Abstract
Wear particles generated with use of total joint replacements incite a chronic macrophage-mediated inflammatory reaction, which leads to implant failure. Macrophage activation may be polarized into two states, with an M1 proinflammatory state dominating an alternatively activated M2 anti-inflammatory state. We hypothesized that IL-4, an activator of M2 macrophages, could modulate polyethylene (PE) particle-induced osteolysis in an experimental murine model. Four animal groups included (a) calvarial saline injection with harvest at 14 days (b) single calvarial injection of PE particles subcutaneously (SC) without IL-4 (c) PE particles placed as in (b), then IL-4 given SC for 14 consecutive days and (d) PE particles as in (b) then IL-4 beginning 7 days after particle injection for 7 days. The calvarial bone volume to total tissue volume was measured using microCT and histomorphometry. Calvaria were cultured for 24 h to assess release of RANKL, OPG, TNF-α, and IL-1ra and isolation and identification of M1 and M2 specific proteins. MicroCT and histomorphometric analysis showed that bone loss was significantly decreased following IL-4 administration to PE treated calvaria for both 7 and 14 days. Western blot analysis showed an increased M1/M2 ratio in the PE treated calvaria, which decreased with addition of IL-4. Cytokine analysis showed that the RANKL/OPG ratio and TNF-α/IL-1ra ratio decreased in PE-treated calvaria following IL-4 addition for 14 days. IL-4 delivery mitigated PE particle-induced osteolysis through macrophage polarization. Modulation of macrophage polarization is a potential treatment strategy for wear particle induced periprosthetic osteolysis.
Keywords: osteolysis, polyethylene particles, interleukin-4, macrophage polarization, murine calvarium
INTRODUCTION
Total joint replacements (TJR) are very successful operations for treatment of patients with disabling arthritis. However, with usage, the implant ultimately wears, creating particulate debris from the articulating surfaces. The wear particles incite a chronic inflammatory reaction, leading to periprosthetic bone loss, loosening of the implant, and ultimately failure.1–9 Up to 15% of the TJRs currently require a revision surgery, with potential severe complications and disabling consequences for patients.2 The need for a nonsurgical treatment or preventative therapy to interfere with the processes of particle-induced inflammation would be of benefit in reducing the number of revision surgeries.
Particle-induced inflammation and subsequent osteolysis is one of the largest contributors to joint replacement failure.10 Wear particles are phagocytosed by the monocyte/macrophage lineage of cells, leading to their activation, proliferation, and differentiation.11 Macrophage activation leads to the release of proinflammatory mediators and cytokines, such as prostaglandin E2 (PGE2), tumor-necrosis factor-α (TNF-α), interleukin 1 beta (IL-1β), and macrophage-colony stimulating factor (M-CSF), which are potent stimulators of osteoclastogenesis.12 A key initiator of osteoclastogenesis is the interaction of receptor activator of nuclear factor kappa-β ligand (RANKL), expressed on the surface of osteoblasts, and receptor activator of nuclear factor kappa-β (RANK), expressed on osteoclast precursors and mature osteoclasts. Following RANKL-RANK contact, signaling molecules including nuclear factor kappa-β (NFκβ) and nuclear factor of activated T-cells, cytoplasmic 1 (NFATc1) are activated. RANKL also activates bone resorptive functions and maintains the survival of mature osteoclasts.1,13–15 Osteoprotegerin (OPG), a soluble decoy receptor of RANKL produced by bone marrow stromal cells and monocyte/macrophages, blocks the RANKL-RANK interaction by competitively binding with RANK.16 The molecular ratio of RANKL, RANK, and OPG expression can be used as a means of assessing bone remodeling.
A current hypothesis suggests that the macrophage response in particle-induced osteolysis may be polarized, with M1 proinflammatory macrophages activated in response to wear debris production dominating the M2 anti-inflammatory response that normally promotes bone healing, debris scavenging, wound healing, and angiogenesis.12,17–21 M1 macrophages, producers of primarily proinflammatory mediators including TNF-α, IL-1, IL-6, express inducible nitric oxide synthase (iNOS) and HLA-DR.22–24 In contrast, M2 macrophages produce primarily anti-inflammatory mediators including IL-4, IL-10, and IL-13 production, and express mammalian chitinase Ym1, Arginase 1, CD163, and chitotriosidase.12,18,25,26 This differential cytokine production and receptor expression can be used to characterize which macrophages are present in a clinical situation. Previous work has shown that polymethylmethacrylate (PMMA) particles polarize macrophages towards an M1 proinflammatory response in vitro.27 However, the addition of inter-leukin-4 (IL-4) to PMMA stimulated macrophages selectively polarized a subset of the macrophages towards an M2 phenotype.27 Thus, IL-4 appears to be a key factor in promoting an anti-inflammatory healing response by inducing macrophage polarization towards an M2 phenotype.
IL-4 is produced by activated T lymphocytes and mast cells, and is a differentiation factor for cells in the hematopoietic lineage as well as stimulating B cell growth.28 IL-4 has been shown to be an anti-osteolytic factor in response to parathyroid hormone (PTH), PTH-related peptide (PTHrP), and IL-1α.29 IL-4 acts to block bone resorption by inhibiting osteoclast formation and survival by multiple mechanisms. IL-4 acts on osteoblasts to suppress cyclooxygenase-2-dependent PGE2 synthesis, enhance the production of osteoprotegerin (OPG), and to decrease the production of RANKL, all of which inhibit osteoclast precursor differentiation and function.28–36 Additionally, IL-4 acts through the transcription factor STAT6 and selectively blocks RANKL-induced action of NFκβ and mitogen-activated protein kinase (MAPK). In the short term, IL-4 blocks RANKL-induced NFATc1, a master osteoclastogenic transcription factor.13 Early studies using inflammatory tissues taken from rheumatoid arthritis patients have shown that IL-4 has anti-inflammatory effects on monocyte/macrophages, endothelial cells, and fibroblasts. IL-4 also inhibited the production of IL-6, TNF-α, LIF, and PGE2.28 IL-4 has also been shown to decrease production of IL-1 and TNF, as well as bone resorption in a polyethylene wear debris-induced osteolysis in a murine air pouch model.37
The goal of this work is to delineate the effect of IL-4 and its effect on macrophage polarization on polyethylene particle induced osteolysis in the murine calvarial model. Our hypothesis is that IL-4 administration will decrease the inflammation induced bone resorption associated with polyethylene particles.37 We administered IL-4 to polyethylene particle treated calvaria for 14 consecutive days, and 7 days after particle implantation for 7 days. This model was used to mimic 2 clinical scenarios: the first simulates exposure to wear debris from the time of prosthesis implantation (similar to the initial “bedding-in” period of high particle production), and the second simulates a time period after the inflammatory reaction has already been established. We used μCT analysis and histomorphometric analysis to assess the bone volume differences, and tartrate resistant acid phosphatase (TRAP) staining to assess osteoclast formation. We also cultured calvaria and used enzyme linked immunosorbant assays (ELISAs) and Western blotting to examine cytokine release and M1 and M2 protein specific expression.
