to be rewatered and kept under high humidity by water spraying and covering with a plastic cover for at least 24 h.
- A-YFPN / B-YFPC / CFP or RFP (sample)
- C-YFPN / B-YFPC / CFP or RFP (negative control)
- A-YFPN / D-YFPC / CFP or RFP (negative control)
- Amutation-YFPN / B-YFPC / CFP or RFP (negative control)
- A-YFPN / Bmutation-YFPC / CFP or RFP (negative control)
- Adeletion-YFPN / B-YFPC / CFP or RFP (negative control)
- A-YFPN / Bdeletion-YFPC / CFP or RFP (negative control)
Here, C and D belong to the respective protein family of A and B and mutation or deletion indicates possible modifications in the respective interaction domains. The BiFC-tag orientation should be determined according to Note 1. For infiltration of N. benthamiana, co-infiltration of the p19 silencing suppressor strain is recommended (97).
For infiltration of N. benthamiana leaves the OD600 of Agrobacteria could be adjusted between 0.05 and 2, where higher OD600 typically leads to higher expression levels.
For infiltration of Arabidopsis leaves the OD600 of Agrobacteria could be adjusted between 0.4 and 0.9 to obtain an appropriate expression level but should not exceed a total OD600 of 1.2 as this induces strong necrosis formations. OD600 of 0.3 and lower on the other hand drastically reduces transient expression efficiencies (95).
The choice of leaves for infiltration is critical for performing adequate semiquantitative BiFC analyses in N. benthamiana. N. benthamiana leaves of different age vary in their transient expression efficiencies. To compare BiFC efficiencies of different construct combinations, it is recommended infiltrating each construct combination into leaves of different plants but of the same age, size and physiological status. The use of different leaves of the same plant is not recommended. Alternatively, two different BiFC combinations could be infiltrated into one leaf on both sides of the leaf mid-vein. More than two different infiltrations per leaf are not recommended as for semiquantitative BiFC analyses samples from the same leaf area are needed (see Note 8).
Semiquantitative BiFC analyses are critical for the evaluation of positive BiFC results. The best system to perform BiFC quantification analyses is N. benthamiana epidermal cells, as these cells are easy to image and exhibit, depending on the proteins to be expressed, a remarkable high expression efficiency when BiFC samples are co-expressed with the silencing suppressor p19. It is recommended to infiltrate samples to be compared into leaves of similar physiological status. Samples for microscopic or western-blot analyses also need to be harvested form similar leaf areas. Expression levels in infiltrated N. benthamiana leaves vary dependent on the time point after infiltration. Usually strongly expressed protein constructs are already detectable at day two after infiltration and expression increases until day five after infiltration. However, it is strongly recommended to perform semiquantitative BiFC analyses only on days 3 – 4 after infiltration because of a maximal signal/noise-ratio during that time phase.
As an example for BiFC analyses in N. benthamiana the well known interaction of the calcineurin B-like (CBL) calcium sensor CBL10 and the CBL interacting protein kinase CIPK24 was investigated (Figure 2, 49, 104). As negative control the CIPK24NAFΔ construct was used, in which the NAF-domain, that is necessary and sufficient for CBL calcium sensor interaction (105), is deleted. Imaging was conducted using an inverted spinning disc confocal microscope. Higher resolution overview images and maximum projections of a z-stack including 32 focal planes were taken with a 60× objective (Figure 2a) and indicate CIPK24/CBL10 complex formations (yellow) at the vacuolar membrane that is clearly distinguishable from SCFP3A (cyan) localization in the cytoplasm and nucleus. For semiquantitative analyses images were taken with a 20× objective and quantified 3 days after infiltration (Figure 2b). After p19 background subtraction BiFC intensities were normalized to CIPK24/CBL10 interaction. Here, the use of the NAF domain deletion construct (CIPK24NAFΔ) resulted in reduced BiFC intensities of 41 % (BiFC constructs only) and 44 % (co-expressed with SCFP3A). Normalization of the BiFC/SCFP3A (Y/C)-ratios resulted in a reduced BiFC efficiency for CIPK24NAFΔ/CBL10 of 57 %. These data indicate that compared to CIPK24 wild type protein CIPK24NAFΔ interacts less efficient with CBL10 (49, 106). For western analyses, proteins were extracted using 1× SDS sample buffer, separated in 10 % Acrylamide/Bisacrylamide SDS gels and blotted onto PVDF membranes. Immuno detection of the different BiFC and SCFP3A construct combinations resulted in comparable expression levels of the respective fusion proteins (Figure 2c). Samples infiltrated only with the p19 strain were used as background controls and did not exhibit a respective signal in the western blots. PageBlue staining of the large RuBisCO subunit was used as loading control.
Taken together, co-expression of a reference FP, here SCFP3A (107), is not necessarily needed for BiFC quantification in N. benthamiana but it could minimize differences in BiFC signals due to differences in expression efficiencies between different leaves or leaf areas. Semiquantitative BiFC analyses in N. benthamiana as presented in Figure 2 are only applicable for PPIs that are localized in the plasma membrane, the cytoplasm, the cytoplasm including the nucleus, the endoplasmatic reticulum and the tonoplast. Such BiFC quantifications are not applicable for smaller compartments or organelles like the nucleus, peroxisomes, chloroplasts, mitochondria and the endosomal compartments as subtraction of the background signals from entire images most likely would lead to undetectable signals. Here, signal intensities of individual organelles from different samples need to be compared.
