Abstract
This review attempts to show that there may be a relationship between inflammatory processes induced by chronic overstimulation of the renin-angiotensin system (RAS) and the worldwide deficiency of vitamin D (VitD) and that both disorders are probably associated with environmental factors. Low VitD levels represent a risk factor for several apparently different diseases, such as infectious, autoimmune, neurodegenerative, and cardiovascular diseases, as well as diabetes, osteoporosis, and cancer. Moreover, VitD insufficiency seems to predispose to hypertension, metabolic syndrome, left ventricular hypertrophy, heart failure, and chronic vascular inflammation. On the other hand, inappropriate stimulation of the RAS has also been associated with the pathogenesis of hypertension, heart attack, stroke, and hypertrophy of the left ventricle and vascular smooth muscle cells. Because VitD receptors (VDRs) and RAS receptors are almost distributed in the same tissues, a possible link between VitD and the RAS is even more plausible. Furthermore, from an evolutionary point of view, both systems were developed simultaneously, actively participating in the regulation of inflammatory and immunological mechanisms. Changes in RAS activity and activation of the VDR seem to be inversely related; thus any changes in one of these systems would have a completely opposite effect on the other, making it possible to speculate that the two systems could have a feedback relationship. In fact, the pandemic of VitD deficiency could be the other face of increased RAS activity, which probably causes lower activity or lower levels of VitD. Finally, from a therapeutic point of view, the combination of RAS blockade and VDR stimulation appears to be more effective than either RAS blockade or VDR stimulation individually.
Keywords: oxidative stress, mitochondria, cardiovascular disease, angiotensin receptor blocker, vitamin D receptor
vitamin D (VitD) deficiency is pandemic. Our hypothesis maintains that this deficiency is probably due to environmental factors, such as diet, sun exposure, sedentary life style, and stress.1 Recent studies suggest that, in addition to its importance in bone metabolism, VitD plays a central role in such basic cell functions as multiplication, differentiation, and metabolism. This may explain why low VitD levels represent a risk factor for several apparently different diseases, such as infective, autoimmune, neurodegenerative, and cardiovascular diseases, as well as diabetes, osteoporosis, and cancer. Accumulating evidence suggests that an adequate intake of VitD may significantly decrease the prevalence and improve the clinical outcomes of these diseases (42, 60, 92, 146). Moreover, VitD insufficiency seems to predispose to hypertension, diabetes, metabolic syndrome, left ventricular hypertrophy, heart failure, and chronic vascular inflammation (57, 99, 107, 181).
The relationship between baseline VitD status, dose of VitD supplements, and cardiovascular events remains to be investigated by ongoing randomized trials; however, increasing evidence suggests that the provision of a simple, well-tolerated, and inexpensive correction of VitD insufficiency favorably affects the morbidity and mortality of cardiovascular disease and prevents the most common chronic degenerative diseases (104).
Evolution
The photosynthesis of VitD evolved over 750 million years ago; the phytoplankton coccolithophor Emeliani huxleii is an early example (55). Nevertheless, the exact role of VitD in early plants and animal forms is unknown. From an evolutionary standpoint, a fascinating example is an ancient vertebrate lacking a calcified skeleton and teeth, the lamprey (Petromyzon marinus), in which a type of VitD receptor (VDR) has been described (131). In this jawless fish, several noncalcemic roles for VitD, including its action as a sensor for endogenous or exogenous toxins and as an inducer of cytochrome P-450 (CYP) enzymes, have been proposed. More interestingly, VitD plays a role in the regulation of the lamprey's primitive immune function (172). Lamprey studies provide valuable insight into the evolution of the adaptive immune system, as these ancient vertebrates possess a convergently evolved adaptive immunity with cells that function like the T cells and B cells in higher vertebrates (141). This could have implications for the anti-inflammatory effect of VitD, which is described later in this review.
Because VitD can be synthesized via a photochemical process only, early vertebrates that ventured onto land had to ingest foods that contained VitD or had to be exposed to sunlight to photosynthesize VitD in their skin to satisfy their bodies' VitD requirements (54).
Also, the renin-angiotensin system (RAS) is found in animals as primitive as the jellyfish, the lamprey, and the crab, among others. However, none of these animals has a closed circulatory system (109). What function does the RAS serve in these animals if they do not have the necessity of retaining sodium and there is no pressure to maintain? Why would the members of these species have a RAS? The RAS is also a system of self-defense. ANG II regulates the synthesis of proinflammatory substances, and inflammation is the most basic mechanism found in any living organism, allowing them to defend themselves against any aggressor (136, 139).
Finally, it could be proposed that VitD and RAS evolved in nature in a similar and parallel way (Fig. 1).
Fig. 1.
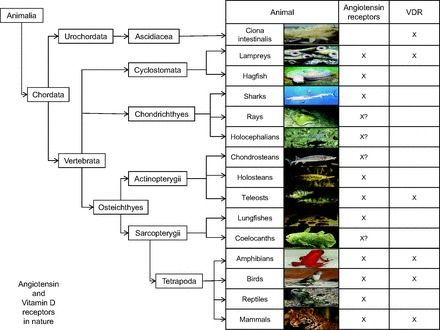
Occurrence of angiotensin receptors and vitamin D (VitD) receptors (VDRs) in nature.
Sources and Synthesis of VitD
Two main sources of VitD are available to humans: sunlight (exposure to solar UV-B radiation) and food (including dietary supplements). VitD exists in two forms: VitD2 (ergocalciferol) and VitD3 (cholecalciferol). VitD2, found in plants, is the product of UV-B (290–315 mm) irradiation of ergosterol and can be consumed as a supplement or in fortified foods (57). VitD3, a product of UV-B irradiation of 7-dehydrocholesterol, is synthesized in the human epidermis or is found in oily fish, fortified foods, and supplements.
VitD is converted in the liver to 25-hydroxyvitamin D [25(OH)D], which is the major circulating metabolite of VitD. Serum 25(OH)D concentrations, which reflect VitD intake and endogenous production, should be measured to clinically assess VitD status (57). In the kidney, 25(OH)D is converted by 1α-hydroxylase to its active form, 1,25-dihydroxyvitamin D [1,25(OH)2D]. VitD in the form of 1,25(OH)2D is a hormone, because it is produced primarily in one organ (the kidney) and then circulates throughout the body, where it exerts wide-ranging effects. Extrarenal synthesis of 1,25(OH)2D occurs through cytokine stimulation (175) and is locally important in the paracrine regulation of cell growth, differentiation, and function (101). This may explain why VitD deficiency has been associated with type 1 diabetes, cancer, and multiple sclerosis (92).
It is now recognized that most cells in the body have a VDR, and they also have the ability to produce 1,25-dihydroxyvitamin D3 [1,25(OH)2D3], which in turn is capable of regulating a wide variety of genes that have important functions in regulating cell growth, modulating immune function, and promoting cardiovascular health (59).
VitD Deficiency
VitD deficiency and VitD insufficiency are prevalent in most of the world's population (41, 57, 114). Although a consensus regarding the optimal level of serum 25(OH)D has not been established, most experts define VitD deficiency as a 25(OH)D level of <20 ng/ml (50 nmol/l) and VitD insufficiency as 21–29 ng/ml. For all studied end points to date, the optimal concentration of 25(OH)D is ≥30 ng/ml (14). A rapidly evolving knowledge base indicates that VitD deficiency is much more prevalent than has previously been recognized and is present in up to 50% of young adults (154) and apparently healthy children (57). The Third National Health and Nutrition Examination Survey (NHANES III), a nationally representative cross-sectional survey of the noninstitutionalized population in the United States carried out from 1988 to 1994, reported the prevalence of VitD deficiency in the United States to be 25–57% of adults (95).
Racial differences in the adequacy of VitD stores have been shown (48). VitD deficiency is seen frequently in African Americans, who also present a high prevalence of hypertension (112). In addition, VitD deficiency throughout life from its earliest phases may adversely affect the microvasculature in African Americans, thereby playing a major role in the genesis and maintenance of hypertension (135). This is probably due to skin pigmentation with melanin, which is a limiting factor in the cutaneous synthesis of VitD. Melanin acts as an effective natural sunscreen; therefore, increased skin pigment can reduce the solar UV-B-mediated cutaneous synthesis of VitD3 by as much as 99% (18).
Epidemiological studies have also recently linked VitD deficiency with an increased risk of major adverse cardiovascular events (169). Accordingly, data from NHANES III show an elevated risk of cardiovascular death (coronary heart disease, heart failure, and stroke) in adults with 25(OH)D serum levels in the lowest quartile (mean = 13.9 ng/ml) compared with those in the three higher quartiles (mean = 21.6, 28.4, and 41.6 ng/ml) (36).
Relationship Between VitD and the RAS
Three decades ago, an original early work suggested a possible link between VitD and the RAS (68).
The RAS is a regulatory cascade that plays a critical role in the regulation of blood pressure and electrolyte and plasma volume homeostasis. Inappropriate stimulation of the RAS has been associated with hypertension, heart attack, stroke, and hypertrophy of the left ventricle and vascular smooth muscle cells (181).
It is well established that renin secretion is regulated by renal perfusion pressure, renal sympathetic nerve activity, and tubular sodium load (8, 49). It is also stimulated by factors such as prostaglandins, nitric oxide (NO), and adrenomedullin and inhibited by other factors, including ANG II (feedback), endothelin, vasopressin, and adenosine (8, 49). These stimulations of renin secretion are often mediated by an increase in intracellular cAMP and are accompanied by increases in renin gene transcription (134). Recent studies have significantly enhanced our knowledge of the regulation of gene expression encoding for renin production on a cellular basis (7, 116, 121). It is interesting to note that, to regulate gene expression, 1,25(OH)2D3 can act as a negative regulator of specific DNA sequences [VitD response element (VDRE)] in the promoter of target genes [inhibition of other transcriptional complexes by the VDR-retinoid X receptor (RXR) heterodimers or the VDR homodimers, interaction of the VDR-RXR heterodimers with corepressors, and binding of the VDR to a negative VDRE]. Therefore, 1,25(OH)2D3 can suppress renin gene expression through a cis-DNA element(s) in the renin gene promoter. The finding that 1,25(OH)2D3 suppresses the expression of renin's gene is of utmost interest, although the exact molecular mechanism has not been elucidated (88). However, one important mechanism underlying this action is transrepression of renin gene transcription by 1,25(OH)2D3 by targeting the cAMP-PKA pathway (174) (Fig. 2).
Fig. 2.
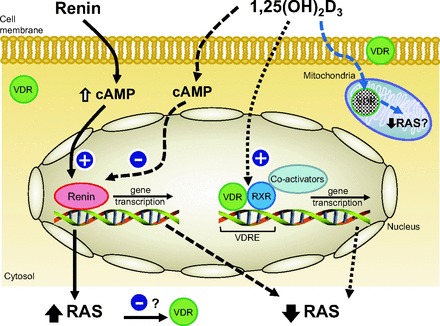
Cellular interactions of angiotensin and VitD receptors. RXR, retinoid X receptor; RAS, renin-angiotensin system; VDRE, VitD response element; 1,25(OH)2D3, 1,25-dihydroxyvitamin D3.
