Abstract
While initial phylogenetic analyses concluded to Guinea 2014 EBOV falling outside the Zaïre lineage (ZEBOV), a recent re-analysis of the same dataset by Dudas and Rambaut (2014) suggested that Guinea 2014 EBOV actually is ZEBOV. Under the same hypothesis as used by these authors (the molecular clock hypothesis), we reinforce their conclusion by providing a statistical assessment of the location of the root of the Zaïre lineage. Our analysis unambiguously supports Guinea 2014 EBOV as a member of the Zaïre lineage. In addition, we also show that some uncertainty exists so as to the location of the root of the genus Ebolavirus. We release the software we used for these re-analyses. RootAnnotator allows for the easy determination of branch root posterior probability from any posterior sample of clocked trees and is freely available at http://sourceforge.net/projects/rootannotator/.
Introduction
Phylogenetic analyses are a very popular and powerful way to extract information from sequences. The end product is a tree-like graph intended to summarize the course of evolution. Sequence relatedness can be discussed on the basis of this graph but only on condition that it is assigned a direction for time. This is achieved by a process known as rooting, that turns a tree-like graph into a proper phylogenetic tree1 .
Dudas and Rambaut (2014) recently demonstrated that improper rooting can end up in supporting strikingly erroneous evolutionary scenarios, which can in turn mislead the formulation of important epidemiological hypotheses2 . These authors contradicted a preliminary report that identified Guinea 2014 Ebolavirus (EBOV) as a divergent lineage falling outside the Zaïre lineage (ZEBOV)3 , and suggested that Guinea 2014 EBOV instead nests within this lineage, i.e. is ZEBOV. Accordingly, Guinea 2014 EBOV is more likely to be the result of a fairly recent introduction of ZEBOV from Central Africa than a long-term endemic in West Africa.
The initial misplacement of Guinea 2014 EBOV was due to unnoticed long-branch attraction to the outgroup4 . As pointed out by Dudas and Rambaut (2014), this phenomenon made the long branch of Guinea 2014 EBOV drift towards the basis of the Zaïre clade as it was attracted to the other (very divergent) EBOV lineages included in the analyses (Bundibugyo, Taï Forest, Reston and Sudan)2 . To identify the location of the root within the Zaïre clade, Dudas and Rambaut (2014) excluded any outgroup and rooted the ingroup tree by minimizing the variance of root-to-tip distances (using Path-O-Gen, available at http://tree.bio.ed.ac.uk/software/pathogen/)2 . By doing so, they made the reasonable hypothesis that ZEBOV sequences evolve according to some kind of molecular clock and that the most likely root location minimizes rate variation across lineages.
This approach is very sensible. However, here we introduce an alternative method which is built under the same hypothesis but additionally allows for a quantitative assessment of the support for any root location. As an illustration, we apply this method to localize the root within the Zaïre clade. Using this same tool, we also investigate the position of the root within the genus Ebolavirus, whose deep branching order is controversial14 , 15 .
Rationale
Over the last decade, one of the most fundamental developments in the field of phylogenetics was the introduction of models employing relaxed molecular clocks5 . These are clock models that recognize some degree of clocklikeness to the substitution process without going to the extreme of a single constant rate of evolution applied to the entire tree. This methodological leap considerably popularized phylogenetic analyses under clock models, which are commonly performed on one of the two leading platforms for Bayesian phylogenetics, BEAST6 , 7 and MrBayes8 , 9 , 10 . These platforms use Markov chain Monte Carlo (MCMC) samplers to approximate the (Bayesian) posterior distribution of all model parameters. Trees (i.e. topology and branch lengths) are among the parameters to estimate and MCMC samplers typically end up generating plausible sets of phylogenetic trees. When the model of evolution incorporates a clock model, MCMC samplers actually generate plausible sets of rooted trees. This can be viewed as generating a plausible set of tree-like graphs on the one hand and a plausible set of root locations on the other. Branch support is typically derived from posterior tree samples by counting the proportion of trees comprising the branch of interest (branch posterior probability). Following others5 , 11 , 12 , we propose to apply the same logic to derive root support. Here, the proportion of posterior trees for which the branch of interest wears the root would be taken as this branch root posterior probability (RPP).
Software
Assessing branch RPP only requires parsing the posterior sample of trees, recording the branches wearing the root and their frequency. Due to the large number of trees, this cannot be done manually. As, to the best of our knowledge, no software is available for this purpose, we developed RootAnnotator, a user-friendly, portable software that collects information on root positions in posterior samples of trees and annotates a target tree with the according RPP (available from www.sourceforge.net/projects/rootannotator/). Among other options, the user can choose that the target tree is the maximum clade credibility (MCC) tree, which RootAnnotator will identify by running TreeAnnotator (distributed with BEAST)6 .
