Abstract
Hibernators periodically undergo profound physiological changes including dramatic reductions in metabolic, heart, and respiratory rates and core body temperature. This review discusses the effect of hypoperfusion and hypothermia observed during hibernation on glomerular filtration and renal plasma flow, as well as specific adaptations in renal architecture, vasculature, the renin-angiotensin system, and upregulation of possible protective mechanisms during the extreme conditions endured by hibernating mammals. Understanding the mechanisms of protection against organ injury during hibernation may provide insights into potential therapies for organ injury during cold storage and reimplantation during transplantation.
Keywords: hibernation, kidney, torpor, metabolism, electrolytes
mammalian hibernators exhibit heterothermy during winter months, cycling through periods of low core body temperature (CBT) for days to weeks (22) during torpor, followed by periodic arousals when CBT is elevated to 37°C for ∼12 h (Fig. 1) (63). CBT during torpor can fall to as low as −3°C in arctic ground squirrels (7) or 2–10°C in temperate-zone hibernators (22). During torpor, hibernators undergo profound physiological changes, reducing their heart rate from a summertime level of 200–300 to 3–5 beats/min (128), their respiratory rate from 100–200 to 4–6 breaths/min (33, 46) and their metabolic rate to as low as 1–5% of basal metabolic rate (47). During arousal, organs undergo rapid metabolic reactivation, reperfusion, and rewarming to near normal levels (22). After maintaining euthermia and high metabolic activity for 12–18 h, the hibernator reenters torpor (Figs. 1 and 2). This review will focus on the renal adaptations employed by hibernators that permit their kidneys to withstand extreme fluctuations in CBT and organ perfusion during winter heterothermy. Studies of hibernation have been conducted for over a century and the nomenclature used to describe the various stages of hibernation, and the criteria used to define stages of hibernation, have evolved. Earlier studies often simply compared “hibernating” with “nonhibernating,” and both terms were variably used. Hibernating often meant mid-winter torpor but was also used to refer to animals that were aroused, either naturally or more often, artificially. Nonhibernating was used to denote mid-summer euthermic animals or winter aroused animals (94). As a further complication in the nomenclatures used, some investigators examine monthly or seasonal changes whereas others examine changes that occur during one torpor-arousal cycle of a few days duration. More recent studies have employed specific definitions of stages, representing both seasonal and torpor-arousal cycles, based on CBT monitoring using radiotelemetry (62, 63, 70, 83) (Fig. 1). In addition, torpor can occur daily (referred to as “daily torpor” in “daily heterotherms”) or over several days to weeks typically during winter in hibernators (46, 125). For the purposes of this review we will 1) focus on renal function in hibernators that experience torpor for at least several consecutive days. For an extensive discussion of daily torpor, the reader is referred to previously published excellent reviews (46, 47); 2) specify the definition used to identify a stage of hibernation if it is available; 3) use “euthermia” to denote a body temperature of ∼37°C and specify the season during which euthermia is described, i.e., summer euthermia or winter euthermia during arousal; and 4) refer to dormancy and winter sleep in bears as hibernation.
Fig. 1.
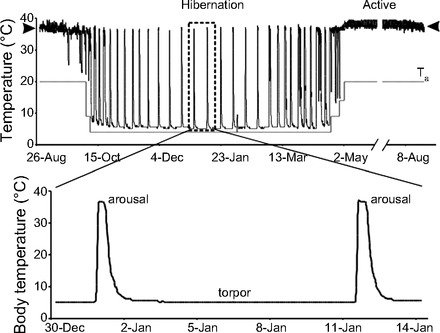
Core body temperature (CBT) changes over a year measured intraperitoneally in a laboratory-housed 13-lined ground squirrel. Top: black line plots CBT, gray line plots ambient temperature. Hibernation is the heterothermic period; arrowheads bracket euthermic CBT in both the homeothermic (active) and heterothermic (hibernation) seasons. Note that the fall transition between homeothermy and heterothermy can begin to occur before ambient temperature is lowered to the winter temperature of 4°C (94). The boxed region of the CBT trace is expanded in the bottom panel to emphasize the torpor arousal cycle. CBT nadirs at ∼4°C for multiple days during the torpor phase of hibernation during which time heart and respiratory rates decline from a summertime level of 200–300 to 3–5 beats/min and respiratory rate from 100–200 to 4–6 breaths/min (22). During periodic arousal, heart, respiratory, and metabolic rates rapidly increase, and CBT returns to euthermia. After ∼12 h, metabolic, heart, and respiratory rates decline, CBT falls again, and the hibernator reenters torpor (53).
Fig. 2.

A 13-lined ground squirrel emerges healthy and alert (A) after months of cycling between torpor (B) with low perfusion and arousal with rapid reperfusion during hibernation.
Measurement of Renal Function During Hibernation
Several lines of evidence indicate that glomerular filtration either ceases completely during torpor in small hibernators such as ground squirrels and dormice (22) or is greatly reduced in marmots and in bears. The following methods have been used to measure renal function in hibernators.
1) Indirect assessments of glomerular filtration rate (GFR) can be made by measuring the clearance of endogenously produced substances, such as creatinine, which is derived from creatine in skeletal muscle at a constant daily rate. Increased serum creatinine (SCr) generally reflects a decrease in GFR but may also result from increased creatine consumption (for example, when eating meat; Ref. 61) or a decrease in creatinine secretion, which, under conditions of normal kidney function, accounts for ∼15% of creatinine excretion (112, 117).
SCr in small hibernators.
SCr was observed to increase significantly during torpor and early arousal before normalizing to summer euthermic values during late arousal in dormice (126), European ground squirrels (101), and 13-lined ground squirrels (62). The increase in SCr during torpor could have been due to 1) a reduction in GFR, 2) an increase in creatinine production related to increased thermogenesis during early arousal, or 3) decreased tubular secretion of creatinine (117). Evidence of creatinine secretion was observed in torpid marmots (129) and in hamsters with a body temperature held at 6°C (33), suggesting that decreased tubular secretion of creatinine is not likely to account for the increased SCr. While it is possible that thermogenesis or decreased secretion may have initially led to an increase in SCr, one would not expect it to persist unless filtration was also concomitantly reduced (61, 117). Taken together, these studies suggest that in a variety of small hibernators SCr is increased during torpor because of a decrease in GFR and then normalizes during arousal when GFR returns.
SCr in bears during hibernation.
A low ratio blood urea to creatinine ratio has been suggested as a biomarker of hibernation in bears. The ratio of blood urea to creatinine was found to be low during hibernation in the American black bear, polar bear, and grizzly bear (56, 79, 105). Nelson et al. (79) also observed a decrease in urea to creatinine ratio in some bears during late summer and early fall, several weeks before denning when food was still available. The authors suggested the bears appeared to be biochemically adapted for hibernation (so called “walking hibernation”) before food became scarce and they entered their dens. The decline in the blood urea to creatinine ratio likely occurs because of decreasing urea and increasing creatinine concentration. Urea levels fall due to decreased urea production and increased recycling during hibernation (105). Hellgren et al. (56) observed a significant increase in SCr in black bears from autumn values of 1.4–1.8 to 2.0 mg/dl 6 days before hibernation, 2.6 mg/dl during the first week, and 3.3 mg/dl at 3 mo after the onset of hibernation. As discussed above, the observed increase in SCr may have been due to an increase in consumption of meat (61), increased release from skeletal muscle or a decrease in creatinine excretion via secretion (117), or decreased filtration (112). Since the bears endured extended fasts and lost an average of 23.1% of peak body mass during the study (56), muscle mass was unlikely to have increased. It seems likely therefore, that the increase in SCr was due to decreased excretion.
2) Direct measurements of GFR are best evaluated by measuring the clearance of exogenously administered agents such as inulin or radiopharmaceuticals such as 99mTechnetium-labeled diethylenetriamine pentaacetic acid (DTPA) or iodohippurate-131I (52). Several studies employing these techniques have assessed GFR during torpor and arousal in small hibernators and marmots and during hibernation in bears.
a) Technetium and iodohippurate-131I renograms in rewarming hypothermic hamsters demonstrated renal perfusion at 8°C, but an absence of glomerular filtration until the rectal temperature had risen to 10–12°C (33, 115).
b) Direct measurements of GFR by inulin clearance and urination in Columbian ground squirrels and jerboa (6, 68, 76) also support the conclusion that glomerular filtration and urine production is absent or greatly reduced during torpor but increases to near normal during arousal.
c) In torpid marmots, plasma inulin clearance decreased to ∼10% of that in euthermic aroused animals. Renal plasma flow (RPF) estimated by PAH clearance decreased to 5% of euthermic aroused values (129).
d) Brown et al. (20) examined renal function in anesthetized American black bears during activity (rectal temperature 36–38.5°C) and again during dormancy (rectal temperature 34.5–35.5°C) in the same animal. During hibernation, renal blood flow (RBF) decreased by ∼36% while GFR declined ∼68% compared with the active state. No difference in urine concentrating ability was observed during hibernation although free water clearance tended to be higher.
3) The following studies of urine output provide further evidence for absent or greatly reduced GFR during torpor in small mammals and during hibernation in bears.
a) Urinary flow was not measurable in jerboa during torpor at a CBT of 7–8°C (6).
b) Studies in the Columbian ground squirrel suggest that urine output ceases during torpor (68, 75, 76) and resumes during arousal and entrance. Since corticomedullary concentration gradients are abolished during torpor, hypertonic urine found in the bladder of a torpid squirrel was presumed to have formed during a prior arousal (76).
c) In Columbian ground squirrels implanted with a ureteral catheter, urine flow was noted to begin only after rewarming during arousal, and the lowest temperature at which urine flow began was 18°C (68).
d) Nelson et al. (80) studied two American black bears and one Himalayan bear before, during and after hibernation. Urine output in dormant bears was noted to be 95% less than during the other active states. The authors postulated that bladder reabsorption of urine permitted the bears to avoid micturition during hibernation.
4) Other indirect evidence of the decrease in GFR during torpor is the observed loss of corticomedullary concentration gradients for sodium and urea in the Columbian ground squirrel (75). A similar loss of the corticomedullary gradient for urea is also observed in hypothermic and hibernating hamsters (113), hedgehogs (26), and jerboa (6).
