High-resolution imaging techniques were used to analyze the relationship between a Wnt-responsive cancer stem cell (CSC)-enriched population and the tumor vasculature using p53-null mouse mammary tumors transduced with a Wnt signaling pathway reporter. The results demonstrate that the combined strategy of monitoring the fluorescently labeled CSCs and vasculature using high-resolution imaging techniques provides a unique opportunity to study the CSC microenvironment.
Keywords: Cancer stem cells, Stem cell microenvironment, In vivo optical imaging, Microvasculature, Signal transduction, p53
Abstract
Cancer stem cells (CSCs, or tumor-initiating cells) may be responsible for tumor formation in many types of cancer, including breast cancer. Using high-resolution imaging techniques, we analyzed the relationship between a Wnt-responsive, CSC-enriched population and the tumor vasculature using p53-null mouse mammary tumors transduced with a lentiviral Wnt signaling reporter. Consistent with their localization in the normal mammary gland, Wnt-responsive cells in tumors were enriched in the basal/myoepithelial population and generally located in close proximity to blood vessels. The Wnt-responsive CSCs did not colocalize with the hypoxia-inducible factor 1α-positive cells in these p53-null basal-like tumors. Average vessel diameter and vessel tortuosity were increased in p53-null mouse tumors, as well as in a human tumor xenograft as compared with the normal mammary gland. The combined strategy of monitoring the fluorescently labeled CSCs and vasculature using high-resolution imaging techniques provides a unique opportunity to study the CSC and its surrounding vasculature.
Introduction
Cancer stem cells (CSCs, or tumor-initiating cells) are defined as a subpopulation of tumor cells that have the potential for self-renewal and differentiation into all cell types within a given tumor, as well as the ability to regenerate a tumor upon transplantation. Currently, enriched CSC populations are isolated by fluorescence-activated cell sorting (FACS) based on expression of specific cell surface markers or enzymatic activity of aldehyde dehydrogenase followed by in vitro sphere and colony formation assays or, ideally, in vivo limiting dilution cell transplantation analysis in many solid tumors [1–3]. The prevailing idea is that CSCs may exhibit an intrinsic resistance to chemo- and radiation therapies [4, 5], therefore contributing to disease recurrence and metastatic dissemination.
The identification of CSCs in different types of tumors has led to the functional characterization of signaling pathways that regulate these cells, including the Wnt signaling pathway. The canonical Wnt/β-catenin signaling pathway is well known for its roles in the regulation of stem cell self-renewal, and its activation is involved in various cancers [6, 7]. Using a subset of p53-null basal-like tumors transduced with TOP-enhanced green fluorescent protein (eGFP) Wnt reporter lentivirus [8], our previous studies have demonstrated that cells with active Wnt signaling overlapped significantly with the CSC subpopulation identified using cell surface markers [9]. Consistent with this observation, limiting dilution transplantation assays confirmed that the Wnt-responsive cells were enriched for the CSC subpopulation. These CSCs demonstrated more efficient DNA damage repair following in vivo ionizing radiation than the bulk of the tumor cells, in part because of activation of canonical Wnt/β-catenin signaling specifically within the CSC subpopulation. In human breast cancer, aberrant activation of the Wnt ligand has also been described [10]. In addition, Wnt activation is often enriched in basal-like breast cancer through tissue microarray analysis on 56 in situ and 134 invasive breast cancer samples and is correlated with poor outcome [11], making Wnt signaling a potential pharmacological target for this aggressive breast cancer subtype [11, 12]. Recently, using a panel of cancer cell lines and patient samples, Lamb et al. [13] reported that canonical Wnt pathway-related genes were increased in tumor cells with stem-like activity. Therapeutic studies targeting the Wnt pathway in cancer are in the early stages of development [14, 15].
Normal adult mammalian stem cells in many tissues reside in specific niche microenvironments to retain their unique properties of self-renewal and the capacity for differentiation, while remaining in an undifferentiated state [16, 17]. Like their normal counterparts, CSCs also appear to depend on an analogous microenvironment, the CSC niche, to retain CSC characteristics [18, 19]. For example, Wnt signaling has been shown to define a CSC population in close proximity to the stromal microenvironment in colon cancer [20].
Among various microenvironmental factors, a vascular niche plays a critical role in the maintenance of skin CSC stemness and the regulation of skin tumor initiation [21]. A reciprocal relationship between the CSCs and the perivascular niche has also been reported in brain tumors [18]. Finally, indirect evidence has suggested that a subgroup of hematopoietic stem cells might be in close apposition with the blood vessels facilitating the release of stem cells and their derivatives into circulation [22]. Although these studies indicated a close relationship between the CSCs and blood vessels, other studies have reported that the CSCs may reside in hypoxic regions within tumors. Hypoxia-inducible factor 1α (HIF1α) plays a critical role in mediating the hypoxic response of tumor cells and has been reported to regulate both hypoxic embryonic neural stem cells and isolated neural stem cells through the Wnt/β-catenin signaling pathway [23]. HIF1α has also been shown to stimulate primary tumor growth and CSC activity in breast cancer using a MMTV-PyMT mouse model harboring conditional alleles of HIF1α [24]; however, the relative localization of the CSCs, the vasculature, and the hypoxic region has not been characterized in these studies.
Fluorescence microscopy-based image analysis has been used to characterize the association of the neural stem cells with the blood vessels in the neurogenic niche in adult mouse (the subependymal zone) [25, 26]. Measurements based on fluorescence imaging have also been used to estimate the population of neural stem cells in the subependymal zone, as well as to calculate the proximity of stem cells to vessels and the ventricle [27]. Studying the localization of the CSCs and their relationship with blood vessels should allow a better understanding of the role of the CSCs in tumor progression, recurrence, and metastasis.
In this study, we have applied high-resolution imaging and image analysis techniques to localize the tumorigenic CSCs expressing the canonical Wnt reporter in several p53-null basal-like mammary tumor models, with specific emphasis on their spatial relationship to the tumor vasculature. We have also investigated the relative localization of Wnt-responsive cells and the HIF1α-positive cells. Finally, we have characterized the vessel morphology using a triple-negative human xenograft tumor model and show that the vasculature in this human xenograft model is qualitatively similar to that of transplantable mouse tumor models. The strategy of monitoring the fluorescently labeled CSCs using high-resolution imaging techniques provides a unique opportunity to study the CSC microenvironment, an important area that has not been explored adequately in solid cancers.
Materials and Methods
Ethics Statement
All animal protocols were reviewed and approved by the Baylor College of Medicine and University of Pittsburgh Institutional Animal Care and Use Committees.
Materials
The Wnt reporter lentivirus, TOP-eGFP, and its control vector, FOP-eGFP, were kind gifts from Dr. Irving Weissman (Stanford University). All antibodies were purchased from commercial sources as listed in the supplemental online data.
Preparation of Single Mammary Tumor Cells
p53-null mammary tumors were generated through transplantation of the p53-null mammary epithelium cells into the epithelium-free “cleared” mammary fat-pad of syngeneic recipient mice whose endogenous epithelium was surgically removed, as described previously [28, 29]. A detailed description for preparation of single mammary tumor cells is available in the supplemental online data.
Lentiviral Transduction
Lentiviral transduction was performed as described previously [9] and in the supplemental online data.
Transplantation Into the Cleared Mammary Fat Pad and Tissue Harvest
Clearance of endogenous mammary epithelial cells and tumor transplantation procedures were performed as described previously [30] and in the supplemental online data.
Tail Vein Injection of Vascular Imaging Agents and Sample Preparation for Imaging
A total amount of 10 μg of DyLight 594-labeled Lycopersicon esculentum (tomato) lectin in a volume of 100 μl per 1 g of mouse (or 100 μl of a 25 mg/ml dextran phosphate-buffered saline solution) was carefully injected through the tail vein using a 27-gauge needle with a syringe. Imaging samples were prepared essentially as described previously [31]. Detailed information is available in the supplemental online data.