MATERIALS AND METHODS
Animals and surgery
40 C57BL/6 male mice 8–10 weeks old (Jackson Laboratories) were housed and fed in our Animal Facility. The experimental design was approved by the Institutional Administration Panel for Laboratory Animal Care. We strictly followed university guidelines for care and use of laboratory animals. Animals were anesthetized with 2–3% isoflurane in 100% oxygen at a flow rate of 1 L/min and were operated on a warm small animal surgery station. An area of skin overlying the skull was carefully shaved and sterilized with betadine scrub. Using sterile technique, a 25-gauge needle was used to inject 100 μL of saline or polyethylene particles resuspended in phosphate buffered saline (PBS) directly over the calvarial bone and periosteum. Ten control mice received saline injections (Group 1, sham), and 10 control animals received 4 × 108 particles resuspended in PBS (Group 2, UHMWPE only). Ten animals received 4 × 108 particles as well as 1 μg of IL-4 at the time of operation and a 1 μg injection of IL-4 every day for 14 days into the subcutaneous bursa overlying the calvarium (Group 3, UHMWPE+IL-4 14 days). Ten different animals received 4 × 108 particles at day 0, and 7 days later began receiving injections of 1 μg of IL-4 for the remaining 7 days (Group 4, UHMWPE+IL-4 7 days) (Table I).38,39 Fourteen days postoperatively, the mice were euthanized, and the calvaria harvested for micro-CT analysis, protein quantification, and histology.
TABLE I.
Experimental Design and Group Designation for Polyethylene Particle Induced Osteolysis on the Murine Calvarium with IL-4 Administration
| Saline | Polyethylene Particles (2 Weeks) | IL-4 | |
|---|---|---|---|
| Group 1 (n = 10) | x | ||
| Group 2 (n = 10) | x | ||
| Group 3 (n = 10) | x | Beginning at day 0 for 14 consecutive days | |
| Group 4 (n = 10) | x | Beginning at day 7 for 7 consecutive days |
Particles
We used conventional nonhighly cross-linked UHMWPE particles (a gift from Dr. Timothy Wright, Hospital for Special Surgery, New York, NY) obtained from knee joint stimulator tests and isolated according to an established protocol. The particles were isolated by density gradient centrifugation and sterilized in 95% ethanol overnight. Frozen aliquots of the particles containing serum were lyophilized for 4–7 days. The dried material was digested in 5M sodium hydroxide at 70°C for 2 h. The digested particle suspension was centrifuged through a 5% sucrose gradient at 40 K rpm at 10°C for 3 h. The collected particles at the surface of the sucrose solution were ultrasonicated and centrifuged again through an isopropanol gradient (0.96 and 0.90 g/cm3) at 40 K rpm at 10°C for 1 h. Particles were resuspended in 95% ethanol, which was evaporated completely. Ultimately, UHMWPE particles were washed in 70% ethanol and resuspended in phosphate buffered saline prior to implantation. The particles tested negative for endotoxin using a Limulus Amebocyte Lysate Kit (BioWhittaker, Walkersville, MD). The mean diameter of the particles was 1.0 ± 0.1 μm (mean ± SE) measured by electron microscopy. The concentration of UHMWPE was 30 mg/mL, and using the density of 0.94 g/mL3, we calculated the appropriate volume to administer 4 × 108 particles to the calvarium of each animal.
MicroCT imaging
The imaging was performed in the Small Animal Imaging Facility at Stanford University (Clark Center). A μCT scan was performed for 10 animals per group in order to detect changes in bone volume (BV). We used a phantom made of an epoxy-based resin, which mimics hydroxyapaptite and contains water and air inclusion for calibration. Animals were placed in the ventral position in the MicroCAT μCT scanner (Imtek, Knoxville, TN) with 40 μm resolution during 35 min. After scanning, we used the MicroCAT software (Imtek, Inc) for acquisition and COBRA Reconstruction interface software (Exxim computing corporation, Pleasanton, CA) for reconstruction. For bone volume fraction (BVF) assessment we used the MicroView software (GE Medical Systems) and a 3D region of interest was created at the level of the parietal bones.40,41
Histology and TRAP staining
Calvaria were harvested as one piece from five animals per group and fixed in 4% paraformaldehyde (PFA) for 3 days, washed in PBS and decalcified in ethylenediaminetetraacetic acid (EDTA) twice for 5 days. Calvaria were then embedded on optimal cutting temperature (OCT) medium and stored at −80°C. Frozen sections of 7 μm were cut using a cryostat (Cambridge Instruments, Buffalo, NY) to include the distal half of the frontal bones and proximal half of the parietal bones, the site of particle injection. Slides were fixed in acetone and stained used hematoxylin and eosin (H&E) (Vector Labs, Burlingame, CA and Sigma, Steinheim, Germany) on the cut histological sections. Histomorphometric analysis was performed by taking the most central section at the midline of the parietal bones and two adjacent sections from each calvaria. Using 20× magnification, the region of interest was defined as the area of soft tissue in continuity with the midline suture. To determine bone thickness, the sections were divided at 1 cm steps from 2 cm to the left and right of the sides of the midline suture using a digital ruler. The total tissue thickness (ThT) and the bone thickness (BT) were then manually measured at these 5 points. The measurements were expressed as a mean ratio of BT/ThT. Osteoclast-like cells were identified using a leukocyte acid phosphatase kit, TRAP (Sigma) as large multinucleated cells located on the bone perimeter within a resorption lacuna.
Calvaria culture
The calvaria were removed as a whole under sterile conditions from five animals per group randomly assigned for culture. Each calvaria was placed into one well of a 12-well plate and cultured with 2 mL Dulbecco’s modified Eagle’s medium (DMEM) with glutamine (Gibco, Grand Island, NY), and 1% Anti-mycotic/Anti-biotic (Gibco) and incubated for 24 h at 37°C with 5% CO2.42 The culture medium was then collected and stored at −80°C for assay of TNF-α, IL-1ra, RANKL, and OPG secretion.