In summary, GFR, RBF, and urine output are profoundly reduced or absent during torpor in small hibernators and during hibernation in bears.
Putative Mechanisms of a Decreased GFR During Torpor
Glomerular filtration across the glomerular capillary into Bowman's space is determined by RPF and the net balance between Starling's forces across the glomerular capillary barrier. The relevant Starling's forces in the kidney are 1) the transcapillary hydraulic pressure gradient, 2) the transcapillary colloid osmotic pressure gradient, and 3) the permeability of the glomerular filtration barrier (generally represented as Kf, the product of the glomerular hydraulic permeability and filtration surface area; Refs. 17, 34, 35, 112). The mechanism by which GFR is reduced during torpor has not been definitively elucidated but several factors may contribute;
The observed decrease in cardiac output and mean arterial pressure (MAP) during torpor may lead to decreased RPF and consequently lower GFR (128). RPF may also be decreased by hypothermia. Hypothermia was found to reduce GFR ex vivo in isolated perfused kidneys (109) and in vivo in nonhibernating mammals due to an increase in preglomerular resistance (19). Cold exposure increases circulating concentrations of epinephrine and norepinephrine in both unanesthetized and anesthetized human subjects (50). As will be discussed later, increased sympathetic activity may mediate renal vasoconstriction in torpid animals (96, 122).
The glomerular transcapillary colloid osmotic pressure is principally determined by plasma proteins and is therefore referred to as the plasma “oncotic” pressure. An increase in plasma oncotic pressure should theoretically oppose hydraulic pressure and reduce glomerular filtration. An increase in plasma protein and oncotic pressure has been proposed as a mechanism of decreased GFR (128), although definitive studies are lacking. Furthermore, studies in rats have suggested that plasma oncotic pressure may vary with Kf or permeability but the mechanism of such an interaction is not known (112). Studies in isolated glomerular basement membranes indicate a biphasic relationship between albumin concentration and permeability, such that permeability at albumin concentrations of 4 g/dl is lower than permeability at albumin concentrations of either 0 or 8 g/dl (28). Whether a similar relationship between plasma oncotic pressure and glomerular permeability exists in hibernators is not known.
Earlier studies suggested that the glomerular basement membrane (GBM) underwent thickening during hibernation (130, 133), which could theoretically decrease Kf. The specific stage of hibernation during which the aforementioned thickening occurred was not described, and it is unclear whether it was reversed during arousal. More recent studies suggest no change occurs in GBM during the torpor-arousal cycle except for focal wrinkling during torpor (126). The differences may be attributable to species differences and use of different fixation procedures. The potential relevance of these structural changes to the glomeruli during hibernation is discussed later in Renal Morphology.
In summary, the most likely cause of decreased GFR during torpor is the observed decrease in RBF that occurs with a decrease in cardiac output, MAP, and renal vasoconstriction associated with hypothermia. The contribution, if any, of changes in the transcapillary colloid osmotic pressure gradient or the permeability of the glomerular filtration barrier remains to be determined.
Do Hibernators Arouse to Restore Kidney Function?
The physiological reason for periodic arousals during hibernation is not known. Several hypotheses have been advanced to explain the need for arousal, including the suggestion that arousal is stimulated in response to declines in plasma glucose (45), to restore metabolic homeostasis (39, and references therein), to facilitate testis growth (8), to permit activation of the immune system (90), and to replenish gene products that are catabolized but not resynthesized during torpor (40). Hypotheses specifically related to the kidney suggest that arousal may occur to clear metabolic waste, to restore water balance, and to restore electrolyte balance (90, 123). Németh et al. (81) observed that administration of the diuretic furosemide to European ground squirrels decreased torpor length and increased the frequency of arousals, presumably to allow recovery of water by fat metabolism and thus to restore water balance. Willis et al. (123) suggested that accumulated potassium loss by excitable tissue during torpor could potentially increase local extracellular potassium concentration, promote depolarization, and trigger an arousal thereby enabling a restoration of potassium balance (123). Each of the aforementioned studies illustrates that specific single events or interventions may trigger an arousal. Indeed, it has been observed that even intraperitoneal administration of 0.9% saline can trigger arousals in over 70% of treated animals (121). Since arousal may therefore be stimulated by a variety of distinct stimuli, it seems unlikely that a single specific trigger or organ system would be responsible for heterothermy during hibernation. Rather, it seems more likely that in an unperturbed hibernator, arousal serves to enable restoration of a multitude of physiological mechanisms involving several systems that are required to maintain homeostasis.
Electrolytes
An essential function of the mammalian kidney is to maintain the volume and composition of bodily fluids. Water, electrolytes, amino acids, glucose, and other endogenous compounds are freely filtered from the blood into Bowman's space and must be reabsorbed to maintain homeostasis. Water and electrolyte balance are also influenced by intake and cellular shift. The effect of decreased intake and variable glomerular filtration on water and electrolyte balance during hibernation is therefore of great interest. Several studies have examined water and electrolyte balance during hibernation as described in the following sections.
Water balance and plasma sodium concentration during hibernation.
Plasma sodium concentration reflects water balance, which is determined primarily by water intake and water loss. Under normal conditions plasma sodium concentration is the primary determinant of plasma osmolality (112). In nonhibernating mammals, excess free water consumption will lead to a decrease in plasma sodium and osmolality, a concomitant decrease antidiuretic hormone (ADH) secretion, urinary dilution, and increased free water clearance that restores plasma sodium concentration to normal. Conversely, decreased water intake will lead to an increase in plasma sodium and osmolality, a release of ADH resulting in urinary concentration and reduced free water clearance, and therefore conservation of water (112).
Water balance during torpor arousal.
There is little information regarding free water clearance or plasma ADH levels during torpor-arousal cycles. It is known, however, that neurohypophyseal ADH is stored during torpor and released only during arousal (60), consistent with the observation that urinary concentrating ability is restored during arousal (68, 104).
In the European ground squirrel, serum sodium and chloride did not significantly change during arousal and torpor compared with euthermic nonhibernating animals (101). Serial sampling of blood from an aortic arch catheter in golden-mantled ground squirrels (89) also revealed that plasma sodium concentration varies little during torpor.
With respect to water loss during hibernation, Deavers et al. (33) determined that water turnover and evaporative water loss are markedly reduced during torpor to 1–2.5% of levels observed during euthermia. Water turnover was ∼50% of that predicted from allometric relationships of water loss and body weight (33). There are no studies directly assessing free water clearance during hibernation, but one can surmise that free water clearance must be reduced or absent during torpor and can only occur during arousal when GFR is restored. The extent to which free water clearance occurs is the consequence of the plasma sodium and osmolality.
Water balance during hibernation in bears.
Nelson et al. (80) suggested that water balance is maintained in male bears throughout hibernation despite a lack of food or water intake. Serum sodium and chloride concentrations did not change significantly in bears over a 6-mo period that included fall hyperphagia and hibernation (54) suggesting that Nelson's hypothesis is correct. The ratio of urine-to-serum osmolality increases significantly in hibernating black bears during hibernation (20), a finding explained in part by the unique ability of bears to reabsorb glomerular filtrate not only in the kidney but also in the bladder (80). It has been proposed that bears metabolize fat as the main source of calories during hibernation (80, 116). Taken together, these studies suggest that water conservation during hibernation in bears is achieved by 1) recycling of water produced primarily by fat metabolism, 2) a decline in basal metabolic rate during hibernation, 3) a reduction in GFR which prevents free water loss, and 4) reabsorption of filtrate by the kidney and bladder. The end result is maintenance of serum sodium at a constant level throughout hibernation. Whether lactating female bears have disordered water balance is not known. The estimated water demand on a black bear nursing three cubs in the first 12 wk is ∼21 kg (54). Serum chemistries, including serum sodium, were not affected significantly by pregnancy or lactation in black bears suggesting that, despite the enormous water demand imposed by lactation, water balance is maintained (56). Whether the latter is achieved by increased bladder reabsorption of filtered free water or increased water production because of increased metabolic water production is not known. For further information regarding the reproductive physiology in bears, the reader is referred to the excellent review by Hellgren (54).
In summary, the observed maintenance of the serum sodium concentration throughout the torpor-arousal cycle and during hibernation in bears suggests tightly regulated plasma osmolality. During torpor, one would expect little change in plasma sodium concentration when there is minimal water turnover and free water clearance. During arousal, increased water turnover, glomerular filtration, urinary concentration, and free water clearance are presumably matched to maintain normal plasma sodium and osmolality. As noted earlier, Németh et al. (81) observed that forced water loss by the diuretic furosemide led to shortened torpor bouts and an increase in the frequency of arousal, possibly to stimulate fat metabolism and restore water balance.
Divalent ion balance during hibernation.
Serum concentrations of phosphate and calcium tend to increase progressively and peak during late torpor but subsequently normalize during late arousal (101). The latter findings are consistent with the observation that smaller hibernators (such as the 13-lined ground squirrel) may excrete calcium liberated from bone during periodic arousals (72). In contrast, hibernating bears do not excrete waste, (72), remain eucalcemic throughout hibernation (102), and do not demonstrate bone loss, possibly because of lower bone turnover (119). For further detailed information regarding bone metabolism the reader is referred to the excellent review by McGee-Lawrence et al. (72).
Serum magnesium has been found to increase during torpor across a variety of species, including 13-lined ground squirrels (93, 131), the European ground squirrel (101), the woodchuck, golden hamster, and bats (93). The observed increase in serum magnesium during hibernation has been postulated to prevent clotting (71), facilitate mitochondrial function (131), and induce and result from hypothermia during hibernation (93).