Several mechanisms have been proposed to explain the relationship between VitD and blood pressure (124). Some of these relationships show that VitD downregulates renin gene expression and inhibits renin synthesis, thereby suppressing the RAS (87, 88, 143, 174).
In VitD-deficient animals, there is an increased incidence of hypertension, left ventricular hypertrophy, and atherosclerosis (143). In normal mice, VitD deficiency stimulates renin expression, whereas injection of 1,25(OH)2D3 reduces renin synthesis. In cell cultures, 1,25(OH)2D directly suppresses renin gene transcription by a VDR-dependent mechanism. Mice lacking the VDR gene develop hyperreninemia, resulting in elevated production of ANG II, leading to hypertension, cardiac hypertrophy, and increased water intake (75, 88, 173).
The increased levels of renin can also act through the prorenin/renin receptor (108) and may, independently of ANG II, cause renal and/or cardiovascular damage (166).
Recent studies show that diabetic VDR-null mice developed more severe nephropathy than did wild-type mice, suggesting that VitD protects against hyperglycemia-induced renal injury by regulating the RAS. It has been further suggested that 1,25(OH)2D3 suppresses hyperglycemia-induced AGT expression in the kidney by blocking NF-κB activation of AGT gene transcription. A functional NF-κB binding site in the AGT gene promoter was bound by the p65/p50 heterodimer in the presence of high glucose, in association with the induction of the promoter activity (177).
VitD's suppression of renin expression is independent of calcium metabolism, the volume- and salt-sensing mechanisms, and ANG II feedback regulation (88, 89).
Using a transgenic mouse model with mice overexpressing the human VDR in renin-producing cells, Kong et al. (76) demonstrated that suppression of renin expression by 1,25(OH)2D in vivo is independent of parathyroid hormone and calcium.
Clinical studies conducted over the last two decades have shown an inverse association between plasma 1,25(OH)2D3 concentration and blood pressure and/or plasma renin activity in normotensive men and patients with essential hypertension (17, 78, 90, 132). In accordance with the aforementioned data, other studies have shown a reduction in blood pressure in patients with primary hypertension who were receiving VitD3 supplements (123), as well as a reduction in blood pressure, plasma renin, and ANG II concentration in patients with secondary hyperparathyroidism (73, 118).
In a study of subjects exposed to UV-B radiation in a tanning bed three times per week for 3 mo, Krause et al. (77) reported a 180% increase in 25(OH)D levels and a 6-mmHg reduction in systolic and diastolic blood pressure. A small, randomized, placebo-controlled study of patients with type 2 diabetes and low baseline 25(OH)D levels showed that a single dose of 100,000 IU of VitD2 reduced systolic blood pressure by a mean of 14 mmHg and significantly improved endothelial function as measured by forearm blood flow (146). In the NHANES III study, the mean systolic blood pressure was ∼3 mmHg lower in individuals in the highest quintile of serum 25(OH)D levels than those in the lowest quintile (137).
Receptors
VDRs.
Even though bone, small intestine, and kidneys are the primary organs responsive to VitD, the effects of VitD in the body are more far-reaching. Increasing experimental data have revealed a broad range of biological actions for the VDR, including induction of cell differentiation (19, 61), inhibition of cell growth (82), immunomodulation (93, 110), and control of other hormonal systems (87, 128). The VDR has been identified in many tissues and organs, including those not typically associated with calcium homeostasis and bone metabolism (58). It is present in a large variety of cell types, including myocytes, cardiomyocytes, pancreatic beta cells, vascular endothelial cells, neurons, immune cells, osteoblasts, and chondrocytes (57, 181) (Table 1).
Table 1.
Systems where VitD and RAS receptors are localized
| System | VitD | RAS |
|---|---|---|
| Endocrine | Thyroid | Thyroid |
| Parathyroid | ||
| Adrenal | ||
| Pancreatic beta cells | Pancreatic beta cells | |
| Posterior pituitary | ||
| Cardiovascular | Vascular smooth muscle cells | Vascular smooth muscle cells |
| Myocardium | Myocardium and myocardial mitochondria | |
| Endothelium | ||
| Connective tissue | ||
| Musculoskeletal | Striated muscle | Striated muscle |
| Osteoblasts | ||
| Chondrocytes | ||
| Gastrointestinal | Intestine | Intestine |
| Stomach | Stomach | |
| Esophagus | ||
| Salivary glands | ||
| Pancreas | Pancreas | |
| Colon | Colon | |
| Hepatic | Liver | Liver |
| Urinary | Kidney (tubules, juxtaglomerular apparatus, podocytes) | Kidney (tubule, juxtaglomerular apparatus, podocytes, mesangial cells) |
| Bladder | ||
| Reproductive | Testis | Testis |
| Ovary | Ovary | |
| Uterus | Uterus | |
| Spermatozoa | Spermatozoa | |
| Prostate | Prostate | |
| Immune | Leukocytes | Leukocytes |
| Bone marrow | Bone marrow | |
| Platelets | Platelets | |
| Spleen | ||
| Thymus | ||
| Respiratory | Lung | Lung |
| Integumentary | Epidermis | Epidermis |
| Dermis | Dermis | |
| Nervous system | Brain | Brain |
| Sensory neurons | Sensory neurons | |
| Other | White and brown adipose tissue | White and brown adipose tissue |
| Placenta | Placenta | |
| Mitochondria | Mitochondria |
The VDR is a steroid hormone nuclear receptor that binds to 1,25(OH)2D with high affinity and mediates transcriptional gene regulation (56). 1,25(OH)2D regulates >200 genes, including those involved in renin production in the kidney, insulin production in the pancreas, release of cytokines from lymphocytes, production of cathelicidin in macrophages, and growth and proliferation of vascular smooth muscle cells and cardiomyocytes (57).
The extranuclear receptor localization is still controversial. Several reports indicate a subcellular distribution in the cytoplasm, in discrete regions of the nucleus, and along the nuclear envelope (10), whereas the membrane-initiated effects are attributed to a plasma membrane-associated receptor (106); in fact, the VDR has been found in caveolae-enriched plasma membrane (62). Moreover, microscopy studies have revealed that the VDR has mitochondrial, membrane-based, cytosolic, and perinuclear localizations (43). Silvagno et al. (142) found that human platelets express the VDR, which is mainly located in the mitochondrial compartment. The anucleated platelets are a good model in which to study the extranuclear VDR localization involved in the nongenomic response to VitD. In agreement with the intracellular distribution suggested by Western blot analysis, an anti-VDR antibody revealed the presence of the VDR in the mitochondrial structures and in the cytosol, without significant labeling of other platelet structures. In their work, they report not only the presence of the VDR in human platelets but also, most interestingly, its mitochondrial localization.
In a recent study, Gonzalez-Pardo et al. (43) also described a mitochondrial VDR. Indeed, fractionation studies demonstrated the presence of the receptor in the mitochondrial compartment, and the observation was confirmed by immunoelectron microscopy analysis of platelets. In agreement and moreover, a recent study from our laboratory showed that low mitochondrial VDR expression was associated with increased ANG II type 1 (AT1) receptor expression in the course of RAS upregulation in an animal experimental model, whereas VDR induction by paricalcitol [19-nor-1,25(OH)2D2, a VDR activator (VDRA)] treatment conditioned a minor AT1 receptor expression linked to VDR upregulation. These results suggest a cytoprotective effect of paricalcitol, revealing for the first time a possible AT1 receptor-dependent protective effect that occurs at the mitochondrial level (39).
Again, the presence of the VDR in several tissues (Table 1) supports the assumption that the VitD endocrine system is involved in various physiological functions beyond calcium-phosphate balance. This notion is additionally confirmed by the presence of CYP27A and CYP27B, the enzymes that catalyze conversion of 25(OH)D to 1,25(OH)2D3 in these tissues (60, 144).
VitD signaling is dependent on the availability and turnover of the active VDR ligand 1,25(OH)2D3 and on the efficiency of VDR transactivation. Transactivation of the VDR depends on the correct molecular structure, effective nuclear translocation, and presence of the unliganded heterodimer partner RXR and other nuclear cofactors (32). In most cases in which 1,25(OH)2D3 acts as a positive regulator, the liganded VDR heterodimerizes with the RXR and binds to specific DNA sequences (VDRE) in the promoter of target genes to regulate gene expression. On the other hand, 1,25(OH)2D3 can also act as a negative regulator, but the mechanism of the negative regulation is more complicated and only partially understood (88).
Finally, the mitochondrial localization of the VDR, which has been recently confirmed (142), suggests a mitochondrial nongenomic activity. Therefore, we suggest that this effect could be associated with the RAS, since kidney mitochondrial injury is attenuated by AT1 receptor blockade in experimental models (25).
RAS receptors.
Components of the RAS [renin, angiotensin-converting enzyme (ACE), angiotensinogen, ANG I, and ANG II] receptors have been found in many areas, including kidney and adrenal tissues, blood vessels, and discrete regions of the brain (22) (Table 1). Distribution of AT1 and ANG II type 2 (AT2) receptors has been mapped by in vitro autoradiography throughout most tissues of many mammals, including humans. The AT1 receptor occurs in sites known to be targets for the physiological actions of angiotensin, such as the adrenal cortex and medulla, renal glomeruli and proximal tubules, vascular and cardiac muscle, and brain circumventricular organs (Table 1). In addition, many new sites of action have been demonstrated. In the kidney, the AT1 receptor occurs in high density in renal medullary interstitial cells. In the heart, the highest densities of the AT1 receptor occur in association with the conduction system and vagal ganglia. In the central nervous system, high AT1 receptor densities occur in many regions behind the blood-brain barrier, supporting a role for neurally derived angiotensin as a neuromodulator (2). The AT1 receptor is also found in hepatocytes and bile duct epithelial cells (64, 84, 115, 170). The AT2 receptor also has a characteristic pattern of distribution in several tissues, including the adrenal gland, heart, and brain. The role of this receptor in physiology is still being elucidated, but it appears to involve inhibition of proliferation and participation in development (2).
ANG II also stimulates mitochondrial oxidant release, leading to energy metabolism depression. By lowering mitochondrial oxidant production, ANG II inhibition enhances energy production and protects mitochondrial structure. This seems to be one of the mechanisms underlying the benefits of ANG II inhibition in hypertension, diabetes, and aging rodent models (28).
Hence, some cellular mechanisms responsible for the protective actions of RAS inhibitors were previously discussed in recent reviews by us (26–28). Moreover, and as mentioned above, we found high levels of mitochondrial AT1 receptor mRNA expression in renal cortexes from rats (39). Some mitochondria were increased in size and contained dilated crests and larger-than-normal spaces in their interiors. These changes were not present with paricalcitol treatment.
