Abstract
We show that increased plasma superoxide dismutase 1 (SOD1) levels are statistically significant predictors of the failure of pentavalent antimony treatment for cutaneous leishmaniasis caused by Leishmania braziliensis. In Leishmania amazonensis–infected patients, host SOD1 levels can be used to discriminate between localized and drug-resistant diffuse cutaneous leishmaniasis. Using in situ transcriptomics (nCounter), we demonstrate a significant positive correlation between host SOD1 and interferon α/β messenger RNA (mRNA) levels, as well as interkingdom correlation between host SOD1 and parasite SOD2/4 mRNA levels. In human macrophages, in vitro treatment with SOD1 increases the parasite burden and induces a diffuse cutaneous leishmaniasis–like morphology. Thus, SOD1 is a clinically relevant biomarker and a therapeutic target in both localized and diffuse cutaneous leishmaniasis.
Keywords: Cutaneous leishmaniasis, Leishmania amazonensis, Leishmania braziliensis, Diffuse cutaneous leishmaniasis, Oxidative burst, Parasite escape mechanism, Interkingdom signaling, Superoxide dismutase, Biomarker, Therapeutic failure
Leishmaniasis is endemic in several parts of the world, with a global prevalence of >12 million cases. Mainly classified in 2 distinct clinical forms, leishmaniasis can affect the skin (cutaneous leishmaniasis [CL]) or viscera (visceral leishmaniasis [VL]). CL, with 1.5–2 million new cases per year, is an emerging infectious disease in several countries, owing to behavioral and environmental changes and human immunodeficiency virus coinfection [1]. The clinical spectrum of CL includes localized CL (LCL), a mild form; diffuse CL (DCL), a disfiguring form; and mucocutaneous leishmaniasis (MCL). Leishmania braziliensis causes LCL and MCL, whereas Leishmania amazonensis causes LCL and, sporadically, DCL [2–4]. A low parasite load and a variable tendency for self-healing characterize LCL, but treatment is strongly recommended, to reduce scarring and prevent progression to severe MCL. Pentavalent antimonials (SbV) are currently the first-line treatment for LCL, but because 24.4% of the treated patients do not achieve cure with a single cycle of SbV, ≥2 cycles are indicated to heal the lesions [5]. DCL is characterized by multiple nonulcerative nodules with an extremely high parasite burden, as well as a nonhealing or relapsing phenotype [1, 3, 4, 5]. DCL is typically refractory to SbV and second-line treatment. Recently, miltefosine treatment induced clinical improvement and reduced parasite burden in a cohort of patients with DCL, but treatment suspension led to relapse [6].
As obligatory intramacrophage parasites, Leishmania species have developed several evasion mechanisms to avoid human macrophage leishmanicidal activity. One such mechanism may involve superoxide dismutase (SOD), a conserved isoenzyme responsible for detoxifying reactive superoxide both in Leishmania and human organisms. Previous studies have demonstrated that SOD-deficient New World Leishmania species, as well as Old World Leishmania species, have enhanced sensitivity to exogenous superoxide in axenic culture and reduced survival in mouse and human macrophages [7, 8]. We have previously shown an interferon β (IFN-β)–induced SOD1-mediated inhibition of superoxide-dependent parasite killing in Leishmania-infected human macrophages in vitro [9]. In addition, patients with CL have increased SOD activity in plasma, compared with healthy subjects [10]. We hypothesized that plasma levels of host SOD1 might reflect clinical status during CL and might therefore represent a candidate biomarker.
