The authors describe a more efficient, simple-to-use, and cost-effective method for generating integration-free human induced pluripotent stem (hiPS) cells from keratinocytes. This improved method, using lipid-mediated transfection, achieved a reprogramming efficiency of ∼0.14% on average. The results support keratinocytes as an easily accessible cell source for efficient generation of patient-specific hiPS cells.
Keywords: Derivation of human induced pluripotent stem cells, Keratinocytes, Integration-free, Episomal vectors, Lipid mediated transfection
Abstract
Keratinocytes represent an easily accessible cell source for derivation of human induced pluripotent stem (hiPS) cells, reportedly achieving higher reprogramming efficiency than fibroblasts. However, most studies utilized a retroviral or lentiviral method for reprogramming of keratinocytes, which introduces undesirable transgene integrations into the host genome. Moreover, current protocols of generating integration-free hiPS cells from keratinocytes are mostly inefficient. In this paper, we describe a more efficient, simple-to-use, and cost-effective method for generating integration-free hiPS cells from keratinocytes. Our improved method using lipid-mediated transfection achieved a reprogramming efficiency of ∼0.14% on average. Keratinocyte-derived hiPS cells showed no integration of episomal vectors, expressed stem cell-specific markers and possessed potentials to differentiate into all three germ layers by in vitro embryoid body formation as well as in vivo teratoma formation. To our knowledge, this represents the most efficient method to generate integration-free hiPS cells from keratinocytes.
Introduction
Human induced pluripotent stem (hiPS) cells provide a valuable source for generating patient-specific stem cells for regenerative medicine and disease modeling [1, 2]. hiPS cells can be generated by reprogramming somatic cells using viral-based methods to deliver various reprogramming factors [3–5]. However, a major concern with using viral based methods to generate hiPS cells is uncontrollable integration of foreign transgenes into the host genome. Recent research has utilized episomal vectors to generate integration-free hiPS cells from skin fibroblasts with an average of <0.03% reprogramming efficiency [6, 7]. Some cell types have reportedly achieved higher reprogramming efficiency, such as cord blood myeloid progenitors (average: 1.4% [8]) and neural stem cells (average: 0.1%–1% [9]), but they are not easily accessible. In contrast, keratinocytes are easily accessible from hair follicles and have demonstrated much higher reprogramming efficiency than fibroblasts using viral-based reprogramming methods [10]. However, the current protocol of generating integration-free hiPS cells from keratinocytes can achieve only low reprogramming efficiency (∼0.001% [11]). In this paper, we provide an improved protocol to generate integration-free hiPS cells from keratinocytes.
Materials and Methods
Cell Culture
The hiPS cells were cultured on mitotically inactivated mouse embryonic fibroblasts (MEFs) in KnockOut Dulbecco’s modified Eagle’s medium: Nutrient Mixture F-12 with 20% knockout serum replacement, 10 ng/ml basic fibroblast growth factor, 1× GlutaMAX, 0.1 mM nonessential amino acids, and 100 μM β-mercaptoethanol (Invitrogen, Carlsbad, CA, http://www.invitrogen.com).
Generation of hiPS Cells From Fibroblasts
BJ fibroblasts (Stemgent, Cambridge, MA, https://www.stemgent.com) were reprogrammed, as described previously [6], with modifications. Episomal vectors [12] (pCXLE-hOCT3/4, pCXLE-hOCT3/4-shp53, pCXLE-hSK, pCXLE-hUL, and pCXLE-eGFP) were obtained from Addgene (Cambridge, MA, https://www.addgene.org). On day 0, 6 × 105 fibroblasts were harvested and nucleofected with 4 μg of vectors (1 μg per vector) using Nucleofector II (Lonza, Walkersville, MD, http://www.lonza.com). Nucleofection was carried out with the Nucleofection kit for primary fibroblasts (Lonza) using program T-016. The nucleofected cells were plated down onto one well of a gelatinized six-well plate. On day 7, 1 × 105 BJ fibroblasts were replated onto a 100-mm dish precoated with 1.5 × 106 MEF feeders in hiPS cell medium. Medium was changed every 2 days.