As transient expression in Arabidopsis is less robust than expression in N. benthamiana, co-expression of a reference FP is recommended for semiquantitative BiFC analyses. In addition, prior to BiFC analyses, it is recommended to infiltrate single FP-fusions of proteins, which should be investigated, to verify proper expression. Infiltration of YN-CIPK24/CBL10-YC into Arabidopsis did not result in detectable BiFC signals, possibly due to silencing or inefficient expression of YN-CIPK24 (data not shown). Type 2C protein phosphatases interact with SnRK2 protein kinases (108–111). Interaction of the SnRK2 kinase OST1 (112) and the PP2C phosphatase ABI1 (108, 110) was exemplarily investigated in infiltrated N. benthamiana and Arabidopsis leaves. YFPN173 was fused to the N-terminus of OST1 (YN-OST1) and YFPC155 was fused to the N-terminus of ABI1 (YC-ABI1), infiltrated into N. benthamiana and Arabidopsis leaves and analyzed four and seven days after infiltration, respectively (Figure 2d). BiFC analyses in both plant species indicate OST1/ABI1 complex formations with similar cytoplasmic and nuclear localization patterns.
A representative cell ideally harbors the nucleus in the center of the cell when looking on the z-axis. The nucleus is the best orientation point in a cell because it allows to distinguish between certain localization patterns. Plasma membrane localization of fluorescent proteins or protein complexes is characterized by a fluorescence signal only at the cell border. Here, plasmolysis may allow the appearance of Hechtian strands. Endoplasmatic reticulum localization displays a ring like structure around the nucleus and a net/mesh-like architecture. The vacuolar membrane is characterized by forming a pocket around the nucleus and most likely pushing the nucleus at the cell border. Here also membraneous invaginations and cytoplasmic strands are visible, forming a tunnel system through the large central vacuole (CIPK24/CBL10 complexes in Figure 2a). In some cases also ring like structures are visible. Cytoplasmic localization is visible by fluorescence of cytoplasmic strands. In addition to that the cytoplasm is forced in close proximity to the cell borders and the nucleus as the central vacuole occupies more than 90 % of the cell volume. Cytoplasmic proteins or protein complexes are often dually localized and also found in the nucleus (SCFP3A in Figure 2a, OST1/ABI1 complexes in Figure 2d), except of those that have a nuclear export signal or are too large to pass the nuclear pores passively. To distinguish between plasma membrane, vacuolar membrane, cytoplasm and endoplasmatic reticulum it is recommended to search for cytoplasmic strands, fluorescence around the nucleus and to carefully analyze the architecture of the fluorescence signal at the cell border in a top or bottom cell view or in a maximum projection (see Figure 2a). The nucleus is the largest organelle in the cell and fluorescence is often excluded from the nucleolus. The second largest organelles are chloroplasts. However compared to mesophyll cells, in epidermal cells chloroplasts are smaller and in lower quantity. Chloroplasts exhibit an elliptical structure and are often localized at the cell borders or around the nucleus. Chloroplasts can be easily visualized by chlorophyll auto-fluorescence. However, chlorophyll auto-fluorescence may also appear if too high excitation energy or exposure times are used, especially when FP or BiFC signals are weak. It is more difficult to identify other small cellular organelles like peroxisomes, the golgi apparatus or other endosomes. Here co-localization studies using fluorescent markers are recommended.
For representative images it is recommended displaying one overview image with the focal plane through the nucleus and the cell center. In addition a maximum projection of a z-stack is the first choice to visualize an entire cell, as here multiple focal planes through the cell are merged in one image (see Figure 2).
The use or modification of the protein extraction buffer enables also additional biochemical analyses like co-affinity purification (e.g. iBiSC), which could be conducted using GFP antibodies or antibodies recognizing additional tags in the BiFC constructs. Alternatively to the use of protein extraction buffer, proteins could be extracted directly in 1× SDS sample buffer. In that case, keep samples after grinding in frozen Retsch Mill buckets, add 100 μL (for N. benthamiana samples) or 75 μL (for Arabidopsis samples) 1× SDS sample buffer and directly boil samples for 5 min at 95 °C. Spin samples for 20 min at 14000 rpm and 4 °C, transfer supernatant to 1.5 mL tube and store at − 20 or − 80 °C. Protein quantification using Amidoblack staining is compatible with SDS sample buffer.