The existence of intramitochondrial AT1 and AT2 receptors and a functional RAS has been subsequently corroborated by Abadir et al. (1). How these receptors are transported to the mitochondria is still controversial. Interestingly, disruption of the AT1 receptor was associated with an increased number of mitochondria and upregulation of the prosurvival genes nicotinamide phosphoribosyltransferase (Nampt) and sirtuin 3 (Sirt3) in the kidney, leading to a marked prolongation of the lifespan of mice (12).
Finally, the RAS appears to be much more complicated than it was thought to be a few years ago. Thus, ANG II-(1–7) exhibits direct and indirect effects, the latter resulting from the ANG II-(1–7)-dependent formation of NO and vasodilatory prostaglandins. ANG II-(1–7) potentiates the hypotensive effect of bradykinin and also plays a major role in control of hydroelectrolytic balance. It possesses its own receptors, AT1, AT2, AT3, AT4, AT5, AT6, and AT7, which are recognizable by [Sar1-Thr8]ANG II or sarthran (24). Also, ANG II and its derivatives, ANG III, ANG IV, and ANG-(1–7), alter vascular contractility with different mechanisms of action (158). New evidence has accumulated showing the existence of several novel receptor-interacting proteins and various ANG II receptor activation mechanisms, such as dimerization and mechanical stretch-induced activation, which differ from classical ANG II receptor signaling. These findings may provide new potent therapeutic targets for the treatment of cardiovascular disease (102).
Finally, it should be noted that the VDR and AT1 receptor are distributed in almost the same tissues (Table 1).
Mechanisms in Which VitD and RAS Interact
RAS activity and VitD levels could be related in several ways. In this review we focus on inflammatory response, oxidative stress, and atherosclerosis.
Inflammatory Response
Association of VitD deficiency with markers of inflammation (such as tumor necrosis factor-α) (122) is evidenced by elevated levels of C-reactive protein and IL-10 (181). Furthermore, administration of 1,25(OH)2D to VitD-deficient individuals downregulated inflammatory markers (e.g., C-reactive protein) and conferred an antiproliferative effect (138). VitD is known to have immune-modulating effects (94). There are four potential ways by which serum VitD can influence T cell function: 1) direct effects on T cells mediated via systemic VitD, 2) indirect effects on antigen presentation to T cells and the intracrine synthesis of VitD, 3) direct effects of VitD on T cells following synthesis of the active form of VitD, a paracrine mechanism, and 4) intracrine conversion of 25(OH)D to VitD by T cells (51). In addition, VitD is required for the development of invariant natural killer T cells and CD8αα T cells. The selective requirement for VitD and the VDR in the development of these two populations of regulatory T cells, and not conventional T cell development, suggests that there may be some common mechanism (113).
The recent finding by Isakova et al. (66) that IL-6 is a potential mediator of the association between low levels of VitD and albuminuria is congruent with prior reports.
Hypertension and proteinuria are the two major factors that induce many inflammatory and mitogenic mediators such as transforming growth factor-β (13). In renal proximal tubular cells, 1,25(OH)2D3 stimulates expression of transforming growth factor-β1, a growth factor with anti-inflammatory and profibrotic actions that plays an important role in the development and progression of nephrosclerosis (171).
The RAS has a fundamental role in the mechanisms of inflammation (161) and defense for the different cells and tissues of organisms. This last function is fulfilled by regulation of oxidative stress at the cytoplasmic and mitochondrial levels. This phenomenon was also shown to be associated with the metabolic syndrome (34), as well as the initiation of renal fibrogenesis during unilateral ureteral obstruction (96).
ANG II induces proinflammatory genes and other proinflammatory substances and increases oxidative stress, which could damage endothelium, myocardium, and renal tissue. Chronic activation of NF-κB (a protein complex that controls the transcription of DNA) and chronic activation of NAD(P)H oxidase are fundamental steps in these proinflammatory mechanisms in which intramitochondrial oxidative stress could play a critical role (34, 127). In this way, NF-κB is a potent inducer of proinflammatory cytokines and oxidative stress in cardiovascular disease (71). Also this chronic stimulus is a well-known event in many other proinflammatory diseases (53).
This sequence of events might explain why reduction of ANG II synthesis by angiotensin-converting enzyme inhibitors and angiotensin receptor blockers has a protective effect against cardiovascular disease (34). From an evolutionary standpoint, this occurred before its role as a regulator of arterial pressure. If we were to consider cardiovascular disease as being inflammatory, then beyond its antihypertensive effect, blockade by the RAS could be seen as an etiological treatment of cardiovascular disease (139).
NF-κB activation leads first to a proinflammatory immune response and then to a VitD-dependent anti-inflammatory response. Binding of the active metabolite 1,25(OH)2D3 to the VDR yields a transcription factor that represses NF-κB activation and, additionally, modulates and downregulates adaptive, but enhances innate immune, responses and improves redox balance, thus counterbalancing inflammation on multiple levels. However, these built-in late counterbalances against inflammation work only when stores of calcium and 25(OH)D3 are abundant (53). Therefore, a connection between lowered VitD metabolism and persistent NF-κB activation can be postulated. Consistent with this notion, it has been reported that the VDR reduces NF-κB activation by interference with NF-κB p65 and p105 (40).
Knowledge about the impact of VitD deficiency on chronic NF-κB activation is growing (53).
Oxidative Stress
Oxidative stress and free radicals result from an increase in production and/or a decrease in clearance. An excess of free radicals is detrimental to cell function [including function of beta cells (50, 52, 70), endothelial cells (4), fat cells (157), muscle cells (120), and nerve cells (20)]. Decreasing production or increasing clearance should reduce the net amount of free radicals and cell damage. Different patients (98) (or the organs, tissues, or cells of an individual patient) may be more or less sensitive to free radicals and have different susceptibility to oxidants or greater antioxidant defenses (3, 6, 33, 129). The same level of oxidative stress may be more or less deleterious, depending on the protective antioxidant enzyme defense system and reparative process.
It is clear that the increased generation of cellular reactive oxygen species (ROS) and the activation of redox-sensitive signaling cascades are critical events involved in ANG II actions (163). After binding to its AT1 receptor, ANG II triggers intracellular superoxide production (46, 72, 103). ANG II also enhances NO generation (126), and since the reaction of NO with superoxide generates peroxynitrite, it can promote the production of ROS and reactive nitrogen species and reduce NO availability (103, 160). Under normal physiological conditions, ANG II-mediated ROS and reactive nitrogen species production and the resulting stimulation of redox-sensitive signaling pathways are closely regulated (160). However, under conditions associated with RAS overactivation, such as hypertension, diabetes (133, 162), and normal aging (11, 47, 156, 168), ANG II-dependent oxidant generation becomes a significant contributor to cell oxidation and tissue damage (28, 117).
Oxidative stress linked to VitD metabolism was initially discussed in 1988 (21). These experiments suggested that an increase in mitochondrial membrane lipid hyperperoxide production resulted in a loss of 1α- and 24-hydroxylase activity in proximal tubule cells (21).
Chang et al. (23) found, in monocytes and vascular smooth muscle cells, that 1,25(OH)2D3 inhibits NO synthase (NOS), which in turn reduces NO and free radical production.
Recently, it has been demonstrated that ANG II upregulation stimulates NOX4-derived ROS via the AT1 receptor (45), that NOX4 is the major isoform expressed in renal cells, and that the VDR attenuates obstructive renal injury, at least in part, by suppressing the RAS (176). More recently, our group showed that paricalcitol has an antioxidant effect in the myocardium, aorta, and kidney tissue (63), as well as in mitochondrial fractions from renal cortexes of obstructed kidneys (39).
Treatment with a VDRA has survival benefits that are probably related to its effects beyond its traditional role in mineral metabolism. Recently, Tanaka et al. (152) showed that VDRA reduces oxidative stress in hemodialysis patients. After 4 wk of treatment with calcitriol (1.5 μg/wk iv), Tanaka et al. reported no significant changes in serum intact parathyroid hormone, calcium, or phosphorus levels; however, the ratio of oxidized to unoxidized albumin was markedly decreased. Furthermore, after calcitriol treatment, the radical scavenging activity of albumin was greater that of untreated albumin (152). Their data suggest that intravenous calcitriol treatment reduces oxidative stress and strengthens antioxidant defenses by inhibiting albumin oxidation.
Atherosclerosis
VitD can inhibit various aspects of inflammation leading to intimal and medial calcification (167).
It is also well known that the immune system actively participates in the inflammatory process. T lymphocytes and macrophages are known stimulators of intimal thickening and plaque formation in arteries that are susceptible to atherosclerosis. Th1 lymphocytes secrete IFN-γ, which is a potent macrophage activator and a Th2 lymphocyte suppressor. Th2 lymphocytes, in turn, are antiatherogenic (through the production of IL-10, which inhibits macrophage activation) (86). The development of CD4+ T cells into T helper (Th) type 1 (Th1) or Th type 2 (Th2) cells determines the outcome of an immune response and is primarily directed by cytokines. VDRAs have potential ameliorating effects on the development of atherosclerosis, acting on several mechanisms. First, they directly affect naive CD4+ T cells by enhancing the development of Th2 lymphocytes (through IL-4 production) (15). Furthermore, treatment with VDRA inhibits the transcription of IFN-γ, which is required for Th1 development or is a product of Th1 cells (15, 145). Moreover, human and mouse naive CD4+ cells differentiate into IL-10-producing T cells after treatment with VDRA and dexamethasone (9). Through these mechanisms, VDRAs may change the Th1-Th2 balance and influence the production of anti-inflammatory mediators.
The stimuli of ANG II not only regulate vascular tone and sodium balance but also activate immune cells and promote cell infiltration into target organs (105).
Moreover, increased proinflammatory cytokines in the vessel wall contribute to immune cell recruitment and modified LDL cholesterol deposition by increasing scavenger receptor expression and cholesteryl ester synthesis and by decreasing cholesterol efflux (155). Active VitD metabolites [1,25(OH)2D3 or its analogs] promote monocyte-macrophage differentiation and diminish proinflammatory cytokine release by immune mononuclear cells, suggesting that 1,25(OH)2D3 signaling may regulate monocyte vascular infiltration and macrophage cholesterol retention in the vessel walls (42, 74).
Foam cells are the result of accumulated oxidized LDL within the macrophages (then phagocytes) and, finally, form the fatty streaks of the atheroma plaques. Oh et al. (111) cultured macrophages in VitD-deficient or -supplemented media and exposed them to modified LDL cholesterol. They found that 1,25(OH)2D3 suppressed foam cell formation by reducing acetylated or oxidized LDL cholesterol uptake. Conversely, deletion of the VDR in macrophages accelerated foam cell formation induced by modified LDL. Their results suggest that reduced VDR signaling is a potential mechanism underlying increased foam cell formation and accelerated cardiovascular disease.
Also dysfunctional mitochondria seem to contribute to the pathophysiology of atherosclerosis. As mentioned above, at the mitochondrial level, RAS overactivation plays a critical role (34).