PATIENTS AND METHODS
This study was approved by the Ethics Committee of the Gonçalo Moniz Research Center. Informed consent was obtained from all patients and healthy controls. CL was diagnosed in patients as described elsewhere [11], according to characteristic lesion morphology, positive skin test results, seropositivity to Leishmania antigen, and/or presence of parasites in the lesion. A total of 58 patients with LCL due to L. braziliensis (27 males; mean age [±SD], 29.6 ± 2.3 years) were recruited and treated in 2 outpatient clinics (Jequié and Jiquiriçá-BA, NE Brazil) covering the same rural area. Ten patients with LCL due to L. amazonensis (6 males; mean age [±SD], 38.8 ± 6 years) were recruited and treated at the Professor Edgar Santos University Hospital (Salvador-BA, NE Brazil). Plasma samples from patients with LCL were collected at diagnosis (before treatment). In addition, a paired sample was obtained from 13 patients at the time of definite clinical cure (range, 68–417 days). Patients with LCL (due to either L. braziliensis or L. amazonensis) received standard intravenous pentavalent antimony (Glucantime; Rhodia, 20 mg/kg/day for 20 days). Cure was defined by the complete scarring of lesions, without induration and relapse during 2 years of follow-up. Although all L. amazonensis–infected patients were cured within 90 days following a single treatment cycle, L. braziliensis–infected patients needed a mean (±SD) of 2.2 ± 0.2 treatment cycles over 190.5 ± 16.0 days to achieve cure, with treatment for 23 of 58 patients (39.7%) classified as failing (>2 cycles). Eight patients with DCL (3 males; mean age [±SD], 31.5 ± 7.1 years) infected with L. amazonensis were recruited and treated at the Presidente Dutra University Hospital–HUPD (São Luís-MA, NE Brazil). Patients with DCL had a mean disease duration (±SD) of 10.9 ± 2.1 years, during which several different therapeutic schemes (mean number [±SD] per patient, 2.7 ± 0.9, including liposomal amphotericin B and combinations of Glucantime, interferon γ, and pentamidine) were used, with no or only a transient clinical effect. Since DCL is a rare clinical manifestation, no samples from untreated patients (at diagnosis) were available for comparison. SOD1 plasma levels were quantified using a human SOD1 enzyme-linked immunosorbent assay kit (Calbiochem). RNA was extracted from lesion biopsy specimens from patients with LCL and those with DCL, as well as skin biopsy specimens from healthy controls, using Trizol, followed by an additional purification using RNeasy (QIAgen Benelux, Venlo, the Netherlands). Using in situ transcriptomics, we simultaneously quantified host and parasite RNAs in lesion biopsy specimens from patients with LCL and those with DCL, as well as skin biopsy specimens from healthy controls, by nCounter technology (NanoString, Seattle, WA), based on molecular bar coding of target RNA transcripts and digital detection at the femtomolar range, using direct hybridization, without reverse transcription or amplification [12]. Human SOD1, SOD2, SOD3, IFNA1, IFNA2, IFNA4, IFNB1, and L. braziliensis and L. amazonensis SOD1-5 messenger RNA (mRNA) levels were quantified in situ, in addition to mRNAs of several housekeeping genes (GUSB, G6PD, GAPDH, and HPRT1), for normalization, as well as leukocyte-specific genes (CD3, CD14, CD19, CD56, and CD45). Quantification of the parasite burden in vitro was performed as described elsewhere [9]. Briefly, monocyte-derived human macrophages were infected with L. amazonensis (MHOM/BR/87/BA125) and treated with SOD1 (Sigma) for 48 hours, followed by extensive washing, staining with hematoxylin/eosin, and counting of intracellular amastigotes (100 cells, with duplicate analysis for each sample). Parametric and nonparametric tests were performed according to Kolmogorov-Smirnov test for normality. For multiple comparisons, the Kruskal–Wallis test with the Dunn posttest and 1-way analysis of variance with a posttest for linear trend were used. For comparison between 2 groups, the F test, the Mann–Whitney U test, the t test, or the Wilcoxon test was used. To identify biomarkers, Receiver Operating Characteristic (ROC) curve analysis was used. All tests were 2 tailed, and differences were considered significant at P values of <.05.
