Abstract
There exist more than 30 different morphological amacrine cell types, but there may be fewer physiological types. Here we studied the amacrine cell outputs by measuring the temporal and spatial properties of feedforward inhibition to four different types of ganglion cells. These ganglion cells, each with concentric receptive field organization, appear to receive a different relative contribution of the same three forms of feed-forward inhibition, namely: local glycinergic, local sustained GABAergic, and broad transient GABAergic inhibition. Two of these inhibitory components, local glycinergic inhibition and local sustained GABAergic inhibition were localized to narrow regions confined to the dendritic fields of the ganglion cells. The third, a broad transient GABAergic inhibition, was driven from regions peripheral to the dendritic area. Each inhibitory component is also correlated with characteristic kinetics expressed in all ganglion cells: broad transient GABAergic inhibition had the shortest latency, local glycinergic inhibition had an intermediate latency, and local sustained GABAergic inhibition had the longest latency. We suggest each of these three inhibitory components represents the output from a distinct class of amacrine cell, mediates a specific visual function, and each forms a basic functional component for the four ganglion cell types. Similar subunits likely exist in the circuits of other ganglion cell types as well.
INTRODUCTION
Earlier morphological studies have identified >10 types of bipolar cells and >30 types of amacrine cells in the mammalian retina (Ghosh et al. 2004; MacNeil et al. 1999, 2004; Masland 2001; Rockhill et al. 2002). Circuit interactions between bipolar and amacrine cells lead to the complex response properties of ganglion cells. The morphologies of the bipolar cells are relatively simple: They are all narrowly ramifying and their axons terminate at ∼10 specific depths within the inner plexiform layer (MacNeil et al. 2004). But the morphologies of amacrine cells are much more diverse. The lateral extents of their processes vary from few micrometers to >1,000 μm (Badea and Nathans 2004; MacNeil and Masland 1998; MacNeil et al. 1999) and their dendrites are mono-, bi-, or multiple-stratified. The functional properties and circuitry of a few specific amacrine cells have been well studied, such as the AII (Volgyi et al. 2002), starburst amacrine cell (Euler et al. 2002; Fried et al. 2002, 2005; Lee and Zhou 2006) and a unique subtype of wide-field amacrine cell, the polyaxonal cell (Bloomfield and Volgyi 2007), but the functional properties and connectivity of most other amacrine cell types remain unclear.
Besides bipolar cells and amacrine cells, there are >10 morphological types of ganglion cells in rabbit retina (Rockhill et al. 2002). The properties of inhibitory inputs to some types of ganglion cells have been studied, such as direction selectivity ganglion cells (DSGCs) (Fried et al. 2002, 2005; Lee and Zhou 2006), local edge detector (LED) (van Wyk et al. 2006), alpha like cells (Manookin et al. 2008; Munch et al. 2009; Zaghloul et al. 2007). But the properties of inhibitory inputs to other types are not well addressed. Here we focused on the temporal and spatial properties of inhibition to four ganglion cell types. All of these ganglion cells show concentric receptive field organization, generate brisk spiking, and have small or mid-sized dendritic spread. We found one glycinergic and two GABAergic feed-forward inhibitory inputs common to all four of these cells, suggesting that these forms of inhibition comprise three common functional subunits. These amacrine cell subunits may also participate in the functional circuitry other of ganglion cell types.
METHODS
Surgery
New Zealand white rabbits (2.5 kg) were anesthetized and killed in accordance with protocols approved by the Office of Laboratory Animal Care at University of California, Berkeley. The eyes were quickly enucleated. Each eye was dissected in dim red light by first removing the vitreous then cutting away the periphery to preserve the visual streak, as described previously (Hsueh et al. 2008; Roska et al. 2006). Before recording, the retina was peeled away from sclera and flat-mounted on Millipore paper containing a 4 mm center hole. Then retina with Millipore paper was transported into a chamber filled with Ames solution. The mounts in the chamber were perfused with Ames solution at 32°C. Both the Ames solutions used for perfusion and for storing the extra visual streak pieces were saturated with a mixture of O2 95% - CO2 5% and buffered with NaCO3 to a pH of 7.4.
Identification of four types of ganglion cell
Spikes evoked by bright spots (100, 200, 300, 400, 600, and 1,000 μm) were recorded by loose patch glass electrode filled with Ames and used to identify cell types first. Only ganglion cells with soma <20 μm were selected for recording. Only ganglion cells showing pure on or off spikes were selected for whole cell patch clamp. If there were any spikes happening in both on and off of bright spots in any sizes, this neuron was excluded. Neurons with significantly sluggish spikes output were also excluded (the latency of the 1st spike was >200 ms). Neurons with brisk sustained spikes were possible on or off beta cells, and neurons with brisk transient spikes were possible on or off parasol cells. Some other types of ganglion cells were excluded based on morphology. They are bistratified on cell (Roska et al. 2006) [or G3 in Rockhill et al. (2002)], type-13 on cell (Roska and Werblin 2003), on or off delta, and on or off alpha cells (Roska et al. 2006) [or G9–G11 in Rockhill et al. (2002)].