References
- 1.Morsy M, Gouthu S, Orchard S, Thorneycroft D, Harper JF, Mittler R, Cushman JC. Charting plant interactomes: possibilities and challenges. Trends Plant Sci. 2008;13:183–191. doi: 10.1016/j.tplants.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 2.Lalonde S, Ehrhardt DW, Loqué D, Chen J, Rhee SY, Frommer WB. Molecular and cellular approaches for the detection of protein-protein interactions: latest techniques and current limitations. Plant J. 2008;53:610–635. doi: 10.1111/j.1365-313X.2007.03332.x. [DOI] [PubMed] [Google Scholar]
- 3.Cusick ME, Yu H, Smolyar A, Venkatesan K, Carvunis AR, Simonis N, Rual JF, Borick H, Braun P, Dreze M, Vandenhaute J, Galli M, Yazaki J, Hill DE, Ecker JR, Roth FP, Vidal M. Literature-curated protein interaction datasets. Nat Methods. 2009;6:39–46. doi: 10.1038/nmeth.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jelesarov I, Bosshard HR. Isothermal titration calorimetry and differential scanning calorimetry as complementary tools to investigate the energetics of biomolecular recognition. J Mol Recognit. 1999;12:3–18. doi: 10.1002/(SICI)1099-1352(199901/02)12:1<3::AID-JMR441>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 5.Szabo A, Stolz L, Granzow R. Surface plasmon resonance and its use in biomolecular interaction analysis (BIA) Curr Opin Struct Biol. 1995;5:699–705. doi: 10.1016/0959-440x(95)80064-6. [DOI] [PubMed] [Google Scholar]
- 6.Dufrêne YF, Hinterdorfer P. Recent progress in AFM molecular recognition studies. Pflugers Arch. 2008;456:237–245. doi: 10.1007/s00424-007-0413-1. [DOI] [PubMed] [Google Scholar]
- 7.Piljic A, Schultz C. Simultaneous recording of multiple cellular events by FRET. ACS Chem Biol. 2008;3:156–160. doi: 10.1021/cb700247q. [DOI] [PubMed] [Google Scholar]
- 8.Villalobos V, Naik S, Piwnica-Worms D. Current state of imaging protein-protein interactions in vivo with genetically encoded reporters. Annu Rev Biomed Eng. 2007;9:321–349. doi: 10.1146/annurev.bioeng.9.060906.152044. [DOI] [PubMed] [Google Scholar]
- 9.Kerppola TK. Bimolecular fluorescence complementation (BiFC) analysis as a probe of protein interactions in living cells. Annu Rev Biophys. 2008;37:465–487. doi: 10.1146/annurev.biophys.37.032807.125842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pollok BA, Heim R. Using GFP in FRET-based applications. Trends Cell Biol. 1999;9:57–60. doi: 10.1016/s0962-8924(98)01434-2. [DOI] [PubMed] [Google Scholar]
- 11.Goedhart J, Vermeer JE, Adjobo-Hermans MJ, van Weeren L, Gadella TW. Sensitive detection of p65 homodimers using red-shifted and fluorescent protein-based FRET couples. PLoS One. 2007;2:e1011. doi: 10.1371/journal.pone.0001011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van der Krogt GN, Ogink J, Ponsioen B, Jalink K. A comparison of donor-acceptor pairs for genetically encoded FRET sensors: application to the Epac cAMP sensor as an example. PLoS One. 2008;3:e1916. doi: 10.1371/journal.pone.0001916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Subramanian C, Xu Y, Johnson CH, von Arnim AG. In vivo detection of protein-protein interaction in plant cells using BRET. Methods Mol Biol. 2004;284:271–286. doi: 10.1385/1-59259-816-1:271. [DOI] [PubMed] [Google Scholar]
- 14.Xu X, Soutto M, Xie Q, Servick S, Subramanian C, von Arnim AG, Johnson CH. Imaging protein interactions with bioluminescence resonance energy transfer (BRET) in plant and mammalian cells and tissues. Proc Natl Acad Sci U S A. 2007;104:10264–10269. doi: 10.1073/pnas.0701987104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhat RA, Lahaye T, Panstruga R. The visible touch: in planta visualization of protein-protein interactions by fluorophore-based methods. Plant Methods. 2006;2:12. doi: 10.1186/1746-4811-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fields S, Song O. A novel genetic system to detect protein-protein interactions. Nature. 1989;340:245–246. doi: 10.1038/340245a0. [DOI] [PubMed] [Google Scholar]
- 17.Ehlert A, Weltmeier F, Wang X, Mayer CS, Smeekens S, Vicente-Carbajosa J, Dröge-Laser W. Two-hybrid protein-protein interaction analysis in Arabidopsis protoplasts: establishment of a heterodimerization map of group C and group S bZIP transcription factors. Plant J. 2006;46:890–900. doi: 10.1111/j.1365-313X.2006.02731.x. [DOI] [PubMed] [Google Scholar]
- 18.Johnsson N, Varshavsky A. Split ubiquitin as a sensor of protein interactions in vivo. Proc Natl Acad Sci U S A. 1994;91:10340–10344. doi: 10.1073/pnas.91.22.10340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stagljar I, Korostensky C, Johnsson N, te Heesen S. A genetic system based on split-ubiquitin for the analysis of interactions between membrane proteins in vivo. Proc Natl Acad Sci U S A. 1998;95:5187–5192. doi: 10.1073/pnas.95.9.5187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wehr MC, Laage R, Bolz U, Fischer TM, Grünewald S, Scheek S, Bach A, Nave KA, Rossner MJ. Monitoring regulated protein-protein interactions using split TEV. Nat Methods. 2006;3:985–993. doi: 10.1038/nmeth967. [DOI] [PubMed] [Google Scholar]