An interesting observation during hibernation in European ground squirrels is that divalent ion levels return to control values earlier than SCr during arousal, suggesting that tubular function is restored before filtration (101). Urine concentrating ability also returns before the restoration of filtration in Columbian ground squirrels (68, 104). How this may occur has not been entirely elucidated. Sandovici et al. (101) observed a significant reduction in expression of glomerular endothelial nitric oxide synthase (eNOS; a protein regulating potent vasodilation) in torpid and arousing ground squirrels vs. euthermic animals. Interstitial eNOS expression did not change. The latter suggests the possibility that peritubular capillary blood supply may be maintained throughout torpor arousal enabling an immediate restoration of tubular function during arousal (101). One may speculate that the restoration of tubular function before the return of filtration would provide a survival advantage to fasting hibernators and prevent electrolyte and water loss that would occur if filtration was restored in the absence of tubular function. It is interesting to note that large electrolyte and water losses have been commonly described in patients recovering from tubular injury in which restoration of filtration precedes tubular function (112). In addition, the tubules of small hibernators may be specifically adapted to function at low temperatures early in the arousal process. Tubular reabsorbtion of urea and urinary concentration occurred as efficiently in arousing and active Columbian ground squirrels and more efficiently than in dogs and rats artificially cooled to 25°C (68, 76).
Potassium balance during hibernation.
In contrast to the aforementioned electrolytes, which are relatively unchanged throughout a torpor-arousal cycle, or normalize by the end of arousal, serum potassium tends to reach a nadir during torpor and increase during arousal (101, 126). Monthly serum potassium and potassium deficit (estimated by the presence of periodic acid Schiff-positive intracytoplasmic granules in collecting tubule and quantified papillary granulation) was studied in woodchucks for 5 yr. Serum potassium decreased in concert with an increase in papillary granulation, which peaked in February-March and reached a nadir in June-August (25). As discussed earlier, it has been suggested that the kidney acts as a potassium “sink” during torpor, taking up potassium that has been lost by excitable tissues, thus maintaining potassium balance and preventing depolarization of excitable tissues and arousal (123).
Renal Morphology
Cold preservation followed by warm reperfusion in both mouse and human kidneys is associated with apoptosis, necrosis, and caspase activation (23, 62, 84, 106). Since hibernators repeatedly experience cold exposure and hypoperfusion followed by warm reperfusion during each torpor-arousal cycle, there has been considerable interest in examining the effects of torpor and arousal on renal architecture in hibernators.
Changes to glomerular structure during hibernation.
Gross examination of glomeruli in 13-lined ground squirrels and dormice during torpor-arousal reveal essentially normal architecture despite cold exposure for several days followed by warm reperfusion (62, 126). Detailed electron microscopic analysis of kidney tissue from summer, torpid, and spring arousing dormice revealed that the ultrastructure of the kidney cortex is well preserved in all of these states (126). Unique glomerular changes during the torpor and spring arousal were confined to focal endothelial cell and podocyte swelling (more prominent during arousal) and Golgi bodies surrounded by several small vesicles. Taken in context, the latter changes were described as “slight structural changes” at the low GFR, temperature, and MAP observed during torpor (126). Earlier studies suggested that the GBM underwent thickening during hibernation (130, 133) whereas more recent studies suggest no change occurs in GBM except for focal wrinkling during hibernation (126). It is not known whether the focal endothelial and podocyte swelling (126) and possible changes in GBM thickness (130, 133) would cause any change in glomerular capillary barrier permeability of sufficient magnitude to result in the precipitous decline in GFR during torpor or permit the restoration of GFR during arousal. In rats, small changes in net glomerular permeability do not significantly affect GFR, as it is the rise in capillary oncotic pressure during filtration that limits the filtration of small solutes and water (16). It seems more likely therefore that alterations in glomerular plasma flow due to low cardiac output and possibly hypothermia-induced vasoconstriction (19) contribute more to the decline in GFR during torpor.
Changes to tubular structure during hibernation.
As with glomeruli, gross examination of tubules in 13-lined ground squirrels and dormice during torpor and arousal reveal essentially normal architecture, with well-preserved brush border and tubular cell apical endocytic apparatus, suggesting a preserved cytoskelelton (62, 126). Taken together these studies indicate that tubular architecture remains grossly normal throughout torpor-arousal cycles, despite the precipitous decline of perfusion and CBT, followed by warm reperfusion.
Detailed electron microscopic examination of proximal tubular cells revealed prominent endocytic apparatus (more so during hibernation than arousal), many basolateral elongated mitochondria, and maintenance of cell polarity (126). Similar changes to tubular cells are observed in garden dormice (107) and the golden-mantled ground squirrel (4). Ultrastructural changes in the endoplasmic reticulum and nuclei of proximal tubular epithelial cells have been described. Compared with controls, kidneys from torpid dormice and 13-lined ground squirrels demonstrate dilation of endoplasmic reticulum cisternae, irregularly shaped nuclei, large intranuclear inclusions, supranuclear vacuoles, saccular cavities, and greater numbers of lysosomes (107, 132). The nuclear changes were attributed to a reduction in nuclear volume in the setting of lowered metabolic function, which would be expected given the torpid state. The saccular cavities, distributed in the apical region in both torpid dormice (107) and 13-lined ground squirrels (132), were postulated to be due to passive reabsorption of tubular fluid and sodium, while the increase in lysosomes was linked to protein reabsorption (107).
Tubular epithelial cells of nonhibernating species demonstrate disruption of microfilaments and fragmentation of microtubules during warm ischemia-reperfusion (1, 66) and cold preservation (18, 118). These changes contribute to loss of cell polarity and cell death.
In contrast, tubular cells of hibernating dormice (126) and torpid 13-lined ground squirrels (62) have fully preserved brush border and apical endocytic apparatus, suggesting that the cytoskeleton is preserved during hibernation. The preservation of tubular architecture during torpor is consistent with the finding that urinary concentration (68, 104) and tubular function (101) may return before glomerular filtration during arousal.
The mechanism by which hibernating mammals preserve cellular architecture during extremes of hypoperfusion and hypothermia is not known. It could be argued that the kidneys during torpor are not truly ischemic, in which case no disruption in cellular architecture would be expected. Conversely, it is possible that hibernators upregulate unknown protective factors, which maintain cellular homeostasis and architecture during torpor arousal. For example, three proteins involved in cell redox homeostasis, peroxiredoxin 3 and 6 and glutathione reductase, are all increased during arousal, suggesting that these proteins may have a protective role during warm reperfusion after cold exposure (63).
In contrast to the well-preserved architecture of small hibernators, glomerular fibrosis has been described in kidney samples taken during the spring and autumn in brown bears (91). Picro-sirius red staining revealed considerable quantities of collagen that were not organized into fibers, situated among podocytes and mesangial cells. Whether the glomerular fibrosis was a consequence of aging, hibernation, or both is not known and highlights the paucity of data regarding the long-term effects of hibernation on organ architecture and function.
In summary, the glomerular and tubular architecture are relatively well preserved during torpor arousal, and alterations in morphology are less likely to account for the decrease in GFR observed during torpor than the observed decrease in blood pressure and renal perfusion (128). Whether exposure to repeated episodes of hibernation over several years leads to kidney fibrosis or changes in renal architecture is not known.
Control of Renal Vasculature During Hibernation
Control of the renal vasculature during hibernation is of considerable interest given the decrease in RPF that occurs during torpor. Reduced RPF may occur because of an increase in total peripheral resistance during torpor in marmots and ground squirrels (69, 129) and an increase in renal vascular resistance in black bears during dormancy (20). Several mechanisms by which the increase in renal vascular resistance may occur have been evaluated as described in the following sections.
Role of the autonomic nervous system.
Saitongdee et al. (96) examined vascular innervation of golden hamster renal arteries during torpor and arousal by staining for markers of sympathetic and parasympathetic innervation. Staining for sympathetic innervation was increased two- to threefold during torpor vs. euthermic animals and nonhibernating cold controls. Within 2 h of arousal, staining for sympathetic innervation was significantly reduced. Staining for parasympathetic innervation was absent in the renal arteries suggesting that renal vasoconstriction during torpor is mediated by the sympathetic nervous system. In support of this theory is the observation that kidney norepinephrine levels during torpor are significantly increased compared with arousal, while levels of the norepinephrine precursor tyrosine are decreased (122).
Similar vascular responses are observed ex vivo. Contractile response to transmural nerve stimulation (TNS) and exogenous norepinephrine were significantly increased in renal arterial rings taken from torpid hamsters compared with controls. Vasoconstriction in response to TNS was blocked by prazosin suggesting that sympathetic neurotransmission in hamster renal artery is mediated by norepinephrine via α1-adrenoceptors. The dilator responses of femoral arterial rings to acetylcholine was no different among torpid, euthermic and cold control hamsters, again suggesting little role for the parasympathetic nervous system in renal vascular control during torpor (64).
Role of NOS.
A decrease in endothelial cell production of vasodilatory substances may also account for an increase in renal vascular resistance during torpor. NOS-positive endothelial cells of renal arteries were found to be significantly decreased in torpid hamsters compared with aroused hamsters and summer animals (95). Sandovici et al. (101) examined the glomerular production of NO and the expression of eNOS in hibernating European ground squirrels during torpor and arousal. Glomerular eNOS staining, whole kidney eNOS mRNA levels, and NOS activity decreased in torpor and remained depressed during arousal, suggesting that ground squirrels prepare their kidney for hibernation by a specific downregulation of glomerular eNOS gene transcription.
Autoregulation.
Autoregulation maintains local RBF and GFR over a wide range of perfusion pressures (78) but will not maintain GFR and RBF at low perfusion pressures, such as <90 Torr in dogs (114). To our knowledge, autoregulatory processes have not been examined in hibernating mammals to date. Tempel et al. (114) suggested that, as in nonhibernating mammals, autoregulation is likely absent when arterial pressure is markedly reduced during torpor.
Local control of glomerular blood flow.
It is possible that local factors play a role in maintaining local control of glomerular perfusion. Indirect evidence for the latter was obtained in an immunohistochemical analysis of hibernating kidneys taken from 13-lined squirrels. Heterogeneity of glomerular staining α2-macroglobulin and albumin was observed in the renal vasculature during late torpor and early arousal, i.e., some glomeruli stained with albumin and α2-macroglobulin whereas adjacent glomeruli did not suggesting highly localized control of glomerular blood flow (63).
In summary, increased renal vascular resistance and reduced RBF during torpor appears to result from increased sympathetic activity and a decrease in NO production. During arousal, sympathetic activity decreases and permitting a return of perfusion and GFR. The contribution of autoregulation and localized control of the preglomerular vessels remains to be defined.
