The authors studied the radiosensitization of breast cancer stem-like cells in vitro after treatment with the most commonly used statin, simvastatin, and examined the influence on local control after postmastectomy radiation among inflammatory breast cancer patients taking statins. This work provides new insight on combination regimens for breast cancer treatment and radiosensitization of this clinically radioresistant disease.
Keywords: Statins, Inflammatory breast cancer, Local recurrence, Radiation
Abstract
Reported rates of local failure after adjuvant radiation for women with inflammatory breast cancer (IBC) and triple-negative non-IBC are higher than those of women with receptor-expressing non-IBC. These high rates of locoregional recurrence are potentially influenced by the contribution of radioresistant cancer stem cells to these cancers. Statins have been shown to target stem cells and improve disease-free survival among IBC patients. We examined simvastatin radiosensitization of multiple subtypes of breast cancer cell lines in vitro in monolayer and mammosphere-based clonogenic assays and examined the therapeutic benefit of statin use on local control after postmastectomy radiation (PMRT) among IBC patients. We found that simvastatin radiosensitizes mammosphere-initiating cells (MICs) of IBC cell lines (MDA-IBC3, SUM149, SUM190) and of the metaplastic, non-IBC triple-negative receptor cell line (SUM159). However, simvastatin radioprotects MICs of non-IBC cell lines MCF-7 and SKBR3. In a retrospective clinical study of 519 IBC patients treated with PMRT, 53 patients used a statin. On univariate analysis, actuarial 3-year local recurrence-free survival (LRFS) was higher among statin users, and on multivariate analysis, triple negative breast cancer, absence of lymphatic invasion, neoadjuvant pathological tumor response to preoperative chemotherapy, and statin use were independently associated with higher LRFS. In conclusion, patients with IBC and triple-negative non-IBC breast cancer have the highest rates of local failure, and there are no available known radiosensitizers. We report significant improvement in local control after PMRT among statin users with IBC and significant radiosensitization across triple-negative and IBC cell lines of multiple subtypes using simvastatin. These data suggest that simvastatin should be justified as a radiosensitizing agent by a prospective clinical trial.
Introduction
Inflammatory breast cancer (IBC) is an aggressive variant of breast cancer characterized by rapid progression, clinically apparent involvement of the skin resulting in erythema, and high rate of resistance to therapy [1]. Furthermore, rapid metastasis and resistance to treatment in IBC are strongly associated with the cancer stem cell hypothesis, which posits that a small population of cells with stem-like biology mediates the resistance and spread of disease. Several studies have demonstrated enriched stem cell phenotypes in specimens from IBC patients and IBC cell lines [2–4].
Postmastectomy radiation (PMRT) is a component of care for virtually all women with locally advanced breast cancer. Resistance to radiation resulting in local or regional recurrence (LRR) has clearly and repeatedly been shown to reduce overall survival [5]. Among women with triple-negative breast cancer and triple-negative IBC, the 5-year actuarial rates of local failure after radiation are 11%–35% and 45%, respectively [6, 7]. This contributes directly to the dismal prognosis for these patients.
Recurrence is thought to be initiated by the migration of surviving cancer stem/progenitors cells from the primary site to distant niches where they establish micrometastatic disease, and we speculate that similar migration through the breast itself contributes to the phenotype and treatment resistance of IBC. We and others have shown evidence that breast cancer stem/progenitor cells are resistant to radiation [8–10] and that chemotherapy can increase the percentage of the cell population with CD44highCD24low surface markers, one putative marker of breast cancer stem/progenitor cells [11, 12]. Because of the therapy-resistant character of cancer stem/progenitor cells, significant effort has been made to identify drugs targeting these types of cells. We adapted the three-dimensional (3D) in vitro mammosphere-based self-renewal assay [13] to screen cancer stem/progenitors cells radiosensitizers [14] and demonstrated that drugs that radiosensitize differentiated cells in standard monolayer clonogenic assays can promote the survival and resistance of cancer stem/progenitors cells in 3D assays [15].
3-Hydroxy-3-methylglutaryl coenzyme A (HMG CoA) reductase inhibitors or statins have been associated with breast cancer incidence [16–18] and with reduced cancer-related mortality [19]. Interestingly, lipophilic statins were associated with a 10% reduced risk of breast cancer recurrence in a nationwide, population-based prospective cohort study of Danish women with invasive breast cancer [20]. The ability of statins to inhibit tumor growth, angiogenesis, and metastasis has been attributed to their effects on inhibition of G-proteins Ras and Rho [21], reduction of metalloproteinases [22], decreased synthesis of inflammatory cytokines [23, 24], decreased circulating vascular endothelial growth factor (VEGF) levels and VEGF-induced signaling [25–27], and very recently, inhibition of lymphangiogenesis [28]. More important, statins have been shown to reduce the “stemness” of cancer cells by shifting colorectal cancer cells from a stem-like state to a more differentiated state [29] and, in breast cancer cells, by decreasing the expression of CD44 protein [30] and by inhibiting the protein geranylgeranylation [31].
Taking together the well-known characteristics of IBC and the described effects of statins on biology associated with IBC, we hypothesized that statins can inhibit local recurrence after PMRT in IBC by sensitizing differentiated cancer cells and cancer stem cells to radiation. We studied the radiosensitization of breast cancer stem-like cells in vitro after treatment with the most commonly used statin, simvastatin, and examined the influence on local control after PMRT among IBC patients taking statins. This work provides new insight on combination regimens for breast cancer treatment and radiosensitization of this clinically radioresistant disease.