Patch Clamp and recording
Electrode filled with intracellular solution was used for whole cell patch. Intracellular solution was cesium-based [in mM: 113 CsMeSO4 (Sigma), 1 MgSO4 (Fisher Scientific), 7.8 × 10−3 CaCl2 (Fisher Scientific), 0.5 BAPTA (Fisher Scientific), 10 HEPES (Sigma), 4 ATP-Na2 (Sigma), 0.5 GTP-Na3 (Sigma), 5 QX314 (Sigma), 7.5 Neurobiotin-Cl (Vector Labs), pH 7.2].
Excitatory currents were measured by voltage clamping the cell at the calculated reversal potential for chloride (−60 mV). Inhibitory currents were recorded by voltage clamping the cell at the cation reversal potential (0 mV). Recordings were digitized and sampled at 10 kHz. Signals were filtered and the sample rate was downgraded to 60 Hz before any other analysis, which is the same as the update rate of the stimulus (frequency of projector, 60 Hz). The valuable signals observed in this article are below this frequency.
Stimuli
A variety of stimuli were presented against a gray background using a standard DLP projector projecting onto a diffuser and then focused onto the photoreceptor layer via a condenser. Two types of stimuli were used here, bright spots and bright annuli. Background luminance was set at 2.3 μW/cm2, and the luminance of spots or annuli was set at 10.2 μW/cm2. The sizes of spot were 100, 200, 300, 400, 600, and 1,000 μm. When bright annuli were used, the outside diameter was always 1,000 μm and the inside diameters were 200, 400, or 600 μm.
Pharmacology
Experiments were repeated in the presence of pharmacological blockers of excitation and inhibition. Blockers were added to Ames medium and perfused across the preparation. Four different blockers were used in this article, and they are 20 μM 2-amino-4-phosphonobutyric acid (APB, l-AP4; Tocris) to block metabotropic glutamate receptors (selectively inactivate the on system), 5 μM SR95531 (Sigma) and 100 μM (1,2,5,6-tetrahydropyridin-4-yl)methylphosphinic acid (TPMPA) (Tocris) to block ionotropic GABAA and GABAC receptors, and 1 μM strychnine (Sigma) to block ionotropic glycinergic receptors. Recordings were repeated under Ames as a control to confirm washout in some cases. In other cases, this control current was unavailable because with all the blockers in perfusion, it was almost impossible to wash all of them out.
Data analysis
All analysis was performed with Matlab (The Mathworks). The evoked current was first averaged over a period (in Fig. 7, 0–1 s after the stimuli on or off; in Fig. 10, 0–200 ms after the stimuli on or off for transient currents, or 400–1,000 ms after the stimuli on or off for sustained currents) and then subtracted the baseline (the average current 500 ms before stimulus on). Next the average evoked currents under the stimuli of different sizes of spot (100, 200, 300, 400, 600, and 1,000 μm) were normalized (divided by the maximal value of these 6 average evoked currents). Then normalized average currents evoked by the spot of same size were averaged over neurons and shown in Figs. 7 and 10. We also used the similar way to show the spatial property of spikes outputs (Fig. 2B). For each neuron, evoked spike rates under the stimuli of different sizes of spots were extracted during 1.5 s after stimuli on (on cells) or off (off cells). Spike rates from six sizes of stimuli in one neuron were normalized (divided by the maximal value of 6 spike rates), and normalized spike rates from the stimuli of the same size were averaged over neurons and shown in Fig. 2B.
Fig. 7.
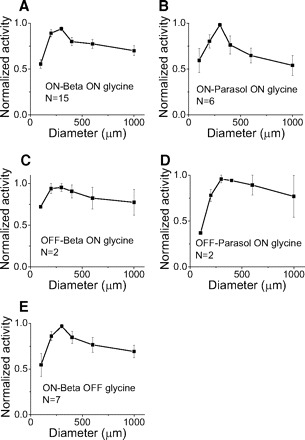
Glycinergic inhibition acts locally. It is elicited only within the dendritic area. Each of the 5 curves shows normalized activity vs. spot size. x axis, size of spots. y axis, normalized activity. Error bar, SE.
Fig. 10.