ELISA analysis of cultured calvaria
ELISA analysis for TNF-α, IL-1ra, RANKL, and OPG secretion from calvaria utilized specific kits (R&D, Minneapolis, MN).
Western blotting
Total cellular protein was extracted using Tri-Reagent Trizol (Invitrogen, Carlsbad, CA) in accordance with the manufacturer’s protocol. Following solubilization, proteins were quantified using BCA protein quantification (Thermo Scientific, Rockford, IL). Totally, 50 μg of protein from each calvaria was loaded into each well and electrophoresed on a 4–20% gradient SDS Tris-Glycine gel (Invitrogen) and transferred onto PVDF membranes (Invitrogen). Primary antibodies directed against rabbit anti-mouse Ym1 monoclonal antibodies (Stem Cell Biotechnologies, Vancouver, BC, Canada) 1:500, and rabbit anti-mouse iNOS (Abcam, Cambridge, MA) 1:500 were used. Detection of GAPDH (Cell Signaling, Danvers, MA) 1:800 was also done as a control. Detection of Ym1, iNOS, and GAPDH were then performed and visualized using an ECL detection system and hyperfilm chemiluminescence.43 Images were analyzed using Image J Software (National Institutes of Health, USA) for densitometry analysis and presented as a percentage of the total size of all the measured peaks.27
Statistical analysis
Data used for statistical analysis were first assessed using a Kolmogorov-Smirnov test to ensure normality and Gaussian distribution using Prism 4.1 (Graphpad Software, La Jolla, CA). For all analyses, a one-way ANOVA was used with a post-hoc Neuman Keuls test to compare each group. Data was reported as mean ± standard error. A p < 0.05 was set as the threshold of significance.
RESULTS
MicroCT analysis
We used μCT analysis to compare the bone volume to total volume fraction (BVF) in the four experimental groups. The BVF for each animal in Groups 2, 3, and 4 was compared with the average BVF for animals from Group 1 for statistical analysis. For the polyethylene only treated group, the BVF was 0.60 ± 0.02, compared with the BVF in the polyethylene treated group with IL-4 for 14 days of 0.91 ± 0.03, and the polyethylene group with IL-4 for 7 days of 0.71 ± 0.05 [Fig. 1(a,b)]. The p value was less than 0.05 between all groups.
FIGURE 1.
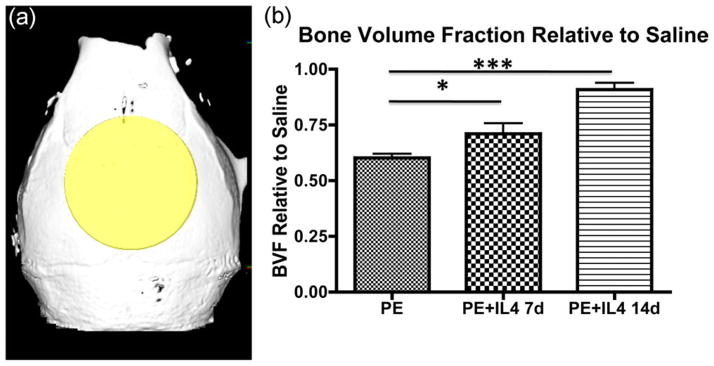
Administration of IL-4 diminishes polyethylene particle induced osteolysis. (a) Polyethylene particle induced osteolysis in C57B6 mice treated with saline, polyethylene particles, polyethylene particles with IL-4 administered 7 days later for 7 days, and polyethylene with IL-4 administered for 14 days was assessed within the volume of interest by longitudinal 3D micro-computed tomography (μCT). The volume of interest is indicated by the yellow shaded region as a 6 mm × 6 mm × 2 mm cylinder. (b) Graphical representation of μCT quantifying the bone volume fraction (BVF) 14 days after PE particle implantation onto calvarium relative to saline control. For the polyethylene only treated group, the BVF was 0.60 ± 0.02, compared with the BVF in the polyethylene group with IL-4 for 7 days of 0.71 ± 0.05, and the polyethylene treated group with IL-4 for 14 days of 0.91 ± 0.03 (p < 0.05 between all groups). *p < 0.05, ***p < 0.001. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Histomorphometry and TRAP analysis
Histomorphometric analysis of the BT/ThT showed a significant difference (p < 0.05) between all groups except between PE+IL4 for 7 days and saline [Fig. 2(a,b)]. The BT/ThT for polyethylene only group was 0.45 ± 0.02, which was significantly lower than saline (0.56 ± 0.03), polyethylene with IL-4 for 14 days group (0.65 ± 0.02), and polyethylene with IL-4 for 7 days group (0.56 ± 0.02) (p < 0.05). The BT/ThT was decreased for polyethylene alone group compared with all other groups, and the BT/ThT was increased in the polyethylene group with IL-4 for 14 days relative to the saline control, as well as all other groups.
FIGURE 2.