Materials and Methods
Cell Culture
Six different breast cancer cell lines of multiple subtypes were used in our studies [32]. IBC cell lines SUM149 and SUM190 were obtained from Asterand (Detroit, MI, https://www.asterand.com), and MDA-IBC3 was generated in our laboratory [33]. Non-IBC cell line MCF-7 was obtained from ATCC (Manassas, VA, http://www.atcc.org); SKBR3 was a generous gift from Dr. Jennifer Mourtada of Christiana Care’s Helen F. Graham Cancer Center in Newark, Delaware; and SUM159 was obtained from Asterand. All IBC cell lines and SUM159 were cultured as monolayers in Ham’s F-12 media supplemented with 10% fetal bovine serum (FBS), 1 μg/ml hydrocortisone, 5 mg/ml insulin, and 1% antibiotic-antimycotic. MCF-7 cells were cultured as monolayer in modified Eagle’s medium (MEM) supplemented with 10% FBS, 0.1 mM nonessential amino acids, 1 mM sodium pyruvate, 5 μg/ml insulin, 1 μg/ml hydrocortisone, and 1% antibiotic-antimycotic. SKBR3 cells were cultured as monolayer in Dulbecco’s MEM supplemented with 10% FBS and 1% penicillin-streptomycin. All cell lines were also propagated in serum-free MEM supplemented with 20 ng/ml basic fibroblast growth factor, 20 ng/ml epidermal growth factor, and B27 in ultra-low attachment plates to enrich for cancer stem/progenitor cell populations [13, 34, 35]. These conditions allow mammosphere formation in a liquid medium (3D culture) of all cell lines used in our studies.
Radiosensitivity Studies
Radiosensitivity of all cell lines was evaluated in both types of culture, monolayer (two-dimensional [2D]) and mammosphere (3D), as described previously [15]. Briefly, cells were trypsinized, counted, and seeded into six-well plates (ultra-low attachment for 3D cultures) with or without simvastatin. Following a short recovery incubation period of 4 hours at 37°C with 5% CO2, cells were exposed to increasing doses of γ-radiation (2 Gy, 4 Gy, and 6 Gy) using a Shepherd Irradiator (J.L. Shepherd and Associates, San Fernando, CA, http://www.jlshepherd.com). Monolayer cultures were incubated between 10 days and 30 days, depending on cell line. Next, colonies were fixed with methanol, stained with crystal violet, and counted. Mammosphere cultures were incubated for 7 days, after which mammospheres were counted with an automated colony counter (Oxford Optronix, Oxford, U.K., http://www.oxford-optronix.com), following addition of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide to increase the contrast and allow automatic detection of mammospheres. Fresh stock solutions of simvastatin (Sigma-Aldrich, St. Louis, MO, http://www.sigmaaldrich.com) were prepared weekly with dimethyl sulfoxide at a concentration of 2 mg/ml and stored at 4°C. Final concentration of simvastatin used to treat the cells varied between 0.5 μM and 2.5 μM, accordingly to cell line sensitivity, such that formation of colonies occurred in control wells. Simvastatin was added to cell cultures in a single dose at seeding time, and culture media was not changed until the experiment finished. All conditions were tested in triplicate in two independent experiments. Survival curves were generated using SigmaPlot version 8.0 (Systat Software Inc., Richmond, CA, http://www.systat.com), and t test was used to compare surviving fractions of groups.
Source Population and Data Collection
In our studies, the IBC database constructed and maintained by the Breast Cancer Management System at MD Anderson Cancer Center was examined. This database includes 1,177 patients diagnosed with IBC between February 24, 1970, and January 27, 2011. We excluded stage IV IBC patients because these patients were previously shown to have no benefit from statin use [36]. Other exclusion criteria include patients diagnosed prior to 1995, patients who did not receive adjuvant postmastectomy radiotherapy, and patients who had a locoregional recurrence prior to radiation. Consequently, 519 patients were included in the final analysis.
The following variables were included in the analysis: age; body mass index (BMI); menopausal status; race (white vs. black/others); clinical/pathologic nodal status; pathologic stage; nuclear grade; status of estrogen receptor (ER) and progesterone receptor (PR); HER2 status; lymphatic/vascular invasion; and use of neoadjuvant, adjuvant, or hormonal therapy. Final HER2 status was determined based on both immunochemistry and fluorescence in situ hybridization. Triple-negative breast cancer (TNBC) status was determined based on proven ER/PR status and final HER2 status.
Treatment
A total of 491 patients (94.6%) received neoadjuvant chemotherapy, the specific regimens of which have been described previously [36]. All patients in the examined cohort received PMRT.
Definition of Outcomes and Statistical Methods
Patient characteristics data were first summarized using descriptive statistics and frequency tabulation. Specific traits were further analyzed and compared between statin usage groups using χ2 and Fisher’s exact test when appropriate. The primary endpoint of this analysis was local recurrence-free survival (LRFS), which was calculated from the date of definitive surgery to the date of local recurrence or last follow-up date. The Kaplan-Meier method was used to assess time to recurrence, and log-rank tests were used to compare patient characteristic groups. Both univariate and multivariate Cox proportional hazard models were used to assess the effects of covariates of interest on time to LRR. All p values <.05 were considered to be significant. Statistical analyses were conducted using either SAS version 9.2 (SAS Institute, Inc., Cary, NC, http://www.sas.com) and S-PLUS 8.0 (TIBCO Software Inc., Palo Alto, CA, http://www.tibco.com) or SPSS version 15 (IBM Corp., Armonk, NY, http://www-01.ibm.com/software/analytics/spss/).
Results
Simvastatin Radiosensitizes Mammosphere-Initiating Cells of IBC Cell Lines
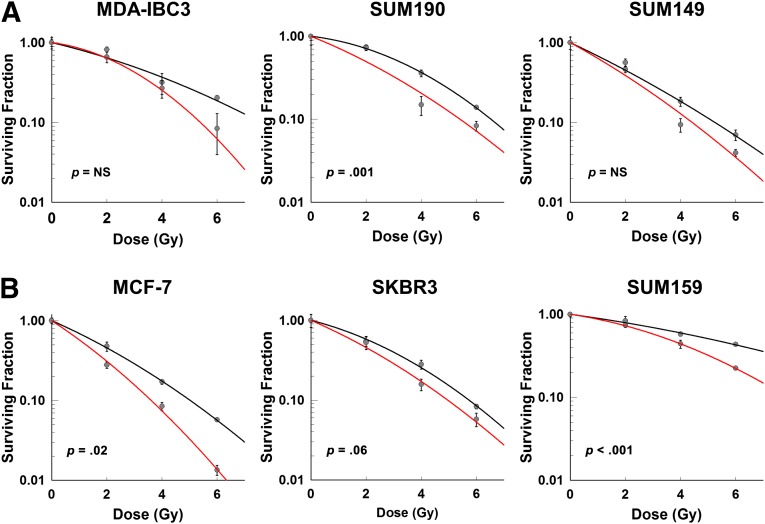
In order to evaluate the effects of simvastatin on in vitro treatment of cancer cells with radiation, we examined the ability to form 2D colonies of six different breast cancer cell lines following treatment with radiation only and in combination with simvastatin. Using standard monolayer clonogenic assays, we demonstrated that simvastatin promoted radiosensitization of IBC and non-IBC cell lines of multiple subtypes (Fig. 1). Among IBC cell lines, the HER2-positive IBC cell line SUM190 had the best response to combined treatment regardless of the radiation dose used (p = .001; Fig. 1A). In contrast, among non-IBC cell lines, HER2-negative cell lines MCF-7 and SUM159 had greater responses to combined treatment regardless of the radiation dose used (p = .02 and p < .001, respectively; Fig. 1B) than HER2-positive line SKBR3.