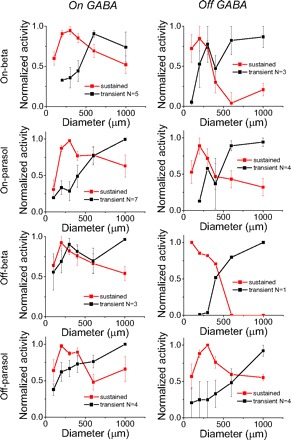
Narrow sustained and broad transient GABAergic components. Transient and sustained GABAergic components show different spatial properties. Red curves, sustained GABA. Black curves, transient GABA. Left: on GABA. Right: off GABA. Error bar, SE.
Fig. 2.
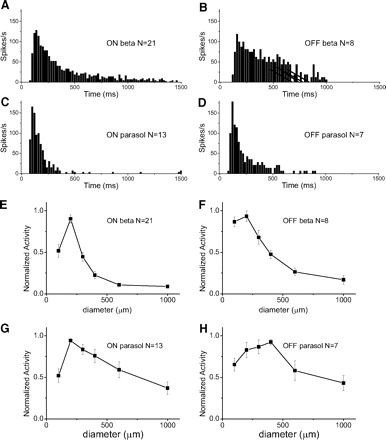
Properties of evoked spikes of 4 types of GCs. A–D: average peristimulus time histogram (PSTH) maps when white spot is on in on beta and on parasol cells, and when white spot is off in off beta and off parasol cells. Zero millisecond indicates the initiation of stimuli. Spot size, 200 μm; Bin size, 10 ms. E–H: spot size vs. normalized activity. Error bar, SE. All cell types show suppression of activity with large spots suggesting surround inhibition.
The latency when the activity of current reaches to peak in any stimulus was called peak latency, and the average peak latencies of different inhibitory components were shown in Fig. 12. Because we downgraded the sample rate to 60 Hz, the accuracy of latency was 16.7 ms, which is short enough for us to extract valuable results.
Fig. 12.
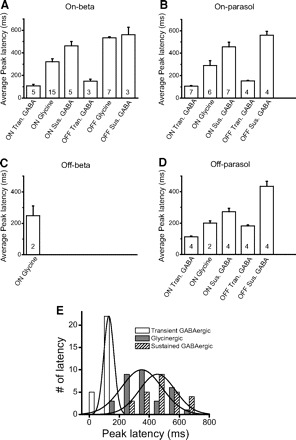
A–D: average peak latencies of different inhibitory currents. In the off beta cell, we only show the latency from on glycinergic currents because the weak GABAergic currents are hard to measure. The numbers in each bar indicate how many cases we average in that condition. Tran., transient; Sus., sustained. E: the distributions of peak latencies of transient GABAergic (open), glycinergic (gray) and sustained GABAergic (hatched) inhibition. Bin size, 100 ms. Three solid lines are the curve fitting of normal distribution.
Morphology
Retina was fixed in 4% paraformaldehyde for 1 h and then incubated in PBS solution overnight with TritonX 100 (0.5%, Sigma, increasing the permeability of membrane), Fluor 488-streptavidin conjugate (0.005 mg/ml, Invitrogen S11223, staining the soma and dendrites) and 4′,6-diamidino-2-phenylindole (DAPI) (0.01 mg/ml, Molecular Probes, staining the nuclear layers). Then retina was washed and sealed with Vectashield. The image was taken by Olympus FV1000. The dendritic size of a neuron was the average of longest diameter and shortest diameter.
Amacrine cells recording and immunocytochemistry
We recorded the excitatory and inhibitory currents with spots stimuli in some amacrine cells in flat mount retina following the same method as we did in ganglion cells. Image of amacrine cell was acquired with a grid confocal imaging system during recording (Fig. 13, D–F). Retina was fixed after recording, and the immunocytochemistry method was used to check the neurotransmitters in amacrine cells. In details, the retina was incubated in primary antibodies against glycine (raised in rat, obtained from D. Pow) and GABA (raised in rabbit, from D. Pow and Abcam) for 24–36 h at 4°C. The dilutions were 1:500 for the glycine antibody, and 1:1,000 for the GABA antibody, in 10% TritonX-100 and PBS. Fluor 488-streptavidin conjugate and DAPI were also included in incubating solution to stain the soma and dendrites of recorded cell, and to stain the nuclear layer. The retina was then washed in PBS and incubated in secondary antibodies (Jackson ImmunoResearch) rat Cy3 (1:100) and rabbit Cy5 (1:100) for 2 h at 25°C, protected from light. The retina was washed again and mounted.
Fig. 13.