Recently, in a model of apolipoprotein E-deficient atherosclerotic mice, our group showed that treatment with paricalcitol and enalapril, alone or in combination, ameliorates inflammatory and oxidative aortic injury in atherosclerotic mice by decreasing monocyte chemoattractant protein-1, TNF-α, cyclooxygenase-2, NADPH oxidase subunit p22 phox, Mn-SOD and inducible NOS protein expression and malondialdehyde levels and by restoring GSH levels and CuZn-SOD and endothelial NOS protein expression. Therefore, atherosclerotic processes can be countered more effectively with the combined use of drugs that act on the VDR (paricalcitol) and RAS receptor (enalapril) (63).
Since the RAS is involved in the pathogenesis of atherosclerosis and is downregulated by VitD, the interaction of paricalcitol and enalapril likely enhances the protective effect against atherosclerosis lesions, probably by means of a more effective amelioration of vascular inflammatory and oxidative injury to the renal and cardiovascular endothelium (63).
Clinical and Epidemiological Studies
Several clinical and epidemiological studies have shown that there may be an association between hypertension and VitD. Scragg et al. (137) recently reported the relationship between serum 25(OH)D concentration and blood pressure. In their study of data from NHANES III, they found a significant inverse association between serum 25(OH)D concentration and blood pressure that was evident even after adjustment for variables including age, gender, ethnicity, and physical activity. Judd et al. (69) also analyzed the NHANES III survey data and determined that there was a statistically significant inverse association between circulating 25(OH)D concentrations and systolic blood pressure. Martins et al. (97) found that a low VitD level was associated with a higher risk of hypertension.
A possible mechanism underlying these findings was studied in the Ludwigshafen Risk and Cardiovascular Health (LURIC) study (159). This work was aimed at documenting a potential association between 25(OH)D, 1,25(OH)2D, and the circulating RAS in a large cohort of patients that had been referred (n = 3,316) for coronary angiography. After measuring 25(OH)D, 1,25(OH)2D, plasma renin, and ANG II concentration, the investigators showed an independent association between them. Their data showed, for the first time in humans, that lower 25(OH)D and 1,25(OH)2D values are independently related to an upregulated circulating RAS (159).
Hypotheses
It seems very possible that a functional interaction between VitD and the RAS exists.
From the evolutionary point of view, both systems developed simultaneously and in parallel, both actively participating in the regulation of inflammatory and immunological mechanisms (Figs. 1 and 3). ANG II is a proinflammatory hormone, and VitD seems to have anti-inflammatory effects. From this, we can speculate that an increased proinflammatory response, generated by various stimuli accompanying diverse pathologies, such as the metabolic syndrome and cardiovascular diseases, could inhibit VitD, that is, an increase in proinflammatory tissue tone that facilitates the development of atherosclerotic (cardiovascular) disease. This would help explain why, in the process of enculturation, humans happened to be the only mammal that developed cardiovascular disease (an inflammatory disease), which also is the number-one cause of mortality in the modern world.
Fig. 3.
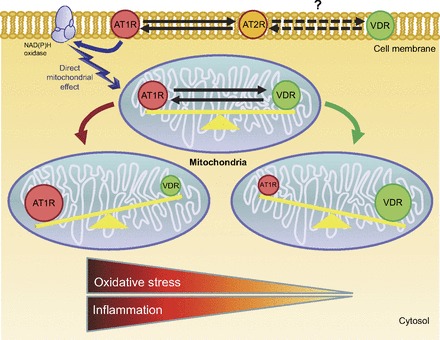
Potential interaction between RAS receptor and VDR, with focus on oxidative stress and inflammatory balance (1, 39). Proposed interaction between angiotensin type 1 receptor (AT1R), angiotensin type 2 receptor (AT2R), and VDR may occur throughout the cell.
In this review we hypothesize that increased RAS activity and lower activation of the VDR can be complementary situations (Figs. 2 and 3), probably underlying a causal relationship. Furthermore, intervening in one factor seems to change, in the opposite direction, the other. It appears quite clear that the RAS can be regulated by VitD, but in turn our novel proposal is that increased ANG II or AT1 receptor may regulate the levels of VitD.
This relationship is also evident in other pathologies that are not discussed in this review but have been very well documented, such as hypertension (73, 91, 118, 123, 124, 143, 173, 180, 181), aging (27, 35, 65, 67, 79–81, 83, 100, 153, 164, 165), diabetes and obesity (29, 37, 38, 76, 85, 88, 125, 148, 151, 174, 176, 178, 179), and chronic kidney disease (66, 130, 149, 150, 176).
In fact, the pandemic of VitD deficiency could be the other face of increased RAS activity, which probably causes lower activity or lower levels of VitD. To make this more likely, a close spatial interaction between the two systems would be necessary. We have shown that almost all tissues that express the VDR also express ANG II receptors (Table 1). This coexpression appears to be present in mitochondria as well, which is basically where cellular energy is generated and where cell signals are triggered, resulting in the oxidative changes associated with chronic inflammatory processes, especially in the cardiovascular and renal systems (Fig. 3). A RAS blockade or VDR stimulation produces similar changes in these oxidative inflammatory disorders.
Finally, at a cellular and mitochondrial level, we have seen that increased ANG II produced a decrease of the VDRs.
In other words, the pandemic of human VitD insufficiency could be seen as an inflammation marker.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.F., F.I., and L.F. are responsible for conception and design of the review; M.F. prepared the figures; M.F., F.I., W.M., and L.F. drafted the manuscript; M.F., F.I., W.M., and L.F. edited and revised the manuscript; M.F., F.I., and L.F. approved the final version of the manuscript.
ACKNOWLEDGMENTS
The authors thank Yma Tomassini and Bob Ritchie (National Center for Research Resources Grant G12 RR-003050/National Institute on Minority Health and Health Disparities Grant 8G12 MD-007579) for help with editing the manuscript.
Footnotes
This article is the topic of an Editorial Focus by R. Brooks Robey and Mardi A. Crane-Godreau (134a).
REFERENCES
- 1. Abadir PM, Foster DB, Crow M, Cooke CA, Rucker JJ, Jain A, Smith BJ, Burks TN, Cohn RD, Fedarko NS, Carey RM, O'Rourke B, Walston JD. Identification and characterization of a functional mitochondrial angiotensin system. Proc Natl Acad Sci USA 108: 14849–14854, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Allen AM, Zhuo J, Mendelsohn FA. Localization and function of angiotensin AT1 receptors. Am J Hypertens 13: 31S–38S, 2000 [DOI] [PubMed] [Google Scholar]
- 3. Anderson JW, Gowri MS, Turner J, Nichols L, Diwadkar VA, Chow CK, Oeltgen PR. Antioxidant supplementation effects on low-density lipoprotein oxidation for individuals with type 2 diabetes mellitus. J Am Coll Nutr 18: 451–461, 1999 [DOI] [PubMed] [Google Scholar]
- 4. Andersson TL, Matz J, Ferns GA, Anggard EE. Vitamin E reverses cholesterol-induced endothelial dysfunction in the rabbit coronary circulation. Atherosclerosis 111: 39–45, 1994 [DOI] [PubMed] [Google Scholar]
- 5. Aquila S, Guido C, Middea E, Perrotta I, Bruno R, Pellegrino M, Ando S. Human male gamete endocrinology: 1α,25-dihydroxyvitamin D3 (1,25(OH)2D3) regulates different aspects of human sperm biology and metabolism. Reprod Biol Endocrinol 7: 140, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Asayama K, Uchida N, Nakane T, Hayashibe H, Dobashi K, Amemiya S, Kato K, Nakazawa S. Antioxidants in the serum of children with insulin-dependent diabetes mellitus. Free Radic Biol Med 15: 597–602, 1993 [DOI] [PubMed] [Google Scholar]
- 7. Bader M, Ganten D. Regulation of renin: new evidence from cultured cells and genetically modified mice. J Mol Med 78: 130–139, 2000 [DOI] [PubMed] [Google Scholar]
- 8. Ballermann BJ, Zeidel ML, Gunning ME, Brenner BM. Vasoactive peptides and the kidney. In: The Kidney, edited by Brenner BM, Rector FC. Philadelphia, PA: Saunders, 1991, p. 510–583 [Google Scholar]
- 9. Barrat FJ, Cua DJ, Boonstra A, Richards DF, Crain C, Savelkoul HF, de Waal-Malefyt R, Coffman RL, Hawrylowicz CM, O'Garra A. In vitro generation of interleukin 10-producing regulatory CD4+ T cells is induced by immunosuppressive drugs and inhibited by T helper type 1 (Th1)- and Th2-inducing cytokines. J Exp Med 195: 603–616, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Barsony J, Renyi I, McKoy W. Subcellular distribution of normal and mutant vitamin D receptors in living cells. Studies with a novel fluorescent ligand. J Biol Chem 272: 5774–5782, 1997 [DOI] [PubMed] [Google Scholar]
- 11. Baylis C, Engels K, Hymel A, Navar LG. Plasma renin activity and metabolic clearance rate of angiotensin II in the unstressed aging rat. Mech Ageing Dev 97: 163–172, 1997 [DOI] [PubMed] [Google Scholar]
- 12. Benigni A, Corna D, Zoja C, Sonzogni A, Latini R, Salio M, Conti S, Rottoli D, Longaretti L, Cassis P, Morigi M, Coffman TM, Remuzzi G. Disruption of the ANG II type 1 receptor promotes longevity in mice. J Clin Invest 119: 524–530, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bernadet-Monrozies P, Rostaing L, Kamar N, Durand D. [The effect of angiotensin-converting enzyme inhibitors on the progression of chronic renal failure]. Presse Med 31: 1714–1720, 2002 [PubMed] [Google Scholar]