Phylogenetic analysis
To investigate the position of the root within the Zaïre clade, we used the alignment of concatenated coding sequences published by Dudas and Rambaut (2014) (available at https://github.com/evogytis/ebolaGuinea2014) from which we removed all sequences that did not belong to the Zaïre lineage2 . This dataset therefore comprised 23 ZEBOV sequences and the three Guinea 2014 EBOV sequences. To assess the position of the root within the genus Ebolavirus, we also used an alignment of concatenated coding sequences derived from Dudas and Rambaut (2014)2 . This alignment included 5 sequences which were selected to represent the five recognized species in the genus.
The first alignment was analyzed in BEAST using a GTR+Γ model of nucleotide substitution and assuming an uncorrelated relaxed clock (lognormal) which was tip-calibrated. We performed these analyses under the same three distinct demographic priors used by Dudas and Rambaut (2014) (constant population size, exponential growth and Bayesian skyride)2 . The second alignment was first analyzed in PhyML13 using a GTR+Γ model of nucleotide substitution. The resulting tree was analysed with Path-O-Gen which did not evidence any strong clocklike signal (a positive correlation of time and root-to-tip distances was only marginally supported; R2: 0.43). The second alignment was therefore analyzed in BEAST using a GTR+Γ model of nucleotide substitution and assuming an uncorrelated relaxed clock (lognormal) which was not tip-calibrated. We performed these analyses under two speciation priors (Yule and Birth-Death process).
Branch RPP were determined using RootAnnotator and plotted on the MCC trees. When a branch appearing at least once as wearing the root in the posterior sample did not appear in the MCC tree, RootAnnotator was used to select a tree containing that branch so as to visualize their RPP (note that the RootAnnotator output also comprises a csv file comprising a list of all branches identified as wearing the root together with the associated RPP).
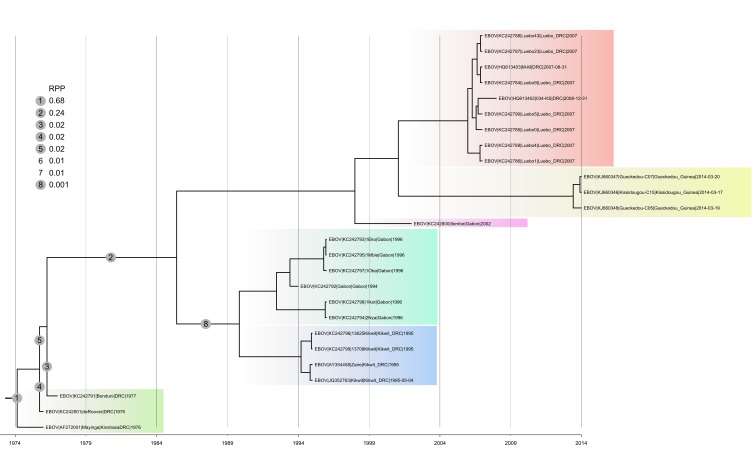
Maximum clade credibility tree of concatenated coding sequences belonging to the Zaïre clade.
MCC tree and branch root posterior probabilities (RPP) derived from the analysis run under a constant population size model (the two other models ended up with generating very similar results) and an uncorrelated relaxed clock (lognormal). In the top left corner the complete list of branches that appeared at least once in the posterior tree sample and the according RPP. Note that two possible root locations (6 and 7) do not appear in the tree as the MCC tree did not comprise the corresponding branches. All internal branches linking coloured clades/groups received very good support (posterior probability: 1.00). The only exception was the branch defining the clade comprising Guinea 2014 EBOV and DRC 2007/2008 EBOV, which was only moderately supported (posterior probability comprised between 0.56 and 0.68).
Results and discussion
For the Zaïre lineage, the posterior tree samples that we analyzed (one sample per demographic model) did not comprise a single tree whose root would be located on the branch leading to Guinea 2014 EBOV (Figure 1). Hence, under the assumption of a relaxed molecular clock it seems extremely unlikely that this virus falls outside the genetic diversity of the Zaïre lineage. The clock rooting approach implemented here therefore provides strong statistical support to the conclusion reached by Dudas and Rambaut (2014)2 . We also note that in our analyses the split of Guinea 2014 EBOV and the closest Central African EBOV was inferred to have taken place in 1999 (Bayesian skyride; 95% HPD interval 1996-2004) or 2001 (constant population size or exponential growth; 95% HPD interval 1996-2003), which comes very close to the GP-based estimates of Dudas and Rambaut (2002; 95% HPD interval 2000-2006)2 .