Immunohistochemistry
Paraffin-embedded sections were stained with the antibodies against green fluorescent protein (GFP), von Willebrand factor (vWF), keratin K5, keratin K8, and HIF1α as described in the supplemental online data.
Fluorescence Imaging
A Zeiss LSM 510 META confocal microscope with 405-, 488-, and 543-nm laser lines was used for imaging nuclei (4′,6-diamidino-2-phenylindole [DAPI]), CSCs (eGFP), and vessels (DyLight 594-labeled L. esculentum lectin). The z-stacks were acquired using the Plan-Apochromat ×20/0.75 NA lens (Zeiss, Jena, Germany, http://www.zeiss.com) and the C-Apochromat ×40/1.2 NA lens (Zeiss). Each image in a z-stack consisted of 512 × 512 pixels with a lateral pixel size of 0.44 μm, and the axial separation between images was approximately 2.00 μm. We also acquired thicker z-stacks using a Zeiss LSM 7 MP two-photon microscope equipped with a titanium-sapphire laser. The 830-nm (DAPI and tomato lectin) and 960-nm laser (eGFP) wavelengths were used to collect three-color two-photon z-stacks. The vessels in tumors were also stained with Texas Red dextran. The 870-nm laser wavelength was used to excite dextran in tumor tissues. The z-stacks were acquired using the W Plan-Apochromat ×20/1.0 NA lens.
Image Analysis
Blood vessel diameters, tortuosities in normal mammary tissues and in tumors, and the proximities of the CSCs with respect to the lectin-labeled vessels were measured from the z-stacks using Imaris (Bitplane AG, Zurich, Switzerland, http://www.bitplane.com). The thickness of the z-stacks used to make the proximity measurements was approximately 40 μm. The population of CSCs that respond to Wnt signaling was measured by taking the ratio of the number of TOP-eGFP nuclei to the number of DAPI stained nuclei from microscope images using the image analysis software FARSIGHT (Badrinath Roysam Laboratory, University of Houston, http://farsight-toolkit.org/wiki/Main_Page) [32–35]. The chi-square tests were done in Microsoft Excel (Microsoft, Redmond, WA, http://www.microsoft.com). All other statistical tests were performed using the MATLAB statistics toolbox (version 7.6, R 2011b; MathWorks, Natick, MA, http://www.mathworks.com). A detailed description is available in the supplemental online data.
Results
Characterization of Wnt-Responsive TOP-eGFP-Positive Cells in p53-Null Mouse Mammary Tumors
The p53-null tumor T1 is a well-differentiated squamous adenocarcinoma that has been characterized previously by gene profiling [36]. FACS analysis has demonstrated that cells expressing TOP-eGFP overlapped with the CSC subpopulation characterized using the cell surface markers CD29 and CD24 [9]. In these previous studies, limiting dilution transplantation assays showed that cells with active Wnt signaling are significantly more tumorigenic than cells not showing Wnt signaling response. Given these results, we were interested in determining whether cells with active Wnt signaling also had a greater potential to generate mammospheres than TOP-eGFP-negative cells. As summarized in Figure 1A (left), TOP-eGFP-positive cells formed significantly more secondary mammospheres as compared with the TOP-eGFP-negative cells.
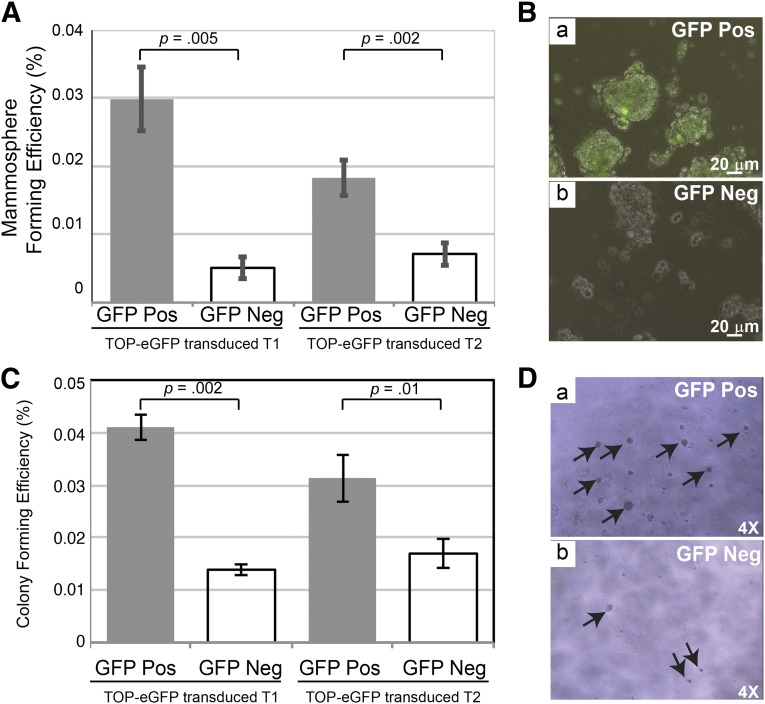
Figure 1.
Mammosphere and Matrigel assays of sorted subpopulations of TOP-eGFP lentivirus-transduced p53-null mammary tumors. (A): Sorted eGFP-positive and -negative cells were plated and grown for 7 days before they were trypsinized and replated at 5,000 cells per well into six-well ultralow attachment plates. Secondary mammospheres were counted on days 7 and 8 from each subpopulation. There are three biological and three technical replicates from each tumor. Left: T1 tumor, p = .005. Right: T2 tumor, p = .002. (B): Pictures were taken on day 8 after plating secondary mammospheres from the TOP-eGFP-positive and -negative cells from TOP-eGFP transduced T2 tumor. Scale bars = 20 μm. (C): Sorted eGFP-positive and -negative cells were plated and grown on growth factor-reduced Matrigel for 6 days at 2,500 cells per well in 96-well plates. There were three biological and three technical replicates from each tumor. Left: T1 tumor, p = .002. Right: T2 tumor, p = .01. (D): Pictures were taken on day 4 after plating the TOP-eGFP-positive and -negative cells from TOP-eGFP transduced T2 tumor. The p values were obtained by paired sample t test. Abbreviations: eGFP, enhanced green fluorescent protein; GFP, green fluorescent protein; Neg, negative; Pos, positive.
In order to determine whether Wnt-responsive cells from phenotypically heterogeneous p53-null tumors also had a greater potential to form mammospheres, cells were isolated from an independently derived p53-null tumor designated T2, which is a well-differentiated, typical papillary adenocarcinoma. As with the T1 model, analysis of the sphere-forming efficiency indicated that the eGFP-positive cells in T2 tumors have a higher potential than eGFP-negative cells or mock sorted total cells (data not shown) to grow under anchorage-independent conditions that have been suggested to enrich for cells with stemness properties (Fig. 1A, right; 1B). eGFP-positive cells from T1 (Fig. 1C, left) and T2 tumors (Fig. 1C, right; 1D) were also plated in the growth factor-reduced Matrigel (BD Biosciences, San Diego, CA, http://www.bdbiosciences.com) to test their efficient growth potential. eGFP-positive cells isolated from both tumors formed a greater number of colonies as compared with the eGFP-negative cells.