RESULTS
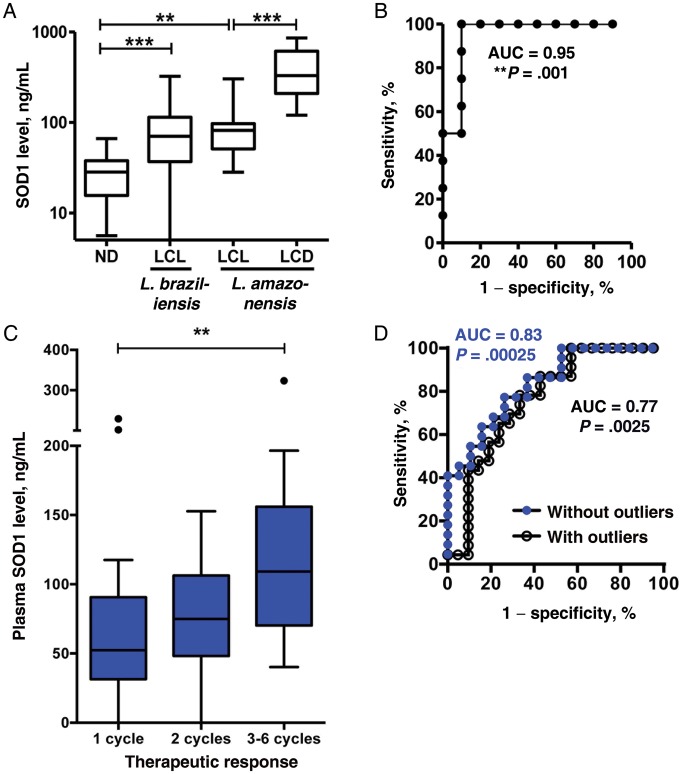
We measured plasma levels of SOD1 in healthy controls, patients with LCL (due to L. braziliensis or L. amazonensis), and patients with DCL (due to L. amazonensis) and investigated its potential role as a biomarker. The mean SOD1 level (±SEM) among patients with LCL due to L. braziliensis (90.6 ± 8.0 pg/mL) was 3-fold greater than that among healthy controls (30.2 ± 3.9 pg/mL; P < .01, by the Kruskal–Wallis; P < .001, by the Dunn posttest), whereas the mean level (±SEM) among patients with LCL due to L. amazonensis (93.1 ± 24.7 pg/mL) was 3.1-fold greater than that among healthy controls (P < .01, by the Kruskal–Wallis test; P < .01, by the Dunn posttest). On the other hand, mean SOD1 levels (±SEM) among patients with DCL (407.3 ± 88.0 pg/mL) were 4.4-fold greater than those among patients with LCL due to L. amazonensis (P = .0005, by the Mann–Whitney U test; Figure 1A). To investigate whether increased SOD1 levels during LCL is related to active disease, the SOD1 level was also measured in paired samples of from 13 patients with LCL due to L. braziliensis before treatment (at diagnosis) and after treatment (at definite clinical cure). SOD1 levels decreased 1.6-fold between pretreatment and posttreatment samples (99.2 ± 20.81 pg/mL vs 60.4 ± 5.2 pg/mL; P = .027, by the Wilcoxon matched pairs test; Supplementary Figure 1A). Since variances differed significantly between controls and patients with LCL due to L. braziliensis (P < .0001, by the F test), we hypothesized that heterogeneous SOD1 levels in patients with LCL might reflect differences in clinical status and/or therapeutic response. SOD1 levels did not significantly correlate with age, sex, disease duration, lesion size, or healing time among patients with LCL (data not shown). However, SOD1 plasma levels were significantly associated with therapeutic response, quantified as the number of treatment cycles required for cure (P = .0069, by the Kruskal–Wallis test; P < .001, by the Dunn posttest; Figure 1C). By use of ROC curve analysis, successful (1 cycle) and failing (>2 cycles) therapeutic response could be significantly discriminated through SOD1 plasma levels (area under the ROC curve, 0.83; P = .00025), authenticating its clinical utility as a biomarker (Figure 1D). Above a cutoff of 122 pg/mL, 9 of 22 patients (40.9%) in whom treatment was failing could be identified with 100% specificity (ie, 0 patients cured with 1 treatment cycle displayed SOD1 levels of >122 pg/mL). Strikingly, at a similar cutoff of 112 pg/mL, patients with DCL could be discriminated from treatable patients with LCL (due to L. amazonensis) with 100% sensitivity and 90% specificity (area under the ROC curve, 0.95; P = .001; Figure 1B).
Figure 1.