- 14. Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr 84: 18–28, 2006 [DOI] [PubMed] [Google Scholar]
- 15. Boonstra A, Barrat FJ, Crain C, Heath VL, Savelkoul HF, O'Garra A. 1α,25-Dihydroxyvitamin D3 has a direct effect on naive CD4+ T cells to enhance the development of Th2 cells. J Immunol 167: 4974–4980, 2001 [DOI] [PubMed] [Google Scholar]
- 16. Bullock GR, Steyaert I, Bilbe G, Carey RM, Kips J, De Paepe B, Pauwels R, Praet M, Siragy HM, de Gasparo M. Distribution of type-1 and type-2 angiotensin receptors in the normal human lung and in lungs from patients with chronic obstructive pulmonary disease. Histochem Cell Biol 115: 117–124, 2001 [DOI] [PubMed] [Google Scholar]
- 17. Burgess ED, Hawkins RG, Watanabe M. Interaction of 1,25-dihydroxyvitamin D and plasma renin activity in high renin essential hypertension. Am J Hypertens 3: 903–905, 1990 [DOI] [PubMed] [Google Scholar]
- 18. Clemens TL, Adams JS, Henderson SL, Holick MF. Increased skin pigment reduces the capacity of skin to synthesise vitamin D3. Lancet 1: 74–76, 1982 [DOI] [PubMed] [Google Scholar]
- 19. Cordero JB, Cozzolino M, Lu Y, Vidal M, Slatopolsky E, Stahl PD, Barbieri MA, Dusso A. 1,25-Dihydroxyvitamin D down-regulates cell membrane growth- and nuclear growth-promoting signals by the epidermal growth factor receptor. J Biol Chem 277: 38965–38971, 2002 [DOI] [PubMed] [Google Scholar]
- 20. Cowell RM, Russell JW. Nitrosative injury and antioxidant therapy in the management of diabetic neuropathy. J Investig Med 52: 33–44, 2004 [DOI] [PubMed] [Google Scholar]
- 21. Crivello JF. Oxidative stress limits vitamin D metabolism by bovine proximal tubule cells in vitro. Arch Biochem Biophys 262: 471–480, 1988 [DOI] [PubMed] [Google Scholar]
- 22. Chai SY, Zhuo J, Mendelsohn FA. Localization of components of the renin-angiotensin system and site of action of inhibitors. Arzneimittelforschung 43: 214–221, 1993 [PubMed] [Google Scholar]
- 23. Chang JM, Kuo MC, Kuo HT, Hwang SJ, Tsai JC, Chen HC, Lai YH. 1α,25-Dihydroxyvitamin D3 regulates inducible nitric oxide synthase messenger RNA expression and nitric oxide release in macrophage-like RAW 264.7 cells. J Lab Clin Med 143: 14–22, 2004 [DOI] [PubMed] [Google Scholar]
- 24. Chansel D, Ardaillou R. [Active metabolites derived from angiotensin II]. Nephrologie 19: 427–432, 1998 [PubMed] [Google Scholar]
- 25. de Cavanagh EM, Ferder L, Toblli JE, Piotrkowski B, Stella I, Fraga CG, Inserra F. Renal mitochondrial impairment is attenuated by AT1 blockade in experimental type 1 diabetes. Am J Physiol Heart Circ Physiol 294: H456–H465, 2008 [DOI] [PubMed] [Google Scholar]
- 26. de Cavanagh EM, Ferder M, Inserra F, Ferder L. Angiotensin II, mitochondria, cytoskeletal, and extracellular matrix connections: an integrating viewpoint. Am J Physiol Heart Circ Physiol 296: H550–H558, 2009 [DOI] [PubMed] [Google Scholar]
- 27. de Cavanagh EM, Inserra F, Ferder L. Angiotensin II blockade: a strategy to slow ageing by protecting mitochondria? Cardiovasc Res 89: 31–40, 2011 [DOI] [PubMed] [Google Scholar]
- 28. de Cavanagh EM, Inserra F, Ferder M, Ferder L. From mitochondria to disease: role of the renin-angiotensin system. Am J Nephrol 27: 545–553, 2007 [DOI] [PubMed] [Google Scholar]
- 29. Deb DK, Chen Y, Zhang Z, Zhang Y, Szeto FL, Wong KE, Kong J, Li YC. 1,25-Dihydroxyvitamin D3 suppresses high glucose-induced angiotensinogen expression in kidney cells by blocking the NF-κB pathway. Am J Physiol Renal Physiol 296: F1212–F1218, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dinh DT, Frauman AG, Johnston CI, Fabiani ME. Angiotensin receptors: distribution, signalling and function. Clin Sci (Lond) 100: 481–492, 2001 [PubMed] [Google Scholar]
- 31. Dirks-Naylor AJ, Lennon-Edwards S. The effects of vitamin D on skeletal muscle function and cellular signaling. J Steroid Biochem Mol Biol 125: 159–168, 2011 [DOI] [PubMed] [Google Scholar]
- 32. Ebert R, Schutze N, Adamski J, Jakob F. Vitamin D signaling is modulated on multiple levels in health and disease. Mol Cell Endocrinol 248: 149–159, 2006 [DOI] [PubMed] [Google Scholar]
- 33. Elangovan V, Shohami E, Gati I, Kohen R. Increased hepatic lipid soluble antioxidant capacity compared with other organs of streptozotocin-induced diabetic rats: a cyclic voltammetry study. Free Radic Res 32: 125–134, 2000 [DOI] [PubMed] [Google Scholar]
- 34. Ferder L, Inserra F, Martinez-Maldonado M. Inflammation and the metabolic syndrome: role of angiotensin II and oxidative stress. Curr Hypertens Rep 8: 191–198, 2006 [DOI] [PubMed] [Google Scholar]
- 35. Ferder LF, Inserra F, Basso N. Advances in our understanding of aging: role of the renin-angiotensin system. Curr Opin Pharmacol 2: 189–194, 2002 [DOI] [PubMed] [Google Scholar]
- 36. Fiscella K, Franks P. Vitamin D, race, and cardiovascular mortality: findings from a national US sample. Ann Fam Med 8: 11–18, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Freundlich M, Quiroz Y, Zhang Z, Zhang Y, Bravo Y, Weisinger JR, Li YC, Rodriguez-Iturbe B. Suppression of renin-angiotensin gene expression in the kidney by paricalcitol. Kidney Int 74: 1394–1402, 2008 [DOI] [PubMed] [Google Scholar]
- 38. Fryer RM, Rakestraw PA, Nakane M, Dixon D, Banfor PN, Koch KA, Wu-Wong JR, Reinhart GA. Differential inhibition of renin mRNA expression by paricalcitol and calcitriol in C57/BL6 mice. Nephron Physiol 106: p76–p81, 2007 [DOI] [PubMed] [Google Scholar]
- 39. Garcia IM, Altamirano L, Mazzei L, Fornes M, Molina MN, Ferder L, Manucha W. Role of mitochondria in paricalcitol-mediated cytoprotection during obstructive nephropathy. Am J Physiol Renal Physiol 302: F1595–F1605, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Geldmeyer-Hilt K, Heine G, Hartmann B, Baumgrass R, Radbruch A, Worm M. 1,25-Dihydroxyvitamin D3 impairs NF-κB activation in human naive B cells. Biochem Biophys Res Commun 407: 699–702, 2011 [DOI] [PubMed] [Google Scholar]
- 41. Ginde AA, Liu MC, Camargo CA., Jr Demographic differences and trends of vitamin D insufficiency in the US population, 1988–2004. Arch Intern Med 169: 626–632, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Giulietti A, van Etten E, Overbergh L, Stoffels K, Bouillon R, Mathieu C. Monocytes from type 2 diabetic patients have a pro-inflammatory profile. 1,25-Dihydroxyvitamin D3 works as anti-inflammatory. Diabetes Res Clin Pract 77: 47–57, 2007 [DOI] [PubMed] [Google Scholar]
- 43. Gonzalez-Pardo V, Boland R, de Boland AR. Vitamin D receptor levels and binding are reduced in aged rat intestinal subcellular fractions. Biogerontology 9: 109–118, 2008 [DOI] [PubMed] [Google Scholar]
- 44. Goodfriend TL. Angiotensin receptors: history and mysteries. Am J Hypertens 13: 442–449, 2000 [DOI] [PubMed] [Google Scholar]
- 45. Gorin Y, Ricono JM, Wagner B, Kim NH, Bhandari B, Choudhury GG, Abboud HE. Angiotensin II-induced ERK1/ERK2 activation and protein synthesis are redox-dependent in glomerular mesangial cells. Biochem J 381: 231–239, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Griendling KK, Ushio-Fukai M. Reactive oxygen species as mediators of angiotensin II signaling. Regul Pept 91: 21–27, 2000 [DOI] [PubMed] [Google Scholar]
- 47. Groban L, Pailes NA, Bennett CD, Carter CS, Chappell MC, Kitzman DW, Sonntag WE. Growth hormone replacement attenuates diastolic dysfunction and cardiac angiotensin II expression in senescent rats. J Gerontol A Biol Sci Med Sci 61: 28–35, 2006 [DOI] [PubMed] [Google Scholar]
- 48. Gutierrez OM, Farwell WR, Kermah D, Taylor EN. Racial differences in the relationship between vitamin D, bone mineral density, and parathyroid hormone in the National Health and Nutrition Examination Survey. Osteoporos Int 22: 1745–1753, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hackenthal E, Paul M, Ganten D, Taugner R. Morphology, physiology, and molecular biology of renin secretion. Physiol Rev 70: 1067–1116, 1990 [DOI] [PubMed] [Google Scholar]
- 50. Haskins K, Kench J, Powers K, Bradley B, Pugazhenthi S, Reusch J, McDuffie M. Role for oxidative stress in the regeneration of islet beta cells? J Investig Med 52: 45–49, 2004 [DOI] [PubMed] [Google Scholar]
- 51. Hewison M. An update on vitamin D and human immunity. Clin Endocrinol (Oxf) 76: 315–325, 2012 [DOI] [PubMed] [Google Scholar]
- 52. Ho E, Bray TM. Antioxidants, NFκB activation, diabetogenesis. Proc Soc Exp Biol Med 222: 205–213, 1999 [DOI] [PubMed] [Google Scholar]
- 53. Hoeck AD, Pall ML. Will vitamin D supplementation ameliorate diseases characterized by chronic inflammation and fatigue? Med Hypotheses 76: 208–213, 2011 [DOI] [PubMed] [Google Scholar]
- 54. Holick MF. Environmental factors that influence the cutaneous production of vitamin D. Am J Clin Nutr 61: 638S–645S, 1995 [DOI] [PubMed] [Google Scholar]
- 55. Holick MF. Phylogenetic and evolutionary aspects of vitamin D from phytoplankton to humans. In: Vertebrate Endocrinology: Fundamentals and Biomedical Implications, edited by Pang PS. Orlando, FL: Academic, 1989, p. 7–43 [Google Scholar]
- 56. Holick MF. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am J Clin Nutr 80: 1678S–1688S, 2004 [DOI] [PubMed] [Google Scholar]
- 57. Holick MF. Vitamin D deficiency. N Engl J Med 357: 266–281, 2007 [DOI] [PubMed] [Google Scholar]