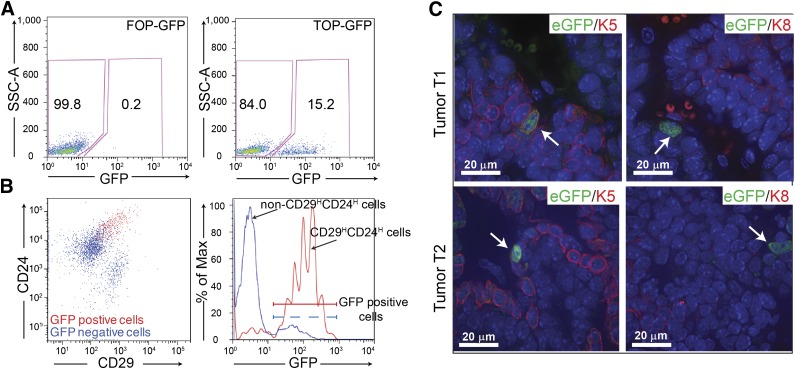
To determine whether cells with active Wnt signaling were also the tumorigenic cells previously identified using cell surface markers CD29 and CD24, enzymatically dissociated T2 tumor cells were transduced in suspension with the Wnt reporter TOP-eGFP lentivirus, and the cells were transplanted into the cleared mammary fat pad of 3-week-old recipient mice. The frequency of the TOP-eGFP-positive cells in the T2 tumor detected by FACS ranged between 11% and 16% (Fig. 2A). FACS analysis demonstrated that a majority of the eGFP-positive cells express CD29 and CD24 cell surface markers used previously to identify the CSC subpopulation in this model, whereas 90% of the CD29HCD24H cells were TOP-eGFP-positive. Approximately 11% of the non-CSC cells (non-CD29H CD24H) from the T2 tumor were also eGFP-positive, indicating a partial overlap of the eGFP-positive Wnt-responsive cells with the CD29HCD24H tumorigenic subpopulation (Fig. 2B). Limiting dilution transplantation analysis showed that the CD29HCD24H Wnt active (eGFP-positive) cells displayed increased tumor initiating ability as compared with the non-CD29HCD24H Wnt active cells (supplemental online Table 1; supplemental online Fig. 1), suggesting the existence of phenotypic heterogeneity within the Wnt active population. Further characterization of the TOP-eGFP Wnt-responsive cells indicated that some of these cells express the basal cell marker keratin K5. No Wnt-responsive cells expressing the luminal cell marker keratin K8 were identified (Fig. 2C, bottom). Similar results were observed for T1 tumor transduced with the TOP-eGFP lentivirus. The Wnt-responsive cells from T1 tumor expressed the basal cell marker keratin K5, whereas no Wnt-responsive cells were found expressing the luminal cell marker keratin K8, suggesting that the canonical Wnt signaling pathway [37] is active primarily in cells with basal/myoepithelial features (Fig. 2C, top). The tumor-forming capacity of eGFP+ and eGFP− within the CD29HCD24H population was also analyzed by mammosphere and colony-forming assays (supplemental online Fig. 2). Within CD29HCD24H cells, eGFP+ has a higher mammosphere and colony-forming efficiency than eGFP−. eGFP+ also formed larger colonies as compared with the eGFP− cells (supplemental online Fig. S2C, S2D), indicating the heterogeneity within the CD29HCD24H tumorigenic population.
Figure 2.
Characterization of the Wnt-responsive cells in the p53-null mammary tumors. (A): Of tumor cells, 11%–16% show GFP expression following transduction with lentiviral TOP-eGFP as compared with little to no expression in the control lentiviral FOP-eGFP-transduced T2 tumor. (B): Fluorescence-activated cell sorting analysis showed that the majority (60%–80%) of eGFP-positive cells were CD29HCD24H (left) and most CD29HCD24Hcells were also eGFP-positive (right) in T2 tumor. (C): Anti-K5 (left) and anti-K8 (right) staining of the TOP-eGFP transduced tumors, T1 and T2. Scale bars = 20 μm. Abbreviations: eGFP, enhanced green fluorescent protein; GFP, green fluorescent protein.
Vessel Morphology
Inspection of the tumor images suggested that vascular morphology in the T1 tumor was markedly different than that of the normal mammary gland. To further investigate this observation, we characterized vascular morphology in normal and tumor tissues by comparing diameter, vessel segment length, and tortuosity [38–42]. Previous studies using transplantable R3230 AC mammary adenocarcinomas have shown that tumors have unique vessel morphologies relative to vessels in granulating subcutaneous tissue that can be identified by measuring properties such as diameter, vessel segment length, and so forth [38, 40]. In the following paragraphs, we present our imaging results and the vessel measurements obtained from mouse normal mammary glands and tumors. To evaluate whether what we observed in mouse was also true for human disease, vessel morphology of a human xenograft tumor was also characterized.
Blood Vessels Differ Significantly Between Normal Mammary Glands and Tumors by Confocal and Two-Photon Imaging
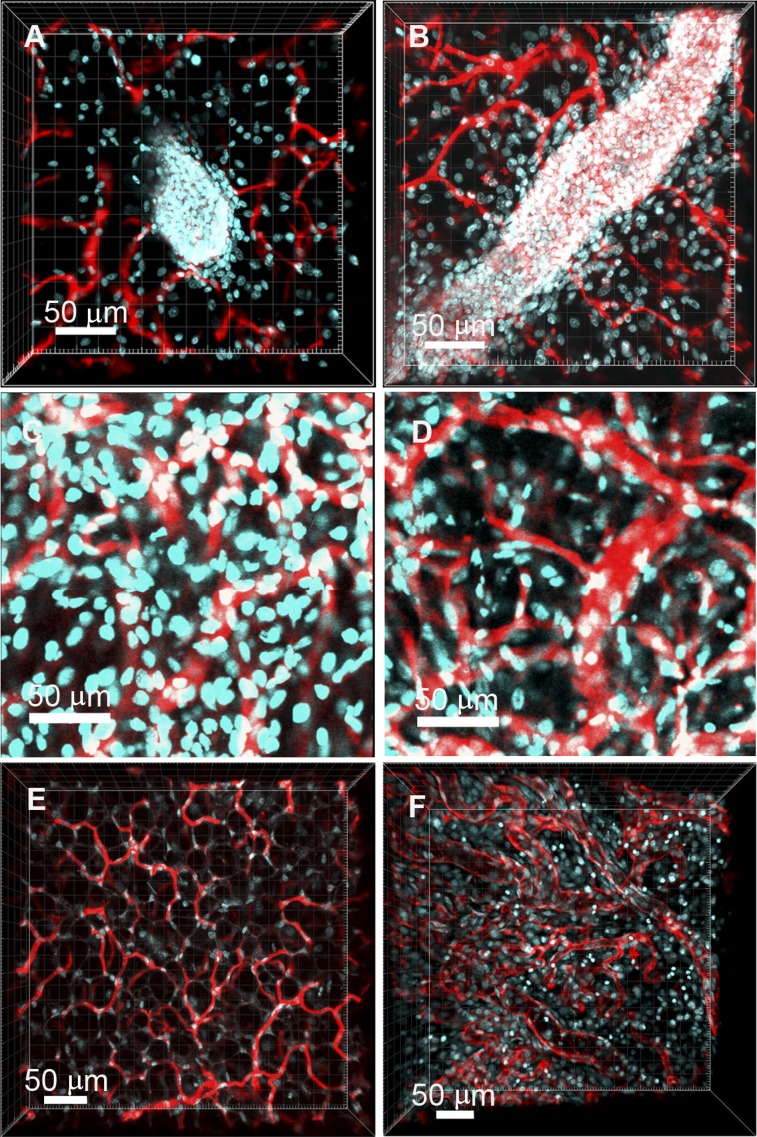
Figure 3A and 3B shows the blood vessels (red) associated with terminal end buds and mature ducts, respectively, in a Balb/c normal mammary gland. Figure 3C and 3D shows images of vessels at different regions in a p53-null T1 tumor. Tiled z-stack images showing nuclear and vascular arrangements in entire sections of the tumor are presented in supplemental online Fig. 3A–3C. Evidence has shown the role of Wnt signaling in basal-like breast cancers; thus we also characterized the vessel morphology in a xenograft BCM-3204 (from a basal-like triple-negative breast cancer patient) [43] growing in a Severe Combined Immunodeficiency/Beige (SCID/Beige) mouse. Three-dimensional (3D) vasculature of both normal Balb/c mammary and p53-null tumor tissues was segmented and reconstructed (supplemental online Figs. 4, 5), again demonstrating significant differences between the normal and tumor tissues. The lectin-labeled vessels from age-matched female normal mammary tissues of SCID/Beige mice were used to measure diameters and tortuosities of vessel segments. Figure 3E and 3F shows z-stacks of vessels in the fat pad of a SCID/Beige mouse and vessels in a xenograft tumor, respectively.