Superoxide dismutase 1 (SOD1) is a biomarker for therapeutic failure in cutaneous leishmaniasis. A, SOD1 measurements in plasma samples from 20 healthy controls, 58 patients with localized cutaneous leishmaniasis (LCL) due to Leishmania braziliensis), 10 patients with LCL due to Leishmania amazonensis, and 8 patients with diffuse cutaneous leishmaniasis (DCL) due to L. amazonensis. Box plots represent the median and interquartile range of each group. P < .001, by the Kruskal–Wallis test; and ***P < .001, by the Dunn posttest, for healthy controls vs patients with LCL due to L. braziliensis; **P < .01, by the Dunn posttest, for healthy controls vs patients with LCL due to L. amazonensis; and ***P < .001, by the Mann–Whitney U test, for patients with LCL due to L. amazonensis vs patients with DCL. B, Plasma SOD1 level significantly discriminates patients with LCL due to L. amazonensis from patients with DCL due to L. amazonensis (area under the receiving operating characteristic [ROC] curve, 0.95; ***P = .001). C, SOD1 measurements in plasma samples from patients with LCL due to L. braziliensis, classified according to number of treatment cycles (P = .0069, by the Kruskal–Wallis test; **P < .001, by the Dunn posttest). Three outliers (by the Tukey test) are shown as single dots. D, Plasma SOD1 level significantly predicts therapeutic response in patients with LCL due to L. braziliensis (success is defined as achievement of cure after 1 treatment cycle; failure is defined as lack of cure after >2 treatment cycles; area under the receiving operating characteristic curve, 0.83; ***P = .00025, without outliers, as in panel C).
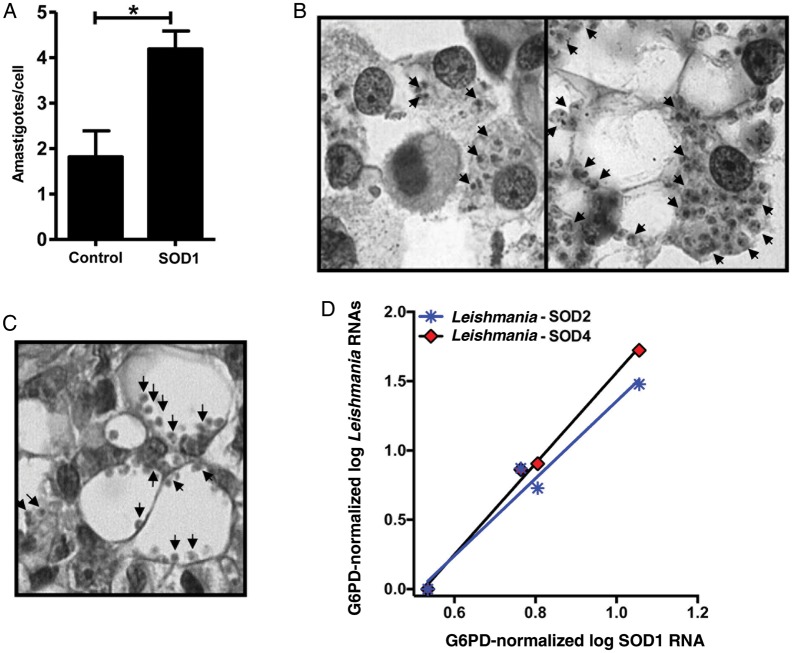
To test whether these high plasma SOD1 levels in patients with CL might be directly related to an increased parasite burden, human macrophages from healthy donors were infected with L. amazonensis (5:1) and cultivated in the absence or presence of recombinant SOD1 protein. As shown in Figure 2A, SOD1 treatment significantly increased parasite load (P = .042, by the t test). Moreover, infected human macrophages treated with recombinant SOD1 protein presented cytomorphological features (Figure 2B) strikingly similar to the vacuolized and highly parasitized macrophages from cutaneous lesions of patients with DCL (Figure 2C).
Figure 2.
Superoxide dismutase 1 (SOD1) correlates to parasite burden in vitro and in situ in diffuse cutaneous leishmaniasis (DCL). A and B, Human macrophages were infected with Leishmania amazonensis (5:1 ratio) for 4 hours and then treated in the absence or presence of SOD1 protein (175 U/mL) for 48 hours. Cells were fixed on glass slides and stained with hematoxylin and eosin. A, Parasite burden was quantified; each bar represents the mean ± standard error of the mean for 3 donors (*P = .042, by the t test). B, Infected macrophages (1000× magnification) left untreated (left panel) or treated with SOD1 (right panel). C, Cutaneous lesion biopsy specimen from a representative patient with DCL stained with hematoxylin and eosin (1000× magnification). D, Positive correlation between human SOD1 and parasite SOD2 (r = 0.98, P = .019) and SOD4 (r = 0.99, P = .0026) messenger RNA (mRNA) levels (normalized to G6PD), quantified by nCounter in situ in biopsy specimens from patients with DCL (n = 4).