- 58. Holick MF. Vitamin D: a millenium perspective. J Cell Biochem 88: 296–307, 2003 [DOI] [PubMed] [Google Scholar]
- 59. Holick MF. Vitamin D: evolutionary, physiological and health perspectives. Curr Drug Targets 12: 4–18, 2011 [DOI] [PubMed] [Google Scholar]
- 60. Holick MF. Vitamin D: the underappreciated D-lightful hormone that is important for skeletal and cellular health. Curr Opin Endocrinol Diabetes Obes 9: 87–98, 2002 [Google Scholar]
- 61. Hsieh JC, Sisk JM, Jurutka PW, Haussler CA, Slater SA, Haussler MR, Thompson CC. Physical and functional interaction between the vitamin D receptor and hairless corepressor, two proteins required for hair cycling. J Biol Chem 278: 38665–38674, 2003 [DOI] [PubMed] [Google Scholar]
- 62. Huhtakangas JA, Olivera CJ, Bishop JE, Zanello LP, Norman AW. The vitamin D receptor is present in caveolae-enriched plasma membranes and binds 1α,25(OH)2-vitamin D3 in vivo and in vitro. Mol Endocrinol 18: 2660–2671, 2004 [DOI] [PubMed] [Google Scholar]
- 63. Husain K, Suarez E, Isidro A, Ferder L. Effects of paricalcitol and enalapril on atherosclerotic injury in mouse aortas. Am J Nephrol 32: 296–304, 2010 [DOI] [PubMed] [Google Scholar]
- 64. Ikura Y, Ohsawa M, Shirai N, Sugama Y, Fukushima H, Suekane T, Hirayama M, Ehara S, Naruko T, Ueda M. Expression of angiotensin II type 1 receptor in human cirrhotic livers: its relation to fibrosis and portal hypertension. Hepatol Res 32: 107–116, 2005 [DOI] [PubMed] [Google Scholar]
- 65. Imura A, Tsuji Y, Murata M, Maeda R, Kubota K, Iwano A, Obuse C, Togashi K, Tominaga M, Kita N, Tomiyama K, Iijima J, Nabeshima Y, Fujioka M, Asato R, Tanaka S, Kojima K, Ito J, Nozaki K, Hashimoto N, Ito T, Nishio T, Uchiyama T, Fujimori T, Nabeshima Y. α-Klotho as a regulator of calcium homeostasis. Science 316: 1615–1618, 2007 [DOI] [PubMed] [Google Scholar]
- 66. Isakova T, Gutierrez OM, Patel NM, Andress DL, Wolf M, Levin A. Vitamin D deficiency, inflammation, and albuminuria in chronic kidney disease: complex interactions. J Ren Nutr 21: 295–302, 2011 [DOI] [PubMed] [Google Scholar]
- 67. Ishizaka N, Mitani H, Nagai R. [Angiotensin II regulates klotho gene expression]. Nippon Rinsho 60: 1935–1939, 2002 [PubMed] [Google Scholar]
- 68. Jensen LP, Ras G, Boes EG. Hypercalcaemia in pregnancy: a case report. S Afr Med J 57: 712–713, 1980 [PubMed] [Google Scholar]
- 69. Judd SE, Nanes MS, Ziegler TR, Wilson PW, Tangpricha V. Optimal vitamin D status attenuates the age-associated increase in systolic blood pressure in white Americans: results from the Third National Health and Nutrition Examination Survey. Am J Clin Nutr 87: 136–141, 2008 [DOI] [PubMed] [Google Scholar]
- 70. Kaneto H, Kajimoto Y, Fujitani Y, Matsuoka T, Sakamoto K, Matsuhisa M, Yamasaki Y, Hori M. Oxidative stress induces p21 expression in pancreatic islet cells: possible implication in beta-cell dysfunction. Diabetologia 42: 1093–1097, 1999 [DOI] [PubMed] [Google Scholar]
- 71. Kang YM, Ma Y, Elks C, Zheng JP, Yang ZM, Francis J. Cross-talk between cytokines and renin-angiotensin in hypothalamic paraventricular nucleus in heart failure: role of nuclear factor-κB. Cardiovasc Res 79: 671–678, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kimura S, Zhang GX, Nishiyama A, Shokoji T, Yao L, Fan YY, Rahman M, Suzuki T, Maeta H, Abe Y. Role of NAD(P)H oxidase- and mitochondria-derived reactive oxygen species in cardioprotection of ischemic reperfusion injury by angiotensin II. Hypertension 45: 860–866, 2005 [DOI] [PubMed] [Google Scholar]
- 73. Kimura Y, Kawamura M, Owada M, Oshima T, Murooka M, Fujiwara T, Hiramori K. Effectiveness of 1,25-dihydroxyvitamin D supplementation on blood pressure reduction in a pseudohypoparathyroidism patient with high renin activity. Intern Med 38: 31–35, 1999 [DOI] [PubMed] [Google Scholar]
- 74. Koeffler HP, Amatruda T, Ikekawa N, Kobayashi Y, DeLuca HF. Induction of macrophage differentiation of human normal and leukemic myeloid stem cells by 1,25-dihydroxyvitamin D3 and its fluorinated analogues. Cancer Res 44: 5624–5628, 1984 [PubMed] [Google Scholar]
- 75. Kong J, Li YC. Effect of ANG II type I receptor antagonist and ACE inhibitor on vitamin D receptor-null mice. Am J Physiol Regul Integr Comp Physiol 285: R255–R261, 2003 [DOI] [PubMed] [Google Scholar]
- 76. Kong J, Qiao G, Zhang Z, Liu SQ, Li YC. Targeted vitamin D receptor expression in juxtaglomerular cells suppresses renin expression independent of parathyroid hormone and calcium. Kidney Int 74: 1577–1581, 2008 [DOI] [PubMed] [Google Scholar]
- 77. Krause R, Buhring M, Hopfenmuller W, Holick MF, Sharma AM. Ultraviolet B and blood pressure. Lancet 352: 709–710, 1998 [DOI] [PubMed] [Google Scholar]
- 78. Kristal-Boneh E, Froom P, Harari G, Ribak J. Association of calcitriol and blood pressure in normotensive men. Hypertension 30: 1289–1294, 1997 [DOI] [PubMed] [Google Scholar]
- 79. Kuro-o M. Klotho. Pflügers Arch 459: 333–343, 2010 [DOI] [PubMed] [Google Scholar]
- 80. Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S, Nagai R, Nabeshima YI. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 390: 45–51, 1997 [DOI] [PubMed] [Google Scholar]
- 81. Kurosu H, Yamamoto M, Clark JD, Pastor JV, Nandi A, Gurnani P, McGuinness OP, Chikuda H, Yamaguchi M, Kawaguchi H, Shimomura I, Takayama Y, Herz J, Kahn CR, Rosenblatt KP, Kuro-o M. Suppression of aging in mice by the hormone klotho. Science 309: 1829–1833, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Lamb J, Ramaswamy S, Ford HL, Contreras B, Martinez RV, Kittrell FS, Zahnow CA, Patterson N, Golub TR, Ewen ME. A mechanism of cyclin D1 action encoded in the patterns of gene expression in human cancer. Cell 114: 323–334, 2003 [DOI] [PubMed] [Google Scholar]
- 83. Lanske B, Razzaque MS. Vitamin D and aging: old concepts and new insights. J Nutr Biochem 18: 771–777, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Leung PS, Suen PM, Ip SP, Yip CK, Chen G, Lai PB. Expression and localization of AT1 receptors in hepatic Kupffer cells: its potential role in regulating a fibrogenic response. Regul Pept 116: 61–69, 2003 [DOI] [PubMed] [Google Scholar]
- 85. Levi M. Nuclear receptors in renal disease. Biochim Biophys Acta 1812: 1061–1067, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Li AC, Glass CK. The macrophage foam cell as a target for therapeutic intervention. Nat Med 8: 1235–1242, 2002 [DOI] [PubMed] [Google Scholar]
- 87. Li YC. Vitamin D regulation of the renin-angiotensin system. J Cell Biochem 88: 327–331, 2003 [DOI] [PubMed] [Google Scholar]
- 88. Li YC, Kong J, Wei M, Chen ZF, Liu SQ, Cao LP. 1,25-Dihydroxyvitamin D3 is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest 110: 229–238, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Li YC, Qiao G, Uskokovic M, Xiang W, Zheng W, Kong J. Vitamin D: a negative endocrine regulator of the renin-angiotensin system and blood pressure. J Steroid Biochem Mol Biol 89–90: 387–392, 2004 [DOI] [PubMed] [Google Scholar]
- 90. Lind L, Hanni A, Lithell H, Hvarfner A, Sorensen OH, Ljunghall S. Vitamin D is related to blood pressure and other cardiovascular risk factors in middle-aged men. Am J Hypertens 8: 894–901, 1995 [DOI] [PubMed] [Google Scholar]
- 91. Lind L, Wengle B, Wide L, Ljunghall S. Reduction of blood pressure during long-term treatment with active vitamin D (α-calcidol) is dependent on plasma renin activity and calcium status. A double-blind, placebo-controlled study. Am J Hypertens 2: 20–25, 1989 [DOI] [PubMed] [Google Scholar]
- 92. Lips P. Vitamin D physiology. Prog Biophys Mol Biol 92: 4–8, 2006 [DOI] [PubMed] [Google Scholar]
- 93. Liu M, Lee MH, Cohen M, Bommakanti M, Freedman LP. Transcriptional activation of the Cdk inhibitor p21 by vitamin D3 leads to the induced differentiation of the myelomonocytic cell line U937. Genes Dev 10: 142–153, 1996 [DOI] [PubMed] [Google Scholar]
- 94. Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, Ochoa MT, Schauber J, Wu K, Meinken C, Kamen DL, Wagner M, Bals R, Steinmeyer A, Zugel U, Gallo RL, Eisenberg D, Hewison M, Hollis BW, Adams JS, Bloom BR, Modlin RL. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science 311: 1770–1773, 2006 [DOI] [PubMed] [Google Scholar]
- 95. Looker AC, Dawson-Hughes B, Calvo MS, Gunter EW, Sahyoun NR. Serum 25-hydroxyvitamin D status of adolescents and adults in two seasonal subpopulations from NHANES III. Bone 30: 771–777, 2002 [DOI] [PubMed] [Google Scholar]
- 96. Manucha W, Oliveros L, Carrizo L, Seltzer A, Valles P. Losartan modulation on NOS isoforms and COX-2 expression in early renal fibrogenesis in unilateral obstruction. Kidney Int 65: 2091–2107, 2004 [DOI] [PubMed] [Google Scholar]
- 97. Martins D, Wolf M, Pan D, Zadshir A, Tareen N, Thadhani R, Felsenfeld A, Levine B, Mehrotra R, Norris K. Prevalence of cardiovascular risk factors and the serum levels of 25-hydroxyvitamin D in the United States: data from the Third National Health and Nutrition Examination Survey. Arch Intern Med 167: 1159–1165, 2007 [DOI] [PubMed] [Google Scholar]
- 98. Maxwell SR, Thomason H, Sandler D, Leguen C, Baxter MA, Thorpe GH, Jones AF, Barnett AH. Antioxidant status in patients with uncomplicated insulin-dependent and non-insulin-dependent diabetes mellitus. Eur J Clin Invest 27: 484–490, 1997 [DOI] [PubMed] [Google Scholar]
- 99. Michos ED, Melamed ML. Vitamin D and cardiovascular disease risk. Curr Opin Clin Nutr Metab Care 11: 7–12, 2008 [DOI] [PubMed] [Google Scholar]
