Abstract
Glioblastoma (GBM) is the most common malignant primary brain tumor in adults. However, the survival of patients with GBM has been dismal after multi-disciplinary treatment with surgery, radiotherapy, and chemotherapy. In the efforts to improve clinical outcome, anti-angiogenic therapy with bevacizumab (Avastin) was introduced to inhibit vascular endothelial growth factor (VEGF) mediated tumor neovascularization. Unfortunately, the results from clinical trials have not lived up to the initial expectations. Patients either fail to respond to anti-angiogenic therapy or develop resistance following an initial response. The failure of anti-angiogenic therapy has led to a frustration among physicians and research community. Recent evidence indicates that the dogma of tumor neovascularization solely dependent on VEGF pathway to be overly simplistic. A realistic model of tumor neovascularization should include alternative pathways that are independent of VEGF signaling. A better understanding of the underlying processes in tumor neovascularization would help in designing successful anti-angiogenic treatment strategies.
Keywords: GBM, VEGF, neovascularization, angiogenesis, vasculogenesis, vascular mimicry
Introduction
Since the concept of angiogenesis-dependent tumor growth was first proposed, improving tumor control with the use of anti-angiogenic (AA) therapy was considered a potential treatment option. Various factors known to play a role in tumor angiogenesis, such as vascular endothelial growth factor (VEGF), have been identified in the past two decades, and different therapeutic targets have been selected. However, results from clinical trials and laboratory experiments have identified the emergence of resistance to AA therapy. Here we briefly discuss the current state AA therapies targeting VEGF and emerging alternative pathways for neovascularization, and future directions for designing novel therapeutic strategies.
VEGF dependent neovascularization
It has been more than four decades since the concept of angiogenesis-dependent tumor growth was first proposed (1). This idea led to a belief that the use of AA therapy would improve tumor control. Various factors known to play a role in tumor angiogenesis have been identified in the past two decades (2). VEGF has been the single most important factor described in tumor angiogenesis to date (3). The discovery of VEGF led to the development of drugs that target VEGF dependent angiogenesis. One of the first agents shown to block tumor growth in vivo against VEGF was a monoclonal antibody, bevacizumab (4). Currently, bevacizumab is being widely used in patients with various types of cancers, including recurrent glioblastoma (GBM) (5, 6). Unfortunately, no significant improvement in overall survival (OS) has been noted with the use of bevacizumab monotherapy (7). In addition to bevacizumab, multi-targeted VEGF receptor tyrosine kinase inhibitors, such as cediranib, sorafenib, sunitinib, and vandetanib have been tested in clinical trials, but without improvement in progression free survival (PFS) or OS (7). Many clinical trials have tested the efficacy of sunitinib in patients with recurrent high-grade glioma with no objective evidence of tumor control (8-10). Similarly, vatalanib was shown to have limited efficacy in the treatment of newly diagnosed GBM (11). A phase III clinical trial in patients with recurrent GBM showed no improvement in PFS with the addition of cediranib alone, or in combination with chemotherapy (12). The failure of the drugs targeting the VEGF pathway in the clinical setting has raised questions on the classical view of tumor neovascularization solely based on angiogenesis.
Resistance to VEGF dependent anti-angiogenic therapy and alternative pathways of neovascularization
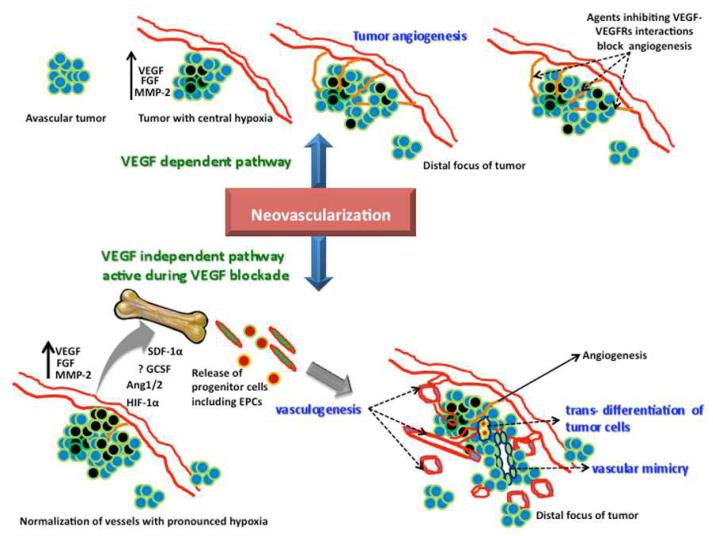
Although many patients experience an initial response to AA therapy, no significant improvement in OS or PFS has been achieved clinically. In some instance, patients do not show any response at all. The initial or acquired resistance to VEGF based AA treatment has been a frustrating clinical problem in the management of GBM patients. One possible mechanism of resistance to VEGF dependent AA therapy might be the activation of alternative angiogenesis signaling pathways, such as the basic fibroblast growth factor (bFGF), Tie-2, stromal-cell derived factor-1α (SDF-1α), and an increase in the invasiveness of the tumor cells due to increased VEGF production (13-15). Another distinct mechanism of resistance might be due to vasculogenesis, where endothelial progenitor cells (EPCs) and pro-angiogenic monocytes are recruited to the tumor site from the bone marrow. AA therapy disturbs tumor vasculature, which