The authors examined impaired globin switching of embryonic ε- and fetal γ-globin to adult β-globin in human erythroid cells by optimizing multicolor flow cytometry to simultaneously follow expression of different globin subtypes using different immunofluorescent probes. Results showed that impaired γ-globin silencing is associated with downregulated BCL11A-L in human pluripotent stem cell-derived erythroblasts and that multicolor staining of globin subtypes is an effective approach to studying globin switching in vitro.
Keywords: Embryonic stem cells, iPS cells, Erythrocyte, Globin switching, BCL11A
Abstract
Adult hemoglobin composed of α- and β-globin reflects a change from expression of embryonic ε- and fetal γ-globin to adult β-globin in human erythroid cells, so-called globin switching. Human pluripotent stem cells (hPSCs) are a potential source for in vitro erythrocyte production, but they show prominent expression of γ-globin with little β-globin expression, which indicates incomplete globin switching. To examine the mechanism of this impaired globin switching, we optimized multicolor flow cytometry to simultaneously follow expression of different globin subtypes using different immunofluorescent probes. This enabled us to detect upregulation of β-globin and the corresponding silencing of γ-globin at the single-cell level during cord blood CD34+ cell-derived erythropoiesis, examined as an endogenous control. Using this approach, we initially characterized the heterogeneous β-globin expression in erythroblasts from several hPSC clones and confirmed the predominant expression of γ-globin. These hPSC-derived erythroid cells also displayed reduced expression of BCL11A-L. However, doxycycline-induced overexpression of BCL11A-L in selected hPSCs promoted γ-globin silencing. These results strongly suggest that impaired γ-globin silencing is associated with downregulated BCL11A-L in hPSC-derived erythroblasts and that multicolor staining of globin subtypes is an effective approach to studying globin switching in vitro.
Introduction
Blood transfusion is an indispensable tool in medicine today. At present, however, the availability of blood for transfusion is entirely dependent on blood collected from donors, the numbers of whom have been declining in most industrialized countries. This makes the safety and stability of the blood supply a significant and continuing concern. To overcome this problem, much effort has gone into developing methods for in vitro generation of human erythrocytes [1, 2], including the use of human pluripotent stem cells (hPSCs; i.e., embryonic stem cells [hESCs] or induced pluripotent stem cells [hiPSCs]) [3, 4]. hiPSCs are particularly advantageous, as blood group type O Rh-negative-derived hiPSCs could potentially serve as a universal source of red blood cell transfusion. The hiPSC technology could also contribute to production of rare types of erythrocytes—for example, D−, Fy(a−b−), Di(b−), and Jr(a−) [5]. However, it is well known that hiPSC clones are heterogeneous with respect to their differentiation potential [6]. Consequently, the ability to distinguish clones suitable for in vitro erythrocyte production from among identical donor-derived hiPSC clones is crucial.
Adult hemoglobin is composed of two α-globin and two β-globin subunits (HbA), whereas fetal hemoglobin is composed of two α-globin and two γ-globin subunits (HbF). The fetal protein binds oxygen with greater affinity than the adult form, giving the developing fetus better access to oxygen from the mother’s bloodstream [7]. Moreover, changes in erythropoiesis, and thus the globin expression profiles, are hallmarks of the developmental stages of erythroblasts [7]. During erythropoiesis from hESCs and hiPSCs, the major globin forms are embryonic ε and fetal γ; little adult β-globin is expressed [7], indicating incomplete globin switching. Why the switching is not accomplished remains unclear, however [8–18].
In the present study, we combined multicolor immunostaining with an improved fixation technique to follow expression of individual globin subtypes during in vitro erythropoiesis from hPSCs and human cord blood (CB) CD34+ cells. This provided the time frame of globin switching during erythropoiesis and enabled selection of suitable clones from among a heterogeneous population of hPSC clones. In addition, we also sought to validate our tracing method by examining the effect of BCL11A-L on γ- and β-globin expression [7, 19]. Our findings indicate that even in hPSC clones deemed suitable for erythropoiesis, γ-globin silencing may be impaired in hPSC-derived erythroid cells, due at least in part to reduced BCL11A-L expression.
Materials and Methods
Cells and Reagents
All reagents were obtained from Sigma-Aldrich (St. Louis, MO, http://www.sigmaaldrich.com) unless indicated otherwise. The KhES-3 hESC line was obtained from the Institute for Frontier Medical Science, Kyoto University (Kyoto, Japan, http://www.frontier.kyoto-u.ac.jp) after approval for hESC use was granted by the Minister of Education, Culture, Sports, Science, and Technology of Japan. The H1 hESC line was obtained from WiCell Research Institute (Madison, WI, http://www.wicell.org) to T. Nakahata. Collection of peripheral blood from healthy volunteers was approved by the Ethical Committee of the Institute of Medical Science at The University of Tokyo and the Kyoto University Committee for Human Sample-Based Experiments. All studies involving the use of human samples were conducted in accordance with the Declaration of Helsinki.
We established hiPSC clones using a Sendai virus harboring four reprogramming factors (OCT3/4, SOX2, KLF4, and cMYC) [20]. hiPSC clones 1 and 2 were derived from human neonatal dermal fibroblasts (Lonza, Walkersville, MD, http://www.lonza.com), clones 3–6 were derived from human CB CD34+ cells (Lonza), and clones 7 and 8 were derived from human adult peripheral blood cells. All hPSCs were maintained as described previously [21–23]. Mouse C3H10T1/2 cells were also cultured as described previously [21–23].
Erythroid Differentiation via Sac Formation From hES/iPSCs
To differentiate hES/hiPSCs into hematopoietic cells, we used our previously established protocol [21–23]. After pretreating mouse mesenchymal feeder C3H10T1/2 cells with mitomycin C, small clumps (approximately 100 cells) of hES/iPSCs (suspended in phosphate-buffered saline [PBS] containing 0.25% trypsin, 1 mM CaCl2, and 20% knockout serum replacement) were transferred onto the C3H10T1/2 cells (8.0 × 105 cells per a 100-mm dish) and cultured in differentiation medium supplemented with 20 ng/ml vascular endothelial growth factor for 14 days (day −14 to day 0). During that period, the medium was replaced every 3 days. On day 0 of culture, hES/hiPSC-derived sacs (hES/iPS sacs) were collected into a 50-ml tube, gently crushed with a pipette, and passed through a 40-μm cell strainer, which yielded a population of disaggregated cells that included hematopoietic progenitor cells (HPCs). These cells were then stained with phycoerythrin (PE)-Cy7-conjugated anti-human CD34 antibody (343516; BioLegend, San Diego, CA, http://www.biolegend.com) for 30 minutes on ice and washed, after which the CD34+ cells were sorted using a flow cytometer (FACSAria II; Becton, Dickinson and Company, Franklin Lakes, NJ, http://www.bd.com). Aliquots (5.0 × 104 cells) of the CD34+ cells were then transferred onto fresh C3H10T1/2 cells (2 × 104 cells per well of a six-well plate) and maintained in differentiation medium supplemented with 50 ng/ml human stem cell factor (SCF; R&D Systems Inc., Minneapolis, MN, http://www.rndsystems.com), 50 ng/ml human thrombopoietin (TPO; R&D Systems Inc.), and 5 IU/ml human erythropoietin (EPO; Kyowa-Kirin, Tokyo, Japan, http://www.kyowa-kirin.co.jp) from day 0 to day 6. Thereafter, the cells were collected, transferred onto C3H10T1/2 cells (8.0 × 105 cells per a 100-mm dish), and cultured in the presence of 5 IU/ml human EPO for another 12 days. Throughout the culture period, the medium was replaced every 3 days, and nonadherent cells were collected and analyzed on days 3, 6, 12, and 18.