Finally, using in situ transcriptomics (nCounter), we demonstrated a significant positive correlation between host SOD1 mRNA level and parasite SOD2 (P = .019, r = 0.98) and SOD4 (P = .0026, r = 0.99) mRNA in DCL. Taken together, these data suggest that systemic SOD1 might play a causal role in both the clinical and anatomopathological phenotype of DCL.
DISCUSSION
Up to now, no noninvasive biomarker for therapeutic response to SbV has been described in CL. In the present work, we demonstrate host SOD1 as a biomarker for therapeutic failure of first-line SbV in New World patients with CL. SOD1 plasma levels could significantly discriminate between successful and failing therapeutic response in patients with LCL (due to L. braziliensis) and between treatable patients with LCL (due to L. amazonensis) and refractory patients with DCL. Quantification of SOD1 plasma level at diagnosis represents a rapid, sensitive, and inexpensive new tool in the clinical management of CL. Further research will be necessary to test its predictive value in CL caused by other Leishmania species, since in Peru failure of antimonial therapy is significantly associated with infections due to L. braziliensis and Leishmania peruviana but not with infection due to Leishmania guyanensis [5]. By identifying, at the time of diagnosis, up to 40.9% of patients (with 100% specificity) in whom antimonial therapy will fail (Figure 1C), second-line treatment (eg, amphotericin B or miltefosine) could be provided as a first choice, thereby diminishing public health costs and avoiding unnecessary and prolonged exposure of the patients to the often severe side effects of intravenous pentavalent antimony therapy.
Although SOD1 is an intracellular antioxidant enzyme, our results suggest a direct role for increased systemic (plasma) SOD1 as a trigger of parasite replication in infected macrophages. Elsewhere, treatment of infected human macrophages with purified SOD1 in vitro led to strongly increased parasite burden and the appearance of large parasitophorous vacuoles, reminiscent of typical nodular lesions in DCL [13]. Moreover, this correlation between SOD1 and parasite burden was recapitulated in situ in DCL lesions, as evidenced by a strong linear relationship (r = 098–0.99) between host (human SOD1) and parasite (L. amazonensis SOD2 and SOD4) SODs. This parallel cross-regulation at the transcriptional level between superoxide-quenching enzymes that are hundreds of millions of years distant in evolution is a striking example of interkingdom signaling. To our knowledge, this is the first description of interkingdom signaling in protozoan-animal interaction. In keeping with our cytomorphological data (Figure 2B), this phenomenon is highly specific for cytosolic SOD1, since cross-kingdom correlations were not observed for mitochondrial SOD2 or extracellular SOD3 with any of the parasite mRNAs. The extremely long (mean disease duration [±SD], 10.9 ± 2.1 years) uncontrolled parasite replication in patients with DCL might have led to selection of SOD-overexpressing parasite clones [7, 8]. Alternatively, increased systemic levels of SOD1 in patients with LCL and those with DCL might reflect a parasite escape mechanism from host leishmanicidal activity mediated by type I IFN, as suggested by our previous work [9] and recently confirmed in murine and human macrophages [14]. In agreement with this hypothesis, we found a significant positive correlation between SOD1 mRNA and total IFN-α/β mRNA levels, using in situ transcriptomics (nCounter; Supplementary Figure 2A).
Finally, since all of our patients with DCL also experienced failure of multiple other treatment schemes (including pentamidine, amphotericin B, and IFN-γ, alone or in combination), an increased plasma SOD1 level truly reflects broad therapeutic failure. The dramatic increase in DCL also reinforces the idea that SOD1 is not a mere surrogate marker but a key molecule in DCL pathogenesis, driving perennial parasite replication and systemic dissemination, as suggested by Figure 2A–D. Hence, SOD1 is also a candidate therapeutic target in CL, reinforcing our previous suggestion of pharmacologic SOD1 inhibition by DETC [15] as a viable therapeutic alternative in LCL and DCL in particular, for which no effective treatment options exist.
In conclusion, our results indicate that host SOD1 is a clinically useful biomarker for therapeutic failure, as well as a possible therapeutic target, in LCL and DCL.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank Jorge Lessa Tolentino and Natali Alexandrino, for excellent technical assistance.
Financial support. This work was supported by the Brazilian National Research Council CNPq (A. B., M. B. N., and J. M. C. are CNPq senior investigators), PRONEX (CNPq-FAPESB), and the Alban Program, European Union Program of High Level Scholarship for Latin America (scholarship E06D103200BR).