Figure 3.
Vascular patterns in normal mammary tissue and in tumors. (A, B): z-Stack images of cells and vessels in normal mammary tissues acquired using two-photon microscopy are shown. Nuclei are labeled with 4′,6-diamidino-2-phenylindole (DAPI, cyan), and vessels are labeled with lectin (red). A mammary bud (A) and duct (B) can be clearly seen in the microscope images. DAPI and tomato lectin were simultaneously excited with an 830-nm laser, and their emission signals were collected using two separate band-pass filters. (C, D): Images from confocal z-stacks taken from serial sections of a T1 tumor. The nuclei are labeled with DAPI (cyan), and the vessels are labeled with tomato lectin (red). (E, F): The two-photon z-stacks show the vascular morphologies in normal mammary tissues ([E], SCID/Beige mice) and in a xenograft BCM-3204 (from a triple-negative breast cancer patient) of SCID/Beige mouse (F). The vessels are labeled with tomato lectin (red), and the nuclei are labeled with DAPI (cyan). DAPI and lectin were simultaneously excited using an 830-nm laser, and the emission signals were collected using two separate band-pass filters. Tumor vessel segments (F) have a broad range of diameters and tortuous vessel segments of different diameters and lengths.
Tumors Demonstrate a Wider Distribution of Vessel Diameter as Compared With Normal Tissues
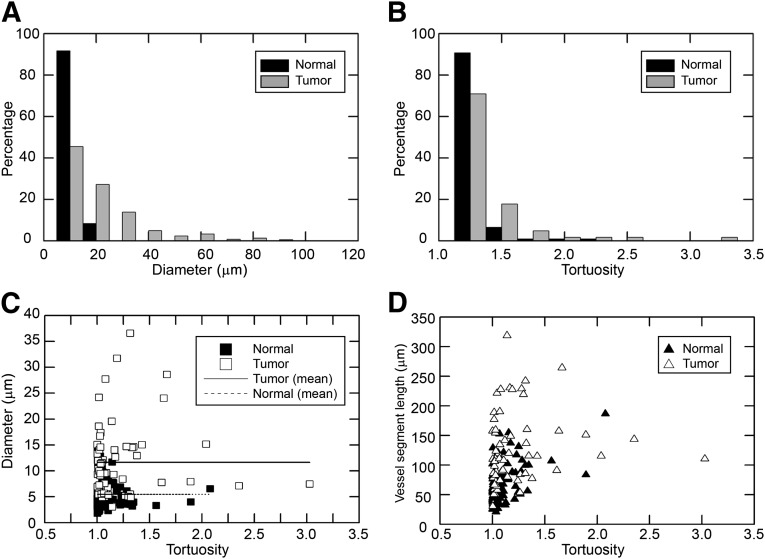
The statistical distributions of diameters of vessel segments associated with buds and ducts in normal mammary glands and p53-null T1 tumors are presented in Figure 4A. Interestingly, the distribution of the diameters of vessel segments in tumors is much broader than the more narrow distribution of diameters of the vessel segments observed in normal mammary tissues. The statistical distribution of vessel diameters in tumors was calculated from 729 measurements taken randomly from vessel segments in three tumors, whereas the distribution of diameters in the normal mammary tissues was calculated from 108 measurements taken randomly from vessel segments associated with buds and ducts in eight mammary glands (n = 3 animals). The two distributions were found to be significantly different (p < .0001, chi-square test). The median diameter of vessel segments in tumors was 11.22 μm, whereas that of the vessel segments associated with buds and ducts in the normal mammary gland was 5.45 μm.
Figure 4.
Morphological characterization of vessel segments in normal mammary tissues and in p53-null tumors. (A): Distributions of vessel diameters in normal mammary glands and T1 tumors. The distribution of vessel diameters in tumors is broad as opposed to the distribution of diameters in normal mammary tissues. The two distributions are significantly different (p < .0001, chi-square test). (B): Distributions of tortuosities of vessel segments in normal mammary tissues and in T1 tumors. The two distributions are not significantly different at α = 0.05 (p = .07, chi-square test). (C): Scatter plot of diameters of vessel segments as a function of their tortuosities. (D): Plot of arc lengths of vessel segments as function of their tortuosities.
The median value of vessel diameters in the xenograft tumor was 11.24 μm (n = 134 random measurements from one tumor), whereas the median diameter of vessel segments in the fat pads of normal mammary tissues in SCID/Beige mice was 5.13 μm (n = 300 random measurements from three mammary tissues). The median values were significantly different (p < .0001, Wilcoxon rank-sum test). In addition, the distributions of vessel diameters in normal mammary tissues and in xenograft tumors (supplemental online Fig. 6) were significantly different (p < .0001, chi-square test). Comparisons of the vessel diameters between T1 and xenograft tumors showed no significant difference, with n = 729 (T1), median = 11.22 μm; n = 134 (xenograft), median = 11.24 μm; p = .19 (Wilcoxon rank-sum test).
To ensure that vessel structure in host mice was not different, we also made a comparison of vessel structure in normal SCID/Beige mice versus the Balb/c mice. The median diameters of vessels in normal Balb/c and SCID/Beige mice are 5.28 μm (n = 581) and 5.13 μm (n = 300), respectively. The diameters are not significantly different (p = .36, Wilcoxon rank-sum test). The median values of tortuosities in normal Balb/c and SCID/Beige mice are 1.13 (n = 35) and 1.15 (n = 18), respectively. The tortuosities are not significantly different (p = .53, Wilcoxon rank-sum test).
Blood Vessel Tortuosity Differs in Tumors From That in Normal Tissues
The statistical distributions of tortuosities of vessel segments in normal mammary tissues and T1 tumors are shown in Figure 4B. The data from normal mammary tissues consist of 108 random measurements made from two-photon z-stacks taken from eight glands (n = 3 animals). The data from tumors are comprised of a total of 62 random measurements made from confocal and two-photon z-stacks taken from four T1 tumors. The two distributions are not significantly different at α = 0.05 (p = .07, chi-square test). The scatter plots of diameters and arc lengths (vessel segment lengths) of vessel segments, respectively, versus their tortuosities are presented in Figure 4C and 4D. The data indicate that vessel segments in tumors are tortuous over a broad range of arc lengths and diameters.
We found similar patterns of variation in the vessel morphology of tumors as compared with their normal counterparts, respectively, from both mouse and xenograft. Although no significant difference was observed in vessel diameters between mouse tumors and xenograft tumors, vessel tortuosities were significantly different between T1 and xenograft tumors, with n = 62 (T1), median = 1.08; n = 23 (xenograft), median = 1.32; p < .0001 (Wilcoxon rank-sum test). The more tortuous vessels in xenograft tumors offer even greater impedance to blood flow than vessels of comparable diameters found in T1 tumors. The vessel segments in the xenograft tumor are also tortuous over a broad range of vessel diameters and vessel segment lengths (data not shown), suggesting that there may exist a more complex vessel morphology in xenograft tumors that may confer more resistance during drug treatment.
