Abstract
Quantitative trait locus (QTL) mapping in animal populations has been a successful strategy for identifying genomic regions that play a role in complex diseases and traits. When conducted in an F2 intercross or backcross population, the resulting QTL is frequently large, often encompassing 30 Mb or more and containing hundreds of genes. To narrow the locus and identify candidate genes, additional strategies are needed. Congenic strains have proven useful but work less well when there are multiple tightly linked loci, frequently resulting in loss of phenotype. As an alternative, we discuss the use of highly recombinant outbred models for directly fine-mapping QTL to only a few megabases. We discuss the use of several currently available models such as the advanced intercross (AI), heterogeneous stocks (HS), the diversity outbred (DO), and commercially available outbred stocks (CO). Once a QTL has been fine-mapped, founder sequence and expression QTL mapping can be used to identify candidate genes. In this regard, the large number of alleles found in outbred stocks can be leveraged to identify causative genes and variants. We end this review by discussing some important statistical considerations when analyzing outbred populations. Fine-resolution mapping in outbred models, coupled with full genome sequence, has already led to the identification of several underlying causative genes for many complex traits and diseases. These resources will likely lead to additional successes in the coming years.
Keywords: intercross, heterogeneous stock, diversity outbred, genetic mapping, high resolution mapping
quantitative trait locus (QTL) mapping in rodents has been a successful strategy for identifying regions of the genome that play a role in many diseases and traits. Initial QTL studies used F2 intercross or backcrosses, in which two strains that differ phenotypically and genetically are bred for two generations (see Fig. 1). With this strategy, F2 progeny are phenotyped and genotyped, and genetic loci linked to the trait of interest are identified. Although this method works extremely well for detecting QTL, it provides only a broad localization, with identified regions tending to be very large (30–40 Mb) and often encompassing hundreds of genes. As a result, although F2 intercrosses and backcrosses have identified thousands of QTL over the past 30 yr, they have proven less useful for identifying the underlying causative genes and variants (see Ref. 30).
Fig. 1.
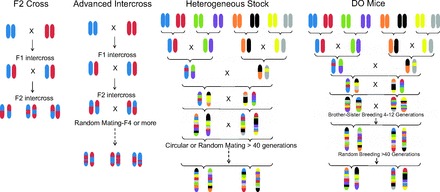
Breeding schemes for outbred populations. A: the F2 intercross in which 2 inbred strains are bred for 2 generations. B: advanced intercross (AI) populations are created by breeding 2 inbred strains together for several generations, thus creating a larger number of historical recombination events than the F2. C: heterogeneous socks (HS) are created by breeding together 8 inbred strains. Once all 8 genomes are combined, the animals are bred by either a circular strategy or random breeding. Existing HS mouse and rat colonies have been bred for >50 generations. D: the diversity outbred (DO) was created from partially inbred Collaborative Cross (CC) mice. The top of this figure depicts how the pre-CC mice were created. After 4–12 generations of inbreeding, a subset of these mice was randomly outbred to create the DO. Not depicted in this figure is that the CC are created with multiple funnels (or multiple combinations of the parental strains), thus leading to a more balanced design and greater genetic diversity.
In an attempt to narrow the QTL regions and identify causative genes, multiple methods have been developed (see Ref. 32). One popular method is to create a congenic strain in which consecutively smaller portions of the QTL from the disease strain are bred onto the genetic background of the disease resistant strain. This strategy has successfully narrowed numerous QTL and has the potential to lead to underlying causative genes (e.g., 7, 43, 70, 75). Although this strategy has several advantages, including the ability to test underlying function once a gene has been identified, this method works less well when there are multiple tightly linked QTL with potentially different effects, often resulting in loss of phenotype when the QTL is moved onto the resistant background and subsequently narrowed.
An alternative approach is to perform linkage disequilibrium mapping in highly recombinant, or outbred, populations. The purpose of the current review is to discuss how highly recombinant rodent models can be used to unravel the underlying genetic basis of complex traits. Such models are advantageous because of the segregation of multiple alleles with relatively low linkage disequilibrium, allowing fine-mapping of many traits to only a few Megabases or less. In the following review, we discuss outbred models with known ancestry such as advanced intercross (AI) populations (created by breeding two inbred strains for many generations) (19) as well as heterogeneous stocks (HS) and the diversity outbred (DO; created by breeding eight inbred strains for many generations) (80, 88). These models are based on breeding strategies as depicted in Fig. 1 and summarized in Table 1. We also discuss the use of outbreds with unknown ancestry such as commercially available outbred (CO) populations (96). We follow this with a discussion of strategies that can be used to identify candidate genes that underlie these QTL and end this review by briefly discussing some of the cautions and concerns regarding the statistical analysis in highly recombinant animal populations.
Table 1.
Comparison of available outbred models
| Model | Description | Advantages | Disadvantages |
|---|---|---|---|
| Advanced intercross (AI) | breeding of two inbred strains for many generations | • mapping resolution is a few Mb | • number of phenotypes that can be mapped is limited by founder strains |
| • parent genotypes are known | |||
| • each marker is informative | |||
| Heterogeneous stocks (HS) | breeding of eight inbred strains for many generations | • mapping resolution is few Mb | • large numbers are needed |
| • hundreds of different phenotypes can be mapped | • functional follow-up is challenging | ||
| • founder strains have been fully sequenced and can be used to identify candidate variants | • statistical challenges including diplotype substitution plots | ||
| • the HS rat may be useful for mapping traits that are hard to replicate in the mouse | |||
| Diversity outbred (DO) | breeding of eight inbred strains for many generations; founder strains are the same as those used to create the Collaborative Cross (CC); founder strains crossed multiple ways (funnels) | • mapping resolution is a few Mb and likely to improve beyond HS | • large numbers are needed• statistical challenges including diplotype substitution plots |
| • more balanced design than HS strategy because multiple funnels were used | |||
| • hundreds of different phenotypes can be mapped | |||
| • founder strains have been fully sequenced and can be used to identify candidate variants | |||
| • ability to tap into CC test of candidate genes | |||
| Commercial outbreds (CO) | outbred models sold by commercial breeders | • potential to map to gene-level resolution | • choosing the correct strain becomes important, as not all traits will map within each strain |
| • underlying genetic structure is similar to inbred strains, such that full sequence can be imputed |
WHY CONSIDER OUTBRED POPULATIONS FOR QTL MAPPING?
There are several advantages of using outbred, or highly recombinant, animal populations for the purpose of QTL mapping. One of the most obvious is the ability to map within only a few megabases or even less. In contrast to studies conducted in F2 intercross or backcrosses, in which there is 40–60 Mb between recombination events, distance between historical recombination events in outbred models is generally <5 Mb. With large sample sizes, the distance between recombination events within the population (as opposed to within an individual animal) is even smaller, leading to fine-mapping of QTL and rapidly narrowing the number of potential candidate genes in the region. Outbred populations also provide the ability to survey many different genetic combinations, thus minimizing the role of genetic background (as significant QTL are identified on a mix of genetic backgrounds). The large degree of genetic variability also results in extremely large phenotypic variability, such that outbred models can be used to fine-map hundreds of different phenotypic traits. Finally, with increased genetic and phenotypic variability, outbred populations offer a closer approximation to human populations than an F2 or backcross.
One may wonder why such animal models are necessary, particularly with the relative success of human genome-wide association studies over the past several years (91). Despite these successes, however, a large proportion of the heritable variance for most complex traits is still unknown (see Ref. 58). Although several methods are currently underway to identify the missing heritability in humans (e.g., meta-analyses to increase power, deep sequencing to identify rare variants, investigation of gene-gene and gene-environment interactions; see Ref. 38), we argue that genetic dissection of complex traits in animal models will complement the human work and aid in more quickly unraveling the genetic basis of this missing heritability (see also Ref. 66). Animal models offer important advantages over work in humans, including the ability to control both genetics and environment, investigate RNA expression levels in tissues that are not readily available in humans, and immediately test underlying function. Below, we discuss multiple outbred populations that will aid in this discovery.