Depending on the demographic model, eight to nine root locations were identified within the Zaïre clade. Irrespective of the demographic model, the same two branches were always identified as receiving the two highest RPP. The external branch leading to the DRC 1976 ZEBOV strain (Mayinga) received RPP comprised between 0.62 and 0.69 whereas for the branch defining the bipartition [DRC 1976/1977 ZEBOV strains|other ZEBOV strains] RPP were between 0.21 and 0.28. These results mostly raise the question of the reciprocal monophyly of early DRC ZEBOV and all other ZEBOV strains (only supported by the second-to-best root location).
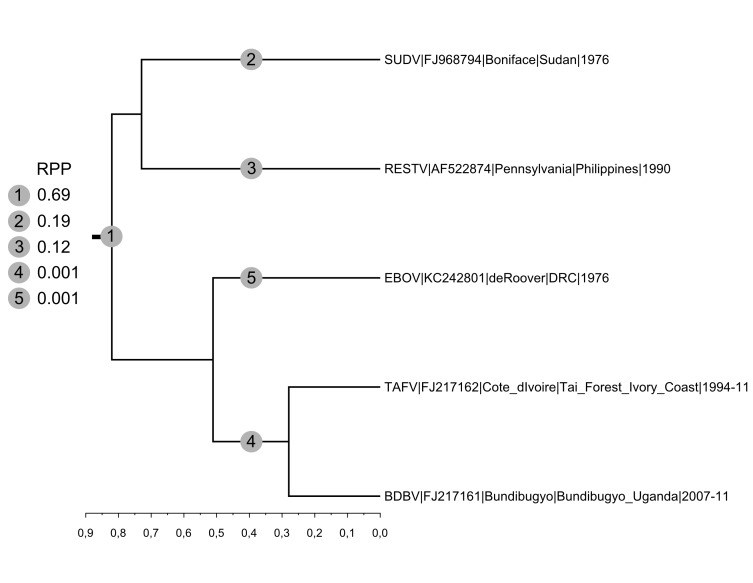
Maximum clade credibility tree of concatenated coding sequences representing the 5 species of the genus Ebolavirus.
MCC tree and branch root posterior probabilities (RPP) derived from the analysis run under a Yule process (a Birth-Death process ended up with generating very similar results) and an uncorrelated relaxed clock (lognormal). The clock was not calibrated and the scale axis therefore is in substitution per site. RPP are reported in the list appearing at the left of the tree. All internal branches received very good support (posterior probability: 1.00).
We also applied the clock rooting approach to the genus Ebolavirus. Two recent articles whose analyses included outgroup sequences (Marburg virus and Lloviu virus) agreed on the monophyly of Bundibugyo, Taï Forest and Zaïre ebolaviruses but supported different sisterships of this clade with Reston and Sudan ebolaviruses. In the phylogeny produced by Lauber and Gorbalenya (2012) Reston ebolavirus was in sistership with the clade comprising Zaïre ebolavirus15 while in the phylogeny by Carroll et al. (2013) Sudan and Reston ebolaviruses formed a sister clade to the clade comprising Zaïre ebolavirus14 .
Under both speciation priors we tested, five root locations were identified and among these three gathered >0.99 RPP (Figure 2). The branch defining the bipartition [Sudan, Reston|Bundibugyo, Taï Forest, Zaïre] received RPP 0.69 and 0.68 (Yule and Birth-Death process, respectively), the external branch leading to Sudan ebolavirus RPP 0.19 and 0.18 and the external branch leading to Reston ebolavirus RPP 0.12 and 0.13. Therefore, while the hypothesis put forward by Carroll et al. (2013) gets more probabilistic support14 , our analyses underlines significant uncertainty and the existence of a third plausible root position. As Reston ebolavirus is the only Asian ebolavirus (all other ebolaviruses are African), we note here that this new rooting scenario would imply a geographical partitioning of the genetic diversity within the genus Ebolavirus.
In our view, these examples highlight the unique ability of clock rooting to capture uncertainty so as to root location. With RootAnnotator it is now easily possible to establish short lists of plausible roots warranting further examination12 , even where no obvious candidate roots exist (e.g. in the absence of appropriate outgroups). We hope that this tool will provide the many biologists using Bayesian phylogenetics under clock models with a rationale to turn even more tree-like graphs into phylogenetic trees.
Competing interests
The authors have declared that no competing interests exist.
Acknowledgments
The authors thank Paul Schäpe for his help with the implementation of BEAST on a local server. SCS thanks the entire group of epidemiology of highly pathogenic microorganisms at the Robert Koch-Institute for stimulating discussions.