Figure 1.
Treatment of cell monolayer cultures with radiation and simvastatin. (A): Inflammatory breast cancer (IBC) cell lines MDA-IBC3 (0.5 μM), SUM190 (2.0 μM), and SUM149 (2.0 μM). (B): Non-IBC cell lines MCF-7 (2.0 μM), SKBR3 (0.2 μM), and SUM159 (0.5 μM). Black line: radiation treatment only. Red line: combination treatment of radiation with simvastatin. Abbreviation: NS, not significant.
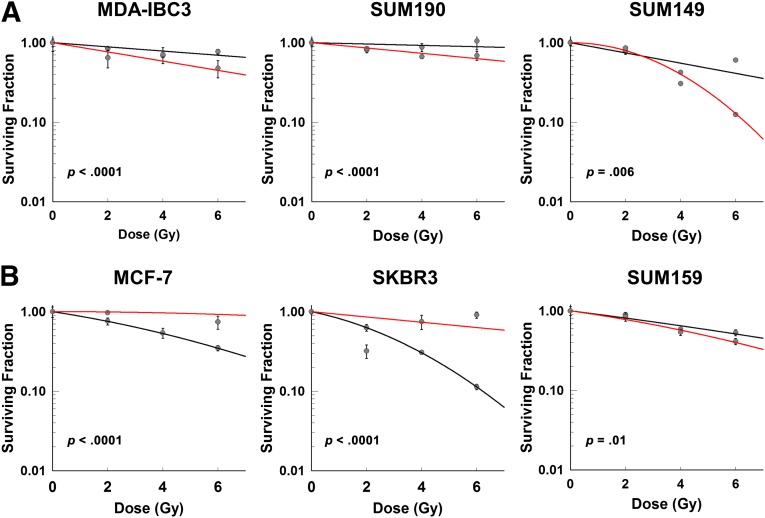
Next, we investigated the effects of simvastatin on 3D mammosphere-based clonogenic assays. Because mammospheres are enriched with mammosphere-initiating cells (MICs) and some drugs that radiosensitize differentiated cells in monolayer cultures promote the resistance of MICs in mammosphere cultures, we hypothesized that simvastatin might be a MIC radiosensitizer. As can be appreciated in Figure 2, simvastatin had a significant effect in all cell lines tested. Concerning IBC cell lines, all had a significantly greater response to combined treatment than to radiation alone, regardless of subtype (MDA-IBC3: p < .0001; SUM190: p < .0001; SUM149: p = .006; Fig. 2A). However, among non-IBC cell lines, simvastatin radioprotected the ER-positive cell line MCF-7 and the HER2-positive cell line SKBR3 (p < .0001 in both cell lines; Fig. 2B) and only radiosensitized the triple-negative cell line SUM159 (p = .01; Fig. 2B).
Figure 2.
Treatment of mammosphere cultures with radiation and simvastatin. (A): Inflammatory breast cancer (IBC) cell lines MDA-IBC3 (2.0 μM), SUM190 (2.0 μM), and SUM149 (2.0 μM). (B): Non-IBC cell lines MCF-7 (2.0 μM), SKBR3 (2.0 μM), and SUM159 (2.0 μM). Black line: radiation treatment only. Red line: combination treatment of radiation with simvastatin.
Patient Characteristics of the Cohort
After exclusionary criteria were applied, a total of 519 patients with stage III IBC who received postmastectomy radiotherapy were analyzed. In this cohort, 53 patients (10.2%) used statins, whereas 466 patients (89.8%) did not. Median follow-up time for the entire cohort was 2.5 years, and median age was 49 years (range: 23–78 years). Table 1 summarizes the baseline patient characteristics stratified by statin usage. Overall, 83% of statin users were older patients (defined as older than 50 years of age) compared with no-statin users, of which only 44% were older than 50 years of age (p < .0001). Consequently, most statin users were postmenopausal (84.6%) in the statin-user group, whereas the percentage of postmenopausal women was significantly smaller in the no-statin-user group (47.2%, p < .0001). As expected, statin users also tended to be obese (63%) compared with no-statin users (41.4%, p = .01).
Table 1.