- 21.Rossi F, Charlton CA, Blau HM. Monitoring protein-protein interactions in intact eukaryotic cells by beta-galactosidase complementation. Proc Natl Acad Sci U S A. 1997;94:8405–8410. doi: 10.1073/pnas.94.16.8405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pelletier JN, Campbell-Valois FX, Michnick SW. Oligomerization domain-directed reassembly of active dihydrofolate reductase from rationally designed fragments. Proc Natl Acad Sci U S A. 1998;95:12141–12146. doi: 10.1073/pnas.95.21.12141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Remy I, Michnick SW. Clonal selection and in vivo quantitation of protein interactions with protein-fragment complementation assays. Proc Natl Acad Sci U S A. 1999;96:5394–5399. doi: 10.1073/pnas.96.10.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galarneau A, Primeau M, Trudeau LE, Michnick SW. Beta-lactamase protein fragment complementation assays as in vivo and in vitro sensors of protein protein interactions. Nat Biotechnol. 2002;20:619–622. doi: 10.1038/nbt0602-619. [DOI] [PubMed] [Google Scholar]
- 25.Wehrman T, Kleaveland B, Her JH, Balint RF, Blau HM. Protein-protein interactions monitored in mammalian cells via complementation of beta-lactamase enzyme fragments. Proc Natl Acad Sci U S A. 2002;99:3469–3474. doi: 10.1073/pnas.062043699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Remy I, Ghaddar G, Michnick SW. Using the beta-lactamase protein-fragment complementation assay to probe dynamic protein-protein interactions. Nat Protoc. 2007;2:2302–2306. doi: 10.1038/nprot.2007.356. [DOI] [PubMed] [Google Scholar]
- 27.Ozawa T, Kaihara A, Sato M, Tachihara K, Umezawa Y. Split luciferase as an optical probe for detecting protein-protein interactions in mammalian cells based on protein splicing. Anal Chem. 2001;73:2516–2521. doi: 10.1021/ac0013296. [DOI] [PubMed] [Google Scholar]
- 28.Paulmurugan R, Umezawa Y, Gambhir SS. Noninvasive imaging of protein-protein interactions in living subjects by using reporter protein complementation and reconstitution strategies. Proc Natl Acad Sci U S A. 2002;99:15608–15613. doi: 10.1073/pnas.242594299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paulmurugan R, Massoud TF, Huang J, Gambhir SS. Molecular imaging of drug-modulated protein-protein interactions in living subjects. Cancer Res. 2004;64:2113–2119. doi: 10.1158/0008-5472.can-03-2972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paulmurugan R, Gambhir SS. Monitoring protein-protein interactions using split synthetic Renilla luciferase protein-fragment-assisted complementation. Anal Chem. 2003;75:1584–1589. doi: 10.1021/ac020731c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luker KE, Smith MC, Luker GD, Gammon ST, Piwnica-Worms H, Piwnica-Worms D. Kinetics of regulated protein-protein interactions revealed with firefly luciferase complementation imaging in cells and living animals. Proc Natl Acad Sci U S A. 2004;101:12288–12293. doi: 10.1073/pnas.0404041101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Remy I, Michnick SW. A highly sensitive protein-protein interaction assay based on Gaussia luciferase. Nat Methods. 2006;3:977–979. doi: 10.1038/nmeth979. [DOI] [PubMed] [Google Scholar]
- 33.Fujikawa Y, Kato N. Split luciferase complementation assay to study protein-protein interactions in Arabidopsis protoplasts. Plant J. 2007;52:185–195. doi: 10.1111/j.1365-313X.2007.03214.x. [DOI] [PubMed] [Google Scholar]
- 34.Chen H, Zou Y, Shang Y, Lin H, Wang Y, Cai R, Tang X, Zhou JM. Firefly luciferase complementation imaging assay for protein-protein interactions in plants. Plant Physiol. 2008;146:368–376. doi: 10.1104/pp.107.111740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gehl C, Kaufholdt D, Hamisch D, Bikker R, Kudla J, Mendel RR, Hänsch R. Quantitative analysis of dynamic protein-protein interactions in planta by a floated-leaf luciferase complementation imaging (FLuCI) assay using binary Gateway vectors. Plant J. 2011;67:542–553. doi: 10.1111/j.1365-313X.2011.04607.x. [DOI] [PubMed] [Google Scholar]
- 36.Ghosh I, Hamilton AD, Regan L. Antiparallel Leucine Zipper-Directed Protein Reassembly: Application to the Green Fluorescent Protein. J Am Chem Soc. 2000;122:5658–5659. [Google Scholar]
- 37.Hu CD, Chinenov Y, Kerppola TK. Visualization of interactions among bZIP and Rel family proteins in living cells using bimolecular fluorescence complementation. Mol Cell. 2002;9:789–798. doi: 10.1016/s1097-2765(02)00496-3. [DOI] [PubMed] [Google Scholar]