ADVANCED INTERCROSS
The simplest outbred model is the AI. AI populations, first proposed by Darvasi and Soller (19), are created by breeding two inbred strains for many generations, resulting in the accumulation of many historical recombination events and allowing for much finer resolution mapping than an F2 (see Fig. 1). Studies have been conducted in populations after a moderate number of crosses such F8 (e.g., Ref. 65) or after many more crosses such as F34 (e.g., Refs. 52, 72). Specifically, the LG/J × SM/J AI population has existed for several years and has thus have been through many generations of breeding (24). Because distance between recombinant events decreases with each additional generation, populations developed from a larger number of crosses enables finer mapping resolution (see Ref. 26), with some studies achieving fine-mapping resolution <1 Mb (see Refs. 14, 52). It is also possible to integrate data from an AI with F2 data, thus achieving both power (from the F2) and mapping resolution (from the AI) (14). When analyzing AI populations, it is important to take into account the complex family relationships (14), and this can be done by using mixed modeling approaches within a recently developed R package, QTLRel (13). AI populations have been used to fine-map multiple traits including obesity (26, 64), metabolic syndrome (50), methamphetamine sensitivity (10, 14, 65), among others (e.g., Refs. 25, 42, 51, 52). When coupled with haplotype analysis of the parent strains and/or RNA expression analysis, AI populations have the potential to lead to underlying candidate genes (e.g., Ref. 25). Although there are several advantages to this relatively simple outbred model (i.e., each marker is informative, founder genotypes are known), it is limited by its ability to identify only those QTL that segregate within the two starting populations.
HETEROGENEOUS STOCKS
HS animals are similar to an AI, but instead of starting with only two inbred strains, the population is created by combining eight inbred strains and then outbreeding in a way that minimizes inbreeding (see Fig. 1). After 50 generations of outbreeding, the genetic make-up of the resulting progeny represents a random mosaic of the founding animals, with the average distance between recombination events approaching a single centimorgan (61), enabling the fine-mapping of QTL to only a few megabases. Unlike an AI, the underlying genetic architecture is more complex and the underlying ancestral haplotypes need to be determined prior to analysis. Determining this underlying structure provides increased information from what is obtained from the genotypes themselves and lends to improved genetic mapping (61). This is done by determining ancestral probabilities with a dynamic programming algorithm initially developed by Mott and colleagues (61). Regression modeling is then conducted on the underlying mosaic structure to identify QTL. Similar to AI populations, it is important to take family structure into account to avoid detecting false QTL, and this can be done either through mixed modeling (14, 46) or resample model averaging (87). Once QTL are identified, founder sequence can be used to narrow potentially causative variants (48).
HS populations were initially created as a source for selection studies and have only recently been used for QTL mapping. Initial breeding designs of HS populations were not balanced (i.e., the founder genomes were not represented equally in the resulting population), leading to certain statistical challenges as well as potential loss of alleles in advanced generations. Despite these challenges, HS mice were first used for genetic mapping in 1999 when Flint and colleagues demonstrated the ability to narrow a previously identified QTL (29) to only 0.8 cM (81). Since that time HS mice have been used to fine-map QTL for hundreds of traits to an average confidence interval of only 2.7 Mb (88). In separate studies, the HS mouse has also been used to fine-map traits such as fear (82), ethanol-induced locomotor activity (20) and arthritis (45).
In addition to the HS mice, there is also an HS rat colony. The HS rat colony (N:NIH-HS) was first established by the National Institutes of Health in 1984 to serve as a source of genetically segregating animals for both experimental and selection studies (36). Similar to the HS mouse, the HS rat has been used to map hundreds of traits to an average of 4.5 Mb, with several causal genes being identified (6). In addition to this large study, the N:NIH-HS rat colony has also been used to fine-map traits involved in fear (44) and diabetes (76, 77). Other studies show that the HS rat will be a promising model for mapping kidney-related traits (78), bone fragility (3), drug abuse behavior (71), as well as behavioral and physiological responses to stress (21, 55, 56) and to ethanol (8, 33, 79). Because of the rich history of the rat in behavioral studies, the HS rat will likely prove a useful model for genetic dissection of behaviors that are not easily modeled in the mouse (see Ref. 63). The utility of the HS rat will be enhanced by the recent availability of gene knockouts and other genetic manipulations now available in the rat (47). Full genome sequence is available for founder strains of the HS mouse (48) and HS rat (6) and will be invaluable for identifying causative genes and variants within fine-mapped QTL. Two HS mouse colonies [the Boulder HS (59) and the Northport HS (20)] and two N:NIH HS rat (one at the Medical College of Wisconsin in the U.S. and the other at the Autonomous University of Barcelona in Spain) currently exist.
Despite the clear successes of using HS mice and rats for genetic fine-mapping, one of the disadvantages of the HS strategy is that, as with an F2 intercross, each animal is genetically and phenotypically distinct. Because of this, each time a new study is started, not only does a new group of animals need to be phenotyped, but all animals also need to be fully genotyped. The highly recombinant nature of these populations requires that relatively large number of animals are needed for sufficient statistical power (see Refs. 60, 61, 89), and high-density genotyping platforms are required (94). Although 1–4,000 SNPs are sufficient in an AI (14, 65), 7,000 SNPs or more are needed for analysis in the HS (88). Because of the need for both large numbers and high-density genotyping, these studies have the potential to be both time-consuming and expensive. It is therefore beneficial to gather as much phenotype information as possible from the same group of animals, so that genotyping only needs to be done once, and this information can be used to map multiple traits (e.g., Refs. 6, 80, 88). Because of practical concerns, however, this is not always feasible. Another disadvantage of the HS strategy is that, because each animal is genetically and phenotypically distinct, it is not possible to conduct follow-up functional studies in specific animal population once a candidate region has been identified (as is possible by using, for example, congenic strains). A further disadvantage is that these populations have been created through a single funnel (i.e., combining founder genomes only once), leading to loss of certain alleles and an unbalanced representation of the founder genomes.
COLLABORATIVE CROSS AND DIVERSITY OUTBRED
In an effort to develop a lasting resource in which to model human disease, the mouse community began the process of developing what is now called the Collaborative Cross (CC), a panel of recombinant inbred lines created from eight inbred founder strains (18). Recombinant inbreds (RI) are created by combining genomes from two or more inbred strains followed by brother-sister mating of the progeny to create inbred strains with differing combinations of the parent genomes. RI strains are advantageous because once they are genotyped, this information can be used for all future mapping studies. Prior to the CC, most RI strains were created using only two founder strains. Because of this, loci generally map to relatively large regions, similar to an F2 intercross, although there are some RI strains that have been bred for several generations prior to inbreeding, leading to increased mapping resolution (see Ref. 68). Combining eight founder strains, as found in the CC, also leads to high resolution mapping. Because the CC have only been through 22 generations prior to becoming inbred, however, this resource does not tend to achieve as fine-resolution mapping as what can be accomplished in the HS or DO.
The CC was developed in an effort to not only map complex traits to relatively fine resolution but also to have a resource of inbred strains to support systems level genetic studies (85). The broad idea of this undertaking was that it would be a shared resource in which investigators from many labs would be able to study their phenotype of interest while being able to utilize the full genetic information, as well as transcriptome, proteome, and metabolome information from each strain (16). The CC is made up of five inbred strains and three wild-derived strains, such that it is the most genetically diverse mouse resource available to date. The CC was established by breeding together the eight strains and then inbreeding the G2:F1 generation. The CC have been created from multiple funnels, such that each line has relatively equal contributions from the eight founder strains (18). To demonstrate utility of this resource, the partially inbred pre-CC have been used to map multiple traits ranging from body weight (5) to influenza pathogenesis (27), among others (e.g., Refs. 9, 23, 49, 83). CC lines are being developed in three different locations: U.S. (University of North Carolina), Israel (Tel Aviv), and Australia (Perth) (18). In 2012, 42 fully inbred lines were available, and this number is expected to significantly increase in the next couple of years (93). Genetic information for each inbred line is fully available at: http://csbio.unc.edu/CCstatus/index.py. Although far fewer lines have been developed than originally proposed (see Ref. 16), this resource will be invaluable for follow-up (validation and functional studies) of QTL identified in the DO (see below).