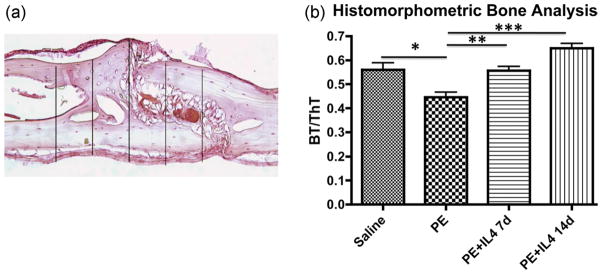
Histomorphometric analysis was performed by assessing the bone thickness (BT) to total tissue thickness (ThT) ratio. (a) Using 20x magnification, the region of interest was defined as the area 2 cm to the left and right of the midline sagittal suture. The area of interest was divided into five parts, and the ThT and BT were measured at these 5 points. Measurements were expressed as a mean ratio of BT/ThT. Shown is a representative image from the polyethylene with IL-4 for 14 days group. (b) The BT/ThT ratio showed a significant difference (p < 0.05) between all groups except between polyethylene +IL4 for 7 days and saline. The BT/ThT for PE only was 0.45 ± 0.02, which was significantly lower than saline (0.56 ± 0.03), polyethylene with IL-4 for 7 days (0.56 ± 0.02), and polyethylene with IL-4 for 14 days (0.65 ± 0.02) (p < 0.05). The BT/ThT was decreased for polyethylene compared to all groups, and the BT/ThT was increased in PE with IL-4 for 14 days relative to the saline control, as well as all other groups. *p < 0.05, **p < 0.01, ***p < 0.001. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
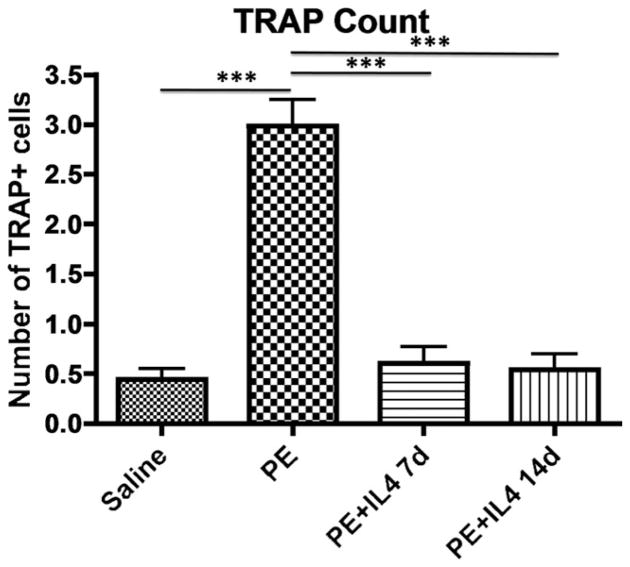
TRAP staining showed a significantly higher number of TRAP positive cells in the calvaria of animals with implanted polyethylene particles alone (2.99 ± 0.26) compared with the saline (0.44 ± 0.11), polyethylene with IL-4 for 14 days (0.54 ± 0.16), and polyethylene with IL-4 for 7 days (0.60 ± 0.16) groups (p < 0.0001) (Fig. 3). There was no difference in TRAP staining between the saline only group and the groups that received IL-4 with polyethylene particles.
FIGURE 3.
Osteoclast-like activity was identified using leukocyte acid phosphatase (TRAP) staining as large multinucleated cells at the bone perimeter within resorption lacuna (images taken at ×20). TRAP analysis shows a predominance of osteoclast-like cells in calvaria treated with polyethylene particles, and is decreased with the administration of IL-4. *** p < 0.001.
Western blot analysis
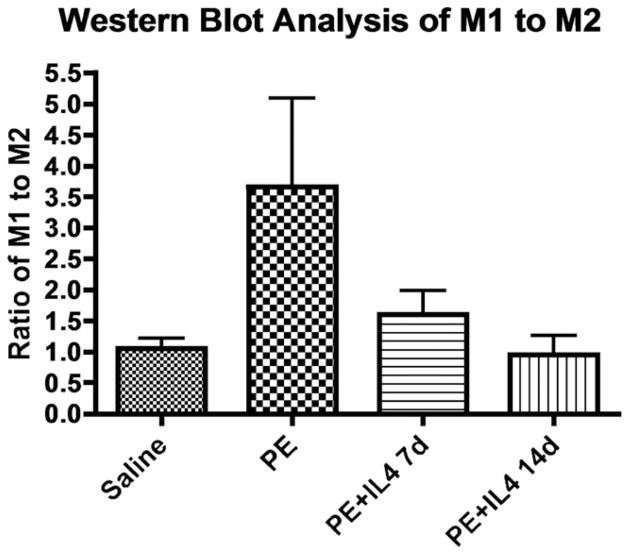
Western blot analysis of the M1/M2 ratio was done by comparing the ratio of iNOS/GAPDH to Ym1/GAPDH. There was a significant difference in the M1/M2 ratio between the four groups using the one-way ANOVA analysis. The average densitometry M1/M2 ratio for the polyethylene only group was 2.33 ± 1.13 as compared with saline (0.89 ± 0.20), polyethylene with IL-4 for 14 days (0.80 ± 0.28), and polyethylene with IL-4 for 7 days (2.03 ± 0.51) groups (Fig. 4). Although there was no significant difference between groups by post-hoc Neuman Keuls analysis, there was a strong trend of a higher M1/M2 ratio in the polyethylene only, and polyethylene with IL-4 for 7 days groups as compared with saline and polyethylene with IL-4 for 14 days.
FIGURE 4.
Total cellular protein in the calvarium of the four groups was used for Western blotting staining for the M1 marker iNOS and the M2 marker, Ym1, using 50 μg of protein per well. M1 and M2 ratios in the calvarium were calculated using densitometry analysis. The average densitometry M1/M2 ratio for the polyethylene only group was 2.33 ± 1.13 as compared with saline (0.89 ± 0.20), polyethylene with IL-4 for 7 days (2.03 ± 0.51), and polyethylene with IL-4 for 14 days (0.80 ± 0.28) groups. This suggests that there is a higher M1/M2 ratio in the polyethylene only treated group, which decreases with the administration of IL-4.
ELISA analysis
Cell culture supernatants of cultured calvaria were analyzed for secretion of RANKL, OPG, TNF-α, and IL-1ra 24 h after culture. There was a significantly higher release of RANKL from the calvaria treated with polyethylene only (24.50 ± 3.00 pg/mL) compared with polyethylene with IL-4 for 14 days (10.36 ± 1.10 pg/mL) (p < 0.05). The RANKL secretion from saline treatment was 16.86 ± 2.50 pg/mL, and the RANKL secretion from polyethylene with IL-4 for 7 days treated calvaria was 17.32 ± 1.91 pg/mL [Fig. 5(a)]. There was no significant difference between other groups as assessed by post-hoc Neuman Keuls.
FIGURE 5.
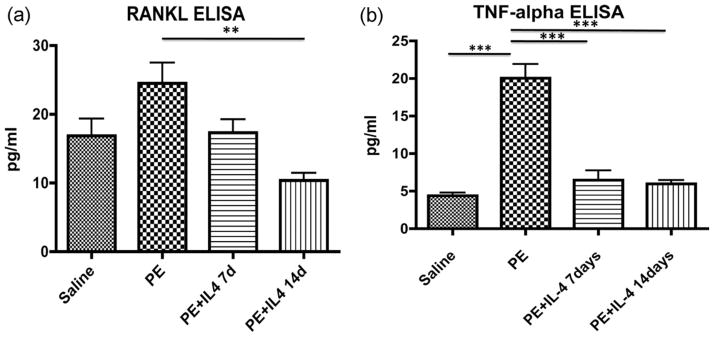
Cytokine release of RANKL and TNF-α showed a significantly higher release of the proinflammatory cytokines following polyethylene particle placement, which was mitigated by IL-4 administration. ELISA analysis was performed on cell culture supernatants of cultured calvarium 24 h after culture (n = 5 per group). Analysis was performed for TNF-α, IL-1ra, RANKL, and OPG. Results are presented as mean ± standard error of the mean. (a) ELISA analysis shows s a significantly higher release of RANKL from the calvaria treated with polyethylene only (24.50 ± 3.00 pg/mL) compared with polyethylene with IL-4 for 14 days (10.36 ± 1.10 pg/mL) (p < 0.05). There was no significant difference between other groups as assessed by post-hoc Neuman Keuls. (b) TNF-α release from the cultured calvaria showed a significantly higher release from the polyethylene treated group (20.04 ± 1.85 pg/mL) compared with saline (4.36 ± 0.41 pg/mL), polyethylene with IL-4 for 7 days (6.46 ± 1.28 pg/mL), and polyethylene with IL-4 for 14 days (5.97 ± 0.50 pg/mL) (p < 0.001). *p < 0.05, **p < 0.01, ***p < 0.001.