Characteristics of patients with stage III inflammatory breast cancer who received adjuvant radiation
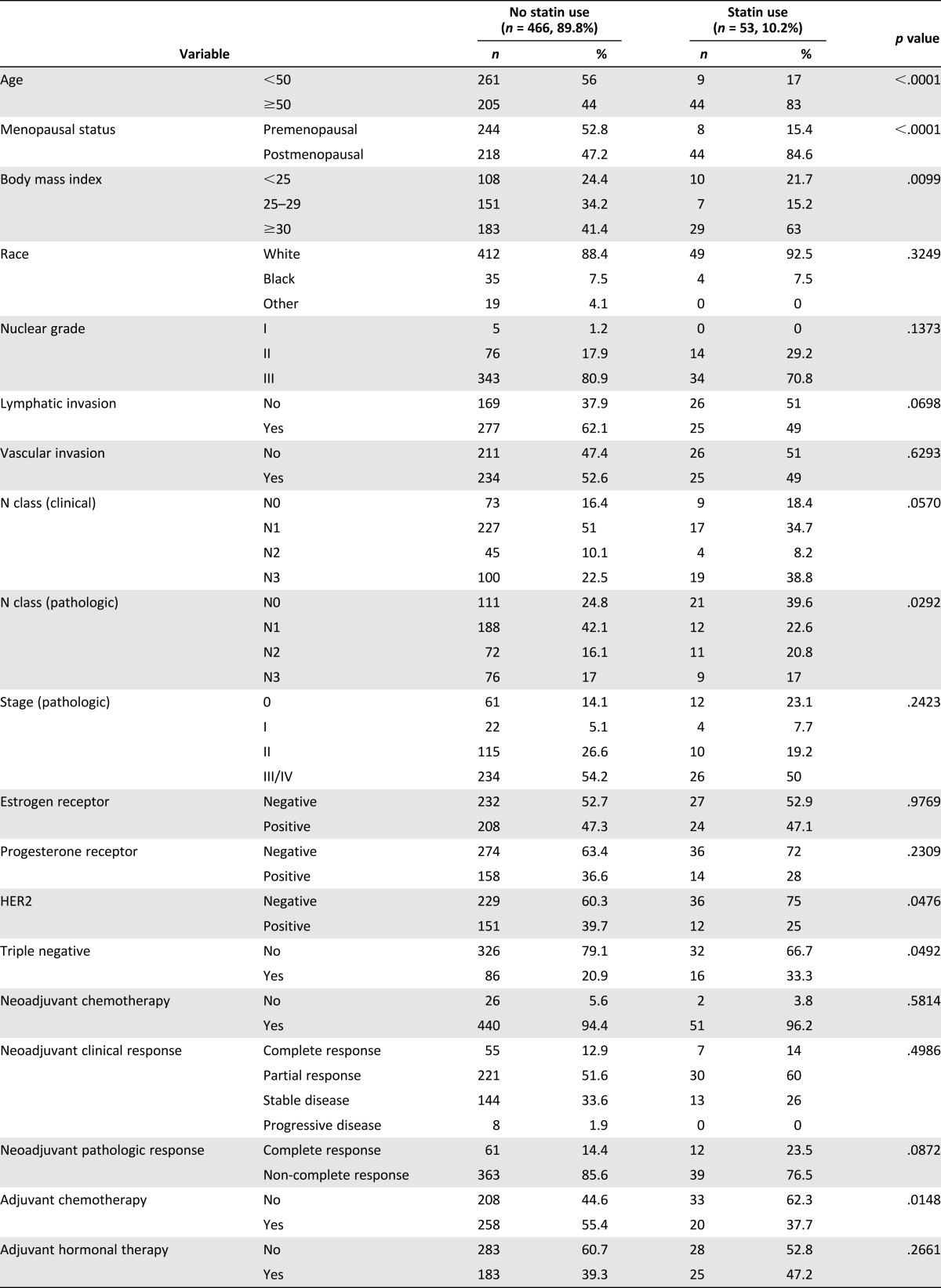
Statin users more commonly had pathologic N0 disease (39.6% vs. 24.8%, p = .029). However, race, clinical nodal status, pathologic stage, hormone receptor positivity, HER2 status, and triple-negative disease were found in similar proportions in both groups (Table 1).
Impact of Statin Usage on Time to Locoregional Recurrence
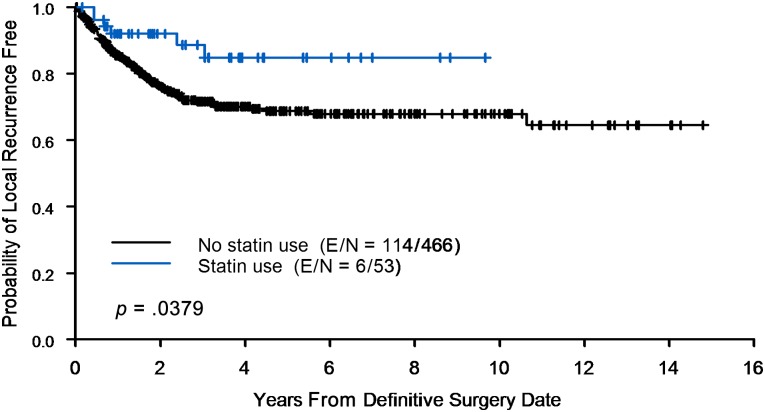
Among the entire 519-patient cohort with stage III IBC who underwent adjuvant radiotherapy, 120 patients (23.1%) experienced LRR. In the statin-usage group, 6 of 53 patients experienced a local recurrence (11.3%) compared with 114 of 466 patients in the no-statin-usage group (24.5%). The Kaplan-Meier estimate of LRR is shown in Figure 3. The actuarial 2- and 5-year local control rates for patients in the no-statin group are 76% and 69%, respectively, and for patients in the statin group are 92% and 85%, respectively.
Figure 3.
Use of statins reduces local recurrence following radiation therapy. Kaplan-Meier curve with time to local recurrence of inflammatory breast cancer patients comparing statin users and no-statin users. Abbreviation: E/N, event/number at risk for event.
The results of univariate and multivariate regression analyses are listed in Tables 2 and 3. Despite the fact that older, postmenopausal patients more frequently used statins, neither age (hazard ratio [HR]: 0.81; 95% confidence interval [CI]: 0.57–1.17; p = .262) nor menopausal status (HR: 1.03; 95% CI: 0.72–1.48; p = .8569) affected time to local recurrence on univariate analysis. Consistent with our recently published report [37], non-triple-negative IBC had a reduced risk of LRR on univariate analysis (HR: 0.44; 95% CI: 0.29–0.67; p = .0001). Consequently, lack of hormone therapy was associated with increased risk of LRR (HR: 1.87; 95% CI: 1.27–2.76; p = .001). Additional variables associated with reduced LRR on univariate analysis included absence of lymphatic or vascular invasion and response to neoadjuvant chemotherapy (Table 2), which are consistent with our previously published findings on IBC [37].
Table 2.
