Abstract
In this pilot study, amygdala connectivity related to trauma symptoms was explored using resting-state functional magnetic resonance imaging (R-fMRI) in 23 healthy adolescents ages 13–17 years with no psychiatric diagnoses. Adolescents completed a self-report trauma symptom checklist and a R-fMRI scan. We examined the relationship of trauma symptoms to resting-state functional connectivity of the amygdala. Increasing self-report of trauma symptoms by adolescents was associated with increasing functional connectivity with the right amygdala and a local limbic cluster and decreasing functional connectivity with the amygdala and a long-range frontoparietal cluster to the left amygdala, which can be a hallmark of immaturity. These pilot findings in adolescents provide preliminary evidence that even mild trauma symptoms can be linked to the configuration of brain networks associated with the amygdala.
Trauma is associated with lifelong neurobiological consequences (Carrion, Wong, & Kletter, 2013; Rinne-Albers, van der Wee, Lamers-Winkleman, & Vermeiren, 2013). Research on brain changes following trauma in human and animal models typically examines those who have high trauma symptoms, as they are at great risk for psychopathology and adverse consequences (De Brito et al., 2013; Garrett et al., 2012; Raineki, Cortés, Belnoue, & Sullivan, 2012). Neuroimaging studies suggest severe trauma symptomatology (e.g., posttraumatic stress disorder) is associated with amygdala dysregulation (e.g., Ahmed, Spottiswoode, Carey, Stein, & Seedat, 2012; Grant, Cannistraci, Hollon, Gore, & Shelton, 2011; Weber et al., 2013), and that a resting-state amygdala network can be functionally defined (Li, Liu, Liu, & Yin, 2013). However, the key to effectively preventing and treating trauma symptoms may not only lie in studying the brains of those who present with clinically significant trauma symptoms. Neural correlates of subclinical trauma symptoms have been largely unexplored and yet may also hold important clues for prevention efforts targeting resilience and treatment aimed at reducing trauma symptoms (Carrion et al., 2013).
This pilot study investigated whether trauma symptoms are related to amygdala functional connectivity in healthy adolescents with no psychiatric diagnoses. Adolescents who experience even mild traumatic events can display a spectrum of reactions, ranging from effective coping to minor increases in inattentive or aggressive behavior (Nooner et al., 2012). As such, we evaluated the correlation of trauma symptoms as reported in the Trauma Symptom Checklist for Children (TSCC; Briere, 1996) to functional connectivity between bilateral amygdala seeds and the rest of the brain.
Method
Participants
Analyses were conducted on 23 healthy right-handed adolescents ages 13–17 years with no psychiatric diagnoses participating in a community-based study of mental health. Participants were determined to have no psychiatric diagnoses through a structured clinical interview (see Measures section). Per parent and adolescent report, participants had no known psychological diagnoses, medical, neurological, or developmental problems in the past or present (see Table 1). Participants had a Full-Scale Intelligence Quotient (IQ) ≥ 70 and were unmedicated. This study had the Nathan Kline Institute for Psychiatric Research Institutional Review Board approval. Parents gave informed consent and participants gave assent prior to participation. For further diagnostic method details, see http://fcon_1000.projects.nitrc.org/indi/pro/nki.html.
Table 1.
Demographic Characteristics of the Sample
| Variable | Na | %a |
|---|---|---|
| Total participants | 23 | 100 |
| Sex: Female/male | 11/12 | 47.8/50.9 |
| Mean age in years (range) | M = 14.47 | SD = 1.62 |
| Traumaa: Present/absent | 11/12 | 47.8/50.9 |
| Trauma typeb | ||
| Death of peer, sibling, or parentc | 4 | 17.4 |
| Serious car accident | 2 | 8.7 |
| Physical abused | 2 | 8.7 |
| Sexual abused | 2 | 8.7 |
| Witness domestic violenced | 1 | 4.3 |
| Psychiatric diagnosisb: Present/absent | 2/23 | 8.7/91.3 |
| Adjustment disorder with anxietyb | 1 | 4.3 |
| Adjustment disorder with depressed moodb | 1 | 4.3 |
| Participants with trauma and diagnosisb | 0 | 0.0 |
Unless otherwise noted.
Trauma and psychiatric diagnosis defined by DSM-IV-TR (American Psychiatric Association, 2000).
Not lesser news such as death of grandparent or minor injury of friend.
Not current or past 6 months. All reporting laws followed.