The DO is an outbred panel created from eight inbred strains, similar to the HS. The major difference is that the DO has been created from the same eight founder strains used to create the CC (80). The DO offers several advantages over the HS including an extraordinarily high degree of genetic and phenotypic diversity as a result of the added wild-derived strains. This resource is also being maintained with 175 breeding pairs (80), as opposed to the 40–50 breeding pairs generally used to maintain the HS (94). In addition to the increased diversity that this resource provides, there is the additional advantage of being able to tap into the CC for both validation and further functional studies once a QTL has been identified. To demonstrate the utility of this resource, 150 DO from the G4 and G5 generation were used to fine-map cholesterol levels to only 2 Mb, containing only 11 genes (80). By using complete genome sequence from the founder strains combined with known founder effects at the QTL, a single candidate gene was identified. The DO have also recently been used to fine-map several behavioral traits to only 1–3 Mb, with several potential candidate genes identified (53). The DO are currently maintained at Jackson Laboratories using a randomized breeding strategy to maintain heterozygosity and control genetic drift, with the expectation that mapping resolution will improve with each generation of breeding (17). In addition to the DO, a second HS_CC outbred population has also been created from the CC founders and has been used to look at striatal gene networks (41).
COMMERCIAL OUTBRED
Outbred lines sold by commercial breeders are often maintained using thousands of lines, offering the potential to map to a much finer resolution than the HS or DO (94). Studies in outbred CD1 mice were the first to show that outbred mice exhibit low linkage disequilibrium and high heterozygosity, indicating they would be useful for genome-wide genetic mapping studies (4). Since that time, successful fine-mapping in CO has been conducted for anxiety (97), metabolic traits (99), and gene expression levels (35). To demonstrate the general utility of CO mice for genetic mapping purposes, Yalcin and colleagues (96) assessed genetic architecture in 66 available outbred mouse colonies. They found that many of the strains exhibit low linkage disequilibrium with some strains exhibiting haplotype block sizes <100 Kb, potentially enabling gene-level resolution of mapping studies. In addition, they found that the underlying genetic structure in CO mice is similar to that found in the classical inbred mouse strains, making it possible to impute full genetic sequence of the CO strains from the available sequence of classical inbred strains. As proof of principle, Yalcin et al. (96) conducted a genome-wide mapping analysis in three different outbred strains. Analysis was conducted by haplotype reconstruction, as previously described for the HS and DO populations, in addition to single marker analysis. They were able to fine-map three different traits and identified H2-Ea as the causative gene (as confirmed by complementation analysis) within a QTL identified for CD4+/CD8+ ratio in lymphocytes (96). Although this strategy holds significant promise for gene-level resolution of mapping studies, choice of strain will significantly affect whether or not a QTL is identified and at what resolution. In general, choosing a strain based on low linkage disequilibrium coupled with a high mean allele frequency is likely to provide good mapping resolution for most traits (94). As genome-wide information becomes available for more outbred strains, this information can be used to aid in choosing an outbred strain that harbors specific alleles of interest.
IDENTIFICATION OF CANDIDATE GENES AND VARIANTS UNDERLYING QTL
Outbred populations offer the ability to fine-map QTL to only a few megabases or less. As mentioned previously, successful mapping is dependent on sufficiently dense genotyping of the population. In the studies described above, various platforms have been used including the Mouse Universal Genotyping Array (MUGA), which contains 7,851 SNPs (see Ref. 80). In rat, a high-density Affymetrix single nucleotide polymorphism (SNP) genotyping array (RATDIV) containing 800K SNPs was used (6). For full utility of these outbred resources, genotyping capabilities need to continue to develop. The MegaMUGA, containing 78K SNPs, has recently been developed in mice and will prove useful for analysis of the DO population. Future studies may also implement whole genome light sequencing (see Ref. 67) or genotype by sequencing methods (see Ref. 28).
While fine-mapping to only a few Mb is a large advantage over F2 intercross or backcross strategies, mapping using outbred models frequently does not lead to single gene resolution and additional strategies are needed to identify candidate genes. In the following paragraphs, we discuss the use of founder sequence and expression QTL (eQTL) mapping for this purpose (see Fig. 2). These methods are not unique to outbred models and have been used previously to narrow candidate genes within QTL mapped using F2 intercross or backcross strategies. There are, however, several advantages when applying them to outbred crosses including 1) The QTL itself is often only a few Mb and so contains far fewer genes and 2) multiple alleles within the outbred models can be leveraged to identify candidate variants.
Fig. 2.
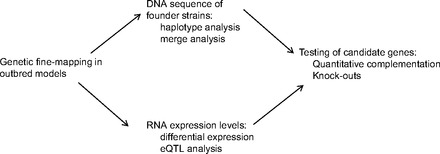
General schematic for moving from quantitative trait locus (QTL) to gene by using highly recombinant rodent populations.
To date, complete genomes have been sequenced in the founder strains of the HS and DO mice (48) as well as the HS rat (6). Relative to the respective reference genomes, >4 million SNPs per strain have been identified in the mouse (48) and >2 million SNPs per strain have been identified in the rat (6), in addition to structural variants and indels. Because a portion of the genome (∼15% in mouse and ∼12% in rat) could not be mapped back to the reference strains, the number of variants identified is likely much larger than reported (6, 48). The available sequence information can be used in several ways to identify candidate genes and/or variants within a fine-mapped QTL. First, full genome sequence of the founders allows one to conduct a haplotype analysis to identify regions in which founder sequence matches founder allele effects at a particular QTL, thereby narrowing the possible causative region within the QTL. This strategy has been used in the DO mice to identify potential causative SNPs within a 2 Mb QTL for plasma cholesterol (80), as well as in the CC to identify candidate genes for hematological parameters (49).
By coupling founder sequence with relatively dense genotyping of the outbred population, it is possible to impute HS or DO genotypes at all possible SNPs within a QTL. This can then be followed by a merge analysis to narrow the potential causative variants within the QTL (95). In brief, a merge analysis uses probabilistically inferred descent to impute genotypes at unobserved loci and then surveys those multiple imputed SNPs for their association with the phenotype. Using this method, one compares two statistical models: the haplotype model and the allelic model. In the haplotype model, the underlying ancestral probabilities at each SNP are used to run the analysis. This haplotype model is compared with one in which only the alleles for that SNP are used (allelic model). In the allelic model, the founder strain alleles are “merged” into two groups for each diallelic SNP: those containing allele “a” at a locus of interest and those containing allele “b” at this locus (95). Potentially causative variants are those in which the allelic model provides a better fit, that is, explaining the same amount but with far fewer parameters, than the haplotype model (see Refs. 45, 95). This method has proven useful in narrowing the number of causative variants within QTL mapped in HS mice (48) and rats (6). Although powerful, it is important to recognize that this strategy does not always lead to a single causative variant, and additional follow-up is often required. Once identified, potential variants can be prioritized by choosing those that fall within gene coding regions or are within regions of high conservation. Additionally, any gene that falls in close proximity to multiple candidate variants could be considered a high-priority candidate gene. Similarly, any gene that lies far away from any potential candidate variant can be ruled out as a potential candidate (6).