Generation of hiPS Cells From Keratinocytes
We plated 1.3–1.5 × 105 epidermal keratinocytes (System Biosciences, Mountain View, CA, http://www.systembio.com) in one well of a gelatinized six-well plate, and they were allowed to grow overnight. On day 0, keratinocytes with >50% confluence were transfected using FuGENE HD (Roche, Indianapolis, IN, http://www.roche.com) using Fugene HD (Roche, Indianapolis, IN, http://www.roche.com) with a 8 μl to μg DNA ratio (0.5 μg per vector) for 4 hours. Second transfection was repeated on day 2. On day 3, keratinocytes were trypsinized, and 90% of the cells were replated onto a 100-mm dish precoated with 4 × 106 MEF feeders in hiPS cell medium. Medium was changed every day.
Quantification of Reprogramming Efficiency
On day 30, reprogrammed cells were immunostained with TRA-1-60 antibodies (Millipore, Billerica, MA, http://www.millipore.com). Reprogramming efficiency is calculated as the number of TRA-1-60 positive colonies divided by the number of starting cells and is adjusted to a passaging ratio when replating onto MEF feeders. Alternatively, quantification of hiPS cell colonies was performed by counting the colonies that resemble human embryonic stem cell-like (ESC-like) morphology and are positive for expression of alkaline phosphatase (AP; detection kit from Sigma-Aldrich, St. Louis, MO, http://www.sigmaaldrich.com).
In Vitro and In Vivo Differentiation of hiPS Cells
For mesoderm and endoderm differentiation, day 7 embryoid bodies (EBs) were plated on gelatinized dishes to differentiate for 4 days. For neural differentiation, day 10 EBs were plated on gelatinized dishes to differentiate for 15 days.
Teratoma were formed in vivo by transplanting ∼2 × 106 hiPS cells into nude rats using a vascularized tissue engineering chamber, as described previously [13, 14]. Teratoma constructs were harvested at 4 weeks after implantation for histological analysis.
Characterization of Derived hiPS Cells
For RT-PCR, 25–30 cycles of polymerase chain reaction (PCR) were performed using primers listed in supplemental online Table 1. For immunocytochemistry, the standard procedure was performed, as described previously [15]. Primary antibodies used are TRA-1-60 (Stemgent or Millipore), OCT4 (Santa Cruz Biotechnology Inc., Santa Cruz, CA, http://www.scbt.com), NANOG (R&D Systems Inc., Minneapolis, MN, http://www.rndsystems.com), α smooth muscle actin (SMA; R&D Systems Inc.), GATA4 (Santa Cruz Biotechnology Inc.), or Nestin (Abcam, Cambridge, U.K., http://www.abcam.com).
Results
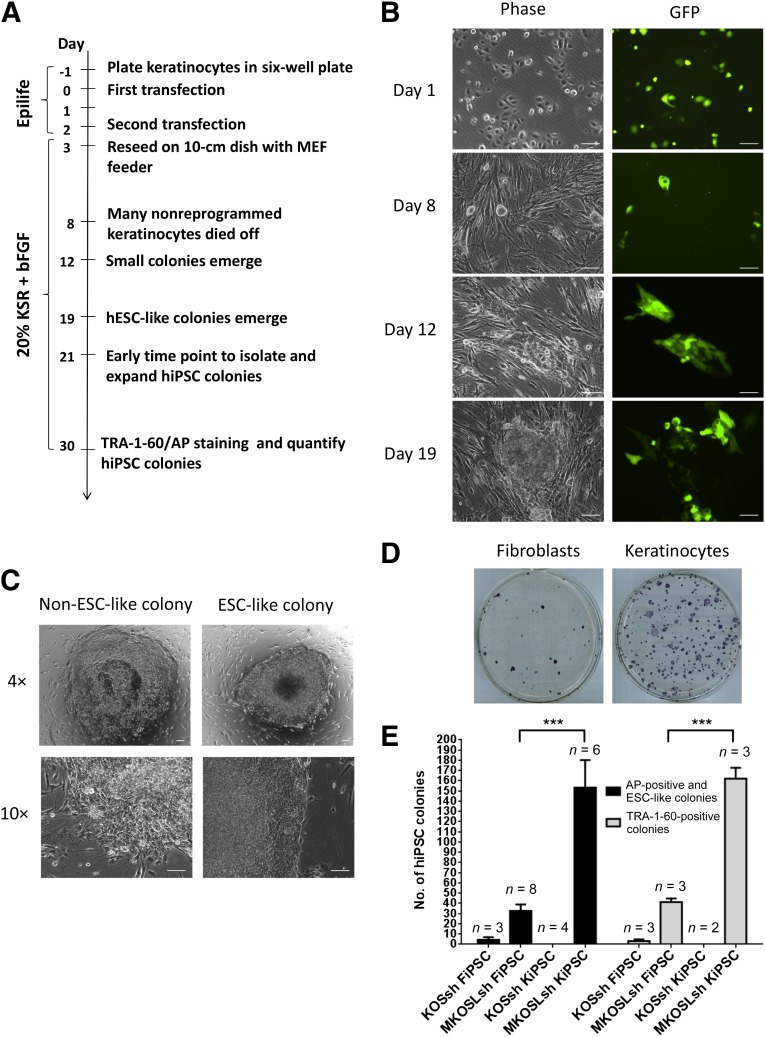