Measures
A structured Diagnostic and Statistical Manual of Mental Disorders (4 ed., text rev.; DSM-IV-TR, American Psychiatric Association, 2000) clinical interview (K-SADS-PL; Birmaher, 2009) was administered to confirm the absence of psychological diagnoses. Two of the 23 participants met criteria for an adjustment disorder and were still included in the present study; the remainder did not meet criteria for a diagnosis (see Table 1). There were no reports of current or ongoing maltreatment.
The 23 participants completed the TSCC (mean total raw score = 27.00, SD = 15.42). There were no significant correlations in TSCC total scores related to age, sex, IQ, socioeconomic status, or diagnosis.
Procedure
Images were acquired on a 3.0 Tesla Siemens Trio Tim MRI scanner. For each participant a T2* BOLD sensitive echo planar image sequence was acquired (time to repetition [TR] = 2500 ms; echo time [TE] = 30 ms; field of view [FOV] = 216; voxel dimensions = 3 mm isometric; number of slices = 38; number of volumes = 260). A T1-weighted anatomical image was acquired using a magnetization prepared gradient echo sequence (TR = 2530 ms; TE = 3.25 ms; inversion time [TI] = 1100 ms; flip angle = 7°; 128 slices; FOV = 256 mm; acquisition voxel size = 1.3 × 1 × 1.3 mm). For full details, see http://fcon_1000.projects.nitrc.org/indi/pro/nki.html.
We used a combination of analysis of functional neuroimages (AFNI version 2.56a; Cox, 1996) and FSL version 5.0 (Jenkinson, Beckmann, Behrens, Woolrich, & Smith, 2012; FMRIB Software Library, www.fmrib.ox.ac.uk) to preprocess the R-fMRI scans (Mennes et al., 2010). Resting-state data preprocessing comprised slice time correction for interleaved slice acquisition, three-dimensional (3D) motion correction, despiking, mean-based intensity normalization of all volumes by the same factor, temporal band-pass filtering (0.009–0.1 Hz), and linear and quadratic detrending. Linear registration of structural images to the Montreal Neurological Institute MNI152 template with 2 × 2 × 2 mm resolution was carried out using the FSL tool FLIRT, and was refined using FNIRT nonlinear registration (Andersson, Jenkinson, & Smith, 2007). Participant resting state data was transformed into MNI space by the concatenation of a linear affine transformation to their anatomical image, and the transformation derived from registration of the anatomical image to MNI space.
For each participant, seed masks for right and left amygdala were derived from structural segmentation of their structural T1 image using Freesurfer (Fischl et al., 2004). Seed masks were transformed into MNI space by applying transforms derived from anatomical to MNI registration (described above). The representative time series for each seed ROI was extracted from their four-dimensional (unsmoothed) standard-space volume by averaging the time-series across all voxels within the ROIs. We calculated the correlation between each seed ROI time series and that of every other voxel, modeling nonneuronal signals derived from white matter, cerebrospinal fluid, global signal, and six motion parameters as nuisance variables (e.g., Kelly et al., 2009) using FEAT from FSL. Specific within-subject contrasts included: main effects of left and right amygdala, mean amygdala (left and right), as well as left versus right comparisons. A post hoc examination of motion correction parameters revealed no significant relationship between mean frame-wise displacement (Power, Barnes, Snyder, Schlaggar, & Petersen, 2012) and z-transformed trauma scores (r = .18, nonsignificant).
Data Analysis
The resulting parameter estimates for mean subject-level amygdala connectivity analyses were forwarded to a group-level analysis under FSL’s FLAME. We regressed z-transformed TSCC total scores against amygdala connectivity maps, allowing us to test for systematic variation in connectivity between amygdala and other brain regions as a function of trauma score. Age and sex were modeled as covariates. We corrected for multiple comparisons at the cluster level (z > 2.33, p < .05; Worsley, 2001).