Renin-Angiotensin System During Hibernation
The renin-angiotensin system (RAS) plays a crucial role in potassium homeostasis and blood pressure control. Renin is secreted by the juxtaglomerular cells of the afferent arteriole, primarily in response to renal hypoperfusion and increased sympathetic activity (112). One might therefore expect an increase in renin secretion during torpor since cardiac output and renal perfusion fall (22, 129) and sympathetic activity is increased (96, 122). Kastner et al. (65) examined seasonal variation of renin and aldosterone in yellow-bellied marmots. In early torpor, plasma renin activity (PRA) was initially the same as nonhibernating spring controls but subsequently increased to levels approximately twofold greater than controls by day 9 of torpor. Plasma aldosterone concentration (PAC) followed a similar pattern, increasing from a level significantly less than controls to levels equivalent to controls by day 9 of torpor. Increasing PAC and PRA was significantly correlated with time in torpor. Aldosterone release was attributed to increasing PRA, which in turn was attributed to reduced afferent arteriolar pressure and decreased delivery of sodium to the macula densa (65).
Consistent with the increase in PAC and PRA are the observations that 1) juxtaglomerular cell activity is increased during torpor in 13-lined ground squirrels (131), and 2) the activity of the zona glomerulosa of the adrenal cortex is increased in dormice during torpor compared with euthermic summer animals. Zona glomerulosa cells from torpid hibernators had an abundance of smooth endoplasmic reticulum, larger smooth endoplasmic reticulum vesicles, and increased size of mitochondria with abundant tubular cristae suggesting an increase in adrenal steroid synthesis (127).
In summary, adrenal zona glomerulosa and juxtaglomerular cell activity increase during torpor, leading to an increase in PRA and PAC. The latter changes may be physiological responses to the reduction in MAP and increased sympathetic activity during torpor. Whether the RAS plays a role in the timing of torpor arousal is not known. Weekley and Harlow (121) found that intraperitoneal injections of Asp1Val5 angiotensin II (ANG II) significantly delayed arousal in adult 13-lined ground squirrels compared with saline injections. The ANG II receptor antagonist saralasin was found to prevent the delayed arousal while inhibition of the ANG I-converting enzyme caused significantly enhanced reentry into torpor (121). The latter results are both counterintuitive and contradictory. Since ANG II increases vascular resistance and MAP (112), one would expect an injection of ANG II to stimulate arousal, not delay it (121). Furthermore, ANG I-converting enzyme inhibition would reduce ANG II levels and therefore allow arousal to persist, rather than enhance entry into torpor. Thus whether the RAS is required for regulating torpor arousal requires further study.
Does Ischemia Occur During Torpor Arousal?
A central question is whether the profound decreases in RPF observed during torpor-arousal cycles result in kidney ischemia. Kidneys of nonhibernating mammals have high metabolic demand and are described, particularly in the outer medulla, as being on the verge of ischemia (3). Ischemia represents a leading cause of ischemic acute kidney injury and is associated with low GFR, tubular apoptosis, and necrosis and high mortality rates of ∼50% (23, 100). The word “ischemia” is derived from the Greek root “isch” meaning “a restriction” and “haema” meaning blood (87a). In a literal sense, the kidneys of torpid hibernators are ischemic relative to their well-perfused, euthermic state in summer or during winter arousals. A clinical, or perhaps pathophysiological. interpretation would suggest that ischemia refers to an imbalance of blood oxygen supply and demand leading to cellular injury or death (15). Whether clinical renal ischemia occurs during hibernation is not known. Clinical ischemia during hibernation has been proposed based on the profound reduction in tissue perfusion during torpor and the prolonged periods of apnea observed in some species (21, 43). An alternate view is that metabolism and core body temperature decrease during torpor in parallel with decreased perfusion, thus ensuring a balance between oxygen supply and demand and preventing ischemia (22). However, a risk of clinical ischemia may occur during entrance into torpor, when cardiac output falls before a significant reduction in core body temperature and cellular reactions are still occurring at euthermic rates (22). During arousal, it is possible that the rapid increase in metabolism may result in an imbalance of oxygen supply/demand in areas where blood flow is not fully restored before elevations in CBT occur (22). In this regard, it is possible that clinical ischemia does occur during entrance into and arousal from torpor and hibernators upregulate protective factors at these times to prevent injury or cell death. For example, Akt protein expression decreases in the intestinal mucosa of 13-lined ground squirrels during torpor and increases in arousal (42). Phosphorylation of Akt at both threonine 308 and serine 473 was observed during entrance and arousal in the livers of 13-lined ground squirrels leading to the suggestion that regulation of Akt phosphorylation was not simply a consequence of changing CBT and its effect on kinases and phosphatases involved in Akt regulation (73). An antiapoptotic role for Akt during these states has been proposed (42, 73).
Data regarding basal metabolic rate, O2 consumption and ATP levels in the kidneys of hibernating species are sparse. While O2 consumption of 13-lined ground squirrels during torpor was found to be 2% of euthermic values (33), in vivo renal O2 consumption has not been defined during torpor-arousal cycles. Kidney slices obtained from active and torpid bats were compared with slices from rats under conditions of euthermia and hypothermia to 9°C. Renal O2 consumption was similar in slices obtained from both active and torpid bats, when cooled to 23°C. When the temperature was reduced to 9°C, O2 consumption leveled off in active bats in a manner similar to kidney slices from rats, whereas renal O2 consumption of torpid bat kidney slices continued to decline, suggesting that respiration fell in concert with decreased temperature (58). Since bats undergo both daily and seasonal torpor (5), it is not certain that these findings are applicable to renal respiration during torpor in seasonal hibernators or whether the in vivo response is the same. Thus the question of whether the kidney becomes ischemic in the clinical sense during torpor-arousal cycles remains unresolved. It seems likely that cellular injury or death during hypoperfusion is prevented by hypometabolism and possibly by the upregulation of cellular protective factors. The following sections outline some of the work that has investigated possible mechanisms by which protection against ischemia, if it occurs, is achieved during hibernation.
Studies of Na+-K+-ATPase During Hibernation
Human donor kidneys (and other donor organs) are subjected to cold storage at 4°C to prolong the time available for organ transport and recipient preparation before transplantation. The duration of cold storage tolerated by donor organs is limited, however, and the rate of kidney transplant nonfunction rises linearly and significantly with cold storage >24 h (103). Donor kidneys subjected to prolonged cold storage demonstrate increased apoptotic tubular cell death both during prolonged preservation (84) and after transplantation (23). It has been proposed that prolonged (>24 h) exposure to cold storage results in dislocation of Na+-K+-ATPase from the tubular cell membrane to the cytoplasm leading to cell and mitochondrial swelling and activation of apoptotic pathways (97). Widely used organ preservation solutions such as the University of Wisconsin solution and histidine-tryptophane-ketoglutarate solution contain impermeants formulated in an attempt to limit cell swelling during cold ischemia (9, 51, 108).
There has consequently been an interest in understanding whether Na+-K+-ATPase behaves differently in hibernating mammals. Ex vivo Na+-K+-ATPase activity is preserved in kidney cortex preparations taken from hibernating hamsters but abolished in rat kidney cortex preparations (124). Furthermore, ex vivo Na+-K+-ATPase activity was found to be twice as great in cortical preparations taken from torpid hamsters vs. hamsters kept active at 23°C (41). In the greater Egyptian jerboa, Na+-K+-ATPase unit number and hydrolytic activity were reduced during cold exposure in the medullary thick ascending limb but increased in cortical and outer medullary collecting ducts vs. controls (10). The Na+-K+-ATPase subunit number was not affected in other nephron segments, and the Vmax of Na+-K+-ATPase did not change during deep hibernation. Segment-specific increases in Na+-K+-ATPase number and activity were related to entry into hibernation but dissociated from specific changes during torpor.
In summary, maintenance of Na+-K+-ATPase activity during cold exposure or low CBT during hibernation may contribute to reduced cell swelling and thus better cell survival in hibernating jerboa and hamsters.
Adaptations in Renal Biochemistry During Hibernation
Evidence suggests that mammalian hibernators respond to the physiological challenges of fasting, and the variable perfusion observed during torpor arousal, by regulating enzyme activity related to gluconeogenesis, energy storage, and protein endocytosis. During hibernation, the enzymes needed for renal gluconeogenesis from a variety of substrates, including of propionate and glycerol, are observed in abundance in the kidneys of 13-lined ground squirrels (49, 63). Green et al. (49) suggested that this allows for rapid resumption of gluconeogenesis whenever metabolic rates are elevated during the arousal phase of hibernation.
An examination of the kidney proteome during the torpor-arousal cycle of 13-lined ground squirrels revealed 150 proteins that differed among 6 physiological stages (63). The protein differences were broadly categorized based on function and revealed: 1) a large group of proteins generally involved with capturing and storing energy that were most abundant in summer; 2) a select subset of these also increased during each arousal from torpor, including those involved with gluconeogenesis; 3) 14 spots increased in torpor and early arousal that were enriched for plasma proteins that enter cells via the endocytic pathway; and 4) a group of proteins that were increased during summer and arousal, including enzymes involved in hexose metabolism and ketone body synthesis. It is noteworthy that the latter enzymes may also be antiapoptotic and suggest that specific responses to protect cells from ischemic stress during hibernation may exist. Hexokinase has been shown to stabilize the outer mitochondrial membrane and thus prevent the mitochondrial pathway of apoptosis (44, 48, 110). Ketone body formation during hibernation may increase the survival of ground squirrels during acute hypoxia (22, 27). The above studies suggest a complex array of renal adaptations for hibernation, some that distinguish summer homeotherms from winter heterotherms and others that alter as a function of torpor-arousal state. The changes associated with torpor are centered on conservation of substrates for energy production and upregulation of pathways that may prevent cell death or mitochondrial dysfunction in the setting of decreased CBT and perfusion.