Another strategy that is often used to identify candidate genes is eQTL analysis, in which RNA transcript levels are used as the phenotypic trait and mapped to the genome. In such analysis, choice of tissue is an important consideration as gene expression levels may be altered only in specific tissues (69, 90). By employing eQTL analysis, one gains an understanding of how alterations in the genome affect transcript expression levels, providing further insight into how both DNA and RNA regulate disease. Through eQTL analysis both local (cis-acting) and distant (trans-acting) QTL are identified. It has been shown that identification of cis-eQTL that reside within previously identified physiological QTL can serve as a means for narrowing candidate genes that reside within the region (e.g., Refs. 2, 40, 74). Employing eQTL analysis in highly recombinant populations has the potential to significantly shorten the time-line to identify potentially causative genes, particularly because there are already far fewer genes within the physiological QTL (see Ref. 5). Using outbred models also offers the advantage being able to map with confidence to within only a few Mb of the transcript itself, as shown in HS (39) and CO mice (5, 35). This can be particularly advantageous when mapping trans-eQTL. Once identified, any correlation between the expression trait and the phenotypic trait can be determined, followed by testing for causality (i.e., Ref. 73). Although not specifically applied to outbred models, these strategies have had several successes at identifying causal genes in humans and animal models (e.g., Refs. 12, 98) and will likely be a promising avenue of research in outbred models in future studies.
PROVING CAUSATION OF CANDIDATE GENES
The strategies outlined above are often used in tandem to identify potentially causative players within a QTL. Even with the advantages of using outbred models, once a candidate gene is identified, follow-up studies are needed to confirm or disprove the role of that candidate in the trait. One of the most popular methods used is to study a knockout model. Such methods have been available in the mouse since 1990 (84) and have recently become available in the rat (34). Although popular because of the relative ease of constructing a knockout, it is important to recognize that showing a change in phenotype in a knockout model neither proves nor disproves a causative role of this gene at the QTL (30), particularly because there is no way to create a knockout on the same background in which the QTL was identified. New gene targeting approaches that allow for changes in single base pairs are now being used and offer a more realistic approach than a full gene knockout (see Ref. 1). Other methods such as quantitative complementation can also be used to test a causal role of the gene or variant. Quantitative complementation was first used in Drosophila melanogaster (54) and involves creating four sets of F1s from the following strains: an inbred strain with the susceptibility allele at the QTL, an inbred strain with a protective allele at the QTL, and two pairs of co-isogenic strains (genetic background is the same everywhere except at a single locus) that are either wild type or mutated at the suspected gene (a nice review is found in Ref. 31). The trait is tested in these four F1 strains and analyzed for an interaction (see Refs. 96, 97). Because quantitative complementation relies on obtaining co-isogenic strains, most investigators rely on testing a knockout strain for their gene of interest, at least as an initial step in determining whether or not to follow up a particular candidate. Without the use of more realistic vector-based approaches or a quantitative complementation test, however, it is important for investigators to assess all available information, including expression, sequence, results from the knockout, as well as possible in vitro, studies to assess the potential causative role of a particular gene and/or variant.
STATISTICAL ANALYSIS: CONCERNS AND CAUTIONS
Although genetic mapping in outbred models offer promise for capturing at least some of the missing heritability of complex traits, the analysis using these populations is not simple and care needs to be taken to ensure the analysis is done correctly, thereby avoiding identification of false QTL. In human studies, one of the most reliable methods is to replicate the QTL in additional studies. Because allele frequencies may vary between populations, however, a QTL may not segregate in all rodent populations and therefore may not be detected in all studies. This can occur in outbred colonies created from different founding inbred strains or in outbreds created from the same founders but maintained for many years in separate locations. Thus, care needs to be taken when comparing QTL studies across populations. In the absence of replication studies, the statistical strategies discussed below can be used to provide confidence in identified QTL.
One particularly important statistical issue is the need to address the complex family relationships of outbred models. This can be done using mixed modeling approaches such as EMMA (46), as applied in AI (14), HS (76, 77), and DO (80), or by using resample model averaging (87). QTLRel, an R package recently developed for this purpose, makes it relatively easy to account for family relationships in highly recombinant animal populations, particularly when full pedigree information is known (13). Resample model averaging approaches make use of genome-wide genotypes to determine genetic relatedness directly and may prove advantageous under certain circumstances, particularly when pedigree information is unknown (87).
In addition to the importance of taking into account family relationships, there are several other statistical concepts that investigators should consider when analyzing outbred populations. One consideration is how to determine significance thresholds. Cheng and Palmer (15) recently compared four different methods used in an AI population. They found that as long as an appropriate statistical model (i.e., one that takes into account the complex family relationships) is used, all methods worked relatively well, with gene-dropping decreasing false QTL even when family is not taken into account. Another consideration is how best to determine the confidence interval of the QTL. Many studies use the 1.5 LOD drop method (e.g., Refs. 53, 72, 77). An alternative approach is to use nonparametric bootstrapping (37), in which the QTL is re-estimated under alternative datasets based on the original, with each alternative dataset created by resampling the individuals with replacement (92). Although this method has been shown to be overly conservative (57), it does provide a complementary estimate of how sensitive the localization of the top QTL peak is to resampling, thus providing insight into whether more than one locus may underlie the QTL (see Ref. 77). Accurate determination of diplotype substitution effects is also an on-going statistical challenge in outbred populations. Several papers have looked at the effects of just the founder allele effects within the QTL (53, 80). This has been useful in conducting haplotype analysis and narrowing the region of the QTL. However, within the HS or DO populations, there are in effect 36 possible diplotype combinations, and founder effects account for only eight of these. We (77) have recently published methods that account for all 36 possible diplotype effects, and work in this area is on-going (see Ref. 22).
A final statistical concern is that of statistical power. Although previous power calculations have been run in multi-founder populations and suggest that 1,000–1,500 animals provide sufficient power for mapping QTL explaining 5% of the variance (60, 61, 89), these simulations do not account for the confounding effects of relatedness (e.g., Refs. 14, 87), or marker ascertainment (e.g., Ref. 86) and are therefore likely overstated. Previous studies in the DO have used as few as 150 mice; however, this study provided sufficient power to map only 11 of 113 traits that were measured (80). Studies in HS populations have used >1,000 animals, successfully mapping most traits analyzed (6, 88) and demonstrating the increased power of these studies. To have more accurate power estimates for future studies in these populations, power calculations will need to account for both family structure and polygenes.
CONCLUSION
This review discusses currently available outbred models for QTL mapping. Such models provide several advantages over traditional F2 intercross or backcross strategies, including increased mapping resolution and greater genetic and phenotypic variability. Upon fine-resolution mapping, haplotype analysis or a statistical merge analysis can be used to narrow the region further, and expression QTL mapping can be used to rapidly identify candidate genes. There have been several successes using these strategies. The most recent is identification of 35 causal genes for 31 traits in HS rats (6). Other successes include using AI mice to identify Cskne1 for methamphetamine sensitivity (11) and Cdh11 for regulating femoral morphology (25). HS mice have been used to identify Rgs2 for anxiety (97), and CO mice have been used to identify H2-Ea for CD4+/CD8+ ratio (96). In addition to these examples, in just the past few years, these populations have led to narrowing of candidate genes and identification of potentially causative variants in hundreds of QTL, and we expect that many of these genes will be confirmed in the next few years. Although several statistical challenges still exist (including determination of significance thresholds, confidence intervals, as well as understanding diplotype effects and determining statistical power), we believe that QTL mapping in outbred populations will provide the ability to identify at least some of the missing heritability for complex traits in humans.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: L.C.S.W. drafted manuscript; L.C.S.W. edited and revised manuscript; L.C.S.W. approved final version of manuscript.
ACKNOWLEDGMENTS
I thank Dr. William Valdar for helpful comments on a previous version of this review and for assistance in responding to reviewer comments. I also thank Katie Holl for creating Fig. 1 and Dr. Jozef Lazar for assistance in responding to a reviewer comment. Finally, I thank Dr. Jonathan Flint for introducing me to outbred animals for QTL fine-mapping.