Results
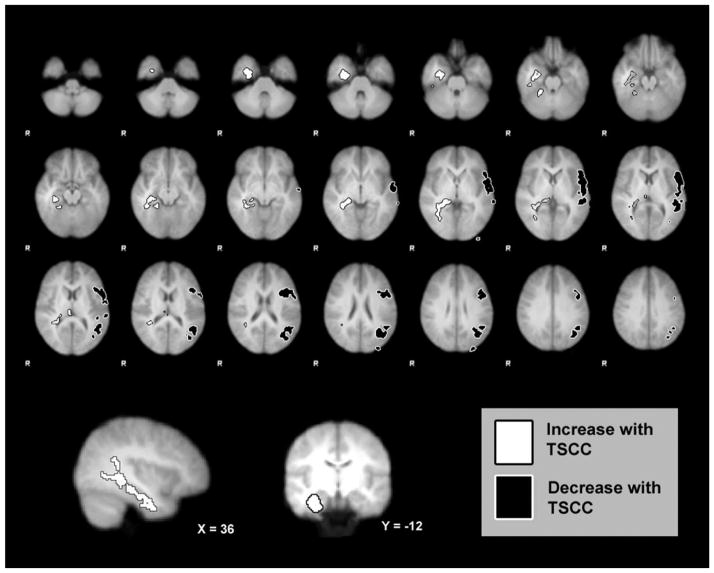
Two functional clusters demonstrated differential connectivity with the mean amygdala (left + right) time course as a function of TSCC score. The TSCC scores were associated with increasing connectivity between the amygdala and a local limbic cluster, largely encompassing the right amygdala, hippocampus, parahippocampal gyrus, and lingual gyrus. Additionally, increasing TSCC scores were associated with decreasing amygdala connectivity with a long-range frontoparietal cluster encompassing the left middle frontal, inferior frontal, and angular gyri, superior and inferior parietal lobes, and lateral occipital cortex (see Figure 1). No significant clusters were found in post hoc exploratory analyses examining the interaction of hemispheric seed (left vs. right) on network connectivity modulation by TSCC scores.
Figure 1.
White cluster: (largely encompassing) right amygdala, hippocampus, parahippocampal gyrus, & lingual gyrus) showed main effect of increasing amygdala functional connectivity with increasing trauma scores; black frontoparietal cluster: (largely encompassing left middle frontal, inferior frontal, angular gyri, superior & inferior parietal lobes, lateral occipital cortex) showed main effect of decreasing amygdala functional connectivity with increasing trauma scores. TSCC = Trauma Symptom Checklist for Children.
Discussion
This work provides preliminary evidence that trauma symptoms are related to amygdala connectivity in healthy adolescents with no psychiatric symptoms. Increasing self-report of trauma symptoms by adolescents was associated with (a) increasing functional connectivity with the right amygdala and a local limbic cluster, and (b) decreasing functional connectivity with the left amygdala and a long-range frontoparietal cluster. Increased local and decreased long-range connectivity can be a hallmark of brain immaturity (Fair et al., 2009; Kelly et al., 2009). Our results suggest that even among adolescents reporting mild to moderate trauma symptoms, there is a measurable positive correlation between trauma symptoms and functional immaturity. Additionally, the TSCC-related increase in right, but not left, local connectivity even in adolescents may suggest a role in selective right-amygdala dysregulation as a basis for trauma symptoms (e.g., Shin et al., 2005). These initial findings should not, of course, be taken as a statement of causal effect.
This pilot study offers preliminary evidence that the impact of trauma on the brain during adolescence may be a dimensional phenomenon even among adolescents with subclinical trauma symptoms. Differences in amygdala connectivity can be observed along a continuum, and not just when trauma symptoms exceed a particular threshold. This suggests that we may be able to track changes in the brain following trauma for a larger range of trauma symptoms than previously appreciated. Monitoring amygdala connectivity could someday provide a way to evaluate the effectiveness of therapeutic and preventative interventions, thereby enhancing and promoting resilience and recovery.
A limitation of the current pilot study is that trauma was not carefully characterized beyond the structured diagnostic interview and that only a community sample was used. As such, it should be regarded an exploratory pilot study. Further research is needed to advance our understanding of trauma and the role of the amygdala and its functional networks in children and adolescents.