Antioxidant Protection During Hibernation
Hibernators are potentially vulnerable to oxidative stress during the hypothermia, hypoperfusion, and warm reperfusion that characterize torpor arousal (22). It is interesting to note that human tubular cells suffer oxidative stress under similar conditions. Human tubular cells exposed to hypothermia experience oxidative stress and cell death that are reduced by antioxidants (99). Rat kidney isografts subjected to cold exposure followed by transplantation experienced a significant reduction in tubular apoptosis and necrosis when treated with the antioxidant deferoxamine (59). If hibernators do experience ischemia and oxidative stress, it is possible that endogenous antioxidants may be employed as a protective mechanism against potentially lethal conditions.
Protein expression of the antioxidant enzyme heme oxygenase 1 (HO-1) and the transcription factor nuclear factor (erythroid-derived 2)-like 2 (Nrf2) were examined in 13-lined ground squirrels (82). Oxidative stress induces Nrf2 to bind the antioxidant response element of target genes and increases the transcription of a number of antioxidative enzymes (88). Nrf2, its heterodimeric partner protein, MafG, and HO-1 protein were increased in torpid kidneys, suggesting an increase in antioxidant defense during hibernation. Proteomic analysis of 13-lined ground squirrel kidneys during arousal revealed an increase of peroxiredoxin 3 and 6 and glutathione reductase, proteins involved in cell redox homeostasis, which may also have a protective role (63). During torpor, plasma levels of the antioxidant ascorbate were found to increase three- to sixfold in arctic and 13-lined ground squirrels, suggesting that an increase in plasma ascorbate concentration during hibernation may be a physiologically significant adaptation (37, 39).
In summary, antioxidant defenses appear to be increased during hibernation and may reflect an attempt to protect against cell death during periods when hibernators might most likely to experience ischemia (i.e., during the hypoperfusion of torpor and the warm reperfusion in arousal). An increase in antioxidants may protect hibernators from a possible increase in oxygen free radicals generated during intense metabolic activity upon rewarming. However, without mechanistic studies, it is difficult to conclude that a state of ischemia requiring antioxidant protection exists during torpor arousal. It is possible, for example, that the increased antioxidant levels observed during torpor arousal simply reflect less consumption of antioxidants. Further mechanistic studies to determine whether observed increases reflect their increased production or decreased utilization will help clarify the role of these molecules during hibernation.
Renal Mitochondria
Both cold storage and warm ischemia/reperfusion result in disturbances of mitochondrial function and apoptosis in nonhibernating mammals. Mitochondrial swelling and apoptosis occurred in human tubular cells exposed to cold storage followed by rewarming (98). Cold ischemia of donor organs is also associated with mitochondrial dysfunction and activation of mitochondrial apoptotic pathways (97). Mitochondrial cytochrome c oxidase subunit 1 (COX1) synthesis and activity were significantly reduced in a rat model of brain warm ischemia/reperfusion (92), and oxidative metabolism through cytochrome oxidase was decreased in a cardiac model of warm ischemia/reperfusion (67). In contrast, gene and protein expression of COX1 and mitochondrial ATP synthase 6/8 were upregulated in 13-lined ground squirrels kidneys during torpor (57), suggesting that mitochondria of hibernating species are resistant to injury during hypothermia and hypoperfusion. In addition, a study of dormice revealed that mitochondrial number was not different between summer and torpid animals (107). These preliminary studies, as well as the finding that enzymes involved in hexose metabolism are upregulated during arousal (63), suggest the possibility that specific pathways of mitochondrial protection may be activated during torpor and arousal.
Clinical Implications of Renal Adaptation During Hibernation
That hibernators can withstand temperature extremes and warm reperfusion after cold exposure has important implications for clinical medicine. Mammalian hibernators are said to demonstrate nature's version of organ preservation (22, 62, 126). Small mammalian hibernators show little or no structural renal injury, tubular apoptosis, necrosis, or caspase-3 activity during torpor, arousal, or ex vivo preservation for 72 h (62, 126).
Attempts to replicate protection during hypoxia, ischemia and cold exposure in nonhibernating mammals have focused on inducing states of hypometabolism by various means. Several early studies (29–32, 77, 85, 87, 111) suggested the existence of a “hibernation trigger” in plasma that could induce hibernation and hypometabolism in both hibernating and nonhibernating species. Subsequently, the hibernation induction assay was proven inadequate (2, 120) and, to date, there are no substances known to induce hibernation. Nevertheless, there appears to be some effect of summer and torpid woodchuck plasma fractions on renal function in monkeys following introduction by infusion. Creatinine clearance (CrCl), urine flow rate, and creatinine production did not differ between control primates and those receiving summer plasma fractions. Primates receiving torpid plasma fractions had a significantly reduced creatinine clearance compared with untreated controls. Urine flow rate was reduced ∼ 30%, but this did not reach significance (86). These studies suggested that some type of hypometabolic state could be induced using plasma fractions from hibernating mammals, although the clinical significance of a reduction in creatinine clearance remains to be determined. These hypometabolic agents, likely opiate peptides, have also been studied in a dog model of multiorgan transplantation and organ preservation. In a study by Chien et al. (24), canine organs were removed for transplantation and treated with plasma obtained from hibernating woodchucks. Survival time and transplant organ function in the recipients of the treated donor organs were significantly improved vs. untreated controls. This preliminary study suggested that something circulating in the hibernator could improve survival and function in organs from nonhibernating species subjected to warm and cold ischemia (24). Despite these intriguing studies, little is known about potential hypometabolic agents, the mechanism(s) by which they may work, or whether they can prevent injury during renal cold and warm ischemia. In addition, the observation that 13-lined ground squirrel kidneys exposed to prolonged ex vivo cold exposure are protected from apoptotic cell death suggests protective mechanisms exist at a cellular level (62). Hydrogen sulfide (H2S) has also been reported to induce a reversible “suspended animation” in mice (11, 12) and to reduce myocardial infarct size and inflammation (38). Treatment of isolated perfused rat kidneys with 1 mM sodium hydrosulfide for 30 min significantly reduced renal O2 consumption and ATP compared with controls. Mice treated with H2S before 30 min of bilateral renal ischemia had significantly improved survival, decreased SCr, less apoptosis, and less tubular injury. Treatment of mice with H2S during reperfusion did not improve survival but reduced apoptosis and tubular damage (13). These findings suggest that the induction of a hypometabolic state affords renal protection in nonhibernating mammals (62). For an extensive discussion of hypometabolic agents as they relate to hibernation, the reader is referred to an excellent recent review by Bouma et al. (14).
In summary, hibernators exhibit a remarkable phenotype that permits tolerance of profound decreases in core body temperature, cardiac output, and MAP. GFR and urine output are significantly decreased during torpor in small hibernators and during hibernation in bears. Decreased filtration is primarily due to a decrease in RPF that results from increases in renal vascular resistance. The latter appears to be the consequence of hypothermia, increased sympathetic activity, and a decrease in NO production. During arousal, sympathetic activity in the renal circuit decreases, resulting in renal vasodilation and increased RPF and GFR. The majority of evidence suggests that the renal architecture is preserved during hibernation and that water balance and electrolyte homeostasis is maintained. Reduced water and electrolyte intake are matched by a coordinated reduction in water turnover and by urinary concentration during arousal. It is possible that hibernators prevent renal ischemia during extreme hypothermia and hypoperfusion by matching organ supply with demand. There is also evidence to suggest that mechanisms to prevent cell death or injury exist in the event that ischemia does occur. Cell volume regulation during hypothermia may be enhanced by continued activity of Na+-K+-ATPase. An increase in antioxidants, enhanced protection of mitochondria, and increase in antiapoptotic molecules such as Akt may all serve to protect cells and organs during hibernation. Whether specific “hibernation triggers” or hypometabolic agents exist is still a matter of debate and a fertile opportunity for future research. An understanding of the mechanisms of protection against kidney injury during hibernation may provide insights into potential therapies to decrease acute kidney injury due to cold storage and reimplantation during transplantation.
GRANTS
This study was supported by Veterans Affairs Merit Award 1I01BX001737–01A1 (to C. L. Edelstein), National Heart, Lung, and Blood Institute Grant HL-089049 (to S. L. Martin), and National Institute of Diabetes and Digestive and Kidney Diseases Grant 1R03-DK-096151-01 (to A. Jani).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: A.J., S.J., and C.L.E. prepared figures; A.J., S.L.M., S.J., D.K., and C.L.E. drafted manuscript; A.J., S.L.M., S.J., D.K., and C.L.E. edited and revised manuscript; A.J. and C.L.E. approved final version of manuscript.