TOP-eGFP-Expressing Population and Proximity to Vessels
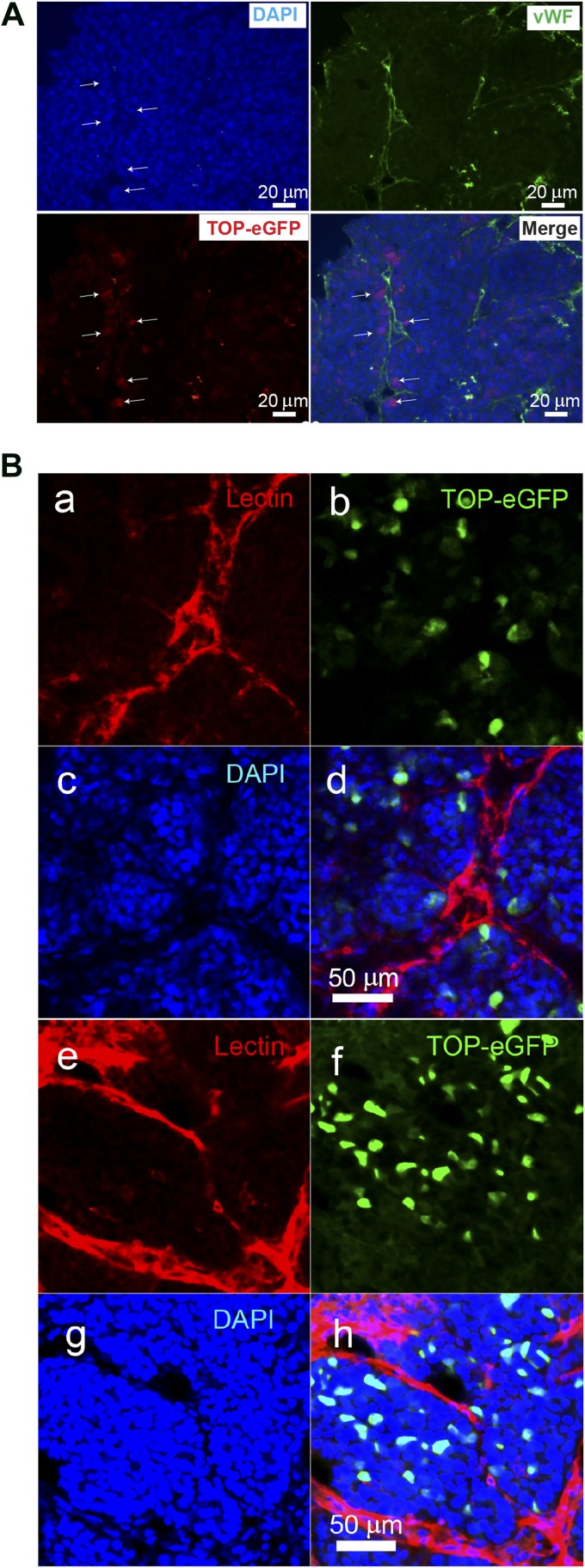
To characterize Wnt-responsive cells and to localize these cells within their microenvironment more precisely, codistribution of vWF, a glycoprotein that lines the inside surface of blood vessels, and the eGFP-positive Wnt-responsive cells was investigated. Our results suggested that at least some of the TOP-eGFP-positive cells were located within 1–2 cell diameters from blood vessels (Fig. 5A).
Figure 5.
Localization of Wnt-responsive cells with respect to vessels in p53-null tumors. (A): Costaining of the vWF and anti-green fluorescent protein on the p53-null T1 tumor. Scale bars = 20 μm. (Ba–Bd): Confocal images of a region in a T1 tumor. The vessels are labeled with tomato lectin (Ba), the Wnt reporter TOP-eGFP labels the cancer stem cells (Bb), the nuclei are labeled with DAPI (Bc), and merge of the vessels, Wnt reporter TOP-eGFP labeled cancer stem cells and nuclei (Bd). (Be–Bh): Confocal images of a region in a T2 tumor. The vessels, CSCs, and nuclei are labeled with lectin (Be), TOP-eGFP (Bf), DAPI (Bg), and merge of lectin, TOP-eGFP and DAPI (Bh). respectively. Abbreviations: DAPI, 4′,6-diamidino-2-phenylindole; eGFP, enhanced green fluorescent protein; vWF, von Willebrand factor.
To localize Wnt-responsive population in situ and to determine whether Wnt-responsive cells show close proximity to blood vessels, confocal and two-photon microscopic images were used. From the analysis of 174 GFP-positive nuclei found within a total population of 5,084 DAPI stained nuclei, we found that the mean population of TOP-eGFP nuclei in the T1 tumors was 3.53% ± 2.27% (three tumors, 13 confocal images), which correlated with the FACS-determined value of 6.37% ± 2.44%. Further analysis showed that the majority of TOP-eGFP cells were found within 10 μm or less of a blood vessel. The mean distance of the GFP-positive nuclei from the closest lectin-labeled vessel (Fig. 5Ba–5Bd) was 5.39 ± 2.06 μm (three tumors, 22 measurements).
In T2 tumors the mean population of GFP-positive nuclei was 5.17% ± 1.18% (two tumors, four confocal images). A total of 96 GFP-positive nuclei from a pool of 1,857 DAPI-stained nuclei were used in the calculations. The mean distance of the GFP-positive nuclei from the closest lectin-labeled vessel (Figs. 5Be–5Bh) was 5.40 ± 3.68 μm (four tumors, 18 measurements). These data indicate that, in both T1 and T2 tumors, there is a subpopulation of Wnt-responsive cells that resides in close proximity to blood vessels (supplemental online Videos 1, 2). A 3D animation of Wnt-responsive cells and vessels showing their localization relative to blood vessels in a T1 tumor is shown in supplemental online Fig. 7 and supplemental online Video 3.
Finally, the proximity of Wnt-responsive cells to the corresponding vessels of different diameters was measured. The vessels are grouped into two sets. In the first group, the mean diameter of vessels is 11.26 ± 3.03 μm (n = 7), whereas the mean diameter of vessels in the second group is 23.21 ± 4.03 μm (n = 5). The diameters of the vessels are significantly different (p = .0025, Wilcoxon rank-sum test). The mean proximities of cells to vessels are 4.92 ± 1.94 and 6.65 ± 3.55 μm, respectively. The proximities of cells to vessels are not significantly different between the groups (p = .34, Wilcoxon rank-sum test).