Biographies
Epidemiology of highly pathogenic microorganisms
Research Group Bioinformatics (NG4)
NG 4 - Bioinformatics
Funding Statement
This work was entirely funded by the Robert Koch-Institute.
Contributor Information
Sébastien Calvignac-Spencer, Robert Koch-Institute, Berlin, Germany.
Jakob M. Schulze, Robert Koch-Institute, Berlin, Germany
Franziska Zickmann, Robert Koch-Institute, Berlin, Germany.
Bernhard Y. Renard, Robert Koch-Institute, Berlin, Germany
References
- 1.Hall BG. Building phylogenetic trees from molecular data with MEGA. Mol Biol Evol. 2013 May;30(5):1229-35. PubMed PMID:23486614. [DOI] [PubMed]
- 2.Dudas G, Rambaut A. Phylogenetic Analysis of Guinea 2014 EBOV Ebolavirus Outbreak. PLOS Currents Outbreaks. 2014 May 2. Edition 1. doi: 10.1371/currents.outbreaks.84eefe5ce43ec9dc0bf0670f7b8b417d. [DOI] [PMC free article] [PubMed]
- 3.Baize S, Pannetier D, Oestereich L, Rieger T, Koivogui L, Magassouba N, Soropogui B, Sow MS, Keïta S, De Clerck H, Tiffany A, Dominguez G, Loua M, Traoré A, Kolié M, Malano ER, Heleze E, Bocquin A, Mély S, Raoul H, Caro V, Cadar D, Gabriel M, Pahlmann M, Tappe D, Schmidt-Chanasit J, Impouma B, Diallo AK, Formenty P, Van Herp M, Günther S. Emergence of Zaire Ebola Virus Disease in Guinea - Preliminary Report. N Engl J Med. 2014 Apr 16. PubMed PMID:24738640. [DOI] [PubMed]
- 4.Bergsten, J. (2005), A review of long-branch attraction. Cladistics, 21: 163–193. doi: 10.1111/j.1096-0031.2005.00059.x 10.1111/j.1096-0031.2005.00059.x [DOI] [PubMed]
- 5.Drummond AJ, Ho SY, Phillips MJ, Rambaut A. Relaxed phylogenetics and dating with confidence. PLoS Biol. 2006 May;4(5):e88. PubMed PMID:16683862. [DOI] [PMC free article] [PubMed]
- 6.Drummond AJ, Suchard MA, Xie D, Rambaut A. Bayesian phylogenetics with BEAUti and the BEAST 1.7. Mol Biol Evol. 2012 Aug;29(8):1969-73. PubMed PMID:22367748. [DOI] [PMC free article] [PubMed]
- 7.Drummond AJ, Rambaut A. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol Biol. 2007 Nov 8;7:214. PubMed PMID:17996036. [DOI] [PMC free article] [PubMed]
- 8.Huelsenbeck JP, Ronquist F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics. 2001 Aug;17(8):754-5. PubMed PMID:11524383. [DOI] [PubMed]
- 9.Ronquist F, Huelsenbeck JP. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003 Aug 12;19(12):1572-4. PubMed PMID:12912839. [DOI] [PubMed]
- 10.Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol. 2012 May;61(3):539-42. PubMed PMID:22357727. [DOI] [PMC free article] [PubMed]
- 11.Boykin LM, Kubatko LS, Lowrey TK. Comparison of methods for rooting phylogenetic trees: a case study using Orcuttieae (Poaceae: Chloridoideae). Mol Phylogenet Evol. 2010 Mar;54(3):687-700. PubMed PMID:19931622. [DOI] [PubMed]
- 12.Renner SS, Grimm GW, Schneeweiss GM, Stuessy TF, Ricklefs RE. Rooting and dating maples (Acer) with an uncorrelated-rates molecular clock: implications for north American/Asian disjunctions. Syst Biol. 2008 Oct;57(5):795-808. PubMed PMID:18853365. [DOI] [PubMed]
- 13.Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, Gascuel O. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol. 2010 May;59(3):307-21. PubMed PMID:20525638. [DOI] [PubMed]
- 14.Carroll SA, Towner JS, Sealy TK, McMullan LK, Khristova ML, Burt FJ, Swanepoel R, Rollin PE, Nichol ST. Molecular evolution of viruses of the family Filoviridae based on 97 whole-genome sequences. J Virol. 2013 Mar;87(5):2608-16. PubMed PMID:23255795. [DOI] [PMC free article] [PubMed]
- 15.Lauber C, Gorbalenya AE. Genetics-based classification of filoviruses calls for expanded sampling of genomic sequences. Viruses. 2012 Sep;4(9):1425-37. PubMed PMID:23170166. [DOI] [PMC free article] [PubMed]