Univariate analysis of locoregional recurrence (stage III inflammatory breast cancer with adjuvant radiation)
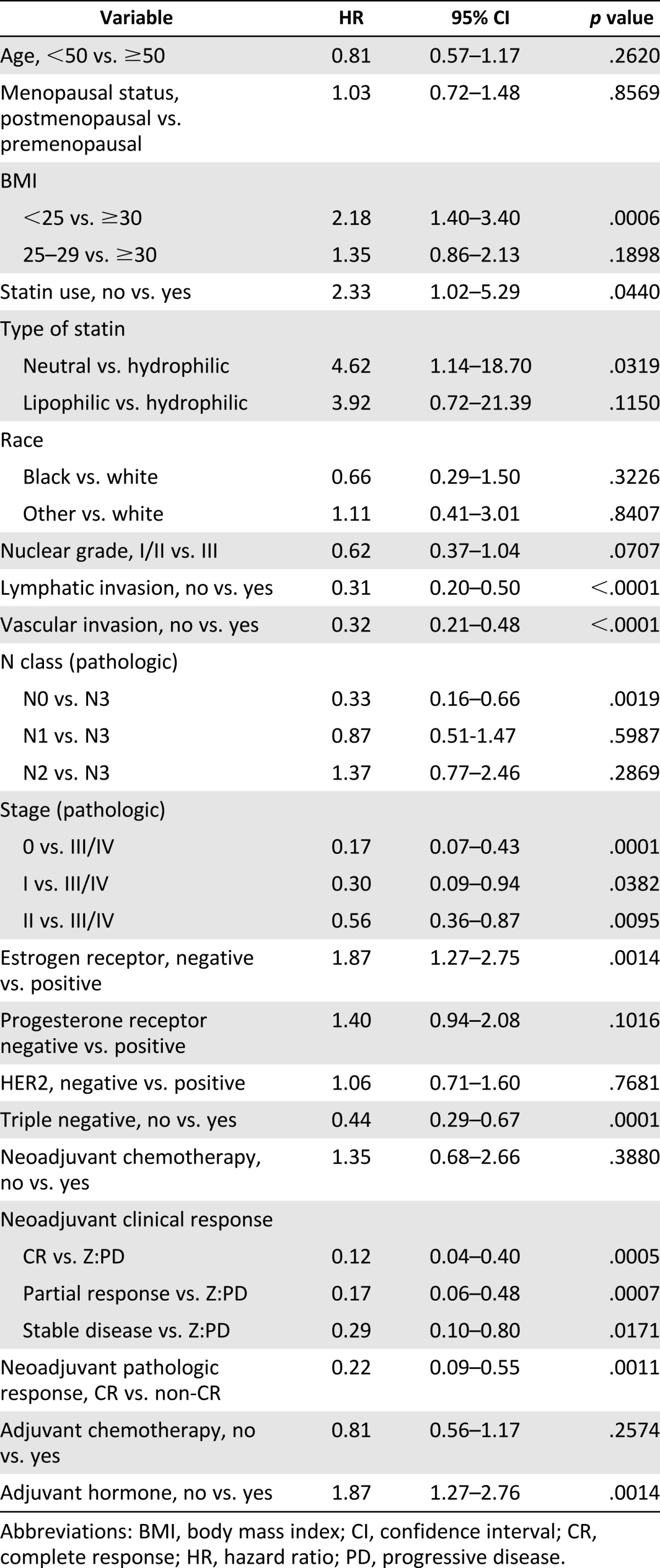
Table 3.
Multivariate analysis of locoregional recurrence (stage III inflammatory breast cancer with adjuvant radiation)
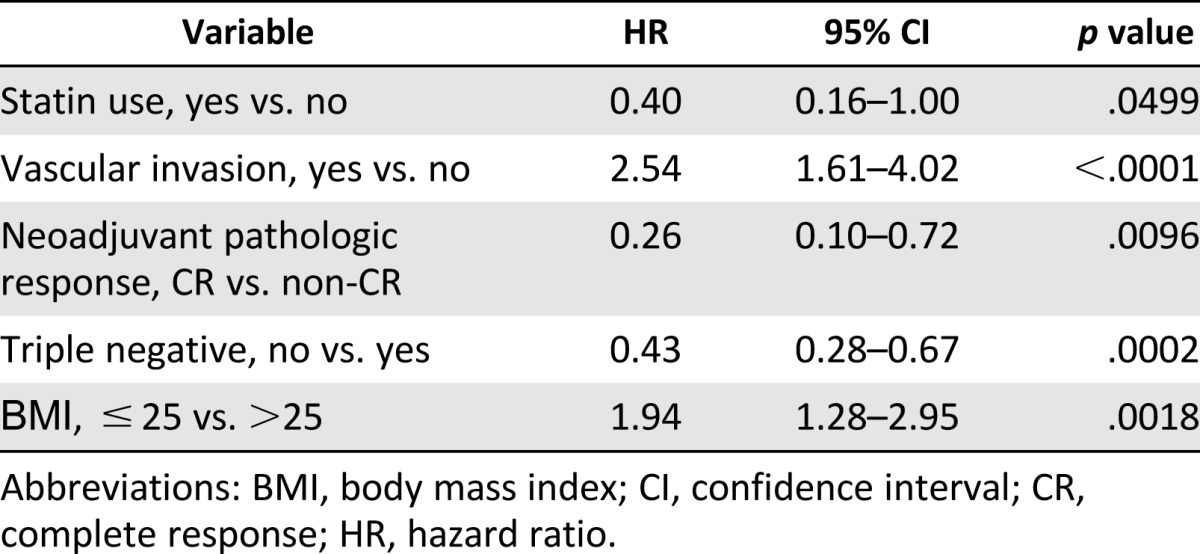
As hypothesized, the use of statins was associated with reduced LRR on multivariate analysis (HR: 0.40; 95% CI: 0.16–1.00; p = .0499). Additional factors associated with reduced LRR on multivariate analysis include non-TNBC (HR: 0.43; 95% CI: 0.28–0.67; p = .0002) and complete pathologic response to neoadjuvant chemotherapy (HR: 0.26; 95% CI: 0.10–0.72; p = .0096). The presence of vascular invasion, as expected, was associated with increased risk of LRR on multivariate analysis (HR: 2.54; 95% CI: 1.61–4.02; p < .0001). BMI was associated with increased LRR in multivariate analysis, but analysis for interaction between use of statins and BMI was negative (HR: 1.94; 95% CI: 1.28–2.95; p = .0018).