- 100. Mitani H, Ishizaka N, Aizawa T, Ohno M, Usui S, Suzuki T, Amaki T, Mori I, Nakamura Y, Sato M, Nangaku M, Hirata Y, Nagai R. In vivo klotho gene transfer ameliorates angiotensin II-induced renal damage. Hypertension 39: 838–843, 2002 [DOI] [PubMed] [Google Scholar]
- 101. Mitsuhashi T, Morris RC, Jr, Ives HE. 1,25-Dihydroxyvitamin D3 modulates growth of vascular smooth muscle cells. J Clin Invest 87: 1889–1895, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Mogi M, Iwai M, Horiuchi M. New insights into the regulation of angiotensin receptors. Curr Opin Nephrol Hypertens 18: 138–143, 2009 [DOI] [PubMed] [Google Scholar]
- 103. Mollnau H, Wendt M, Szocs K, Lassegue B, Schulz E, Oelze M, Li H, Bodenschatz M, August M, Kleschyov AL, Tsilimingas N, Walter U, Forstermann U, Meinertz T, Griendling K, Munzel T. Effects of angiotensin II infusion on the expression and function of NAD(P)H oxidase and components of nitric oxide/cGMP signaling. Circ Res 90: E58–E65, 2002 [DOI] [PubMed] [Google Scholar]
- 104. Muller DN, Kleinewietfeld M, Kvakan H. Vitamin D review. J Renin Angiotensin Aldosterone Syst 12: 125–128, 2011 [DOI] [PubMed] [Google Scholar]
- 105. Muller DN, Kvakan H, Luft FC. Immune-related effects in hypertension and target-organ damage. Curr Opin Nephrol Hypertens 20: 113–117, 2011 [DOI] [PubMed] [Google Scholar]
- 106. Nemere I, Dormanen MC, Hammond MW, Okamura WH, Norman AW. Identification of a specific binding protein for 1α,25-dihydroxyvitamin D3 in basal-lateral membranes of chick intestinal epithelium and relationship to transcaltachia. J Biol Chem 269: 23750–23756, 1994 [PubMed] [Google Scholar]
- 107. Nemerovski CW, Dorsch MP, Simpson RU, Bone HG, Aaronson KD, Bleske BE. Vitamin D and cardiovascular disease. Pharmacotherapy 29: 691–708, 2009 [DOI] [PubMed] [Google Scholar]
- 108. Nguyen G, Delarue F, Burckle C, Bouzhir L, Giller T, Sraer JD. Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. J Clin Invest 109: 1417–1427, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Nishimura H. Angiotensin receptors—evolutionary overview and perspectives. Comp Biochem Physiol A Mol Integr Physiol 128: 11–30, 2001 [DOI] [PubMed] [Google Scholar]
- 110. O'Kelly J, Hisatake J, Hisatake Y, Bishop J, Norman A, Koeffler HP. Normal myelopoiesis but abnormal T lymphocyte responses in vitamin D receptor knockout mice. J Clin Invest 109: 1091–1099, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Oh J, Weng S, Felton SK, Bhandare S, Riek A, Butler B, Proctor BM, Petty M, Chen Z, Schechtman KB, Bernal-Mizrachi L, Bernal-Mizrachi C. 1,25(OH)2 vitamin D inhibits foam cell formation and suppresses macrophage cholesterol uptake in patients with type 2 diabetes mellitus. Circulation 120: 687–698, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Ong KL, Cheung BM, Man YB, Lau CP, Lam KS. Prevalence, awareness, treatment, and control of hypertension among United States adults 1999–2004. Hypertension 49: 69–75, 2007 [DOI] [PubMed] [Google Scholar]
- 113. Ooi JH, Chen J, Cantorna MT. Vitamin D regulation of immune function in the gut: why do T cells have vitamin D receptors? Mol Aspects Med 33: 77–82, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Oren Y, Shapira Y, Agmon-Levin N, Kivity S, Zafrir Y, Altman A, Lerner A, Shoenfeld Y. Vitamin D insufficiency in a sunny environment: a demographic and seasonal analysis. Isr Med Assoc J 12: 751–756, 2010 [PubMed] [Google Scholar]
- 115. Paizis G, Cooper ME, Schembri JM, Tikellis C, Burrell LM, Angus PW. Up-regulation of components of the renin-angiotensin system in the bile duct-ligated rat liver. Gastroenterology 123: 1667–1676, 2002 [DOI] [PubMed] [Google Scholar]
- 116. Pan L, Gross KW. Transcriptional regulation of renin: an update. Hypertension 45: 3–8, 2005 [DOI] [PubMed] [Google Scholar]
- 117. Paravicini TM, Touyz RM. Redox signaling in hypertension. Cardiovasc Res 71: 247–258, 2006 [DOI] [PubMed] [Google Scholar]
- 118. Park CW, Oh YS, Shin YS, Kim CM, Kim YS, Kim SY, Choi EJ, Chang YS, Bang BK. Intravenous calcitriol regresses myocardial hypertrophy in hemodialysis patients with secondary hyperparathyroidism. Am J Kidney Dis 33: 73–81, 1999 [DOI] [PubMed] [Google Scholar]
- 119. Paul M, Poyan Mehr A, Kreutz R. Physiology of local renin-angiotensin systems. Physiol Rev 86: 747–803, 2006 [DOI] [PubMed] [Google Scholar]
- 120. Pennathur S, Wagner JD, Leeuwenburgh C, Litwak KN, Heinecke JW. A hydroxyl radical-like species oxidizes cynomolgus monkey artery wall proteins in early diabetic vascular disease. J Clin Invest 107: 853–860, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Persson PB, Skalweit A, Mrowka R, Thiele BJ. Control of renin synthesis. Am J Physiol Regul Integr Comp Physiol 285: R491–R497, 2003 [DOI] [PubMed] [Google Scholar]
- 122. Peterson CA, Heffernan ME. Serum tumor necrosis factor-α concentrations are negatively correlated with serum 25(OH)D concentrations in healthy women. J Inflamm 5: 10, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Pfeifer M, Begerow B, Minne HW, Nachtigall D, Hansen C. Effects of a short-term vitamin D3 and calcium supplementation on blood pressure and parathyroid hormone levels in elderly women. J Clin Endocrinol Metab 86: 1633–1637, 2001 [DOI] [PubMed] [Google Scholar]
- 124. Pilz S, Tomaschitz A, Ritz E, Pieber TR. Vitamin D status and arterial hypertension: a systematic review. Nat Rev Cardiol 6: 621–630, 2009 [DOI] [PubMed] [Google Scholar]
- 125. Porsti IH. Expanding targets of vitamin D receptor activation: downregulation of several RAS components in the kidney. Kidney Int 74: 1371–1373, 2008 [DOI] [PubMed] [Google Scholar]
- 126. Pueyo ME, Arnal JF, Rami J, Michel JB. Angiotensin II stimulates the production of NO and peroxynitrite in endothelial cells. Am J Physiol Cell Physiol 274: C214–C220, 1998 [DOI] [PubMed] [Google Scholar]
- 127. Queisser N, Oteiza PI, Stopper H, Oli RG, Schupp N. Aldosterone induces oxidative stress, oxidative DNA damage and NF-κB activation in kidney tubule cells. Mol Carcinog 50: 123–135, 2011 [DOI] [PubMed] [Google Scholar]
- 128. Quesada JM, Martin-Malo A, Santiago J, Hervas F, Martinez ME, Castillo D, Barrio V, Aljama P. Effect of calcitriol on insulin secretion in uraemia. Nephrol Dial Transplant 5: 1013–1017, 1990 [DOI] [PubMed] [Google Scholar]
- 129. Rema M, Mohan V, Bhaskar A, Shanmugasundaram KR. Does oxidant stress play a role in diabetic retinopathy? Indian J Ophthalmol 43: 17–21, 1995 [PubMed] [Google Scholar]
- 130. Remuzzi G, Bertani T. Pathophysiology of progressive nephropathies. N Engl J Med 339: 1448–1456, 1998 [DOI] [PubMed] [Google Scholar]
- 131. Reschly EJ, Bainy AC, Mattos JJ, Hagey LR, Bahary N, Mada SR, Ou J, Venkataramanan R, Krasowski MD. Functional evolution of the vitamin D and pregnane X receptors. BMC Evol Biol 7: 222, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Resnick LM, Muller FB, Laragh JH. Calcium-regulating hormones in essential hypertension. Relation to plasma renin activity and sodium metabolism. Ann Intern Med 105: 649–654, 1986 [DOI] [PubMed] [Google Scholar]
- 133. Rincon-Choles H, Kasinath BS, Gorin Y, Abboud HE. Angiotensin II and growth factors in the pathogenesis of diabetic nephropathy. Kidney Int Suppl: 8–11, 2002 [DOI] [PubMed] [Google Scholar]
- 134. Ritthaler T, Scholz H, Ackermann M, Riegger G, Kurtz A, Kramer BK. Effects of endothelins on renin secretion from isolated mouse renal juxtaglomerular cells. Am J Physiol Renal Fluid Electrolyte Physiol 268: F39–F45, 1995 [DOI] [PubMed] [Google Scholar]
- 134a. Robey RB, Crane-Godreau MA. “Does sunscreen promote hypertension?” and other questions. Novel interactions between vitamin D and the renin-angiotensin axis. Focus on “The world pandemic of vitamin D deficiency could possibly be explained by cellular inflammatory response activity induced by the renin-angiotensin system.” Am J Physiol Cell Physiol (April 10, 2013). 10.1152/ajpcell.00090.2013 [DOI] [PubMed] [Google Scholar]
- 135. Rostand SG. Vitamin D, blood pressure, and African Americans: toward a unifying hypothesis. Clin J Am Soc Nephrol 5: 1697–1703, 2010 [DOI] [PubMed] [Google Scholar]
- 136. Salzet M, Deloffre L, Breton C, Vieau D, Schoofs L. The angiotensin system elements in invertebrates. Brain Res Brain Res Rev 36: 35–45, 2001 [DOI] [PubMed] [Google Scholar]
- 137. Scragg R, Sowers M, Bell C. Serum 25-hydroxyvitamin D, ethnicity, and blood pressure in the Third National Health and Nutrition Examination Survey. Am J Hypertens 20: 713–719, 2007 [DOI] [PubMed] [Google Scholar]
- 138. Schleithoff SS, Zittermann A, Tenderich G, Berthold HK, Stehle P, Koerfer R. Vitamin D supplementation improves cytokine profiles in patients with congestive heart failure: a double-blind, randomized, placebo-controlled trial. Am J Clin Nutr 83: 754–759, 2006 [DOI] [PubMed] [Google Scholar]
- 139. Serrano GL, Ritchie B, Hoffman D, Ferder L. A new concept for an old system: the anti-inflammatory paradigm of the renin-angiotensin system. Med Hypotheses 72: 584–588, 2009 [DOI] [PubMed] [Google Scholar]
- 140. Shahbazi M, Jeddi-Tehrani M, Zareie M, Salek-Moghaddam A, Akhondi MM, Bahmanpoor M, Sadeghi MR, Zarnani AH. Expression profiling of vitamin D receptor in placenta, decidua and ovary of pregnant mice. Placenta 32: 657–664, 2011 [DOI] [PubMed] [Google Scholar]