REFERENCES
- 1.Adams DJ, van der Weyden L. Contemporary approaches for modifying the mouse genome. Physiol Genomics 34: 225–238, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aitman TJ, Glazier AM, Wallace CA, Cooper LD, Norsworthy PJ, Wahid FN, Al-Majali KM, Trembling PM, Mann CJ, Shoulders CC, Graf D, St Lezin E, Kurtz TW, Kren V, Pravenec M, Ibrahimi A, Abumrad NA, Stanton LW, Scott J. Identification of Cd36 (Fat) as an insulin-resistance gene causing defective fatty acid and glucose metabolism in hypertensive rats. Nat Genet 21: 76–83, 1999 [DOI] [PubMed] [Google Scholar]
- 3.Alam I, Koller DL, Sun Q, Roeder RK, Canete T, Blazquez G, Lopez-Aumatell R, Martinez-Membrives E, Vicens-Costa E, Mont C, Diaz S, Tobena A, Fernandez-Teruel A, Whitley A, Strid P, Diez M, Johannesson M, Flint J, Econs MJ, Turner CH, Foroud T. Heterogeneous stock rat: a unique animal model for mapping genes influencing bone fragility. Bone 48: 1169–1177, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aldinger KA, Sokoloff G, Rosenberg DM, Palmer AA, Millen KJ. Genetic variation and population substructure in outbred CD-1 mice: implications for genome-wide association studies. PLoS One 4: e4729, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aylor DL, Valdar W, Foulds-Mathes W, Buus RJ, Verdugo RA, Baric RS, Ferris MT, Frelinger JA, Heise M, Frieman MB, Gralinski LE, Bell TA, Didion JD, Hua K, Nehrenberg DL, Powell CL, Steigerwalt J, Xie Y, Kelada SN, Collins FS, Yang IV, Schwartz DA, Branstetter LA, Chesler EJ, Miller DR, Spence J, Liu EY, McMillan L, Sarkar A, Wang J, Wang W, Zhang Q, Broman KW, Korstanje R, Durrant C, Mott R, Iraqi FA, Pomp D, Threadgill D, de Villena FP, Churchill GA. Genetic analysis of complex traits in the emerging Collaborative Cross. Genome Res 21: 1213–1222, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baud A, Hermsen R, Guryev V, Stridh P, Graham D, McBride MW, Foroud T, Calderari S, Diez M, Ockinger J, Beyeen AD, Gillett A, Abdelmagid N, Guerreiro-Cacais AO, Jagodic M, Tuncel J, Norin U, Beattie E, Huynh N, Miller WH, Koller DL, Alam I, Falak S, Osborne-Pellegrin M, Martinez-Membrives E, Canete T, Blazquez G, Vicens-Costa E, Mont-Cardona C, Diaz-Moran S, Tobena A, Hummel O, Zelenika D, Saar K, Patone G, Bauerfeind A, Bihoreau MT, Heinig M, Lee YA, Rintisch C, Schulz H, Wheeler DA, Worley KC, Muzny DM, Gibbs RA, Lathrop M, Lansu N, Toonen P, Ruzius FP, de Bruijn E, Hauser H, Adams DJ, Keane T, Atanur SS, Aitman TJ, Flicek P, Malinauskas T, Jones EY, Ekman D, Lopez-Aumatell R, Dominiczak AF, Johannesson M, Holmdahl R, Olsson T, Gauguier D, Hubner N, Fernandez-Teruel A, Cuppen E, Mott R, Flint J. Combined sequence-based and genetic mapping analysis of complex traits in outbred rats. Nat Genet 45: 767–775, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhatnagar S, Oler AT, Rabaglia ME, Stapleton DS, Schueler KL, Truchan NA, Worzella SL, Stoehr JP, Clee SM, Yandell BS, Keller MP, Thurmond DC, Attie AD. Positional cloning of a type 2 diabetes quantitative trait locus; tomosyn-2, a negative regulator of insulin secretion. PLoS Genet 7: e1002323, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bice PJ, Liang T, Zhang L, Graves TJ, Carr LG, Lai D, Kimpel MW, Foroud T. Fine mapping and expression of candidate genes within the chromosome 10 QTL region of the high and low alcohol-drinking rats. Alcohol 44: 477–485, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bottomly D, Ferris MT, Aicher LD, Rosenzweig E, Whitmore A, Aylor DL, Haagmans BL, Gralinski LE, Bradel-Tretheway BG, Bryan JT, Threadgill DW, de Villena FP, Baric RS, Katze MG, Heise M, McWeeney SK. Expression quantitative trait Loci for extreme host response to influenza a in pre-collaborative cross mice. G3 (Bethesda) 2: 213–221, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bryant CD, Kole LA, Guido MA, Cheng R, Palmer AA. Methamphetamine-induced conditioned place preference in LG/J and SM/J mouse strains and an F45/F46 advanced intercross line. Front Genet 3: 126, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bryant CD, Parker CC, Zhou L, Olker C, Chandrasekaran RY, Wager TT, Bolivar VJ, Loudon AS, Vitaterna MH, Turek FW, Palmer AA. Csnk1e is a genetic regulator of sensitivity to psychostimulants and opioids. Neuropsychopharmacology 37: 1026–1035, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen Y, Zhu J, Lum PY, Yang X, Pinto S, MacNeil DJ, Zhang C, Lamb J, Edwards S, Sieberts SK, Leonardson A, Castellini LW, Wang S, Champy MF, Zhang B, Emilsson V, Doss S, Ghazalpour A, Horvath S, Drake TA, Lusis AJ, Schadt EE. Variations in DNA elucidate molecular networks that cause disease. Nature 452: 429–435, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng R, Abney M, Palmer AA, Skol AD. QTLRel: an R package for genome-wide association studies in which relatedness is a concern. BMC Genet 12: 66, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng R, Lim JE, Samocha KE, Sokoloff G, Abney M, Skol AD, Palmer AA. Genome-wide association studies and the problem of relatedness among advanced intercross lines and other highly recombinant populations. Genetics 185: 1033–1044, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng R, Palmer AA. A simulation study of permutation, bootstrap, and gene dropping for assessing statistical significance in the case of unequal relatedness. Genetics 193: 1015–1018, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Churchill GA, Airey DC, Allayee H, Angel JM, Attie AD, Beatty J, Beavis WD, Belknap JK, Bennett B, Berrettini W, Bleich A, Bogue M, Broman KW, Buck KJ, Buckler E, Burmeister M, Chesler EJ, Cheverud JM, Clapcote S, Cook MN, Cox RD, Crabbe JC, Crusio WE, Darvasi A, Deschepper CF, Doerge RW, Farber CR, Forejt J, Gaile D, Garlow SJ, Geiger H, Gershenfeld H, Gordon T, Gu J, Gu W, de Haan G, Hayes NL, Heller C, Himmelbauer H, Hitzemann R, Hunter K, Hsu HC, Iraqi FA, Ivandic B, Jacob HJ, Jansen RC, Jepsen KJ, Johnson DK, Johnson TE, Kempermann G, Kendziorski C, Kotb M, Kooy RF, Llamas B, Lammert F, Lassalle JM, Lowenstein PR, Lu L, Lusis A, Manly KF, Marcucio R, Matthews D, Medrano JF, Miller DR, Mittleman G, Mock BA, Mogil JS, Montagutelli X, Morahan G, Morris DG, Mott R, Nadeau JH, Nagase H, Nowakowski RS, O'Hara BF, Osadchuk AV, Page GP, Paigen B, Paigen K, Palmer AA, Pan HJ, Peltonen-Palotie L, Peirce J, Pomp D, Pravenec M, Prows DR, Qi Z, Reeves RH, Roder J, Rosen GD, Schadt EE, Schalkwyk LC, Seltzer Z, Shimomura K, Shou S, Sillanpaa MJ, Siracusa LD, Snoeck HW, Spearow JL, Svenson K, Tarantino LM, Threadgill D, Toth LA, Valdar W, de Villena FP, Warden C, Whatley S, Williams RW, Wiltshire T, Yi N, Zhang D, Zhang M, Zou F. The Collaborative Cross, a community resource for the genetic analysis of complex traits. Nat Genet 36: 1133–1137, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Churchill GA, Gatti DM, Munger SC, Svenson KL. The Diversity Outbred mouse population. Mamm Genome 23: 713–718, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Consortium CC. The genome architecture of the Collaborative Cross mouse genetic reference population. Genetics 190: 389–401, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Darvasi A, Soller M. Advanced intercross lines, an experimental population for fine genetic mapping. Genetics 141: 1199–1207, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Demarest K, Koyner J, McCaughran J, Jr, Cipp L, Hitzemann R. Further characterization and high-resolution mapping of quantitative trait loci for ethanol-induced locomotor activity. Behav Genet 31: 79–91, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Diaz-Moran S, Palencia M, Mont-Cardona C, Canete T, Blazquez G, Martinez-Membrives E, Lopez-Aumatell R, Tobena A, Fernandez-Teruel A. Coping style and stress hormone responses in genetically heterogeneous rats: comparison with the Roman rat strains. Behav Brain Res 228: 203–210, 2012 [DOI] [PubMed] [Google Scholar]