Discussion
We have previously reported radioresistance of IBC cell lines and of stem cell surrogates [15] and comparatively higher rates of LRR among patients with IBC [37]. In this paper, we report for the first time that simvastatin promoted radiosensitization of monolayer cultures across IBC and non-IBC cell lines of multiple subtypes and radiosensitization of MICs of IBC and non-IBC triple-negative cell lines. Furthermore, statin use was independently associated with significant improvement in local control after PMRT among IBC patients.
Statins have a well-described safety and toxicity profile and have been used since the 1980s; very recently, large retrospective studies from Denmark [19, 20] reopened the interest of the scientific community in cholesterol-independent and pleiotropic effects (antiproliferative and proapoptotic) of statins on breast cancer. Our team works on a unique subtype of breast cancer and reported previously that statins are associated with improved progression-free survival in patients with IBC [36]. Treatment-resistant breast cancer cells or breast cancer stem-like cells have become the therapeutic focal point of numerous recent studies. Such kinds of cells have been targeted successfully using simvastatin and γ-tocotrienol combined therapies via inhibition of the mevalonate pathway [38]. Furthermore, the inhibition of the protein geranylgeranylation by simvastatin reduced the cancer stem-like cell populations in basal/mesenchymal mammospheres (including SUM149, SUM159, and SUM190) but not in luminal mammospheres (including MFC-7) [31].
In the present study, we observed that combined treatment with simvastatin and radiation had distinct effects on IBC and non-IBC cell lines cultured as monolayer or mammospheres. The HER2-positive cell line SUM190 was the only IBC cell line that responded to combined treatment when cultured as monolayer; however, all IBC cell lines, regardless of subtype, cultured as mammospheres responded to combined therapy. In contrast, the non-IBC cell lines with greater response to combined therapy were HER2-negative MCF-7 and SUM159 when cultured as monolayer and only the triple-negative SUM159 when cultured as mammospheres. Given the lack of reduction in stem-like cells in luminal cell lines examined by Ginestier et al., the radioprotection offered by simvastatin to the cell lines MCF-7 and SKBR3 (both luminal) that we observed is possibly related to the lack of a specific signaling pathway associated with these cell lines when cultured as a monolayer or mammosphere [31].
It has been reported that cancer cells from different organs are more responsive to simvastatin than pravastatin [39] and that simvastatin induces death of HER2-overexpressing cell lines, such as MDA-MB-361, SK-Ov3, and SKBR3, and inhibits the activity of the HER2 promoter [40]. We did not observe this outcome with the HER2-positive cell lines used in this study, yet activation of different pathways by radiation treatment might be a reason for such outcome. Interestingly, the use of lipophilic statins has been associated with a reduction in the proportion of hormone receptor-negative breast cancers [41] and treatment of triple-negative cell lines with simvastatin has been reported to induce cell death through the PI3K pathway [42]. In our study, triple-negative cell lines did not consistently correlate with response to combined treatment when monolayer cultures were used; however, when mammosphere cultures were used, both TNBC cell lines (IBC SUM149 and non-IBC SUM159) were radiosensitized by simvastatin.
IBC cells retain intracellular cholesterol esters, free cholesterol, and triglycerides in lipid-deficient environments [43]; increased angiogenesis and lymphangiogenesis are associated with IBC [44]; and VEGF-A, a lymphangiogenesis mediator, was recently shown to be a prognostic indicator in IBC [45]. All of these described characteristics of IBC may also contribute to the differences in radiosensitization that we found between mammospheres of IBC and non-IBC cell lines. Lymphangiogenesis is important for IBC invasion and metastasis, and very recently, a study on corneal and cutaneous lymphangiogenesis in vivo demonstrated that statins are potent inhibitors of lymphangiogenesis, with simvastatin showing the strongest effect [28].
This work includes a retrospective study and is limited by the biases inherent in all retrospective work. Statin use was primarily in women with hyperlipidemia as well as history of coronary artery disease, and other hidden biases may exist that are unaccounted for in the study. Statin use was extracted from the chart, and complete details regarding duration and dosage were not available in all cases. Patients who did not receive PMRT were excluded, thus representing a bias excluding those who progressed on chemotherapy without surgery or preoperative radiation and those who progressed after surgery and before chemotherapy. Nevertheless, considering all known, extractable variables, the findings are of considerable interest, are congruent with the preclinical findings, and are worthy of additional consideration in the prospective setting for patients with nonluminal IBC and triple-negative breast cancer.
Conclusion
Statins represent a potential new therapeutic strategy to reduce LRR among patients with IBC when used in combination with radiation. Specifically, simvastatin is approved worldwide by different U.S. Food and Drug Administration-equivalent organizations, has a safe toxicity profile, and is commercially available in generic forms. Randomized trials evaluating statins in this disease should test radiosensitization in their design.
Acknowledgments
This work was supported by the National Institutes of Health Grants R01CA138239-01 and 1R01CA180061-01, and the State of Texas Grant for Rare and Aggressive Breast Cancer Research Program. L. Lacerda, B.G.D., and W.X. are recipients of Susan G. Komen for the Cure postdoctoral fellowships (PDF12226438, KG101478, and KG111387, respectively). We thank Christine Wogan in the Department of Radiation Oncology at The University of Texas MD Anderson Cancer Center for editing this article and Dr. Jeffrey Rosen at Baylor College of Medicine for constructive criticism of this article.