- 141. Shintani S, Terzic J, Sato A, Saraga-Babic M, O'HUigin C, Tichy H, Klein J. Do lampreys have lymphocytes? The Spi evidence. Proc Natl Acad Sci USA 97: 7417–7422, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Silvagno F, De Vivo E, Attanasio A, Gallo V, Mazzucco G, Pescarmona G. Mitochondrial localization of vitamin D receptor in human platelets and differentiated megakaryocytes. PLos One 5: e8670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Simpson RU, Hershey SH, Nibbelink KA. Characterization of heart size and blood pressure in the vitamin D receptor knockout mouse. J Steroid Biochem Mol Biol 103: 521–524, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Somjen D, Weisman Y, Kohen F, Gayer B, Limor R, Sharon O, Jaccard N, Knoll E, Stern N. 25-Hydroxyvitamin D3-1α-hydroxylase is expressed in human vascular smooth muscle cells and is upregulated by parathyroid hormone and estrogenic compounds. Circulation 111: 1666–1671, 2005 [DOI] [PubMed] [Google Scholar]
- 145. Staeva-Vieira TP, Freedman LP. 1,25-Dihydroxyvitamin D3 inhibits IFN-γ and IL-4 levels during in vitro polarization of primary murine CD4+ T cells. J Immunol 168: 1181–1189, 2002 [DOI] [PubMed] [Google Scholar]
- 146. Sugden JA, Davies JI, Witham MD, Morris AD, Struthers AD. Vitamin D improves endothelial function in patients with type 2 diabetes mellitus and low vitamin D levels. Diabet Med 25: 320–325, 2008 [DOI] [PubMed] [Google Scholar]
- 147. Swami S, Krishnan AV, Feldman D. Vitamin D metabolism and action in the prostate: implications for health and disease. Mol Cell Endocrinol 347: 61–69, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Takiishi T, Gysemans C, Bouillon R, Mathieu C. Vitamin D and diabetes. Endocrinol Metab Clin North Am 39: 419–446, 2010 [DOI] [PubMed] [Google Scholar]
- 149. Tan X, He W, Liu Y. Combination therapy with paricalcitol and trandolapril reduces renal fibrosis in obstructive nephropathy. Kidney Int 76: 1248–1257, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Tan X, Li Y, Liu Y. Paricalcitol attenuates renal interstitial fibrosis in obstructive nephropathy. J Am Soc Nephrol 17: 3382–3393, 2006 [DOI] [PubMed] [Google Scholar]
- 151. Tan X, Wen X, Liu Y. Paricalcitol inhibits renal inflammation by promoting vitamin D receptor-mediated sequestration of NF-κB signaling. J Am Soc Nephrol 19: 1741–1752, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Tanaka M, Tokunaga K, Komaba H, Itoh K, Matsushita K, Watanabe H, Kadowaki D, Maruyama T, Otagiri M, Fukagawa M. Vitamin D receptor activator reduces oxidative stress in hemodialysis patients with secondary hyperparathyroidism. Ther Apher Dial 15: 161–168, 2011 [DOI] [PubMed] [Google Scholar]
- 153. Tang R, Zhou Q, Shu J, Tang T, Ao X, Peng W, Zhang Y. [Effect of Cordyceps sinensis extract on klotho expression and apoptosis in renal tubular epithelial cells induced by angiotensin II]. Zhong Nan Da Xue Xue Bao Yi Xue Ban 34: 300–307, 2009 [PubMed] [Google Scholar]
- 154. Tangpricha V, Pearce EN, Chen TC, Holick MF. Vitamin D insufficiency among free-living healthy young adults. Am J Med 112: 659–662, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Tedgui A, Mallat Z. Cytokines in atherosclerosis: pathogenic and regulatory pathways. Physiol Rev 86: 515–581, 2006 [DOI] [PubMed] [Google Scholar]
- 156. Thompson MM, Oyama TT, Kelly FJ, Kennefick TM, Anderson S. Activity and responsiveness of the renin-angiotensin system in the aging rat. Am J Physiol Regul Integr Comp Physiol 279: R1787–R1794, 2000 [DOI] [PubMed] [Google Scholar]
- 157. Ting HH, Timimi FK, Boles KS, Creager SJ, Ganz P, Creager MA. Vitamin C improves endothelium-dependent vasodilation in patients with non-insulin-dependent diabetes mellitus. J Clin Invest 97: 22–28, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Toda N, Ayajiki K, Okamura T. Interaction of endothelial nitric oxide and angiotensin in the circulation. Pharmacol Rev 59: 54–87, 2007 [DOI] [PubMed] [Google Scholar]
- 159. Tomaschitz A, Pilz S, Ritz E, Grammer T, Drechsler C, Boehm BO, Marz W. Independent association between 1,25-dihydroxyvitamin D, 25-hydroxyvitamin D and the renin-angiotensin system: the Ludwigshafen Risk and Cardiovascular Health (LURIC) study. Clin Chim Acta 411: 1354–1360, 2010 [DOI] [PubMed] [Google Scholar]
- 160. Touyz RM. Activated oxygen metabolites: do they really play a role in angiotensin II-regulated vascular tone? J Hypertens 21: 2235–2238, 2003 [DOI] [PubMed] [Google Scholar]
- 161. Touyz RM. Molecular and cellular mechanisms in vascular injury in hypertension: role of angiotensin II. Curr Opin Nephrol Hypertens 14: 125–131, 2005 [DOI] [PubMed] [Google Scholar]
- 162. Touyz RM. Oxidative stress and vascular damage in hypertension. Curr Hypertens Rep 2: 98–105, 2000 [DOI] [PubMed] [Google Scholar]
- 163. Touyz RM. Reactive oxygen species in vascular biology: role in arterial hypertension. Expert Rev Cardiovasc Ther 1: 91–106, 2003 [DOI] [PubMed] [Google Scholar]
- 164. Tsujikawa H, Kurotaki Y, Fujimori T, Fukuda K, Nabeshima Y. Klotho, a gene related to a syndrome resembling human premature aging, functions in a negative regulatory circuit of vitamin D endocrine system. Mol Endocrinol 17: 2393–2403, 2003 [DOI] [PubMed] [Google Scholar]
- 165. Tuohimaa P. Vitamin D and aging. J Steroid Biochem Mol Biol 114: 78–84, 2009 [DOI] [PubMed] [Google Scholar]
- 166. Veniant M, Menard J, Bruneval P, Morley S, Gonzales MF, Mullins J. Vascular damage without hypertension in transgenic rats expressing prorenin exclusively in the liver. J Clin Invest 98: 1966–1970, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Verhave G, Siegert CE. Role of vitamin D in cardiovascular disease. Neth J Med 68: 113–118, 2010 [PubMed] [Google Scholar]
- 168. Wang M, Takagi G, Asai K, Resuello RG, Natividad FF, Vatner DE, Vatner SF, Lakatta EG. Aging increases aortic MMP-2 activity and angiotensin II in nonhuman primates. Hypertension 41: 1308–1316, 2003 [DOI] [PubMed] [Google Scholar]
- 169. Wang TJ, Pencina MJ, Booth SL, Jacques PF, Ingelsson E, Lanier K, Benjamin EJ, D'Agostino RB, Wolf M, Vasan RS. Vitamin D deficiency and risk of cardiovascular disease. Circulation 117: 503–511, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Wei HS, Li DG, Lu HM, Zhan YT, Wang ZR, Huang X, Zhang J, Cheng JL, Xu QF. Effects of AT1 receptor antagonist, losartan, on rat hepatic fibrosis induced by CCl4. World J Gastroenterol 6: 540–545, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Weinreich T, Landolt M, Booy C, Wuthrich R, Binswanger U. 1,25-Dihydroxyvitamin D3 stimulates transforming growth factor-β1 synthesis by mouse renal proximal tubular cells. Kidney Blood Press Res 22: 99–105, 1999 [DOI] [PubMed] [Google Scholar]
- 172. Whitfield GK, Dang HT, Schluter SF, Bernstein RM, Bunag T, Manzon LA, Hsieh G, Dominguez CE, Youson JH, Haussler MR, Marchalonis JJ. Cloning of a functional vitamin D receptor from the lamprey (Petromyzon marinus), an ancient vertebrate lacking a calcified skeleton and teeth. Endocrinology 144: 2704–2716, 2003 [DOI] [PubMed] [Google Scholar]
- 173. Xiang W, Kong J, Chen S, Cao LP, Qiao G, Zheng W, Liu W, Li X, Gardner DG, Li YC. Cardiac hypertrophy in vitamin D receptor knockout mice: role of the systemic and cardiac renin-angiotensin systems. Am J Physiol Endocrinol Metab 288: E125–E132, 2005 [DOI] [PubMed] [Google Scholar]
- 174. Yuan W, Pan W, Kong J, Zheng W, Szeto FL, Wong KE, Cohen R, Klopot A, Zhang Z, Li YC. 1,25-Dihydroxyvitamin D3 suppresses renin gene transcription by blocking the activity of the cyclic AMP response element in the renin gene promoter. J Biol Chem 282: 29821–29830, 2007 [DOI] [PubMed] [Google Scholar]
- 175. Zehnder D, Bland R, Chana RS, Wheeler DC, Howie AJ, Williams MC, Stewart PM, Hewison M. Synthesis of 1,25-dihydroxyvitamin D3 by human endothelial cells is regulated by inflammatory cytokines: a novel autocrine determinant of vascular cell adhesion. J Am Soc Nephrol 13: 621–629, 2002 [DOI] [PubMed] [Google Scholar]
- 176. Zhang Y, Kong J, Deb DK, Chang A, Li YC. Vitamin D receptor attenuates renal fibrosis by suppressing the renin-angiotensin system. J Am Soc Nephrol 21: 966–973, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Zhang Z, Sun L, Wang Y, Ning G, Minto AW, Kong J, Quigg RJ, Li YC. Renoprotective role of the vitamin D receptor in diabetic nephropathy. Kidney Int 73: 163–171, 2008 [DOI] [PubMed] [Google Scholar]
- 178. Zhang Z, Yuan W, Sun L, Szeto FL, Wong KE, Li X, Kong J, Li YC. 1,25-Dihydroxyvitamin D3 targeting of NF-κB suppresses high glucose-induced MCP-1 expression in mesangial cells. Kidney Int 72: 193–201, 2007 [DOI] [PubMed] [Google Scholar]
- 179. Zhang Z, Zhang Y, Ning G, Deb DK, Kong J, Li YC. Combination therapy with AT1 blocker and vitamin D analog markedly ameliorates diabetic nephropathy: blockade of compensatory renin increase. Proc Natl Acad Sci USA 105: 15896–15901, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Zhou C, Lu F, Cao K, Xu D, Goltzman D, Miao D. Calcium-independent and 1,25(OH)2D3-dependent regulation of the renin-angiotensin system in 1α-hydroxylase knockout mice. Kidney Int 74: 170–179, 2008 [DOI] [PubMed] [Google Scholar]
- 181. Zittermann A. Vitamin D and disease prevention with special reference to cardiovascular disease. Prog Biophys Mol Biol 92: 39–48, 2006 [DOI] [PubMed] [Google Scholar]