- 38.Hu CD, Kerppola TK. Simultaneous visualization of multiple protein interactions in living cells using multicolor fluorescence complementation analysis. Nat Biotechnol. 2003;21:539–545. doi: 10.1038/nbt816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Robida AM, Kerppola TK. Bimolecular fluorescence complementation analysis of inducible protein interactions: effects of factors affecting protein folding on fluorescent protein fragment association. J Mol Biol. 2009;394:391–409. doi: 10.1016/j.jmb.2009.08.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nagai T, Ibata K, Park ES, Kubota M, Mikoshiba K, Miyawaki A. A variant of yellow fluorescent protein with fast and efficient maturation for cell-biological applications. Nat Biotechnol. 2002;20:87–90. doi: 10.1038/nbt0102-87. [DOI] [PubMed] [Google Scholar]
- 41.Kodama Y, Hu CD. An improved bimolecular fluorescence complementation assay with a high signal-to-noise ratio. Biotechniques. 2010;49:793–805. doi: 10.2144/000113519. [DOI] [PubMed] [Google Scholar]
- 42.Li M, Doll J, Weckermann K, Oecking C, Berendzen KW, Schöffl F. Detection of in vivo interactions between Arabidopsis class A-HSFs, using a novel BiFC fragment, and identification of novel class B-HSF interacting proteins. Eur J Cell Biol. 2010;89:126–132. doi: 10.1016/j.ejcb.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 43.Nakagawa C, Inahata K, Nishimura S, Sugimoto K. Improvement of a Venus-based bimolecular fluorescence complementation assay to visualize bFos-bJun interaction in living cells. Biosci Biotechnol Biochem. 2011;75:1399–1401. doi: 10.1271/bbb.110189. [DOI] [PubMed] [Google Scholar]
- 44.Lin J, Wang N, Li Y, Liu Z, Tian S, Zhao L, Zheng Y, Liu S, Li S, Jin C, Xia B. LEC-BiFC: a new method for rapid assay of protein interaction. Biotech Histochem. 2011;86:272–279. doi: 10.3109/10520295.2010.483068. [DOI] [PubMed] [Google Scholar]
- 45.Kerppola TK. Design and implementation of bimolecular fluorescence complementation (BiFC) assays for the visualization of protein interactions in living cells. Nat Protoc. 2006;1:1278–1286. doi: 10.1038/nprot.2006.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ohad N, Shichrur K, Yalovsky S. The analysis of protein-protein interactions in plants by bimolecular fluorescence complementation. Plant Physiol. 2007;145:1090–1099. doi: 10.1104/pp.107.107284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Citovsky V, Gafni Y, Tzfira T. Localizing protein-protein interactions by bimolecular fluorescence complementation in planta. Methods. 2008;45:196–206. doi: 10.1016/j.ymeth.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 48.Shyu YJ, Liu H, Deng X, Hu CD. Identification of new fluorescent protein fragments for bimolecular fluorescence complementation analysis under physiological conditions. Biotechniques. 2006;40:61–66. doi: 10.2144/000112036. [DOI] [PubMed] [Google Scholar]
- 49.Waadt R, Schmidt LK, Lohse M, Hashimoto K, Bock R, Kudla J. Multicolor bimolecular fluorescence complementation reveals simultaneous formation of alternative CBL/CIPK complexes in planta. Plant J. 2008;56:505–516. doi: 10.1111/j.1365-313X.2008.03612.x. [DOI] [PubMed] [Google Scholar]
- 50.Remy I, Michnick SW. A cDNA library functional screening strategy based on fluorescent protein complementation assays to identify novel components of signaling pathways. Methods. 2004;32:381–388. doi: 10.1016/j.ymeth.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 51.Wilson CG, Magliery TJ, Regan L. Detecting protein-protein interactions with GFP-fragment reassembly. Nat Methods. 2004;1:255–262. doi: 10.1038/nmeth1204-255. [DOI] [PubMed] [Google Scholar]
- 52.Magliery TJ, Wilson CG, Pan W, Mishler D, Ghosh I, Hamilton AD, Regan L. Detecting protein-protein interactions with a green fluorescent protein fragment reassembly trap: scope and mechanism. J Am Chem Soc. 2005;127:146–157. doi: 10.1021/ja046699g. [DOI] [PubMed] [Google Scholar]
- 53.Beauchemin C, Boutet N, Laliberté JF. Visualization of the interaction between the precursors of VPg, the viral protein linked to the genome of turnip mosaic virus, and the translation eukaryotic initiation factor iso 4E in planta. J Virol. 2007;81:775–782. doi: 10.1128/JVI.01277-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kojima T, Karasawa S, Miyawaki A, Tsumuraya T, Fujii I. Novel screening system for protein-protein interactions by bimolecular fluorescence complementation in Saccharomyces cerevisiae. J Biosci Bioeng. 2011;111:397–401. doi: 10.1016/j.jbiosc.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 55.Zhou J, Lin J, Zhou C, Deng X, Xia B. An improved bimolecular fluorescence complementation tool based on superfolder green fluorescent protein. Acta Biochim Biophys Sin (Shanghai) 2011;43:239–244. doi: 10.1093/abbs/gmq128. [DOI] [PubMed] [Google Scholar]
- 56.Bracha-Drori K, Shichrur K, Katz A, Oliva M, Angelovici R, Yalovsky S, Ohad N. Detection of protein-protein interactions in plants using bimolecular fluorescence complementation. Plant J. 2004;40:419–427. doi: 10.1111/j.1365-313X.2004.02206.x. [DOI] [PubMed] [Google Scholar]