REFERENCES
- 1.Abbate M, Bonventre JV, Brown D. The microtubule network of renal epithelial cells is disrupted by ischemia and reperfusion. Am J Physiol Renal Fluid Electrolyte Physiol 267: F971–F978, 1994 [DOI] [PubMed] [Google Scholar]
- 2.Abbotts B, Wang LC, Glass JD. Absence of evidence for a hibernation “trigger” in blood dialyzate of Richardson's ground squirrel. Cryobiology 16: 179–183, 1979 [DOI] [PubMed] [Google Scholar]
- 3.Alpern RJ, Hebert SC. Seldin and Giebisch's the Kidney: Physiology Pathophysiology 1–2. New York: Academic, 2007 [Google Scholar]
- 4.Anderson DG. Changes in renal morphology and renin secretion in the golden-mantled ground squirrel (Spermophilus lateralis) during activity and hibernation. Cell Tissue Res 262: 99–104, 1990 [DOI] [PubMed] [Google Scholar]
- 5.Audet D, Fenton MB. Heterothermy and the use of torpor by the bat eptesicus fuscus (chiroptera: vespertilionidae): a field study. Physiol Zool 61: 197–204, 1988 [Google Scholar]
- 6.Baddouri K, Hilali M. [Secretion of antidiuretic hormone and renal function during the awakening of Jaculus orientalis from hibernation]. J Physiol (Paris) 81: 202–208, 1986 [PubMed] [Google Scholar]
- 7.Barnes BM. Freeze avoidance in a mammal: body temperatures below 0 degree C in an Arctic hibernator. Science 244: 1593–1595, 1989 [DOI] [PubMed] [Google Scholar]
- 8.Barnes BM, Kretzmann M, Licht P, Zucker I. The influence of hibernation on testis growth and spermatogenesis in the golden-mantled ground squirrel, Spermophilus lateralis. Biol Reprod 35: 1289–1297, 1986 [DOI] [PubMed] [Google Scholar]
- 9.Belzer F. Organ Preservation: a Personal Perspective: Early Experience In Kidney Transplantation. (Online) http://www.stanford.edu/dept/HPS/transplant/html
- 10.Bennis C, Cheval L, Barlet-Bas C, Marsy S, Doucet A. Effects of cold exposure and hibernation on renal Na,K-ATPase of the jerboa Jaculus orientalis. Pflügers Arch 430: 471–476, 1995 [DOI] [PubMed] [Google Scholar]
- 11.Blackstone E, Morrison M, Roth MB. H2S induces a suspended animation-like state in mice. Science 308: 518, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Blackstone E, Roth MB. Suspended animation-like state protects mice from lethal hypoxia. Shock 27: 370–372, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Bos EM, Leuvenink HG, Snijder PM, Kloosterhuis NJ, Hillebrands JL, Leemans JC, Florquin S, van Goor H. Hydrogen sulfide-induced hypometabolism prevents renal ischemia/reperfusion injury. J Am Soc Nephrol 20: 1901–1905, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bouma HR, Verhaag EM, Otis JP, Heldmaier G, Swoap SJ, Strijkstra AM, Henning RH, Carey HV. Induction of torpor: mimicking natural metabolic suppression for biomedical applications. J Cell Physiol 227: 1285–1290, 2012 [DOI] [PubMed] [Google Scholar]
- 15.Braunwald E, Bonow RO. Braunwald's Heart Disease: a Textbook of Cardiovascular Medicine. Philadelphia, PA: Saunders, 2012,. p. xxiv [Google Scholar]
- 16.Brenner BM, Humes HD. Mechanics of glomerular ultrafiltration. N Engl J Med 297: 148–154, 1977 [DOI] [PubMed] [Google Scholar]
- 17.Brenner BM, Troy JL, Daugharty TM, Deen WM, Robertson CR. Dynamics of glomerular ultrafiltration in the rat. II. Plasma-flow dependence of GFR. Am J Physiol 223: 1184–1190, 1972 [DOI] [PubMed] [Google Scholar]
- 18.Breton S, Brown D. Cold-induced microtubule disruption and relocalization of membrane proteins in kidney epithelial cells. J Am Soc Nephrol 9: 155–166, 1998 [DOI] [PubMed] [Google Scholar]
- 19.Broman M, Kallskog O, Kopp UC, Wolgast M. Influence of the sympathetic nervous system on renal function during hypothermia. Acta Physiol Scand 163: 241–249, 1998 [DOI] [PubMed] [Google Scholar]
- 20.Brown DC, Mulhausen RO, Andrew DJ, Seal US. Renal function in anesthetized dormant and active bears. Am J Physiol 220: 293–298, 1971 [DOI] [PubMed] [Google Scholar]
- 21.Bullard RW, Funkhouser GE. Estimated regional blood flow by rubidium 86 distribution during arousal from hibernation. Am J Physiol 203: 266–270, 1962 [DOI] [PubMed] [Google Scholar]
- 22.Carey HV, Andrews MT, Martin SL. Mammalian hibernation: cellular and molecular responses to depressed metabolism and low temperature. Physiol Rev 83: 1153–1181, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Castaneda MP, Swiatecka-Urban A, Mitsnefes MM, Feuerstein D, Kaskel FJ, Tellis V, Devarajan P. Activation of mitochondrial apoptotic pathways in human renal allografts after ischemiareperfusion injury. Transplantation 76: 50–54, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Chien S, Oeltgen PR, Diana JN, Shi X, Nilekani SP, Salley R. Two-day preservation of major organs with autoperfusion multiorgan preparation and hibernation induction trigger. A preliminary report. J Thorac Cardiovasc Surg 102: 224–234, 1991 [PubMed] [Google Scholar]
- 25.Christian JJ. Potassium deficiency in marmots during hibernation. Science 134: 390–391, 1961 [DOI] [PubMed] [Google Scholar]
- 26.Clausen G, Storesund A. Electrolyte distribution and renal function in the hibernating hedgehog. Acta Physiol Scand 83: 4–12, 1971 [DOI] [PubMed] [Google Scholar]
- 27.D'Alecy LG, Lundy EF, Kluger MJ, Harker CT, LeMay DR, Shlafer M. Beta-hydroxybutyrate and response to hypoxia in the ground squirrel, Spermophilus tridecimlineatus. Comp Biochem Physiol B 96: 189–193, 1990 [DOI] [PubMed] [Google Scholar]
- 28.Daniels BS, Hauser EB, Deen WM, Hostetter TH. Glomerular basement membrane: in vitro studies of water and protein permeability. Am J Physiol Renal Fluid Electrolyte Physiol 262: F919–F926, 1992 [DOI] [PubMed] [Google Scholar]
- 29.Dawe AR, Spurrier WA. The blood-borne “trigger” for natural mammalian hibernation in the 13-lined ground squirrel and the woodchuck. Cryobiology 9: 163–172, 1972 [DOI] [PubMed] [Google Scholar]
- 30.Dawe AR, Spurrier WA. Hibernation induced in ground squirrels by blood transfusion. Science 163: 298–299, 1969 [DOI] [PubMed] [Google Scholar]
- 31.Dawe AR, Spurrier WA. Summer hibernation of infant (six week old) 13-lined ground squirrels, Citellus tridecemlineatus. Cryobiology 11: 33–43, 1974 [DOI] [PubMed] [Google Scholar]
- 32.Dawe AR, Spurrier WA, Armour JA. Summer hibernation induced by cryogenically preserved blood “trigger”. Science 168: 497–498, 1970 [DOI] [PubMed] [Google Scholar]
- 33.Deavers DR, Musacchia XJ. Water metabolism and renal function during hibernation and hypothermia. Fed Proc 39: 2969–2973, 1980 [PubMed] [Google Scholar]
- 34.Deen WM, Lazzara MJ, Myers BD. Structural determinants of glomerular permeability. Am J Physiol Renal Physiol 281: F579–F596, 2001 [DOI] [PubMed] [Google Scholar]
- 35.Deen WM, Troy JL, Robertson CR, Brenner BM. Dynamics of glomerular ultrafiltration in the rat. IV. Determination of the ultrafiltration coefficient. J Clin Invest 52: 1500–1508, 1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Drew KL, Toien O, Rivera PM, Smith MA, Perry G, Rice ME. Role of the antioxidant ascorbate in hibernation and warming from hibernation. Comp Biochem Physiol C Toxicol Pharmacol 133: 483–492, 2002 [DOI] [PubMed] [Google Scholar]
- 38.Elrod JW, Calvert JW, Morrison J, Doeller JE, Kraus DW, Tao L, Jiao X, Scalia R, Kiss L, Szabo C, Kimura H, Chow CW, Lefer DJ. Hydrogen sulfide attenuates myocardial ischemia-reperfusion injury by preservation of mitochondrial function. Proc Natl Acad Sci USA 104: 15560–15565, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Epperson LE, Karimpour-Fard A, Hunter LE, Martin SL. Metabolic cycles in a circannual hibernator. Physiol Genomics 43: 799–807, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Epperson LE, Martin SL. Quantitative assessment of ground squirrel mRNA levels in multiple stages of hibernation. Physiol Genomics 10: 93–102, 2002 [DOI] [PubMed] [Google Scholar]
- 41.Fang LS, Willis JS. Increase of Na-K-ATPase activity in renal cortex of hamster (Mesocricetus auratus) during pre-hibernation cold exposure. Comp Biochem Physiol A Comp Physiol 48: 687–698, 1974 [DOI] [PubMed] [Google Scholar]
- 42.Fleck CC, Carey HV. Modulation of apoptotic pathways in intestinal mucosa during hibernation. Am J Physiol Regul Integr Comp Physiol 289: R586–R595, 2005 [DOI] [PubMed] [Google Scholar]
- 43.Frerichs KU, Kennedy C, Sokoloff L, Hallenbeck JM. Local cerebral blood flow during hibernation, a model of natural tolerance to “cerebral ischemia”. J Cereb Blood Flow Metab 14: 193–205, 1994 [DOI] [PubMed] [Google Scholar]
- 44.Gall JM, Wong V, Pimental DR, Havasi A, Wang Z, Pastorino JG, Bonegio RG, Schwartz JH, Borkan SC. Hexokinase regulates Bax-mediated mitochondrial membrane injury following ischemic stress. Kidney Int 79: 1207–1216, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Galster WA, Morrison P. Cyclic changes in carbohydrate concentrations during hibernation in the arctic ground squirrel. Am J Physiol 218: 1228–1232, 1970 [DOI] [PubMed] [Google Scholar]
- 46.Geiser F. Hibernation. Curr Biol 23: R188–193, 2013 [DOI] [PubMed] [Google Scholar]
- 47.Geiser F. Metabolic rate and body temperature reduction during hibernation and daily torpor. Annu Rev Physiol 66: 239–274, 2004 [DOI] [PubMed] [Google Scholar]