Expansion and Differentiation of Erythroblasts From Human Cord Blood CD34+ Cells
To expand and differentiate human CB CD34+ cells, they were applied to C3H10T1/2 cells (8.0 × 105 cells per 100-mm dish) and cultured in hematopoietic differentiation medium supplemented with 50 ng/ml human SCF, 50 ng/ml human TPO, and 5 IU/ml human EPO for 6 days, after which they were cultured with 5 IU/ml human EPO alone for another 12 days. Throughout the culture period, the medium was replaced every 3 days, and nonadherent cells were collected and analyzed on days 3, 6, 12, and 18.
Flow Cytometric Analysis of Cell Surface Antigens
Nonadherent cells were prepared in PBS containing 3% fetal bovine serum (FBS) and stained with combinations of antibodies for 30 minutes on ice. The antibodies used were Pacific Blue-conjugated anti-human CD235a (306612; BioLegend), PE-conjugated anti-human CD71 (334106; BioLegend), and allophycocyanin-conjugated anti-human CD43 (343206; BioLegend). Samples were then washed with PBS and analyzed using flow cytometry.
To detect stage-specific embryonic antigen-4 (SSEA-4) expression in hES/iPSCs, the trypsinized cells were collected and stained with PE-conjugated anti-SSEA-4 antibody (FAB1435P; R&D Systems Inc.) and analyzed using flow cytometry.
Intracellular Detection of Globin Subtypes Using Flow Cytometry
For flow cytometric detection of human globin subtypes, cells in suspension at a concentration of 1.0 × 106 cells per milliliter were fixed in 4% paraformaldehyde (PFA; Wako Pure Chemical Industries, Ltd., Tokyo, Japan, http://www.wako-chem.co.jp/english/) for 60 minutes at room temperature and then treated with ice-cold 100% methanol for 5 minutes. The fixed cells were then permeabilized with 0.5% saponin for 10 minutes at room temperature, after which aliquots (2.0 × 105 cells) were stained for 30 minutes on ice using 1 μg (20 μl) of PE-conjugated anti-human hemoglobin β (mouse monoclonal IgG1, sc-21757 PE; Santa Cruz Biotechnology Inc., Santa Cruz, CA, http://www.scbt.com), and/or 5 μl of TRI-COLOR-conjugated (tandem R-PE-Cy5-conjugated) anti-human fetal hemoglobin (HbF; mouse monoclonal IgG1, HFH-06; Invitrogen, Carlsbad, CA, http://www.invitrogen.com), and/or 1 μg (0.1 μl) of fluorescein isothiocyanate (FITC)-conjugated anti-human hemoglobin ε (mouse monoclonal IgG1 κ, 61C-CR8008M1F; Fitzgerald, Acton, MA, http://www.fitzgerald-fii.com). Finally, the cells were stained with Pacific Blue-conjugated anti-human CD235a (349108; BioLegend; mouse IgG2a κ) for 30 minutes on ice. Using flow cytometry, we then analyzed the samples for CD235a+ cell populations. In addition, 20 μl of normal mouse IgG1-PE-Cy5 (sc-2877; Santa Cruz Biotechnology Inc.), normal mouse IgG1-PE (sc-2866; Santa Cruz Biotechnology Inc.), and normal mouse IgG1-FITC (sc-2855; Santa Cruz Biotechnology Inc.) were used as isotype controls, and all procedures were performed after washing the cells with PBS containing 3% FBS.
To detect green fluorescent protein (GFP) expression in the fixed/permeabilized cells, we used anti-GFP Alexa Fluor 488 (sc-9996 AF488; Santa Cruz Biotechnology Inc.).
Real-Time Reverse Transcription-Polymerase Chain Reaction
Total RNA was extracted from cells using an RNeasy Micro kit (Qiagen, Hilden, Germany, http://www.qiagen.com), after which complementary DNAs were generated using a reverse transcription-polymerase chain reaction (RT-PCR) system (Thermo Fisher Scientific, Waltham, MA, http://www.thermofisher.com) with oligo(dT) primers (Invitrogen). RT-PCR was performed using a kit (SYBR Premix DimerEraser; Takara Bio, Shiga, Japan, http://www.takara-bio.com) according to the manufacturer’s instructions. Signals were detected using an ABI7900HT Real-Time PCR system (Life Technologies, Rockville, MD, http://www.lifetech.com). GAPDH was used as an internal control. Fold changes were calculated using the ΔΔCT method, with day 18 human CB CD34+ cell-derived erythroblasts serving as a calibrator. The primer sets are listed in supplemental online Table 1.
Doxycycline-Inducible BCL11A Lentiviral Vector and Transfection Methods
Human BCL11A-L gene-inducible lentiviral vector was based on an all-in-one inducible lentiviral vector (Ai-LV) [24] from Dr. T. Yamaguchi (University of Tokyo). Using PCR, human BCL11A was cloned from human CB-derived CD34+ erythroblasts and used to replace the mOKS cassette in the lentiviral vector, thereby enabling doxycycline (DOX)-dependent induction of BCL11A-L. Viral supernatant was generated as described previously [25]. Virus-transfected hES/iPSCs were cloned and maintained as previously described.
Image Analysis
Phase-contrast and fluorescence images were captured using a BZ-9000 microscope (Keyence, Osaka, Japan, http://www.keyence.com), after which the images were analyzed using BZ-Analyzer software (Keyence).