TOP-eGFP-Expressing Population Is Not Located Within Hypoxic Regions
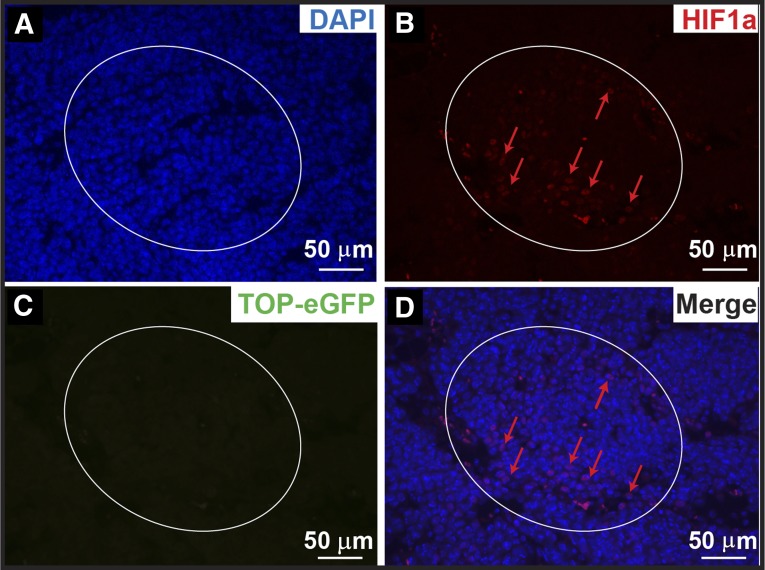
Hypoxic regions exist throughout solid tumors because of aberrant vessel morphologies and altered blood flow. Targeting HIF1α in a mouse lymphoma and serially transplantable human acute myeloid leukemia xenograft model reduced CSC malignancies [44]; however, the relative localizations of the CSCs and hypoxia regions are in general not well characterized. Given the close proximity of Wnt-responsive cells to blood vessels, it seemed unlikely that these cells would lie in a hypoxic microenvironment, as has been hypothesized. To evaluate this likelihood, we analyzed HIF1α expression by coimmunofluorescence microscopy. Consistent with their close proximity to blood vessels, coimmunofluorescence staining did not show any colocalization of the HIF1α-positive cells and the Wnt-responsive cells in these tumor models, although occasionally Wnt-responsive cells were found neighboring hypoxic regions (Fig. 6; supplemental online Fig. 8). Ten HIF1α-positive areas (∼300–400 cells per area, ∼50%–80% cells positive for HIF1α staining) from ×20 magnification images were counted. Less than 1% of the cells were positive for Wnt activity, and those that were positive for HIF1α exhibited very low expression of GFP, i.e., low Wnt pathway reporter activity. In other words, GFPHigh Wnt-responsive cells and HIF1α-positive cells appeared to be mutually exclusive. Thus, the GFPHigh cells are not within the area of hypoxic regions in the tumors analyzed.
Figure 6.
Wnt-responsive cells are not colocalized with the HIF1α-positive cells in the p53-null mammary tumors. (A): DAPI staining. (B): HIF1α-positive regions (circled) and representative HIF1α-positive cells (red arrows). (C): eGFP-positive Wnt-responsive cells (none was observed within this hypoxic region). (D): Merged images. Scale bars = 50 μm. Abbreviations: DAPI, 4′,6-diamidino-2-phenylindole; eGFP, enhanced green fluorescent protein; HIF, hypoxia-inducible factor.
In order to test the ability of FACS sorted Wnt-responsive cells to express HIF1α in response to hypoxia, Wnt-responsive cells grown in suspension or on plastic were immediately fixed and immunostained with the anti-HIF1α antibody. More than 90% of the cells expressed HIF1α 48 hours after induction of hypoxia (supplemental online Fig. 9), indicating that these CSCs were able to express HIF1α in a hypoxic environment.
Discussion
The use of the signaling pathway reporter provides a complementary approach to the use of cell surface markers for the identification of CSCs. Labeling tumor CSCs with the Wnt pathway reporter tagged with GFP allows for visualization and localization in both primary tumor sections and lung metastases (M. Zhang and J.M. Rosen, unpublished data), thus providing a unique opportunity to study the local microenvironment. Tumor vasculature is critical for tumor development and maintenance. Dissecting the relationship between CSCs and vasculature is critical for drug delivery and development in cancer therapy.
Using the p53-null mammary tumor T1 model, eGFP-positive cells derived by transduction with the TOP-eGFP lentivirus (a canonical Wnt-pathway reporter) showed a 70-fold enrichment in CSC frequency as compared with eGFP-negative cells through limiting dilution transplantation assay [9]. By applying an in vitro surrogate assay for stem cell self-renewal, mammosphere assay, and colony-forming assay, we have shown that the TOP-eGFP-positive cells have a higher self-renewal potential than the negative cells in T1 tumor, consistent with the in vivo functional results. Using an independently derived p53-null mammary T2 tumor, we also showed that the eGFP-positive cells derived from TOP-eGFP lentivirus transduced tumors are enriched in the CSC subpopulation through in vitro mammosphere and colony-forming assays, demonstrating again the role of the Wnt signaling pathway in stem cell self-renewal in these basal-like tumors. Although the TOP-eGFP-positive population is enriched for CSCs, it is still heterogeneous. Therefore, not every eGFP-positive cell is a CSC. In T2 p53-null tumors, there existed a population of non-CD29HCD24H that also displayed Wnt pathway activity (Fig. 2B, non-CD29HCD24H GFP+, dotted line). This non-CSC eGFP-positive population, which was significantly less tumorigenic than the population of CSC eGFP-positive cells (supplemental online Table 1), might represent the cells in the interior of the tumor located further away from blood vessels, whereas the CSC eGFP-positive population follows the example of the T1 model.
The frequency of the TOP-eGFP-positive cells detected by FACS varied among the different p53-null tumors that represent several different human breast cancer subtypes from 0% to 20%, suggesting that not every tumor subtype has a detectable subpopulation of cells with active Wnt pathway signaling. Different signaling pathways may be active in CSCs of different tumor subtypes, reflecting the roles of these pathways in the cell of origin of various tumors. Thus, the roles of Notch, Hedgehog, and the Jak/Stat signaling pathways are being investigated in tumorigenesis of the heterogeneous p53-null mammary tumors.
Fluorescence microscopy-based image analysis makes possible quantitative measurements in the stem cell microenvironment of p53-null tumors such as diameters and tortuosities of lectin-labeled vessels, the population of Wnt-responsive cells, and the proximities of Wnt-responsive cells to lectin-labeled vessels. These measurements, in addition to enhancing our understanding of the stem cell microenvironment, can also be used to construct multiscale in silico models [45] of p53-null tumors. The broad distribution of diameters in tumors leads to a heterogeneous blood flow pattern such that there will be some regions with elevated blood flow and other regions with reduced blood flow as opposed to a more homogeneous blood flow pattern in normal tissues characterized by a narrow distribution of vessel diameters [46]. Additionally, vessel segments that are tortuous over a broad range of diameters and arc lengths contribute to increased resistance to blood flow along the segments because blood will have to travel greater distances between the endpoints of the segments. The structural resistance to blood flow (supplemental online Fig. 10) resulting from the heterogeneities in blood flow discussed above can lead to inefficient transport of oxygen, nutrients, and drugs by the tumor vasculature [41]. The resistance to blood flow offered by the tumor vasculature is different from the drug resistance caused by cancer stem cells. The p53-null mouse model combined with fluorescence imaging provides an ideal platform to investigate the various sources of drug resistance in tumors.
Conley et al. [47] have demonstrated that breast CSC ALDH1+ cells are enriched within hypoxic regions of tumors after sunitinib treatment as compared with the randomly distributed CSCs in the control tumors. Whether the inhibition of sunitinib to the receptor tyrosine kinases critical for tumor angiogenesis kills the CSCs close to vessels or induces the self-renewal of CSCs in hypoxic regions remains unclear. Our data indicate that in both T1 and T2 tumors there is a subpopulation of Wnt-responsive CSCs that are closely associated with the vessels. It is possible that these cells can participate in intravasation and extravasation leading to tumor metastasis. Cells with high Wnt pathway activity were also observed but not colocalized in close proximity to hypoxic regions characterized by high levels of HIF1α expression. We hypothesize that there may be two or more subpopulations within an already stem cell-enriched population as indicated by our single cell gene expression analyses using the stem cell-enriched claudin-low p53-null tumors (data not published). Gilbertson and Rich [48] have shown that tumor CSCs may reside in both perivascular and the surrounding necrotic areas. An attractive feature of the fluorescence microscopy-based 3D analysis is that it is possible to investigate the association of cells to vessels of different diameters in the tumor microenvironment. In the population of cells considered to investigate the association of cells to vessels of different diameters in the tumor microenvironment, we do not see a difference in the association of cells to small and large vessels. The blood flow rate increases with diameter even in small diameter vessels similar to the ones discussed here [49, 50]. The association of cells to vessels based on blood flow rate through vessels of different diameters is worth exploring. These studies should also be extended to thicker z-stacks and more cells to get the full picture.