Analysis of TNF-α release from the cultured calvaria showed a significantly higher release from the polyethylene treated group (20.04 ± 1.85 pg/mL) compared with saline (4.36 ± 0.41 pg/mL), polyethylene with IL-4 for 14 days (5.97 ± 0.50 pg/mL), and polyethylene with IL-4 for 7 days (6.46 ± 1.28 pg/mL) groups (p < 0.001). There was no significant difference between the other three groups [Fig. 5(b)].
Analysis of OPG secretion showed that there was a significantly decreased secretion in the polyethylene with IL-4 for 7 days group (2160.98 ± 201.20 pg/mL) compared with saline only (3046.19 ± 41.20 pg/mL), polyethylene only (2872.42 ± 96.0 pg/mL), and polyethylene with IL-4 for 14 days (2160.98 ± 204.20 pg/mL) groups (p < 0.001). There was no significant difference between the other three groups [Fig. 6(a)]. Analysis of the RANKL/OPG ratio showed that both saline (0.0055 ± 0.0008) and polyethylene with IL-4 for 14 days (0.0035 ± 0.0004) were significantly decreased compared to polyethylene (0.0085 ± 0.001) and polyethylene with IL-4 for 7 days (0.008 ± 0.0009) groups (p < 0.05) [Fig. 6(b)].
FIGURE 6.
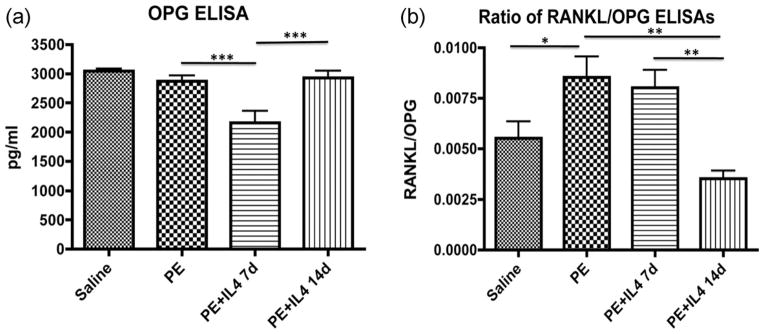
Cytokine release of OPG and the ratio of RANKL/OPG showed that IL-4 helped to decrease the relative release of proinflammatory mediators. (a) ELISA OPG analysis showed a decreased section in the polyethylene with IL-4 for 7 days group compared to all other groups (p < 0.0001). (b) Analysis of the RANKL/OPG ratio showed that both saline (0.0055 ± 0.0008) and polyethylene with IL-4 for 14 days (0.0035 ± 0.0004) were significantly decreased compared with polyethylene (0.0085 ± 0.001) and polyethylene with IL-4 for 7 days (0.008 ± 0.0009) groups (p < 0.05). *p < 0.05, **p < 0.01, ***p < 0.001.
Analysis of the IL-1ra release showed a significant difference between all groups (p < 0.05) except for polyethylene with IL-4 for 14 days compared to polyethylene alone. The IL-1ra release from the saline group was 2117 ± 351.6 pg/mL, from the polyethylene group 1349 ± 109.7 pg/mL, from polyethylene with IL-4 for 14 days group 1150 ± 92.95 pg/mL, and from polyethylene with IL-4 for 7 days group 466.2 ± 46.42 pg/mL [Fig. 7(a)]. Analysis of the IL-1ra to TNF-α ratio showed a significant increase in the ratio in the polyethylene with IL-4 for 14 days (178.68 ± 18.01) group compared with polyethylene only (67.85 ± 5.64) and polyethylene with IL-4 for 7 days groups (74.84 ± 6.88) (p < 0.001) [Fig. 7(b)].
FIGURE 7.
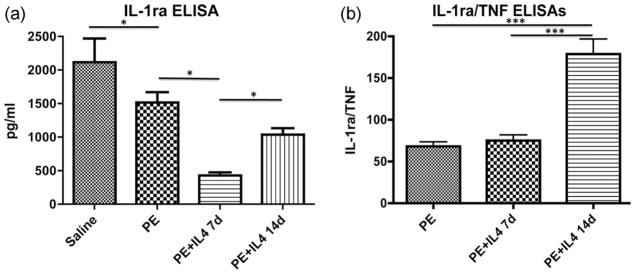
Cytokine release of IL-1ra, a predominant M2 macrophage cytokine, and the ratio of IL-1ra/TNF-α showed a significant increase in the relative amount of the anti-inflammatory mediator after 14 days of IL-4 administration. (a) Analysis of the IL-1ra release showed a significant difference between all groups (p < 0.05) except for PE with IL-4 for 14 days compared to polyethylene alone. (b) Analysis of the IL-1ra to TNF-α ratio showed a significant increase in the ratio in the polyethylene with IL-4 for 14 days (178.68 ± 18.01) compared with polyethylene only (67.85 ± 5.64) and polyethylene with IL-4 for 7 days (74.84 ± 6.88) (p < 0.001). *p < 0.05, **p < 0.01, ***p < 0.001.
DISCUSSION
The purpose of this study was to determine the effect of IL-4 and macrophage polarization on polyethylene-particle induced osteolysis using the murine calvarial model. Although not a long bone, wear debris induced osteolysis on the calvaria allows for preclinical preliminary studies of particle effects, and the assessment of potential treatments such as IL-4.44–47 In addition, the model allowed for daily administration of IL-4, which can more closely mimic the desired clinical administration such as with a scaffold, coating or other drug delivery device. The model is only 2 weeks in time, whereas the typical clinical situation of osteolysis following TJR may take place over years. Therefore, local IL-4 delivery to patients with wear debris induced osteolysis may require a longer time course or different onset of administration relative to this murine calvarial model.