Cation-Exchange High-Performance Liquid Chromatography
The supernatant collected from lysed erythroid cells was analyzed using cation-exchange high-performance liquid chromatography (CE-HPLC) (HLC-723G8, β-thalassemia mode; Tosoh Co. Ltd., Ayase, Kanagawa, Japan, http://www.tosoh.co.jp) as previously described [26]. Briefly, 2.0 × 107 sorted hiPSC 8-BCL11A-L-GFP-derived CD235a+GFP+ cells generated using protocol i (control, no overexpression) or protocol iii (overexpression of BCL11A-L) were evaluated on day 18 of culture for relative expression of hemoglobin A (HbA, α2β2), hemoglobin F (HbF, α2γ2), and hemoglobin E (HbE, α2ε2) [4].
Statistical Analysis
All experiments were performed in triplicate and repeated at least twice. Figures show the results from one representative experiment. All data are presented as mean ± SD. Values of p < .05 were considered significant.
Results
Optimization of Cell Fixation for Tracing Expression of Individual Globins During Erythropoiesis From hPSCs and CB-Derived CD34+ Hematopoietic Progenitors
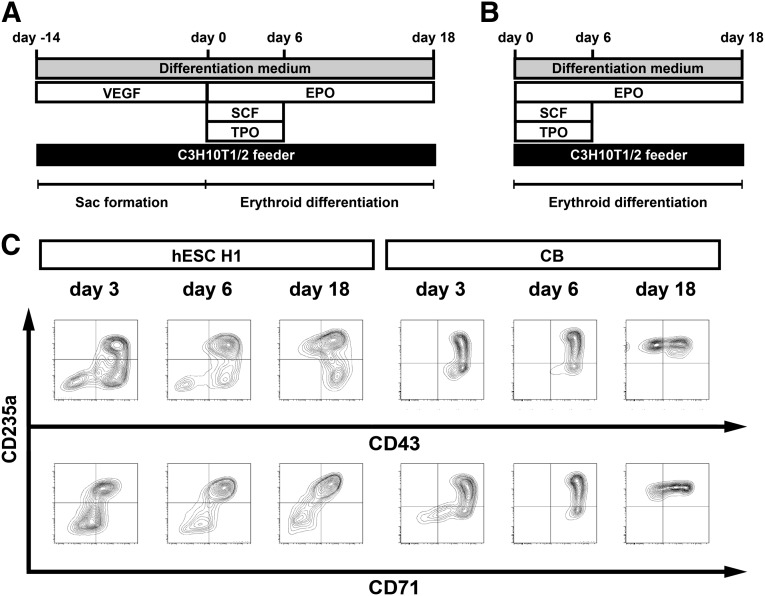
We have developed a coculture system with which human ESCs or iPSCs can be differentiated into multipotent hematopoietic progenitors capable of yielding megakaryocytes, erythroblasts, or lymphocytes [21–23, 27]. Using this culture system, we first sought to generate erythroblasts from the H1 and KhES-3 hESC lines using the protocol diagrammed in Figure 1A and from human CB-CD34+ cells using the protocol diagrammed in Figure 1B. Thereafter, we used flow cytometry to characterize several cell surface markers (e.g., CD235a, CD43, and CD71), which revealed the differentiation capabilities and time frame of the in vitro differentiation from the respective sources. We found that we were able to differentiate hESC H1 and CB-CD34+ cells into CD235a+CD71+ and CD235a+CD71− erythroid cells (Fig. 1C).
Figure 1.
Erythroid differentiation of human pluripotent stem cells. (A): Schematic diagram of the protocol used for in vitro differentiation via sac formation used with hESCs and hiPSCs. hESCs and hiPSCs were differentiated into CD34+CD43+ hematopoietic progenitor cells within the sac structure in the presence of VEGF. (B): Schematic diagram of the differentiation protocol used for human CB CD34+ cell-derived erythroid cell differentiation. (C): Representative flow cytometric analysis of cell surface markers (CD235a, CD43, and CD71) in hESC H1-derived and CB CD34+ cell-derived erythroid cells. Days in culture are indicated above the plots. Abbreviations: CB, cord blood; EPO, erythropoietin; hESCs, human embryonic stem cells; hiPSCs, human induced pluripotent stem cells; SCF, stem cell factor; TPO, thrombopoietin; VEGF, vascular endothelial growth factor.
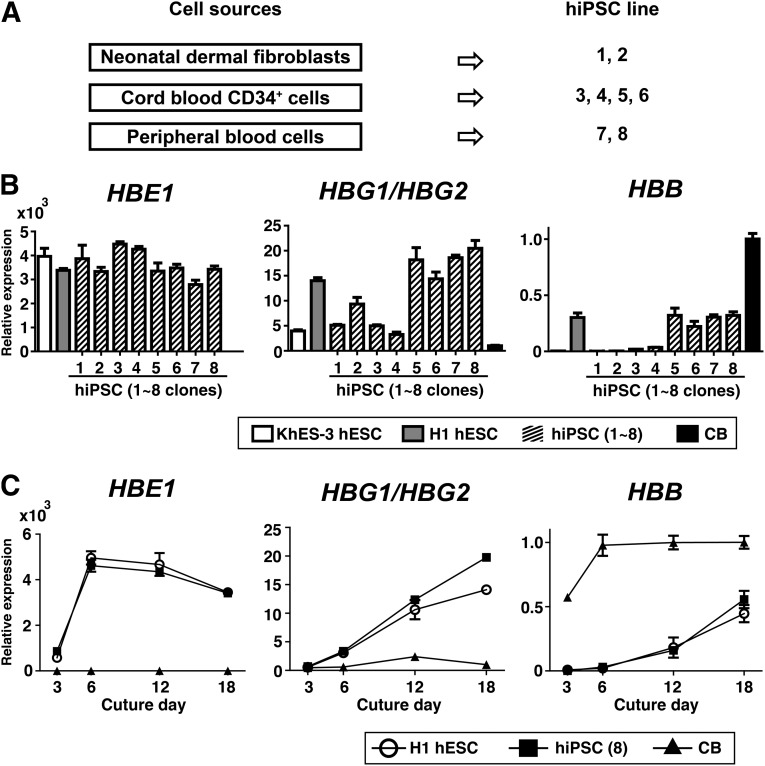
To examine globin switching during erythropoiesis in several hPSC and CB clones, we compared the mRNA levels for globin subtypes encoded in the β-globin locus (HBE1, HBG1/HBG2, or HBB). Because epigenetic memory must be taken into consideration [28, 29], we generated eight different hiPSC clones from different sources using a Sendai-viral system in which nonintegration of transgenes was validated (hiPSCs 1–8). Of these, hiPSCs 1 and 2 were generated from human neonatal dermal fibroblasts, hiPSCs 3–6 from human CB CD34+ cells, and hiPSCs 7 and 8 from human adult peripheral blood cells (Fig. 2A). As shown in Figure 2B, blood-derived hiPSC clones (i.e., hiPSCs 5, 6, 7, and 8) expressed higher levels of HBB mRNA than fibroblast-derived clones, but lower levels than were expressed by human CB CD34+ cell-derived erythroblasts on day 18 of culture. Because hESC H1- and hiPSC 8-derived erythroid cells exhibited similar upregulation of HBB, which was stronger than that seen in hESC KhES-3- or hiPSC 1–4-derived cells (Fig. 2C), we mainly used these clones in subsequent analyses of globin expression.