Finally, evidence has begun to accumulate demonstrating the importance of Wnt signaling in human diseases [51]. Human patient-derived xenograft breast tumor models are commonly used to investigate the pathophysiology of human breast cancer. We believe our results using genetically engineered mouse models can help construct new multiscale models of p53-null tumors that can then be further applied to human xenograft tumor model systems. Studies are underway to develop methodologies to efficiently introduce Wnt pathway reporters into the patient-derived xenograft models. Because of the complexity of various tumor subtypes, the role of the Wnt signaling pathway in human breast cancer remains to be determined. Canonical Wnt signaling has been suggested to function both as a promoting factor in one subtype of patient-derived breast cancer and as an inhibitory factor in another subtype [15], but many more tumors need to be analyzed before definitive conclusions can be drawn. Even though no differences of the median diameters of vessels and the values of tortuosities were observed in normal Balb/c and SCID/Beige mice, the impact of human tumor cells on the vasculature produced by SCID/Beige mice is not quite comparable to the mouse tumors cells on the vasculature of mouse origin. Therefore, it is imperative to develop a model with ideal human microenvironmental factors to study the interaction between human tumor cells and their microenvironment.
Conclusion
Using a well-characterized p53-null mammary tumor model in which the tumor-initiating cells were labeled with fluorescence reporters along with the high-resolution imaging techniques, we have characterized the relative localization between the CSCs-enriched Wnt-responsive cells and the vascular microenvironment. Most Wnt-responsive cells are located within close proximity to blood vessels and did not colocalize with hypoxic regions as detected by staining for HIF1α. Vessels within the tumor have a significantly different morphology than vessels of the normal mammary gland, with the tumor vessels showing a wider variation in vessel diameter and tortuosity than in the normal gland. This will collectively favor inefficient blood transport [41] and therefore impair drug delivery, potentially contributing to chemoresistance. We have also observed a percentage of the Wnt-responsive cells determined by confocal imaging comparable to that detected using more conventional FACS analysis, suggesting that our measurements have the potential to be used to construct new multiscale models of p53-null tumors, as well as to be compared with other tumor models.
Supplementary Material
Acknowledgments
We thank Dr. Irving Weissman for providing the TOP-eGFP and FOP-eGFP Wnt signaling pathway reporter system, as well as the staff of the Pathology Core Laboratory of the Lester and Sue Smith Breast Center for technical assistance. We also thank the Cytometry and Cell Sorting Core at Baylor College of Medicine (BCM) and the expert assistance of the Core Director, Joel Sederstrom. M.E.D. and T.J.V. were also supported by the Optical Imaging and Vital Microscopy Core at BCM. This work was supported by NIH/NCI Grants U54 CA149196 (S.T.C.W., M.T.L., and J.M.R.), CA142898 (M.Z.), and CA148761 (J.M.R.), BCM Cancer Center Grant P30 CA125123 (M.T.L.), and BCM Breast Cancer SPORE Grant P50 CA50183 (M.T.L.). This project also used the University of Pittsburgh Cancer Institute (UPCI) Cytometry Facility, which is supported in part by Award P30CA047904.
Author Contributions
T.J.V. and M.Z.: conception/design, collection and/or assembly of data, data analysis and interpretation, manuscript writing; J.D.L., W.B., and W.W.: collection and/or assembly of data; F.L. and S.T.C.W.: data analysis and interpretation; M.E.D., J.M.R., and M.T.L.: conception/design, manuscript writing.
Disclosure of Potential Conflicts of Interest
M.E.D. has uncompensated intellectual property rights and an uncompensated consultant/advisory role with Carl Zeiss, Inc. M.T.L. has uncompensated employment with StemMed Ltd. and uncompensated intellectual property rights with Baylor College of Medicine.
References
- 1.Lapidot T, Sirard C, Vormoor J, et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645–648. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- 2.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 3.Al-Hajj M, Wicha MS, Benito-Hernandez A, et al. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li X, Lewis MT, Huang J, et al. Intrinsic resistance of tumorigenic breast cancer cells to chemotherapy. J Natl Cancer Inst. 2008;100:672–679. doi: 10.1093/jnci/djn123. [DOI] [PubMed] [Google Scholar]
- 5.Atkinson RL, Zhang M, Diagaradjane P, et al. Thermal enhancement with optically activated gold nanoshells sensitizes breast cancer stem cells to radiation therapy. Sci Transl Med. 2010;2:55ra79. doi: 10.1126/scitranslmed.3001447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843–850. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- 7.van Amerongen R, Bowman AN, Nusse R. Developmental stage and time dictate the fate of Wnt/β-catenin-responsive stem cells in the mammary gland. Cell Stem Cell. 2012;11:387–400. doi: 10.1016/j.stem.2012.05.023. [DOI] [PubMed] [Google Scholar]
- 8.Reya T, Duncan AW, Ailles L, et al. A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature. 2003;423:409–414. doi: 10.1038/nature01593. [DOI] [PubMed] [Google Scholar]
- 9.Zhang M, Atkinson RL, Rosen JM. Selective targeting of radiation-resistant tumor-initiating cells. Proc Natl Acad Sci USA. 2010;107:3522–3527. doi: 10.1073/pnas.0910179107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ni M, Chen Y, Lim E, et al. Targeting androgen receptor in estrogen receptor-negative breast cancer. Cancer Cell. 2011;20:119–131. doi: 10.1016/j.ccr.2011.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khramtsov AI, Khramtsova GF, Tretiakova M, et al. Wnt/beta-catenin pathway activation is enriched in basal-like breast cancers and predicts poor outcome. Am J Pathol. 2010;176:2911–2920. doi: 10.2353/ajpath.2010.091125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsuda Y, Schlange T, Oakeley EJ, et al. WNT signaling enhances breast cancer cell motility and blockade of the WNT pathway by sFRP1 suppresses MDA-MB-231 xenograft growth. Breast Cancer Res. 2009;11:R32. doi: 10.1186/bcr2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lamb R, Ablett MP, Spence K, et al. Wnt pathway activity in breast cancer sub-types and stem-like cells. PLoS One. 2013;8:e67811. doi: 10.1371/journal.pone.0067811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zimmerman ZF, Moon RT, Chien AJ. Targeting Wnt pathways in disease. Cold Spring Harb Perspect Biol. 2012;4:a008086. doi: 10.1101/cshperspect.a008086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Green JL, La J, Yum KW, et al. Paracrine Wnt signaling both promotes and inhibits human breast tumor growth. Proc Natl Acad Sci USA. 2013;110:6991–6996. doi: 10.1073/pnas.1303671110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blanpain C, Fuchs E. Epidermal homeostasis: A balancing act of stem cells in the skin. Nat Rev Mol Cell Biol. 2009;10:207–217. doi: 10.1038/nrm2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Radtke F, Clevers H. Self-renewal and cancer of the gut: Two sides of a coin. Science. 2005;307:1904–1909. doi: 10.1126/science.1104815. [DOI] [PubMed] [Google Scholar]