Figure 1A illustrates the timeline of hiPS cell generation from keratinocytes. We utilized episomal vectors, described previously [6], to deliver various reprogramming factors. In contrast to previous reports that utilized electroporation or nucleofection to deliver episomal vectors [6, 16], we found that lipid-mediated transfection using FuGENE HD is highly efficient in keratinocytes, achieving ∼70% efficiency after a single transfection, as determined by green fluorescent protein (GFP) expression (Fig. 1B). Two transfections were carried out to deliver episomal vectors carrying six reprogramming factors (MKOSLsh: L-MYC, KLF4, OCT4, SOX2, LIN28, and short hairpin RNA [shRNA] for p53). By day 8, nonreprogrammed keratinocytes would slowly detached from the culture. We observed small colonies emerge from day 12, and some of these colonies acquired a human ESC-like morphology by day 19 (Fig. 1B), forming tight colonies with defined borders (Fig. 1C). These hiPS cell colonies could be picked and expanded from days 21–30.
Figure 1.
hiPSC generation from keratinocytes using episomal vectors. (A): Schematic timeline. (B): Cell morphology and GFP expression of keratinocytes during reprogramming at days 1, 8, 12, and 19 using six reprogramming factors (MKOSLsh) and GFP. Scale bar = 100 μm. (C): Representative morphology of a non-ESC-like colony and an ESC-like colony reprogrammed from keratinocytes. Scale bar = 100 μm. (D): Representative image of AP staining of FiPSCs and KiPSCs in a 100-mm dish. (E): Quantification of FiPSCs or KiPSCs using four reprogramming factors (KOSsh) or six reprogramming factors (MKOSLsh). ∗∗∗ = p < .05, unpaired two-tailed t test. Abbreviations: AP, alkaline phosphatase; bFGF, basic fibroblast growth factor; ESC-like, embryonic stem cell-like; FiPSC, hiPSC colonies generated from fibroblasts; GFP, green fluorescent protein; hESC-like, human embryonic stem cell-like; hiPSC, human induced pluripotent stem cell; KiPSC, hiPSC colonies generated from keratinocytes; KOSsh, KLF4, OCT4, SOX2, and short hairpin RNA for p53; KSR, knockout serum replacement; MEF, mouse embryonic fibroblast; MKOSLsh, L-MYC, KLF4, OCT4, SOX2, LIN28, and short hairpin RNA for p53.
We compared the reprogramming efficiency using episomal vectors on keratinocytes (this protocol) to fibroblasts following a previous protocol [6]. Fully reprogrammed hiPS cell colonies can be identified by TRA-1-60 expression [17] or human ESC-like morphology [5, 6, 18]. Figure 1D showed a representative picture of hiPS cell colonies generated from keratinocytes or fibroblasts. We showed that our protocol to reprogram keratinocytes using six reprogramming factors (MKOSLsh) yielded 162 ± 11 TRA-1-60-positive colonies (n = 3), compared with 41 ± 4 TRA-1-60-positive colonies (n = 3) from fibroblasts (Fig. 1E). In addition, quantification of hiPS cell colonies defined by human ESC-like morphology and AP expression yielded similar results (153 ± 27 colonies from keratinocytes compared with 33 ± 6 colonies from fibroblasts).