Figure 2.
Globin expression in erythroid cells derived from several hPSC clones or CB. (A): Cell sources for hiPSC generation using Sendai virus-mediated transduction. Cell sources are indicated on the left, and the derived clones are on the right. (B): mRNA expression of human ε-globin, γ-globin, and β-globin on day 18 of culture in erythroid cells derived from hESCs (KhES-3 or H1), hiPSCs (clones 1–8), and CB. (C): Time course of the changes in the indicated globins over the 18-day culture period (n = 3, symbols are means ± SD from three independent experiments). Abbreviations: CB, cord blood; HBB, β-globin; human HBE1, human ε-globin; HBG1/HBG2, human γ-globin; hESCs, human embryonic stem cells; hiPSCs, human induced pluripotent stem cells.
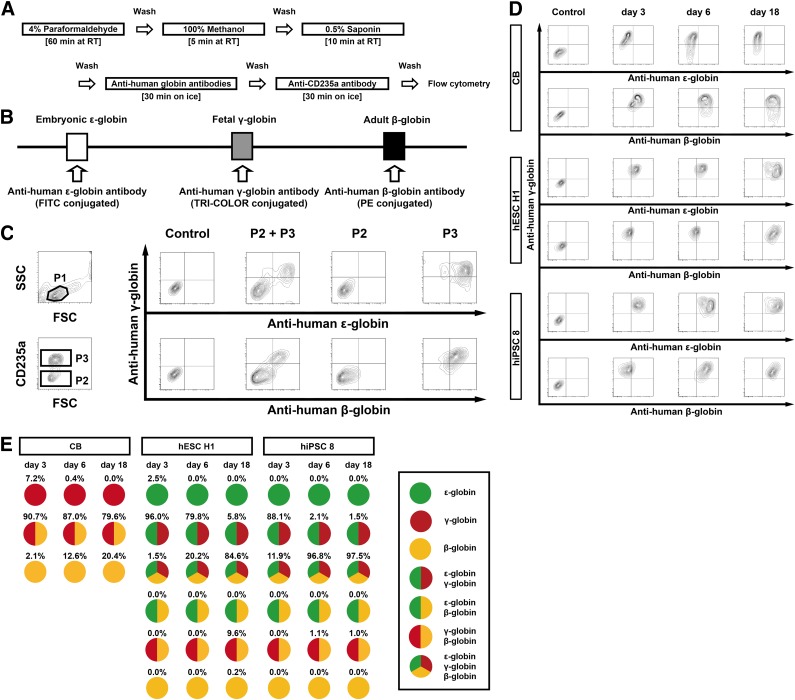
We next sought to optimize the fixation conditions so that they would enable us to trace the expression patterns of individual globin subtypes during development of hPSC-derived erythroblasts and determine the levels of each protein. As shown in Figure 3A, initial fixation with 4% PFA, followed by 100% methanol and permeabilization using 0.5% saponin were most suitable for simultaneous flow cytometric detection of individual globin subtypes as well as erythroblast surface markers (e.g., CD235a). The different intracellular globin subtypes were then labeled using FITC-, TRI-COLOR-, and PE-conjugated anti-human globin subtype antibodies (Fig. 3B). For example, cell populations detectable as side scatter (SSC) and forward scatter (FSC) on flow cytometry (P1) were subsequently selected as Pacific Blue-conjugated CD235a+ cells (P3), which were also evaluated based on the anti-human globin immunofluorescence intensities (Fig. 3C). With this methodology, we were able to distinguish different anti-human globin immunofluorescence intensities, as evaluated based on mean fluorescence intensity. We ruled out nonspecific antibody binding using Jurkat cells, hESC H1-derived erythroblasts on day 6, human CB CD34+ cell-derived erythroblasts on day 6, and fresh red blood cells (RBCs) (supplemental online Fig. 1). hESC H1-derived erythroblasts showed positivity for human ε-globin along with γ-globin or β-globin (Fig. 3D), and CB-erythroblasts (Fig. 3D) and fresh RBCs (supplemental online Fig. 1) showed profiles consistent with earlier reports [7, 30]. The results from repeated experiments using this approach are summarized in Figure 3E. Collectively, these findings indicate that on day 18 of culture most hESC H1- and hiPSC 8-derived erythroblasts simultaneously express ε-, γ-, and β-globin. However, a small population of ε-globin-negative cells was also observed, whereas few or no γ-globin-negative cells were seen (Fig. 3E). This indicates that hPSC-derived erythroblasts coexpress fetal γ- and adult β-globin. However, this experiment does not reveal why globin switching from fetal γ- to adult β-globin is impaired despite selection of good hPSC clones.
Figure 3.
Intracellular staining of globin subtypes for flow cytometric analysis. (A): Schematic diagram of the protocols used for sample preparation and intracellular staining. (B): Fluorescently labeled antibodies used in combination to detect the indicated human globin subtypes. (C): Flow cytometric data illustrating the gating strategy. To discriminate between healthy and damaged or dying cells, side scatter and forward scatter (linear) were gated as P1. Then Pacific Blue-conjugated anti-CD235a+ and CD235a− cells, detected by excitation at 350 nm, were gated as P3 and P2, respectively. In addition, FITC+ cells (ε-globin) were detected by excitation at 488 nm; TRI-COLOR+ cells (γ-globin) were detected by excitation at 633 nm; and PE+ cells (β-globin) were detected by excitation at 561 nm. As isotype controls, cells were stained with normal mouse IgG1-FITC, normal mouse IgG1-PE-Cy5, and normal mouse IgG1-PE. (D): Flow cytometric detection of anti-human ε-, γ-, and β-globin immunofluorescence in CB-, hESC H1-, and hiPSC 8-derived erythroid cells. Days in culture are indicated above the plots. (E): Pattern diagrams for cells showing anti-human globin immunofluorescence. Results were means from three independent experiments. Abbreviations: CB, cord blood; FITC, fluorescein isothiocyanate; FSC, forward scatter; hESC, human embryonic stem cell; hiPSC, human induced pluripotent stem cell; P1, gate containing most erythroid progenitor; P2; CD235a-negative gate; P3; CD235a-positive gate; PE, phycoerythrin; RT, room temperature; SSC, side scatter.