- 22.Durrant C, Mott R. Bayesian quantitative trait locus mapping using inferred haplotypes. Genetics 184: 839–852, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Durrant C, Tayem H, Yalcin B, Cleak J, Goodstadt L, de Villena FP, Mott R, Iraqi FA. Collaborative Cross mice and their power to map host susceptibility to Aspergillus fumigatus infection. Genome Res 21: 1239–1248, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ehrich TH, Hrbek T, Kenney-Hunt JP, Pletscher LS, Wang B, Semenkovich CF, Cheverud JM. Fine-mapping gene-by-diet interactions on chromosome 13 in a LG/J × SM/J murine model of obesity. Diabetes 54: 1863–1872, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Farber CR, Kelly SA, Baruch E, Yu D, Hua K, Nehrenberg DL, de Villena FP, Buus RJ, Garland T, Jr, Pomp D. Identification of quantitative trait loci influencing skeletal architecture in mice: emergence of Cdh11 as a primary candidate gene regulating femoral morphology. J Bone Miner Res 26: 2174–2183, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fawcett GL, Jarvis JP, Roseman CC, Wang B, Wolf JB, Cheverud JM. Fine-mapping of obesity-related quantitative trait loci in an F9/10 advanced intercross line. Obesity (Silver Spring) 18: 1383–1392, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferris MT, Aylor DL, Bottomly D, Whitmore AC, Aicher LD, Bell TA, Bradel-Tretheway B, Bryan JT, Buus RJ, Gralinski LE, Haagmans BL, McMillan L, Miller DR, Rosenzweig E, Valdar W, Wang J, Churchill GA, Threadgill DW, McWeeney SK, Katze MG, Pardo-Manuel de Villena F, Baric RS, Heise MT. Modeling host genetic regulation of influenza pathogenesis in the collaborative cross. PLoS Pathog 9: e1003196, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fitzpatrick CJ, Gopalakrishnan S, Cogan ES, Yager LM, Meyer PJ, Lovic V, Saunders BT, Parker CC, Gonzales NM, Aryee E, Flagel SB, Palmer AA, Robinson TE, Morrow JD. Variation in the form of Pavlovian conditioned approach behavior among outbred male Sprague-Dawley rats from different vendors and colonies: sign-tracking vs. goal-tracking. PLoS One 8: e75042, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Flint J, Corley R, DeFries JC, Fulker DW, Gray JA, Miller S, Collins AC. A simple genetic basis for a complex psychological trait in laboratory mice. Science 269: 1432–1435, 1995 [DOI] [PubMed] [Google Scholar]
- 30.Flint J, Eskin E. Genome-wide association studies in mice. Nat Rev Genet 13: 807–817, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Flint J, Mott R. Finding the molecular basis of quantitative traits: successes and pitfalls. Nat Rev Genet 2: 437–445, 2001 [DOI] [PubMed] [Google Scholar]
- 32.Flint J, Valdar W, Shifman S, Mott R. Strategies for mapping and cloning quantitative trait genes in rodents. Nat Rev Genet 6: 271–286, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Foroud T, Bice P, Castelluccio P, Bo R, Miller L, Ritchotte A, Lumeng L, Li TK, Carr LG. Identification of quantitative trait loci influencing alcohol consumption in the high alcohol drinking and low alcohol drinking rat lines. Behav Genet 30: 131–140, 2000 [DOI] [PubMed] [Google Scholar]
- 34.Geurts AM, Cost GJ, Freyvert Y, Zeitler B, Miller JC, Choi VM, Jenkins SS, Wood A, Cui X, Meng X, Vincent A, Lam S, Michalkiewicz M, Schilling R, Foeckler J, Kalloway S, Weiler H, Menoret S, Anegon I, Davis GD, Zhang L, Rebar EJ, Gregory PD, Urnov FD, Jacob HJ, Buelow R. Knockout rats via embryo microinjection of zinc-finger nucleases. Science 325: 433, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ghazalpour A, Doss S, Kang H, Farber C, Wen PZ, Brozell A, Castellanos R, Eskin E, Smith DJ, Drake TA, Lusis AJ. High-resolution mapping of gene expression using association in an outbred mouse stock. PLoS Genet 4: e1000149, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hansen C, Spuhler K. Development of the National Institutes of Health genetically heterogeneous rat stock. Alcohol Clin Exp Res 8: 477–479, 1984 [DOI] [PubMed] [Google Scholar]
- 37.Hastie T, Tibshirani R, Friedman J. The Elements of Statistical Learning: Data Mining, Inference, and Prediction (2nd ed.). New York: Springer, 2009 [Google Scholar]
- 38.Hu X, Daly M. What have we learned from six years of GWAS in autoimmune diseases, and what is next? Curr Opin Immunol 24: 571–575, 2012 [DOI] [PubMed] [Google Scholar]
- 39.Huang GJ, Shifman S, Valdar W, Johannesson M, Yalcin B, Taylor MS, Taylor JM, Mott R, Flint J. High resolution mapping of expression QTLs in heterogeneous stock mice in multiple tissues. Genome Res 19: 1133–1140, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hubner N, Wallace CA, Zimdahl H, Petretto E, Schulz H, Maciver F, Mueller M, Hummel O, Monti J, Zidek V, Musilova A, Kren V, Causton H, Game L, Born G, Schmidt S, Muller A, Cook SA, Kurtz TW, Whittaker J, Pravenec M, Aitman TJ. Integrated transcriptional profiling and linkage analysis for identification of genes underlying disease. Nat Genet 37: 243–253, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Iancu OD, Darakjian P, Walter NA, Malmanger B, Oberbeck D, Belknap J, McWeeney S, Hitzemann R. Genetic diversity and striatal gene networks: focus on the heterogeneous stock-collaborative cross (HS-CC) mouse. BMC Genom 11: 585, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jarvis JP, Cheverud JM. Mapping the epistatic network underlying murine reproductive fatpad variation. Genetics 187: 597–610, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Joe B, Saad Y, Dhindaw S, Lee NH, Frank BC, Achinike OH, Luu TV, Gopalakrishnan K, Toland EJ, Farms P, Yerga-Woolwine S, Manickavasagam E, Rapp JP, Garrett MR, Coe D, Apte SS, Rankinen T, Perusse L, Ehret GB, Ganesh SK, Cooper RS, O'Connor A, Rice T, Weder AB, Chakravarti A, Rao DC, Bouchard C. Positional identification of variants of Adamts16 linked to inherited hypertension. Hum Mol Genet 18: 2825–2838, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Johannesson M, Lopez-Aumatell R, Stridh P, Diez M, Tuncel J, Blazquez G, Martinez-Membrives E, Canete T, Vicens-Costa E, Graham D, Copley RR, Hernandez-Pliego P, Beyeen AD, Ockinger J, Fernandez-Santamaria C, Gulko PS, Brenner M, Tobena A, Guitart-Masip M, Gimenez-Llort L, Dominiczak A, Holmdahl R, Gauguier D, Olsson T, Mott R, Valdar W, Redei EE, Fernandez-Teruel A, Flint J. A resource for the simultaneous high-resolution mapping of multiple quantitative trait loci in rats: the NIH heterogeneous stock. Genome Res 19: 150–158, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Johnsen