- 57.Walter M, Chaban C, Schütze K, Batistič O, Weckermann K, Näke C, Blazevic D, Grefen C, Schumacher K, Oecking C, Harter K, Kudla J. Visualization of protein interactions in living plant cells using bimolecular fluorescence complementation. Plant J. 2004;40:428–438. doi: 10.1111/j.1365-313X.2004.02219.x. [DOI] [PubMed] [Google Scholar]
- 58.Ottmann C, Weyand M, Wolf A, Kuhlmann J. Applicability of superfolder YFP bimolecular fluorescence complementation in vitro. Biol Chem. 2009;390:81–90. doi: 10.1515/BC.2009.008. [DOI] [PubMed] [Google Scholar]
- 59.Jach G, Pesch M, Richter K, Frings S, Uhrig JF. An improved mRFP1 adds red to bimolecular fluorescence complementation. Nat Methods. 2006;3:597–600. doi: 10.1038/nmeth901. [DOI] [PubMed] [Google Scholar]
- 60.Fan JY, Cui ZQ, Wei HP, Zhang ZP, Zhou YF, Wang YP, Zhang XE. Split mCherry as a new red bimolecular fluorescence complementation system for visualizing protein-protein interactions in living cells. Biochem Biophys Res Commun. 2008;367:47–53. doi: 10.1016/j.bbrc.2007.12.101. [DOI] [PubMed] [Google Scholar]
- 61.Chu J, Zhang Z, Zheng Y, Yang J, Qin L, Lu J, Huang ZL, Zeng S, Luo Q. A novel far-red bimolecular fluorescence complementation system that allows for efficient visualization of protein interactions under physiological conditions. Biosens Bioelectron. 2009;25:234–239. doi: 10.1016/j.bios.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 62.Kodama Y, Wada M. Simultaneous visualization of two protein complexes in a single plant cell using multicolor fluorescence complementation analysis. Plant Mol Biol. 2009;70:211–217. doi: 10.1007/s11103-009-9467-0. [DOI] [PubMed] [Google Scholar]
- 63.Zilian E, Maiss E. An optimized mRFP-based bimolecular fluorescence complementation system for the detection of protein-protein interactions in planta. J Virol Methods. 2011;174:158–165. doi: 10.1016/j.jviromet.2011.03.032. [DOI] [PubMed] [Google Scholar]
- 64.Lee YR, Park JH, Hahm SH, Kang LW, Chung JH, Nam KH, Hwang KY, Kwon IC, Han YS. Development of bimolecular fluorescence complementation using Dronpa for visualization of protein-protein interactions in cells. Mol Imaging Biol. 2010;12:468–478. doi: 10.1007/s11307-010-0312-2. [DOI] [PubMed] [Google Scholar]
- 65.Rebois RV, Robitaille M, Galés C, Dupré DJ, Baragli A, Trieu P, Ethier N, Bouvier M, Hébert TE. Heterotrimeric G proteins form stable complexes with adenylyl cyclase and Kir3.1 channels in living cells. J Cell Sci. 2006;119:2807–2818. doi: 10.1242/jcs.03021. [DOI] [PubMed] [Google Scholar]
- 66.Gandia J, Galino J, Amaral OB, Soriano A, Lluís C, Franco R, Ciruela F. Detection of higher-order G protein-coupled receptor oligomers by a combined BRET-BiFC technique. FEBS Lett. 2008;582:2979–2984. doi: 10.1016/j.febslet.2008.07.045. [DOI] [PubMed] [Google Scholar]
- 67.Shyu YJ, Suarez CD, Hu CD. Visualization of AP-1 NF-kappaB ternary complexes in living cells by using a BiFC-based FRET. Proc Natl Acad Sci U S A. 2008;105:151–156. doi: 10.1073/pnas.0705181105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kwaaitaal M, Keinath NF, Pajonk S, Biskup C, Panstruga R. Combined bimolecular fluorescence complementation and Forster resonance energy transfer reveals ternary SNARE complex formation in living plant cells. Plant Physiol. 2010;152:1135–1147. doi: 10.1104/pp.109.151142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rebois RV, Robitaille M, Pétrin D, Zylbergold P, Trieu P, Hébert TE. Combining protein complementation assays with resonance energy transfer to detect multipartner protein complexes in living cells. Methods. 2008;45:214–218. doi: 10.1016/j.ymeth.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 70.Vidi PA, Watts VJ. Fluorescent and bioluminescent protein-fragment complementation assays in the study of G protein-coupled receptor oligomerization and signaling. Mol Pharmacol. 2009;75:733–739. doi: 10.1124/mol.108.053819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fang D, Kerppola TK. Ubiquitin-mediated fluorescence complementation reveals that Jun ubiquitinated by Itch/AIP4 is localized to lysosomes. Proc Natl Acad Sci U S A. 2004;101:14782–14787. doi: 10.1073/pnas.0404445101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ikeda H, Kerppola TK. Lysosomal localization of ubiquitinated Jun requires multiple determinants in a lysine-27-linked polyubiquitin conjugate. Mol Biol Cell. 2008;19:4588–4601. doi: 10.1091/mbc.E08-05-0496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rackham O, Brown CM. Visualization of RNA-protein interactions in living cells: FMRP and IMP1 interact on mRNAs. EMBO J. 2004;23:3346–3355. doi: 10.1038/sj.emboj.7600341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Valencia-Burton M, McCullough RM, Cantor CR, Broude NE. RNA visualization in live bacterial cells using fluorescent protein complementation. Nat Methods. 2007;4:421–427. doi: 10.1038/nmeth1023. [DOI] [PubMed] [Google Scholar]