leads to tumor hypoxia. Hypoxia leads to up-regulation of hypoxia-inducible factor 1-alpha (HIF-1α), which in turn leads to the up-regulation of SDF-1α. SDF-1α is a potent chemo-attractant for bone marrow-derived EPCs, due to the presence of CXCR4 receptors in these cells (16, 17). Any treatment that recruits EPCs to the tumor site might promote neovascularization and tumor growth. Thus, the use of VEGF inhibitory therapy could paradoxically enhance an unwanted angiogenic and pro-growth response. Activation of the SDF-1α-CXCR4 pathway provides a mechanistic explanation for the role of hypoxia in promoting resistance to anti-VEGF therapy. Our recent work with rat orthotopic human glioma model showed a paradoxical increase in the production of VEGF at the peripheral part of the tumors, as well as an elevated expression of HIF-1α and SDF-1 α, and a significant increase in the number of dilated blood vessels in animals that underwent two weeks of PTK787 (small molecule tyrosine kinase inhibitor; vatalanib) treatment (18). We also observed increased production of granulocyte colony stimulating factor (G-CSF) in glioma treated with vatalanib. GCSF is known to mobilize bone marrow cells. We have also shown the involvement of bone marrow progenitor cells in promoting GBM growth (19). Other VEGF-independent mechanisms of tumor neovascularization include vascular co-option, vascular mimicry, and GBM endothelial cell trans-differentiation (20). Vascular co-option precedes tumor angiogenesis and involves infiltration of tumor cells around pre-existing micro vessels (21). Vascular mimicry is a process by which GBM cells form functional vascular networks in the tumor (22). Trans-differentiation of glioma stem cells into endothelial cells is another mechanism of tumor neovascularization unaffected by VEGF signaling (23). These processes may be responsible to a varied extent in reducing tumor sensitivity to anti-VEGF drugs. Figure 1 shows a schematic of VEGF dependent and VEGF-independent pathways in tumor neovascularization. Apart from treatment resistance, the use of bevacizumab has been noted to enhance tumor invasiveness and metastatic potential in patients with relapsed GBM (24). Also, VEGF inhibition has been shown to paradoxically increase co-option and vasculogenesis (25, 26).
Figure 1. Schematic of VEGF-dependent and VEGF-independent pathways in GBM neovascularization.
Therapeutic approach based on alternative pathways of neovascularization
There has been a considerable effort in recent years to develop drugs that target VEGF-independent angiogenesis. These include agents that target the angiopoietin/Tie2 pathway, which is involved in vessel stability (27). One such drug, AMG 386 (Trebanabnib), is currently being tested in a phase II clinical trial for recurrent GBM (7). Other agents such as ramucirumab (monoclonal antibody targeting PDGFα), XL184 (pan-tyrosine kinase inhibitor), Tandutinib (inhibitor of type III receptor tyrosine kinases including PDGFR-β, FLT-3, and c-Kit), Aflibercept (VEGF-Trap), and many other agents have been tested or are undergoing investigation in clinical trials (7, 27). Many of the clinical trials were stopped prematurely due to significant drug related toxicity. To date, none of these agents have demonstrated a survival benefit or gained FDA approval for clinical use.
Conclusion and future directions
In addition to VEGF based therapy, future improvements in AA therapy for GBM should include modulating the various processes involved in tumor neovascularization. This would entail a broad approach of using combination agents to block multiple pathways. One strategy would be to use drugs that block tumor invasion in combination with AA agents to overcome treatment induced invasive phenotypes. In addition, future efforts should be directed towards developing agents that block VEGF-independent processes in tumor neovascularization. One such mechanism could be to block SDF-1α-CXCR4 signaling to prevent vasculogenesis. AMD3100, a CXCR4 receptor antagonist, was initially developed as an anti-HIV drug and later used to mobilize CD34+ hematopoietic stem cells to the peripheral circulation (28). Although AMD3100 increases the number of peripheral CD34+ cells, recent investigations point towards inhibition of tumor vasculogenesis following continuous treatment with AMD3100 or similar CXCR4 receptor antagonists (28, 29). On a physiological level, as hypoxia is known to induce treatment resistance, efforts should be made to improve oxygen saturation in the tumor microenvironment. The latest results from clinical trials employing agents that target VEGF-independent pathways (angiopoietin/Tie2 pathway) are eagerly awaited and could lead to a paradigm shift in AA therapy of GBM.