Reduced BCL11A-Mediated Silencing of γ-Globin Expression in PSC-Derived Erythroid Cells May Be Associated With Impaired Globin Switching
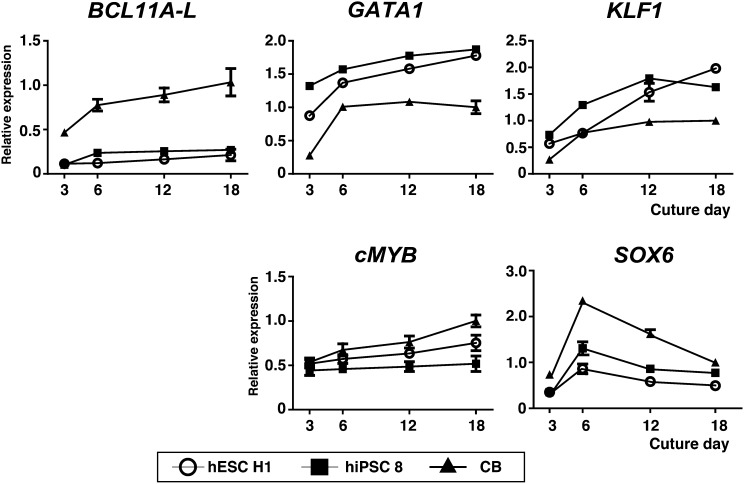
It has been postulated that BCL11A facilitates γ-globin silencing [19, 31]. In that regard, we found that the mRNA level for one BCL11A isoform, BCL11A-L, was significantly lower in hESC H1- and hiPSC 8-derived erythroid cells than in the CB-derived cells (Fig. 4). By contrast, mRNA expression of KLF1, an accelerator for β-globin upregulation [32], and its upstream molecules in hPSC-derived cells, GATA1 and cMYB, was comparable to or higher than in CB-erythroid cells (Fig. 4) [33]. In addition, we noticed that expression of SOX6, which is essential for formation of the BCL11A signaling complex [33, 34], was also lower in hPSC-derived erythroid cells (Fig. 4). With those findings in mind, we hypothesized that downregulated BCL11A-L expression was associated with the impaired γ-globin switching seen in hPSC-derived erythroid cells.
Figure 4.
Time course of endogenous expression of genes essential for globin switching. mRNA levels of BCL11A-L, GATA1, KLF1, cMYB, and SOX6 were examined in differentiated erythroid cells derived from hESC H1, hiPSC 8, and CB (n = 3, symbols are means ± SD from three independent experiments). Abbreviations: CB, cord blood; hESC, human embryonic stem cell; hiPSC, human induced pluripotent stem cell.
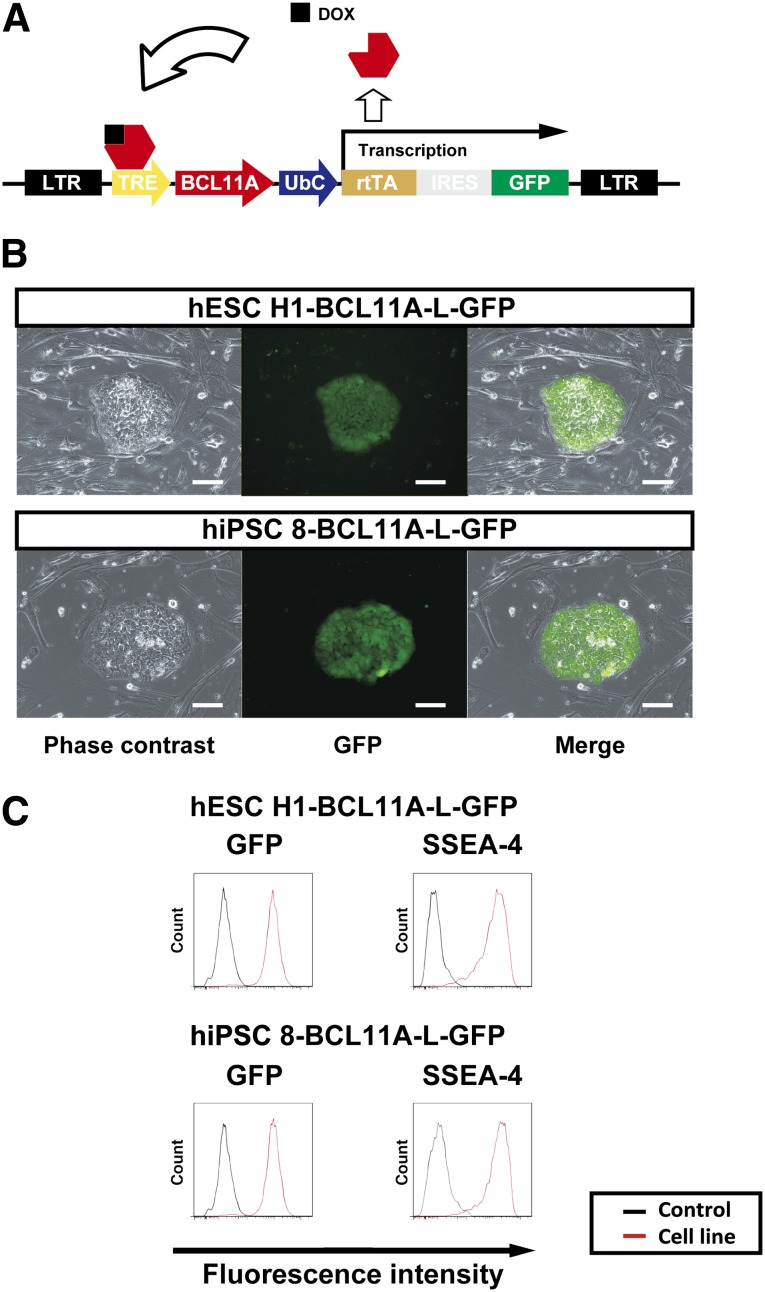
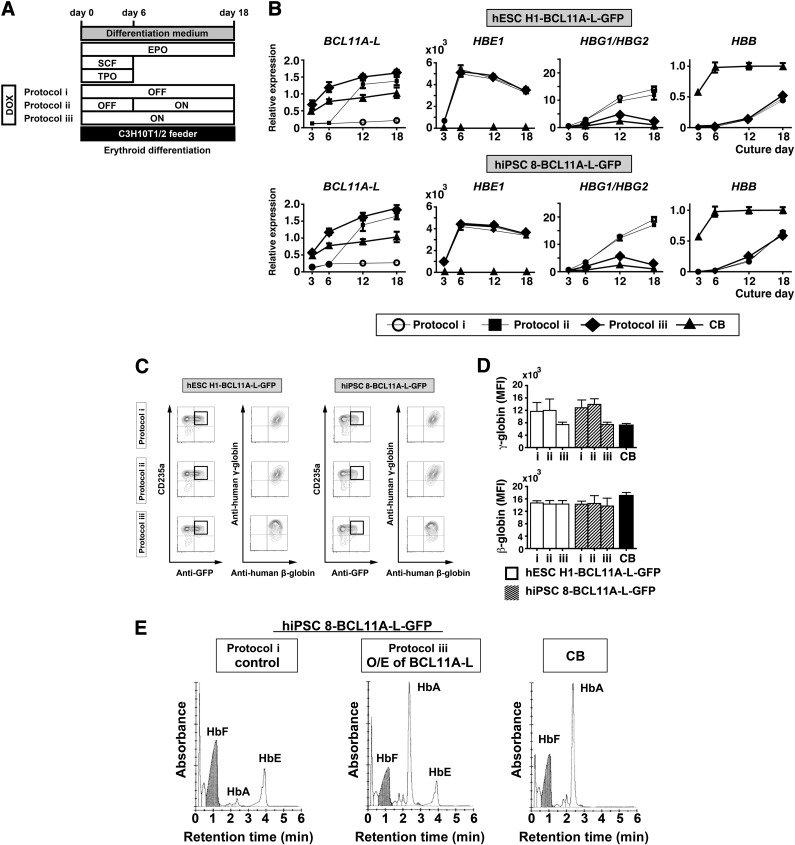
To test that idea, we used a lentiviral vector harboring a DOX-inducible overexpression system (Fig. 5A) to establish hPSC clones (hESC H1-BCL11A-L-GFP and hiPSC 8-BCL11A-L-GFP) expressing BCL11A-L plus GFP as a marker, and the pluripotent state was indicated by SSEA-4 positivity (Fig. 5B, 5C). With this system, the DOX-OFF and DOX-ON states showed no expression and overexpression of BCL11A-L plus GFP, respectively. We then tested the three protocols depicted in Figure 6A (protocols i, ii, and iii). Flow cytometric analysis showed that differentiation phase-dependent changes in BCL11A-L levels were associated with reductions in the expression of both γ-globin mRNA (Fig. 6B) and protein (Fig. 6C) in hESC H1-BCL11A-L-GFP- and hiPSC 8-BCL11A-L-GFP-derived erythroid cells. Interestingly, protocol iii induced substantial silencing of γ-globin expression without affecting β-globin expression (Fig. 6C, 6D, day 18 of culture), which is consistent with earlier reports [19, 31]. These results strongly suggest that downregulated expression of BCL11A-L and its signaling complex is associated with impaired γ-globin silencing, whereas β-globin expression is unaffected.
Figure 5.
Overexpression of BCL11A-L in hESC and hiPSC-derived erythroid cells. (A): Schematic diagram of the DOX-inducible system for expression of human BCL11A-L. (B): Photomicrographs of GFP+ hESC H1 (hESC H1-BCL11A-L-GFP) and hiPSC 8 (hiPSC 8-BCL11A-L-GFP) cells on mouse embryonic feeder cells: left, phase contrast; middle, GFP; right, merged image. Scale bars = 100 μm. (C): Both cell lines were similarly GFP+ and SSEA-4+. Abbreviations: DOX, doxycycline; GFP, green fluorescent protein; hESC, human embryonic stem cell; hiPSC, human induced pluripotent stem cell; SSEA-4, stage-specific embryonic antigen-4.
Figure 6.