Author Contributions
L. Lacerda: conception and design, collection and assembly of data, data analysis and interpretation, manuscript writing; J.P.R.: data analysis and interpretation, manuscript writing; D.L.: data analysis and interpretation; R.L., L. Li, B.G.D., W.X.: collection and assembly of data; H.M., T.B.: collection and assembly of data, provision of study material or patients; G.N.H., T.A.B.: financial and administrative support; N.T.U.: financial support, provision of study material or patients; W.A.W.: conception and design, financial support, provision of study material or patients, data analysis and interpretation, manuscript writing and final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
References
- 1.Dawood S, Merajver SD, Viens P, et al. International expert panel on inflammatory breast cancer: Consensus statement for standardized diagnosis and treatment. Ann Oncol. 2011;22:515–523. doi: 10.1093/annonc/mdq345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Charafe-Jauffret E, Ginestier C, Iovino F, et al. Aldehyde dehydrogenase 1-positive cancer stem cells mediate metastasis and poor clinical outcome in inflammatory breast cancer. Clin Cancer Res. 2010;16:45–55. doi: 10.1158/1078-0432.CCR-09-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosenthal DT, Zhang J, Bao L, et al. RhoC impacts the metastatic potential and abundance of breast cancer stem cells. PLoS One. 2012;7:e40979. doi: 10.1371/journal.pone.0040979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xiao Y, Ye Y, Yearsley K, et al. The lymphovascular embolus of inflammatory breast cancer expresses a stem cell-like phenotype. Am J Pathol. 2008;173:561–574. doi: 10.2353/ajpath.2008.071214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clarke M, Collins R, Darby S, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: An overview of the randomised trials. Lancet. 2005;366:2087–2106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 6.Meyers MO, Klauber-Demore N, Ollila DW, et al. Impact of breast cancer molecular subtypes on locoregional recurrence in patients treated with neoadjuvant chemotherapy for locally advanced breast cancer. Ann Surg Oncol. 2011;18:2851–2857. doi: 10.1245/s10434-011-1665-8. [DOI] [PubMed] [Google Scholar]
- 7.Panoff JE, Hurley J, Takita C, et al. Risk of locoregional recurrence by receptor status in breast cancer patients receiving modern systemic therapy and post-mastectomy radiation. Breast Cancer Res Treat. 2011;128:899–906. doi: 10.1007/s10549-011-1495-1. [DOI] [PubMed] [Google Scholar]
- 8.Chen MS, Woodward WA, Behbod F, et al. Wnt/beta-catenin mediates radiation resistance of Sca1+ progenitors in an immortalized mammary gland cell line. J Cell Sci. 2007;120:468–477. doi: 10.1242/jcs.03348. [DOI] [PubMed] [Google Scholar]
- 9.Phillips TM, McBride WH, Pajonk F. The response of CD24(-/low)/CD44+ breast cancer-initiating cells to radiation. J Natl Cancer Inst. 2006;98:1777–1785. doi: 10.1093/jnci/djj495. [DOI] [PubMed] [Google Scholar]
- 10.Woodward WA, Chen MS, Behbod F, et al. WNT/beta-catenin mediates radiation resistance of mouse mammary progenitor cells. Proc Natl Acad Sci USA. 2007;104:618–623. doi: 10.1073/pnas.0606599104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Creighton CJ, Li X, Landis M, et al. Residual breast cancers after conventional therapy display mesenchymal as well as tumor-initiating features. Proc Natl Acad Sci USA. 2009;106:13820–13825. doi: 10.1073/pnas.0905718106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li X, Lewis MT, Huang J, et al. Intrinsic resistance of tumorigenic breast cancer cells to chemotherapy. J Natl Cancer Inst. 2008;100:672–679. doi: 10.1093/jnci/djn123. [DOI] [PubMed] [Google Scholar]
- 13.Dontu G, Abdallah WM, Foley JM, et al. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev. 2003;17:1253–1270. doi: 10.1101/gad.1061803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Woodward WA, Bristow RG. Radiosensitivity of cancer-initiating cells and normal stem cells (or what the Heisenberg uncertainly principle has to do with biology) Semin Radiat Oncol. 2009;19:87–95. doi: 10.1016/j.semradonc.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Debeb BG, Xu W, Mok H, et al. Differential radiosensitizing effect of valproic acid in differentiation versus self-renewal promoting culture conditions. Int J Radiat Oncol Biol Phys. 2010;76:889–895. doi: 10.1016/j.ijrobp.2009.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Graaf MR, Beiderbeck AB, Egberts AC, et al. The risk of cancer in users of statins. J Clin Oncol. 2004;22:2388–2394. doi: 10.1200/JCO.2004.02.027. [DOI] [PubMed] [Google Scholar]
- 17.Blais L, Desgagné A, LeLorier J. 3-Hydroxy-3-methylglutaryl coenzyme A reductase inhibitors and the risk of cancer: A nested case-control study. Arch Intern Med. 2000;160:2363–2368. doi: 10.1001/archinte.160.15.2363. [DOI] [PubMed] [Google Scholar]
- 18.Cauley JA, Zmuda JM, Lui LY, et al. Lipid-lowering drug use and breast cancer in older women: A prospective study. J Womens Health (Larchmt) 2003;12:749–756. doi: 10.1089/154099903322447710. [DOI] [PubMed] [Google Scholar]
- 19.Nielsen SF, Nordestgaard BG, Bojesen SE. Statin use and reduced cancer-related mortality. N Engl J Med. 2012;367:1792–1802. doi: 10.1056/NEJMoa1201735. [DOI] [PubMed] [Google Scholar]
- 20.Ahern TP, Pedersen L, Tarp M, et al. Statin prescriptions and breast cancer recurrence risk: A Danish nationwide prospective cohort study. J Natl Cancer Inst. 2011;103:1461–1468. doi: 10.1093/jnci/djr291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Denoyelle C, Vasse M, Körner M, et al. Cerivastatin, an inhibitor of HMG-CoA reductase, inhibits the signaling pathways involved in the invasiveness and metastatic properties of highly invasive breast cancer cell lines: An in vitro study. Carcinogenesis. 2001;22:1139–1148. doi: 10.1093/carcin/22.8.1139. [DOI] [PubMed] [Google Scholar]
- 22.Luan Z, Chase AJ, Newby AC. Statins inhibit secretion of metalloproteinases-1, -2, -3, and -9 from vascular smooth muscle cells and macrophages. Arterioscler Thromb Vasc Biol. 2003;23:769–775. doi: 10.1161/01.ATV.0000068646.76823.AE. [DOI] [PubMed] [Google Scholar]