Acknowledgment
This work is supported by NIH grants R01CA160216 and R01CA172048
References
- 1.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285(21):1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 2.Weis SM, Cheresh DA. Tumor angiogenesis: molecular pathways and therapeutic targets. Nat Med. 2011 Nov 7;17(11):1359–70. doi: 10.1038/nm.2537. Review. [DOI] [PubMed] [Google Scholar]
- 3.Ferrara N, Houck K, Jakeman L, Leung DW. Molecular and biological properties of the vascular endothelial growth factor family of proteins. Endocr Rev. 1992 Feb;13(1):18–32. doi: 10.1210/edrv-13-1-18. [DOI] [PubMed] [Google Scholar]
- 4.Kim KJ, Li B, Winer J, Armanini M, Gillett N, Phillips HS, Ferrara N. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature. 1993;362(6423):841–844. doi: 10.1038/362841a0. [DOI] [PubMed] [Google Scholar]
- 5.Friedman HS, Prados MD, Wen PY, Mikkelsen T, Schiff D, Abrey LE, Yung WK, Paleologos N, Nicholas MK, Jensen R, Vredenburgh J, Huang J, Zheng M, Cloughesy T. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009 Oct 1;27(28):4733–40. doi: 10.1200/JCO.2008.19.8721. [DOI] [PubMed] [Google Scholar]
- 6.Vredenburgh JJ, Desjardins A, Herndon JE, 2nd, Marcello J, Reardon DA, Quinn JA, Rich JN, Sathornsumetee S, Gururangan S, Sampson J, Wagner M, Bailey L, Bigner DD, Friedman AH, Friedman HS. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol. 2007 Oct 20;25(30):4722–9. doi: 10.1200/JCO.2007.12.2440. [DOI] [PubMed] [Google Scholar]
- 7.Taylor J, Gerstner ER. Anti-Angiogenic Therapy in High-Grade Glioma (Treatment and Toxicity) Curr Treat Options Neurol. 2013 doi: 10.1007/s11940-013-0224-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neyns B, Sadones J, Chaskis C, Dujardin M, Everaert H, Lv S, Duerinck J, Tynninen O, Nupponen N, Michotte A, De Greve J. Phase II study of sunitinib malate in patients with recurrent high-grade glioma. J Neurooncol. 2011103:491–01. doi: 10.1007/s11060-010-0402-7. [DOI] [PubMed] [Google Scholar]
- 9.Reardon DA, Vredenburgh JJ, Coan A, Desjardins A, Peters KB, Gururangan S, Sathornsumetee S, Rich JN, Herndon JE, Friedman HS. Phase I study of sunitinib and irinotecan for patients with recurrent malignant glioma. J Neurooncol. 2011;105:621–7. doi: 10.1007/s11060-011-0631-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pan E, Yu D, Yue B, Potthast L, Chowdhary S, Smith P, Chamberlain M. A prospective phase II single-institution trial of sunitinib for recurrent malignant glioma. J Neurooncol. 2012;110:111–18. doi: 10.1007/s11060-012-0943-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brandes AA, Stupp R, Hau P, Lacombe D, Gorlia T, Tosoni A, Mirimanoff RO, Kros JM, van den Bent MJ. EORTC study 26041-22041: phase I/II study on concomitant and adjuvant temozolomide (TMZ) and radiotherapy (RT) with PTK787/ZK222584 (PTK/ZK) in newly diagnosed glioblastoma. Eur J Cancer. 2010;46:348–54. doi: 10.1016/j.ejca.2009.10.029. [DOI] [PubMed] [Google Scholar]
- 12.Batchelor TT, Mulholland P, Neyns P, Nabors LB, et al. The efficacy of cediranib as monotherapy and in combination with lomustine compared to lomustine alone in patients with recurrent glioblastoma: a phase III randomized study (abstract) Neuro Oncol. 2010;12(75):OT–25. [Google Scholar]
- 13.Kerbel RS. Tumor angiogenesis. N Engl J Med. 2008;358:2039–2049. doi: 10.1056/NEJMra0706596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Batchelor TT, Sorensen AG, di Tomaso E, Zhang WT, Duda DG, et al. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell. 2007;11:83–95. doi: 10.1016/j.ccr.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Norden AD, Drappatz J, Wen PY. Novel anti-angiogenic therapies for malignant gliomas. Lancet Neurol. 2008;7:1152–1160. doi: 10.1016/S1474-4422(08)70260-6. [DOI] [PubMed] [Google Scholar]