Effect of BCL11A-L overexpression in hESC and hiPSC-derived erythroid cells. (A): Schematic diagram of the protocols: protocol i, no overexpression during the differentiation period; protocol ii, overexpression of BCL11A-L from day 6 to day 18; protocol iii, overexpression of BCL11A-L during the entire differentiation period. (B): Relative expression of BCL11A-L, HBE1, HBG1/HBG2, and HBB. The fold changes were calculated using the ΔΔCT method with human CB CD34+ cell-derived erythroid cells (day 18) as a calibrator. (C): Globin expression analysis in hESC H1-BCL11A-L-GFP- and hiPSC 8-BCL11A-L-GFP-derived erythroid cells after culture for 18 days under the indicated protocols. Flow cytometry and multicolor staining were used for the analysis, and CD235a+ anti-GFP+ cells were gated. (D): Summarized results showing MFI levels (n = 3). Bars are means ± SD from three independent experiments. (E): Representative chromatograms showing cation-exchange high-performance liquid chromatography analyses of hemoglobin generated in hiPSC 8-derived erythroid cells, with (protocol iii) or without (protocol i) overexpression of BCL11A-L, or in CB-derived erythroid cells. Cells were used on day 18 of culture. Abbreviations: CB, cord blood; DOX, doxycycline; GFP, green fluorescent protein; hESC, human embryonic stem cell; hiPSC, human induced pluripotent stem cell; MFI, mean fluorescence intensity; O/E, overexpression; SCF, stem cell factor; TPO, thrombopoietin.
We also conducted CE-HPLC studies to detect different hemoglobin chain types. Sorted hiPSC 8-BCL11A-L-GFP-derived CD235a+GFP+ cells (2.0 × 107 cells) generated using protocol i (DOX-OFF) or protocol iii (DOX-ON from day 0 to day 18) were evaluated on day 18 of culture. The majority of the hemoglobin in cells generated using protocol i was HbF (α2γ2) and HbE (α2ε2), although small amounts of HbA (α2β2) were also present. Sustained overexpression of BCL11A-L in protocol iii altered the hemoglobin expression pattern, as exemplified by the reduction in HbF (α2γ2) and the increase in HbA (α2β2). This profile was similar to that of CB-derived erythroblasts, except that HbE (α2ε2) was also retained in hiPSC 8-derived erythroid cells (Fig. 6E).
Discussion
The globin expression profile is a hallmark of the developmental stages of erythroblasts [7, 33]. In adults, human hemoglobin is composed of α- and β-globins. The human β-globin gene cluster contains five functional genes that are expressed in the order of their arrangement within the locus (ε-Gγ-Aγ-δ-β) on chromosome 11. In humans, these genes undergo two major transitions in expression during ontogeny; the first is the transition from embryonic (ε) to fetal (Gγ, Aγ) globins, and the second is from fetal to adult (δ, β) globins [7]. The human system does not always correspond to the murine one, however [35, 36]; consequently, tracing globin switching using hPSC-derived erythroblasts could be an effective approach to characterizing human hemoglobin ontogeny. We therefore sought to use hPSC clones (i.e., hESCs and hiPSCs) to trace the changes in the expression of β-globin during in vitro differentiation toward erythrocytes. To accomplish this, we optimized multicolor flow cytometric analysis to simultaneously follow the expression of individual globin subtypes using several immunofluorescent probes. Initially, we used human CB CD34+ cell-derived and hPSC-derived erythroid cells to determine the optimal methodology for cell fixation and the most suitable probes for detection of the protein expression of individual globin subtypes (Figs. 1–3). Most of the hES/iPSC clone-derived erythroblasts showed prominent expression of ε- and γ-globin, but little expression of β-globin, likely reflecting primitive erythropoiesis. By contrast, clones established from human blood cells (CB CD34+ cells or peripheral blood cells) were strongly positive for β-globin expression (Fig. 3D, 3E). This suggests that “epigenetic memory”-based mechanisms may influence definitive erythropoiesis, keeping it at a primitive stage [28].