- 23.Davignon J. Beneficial cardiovascular pleiotropic effects of statins. Circulation. 2004;109(suppl 1):III39–III43. doi: 10.1161/01.CIR.0000131517.20177.5a. [DOI] [PubMed] [Google Scholar]
- 24.Hakamada-Taguchi R, Uehara Y, Kuribayashi K, et al. Inhibition of hydroxymethylglutaryl-coenzyme a reductase reduces Th1 development and promotes Th2 development. Circ Res. 2003;93:948–956. doi: 10.1161/01.RES.0000101298.76864.14. [DOI] [PubMed] [Google Scholar]
- 25.Feleszko W, Bałkowiec EZ, Sieberth E, et al. Lovastatin and tumor necrosis factor-alpha exhibit potentiated antitumor effects against Ha-ras-transformed murine tumor via inhibition of tumor-induced angiogenesis. Int J Cancer. 1999;81:560–567. doi: 10.1002/(sici)1097-0215(19990517)81:4<560::aid-ijc10>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 26.Alber HF, Dulak J, Frick M, et al. Atorvastatin decreases vascular endothelial growth factor in patients with coronary artery disease. J Am Coll Cardiol. 2002;39:1951–1955. doi: 10.1016/s0735-1097(02)01884-3. [DOI] [PubMed] [Google Scholar]
- 27.Hata Y, Miura M, Asato R, et al. Antiangiogenic mechanisms of simvastatin in retinal endothelial cells. Graefes Arch Clin Exp Ophthalmol. 2010;248:667–673. doi: 10.1007/s00417-009-1282-4. [DOI] [PubMed] [Google Scholar]
- 28.Schulz MM, Reisen F, Zgraggen S, et al. Phenotype-based high-content chemical library screening identifies statins as inhibitors of in vivo lymphangiogenesis. Proc Natl Acad Sci USA. 2012;109:E2665–E2674. doi: 10.1073/pnas.1206036109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kodach LL, Jacobs RJ, Voorneveld PW, et al. Statins augment the chemosensitivity of colorectal cancer cells inducing epigenetic reprogramming and reducing colorectal cancer cell ‘stemness’ via the bone morphogenetic protein pathway. Gut. 2011;60:1544–1553. doi: 10.1136/gut.2011.237495. [DOI] [PubMed] [Google Scholar]
- 30.Mandal CC, Ghosh-Choudhury N, Yoneda T, et al. Simvastatin prevents skeletal metastasis of breast cancer by an antagonistic interplay between p53 and CD44. J Biol Chem. 2011;286:11314–11327. doi: 10.1074/jbc.M110.193714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ginestier C, Monville F, Wicinski J, et al. Mevalonate metabolism regulates basal breast cancer stem cells and is a potential therapeutic target. Stem Cells. 2012;30:1327–1337. doi: 10.1002/stem.1122. [DOI] [PubMed] [Google Scholar]
- 32.Kao J, Salari K, Bocanegra M, et al. Molecular profiling of breast cancer cell lines defines relevant tumor models and provides a resource for cancer gene discovery. PLoS One. 2009;4:e6146. doi: 10.1371/journal.pone.0006146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klopp AH, Lacerda L, Gupta A, et al. Mesenchymal stem cells promote mammosphere formation and decrease E-cadherin in normal and malignant breast cells. PLoS One. 2010;5:e12180. doi: 10.1371/journal.pone.0012180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fillmore CM, Kuperwasser C. Human breast cancer cell lines contain stem-like cells that self-renew, give rise to phenotypically diverse progeny and survive chemotherapy. Breast Cancer Res. 2008;10:R25. doi: 10.1186/bcr1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gupta PB, Onder TT, Jiang G, et al. Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell. 2009;138:645–659. doi: 10.1016/j.cell.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brewer TM, Masuda H, Liu DD, et al. Statin use in primary inflammatory breast cancer: A cohort study. Br J Cancer. 2013;109:318–324. doi: 10.1038/bjc.2013.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li J, Gonzalez-Angulo AM, Allen PK, et al. Triple-negative subtype predicts poor overall survival and high locoregional relapse in inflammatory breast cancer. The Oncologist. 2011;16:1675–1683. doi: 10.1634/theoncologist.2011-0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gopalan A, Yu W, Sanders BG, et al. Eliminating drug resistant breast cancer stem-like cells with combination of simvastatin and gamma-tocotrienol. Cancer Lett. 2013;328:285–296. doi: 10.1016/j.canlet.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 39.Menter DG, Ramsauer VP, Harirforoosh S, et al. Differential effects of pravastatin and simvastatin on the growth of tumor cells from different organ sites. PLoS One. 2011;6:e28813. doi: 10.1371/journal.pone.0028813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao Z, Cao X, Pan Y, et al. Simvastatin downregulates HER2 via upregulation of PEA3 to induce cell death in HER2-positive breast cancer cells. Oncol Res. 2012;20:187–195. doi: 10.3727/096504013x13589503482699. [DOI] [PubMed] [Google Scholar]
- 41.Kumar AS, Benz CC, Shim V, et al. Estrogen receptor-negative breast cancer is less likely to arise among lipophilic statin users. Cancer Epidemiol Biomarkers Prev. 2008;17:1028–1033. doi: 10.1158/1055-9965.EPI-07-0726. [DOI] [PubMed] [Google Scholar]
- 42.Park YH, Jung HH, Ahn JS, et al. Statin induces inhibition of triple negative breast cancer (TNBC) cells via PI3K pathway. Biochem Biophys Res Commun. 2013;439:275–279. doi: 10.1016/j.bbrc.2013.08.043. [DOI] [PubMed] [Google Scholar]
- 43.Martin BJ, van Golen KL. A comparison of cholesterol uptake and storage in inflammatory and noninflammatory breast cancer cells. Int J Breast Cancer. 2012:412581. doi: 10.1155/2012/412581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Van der Auwera I, Van Laere SJ, Van den Eynden GG, et al. Increased angiogenesis and lymphangiogenesis in inflammatory versus noninflammatory breast cancer by real-time reverse transcriptase-PCR gene expression quantification. Clin Cancer Res. 2004;10:7965–7971. doi: 10.1158/1078-0432.CCR-04-0063. [DOI] [PubMed] [Google Scholar]
- 45.Arias-Pulido H, Chaher N, Gong Y, et al. Tumor stromal vascular endothelial growth factor A is predictive of poor outcome in inflammatory breast cancer. BMC Cancer. 2012;12:298. doi: 10.1186/1471-2407-12-298. [DOI] [PMC free article] [PubMed] [Google Scholar]