Acknowledgments
Thank you Nathan Kline Institute outpatient and imaging staff.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: Author; 2000. text rev. [Google Scholar]
- Ahmed F, Spottiswoode BS, Carey PD, Stein DJ, Seedat S. Relationship between neurocognition and regional brain volumes in traumatized adolescents with and without posttraumatic stress disorder. Neuropsychobiology. 2012;66:174–184. doi: 10.1159/000339558. [DOI] [PubMed] [Google Scholar]
- Andersson JLR, Jenkinson M, Smith S. TR07JA2: Non-linear registration, aka spatial normalisation. FMRIB Analysis Group Technical Reports. Oxford, England: University of Oxford, The Oxford Centre for Functional MRI of the Brain, Nuffield Department of Clinical Neurosciences; 2007. [Google Scholar]
- Birmaher B, Ehmann M, Axelson D, Goldstein B, Monk K, Kalas C, Brent DA. Schedule for affective disorders and schizophrenia for school-age children (K-SADS-PL) for the assessment of preschool children—a preliminary psychometric study. Journal of Psychiatric Research. 2009;43:680–686. doi: 10.1016/j.jpsychires.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briere J. Trauma Symptom Checklist for Children: Professional manual. Odessa, FL: Psychological Assessment Resources; 1996. [Google Scholar]
- Carrion VG, Wong SS, Kletter H. Update on neuroimaging and cognitive functioning in maltreatment-related pediatric PTSD: Treatment implications. Journal of Family Violence. 2013;28:53–61. doi: 10.1007/s10896-012-9489-2. [DOI] [Google Scholar]
- Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computational Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- De Brito SA, Viding E, Sebastian CL, Kelly PA, Mechelli A, Maris H, McCrory EJ. Reduced orbitofrontal and temporal grey matter in a community sample of maltreated children. Journal of Child Psychology and Psychiatry. 2013;54:105–112. doi: 10.1111/j.1469-7610.2012.02597.x. [DOI] [PubMed] [Google Scholar]
- Fair DA, Cohen AL, Power JD, Dosenbach NU, Church JA, Miezin FM, Petersen SE. Functional brain networks develop from a “local to distributed” organization. PLoS Comput Biol. 2009;5:e1000381. doi: 10.1371/journal.pcbi.1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Salat DH, van der Kouwe AJW, Makris N, Segonne F, Quinn BT, Dale AM. Sequence-independent segmentation of magnetic resonance images. Neuroimage. 2004;23:S69–S84. doi: 10.1016/j.neuroimage.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Garrett AS, Carrion V, Kletter H, Karchemskiy A, Weems CF, Reiss A. Brain activation to facial expressions in youth with PTSD symptoms. Depression and Anxiety. 2012;29:449–459. doi: 10.1002/da.21892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant MM, Cannistraci C, Hollon SD, Gore J, Shelton R. Childhood trauma history differentiates amygdala response to sad faces within MDD. Journal of Psychiatric Research. 2011;45:886–895. doi: 10.1016/j.jpsychires.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. FSL. NeuroImage. 2012;62:782–790. doi: 10.1016/j.neuroimage.2011.09015. [DOI] [PubMed] [Google Scholar]
- Kelly AM, Di Martino A, Uddin LQ, Shehzad Z, Gee DG, Reiss PT, Milham MP. Development of anterior cingulate functional connectivity from late childhood to early adulthood. Cerebral Cortex. 2009;19:640–657. doi: 10.1093/cercor/bhn117. [DOI] [PubMed] [Google Scholar]
- Li B, Liu J, Liu Y, Lu HB, Yin H. Altered resting-state functional connectivity in post-traumatic stress disorder: A perfusion MRI study. SPIE Medical Imaging. 2013:867318–867318. doi: 10.1117/12.2008168. [DOI] [Google Scholar]
- Mennes M, Kelly C, Zuo XN, Di Martino A, Biswal BB, Castellanos FX, Milham MP. Inter-individual differences in resting-state functional connectivity predict task-induced BOLD activity. Neuroimage. 2010;50:1690–1701. doi: 10.1016/j.neuroimage.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nooner K, Linares LO, Batinjane J, Kramer RA, Silva R, Cloitre M. Factors related to posttraumatic stress disorder in adolescence. Trauma, Violence, & Abuse. 2012;13:153–166. doi: 10.1177/1524838012447698. [DOI] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raineki C, Cortés MR, Belnoue L, Sullivan RM. Effects of early-life abuse differ across development: Infant social behavior deficits are followed by adolescent depressive-like behaviors mediated by the amygdala. The Journal of Neuroscience. 2012;32:7758–7765. doi: 10.1523/JNEUROSCI.5843-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinne-Albers MA, van der Wee NJ, Lamers-Winkelman F, Vermeiren RR. Neuroimaging in children, adolescents and young adults with psychological trauma. European Child & Adolescent Psychiatry. 2013:1–11. doi: 10.1007/s00787-013-0410-1. [DOI] [PubMed] [Google Scholar]
- Shin LM, Wright CI, Cannistraro PA, Wedig MM, McMullin K, Martis B, Rauch SL. A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Archives of General Psychiatry. 2005;62:273–281. doi: 10.1001/archpsyc.62.3.273. [DOI] [PubMed] [Google Scholar]
- Weber M, Killgore WD, Rosso IM, Britton JC, Schwab ZJ, Weiner MR, Rauch SL. Voxel-based morphometric gray matter correlates of posttraumatic stress disorder. Journal of Anxiety Disorders. 2013;27:413–419. doi: 10.1016/j.janxdis.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worsley KJ. Statistical analysis of activation images. Functional MRI: An Introduction to Methods. 2001;14:251–270. doi: 10.1093/acprof:oso/9780192630711.003.0014. [DOI] [Google Scholar]