- 18.Calabrese C, Poppleton H, Kocak M, et al. A perivascular niche for brain tumor stem cells. Cancer Cell. 2007;11:69–82. doi: 10.1016/j.ccr.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 19.Konopleva MY, Jordan CT. Leukemia stem cells and microenvironment: Biology and therapeutic targeting. J Clin Oncol. 2011;29:591–599. doi: 10.1200/JCO.2010.31.0904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vermeulen L, De Sousa E Melo F, van der Heijden M, et al. Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nat Cell Biol. 2010;12:468–476. doi: 10.1038/ncb2048. [DOI] [PubMed] [Google Scholar]
- 21.Beck B, Driessens G, Goossens S, et al. A vascular niche and a VEGF-Nrp1 loop regulate the initiation and stemness of skin tumours. Nature. 2011;478:399–403. doi: 10.1038/nature10525. [DOI] [PubMed] [Google Scholar]
- 22.Heissig B, Hattori K, Dias S, et al. Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand. Cell. 2002;109:625–637. doi: 10.1016/s0092-8674(02)00754-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mazumdar J, O’Brien WT, Johnson RS, et al. O2 regulates stem cells through Wnt/β-catenin signalling. Nat Cell Biol. 2010;12:1007–1013. doi: 10.1038/ncb2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwab LP, Peacock DL, Majumdar D, et al. Hypoxia-inducible factor 1α promotes primary tumor growth and tumor-initiating cell activity in breast cancer. Breast Cancer Res. 2012;14:R6. doi: 10.1186/bcr3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shen Q, Wang Y, Kokovay E, et al. Adult SVZ stem cells lie in a vascular niche: A quantitative analysis of niche cell-cell interactions. Cell Stem Cell. 2008;3:289–300. doi: 10.1016/j.stem.2008.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tavazoie M, Van der Veken L, Silva-Vargas V, et al. A specialized vascular niche for adult neural stem cells. Cell Stem Cell. 2008;3:279–288. doi: 10.1016/j.stem.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kazanis I, Lathia JD, Vadakkan TJ, et al. Quiescence and activation of stem and precursor cell populations in the subependymal zone of the mammalian brain are associated with distinct cellular and extracellular matrix signals. J Neurosci. 2010;30:9771–9781. doi: 10.1523/JNEUROSCI.0700-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jerry DJ, Kittrell FS, Kuperwasser C, et al. A mammary-specific model demonstrates the role of the p53 tumor suppressor gene in tumor development. Oncogene. 2000;19:1052–1058. doi: 10.1038/sj.onc.1203270. [DOI] [PubMed] [Google Scholar]
- 29.Zhang M, Behbod F, Atkinson RL, et al. Identification of tumor-initiating cells in a p53-null mouse model of breast cancer. Cancer Res. 2008;68:4674–4682. doi: 10.1158/0008-5472.CAN-07-6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deome KB, Faulkin LJ, Jr, Bern HA, et al. Development of mammary tumors from hyperplastic alveolar nodules transplanted into gland-free mammary fat pads of female C3H mice. Cancer Res. 1959;19:515–520. [PubMed] [Google Scholar]
- 31.Landua JD, Visbal AP, Lewis MT. Methods for preparing fluorescent and neutral red-stained whole mounts of mouse mammary glands. J Mammary Gland Biol Neoplasia. 2009;14:411–415. doi: 10.1007/s10911-009-9155-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin G, Adiga U, Olson K, et al. A hybrid 3D watershed algorithm incorporating gradient cues and object models for automatic segmentation of nuclei in confocal image stacks. Cytometry A. 2003;56:23–36. doi: 10.1002/cyto.a.10079. [DOI] [PubMed] [Google Scholar]
- 33.Lin G, Chawla MK, Olson K, et al. Hierarchical, model-based merging of multiple fragments for improved three-dimensional segmentation of nuclei. Cytometry A. 2005;63:20–33. doi: 10.1002/cyto.a.20099. [DOI] [PubMed] [Google Scholar]
- 34.Lin G, Chawla MK, Olson K, et al. A multi-model approach to simultaneous segmentation and classification of heterogeneous populations of cell nuclei in 3D confocal microscope images. Cytometry A. 2007;71:724–736. doi: 10.1002/cyto.a.20430. [DOI] [PubMed] [Google Scholar]
- 35.Al-Kofahi Y, Lassoued W, Lee W, et al. Improved automatic detection and segmentation of cell nuclei in histopathology images. IEEE Trans Biomed Eng. 2010;57:841–852. doi: 10.1109/TBME.2009.2035102. [DOI] [PubMed] [Google Scholar]
- 36.Herschkowitz JI, Zhao W, Zhang M, et al. Comparative oncogenomics identifies breast tumors enriched in functional tumor-initiating cells. Proc Natl Acad Sci USA. 2012;109:2778–2783. doi: 10.1073/pnas.1018862108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zeng YA, Nusse R. Wnt proteins are self-renewal factors for mammary stem cells and promote their long-term expansion in culture. Cell Stem Cell. 2010;6:568–577. doi: 10.1016/j.stem.2010.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Less JR, Skalak TC, Sevick EM, et al. Microvascular architecture in a mammary carcinoma: Branching patterns and vessel dimensions. Cancer Res. 1991;51:265–273. [PubMed] [Google Scholar]
- 39.Hashizume H, Baluk P, Morikawa S, et al. Openings between defective endothelial cells explain tumor vessel leakiness. Am J Pathol. 2000;156:1363–1380. doi: 10.1016/S0002-9440(10)65006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dewhirst MW, Tso CY, Oliver R, et al. Morphologic and hemodynamic comparison of tumor and healing normal tissue microvasculature. Int J Radiat Oncol Biol Phys. 1989;17:91–99. doi: 10.1016/0360-3016(89)90375-1. [DOI] [PubMed] [Google Scholar]
- 41.Baish JW, Gazit Y, Berk DA, et al. Role of tumor vascular architecture in nutrient and drug delivery: An invasion percolation-based network model. Microvasc Res. 1996;51:327–346. doi: 10.1006/mvre.1996.0031. [DOI] [PubMed] [Google Scholar]
- 42.Baish JW, Jain RK. Fractals and cancer. Cancer Res. 2000;60:3683–3688. [PubMed] [Google Scholar]
- 43.Zhang X, Claerhout S, Prat A, et al. A renewable tissue resource of phenotypically stable, biologically and ethnically diverse, patient-derived human breast cancer xenograft models. Cancer Res. 2013;73:4885–4897. doi: 10.1158/0008-5472.CAN-12-4081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Y, Liu Y, Malek SN, et al. Targeting HIF1α eliminates cancer stem cells in hematological malignancies. Cell Stem Cell. 2011;8:399–411. doi: 10.1016/j.stem.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Anderson AR, Quaranta V. Integrative mathematical oncology. Nat Rev Cancer. 2008;8:227–234. doi: 10.1038/nrc2329. [DOI] [PubMed] [Google Scholar]
- 46.McDonald DM, Baluk P. Significance of blood vessel leakiness in cancer. Cancer Res. 2002;62:5381–5385. [PubMed] [Google Scholar]
- 47.Conley SJ, Gheordunescu E, Kakarala P, et al. Antiangiogenic agents increase breast cancer stem cells via the generation of tumor hypoxia. Proc Natl Acad Sci USA. 2012;109:2784–2789. doi: 10.1073/pnas.1018866109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gilbertson RJ, Rich JN. Making a tumour’s bed: Glioblastoma stem cells and the vascular niche. Nat Rev Cancer. 2007;7:733–736. doi: 10.1038/nrc2246. [DOI] [PubMed] [Google Scholar]
- 49.Lipowsky HH, Usami S, Chien S. In vivo measurements of “apparent viscosity” and microvessel hematocrit in the mesentery of the cat. Microvasc Res. 1980;19:297–319. doi: 10.1016/0026-2862(80)90050-3. [DOI] [PubMed] [Google Scholar]
- 50.Lipowsky HH, Zweifach BW. Application of the “two-slit” photometric technique to the measurement of microvascular volumetric flow rates. Microvasc Res. 1978;15:93–101. doi: 10.1016/0026-2862(78)90009-2. [DOI] [PubMed] [Google Scholar]
- 51.Styrkarsdottir U, Thorleifsson G, Sulem P, et al. Nonsense mutation in the LGR4 gene is associated with several human diseases and other traits. Nature. 2013;497:517–520. doi: 10.1038/nature12124. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.