AK, Valdar W, Golden L, Ortiz-Lopez A, Hitzemann R, Flint J, Mathis D, Benoist C. Genome-wide and species-wide dissection of the genetics of arthritis severity in heterogeneous stock mice. Arthritis Rheum 63: 2630–2640, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kang HM, Zaitlen NA, Wade CM, Kirby A, Heckerman D, Daly MJ, Eskin E. Efficient control of population structure in model organism association mapping. Genetics 178: 1709–1723, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Katter K, Geurts AM, Hoffmann O, Mates L, Landa V, Hiripi L, Moreno C, Lazar J, Bashir S, Zidek V, Popova E, Jerchow B, Becker K, Devaraj A, Walter I, Grzybowksi M, Corbett M, Filho AR, Hodges MR, Bader M, Ivics Z, Jacob HJ, Pravenec M, Bosze Z, Rulicke T, Izsvak Z. Transposon-mediated transgenesis, transgenic rescue, and tissue-specific gene expression in rodents and rabbits. FASEB J 27: 930–941, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Keane TM, Goodstadt L, Danecek P, White MA, Wong K, Yalcin B, Heger A, Agam A, Slater G, Goodson M, Furlotte NA, Eskin E, Nellaker C, Whitley H, Cleak J, Janowitz D, Hernandez-Pliego P, Edwards A, Belgard TG, Oliver PL, McIntyre RE, Bhomra A, Nicod J, Gan X, Yuan W, van der Weyden L, Steward CA, Bala S, Stalker J, Mott R, Durbin R, Jackson IJ, Czechanski A, Guerra-Assuncao JA, Donahue LR, Reinholdt LG, Payseur BA, Ponting CP, Birney E, Flint J, Adams DJ. Mouse genomic variation and its effect on phenotypes and gene regulation. Nature 477: 289–294, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kelada SN, Aylor DL, Peck BC, Ryan JF, Tavarez U, Buus RJ, Miller DR, Chesler EJ, Threadgill DW, Churchill GA, Pardo-Manuel de Villena F, Collins FS. Genetic analysis of hematological parameters in incipient lines of the collaborative cross. G3 (Bethesda) 2: 157–165, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lawson HA, Cady JE, Partridge C, Wolf JB, Semenkovich CF, Cheverud JM. Genetic effects at pleiotropic loci are context-dependent with consequences for the maintenance of genetic variation in populations. PLoS Genet 7: e1002256, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lawson HA, Lee A, Fawcett GL, Wang B, Pletscher LS, Maxwell TJ, Ehrich TH, Kenney-Hunt JP, Wolf JB, Semenkovich CF, Cheverud JM. The importance of context to the genetic architecture of diabetes-related traits is revealed in a genome-wide scan of a LG/J × SM/J murine model. Mamm Genome 22: 197–208, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lionikas A, Cheng R, Lim JE, Palmer AA, Blizard DA. Fine-mapping of muscle weight QTL in LG/J and SM/J intercrosses. Physiol Genomics 42A: 33–38, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Logan RW, Robledo RF, Recla JM, Philip VM, Bubier JA, Jay JJ, Harwood C, Wilcox T, Gatti DM, Bult CJ, Churchill GA, Chesler EJ. High-precision genetic mapping of behavioral traits in the diversity outbred mouse population. Genes Brain Behav 12: 424–437, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Long AD, Mullaney SL, Mackay TF, Langley CH. Genetic interactions between naturally occurring alleles at quantitative trait loci and mutant alleles at candidate loci affecting bristle number in Drosophila melanogaster. Genetics 144: 1497–1510, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lopez-Aumatell R, Guitart-Masip M, Vicens-Costa E, Gimenez-Llort L, Valdar W, Johannesson M, Flint J, Tobena A, Fernandez-Teruel A. Fearfulness in a large N/Nih genetically heterogeneous rat stock: differential profiles of timidity and defensive flight in males and females. Behav Brain Res 188: 41–55, 2008 [DOI] [PubMed] [Google Scholar]
- 56.Lopez-Aumatell R, Vicens-Costa E, Guitart-Masip M, Martinez-Membrives E, Valdar W, Johannesson M, Canete T, Blazquez G, Driscoll P, Flint J, Tobena A, Fernandez-Teruel A. Unlearned anxiety predicts learned fear: a comparison among heterogeneous rats and the Roman rat strains. Behav Brain Res 202: 92–101, 2009 [DOI] [PubMed] [Google Scholar]
- 57.Manichaikul A, Dupuis J, Sen S, Broman KW. Poor performance of bootstrap confidence intervals for the location of a quantitative trait locus. Genetics 174: 481–489, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Manolio TA, Collins FS, Cox NJ, Goldstein DB, Hindorff LA, Hunter DJ, McCarthy MI, Ramos EM, Cardon LR, Chakravarti A, Cho JH, Guttmacher AE, Kong A, Kruglyak L, Mardis E, Rotimi CN, Slatkin M, Valle D, Whittemore AS, Boehnke M, Clark AG, Eichler EE, Gibson G, Haines JL, Mackay TF, McCarroll SA, Visscher PM. Finding the missing heritability of complex diseases. Nature 461: 747–753, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McClearn GE, Wilson JR, Meredith W. The use of isogenic and heterogenic mouse stocks in behavioral research. In: Contributions to Behavior-Genetic Analysis: the Mouse as a Prototype, edited by Lindzey G, Thiessen D. New York: Appleton Century Crofts, 1970, p. 3–22 [Google Scholar]
- 60.Mott R, Flint J. Simultaneous detection and fine mapping of quantitative trait loci in mice using heterogeneous stocks. Genetics 160: 1609–1618, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mott R, Talbot CJ, Turri MG, Collins AC, Flint J. A method for fine mapping quantitative trait loci in outbred animal stocks. Proc Natl Acad Sci USA 97: 12649–12654, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Parker CC, Chen H, Flagel SB, Geurts AM, Richards JB, Robinson TE, Solberg Woods LC, Palmer AA. Rats are the smart choice: rationale for a renewed focus on rats in behavioral genetics. Neuropharmacology 76B: 250–258 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Parker CC, Cheng R, Sokoloff G, Lim JE, Skol AD, Abney M, Palmer AA. Fine-mapping alleles for body weight in LG/J × SM/J F(2) and F(34) advanced intercross lines. Mamm Genome 22: 563–571, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Parker CC, Cheng R, Sokoloff G, Palmer AA. Genome-wide association for methamphetamine sensitivity in an advanced intercross mouse line. Genes Brain Behav 11: 52–61, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Parker CC, Palmer AA. Dark matter: are mice the solution to missing heritability? Front Genet 2: 32, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pasaniuc B, Rohland N, McLaren PJ, Garimella K, Zaitlen N, Li H, Gupta N, Neale BM, Daly MJ, Sklar P, Sullivan PF, Bergen S, Moran JL, Hultman CM, Lichtenstein P, Magnusson P, Purcell SM, Haas DW, Liang L, Sunyaev S, Patterson N, de Bakker PI, Reich D, Price AL. Extremely low-coverage sequencing and imputation increases power for genome-wide association studies. Nat Genet 44: 631–635, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Peirce JL, Lu L, Gu J, Silver LM, Williams RW. A new set of BXD recombinant inbred lines from advanced intercross populations in mice. BMC Genet 5: 7, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Petretto E, Mangion J, Dickens NJ, Cook SA, Kumaran MK, Lu H, Fischer J, Maatz H, Kren V, Pravenec M, Hubner N, Aitman TJ. Heritability and tissue specificity of expression quantitative trait loci. PLoS Genet 2: e172, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rangel-Filho A, Sharma M, Datta YH, Moreno C, Roman RJ, Iwamoto Y, Provoost AP, Lazar J, Jacob HJ. RF-2 gene modulates proteinuria and albuminuria independently of changes in glomerular permeability in the fawn-hooded hypertensive rat. J Am Soc Nephrol 16: 852–856, 2005 [DOI] [PubMed] [Google Scholar]