- 48.Gogvadze V, Orrenius S, Zhivotovsky B. Mitochondria in cancer cells: what is so special about them? Trends Cell Biol 18: 165–173, 2008 [DOI] [PubMed] [Google Scholar]
- 49.Green CJ, Brosnan JT, Fuller BJ, Lowry M, Stubbs M, Ross BD. Effect of hibernation on liver and kidney metabolism in 13-lined ground squirrels. Comp Biochem Physiol B 79: 167–171, 1984 [DOI] [PubMed] [Google Scholar]
- 50.Greif R, Laciny S, Rajek A, Doufas AG, Sessler DI. Blood pressure response to thermoregulatory vasoconstriction during isoflurane and desflurane anesthesia. Acta Anaesthesiol Scand 47: 847–852, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guibert EE, Petrenko AY, Balaban CL, Somov AY, Rodriguez JV, Fuller BJ. Organ preservation: current concepts and new strategies for the next decade. Transfus Med Hemother 38: 125–142, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.He W, Fischman AJ. Nuclear imaging in the genitourinary tract: recent advances and future directions. Radiol Clin North Am 46: 25–43, 2008 [DOI] [PubMed] [Google Scholar]
- 53.Heldmaier G, Ortmann S, Elvert R. Natural hypometabolism during hibernation and daily torpor in mammals. Respir Physiol Neurobiol 141: 317–329, 2004 [DOI] [PubMed] [Google Scholar]
- 54.Hellgren E. Physiology of hibernation in bears (available at: http://www.bearbiology.com/fileadmin/tpl/Downloads/URSUS/Vol_10/Hellgren_Vol_10.pdf). Ursus 10: 467–477, 1995 [Google Scholar]
- 56.Hellgren EC, Vaughan MR, Kirkpatrick RL, Scanlon PF. Serial changes in metabolic correlates of hibernation in female black bears. J Mammal 71: 291–300 [Google Scholar]
- 57.Hittel DS, Storey KB. Differential expression of mitochondria-encoded genes in a hibernating mammal. J Exp Biol 205: 1625–1631, 2002 [DOI] [PubMed] [Google Scholar]
- 58.Horwitz BA. Temperature effects on oxygen uptake of liver and kidney tissues of a hibernating and a nonhibernating mammal. Physiol Zool 37: 231–239, 1964 [Google Scholar]
- 59.Huang H, He Z, Roberts LJ, 2nd, Salahudeen AK. Deferoxamine reduces cold-ischemic renal injury in a syngeneic kidney transplant model. Am J Transplant 3: 1531–1537, 2003 [DOI] [PubMed] [Google Scholar]
- 60.Hudson JW, Wang LC. Hibernation: endocrinologic aspects. Annu Rev Physiol 41: 287–303, 1979 [DOI] [PubMed] [Google Scholar]
- 61.Jacobsen FK, Christensen CK, Mogensen CE, Andreasen F, Heilskov NS. Pronounced increase in serum creatinine concentration after eating cooked meat. Br Med J 1: 1049–1050, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jani A, Epperson E, Martin J, Pacic A, Ljubanovic D, Martin SL, Edelstein CL. Renal protection from prolonged cold ischemia and warm reperfusion in hibernating squirrels. Transplantation 92: 1215–1221, 2011 [DOI] [PubMed] [Google Scholar]
- 63.Jani A, Orlicky DJ, Karimpour-Fard A, Epperson LE, Russell RL, Hunter LE, Martin SL. The changing kidney proteome provides evidence for dynamic metabolism and regional redistribution of plasma proteins during torpor-arousal cycles of hibernation. Physiol Genomics 44: 717–727, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Karoon P, Knight G, Burnstock G. Enhanced vasoconstrictor responses in renal and femoral arteries of the golden hamster during hibernation. J Physiol 512: 927–938, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kastner PR, Zatzman ML, South FE, Johnson JA. Renin-angiotensin-aldosterone system of the hibernating marmot. Am J Physiol Regul Integr Comp Physiol 234: R178–R182, 1978 [DOI] [PubMed] [Google Scholar]
- 66.Kellerman PS, Bogusky RT. Microfilament disruption occurs very early in ischemic proximal tubule cell injury. Kidney Int 42: 896–902, 1992 [DOI] [PubMed] [Google Scholar]
- 67.Lesnefsky EJ, Chen Q, Slabe TJ, Stoll MS, Minkler PE, Hassan MO, Tandler B, Hoppel CL. Ischemia, rather than reperfusion, inhibits respiration through cytochrome oxidase in the isolated, perfused rabbit heart: role of cardiolipin. Am J Physiol Heart Circ Physiol 287: H258–H267, 2004 [DOI] [PubMed] [Google Scholar]
- 68.Lesser RW, Moy R, Passmore JC, Pfeiffer EW. Renal regulation of urea excretion in arousing and homeothermic ground squirrels (Citellus columbianus). Comp Biochem Physiol 36: 291–296, 1970 [DOI] [PubMed] [Google Scholar]
- 69.Lyman CP, O'Brien RC. Autonomic control of circulation during the hibernating cycle in ground squirrels. J Physiol 168: 477–499, 1963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Martin SL, Epperson LE, Rose JC, Kurtz CC, Ane C, Carey HV. Proteomic analysis of the winter-protected phenotype of hibernating ground squirrel intestine. Am J Physiol Regul Integr Comp Physiol 295: R316–R328, 2008 [DOI] [PubMed] [Google Scholar]
- 71.Masat RJ, Luyet BJ. The effect of magnesium ion on blood-clotting times in an active-phase hibernator and a nonhibernator. Comp Biochem Physiol 22: 29–31, 1967 [DOI] [PubMed] [Google Scholar]
- 72.McGee-Lawrence ME, Stoll DM, Mantila ER, Fahrner BK, Carey HV, Donahue SW. Thirteen-lined ground squirrels (Ictidomys tridecemlineatus) show microstructural bone loss during hibernation but preserve bone macrostructural geometry and strength. J Exp Biol 214: 1240–1247, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McMullen DC, Hallenbeck JM. Regulation of Akt during torpor in the hibernating ground squirrel, Ictidomys tridecemlineatus. J Comp Physiol B 180: 927–934, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Moy RM. Renal function in the hibernating ground squirrel Spermophilus columbianus. Am J Physiol 220: 747–753, 1971 [DOI] [PubMed] [Google Scholar]
- 76.Moy RM, Lesser RW, Pfeiffer EW. Urine concentrating ability of arousing and normothermic ground squirrels (Spermophilus columbianus). Comp Biochem Physiol A Comp Physiol 41: 327–337, 1972 [DOI] [PubMed] [Google Scholar]
- 77.Myers RD, Oeltgen PR, Spurrier WA. Hibernation “trigger” injected in brain induces hypothermia and hypophagia in the monkey. Brain Res Bull 7: 691–695, 1981 [DOI] [PubMed] [Google Scholar]
- 78.Navar LG. Renal autoregulation: perspectives from whole kidney and single nephron studies. Am J Physiol Renal Fluid Electrolyte Physiol 234: F357–F370, 1978 [DOI] [PubMed] [Google Scholar]
- 79.Nelson RA, Beck TD, Steiger DL. Ratio of serum urea to serum creatinine in wild black bears. Science 226: 841–842, 1984 [DOI] [PubMed] [Google Scholar]
- 80.Nelson RA, Wahner HW, Jones JD, Ellefson RD, Zollman PE. Metabolism of bears before, during, and after winter sleep. Am J Physiol 224: 491–496, 1973 [DOI] [PubMed] [Google Scholar]
- 81.Nemeth I, Nyitrai V, Nemeth A, Altbacker V. Diuretic treatment affects the length of torpor bouts in hibernating European ground squirrels (Spermophilus citellus). J Comp Physiol B 180: 457–464, 2010 [DOI] [PubMed] [Google Scholar]
- 82.Ni Z, Storey KB. Heme oxygenase expression and Nrf2 signaling during hibernation in ground squirrels. Can J Physiol Pharmacol 88: 379–387, 2010 [DOI] [PubMed] [Google Scholar]
- 83.O'Hara BF, Watson FL, Srere HK, Kumar H, Wiler SW, Welch SK, Bitting L, Heller HC, Kilduff TS. Gene expression in the brain across the hibernation cycle. J Neurosci 19: 3781–3790, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Oberbauer R, Rohrmoser M, Regele H, Muhlbacher F, Mayer G. Apoptosis of tubular epithelial cells in donor kidney biopsies predicts early renal allograft function. J Am Soc Nephrol 10: 2006–2013, 1999 [DOI] [PubMed] [Google Scholar]
- 85.Oeltgen PR, Bergmann LC, Spurrier WA, Jones SB. Isolation of a hibernation inducing trigger(s) from the plasma of hibernating woodchucks. Prep Biochem 8: 171–188, 1978 [DOI] [PubMed] [Google Scholar]
- 86.Oeltgen PR, Blouin RA, Spurrier WA, Myers RD. Hibernation “trigger” alters renal function in the primate. Physiol Behav 34: 79–81, 1985 [DOI] [PubMed] [Google Scholar]
- 87.Oeltgen PR, Walsh JW, Hamann SR, Randall DC, Spurrier WA, Myers RD. Hibernation “trigger”: opioid-like inhibitory action on brain function of the monkey. Pharmacol Biochem Behav 17: 1271–1274, 1982 [DOI] [PubMed] [Google Scholar]
- 87a.Oxford English Dictionary. Oxford: Oxford Univ. Press, 1989 [Google Scholar]
- 88.Pearson KJ, Lewis KN, Price NL, Chang JW, Perez E, Cascajo MV, Tamashiro KL, Poosala S, Csiszar A, Ungvari Z, Kensler TW, Yamamoto M, Egan JM, Longo DL, Ingram DK, Navas P, de Cabo R. Nrf2 mediates cancer protection but not prolongevity induced by caloric restriction. Proc Natl Acad Sci USA 105: 2325–2330, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pengelley ET, Asmundson SJ, Uhlman C. Homeostasis during hibernation in the golden-mantled ground squirrel, Citellus lateralis. Comp Biochem Physiol A Comp Physiol 38: 645–653, 1971 [DOI] [PubMed] [Google Scholar]
- 90.Prendergast BJ, Freeman DA, Zucker I, Nelson RJ. Periodic arousal from hibernation is necessary for initiation of immune responses in ground squirrels. Am J Physiol Regul Integr Comp Physiol 282: R1054–R1062, 2002 [DOI] [PubMed] [Google Scholar]
- 91.Prunescu C, Serban-Parau N, Brock JH, Vaughan DM, Prunescu P. Liver and kidney structure and iron content in romanian brown bears (Ursus arctos) before and after hibernation. Comp Biochem Physiol A Mol Integr Physiol 134: 21–26, 2003 [DOI] [PubMed] [Google Scholar]