- 75.Ozawa T, Natori Y, Sato M, Umezawa Y. Imaging dynamics of endogenous mitochondrial RNA in single living cells. Nat Methods. 2007;4:413–419. doi: 10.1038/nmeth1030. [DOI] [PubMed] [Google Scholar]
- 76.Vincenz C, Kerppola TK. Different polycomb group CBX family proteins associate with distinct regions of chromatin using nonhomologous protein sequences. Proc Natl Acad Sci U S A. 2008;105:16572–16577. doi: 10.1073/pnas.0805317105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zou P, Pinotsis N, Lange S, Song YH, Popov A, Mavridis I, Mayans OM, Gautel M, Wilmanns M. Palindromic assembly of the giant muscle protein titin in the sarcomeric Z-disk. Nature. 2006;439:229–233. doi: 10.1038/nature04343. [DOI] [PubMed] [Google Scholar]
- 78.Jeong J, Kim SK, Ahn J, Park K, Jeong EJ, Kim M, Chung BH. Monitoring of conformational change in maltose binding protein using split green fluorescent protein. Biochem Biophys Res Commun. 2006;339:647–651. doi: 10.1016/j.bbrc.2005.11.056. [DOI] [PubMed] [Google Scholar]
- 79.Zamyatnin AA, Solovyev AG, Bozhkov PV, Valkonen JP, Morozov SY, Savenkov EI. Assessment of the integral membrane protein topology in living cells. Plant J. 2006;46:145–154. doi: 10.1111/j.1365-313X.2006.02674.x. [DOI] [PubMed] [Google Scholar]
- 80.Zhang S, Ma C, Chalfie M. Combinatorial marking of cells and organelles with reconstituted fluorescent proteins. Cell. 2004;119:137–144. doi: 10.1016/j.cell.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 81.Morell M, Espargaro A, Aviles FX, Ventura S. Study and selection of in vivo protein interactions by coupling bimolecular fluorescence complementation and flow cytometry. Nat Protoc. 2008;3:22–33. doi: 10.1038/nprot.2007.496. [DOI] [PubMed] [Google Scholar]
- 82.Waadt R, Kudla J. In Planta Visualization of Protein Interactions Using Bimolecular Fluorescence Complementation (BiFC) CSH Protoc. 2008 doi: 10.1101/pdb.prot4995. 2008:pdb.prot4995. [DOI] [PubMed] [Google Scholar]
- 83.Schütze K, Harter K, Chaban C. Bimolecular fluorescence complementation (BiFC) to study protein-protein interactions in living plant cells. Methods Mol Biol. 2009;479:189–202. doi: 10.1007/978-1-59745-289-2_12. [DOI] [PubMed] [Google Scholar]
- 84.Hollender CA, Liu Z. Bimolecular fluorescence complementation (BiFC) assay for protein-protein interaction in onion cells using the helios gene gun. J Vis Exp. 2010 doi: 10.3791/1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fang Y, Spector DL. BiFC imaging assay for plant protein-protein interactions. Cold Spring Harb Protoc. 2010 doi: 10.1101/pdb.prot5380. 2010:pdb.prot5380. [DOI] [PubMed] [Google Scholar]
- 86.Ohad N, Yalovsky S. Utilizing bimolecular fluorescence complementation (BiFC) to assay protein-protein interaction in plants. Methods Mol Biol. 2010;655:347–358. doi: 10.1007/978-1-60761-765-5_23. [DOI] [PubMed] [Google Scholar]
- 87.Pusch S, Dissmeyer N, Schnittger A. Bimolecular-fluorescence complementation assay to monitor kinase-substrate interactions in vivo. Methods Mol Biol. 2011;779:245–257. doi: 10.1007/978-1-61779-264-9_14. [DOI] [PubMed] [Google Scholar]
- 88.Pattanaik S, Werkman JR, Yuan L. Bimolecular fluorescence complementation as a tool to study interactions of regulatory proteins in plant protoplasts. Methods Mol Biol. 2011;754:185–193. doi: 10.1007/978-1-61779-154-3_10. [DOI] [PubMed] [Google Scholar]
- 89.Sakuragi Y, Nørholm MH, Scheller HV. Visual mapping of cell wall biosynthesis. Methods Mol Biol. 2011;715:153–167. doi: 10.1007/978-1-61779-008-9_11. [DOI] [PubMed] [Google Scholar]
- 90.Citovsky V, Lee LY, Vyas S, Glick E, Chen MH, Vainstein A, Gafni Y, Gelvin SB, Tzfira T. Subcellular localization of interacting proteins by bimolecular fluorescence complementation in planta. J Mol Biol. 2006;362:1120–1131. doi: 10.1016/j.jmb.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 91.Stolpe T, Süsslin C, Marrocco K, Nick P, Kretsch T, Kircher S. In planta analysis of protein-protein interactions related to light signaling by bimolecular fluorescence complementation. Protoplasma. 2005;226:137–146. doi: 10.1007/s00709-005-0122-6. [DOI] [PubMed] [Google Scholar]
- 92.Lee LY, Fang MJ, Kuang LY, Gelvin SB. Vectors for multi-color bimolecular fluorescence complementation to investigate protein-protein interactions in living plant cells. Plant Methods. 2008;4:24. doi: 10.1186/1746-4811-4-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gehl C, Waadt R, Kudla J, Mendel RR, Hänsch R. New GATEWAY vectors for high throughput analyses of protein-protein interactions by bimolecular fluorescence complementation. Mol Plant. 2009;2:1051–1058. doi: 10.1093/mp/ssp040. [DOI] [PubMed] [Google Scholar]
- 94.Lee MW, Yang Y. Transient expression assay by agroinfiltration of leaves. Methods Mol Biol. 2006;323:225–229. doi: 10.1385/1-59745-003-0:225. [DOI] [PubMed] [Google Scholar]