Metal-on-UHWMPE implants account for at least 50–70% of the bearing surfaces used in TJR in the USA.48,49 Therefore, particle wear debris and their biological sequelae impact implant survival, creating a need for modulation of the inflammatory process to improve implant longevity.50 This is even more important in clinical scenarios such as total knee replacement in which the majority of implants consist of conventional polyethylene.
Our current study has demonstrated an increase in the BVF and BT/ThT in murine calvaria treated with IL-4 following PE particle implantation, suggesting that addition of IL-4 decreased bone resorption associated with PE particle-induced. This result was seen with both 14 days of acute, continuous administration and 7 days of delayed IL-4 administration after an inflammatory environment had already been established, suggesting that IL-4 can manipulate osteoclastogenesis and bone resorption in both a preventative and reparative manner respectively. TRAP staining also found a decrease in the number of osteoclasts and precursors in the calvaria of animals treated with IL-4, further suggesting that IL-4 was able to decrease osteoclastogenesis. This may be through enhancement of OPG production, as well as inhibition of RANKL production, which were shown by ELISAs after IL-4 treatment for 14 days. As seen with Western blotting, IL-4 addition also decreased the M1/M2 ratio compared to PE alone, suggesting that IL-4 may act through multiple mechanisms, including inducing an M2 phenotype, to decrease osteoclastogenesis and inflammation. IL-4 administration also increased the IL-1ra to TNF-α ratio, suggesting an induced anti-inflammatory state rather than the inflammatory state associated with PE-particle induced osteolysis.
Previous studies have reported that there was a higher ratio of M1 to M2 macrophages in retrieved periprosthetic tissues from failed implants.27 Furthermore, PMMA particles could polarize macrophages towards an M1 response, which could be driven towards an M2 phenotype with the addition of IL-4 in vitro.27 We found that polyethylene particles preferentially polarize macrophages towards an M1 response, but that the addition of IL-4 in both an acute and chronic model can polarize these M1 macrophages towards an M2 response, mitigating the polyethylene particle induced osteolysis. In total these 3 studies show that polyethylene and PMMA particles in in vivo, in vitro, and clinical studies all selectively activated M1 macrophages, inciting a proinflammatory response, mediated in part through the release of IL-1 and TNF-α. However, the addition of IL-4 was able to modulate this response both in vivo and in vitro, polarizing the M1 macrophages towards an M2 anti-inflammatory response through the release of IL-10, IL-13, and IL-4. This suggests that macrophage polarization plays a significant role in particle-induced osteolysis, but that this effect may be modulated through the addition of IL-4, an M2 promoter.
Our study has implications for the use of IL-4 as a biological agent to modulate the inflammatory reaction that occurs in response to wear particle production. Preliminary trials have been initiated to examine the effect of manipulating macrophage polarization on disease states such as atherosclerosis, obesity, asthma, and chronic inflammation.21 Use of an agent, such as a CD40 antagonist, under trial to inhibit M1 pathways in atherosclerosis, in addition to IL-4 and PPARγ agonists to promote M2 pathways may be of interest in controlling the macrophage response and subsequent inflammatory response to wear debris.21,51–54 Additionally, work by Wang et al. found a decrease in bone resorption with the addition of recombinant IL-4 together with OPG in polyethylene particle induced osteolysis.37 Future work might examine the potential to create an IL-4 eluting scaffold or drug delivery device to alter the macrophage response to wear debris towards an anti-inflammatory, bone healing response.
Acknowledgments
This work was supported in part by NIH (NIAMS) Grants 2RO1 AR055650-05 and 1RO1 AR063717-01.
References
- 1.Koulouvaris P, Ly K, Ivashkiv LB, Bostrom MP, Nestor BJ, Sculco TP, Purdue PE. Expression profiling reveals alternative macrophage activation and impaired osteogenesis in periprosthetic osteolysis. J Orthop Res. 2008;26:106–116. doi: 10.1002/jor.20486. [DOI] [PubMed] [Google Scholar]
- 2.Ma T, Goodman SB. Biological effects of wear debris from joint arthroplasties. In: Wnek GE, Gary LB, editors. Encyclopedia of Biomaterials and Biomedical Engineering. London: Informa Healthcare; 2008. [Google Scholar]
- 3.Purdue PE, Koulouvaris P, Nestor BJ, Sculco TP. The central role of wear debris in periprosthetic osteolysis. Hss J. 2006;2:102–113. doi: 10.1007/s11420-006-9003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chiu R, Ma T, Smith RL, Goodman SB. Ultrahigh molecular weight polyethylene wear debris inhibits osteoprogenitor proliferation and differentiation in vitro. J Biomed Mater Res A. 2009;89:242–247. doi: 10.1002/jbm.a.32001. [DOI] [PubMed] [Google Scholar]
- 5.Chiu R, Smith KE, Ma GK, Ma T, Smith RL, Goodman SB. Polymethylmethacrylate particles impair osteoprogenitor viability and expression of osteogenic transcription factors Runx2, osterix, and Dlx5. J Orthop Res. 2010;28:571–577. doi: 10.1002/jor.21035. [DOI] [PubMed] [Google Scholar]
- 6.Wooley PH, Schwarz EM. Aseptic loosening. Gene Ther. 2004;11:402–407. doi: 10.1038/sj.gt.3302202. [DOI] [PubMed] [Google Scholar]
- 7.Marshall A, Ries MD, Paprosky W. How prevalent are implant wear and osteolysis, and how has the scope of osteolysis changed since 2000? J Am Acad Orthop Surg. 2008;16 (Suppl 1):S1–S6. doi: 10.5435/00124635-200800001-00003. [DOI] [PubMed] [Google Scholar]