- 71.Richards JB, Lloyd DR, Kuehlewind B, Militello L, Paredez M, Solberg Woods L, Palmer AA. Strong genetic influences on measures of behavioral-regulation among inbred rat strains. Genes Brain Behav 12: 490–502, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Samocha KE, Lim JE, Cheng R, Sokoloff G, Palmer AA. Fine mapping of QTL for prepulse inhibition in LG/J and SM/J mice using F(2) and advanced intercross lines. Genes Brain Behav 9: 759–767, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schadt EE, Lamb J, Yang X, Zhu J, Edwards S, Guhathakurta D, Sieberts SK, Monks S, Reitman M, Zhang C, Lum PY, Leonardson A, Thieringer R, Metzger JM, Yang L, Castle J, Zhu H, Kash SF, Drake TA, Sachs A, Lusis AJ. An integrative genomics approach to infer causal associations between gene expression and disease. Nat Genet 37: 710–717, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schadt EE, Monks SA, Drake TA, Lusis AJ, Che N, Colinayo V, Ruff TG, Milligan SB, Lamb JR, Cavet G, Linsley PS, Mao M, Stoughton RB, Friend SH. Genetics of gene expression surveyed in maize, mouse and man. Nature 422: 297–302, 2003 [DOI] [PubMed] [Google Scholar]
- 75.Shirley RL, Walter NA, Reilly MT, Fehr C, Buck KJ. Mpdz is a quantitative trait gene for drug withdrawal seizures. Nat Neurosci 7: 699–700, 2004 [DOI] [PubMed] [Google Scholar]
- 76.Solberg Woods LC, Holl K, Tschannen M, Valdar W. Fine-mapping a locus for glucose tolerance using heterogeneous stock rats. Physiol Genomics 41: 102–108, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Solberg Woods LC, Holl KL, Oreper D, Xie Y, Tsaih SW, Valdar W. Fine-mapping diabetes-related traits, including insulin resistance, in heterogeneous stock rats. Physiol Genomics 44: 1013–1026, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Solberg Woods LC, Stelloh C, Regner KR, Schwabe T, Eisenhauer J, Garrett MR. Heterogeneous stock rats: a new model to study the genetics of renal phenotypes. Am J Physiol Renal Physiol 298: F1484–F1491, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Spuhler K, Deitrich RA. Correlative analysis of ethanol-related phenotypes in rat inbred strains. Alcohol Clin Exp Res 8: 480–484, 1984 [DOI] [PubMed] [Google Scholar]
- 80.Svenson KL, Gatti DM, Valdar W, Welsh CE, Cheng R, Chesler EJ, Palmer AA, McMillan L, Churchill GA. High-resolution genetic mapping using the Mouse Diversity outbred population. Genetics 190: 437–447, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Talbot CJ, Nicod A, Cherny SS, Fulker DW, Collins AC, Flint J. High-resolution mapping of quantitative trait loci in outbred mice. Nat Genet 21: 305–308, 1999 [DOI] [PubMed] [Google Scholar]
- 82.Talbot CJ, Radcliffe RA, Fullerton J, Hitzemann R, Wehner JM, Flint J. Fine scale mapping of a genetic locus for conditioned fear. Mamm Genome 14: 223–230, 2003 [DOI] [PubMed] [Google Scholar]
- 83.Thaisz J, Tsaih SW, Feng M, Philip VM, Zhang Y, Yanas L, Sheehan S, Xu L, Miller DR, Paigen B, Chesler EJ, Churchill GA, Dipetrillo K. Genetic analysis of albuminuria in collaborative cross and multiple mouse intercross populations. Am J Physiol Renal Physiol 303: F972–F981, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Thomas KR, Capecchi MR. Targeted disruption of the murine int-1 proto-oncogene resulting in severe abnormalities in midbrain and cerebellar development. Nature 346: 847–850, 1990 [DOI] [PubMed] [Google Scholar]
- 85.Threadgill DW, Churchill GA. Ten years of the collaborative cross. G3 (Bethesda) 2: 153–156, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Valdar W, Flint J, Mott R. Simulating the collaborative cross: power of quantitative trait loci detection and mapping resolution in large sets of recombinant inbred strains of mice. Genetics 172: 1783–1797, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Valdar W, Holmes CC, Mott R, Flint J. Mapping in structured populations by resample model averaging. Genetics 182: 1263–1277, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Valdar W, Solberg LC, Gauguier D, Burnett S, Klenerman P, Cookson WO, Taylor MS, Rawlins JN, Mott R, Flint J. Genome-wide genetic association of complex traits in heterogeneous stock mice. Nat Genet 38: 879–887, 2006 [DOI] [PubMed] [Google Scholar]
- 89.Valdar WS, Flint J, Mott R. QTL fine-mapping with recombinant-inbred heterogeneous stocks and in vitro heterogeneous stocks. Mamm Genome 14: 830–838, 2003 [DOI] [PubMed] [Google Scholar]
- 90.van Nas A, Ingram-Drake L, Sinsheimer JS, Wang SS, Schadt EE, Drake T, Lusis AJ. Expression quantitative trait loci: replication, tissue- and sex-specificity in mice. Genetics 185: 1059–1068, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Visscher PM, Brown MA, McCarthy MI, Yang J. Five years of GWAS discovery. Am J Hum Genet 90: 7–24, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Visscher PM, Thompson R, Haley CS. Confidence intervals in QTL mapping by bootstrapping. Genetics 143: 1013–1020, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Welsh CE, Miller DR, Manly KF, Wang J, McMillan L, Morahan G, Mott R, Iraqi FA, Threadgill DW, de Villena FP. Status and access to the Collaborative Cross population. Mamm Genome 23: 706–712, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yalcin B, Flint J. Association studies in outbred mice in a new era of full-genome sequencing. Mamm Genome 23: 719–726, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yalcin B, Flint J, Mott R. Using progenitor strain information to identify quantitative trait nucleotides in outbred mice. Genetics 171: 673–681, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yalcin B, Nicod J, Bhomra A, Davidson S, Cleak J, Farinelli L, Osteras M, Whitley A, Yuan W, Gan X, Goodson M, Klenerman P, Satpathy A, Mathis D, Benoist C, Adams DJ, Mott R, Flint J. Commercially available outbred mice for genome-wide association studies. PLoS Genet 6: e1001085, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yalcin B, Willis-Owen SA, Fullerton J, Meesaq A, Deacon RM, Rawlins JN, Copley RR, Morris AP, Flint J, Mott R. Genetic dissection of a behavioral quantitative trait locus shows that Rgs2 modulates anxiety in mice. Nat Genet 36: 1197–1202, 2004 [DOI] [PubMed] [Google Scholar]
- 98.Yang X, Deignan JL, Qi H, Zhu J, Qian S, Zhong J, Torosyan G, Majid S, Falkard B, Kleinhanz RR, Karlsson J, Castellani LW, Mumick S, Wang K, Xie T, Coon M, Zhang C, Estrada-Smith D, Farber CR, Wang SS, van Nas A, Ghazalpour A, Zhang B, Macneil DJ, Lamb JR, Dipple KM, Reitman ML, Mehrabian M, Lum PY, Schadt EE, Lusis AJ, Drake TA. Validation of candidate causal genes for obesity that affect shared metabolic pathways and networks. Nat Genet 41: 415–423, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhang W, Korstanje R, Thaisz J, Staedtler F, Harttman N, Xu L, Feng M, Yanas L, Yang H, Valdar W, Churchill GA, Dipetrillo K. Genome-wide association mapping of quantitative traits in outbred mice. G3 (Bethesda) 2: 167–174, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]