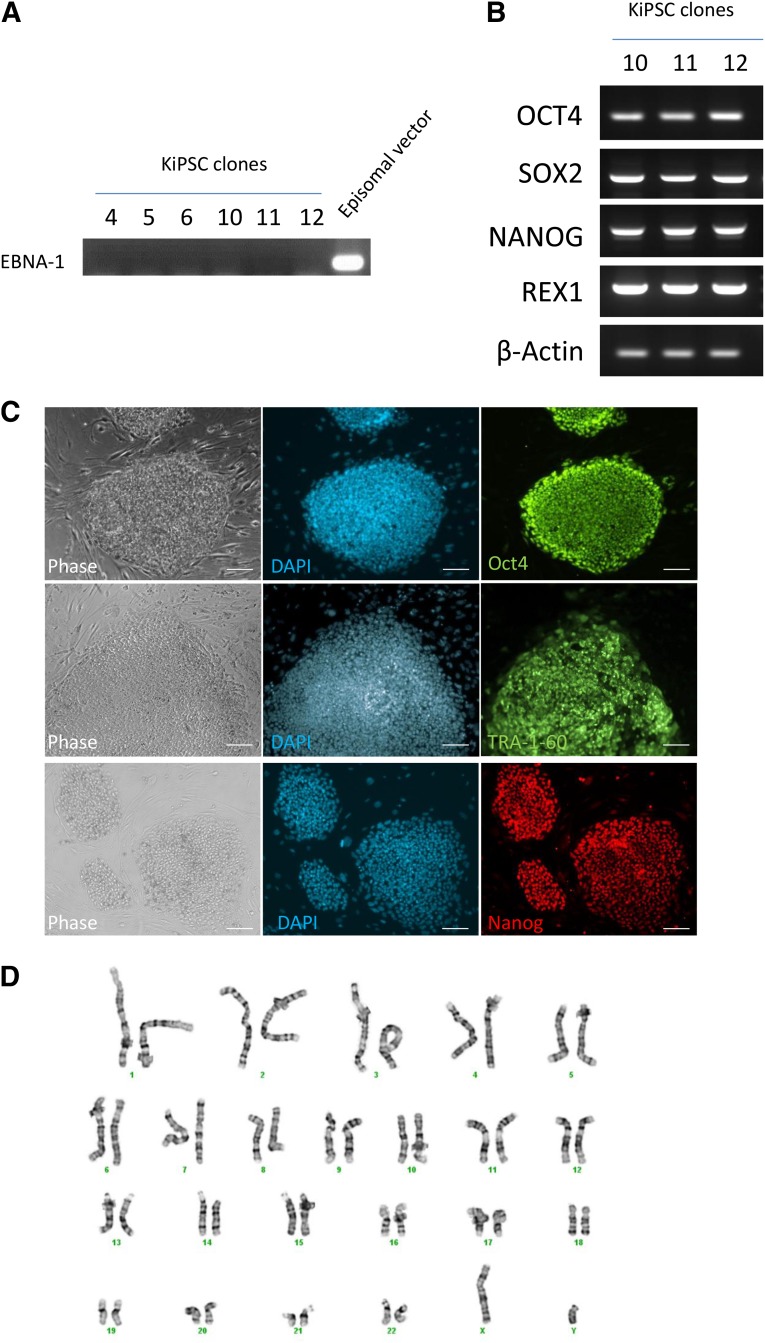
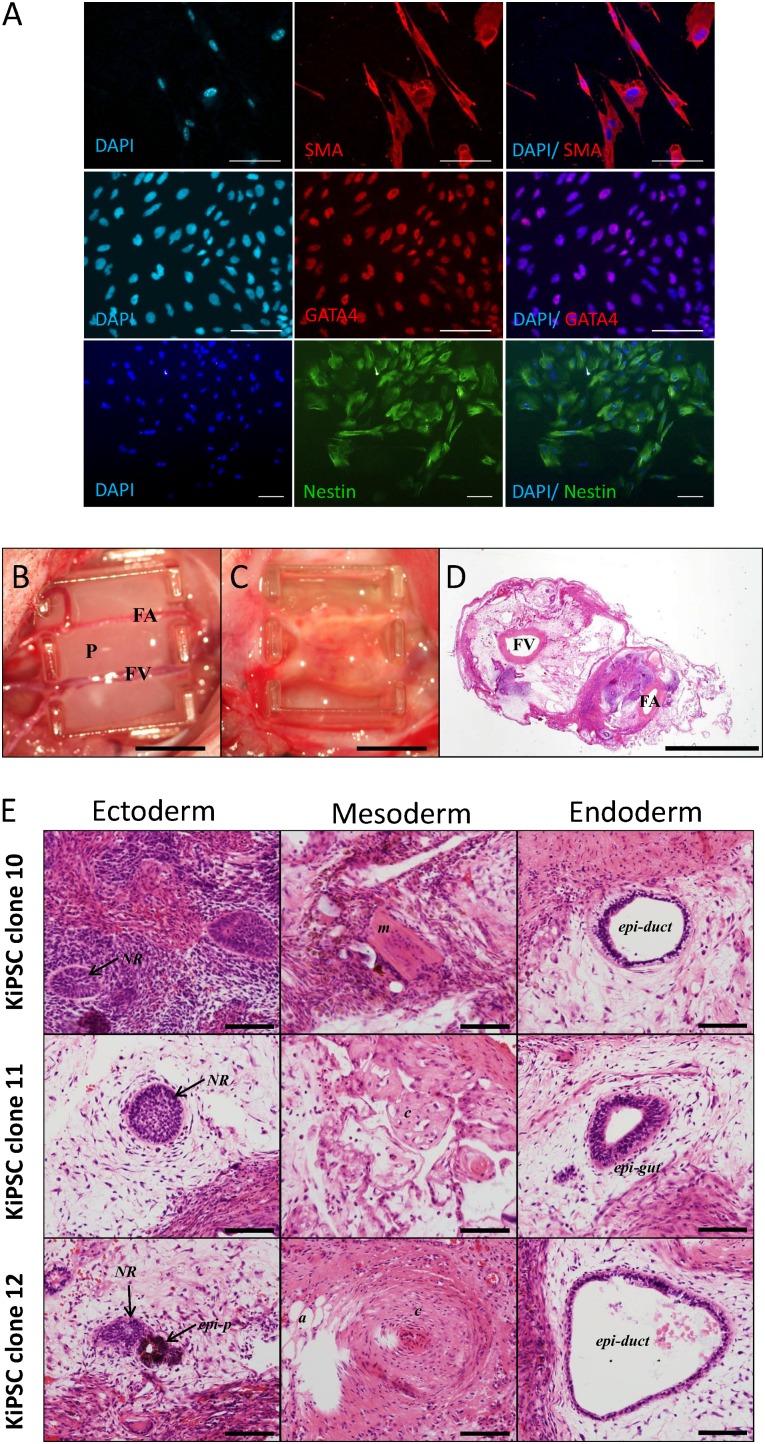
Next, we isolated six hiPS cell clones derived from keratinocytes using six reprogramming factors (MKOSLsh) and performed further characterization. All six clones showed no integration of the episomal vectors to the genome (Fig. 2A). Keratinocyte-derived hiPS cells showed expression of pluripotent genes OCT4, SOX2, NANOG, and REX1 (Fig. 2B). Furthermore, immunocytochemistry analysis showed that keratinocyte-derived hiPSCs expressed pluripotent markers OCT4, TRA-1-60, and NANOG (Fig. 2C) and retained a normal karyotype (Fig. 2D). The keratinocyte-derived hiPS cells formed EBs and differentiated into cells of the endodermal lineage (GATA4 positive), mesodermal lineage (SMA positive), and ectodermal lineage (Nestin positive, Fig. 3A). Moreover, we transplanted three clonal keratinocyte-derived hiPS cell lines into nude rats for teratoma formation using a vascularized tissue engineering chamber [13] (Fig. 3B, 3C). All three keratinocyte-derived hiPS cell lines formed teratoma consisting of cells representative of the germ layers (Fig. 3D, 3E). Together, these results confirmed that keratinocyte-derived hiPS cells derived from this protocol are bona fide hiPS cells.
Figure 2.