- 8.Goodman S. Wear particulate and osteolysis. Orthop Clin North Am. 2005;36:41–8. vi. doi: 10.1016/j.ocl.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 9.Goodman SB. Wear particles, periprosthetic osteolysis and the immune system. Biomaterials. 2007;28:5044–5048. doi: 10.1016/j.biomaterials.2007.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tuan RS, Lee FY, YTK, Wilkinson JM, Smith RL. What are the local and systemic biologic reactions and mediators to wear debris, and what host factors determine or modulate the biologic response to wear particles? J Am Acad Orthop Surg. 2008;16 (Suppl 1):S42–S48. doi: 10.5435/00124635-200800001-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ingham E, Fisher J. The role of macrophages in osteolysis of total joint replacement. Biomaterials. 2005;26:1271–1286. doi: 10.1016/j.biomaterials.2004.04.035. [DOI] [PubMed] [Google Scholar]
- 12.Ho VW, Sly LM. Derivation and characterization of murine alternatively activated (M2) macrophages. Methods Mol Biol. 2009;531:173–185. doi: 10.1007/978-1-59745-396-7_12. [DOI] [PubMed] [Google Scholar]
- 13.Cheng J, Liu J, Shi Z, Xu D, Luo S, Siegal GP, Feng X, Wei S. Interleukin-4 inhibits RANKL-induced NFATc1 expression via STAT6: A novel mechanism mediating its blockade of osteoclastogenesis. J Cell Biochem. 2011;112:3385–3392. doi: 10.1002/jcb.23269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu J, Wu HF, Ang ES, Yip K, Woloszyn M, Zheng MH, Tan RX. NF-kappaB modulators in osteolytic bone diseases. Cytokine Growth Factor Rev. 2009;20:7–17. doi: 10.1016/j.cytogfr.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 15.Granchi D, Amato I, Battistelli L, Ciapetti G, Pagani S, Avnet S, Baldini N, Giunti A. Molecular basis of osteoclastogenesis induced by osteoblasts exposed to wear particles. Biomaterials. 2005;26:2371–2379. doi: 10.1016/j.biomaterials.2004.07.045. [DOI] [PubMed] [Google Scholar]
- 16.Goater JJ, O’Keefe RJ, Rosier RN, Puzas JE, Schwarz EM. Efficacy of ex vivo OPG gene therapy in preventing wear debris induced osteolysis. J Orthop Res. 2002;20:169–173. doi: 10.1016/S0736-0266(01)00083-3. [DOI] [PubMed] [Google Scholar]
- 17.Brown BN, Valentin JE, Stewart-Akers AM, McCabe GP, Badylak SF. Macrophage phenotype and remodeling outcomes in response to biologic scaffolds with and without a cellular component. Biomaterials. 2009;30:1482–1491. doi: 10.1016/j.biomaterials.2008.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 19.Brown BN, Ratner BD, Goodman SB, Amar S, Badylak SF. Macrophage polarization: An opportunity for improved outcomes in bio-materials and regenerative medicine. Biomaterials. 2012;33:3792–3802. doi: 10.1016/j.biomaterials.2012.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Purdue PE. Alternative macrophage activation in periprosthetic osteolysis. Autoimmunity. 2008;41:212–217. doi: 10.1080/08916930701694626. [DOI] [PubMed] [Google Scholar]
- 21.Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2010;122:787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sharda DR, Yu S, Ray M, Squadrito ML, De Palma M, Wynn TA, Morris SM, Jr, Hankey PA. Regulation of macrophage arginase expression and tumor growth by the ron receptor tyrosine kinase. J Immunol. 187:2181–2192. doi: 10.4049/jimmunol.1003460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kawanishi N, Yano H, Yokogawa Y, Suzuki K. Exercise training inhibits inflammation in adipose tissue via both suppression of macrophage infiltration and acceleration of phenotypic switching from M1 to M2 macrophages in high-fat-diet-induced obese mice. Exerc Immunol Rev. 2010;16:105–118. [PubMed] [Google Scholar]
- 24.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raes G, Noel W, Beschin A, Brys L, de Baetselier P, Hassanzadeh GH. FIZZ1 and Ym as tools to discriminate between differentially activated macrophages. Dev Immunol. 2002;9:151–159. doi: 10.1080/1044667031000137629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jensen TO, Schmidt H, Moller HJ, Hoyer M, Maniecki MB, Sjoegren P, Christensen IJ, Steiniche T. Macrophage markers in serum and tumor have prognostic impact in American Joint Committee on Cancer stage I/II melanoma. J Clin Oncol. 2009;27:3330–3337. doi: 10.1200/JCO.2008.19.9919. [DOI] [PubMed] [Google Scholar]
- 27.Rao AJ, Gibon E, Ma T, Yao Z, Lane Smith R, Goodman SB. Revision joint replacement, wear particles, and macrophage polarization. Acta Biomater. 2012;8:2815–2823. doi: 10.1016/j.actbio.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chomarat P, Banchereau J. An update on interleukin-4 and its receptor. Eur Cytokine Netw. 1997;8:333–344. [PubMed] [Google Scholar]
- 29.Watanabe K, Tanaka Y, Morimoto I, Yahata K, Zeki K, Fujihira T, Yamashita U, Eto S. Interleukin-4 as a potent inhibitor of bone resorption. Biochem Biophys Res Commun. 1990;172:1035–1041. doi: 10.1016/0006-291x(90)91550-c. [DOI] [PubMed] [Google Scholar]
- 30.Kasono K, Sato K, Sato Y, Tsushima T, Shizume K, Demura H. Inhibitory effect of interleukin-4 on osteoclast-like cell formation in mouse bone marrow culture. Bone Miner. 1993;21:179–188. doi: 10.1016/s0169-6009(08)80229-2. [DOI] [PubMed] [Google Scholar]
- 31.Yamada A, Takami M, Kawawa T, Yasuhara R, Zhao B, Mochizuki A, Miyamoto Y, Eto T, Yasuda H, Nakamichi Y, et al. Interleukin-4 inhibition of osteoclast differentiation is stronger than that of interleukin-13 and they are equivalent for induction of osteoprotegerin production from osteoblasts. Immunology. 2007;120:573–579. doi: 10.1111/j.1365-2567.2006.02538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bizzarri C, Shioi A, Teitelbaum SL, Ohara J, Harwalkar VA, Erdmann JM, Lacey DL, Civitelli R. Interleukin-4 inhibits bone resorption and acutely increases cytosolic Ca2+ in murine osteoclasts. J Biol Chem. 1994;269:13817–13824. [PubMed] [Google Scholar]
- 33.Miossec P, Briolay J, Dechanet J, Wijdenes J, Martinez-Valdez H, Banchereau J. Inhibition of the production of proinflammatory cytokines and immunoglobulins by interleukin-4 in an ex vivo model of rheumatoid synovitis. Arthritis Rheum. 1992;35:874–883. doi: 10.1002/art.1780350805. [DOI] [PubMed] [Google Scholar]