We also found that the heterogeneous β-globin expression among hESC- and hiPSC-derived erythroid cells might reflect differences in the endogenous expression of BCL11A-L, the full-length form of BCL11A (Fig. 4). BCL11A is a zinc-finger transcription factor that acts as a regulator of HbF expression [31]. It is also suspected that BCL11A levels correlate with developmental stage such that high levels of γ-globin are evident in the absence of the full-length forms of BCL11A [7, 19, 30]. Abundant expression of BCL11A-L is developmentally restricted to adult erythroid cells (i.e., primary BM-erythroblasts), strongly indicating that BCL11A-L acts to repress γ-globin gene expression [19]. In the present study, γ-globin expression was silenced in human CB CD34+ cell-derived erythroid cells, but that silencing was incomplete in hES/iPSC clone-derived cells (Fig. 3E). Furthermore, overexpressed BCL11A-L in hPSC-derived erythroid cells appeared to downregulate γ-globin expression at both the mRNA (Fig. 6B) and protein (Fig. 6C, 6D) levels without affecting β-globin (Fig. 6D, left). Most interesting was the observation that continuous overexpression of BCL11A-L in hPSC-derived CD34+CD43+ multipotent progenitors selectively silenced γ-globin expression (Fig. 6D).
CE-HPLC analysis confirmed that ectopic expression of BCL11A-L in hiPSC-derived erythroid cells upregulated HbA and downregulated HbF, as compared with control cells without overexpression of BCL11A-L. The relative upregulation of HbA observed in the CE-HPLC analysis is thought to result from the downregulation of HbF. These hemoglobin composition patterns are similar to those of human CB CD34+ cell-derived erythroid cells (Fig. 6E), confirming the flow cytometry results.
This suggests that BCL11A-L must be upregulated from the CD34+CD43+ multipotent progenitor stage, as is seen in CB CD34+ cell-derived cells (Fig. 4). Based on these findings, we suggest that future identification of the mechanism underlying the reduced expression of BCL11A-L along with reduced formation of the BCL11A signaling complex [33, 34] in hPSC-derived cells will be a valuable step toward in vitro production of functional erythrocytes.
Conclusion
Our multicolor staining method used with flow cytometry enabled us to shed new light on the mechanism underlying the impaired γ-globin silencing in hPSC-derived erythroid cells. Moreover, hPSC-derived erythroid cells have the potential to provide an infinite supply of erythrocytes by using immortalized erythroblast cell lines from hPSCs [26], thereby replacing donor-dependent transfusion therapy, and our method could be useful for choosing the best clone for generating adult type erythrocytes after solution of globin switching.
Supplementary Material
Acknowledgments
We thank Drs. N. Nakatsuji and H. Suemori (Institute for Frontier Medical Sciences, Kyoto University) for providing human KhES-3 cell lines and Y. Niwa (Tosoh Co. Ltd.) for analyzing CE-HPLC data. This work was supported by Core Research for Evolutionary Science and Technology from the Japan Science and Technology Agency (JST) (to K.E.), and by grants-in-aid (Kiban B to K.E. and Scientific Research on Innovative Areas, Bio Assembler Grant to K.E. and H.N.) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (MEXT), the Project of Realization of Regenerative Medicine and Highway from MEXT/JST (to K.E.), the Initiative for Accelerating Regulatory Science in Innovative Drug, Medical Device, and Regenerative Medicine from the Ministry of Health, Labor, and Welfare (to K.E.), and from the Funding Program for World-Leading Innovative Research and Development on Science and Technology from the Japan Society for the Promotion of Science (to N.T.).
Author Contributions
K.O.: conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript; N.T.: conception and design; S.H. and H.N.: valuable discussion; T.N.: provision of study materials; K.E.: conception and design, data interpretation, manuscript writing and editing.
Disclosures of Potential Conflicts of Interest
H.N. has uncompensated intellectual property rights with the University of Tokyo, a consultant/advisory role with Megakaryon Corp., and an ownership interest with Megakaryon Corp. and Reprocell Inc. K.E. has an uncompensated consultant advisory role with Megakaryon Corp.
References
- 1.Miharada K, Hiroyama T, Sudo K, et al. Efficient enucleation of erythroblasts differentiated in vitro from hematopoietic stem and progenitor cells. Nat Biotechnol. 2006;24:1255–1256. doi: 10.1038/nbt1245. [DOI] [PubMed] [Google Scholar]
- 2.Giarratana MC, Rouard H, Dumont A, et al. Proof of principle for transfusion of in vitro-generated red blood cells. Blood. 2011;118:5071–5079. doi: 10.1182/blood-2011-06-362038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Migliaccio AR, Whitsett C, Migliaccio G. Erythroid cells in vitro: From developmental biology to blood transfusion products. Curr Opin Hematol. 2009;16:259–268. doi: 10.1097/MOH.0b013e32832bcaa2. [DOI] [PubMed] [Google Scholar]
- 4.Kobari L, Yates F, Oudrhiri N, et al. Human induced pluripotent stem cells can reach complete terminal maturation: In vivo and in vitro evidence in the erythropoietic differentiation model. Haematologica. 2012;97:1795–1803. doi: 10.3324/haematol.2011.055566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nance ST. How to find, recruit and maintain rare blood donors. Curr Opin Hematol. 2009;16:503–508. doi: 10.1097/MOH.0b013e3283316bed. [DOI] [PubMed] [Google Scholar]
- 6.Kajiwara M, Aoi T, Okita K, et al. Donor-dependent variations in hepatic differentiation from human-induced pluripotent stem cells. Proc Natl Acad Sci USA. 2012;109:12538–12543. doi: 10.1073/pnas.1209979109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sankaran VG, Xu J, Orkin SH. Advances in the understanding of haemoglobin switching. Br J Haematol. 2010;149:181–194. doi: 10.1111/j.1365-2141.2010.08105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cerdan C, Rouleau A, Bhatia M. VEGF-A165 augments erythropoietic development from human embryonic stem cells. Blood. 2004;103:2504–2512. doi: 10.1182/blood-2003-07-2563. [DOI] [PubMed] [Google Scholar]
- 9.Dias J, Gumenyuk M, Kang H, et al. Generation of red blood cells from human induced pluripotent stem cells. Stem Cells Dev. 2011;20:1639–1647. doi: 10.1089/scd.2011.0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaufman DS, Hanson ET, Lewis RL, et al. Hematopoietic colony-forming cells derived from human embryonic stem cells. Proc Natl Acad Sci USA. 2001;98:10716–10721. doi: 10.1073/pnas.191362598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klimchenko O, Mori M, Distefano A, et al. A common bipotent progenitor generates the erythroid and megakaryocyte lineages in embryonic stem cell-derived primitive hematopoiesis. Blood. 2009;114:1506–1517. doi: 10.1182/blood-2008-09-178863. [DOI] [PubMed] [Google Scholar]