Characterization of KiPSC clones. (A): Reverse transcription polymerase chain reaction (RT-PCR) analysis of EBNA-1 integration in isolated keratinocyte-derived hiPSC clones. The episomal vector was used as a positive control. (B): RT-PCR analysis of pluripotent gene expression OCT4, SOX2, NANOG, and REX1 in KiPSC clone 10-12. β-actin was used as loading control. (C): Immunocytochemistry analysis of pluripotent markers OCT4 (green), TRA-1-60 (green), NANOG (red), and DAPI counterstain (blue) in established KiPSCs. Scale bar = 100 μm. (D): G-band karyotyping of KiPSCs (passage 6). Abbreviations: DAPI, 4′,6-diamidino-2-phenylindole; KiPSC, hiPSC colonies generated from keratinocytes.
Figure 3.
KiPSC clones retain in vitro and in vivo differentiation potentials. (A): Immunocytochemistry analysis of in vitro differentiation potential of established KiPSCs into the three germ layers: endodermal (GATA4), mesodermal (SMA), and ectodermal (Nestin) lineages. Scale bar = 100 μm. (B, C): Image of the tissue engineering chamber implanted with KiPSCs (B) and teratoma formation after 4 weeks (C). Scale bar = 5 cm. (D): Hematoxylin and eosin staining of teratoma constructs harvested at 4 weeks after implantation. Scale bar = 2 mm. (E): In vivo teratoma formation of established KiPSCs showing cells representative of the three germ layers. Scale bar = 100 μm. Abbreviations: a, adipose; c, cartilaginous structure; DAPI, 4′,6-diamidino-2-phenylindole; epi-duct, epithelial-lined duct structure; epi-gut, gut-like epithelium; epi-p, pigment epithelium; FA, femoral artery; FV, femoral vein; KiPSC, hiPSC colonies generated from keratinocytes; m, muscle; NR, neural rosette structure; P, rat plasma clot containing cells.
Discussion
Our hiPS cell derivation protocol using FuGENE HD has the advantage of being more cost-effective compared with previous protocols that require expensive apparatus such as a nucleofector or electroporator. Importantly, our protocols using FuGENE HD achieved a higher reprogramming efficiency of ∼0.14% on average in keratinocytes, compared with those previously reported in fibroblasts (∼0.0006%–0.03% [6, 7]), peripheral blood or bone marrow progenitors (∼0.0006%–0.0009% [16]), and hair keratinocytes (∼0.001% [11]). Also, previous study has shown that KLF4, OCT4, and SOX2 were sufficient to reprogram keratinocytes into hiPS cells using viral methods [10], albeit with low reprogramming efficiency. We attempted to derive hiPS cells using 4 reprogramming factors KOSsh (KLF4, OCT4, SOX2, shRNA for P53) with our protocol, but this did not yield any hiPS cell colony from keratinocytes (Fig. 1E). Since episomal vectors are generally less efficient in hiPS cell generation compared with viral-mediated methods, it is possible that elimination of MYC and LIN28 made it insufficient to reprogram keratinocytes using episomal vectors.
Conclusion
This paper describes an improved method for generating integration-free hiPS cells from keratinocytes by FuGENE HD transfection that is simple to use and cost-effective compared with electroporation and nucleofection. To our knowledge, this improved protocol is the most efficient protocol for generating integration-free hiPS cells from keratinocytes. Our results support keratinocytes as an easily accessible cell source for efficient generation of patient-specific hiPS cells.
Supplementary Material
Acknowledgments
The authors thank Hong Yu (National Institute on Aging, National Institutes of Health [NIA/NIH]) and Priyadharshini Sivakumaran for technical assistance. This work was supported in part by the NIA/NIH Intramural Research Program (Y.P., S.S.-C.H., R.C.-B.W., M.S.H.K.) and the Cranbourne Foundation Fellowship (R.C.-B.W.).
Author Contributions
Y.P. and S.S.-C.H.: conception and design, collection and/or assembly of data, data analysis and interpretation, final approval of manuscript; S.Y.L.: conception and design, collection and/or assembly of data, data analysis and interpretation; R.C.-B.W.: conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript, financial support; M.S.H.K.: conception and design, data analysis and interpretation, manuscript writing, final approval of manuscript, financial support.
Disclosure of Potential Conflicts of Interest
S.S.-C.H. and R.C.-B.W. have compensated employment with Elixirgen LLC.