- 92.Racay P, Tatarkova Z, Drgova A, Kaplan P, Dobrota D. Ischemia-reperfusion induces inhibition of mitochondrial protein synthesis and cytochrome c oxidase activity in rat hippocampus. Physiol Res 58: 127–138, 2009 [DOI] [PubMed] [Google Scholar]
- 93.Riedesel ML. The internal environment during hibernation. Bull Musc Comp Zool 124: 421–435, 1960 [Google Scholar]
- 94.Russell RL, O'Neill PH, Epperson LE, Martin SL. Extensive use of torpor in 13-lined ground squirrels in the fall before cold exposure. J Comp Physiol B 180: 1165–1172, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Saitongdee P, Loesch A, Knight G, Milner P, Burnstock G. Ultrastructural localization of nitric oxide synthase and endothelin in the renal and mesenteric arteries of the golden hamster: differences during and after arousal from hibernation. Endothelium 6: 197–207, 1999 [DOI] [PubMed] [Google Scholar]
- 96.Saitongdee P, Milner P, Loesch A, Knight G, Burnstock G. Electron-immunocytochemical studies of perivascular nerves of mesenteric and renal arteries of golden hamsters during and after arousal from hibernation. J Anat 195: 121–130, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Salahudeen AK. Cold ischemic injury of transplanted kidneys: new insights from experimental studies. Am J Physiol Renal Physiol 287: F181–F187, 2004 [DOI] [PubMed] [Google Scholar]
- 98.Salahudeen AK, Huang H, Joshi M, Moore NA, Jenkins JK. Involvement of the mitochondrial pathway in cold storage and rewarming-associated apoptosis of human renal proximal tubular cells. Am J Transplant 3: 273–280, 2003 [DOI] [PubMed] [Google Scholar]
- 99.Salahudeen AK, Huang H, Patel P, Jenkins JK. Mechanism and prevention of cold storage-induced human renal tubular cell injury. Transplantation 70: 1424–1431, 2000 [DOI] [PubMed] [Google Scholar]
- 100.Salom MG, Ceron SN, Rodriguez F, Lopez B, Hernandez I, Martinez JG, Losa AM, Fenoy FJ. Heme oxygenase-1 induction improves ischemic renal failure: role of nitric oxide and peroxynitrite. Am J Physiol Heart Circ Physiol 293: H3542–H3549, 2007 [DOI] [PubMed] [Google Scholar]
- 101.Sandovici M, Henning RH, Hut RA, Strijkstra AM, Epema AH, van Goor H, Deelman LE. Differential regulation of glomerular and interstitial endothelial nitric oxide synthase expression in the kidney of hibernating ground squirrel. Nitric Oxide 11: 194–200, 2004 [DOI] [PubMed] [Google Scholar]
- 102.Seger RL, Cross RA, Rosen CJ, Causey RC, Gundberg CM, Carpenter TO, Chen TC, Halteman WA, Holick MF, Jakubas WJ, Keisler DH, Seger RM, Servello FA. Investigating the mechanism for maintaining eucalcemia despite immobility and anuria in the hibernating American black bear (Ursus americanus). Bone 49: 1205–1212, 2011 [DOI] [PubMed] [Google Scholar]
- 103.Shoskes DA, Cecka JM. Deleterious effects of delayed graft function in cadaveric renal transplant recipients independent of acute rejection. Transplantation 66: 1697–1701, 1998 [DOI] [PubMed] [Google Scholar]
- 104.Singer MA. Of mice and men and elephants: metabolic rate sets glomerular filtration rate. Am J Kidney Dis 37: 164–178, 2001 [DOI] [PubMed] [Google Scholar]
- 105.Singer MA. Vampire bat, shrew, and bear: comparative physiology and chronic renal failure. Am J Physiol Regul Integr Comp Physiol 282: R1583–R1592, 2002 [DOI] [PubMed] [Google Scholar]
- 106.Solez K, Racusen LC, Marcussen N, Slatnik I, Keown P, Burdick JF, Olsen S. Morphology of ischemic acute renal failure, normal function, and cyclosporine toxicity in cyclosporine-treated renal allograft recipients. Kidney Int 43: 1058–1067, 1993 [DOI] [PubMed] [Google Scholar]
- 107.Soria-Milla MA, Coca-Garcia MC. Ultrastructure of the proximal convoluted tubule in the hibernating garden dormouse (Eliomys quercinus L). Cryobiology 23: 537–542, 1986 [DOI] [PubMed] [Google Scholar]
- 108.Southard JH, Belzer FO. Organ preservation. Ann Rev Med 46: 235–247, 1995 [DOI] [PubMed] [Google Scholar]
- 109.Southard JH, Rice MJ, Ametani MS, Belzer FO. Effects of short-term hypothermic perfusion and cold storage on function of the isolated-perfused dog kidney. Cryobiology 22: 147–155, 1985 [DOI] [PubMed] [Google Scholar]
- 110.Southworth R, Davey KA, Warley A, Garlick PB. A reevaluation of the roles of hexokinase I and II in the heart. Am J Physiol Heart Circ Physiol 292: H378–H386, 2007 [DOI] [PubMed] [Google Scholar]
- 111.Spurrier WA, Folk GE, Jr, Dawe AR. Induction of summer hibernation in the 13-lined ground squirrel shown by comparative serum transfusions from arctic mammals. Cryobiology 13: 368–374, 1976 [DOI] [PubMed] [Google Scholar]
- 112.Taal MW, Brenner BM, Rector FC. Brenner & Rector's the Kidney. Philadelphia, PA: Elsevier/Saunders, 2012 [Google Scholar]
- 113.Tempel GE, Musacchia XJ. Renal function in the hibernating, and hypothermic hamster Mesocricetus auratus. Am J Physiol 228: 602–607, 1975 [DOI] [PubMed] [Google Scholar]
- 114.Tempel GE, Musacchia XJ, Jones SB. Mechanisms responsible for decreased glomerular filtration in hibernation and hypothermia. J Appl Physiol 42: 420–425, 1977 [DOI] [PubMed] [Google Scholar]
- 115.Tempel GE, Volkert W, Musacchia XJ. Renal function in hypothermic and hibernating golden hamster. Fed Proc 33: 423, 1974 [Google Scholar]
- 116.Toien O, Blake J, Edgar DM, Grahn DA, Heller HC, Barnes BM. Hibernation in black bears: independence of metabolic suppression from body temperature. Science 331: 906–909, 2011 [DOI] [PubMed] [Google Scholar]
- 117.van Acker BA, Koomen GC, Koopman MG, de Waart DR, Arisz L. Creatinine clearance during cimetidine administration for measurement of glomerular filtration rate. Lancet 340: 1326–1329, 1992 [DOI] [PubMed] [Google Scholar]
- 118.Verrey F, Groscurth P, Bolliger U. Cytoskeletal disruption in A6 kidney cells: impact on endo/exocytosis and NaCl transport regulation by antidiuretic hormone. J Membr Biol 145: 193–204, 1995 [DOI] [PubMed] [Google Scholar]
- 119.Vestergaard P, Stoen OG, Swenson JE, Mosekilde L, Heickendorff L, Frobert O. Vitamin D status and bone and connective tissue turnover in brown bears (Ursus arctos) during hibernation and the active state. PLoS One 6: e21483, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wang LC, Belke D, Jourdan ML, Lee TF, Westly J, Nurnberger F. The “hibernation induction trigger”: specificity and validity of bioassay using the 13-lined ground squirrel. Cryobiology 25: 355–362, 1988 [DOI] [PubMed] [Google Scholar]
- 121.Weekley B, Harlow HJ. Effects of pharmacological manipulation of the renin-angiotensin system on the hibernation cycle of the 13-lined ground squirrel (Spermophilus tridecemlineatus). Physiol Behav 34: 147–149, 1985 [DOI] [PubMed] [Google Scholar]
- 122.Weekley BL, Harlow HJ. Altered tyrosine and tryptophan metabolism during hypothermic hibernation in the 13-lined ground squirrel (Spermophilus tridecemlineatus). Cryobiology 24: 504–512, 1987 [DOI] [PubMed] [Google Scholar]
- 123.Willis JS, Goldman SS, Foster RF. Tissue K concentration in relation to the role of the kidney in hibernation and the cause of periodic arousal. Comp Biochem Physiol A Comp Physiol 39: 437–445, 1971 [DOI] [PubMed] [Google Scholar]
- 124.Willis JS, Ma Li N. Cold resistance of Na- K-ATPase of renal cortex of the hamster, a hibernating mammal. Am J Physiol 217: 321–326, 1969 [DOI] [PubMed] [Google Scholar]
- 125.Wilz M, Heldmaier G. Comparison of hibernation, estivation and daily torpor in the edible dormouse, Glis glis. J Comp Physiol B 170: 511–521, 2000 [DOI] [PubMed] [Google Scholar]
- 126.Zancanaro C, Malatesta M, Mannello F, Vogel P, Fakan S. The kidney during hibernation and arousal from hibernation. A natural model of organ preservation during cold ischaemia and reperfusion. Nephrol Dial Transplant 14: 1982–1990, 1999 [DOI] [PubMed] [Google Scholar]
- 127.Zancanaro C, Malatesta M, Vogel P, Fakan S. Ultrastructure of the adrenal cortex of hibernating, arousing, and euthermic dormouse, Muscardinus avellanarius. Anat Rec 249: 359–364, 1997 [DOI] [PubMed] [Google Scholar]
- 128.Zatzman ML. Renal and cardiovascular effects of hibernation and hypothermia. Cryobiology 21: 593–614, 1984 [DOI] [PubMed] [Google Scholar]
- 129.Zatzman ML, South FE. Renal function of the awake and hibernating marmot Marmota flaviventris. Am J Physiol 222: 1035–1039, 1972 [DOI] [PubMed] [Google Scholar]
- 130.Zimny ML. Glomerular ultrastructure in kidneys from some northern mammals. Comp Biochem Physiol 27: 859–863, 1968 [DOI] [PubMed] [Google Scholar]
- 131.Zimny ML, Franco EE, St Onge M, Pearson J. Ultrastructure of juxtaglomerular cells correlated with biochemical parameters in a hibernator. Comp Biochem Physiol A Comp Physiol 78: 229–235, 1984 [DOI] [PubMed] [Google Scholar]
- 132.Zimny ML, Hollier L, Clement R. Ultrastructure of the proximal convoluted tubule of a hibernator correlated with renal enzymology. Comp Biochem Physiol A Comp Physiol 40: 405–414, 1971 [DOI] [PubMed] [Google Scholar]
- 133.Zimny ML, Rigamer E. Glomerular ultrastructure in the kidney of a hibernating animal. Anat Rec 154: 87–94, 1966 [DOI] [PubMed] [Google Scholar]