- 34.Riancho JA, Zarrabeitia MT, Mundy GR, Yoneda T, Gonzalez-Macias J. Effects of interleukin-4 on the formation of macrophages and osteoclast-like cells. J Bone Miner Res. 1993;8:1337–1344. doi: 10.1002/jbmr.5650081108. [DOI] [PubMed] [Google Scholar]
- 35.Miossec P, Chomarat P, Dechanet J, Moreau JF, Roux JP, Delmas P, Banchereau J. Interleukin-4 inhibits bone resorption through an effect on osteoclasts and proinflammatory cytokines in an ex vivo model of bone resorption in rheumatoid arthritis. Arthritis Rheum. 1994;37:1715–1722. doi: 10.1002/art.1780371202. [DOI] [PubMed] [Google Scholar]
- 36.Lubberts E, Joosten LA, Chabaud M, van Den Bersselaar L, Oppers B, Coenen-De Roo CJ, Richards CD, Miossec P, van Den Berg WB. IL-4 gene therapy for collagen arthritis suppresses synovial IL-17 and osteoprotegerin ligand and prevents bone erosion. J Clin Invest. 2000;105:1697–1710. doi: 10.1172/JCI7739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Y, Zhou R, Wu NN, Mou YQ, Li RD, Deng ZL. Interleukin-4 and osteoprotegerin suppress polyethylene wear debris-induced osteolysis in a murine air pouch model. Nan Fang Yi Ke Da Xue Xue Bao. 2011;31:1709–1713. [PubMed] [Google Scholar]
- 38.Lange C, Schuler T, Blankenstein T. Interleukin 4 gene-defective mice reconstituted with wild-type bone marrow fail to produce normal immunoglobulin E levels. J Exp Med. 1998;187:1487–1493. doi: 10.1084/jem.187.9.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goldhill J, Morris SC, Maliszewski C, Urban JF, Jr, Funk CD, Finkelman FD, Shea-Donohue T. Interleukin-4 modulates cholinergic neural control of mouse small intestinal longitudinal muscle. Am J Physiol. 1997;272(Part 1):G1135–G1140. doi: 10.1152/ajpgi.1997.272.5.G1135. [DOI] [PubMed] [Google Scholar]
- 40.Pearl JI, Ma T, Irani AR, Huang Z, Robinson WH, Smith RL, Goodman SB. Role of the Toll-like receptor pathway in the recognition of orthopedic implant wear-debris particles. Biomaterials. 2011;32:5535–5542. doi: 10.1016/j.biomaterials.2011.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gibon E, Yao Z, Rao AJ, Zwingenberger S, Batke B, Valladares R, Smith RL, Biswal S, Gambhir SS, Goodman SB. Effect of a CCR1 receptor antagonist on systemic trafficking of MSCs and polyethylene particle-associated bone loss. Biomaterials. 2012;33:3632–3638. doi: 10.1016/j.biomaterials.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nich C, Langlois J, Marchadier A, Vidal C, Cohen-Solal M, Petite H, Hamadouche M. Oestrogen deficiency modulates particle-induced osteolysis. Arthritis Res Ther. 2011;13:R100. doi: 10.1186/ar3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.da Silva RP, Gordon S. Phagocytosis stimulates alternative glycosylation of macrosialin (mouse CD68), a macrophage-specific endosomal protein. Biochem J. 1999;338(Part 3):687–694. [PMC free article] [PubMed] [Google Scholar]
- 44.Langlois J, Hamadouche M. New animal models of wear-particle osteolysis. Int Orthop. 2011;35:245–251. doi: 10.1007/s00264-010-1143-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schwarz EM, Benz EB, Lu AP, Goater JJ, Mollano AV, Rosier RN, Puzas JE, Okeefe RJ. Quantitative small-animal surrogate to evaluate drug efficacy in preventing wear debris-induced osteolysis. J Orthop Res. 2000;18:849–855. doi: 10.1002/jor.1100180602. [DOI] [PubMed] [Google Scholar]
- 46.von Knoch F, Heckelei A, Wedemeyer C, Saxler G, Hilken G, Brankamp J, Sterner T, Landgraeber S, Henschke F, Loer F, et al. Suppression of polyethylene particle-induced osteolysis by exogenous osteoprotegerin. J Biomed Mater Res A. 2005;75:288–294. doi: 10.1002/jbm.a.30441. [DOI] [PubMed] [Google Scholar]
- 47.Greenfield EM, Tatro JM, Smith MV, Schnaser EA, Wu D. PI3K-gamma deletion reduces variability in the in vivo osteolytic response induced by orthopaedic wear particles. J Orthop Res. 2011;29:1649–1653. doi: 10.1002/jor.21440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bozic KJ, Kurtz S, Lau E, Ong K, Chiu V, Vail TP, Rubash HE, Berry DJ. The epidemiology of bearing surface usage in total hip arthroplasty in the United States. J Bone Joint Surg Am. 2009;91:1614–1620. doi: 10.2106/JBJS.H.01220. [DOI] [PubMed] [Google Scholar]
- 49.Bozic KJ, Ong K, Lau E, Kurtz SM, Vail TP, Rubash HE, Berry DJ. Risk of complication and revision total hip arthroplasty among Medicare patients with different bearing surfaces. Clin Orthop Relat Res. 2010;468:2357–2362. doi: 10.1007/s11999-010-1262-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gibon E, Ma T, Ren PG, Fritton K, Biswal S, Yao Z, Smith L, Goodman SB. Selective inhibition of the MCP-1-CCR2 ligand-receptor axis decreases systemic trafficking of macrophages in the presence of UHMWPE particles. J Orthop Res. 2012;30:547–553. doi: 10.1002/jor.21548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Beatty GL, Chiorean EG, Fishman MP, Saboury B, Teitelbaum UR, Sun W, Huhn RD, Song W, Li D, Sharp LL, et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science. 2011;331:1612–1616. doi: 10.1126/science.1198443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stienstra R, Duval C, Keshtkar S, van der Laak J, Kersten S, Muller M. Peroxisome proliferator-activated receptor gamma activation promotes infiltration of alternatively activated macrophages into adipose tissue. J Biol Chem. 2008;283:22620–22627. doi: 10.1074/jbc.M710314200. [DOI] [PubMed] [Google Scholar]
- 53.Lu M, Sarruf DA, Talukdar S, Sharma S, Li P, Bandyopadhyay G, Nalbandian S, Fan W, Gayen JR, Mahata SK, et al. Brain PPAR-gamma promotes obesity and is required for the insulin-sensitizing effect of thiazolidinediones. Nat Med. 2011;17:618–622. doi: 10.1038/nm.2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Charo IF. Macrophage polarization and insulin resistance: PPAR-gamma in control. Cell Metab. 2007;6:96–98. doi: 10.1016/j.cmet.2007.07.006. [DOI] [PubMed] [Google Scholar]