References
- 1.Hayashi Y, Saitou M, Yamanaka S. Germline development from human pluripotent stem cells toward disease modeling of infertility. Fertil Steril. 2012;97:1250–1259. doi: 10.1016/j.fertnstert.2012.04.037. [DOI] [PubMed] [Google Scholar]
- 2.Robinton DA, Daley GQ. The promise of induced pluripotent stem cells in research and therapy. Nature. 2012;481:295–305. doi: 10.1038/nature10761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 4.Yu J, Vodyanik MA, Smuga-Otto K, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 5.Maekawa M, Yamaguchi K, Nakamura T, et al. Direct reprogramming of somatic cells is promoted by maternal transcription factor Glis1. Nature. 2011;474:225–229. doi: 10.1038/nature10106. [DOI] [PubMed] [Google Scholar]
- 6.Okita K, Matsumura Y, Sato Y, et al. A more efficient method to generate integration-free human iPS cells. Nat Methods. 2011;8:409–412. doi: 10.1038/nmeth.1591. [DOI] [PubMed] [Google Scholar]
- 7.Yu J, Hu K, Smuga-Otto K, et al. Human induced pluripotent stem cells free of vector and transgene sequences. Science. 2009;324:797–801. doi: 10.1126/science.1172482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burridge PW, Thompson S, Millrod MA, et al. A universal system for highly efficient cardiac differentiation of human induced pluripotent stem cells that eliminates interline variability. PLoS One. 2011;6:e18293. doi: 10.1371/journal.pone.0018293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marchetto MC, Yeo GW, Kainohana O, et al. Transcriptional signature and memory retention of human-induced pluripotent stem cells. PLoS One. 2009;4:e7076. doi: 10.1371/journal.pone.0007076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aasen T, Raya A, Barrero MJ, et al. Efficient and rapid generation of induced pluripotent stem cells from human keratinocytes. Nat Biotechnol. 2008;26:1276–1284. doi: 10.1038/nbt.1503. [DOI] [PubMed] [Google Scholar]
- 11.Peters A, Zambidis E. Generation of nonviral integration-free induced pluripotent stem cells from plucked human hair follicles. In: Ye K, Jin S, editors. Human Embryonic and Induced Pluripotent Stem Cells: Lineage-Specific Differentiation Protocols. New York, NY: Springer; 2012. pp. 203–227. [Google Scholar]
- 12.Niwa H, Yamamura K, Miyazaki J. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene. 1991;108:193–199. doi: 10.1016/0378-1119(91)90434-d. [DOI] [PubMed] [Google Scholar]
- 13.Lim SY, Lee DG, Sivakumaran P, et al. In vivo tissue engineering chamber supports human induced pluripotent stem cell survival and rapid differentiation. Biochem Biophys Res Commun. 2012;422:75–79. doi: 10.1016/j.bbrc.2012.04.108. [DOI] [PubMed] [Google Scholar]
- 14.Lim SY, Sivakumaran P, Crombie DE, et al. Trichostatin A enhances differentiation of human induced pluripotent stem cells to cardiogenic cells for cardiac tissue engineering. Stem Cells Translational Medicine. 2013;2:715–725. doi: 10.5966/sctm.2012-0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wong RC, Pollan S, Fong H, et al. A novel role for an RNA polymerase III subunit POLR3G in regulating pluripotency in human embryonic stem cells. Stem Cells. 2011;29:1517–1527. doi: 10.1002/stem.714. [DOI] [PubMed] [Google Scholar]
- 16.Chou BK, Mali P, Huang X, et al. Efficient human iPS cell derivation by a non-integrating plasmid from blood cells with unique epigenetic and gene expression signatures. Cell Res. 2011;21:518–529. doi: 10.1038/cr.2011.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chan EM, Ratanasirintrawoot S, Park IH, et al. Live cell imaging distinguishes bona fide human iPS cells from partially reprogrammed cells. Nat Biotechnol. 2009;27:1033–1037. doi: 10.1038/nbt.1580. [DOI] [PubMed] [Google Scholar]
- 18.Chen G, Gulbranson DR, Hou Z, et al. Chemically defined conditions for human iPSC derivation and culture. Nat Methods. 2011;8:424–429. doi: 10.1038/nmeth.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.