- 95.Kim MJ, Baek K, Park CM. Optimization of conditions for transient Agrobacterium-mediated gene expression assays in Arabidopsis. Plant Cell Rep. 2009;28:1159–1167. doi: 10.1007/s00299-009-0717-z. [DOI] [PubMed] [Google Scholar]
- 96.Koncz C, Schell J. The promoter of TL-DNA gene 5 controls the tissue-specific expression of chimaeric genes carriedby a novel type of Agrobacterium binary vector. Mol Gen Genet. 1986;204:383–396. [Google Scholar]
- 97.Voinnet O, Rivas S, Mestre P, Baulcombe D. An enhanced transient expression system in plants based on suppression of gene silencing by the p19 protein of tomato bushy stunt virus. Plant J. 2003;33:949–956. doi: 10.1046/j.1365-313x.2003.01676.x. [DOI] [PubMed] [Google Scholar]
- 98.Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol Plant. 1962;15:473–497. [Google Scholar]
- 99.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 100.Schwacke R, Schneider A, van der Graaff E, Fischer K, Catoni E, Desimone M, Frommer WB, Flügge UI, Kunze R. ARAMEMNON, a novel database for Arabidopsis integral membrane proteins. Plant Physiol. 2003;131:16–26. doi: 10.1104/pp.011577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gasteiger E, Gattiker A, Hoogland C, Ivanyi I, Appel RD, Bairoch A. ExPASy: The proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res. 2003;31:3784–3788. doi: 10.1093/nar/gkg563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tsuda K, Qi Y, Nguyen LV, Bethke G, Tsuda Y, Glazebrook J, Katagiri F. An efficient Agrobacterium-mediated transient transformation of Arabidopsis. Plant J. 2011;69:713–719. doi: 10.1111/j.1365-313X.2011.04819.x. [DOI] [PubMed] [Google Scholar]
- 103.Peragine A, Yoshikawa M, Wu G, Albrecht HL, Poethig RS. SGS3 and SGS2/SDE1/RDR6 are required for juvenile development and the production of trans-acting siRNAs in Arabidopsis. Genes Dev. 2004;18:2368–2379. doi: 10.1101/gad.1231804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kim BG, Waadt R, Cheong YH, Pandey GK, Dominguez-Solis JR, Schültke S, Lee SC, Kudla J, Luan S. The calcium sensor CBL10 mediates salt tolerance by regulating ion homeostasis in Arabidopsis. Plant J. 2007;52:473–484. doi: 10.1111/j.1365-313X.2007.03249.x. [DOI] [PubMed] [Google Scholar]
- 105.Albrecht V, Ritz O, Linder S, Harter K, Kudla J. The NAF domain defines a novel protein-protein interaction module conserved in Ca2+-regulated kinases. EMBO J. 2001;20:1051–1063. doi: 10.1093/emboj/20.5.1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hashimoto K, Eckert C, Anschütz U, Scholz M, Held K, Waadt R, Reyer A, Hippler M, Becker D, Kudla J. Phosphorylation of calcineurin B-like (CBL) calcium sensor proteins by their CBL-interacting protein kinases (CIPKs) is required for full activity of CBL-CIPK complexes toward their target proteins. J Biol Chem. 2012;287:7956–7968. doi: 10.1074/jbc.M111.279331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kremers GJ, Goedhart J, van Munster EB, Gadella TW. Cyan and yellow super fluorescent proteins with improved brightness, protein folding, and FRET Förster radius. Biochemistry. 2006;45:6570–6580. doi: 10.1021/bi0516273. [DOI] [PubMed] [Google Scholar]
- 108.Yoshida R, Umezawa T, Mizoguchi T, Takahashi S, Takahashi F, Shinozaki K. The regulatory domain of SRK2E/OST1/SnRK2.6 interacts with ABI1 and integrates abscisic acid (ABA) and osmotic stress signals controlling stomatal closure in Arabidopsis. J Biol Chem. 2006;281:5310–5318. doi: 10.1074/jbc.M509820200. [DOI] [PubMed] [Google Scholar]
- 109.Vlad F, Rubio S, Rodrigues A, Sirichandra C, Belin C, Robert N, Leung J, Rodriguez PL, Laurière C, Merlot S. Protein phosphatases 2C regulate the activation of the Snf1-related kinase OST1 by abscisic acid in Arabidopsis. Plant Cell. 2009;21:3170–3184. doi: 10.1105/tpc.109.069179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Umezawa T, Sugiyama N, Mizoguchi M, Hayashi S, Myouga F, Yamaguchi-Shinozaki K, Ishihama Y, Hirayama T, Shinozaki K. Type 2C protein phosphatases directly regulate abscisic acid-activated protein kinases in Arabidopsis. Proc Natl Acad Sci U S A. 2009;106:17588–17593. doi: 10.1073/pnas.0907095106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nishimura N, Sarkeshik A, Nito K, Park SY, Wang A, Carvalho PC, Lee S, Caddell DF, Cutler SR, Chory J, Yates JR, Schroeder JI. PYR/PYL/RCAR family members are major in-vivo ABI1 protein phosphatase 2C-interacting proteins in Arabidopsis. Plant J. 2010;61:290–299. doi: 10.1111/j.1365-313X.2009.04054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mustilli AC, Merlot S, Vavasseur A, Fenzi F, Giraudat J. Arabidopsis OST1 protein kinase mediates the regulation of stomatal aperture by abscisic acid and acts upstream of reactive oxygen species production. Plant Cell. 2002;14:3089–3099. doi: 10.1105/tpc.007906. [DOI] [PMC free article] [PubMed] [Google Scholar]