Potential conflict of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Desjeux P. Leishmaniasis: Current situation and new perspectives. Comp Immunol Microbiol Infect Dis. 2004;27:305–18. doi: 10.1016/j.cimid.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 2.Murray HW, Berman JD, Davies CR, Saravia NG. Advances in leishmaniasis. Lancet. 2005;366:1561–77. doi: 10.1016/S0140-6736(05)67629-5. [DOI] [PubMed] [Google Scholar]
- 3.Reithinger R, Dujardin JC, Louzir H, Pirmez C, Alexander B, Brooker S. Cutaneous Leishmaniasis. Lancet Infect Dis. 2007;7:581–96. doi: 10.1016/S1473-3099(07)70209-8. [DOI] [PubMed] [Google Scholar]
- 4.Modabber F, Buffet PA, Torreele E, Milon G, Croft SL. Consultative meeting to develop a strategy for treatment of cutaneous leishmaniasis. Kinetoplastid Biol Dis. 2007;6:3. doi: 10.1186/1475-9292-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Llanos-Cuentas A, Tulliano G, Araujo-Castillo R, et al. Clinical and parasite species risk factors for pentavalent antimonial treatment failure in cutaneous leishmaniasis in Peru. Clin Infect Dis. 2008;46:223–31. doi: 10.1086/524042. [DOI] [PubMed] [Google Scholar]
- 6.Zerpa O, Ulrich M, Blanco B, et al. Diffuse cutaneous leishmaniasis responds to miltefosine but then relapses. Br J Dermatol. 2007;156:1328–35. doi: 10.1111/j.1365-2133.2007.07872.x. [DOI] [PubMed] [Google Scholar]
- 7.Plewes KA, Barr SD, Gedamu L. Iron superoxide dismutases targeted to the glycosomes of Leishmania chagasi are important for survival. Infect Immun. 2003;71:5910–20. doi: 10.1128/IAI.71.10.5910-5920.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghosh S, Goswami S, Adhya S. Role of superoxide dismutase in survival of Leishmania within the macrophage. Biochem J. 2003;369:447. doi: 10.1042/BJ20021684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khouri R, Bafica A, Pereira Silva MP, et al. IFN-β impairs superoxide-dependent parasite killing in human macrophages: evidence for a deleterious role of SOD1 in cutaneous leishmaniasis. J Immunol. 2009;182:2525–31. doi: 10.4049/jimmunol.0802860. [DOI] [PubMed] [Google Scholar]
- 10.Kocyigit A, Gurel M, Ulukanligil M. Erythrocyte antioxidative enzyme activities and lipid peroxidation levels in patients with cutaneous leishmaniasis. Parasite. 2003;10:277–81. doi: 10.1051/parasite/2003103277. [DOI] [PubMed] [Google Scholar]
- 11.Soares G, Barral A, Costa JM, Barral-Netto M, Van Weyenbergh J. CD16+ monocytes in human cutaneous leishmaniasis: increased ex vivo levels and correlation with clinical data. J Leukoc Biol. 2006;79:36–9. doi: 10.1189/jlb.0105040. [DOI] [PubMed] [Google Scholar]
- 12.Geiss GK, Bumgarner RE, Birditt B, et al. Direct multiplexed measurement of gene expression with color-coded probe pairs. Nat Biotechnol. 2008;26:317–25. doi: 10.1038/nbt1385. [DOI] [PubMed] [Google Scholar]
- 13.Barral A, Costa JM, Bittencourt AL, Barral-Netto M, Carvalho EM. Polar and subpolar diffuse cutaneous leishmaniasis in Brazil: clinical and immunopathologic aspects. Int J Dermatol. 1995;34:474–9. doi: 10.1111/j.1365-4362.1995.tb00613.x. [DOI] [PubMed] [Google Scholar]
- 14.Vivarini Ade C, Pereira Rde M, Teixeira KL, et al. Human cutaneous leishmaniasis: interferon-dependent expression of double-stranded RNA-dependent protein kinase (PKR) via TLR2. FASEB J. 2011;25:4162–73. doi: 10.1096/fj.11-185165. [DOI] [PubMed] [Google Scholar]
- 15.Khouri R, Novais F, Santana G, et al. DETC induces Leishmania parasite killing in human in vitro and murine in vivo models: a promising therapeutic alternative in Leishmaniasis. PLoS One. 2010;5:e14394. doi: 10.1371/journal.pone.0014394. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.