- 12.Ledran MH, Krassowska A, Armstrong L, et al. Efficient hematopoietic differentiation of human embryonic stem cells on stromal cells derived from hematopoietic niches. Cell Stem Cell. 2008;3:85–98. doi: 10.1016/j.stem.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 13.Ma F, Ebihara Y, Umeda K, et al. Generation of functional erythrocytes from human embryonic stem cell-derived definitive hematopoiesis. Proc Natl Acad Sci USA. 2008;105:13087–13092. doi: 10.1073/pnas.0802220105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Migliaccio AR, Whitsett C, Papayannopoulou T, et al. The potential of stem cells as an in vitro source of red blood cells for transfusion. Cell Stem Cell. 2012;10:115–119. doi: 10.1016/j.stem.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olivier EN, Qiu C, Velho M, et al. Large-scale production of embryonic red blood cells from human embryonic stem cells. Exp Hematol. 2006;34:1635–1642. doi: 10.1016/j.exphem.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 16.Peyrard T, Bardiaux L, Krause C, et al. Banking of pluripotent adult stem cells as an unlimited source for red blood cell production: Potential applications for alloimmunized patients and rare blood challenges. Transfus Med Rev. 2011;25:206–216. doi: 10.1016/j.tmrv.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 17.Qiu C, Olivier EN, Velho M, et al. Globin switches in yolk sac-like primitive and fetal-like definitive red blood cells produced from human embryonic stem cells. Blood. 2008;111:2400–2408. doi: 10.1182/blood-2007-07-102087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang CJ, Mitra K, Koya M, et al. Production of embryonic and fetal-like red blood cells from human induced pluripotent stem cells. PLoS One. 2011;6:e25761. doi: 10.1371/journal.pone.0025761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sankaran VG, Xu J, Ragoczy T, et al. Developmental and species-divergent globin switching are driven by BCL11A. Nature. 2009;460:1093–1097. doi: 10.1038/nature08243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nishimura K, Sano M, Ohtaka M, et al. Development of defective and persistent Sendai virus vector: A unique gene delivery/expression system ideal for cell reprogramming. J Biol Chem. 2011;286:4760–4771. doi: 10.1074/jbc.M110.183780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takayama N, Nishikii H, Usui J, et al. Generation of functional platelets from human embryonic stem cells in vitro via ES-sacs, VEGF-promoted structures that concentrate hematopoietic progenitors. Blood. 2008;111:5298–5306. doi: 10.1182/blood-2007-10-117622. [DOI] [PubMed] [Google Scholar]
- 22.Takayama N, Nishimura S, Nakamura S, et al. Transient activation of c-MYC expression is critical for efficient platelet generation from human induced pluripotent stem cells. J Exp Med. 2010;207:2817–2830. doi: 10.1084/jem.20100844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nishimura T, Kaneko S, Kawana-Tachikawa A, et al. Generation of rejuvenated antigen-specific T cells by reprogramming to pluripotency and redifferentiation. Cell Stem Cell. 2013;12:114–126. doi: 10.1016/j.stem.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 24.Kobayashi T, Yamaguchi T, Hamanaka S, et al. Generation of rat pancreas in mouse by interspecific blastocyst injection of pluripotent stem cells. Cell. 2010;142:787–799. doi: 10.1016/j.cell.2010.07.039. [DOI] [PubMed] [Google Scholar]
- 25.Eto K, Nishikii H, Ogaeri T, et al. The WAVE2/Abi1 complex differentially regulates megakaryocyte development and spreading: Implications for platelet biogenesis and spreading machinery. Blood. 2007;110:3637–3647. doi: 10.1182/blood-2007-04-085860. [DOI] [PubMed] [Google Scholar]
- 26.Hirose S, Takayama N, Nakamura S, et al. Immortalization of erythroblasts by c-MYC and BCL-XL enables large-scale erythrocyte production from human pluripotent stem cells. Stem Cell Reports. 2013;1:499–508. doi: 10.1016/j.stemcr.2013.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hirata S, Takayama N, Jono-Ohnishi R, et al. Congenital amegakaryocytic thrombocytopenia iPS cells exhibit defective MPL-mediated signaling. J Clin Invest. 2013;123:3802–3814. doi: 10.1172/JCI64721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim K, Zhao R, Doi A, et al. Donor cell type can influence the epigenome and differentiation potential of human induced pluripotent stem cells. Nat Biotechnol. 2011;29:1117–1119. doi: 10.1038/nbt.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim K, Doi A, Wen B, et al. Epigenetic memory in induced pluripotent stem cells. Nature. 2010;467:285–290. doi: 10.1038/nature09342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sankaran VG, Orkin SH. The switch from fetal to adult hemoglobin. Cold Spring Harb Perspect Med. 2013;3:a011643. doi: 10.1101/cshperspect.a011643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sankaran VG, Menne TF, Xu J, et al. Human fetal hemoglobin expression is regulated by the developmental stage-specific repressor BCL11A. Science. 2008;322:1839–1842. doi: 10.1126/science.1165409. [DOI] [PubMed] [Google Scholar]
- 32.Zhou D, Liu K, Sun CW, et al. KLF1 regulates BCL11A expression and gamma- to beta-globin gene switching. Nat Genet. 2010;42:742–744. doi: 10.1038/ng.637. [DOI] [PubMed] [Google Scholar]
- 33.Bauer DE, Kamran SC, Orkin SH. Reawakening fetal hemoglobin: Prospects for new therapies for the β-globin disorders. Blood. 2012;120:2945–2953. doi: 10.1182/blood-2012-06-292078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu J, Sankaran VG, Ni M, et al. Transcriptional silencing of γ-globin by BCL11A involves long-range interactions and cooperation with SOX6. Genes Dev. 2010;24:783–798. doi: 10.1101/gad.1897310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kingsley PD, Malik J, Emerson RL, et al. “Maturational” globin switching in primary primitive erythroid cells. Blood. 2006;107:1665–1672. doi: 10.1182/blood-2005-08-3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hill A, Hardies SC, Phillips SJ, et al. Two mouse early embryonic beta-globin gene sequences. Evolution of the nonadult beta-globins. J Biol Chem. 1984;259:3739–3747. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.