- 16.Jin DK, Shido K, Kopp HG, Petit I, Shmelkov SV, et al. Cytokine-mediated deployment of SDF-1 induces revascularization through recruitment of CXCR4(+) hemangiocytes. Nat Med. 2006;12:557–567. doi: 10.1038/nm1400. Epub 2006 Apr 2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arbab AS, Janic B, Knight RA, Anderson SA, Pawelczyk E, et al. Detection of migration of locally implanted AC133+ stem cells by cellular magnetic resonance imaging with histological findings. FASEB J. 2008;22:3234–3246. doi: 10.1096/fj.07-105676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ali MM, Janic B, Babajani-Feremi A, Varma NR, Iskander AS, et al. Changes in vascular permeability and expression of different angiogenic factors following anti-angiogenic treatment in rat glioma. PLoS ONE. 2010;5:e8727. doi: 10.1371/journal.pone.0008727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arbab AS. Activation of alternative pathways of angiogenesis and involvement of stem cells following anti-angiogenesis treatment in glioma. Histol Histopathol. 2012;27:549–557. doi: 10.14670/hh-27.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hardee ME, Zagzag D. Mechanisms of glioma-associated neovascularization. Am J Pathol. 2012 Oct;181(4):1126–41. doi: 10.1016/j.ajpath.2012.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holash J, Maisonpierre PC, Compton D, Boland P, Alexander CR, Zagzag D, Yancopoulos GD, Wiegand SJ. Vessel cooption, regression, and growth in tumors mediated by angiopoietins and VEGF. Science. 1999;284:1994–1998. doi: 10.1126/science.284.5422.1994. [DOI] [PubMed] [Google Scholar]
- 22.Yue WY, Chen ZP. Does vasculogenic mimicry exist in astrocytoma? J Histochem Cytochem. 2005;53:997–1002. doi: 10.1369/jhc.4A6521.2005. [DOI] [PubMed] [Google Scholar]
- 23.Ricci-Vitiani L, Pallini R, Biffoni M, Todaro M, Invernici G, Cenci T, Maira G, Parati EA, Stassi G, Larocca LM, De Maria R. Tumour vascularization via endothelial differentiation of glioblastoma stemlike cells. Nature. 2010;468:824–828. doi: 10.1038/nature09557. [DOI] [PubMed] [Google Scholar]
- 24.Narayana A, Kelly P, Golfinos J, Parker E, Johnson G, Knopp E, Zagzag D, Fischer I, Raza S, Medabalmi P, Eagan P, Gruber ML. Antiangiogenic therapy using bevacizumab in recurrent high-grade glioma: impact on local control and patient survival. J Neurosurg. 2009;110:173–180. doi: 10.3171/2008.4.17492. [DOI] [PubMed] [Google Scholar]
- 25.Zuniga RM, Torcuator R, Jain R, Anderson J, Doyle T, Ellika S, Schultz L, Mikkelsen T. Efficacy, safety and patterns of response and recurrence in patients with recurrent high-grade gliomas treated with bevacizumab plus irinotecan. J Neurooncol. 2009;91:329–336di. doi: 10.1007/s11060-008-9718-y. [DOI] [PubMed] [Google Scholar]
- 26.Tomaso E, Snuderl M, Kamoun WS, Duda DG, Auluck PK, Fazlollahi L, Andronesi OC, Frosch MP, Wen PY, Plotkin SR, Hedley-Whyte ET, Sorensen AG, Batchelor TT, Jain RK. Glioblastoma recurrence after cediranib therapy in patients: lack of “rebound” revascularization as mode of escape. Cancer Res. 2011;71:19–28. doi: 10.1158/0008-5472.CAN-10-2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cascone T, Heymach JV. Targeting the angiopoietin/Tie2 pathway: cutting tumor vessels with a double-edged sword? J. Clin. Oncol. 2012;30:441–444. doi: 10.1200/JCO.2011.38.7621. [DOI] [PubMed] [Google Scholar]
- 28.Petit I, Jin D, Rafii S. The SDF-1-CXCR4 signaling pathway: a molecular hub modulating neo-angiogenesis. Trends in Immunology. 2007;28:299–307. doi: 10.1016/j.it.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kioi M, Vogel H, Schultz G, Hoffman RM, Harsh GR, et al. Inhibition of vasculogenesis, but not angiogenesis, prevents the recurrence of glioblastoma after irradiation in mice. J Clin Invest. 2010;120:694–705. doi: 10.1172/JCI40283. [DOI] [PMC free article] [PubMed] [Google Scholar]