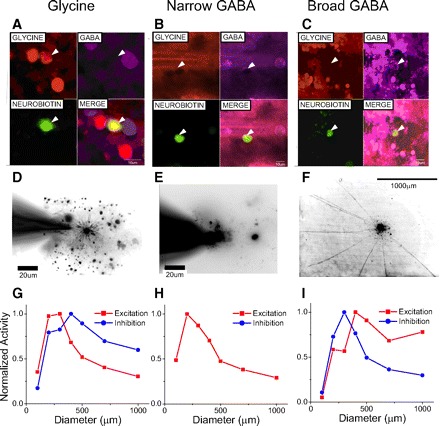
Correlation of immunostaining morphology and receptive field dimensions for diverse amacrine cells. A: immunostaining for glycine. B and C: immunostaining for GABA. In A–C, white arrowheads denote the location of the cell of interest. Top left panels: red channel indicating glycine positive cells; top right panels, purple channel indicating GABA positive cells; bottom left panels, green channel indicating the recorded cell; bottom right panels, the merge of all channels, indicating that the Alex Fluor 488 staining co-localizes with the glycine staining, but not GABA staining in A, and with GABA staining but not glycine staining in B and C. D: the morphology of an on-off narrow-field cell revealed by Alexa Fluor 488 staining. The processes of this cell terminated in the middle of the inner plexiform layer (not shown here) and spanned no more than 200 μm. E: GABA-containing narrow-field on-off amacrine cell whose spread extended for <200 μm. F: GABA-containing wide-field on amacrine cell the spread of which extended for >1,000 μm. G–I: receptive fields of excitation and inhibition. Currents are averaged 1 s after stimuli and then normalized with the peak value. If a neuron show both on and off currents, spatial-activity curves are averaged from both of them. Here glycinergic amacrine cell receives on inhibition, narrow GABAergic amacrine cell doesn't receive strong inhibition, and broad GABAergic amacrine cell receives both on and off inhibition. Both glycinergic amacrine cells and narrow GABAergic amacrine cells show narrow excitation but the broad GABAergic amacrine cells show middle excitation and doesn't receive strong surround inhibition.
RESULTS
We studied the spatial and temporal properties of inhibition in four types of ganglion cell with small and medium size concentric receptive fields. Following the classification of Roska et al. (2006), we identified them as on beta, on parasol, off beta and off parasol cells. We first measured the basic morphological and physiological properties of these four types (Figs. 1 and 2). Then we dissected the separate glycinergic and GABAergic inputs to these cells using different combinations of the blockers APB, strychnine, SR95531, and TPMPA (Figs. 3–6). Afterward, we measured the spatial properties of each of the different inhibitory components (Figs. 7–10). Finally, we measured the temporal properties of each component in Fig. 12. In total we studied inhibition to 29 on beta, 18 on parasol, 9 off beta, and 8 off parasol cells.
Fig. 1.
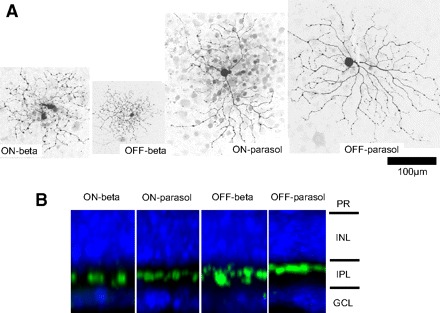
Morphologies of four types of ganglion cell. A: flat-mount view. Scale bar, 100 μm. B: stratification of dendrites. PR, photoreceptor; INL inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer.
Fig. 3.
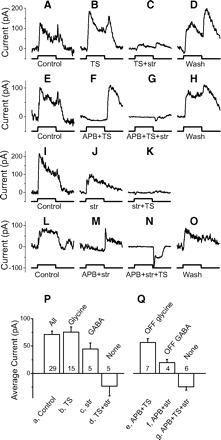
On beta cells receive both on and off glycinergic and on and off GABAergic feedforward inhibition. A–O: inhibitory currents with different combinations of drugs in 4 on beta cells. Each row represents recordings from 1 cell. Top 2 rows: isolating the glycinergic currents. Bottom 2 rows: isolating the GABAergic currents. Spots are 400 μm. In this and subsequent figures, stimulus timing is shown below each trace indicating the onset of bright spots for 1.5 s. Blockers used in perfusion were shown under stimulus timing. TS, TPMPA+SR95531; str, strychnine. P: average on currents; Q: average off currents for each transmitter. In this and subsequent figures, transmitters are shown above each bar and blockers are shown below each bar. Numbers indicate how many cases are included. The average results are only from the stimuli with 400 μm spots.
Fig. 6.
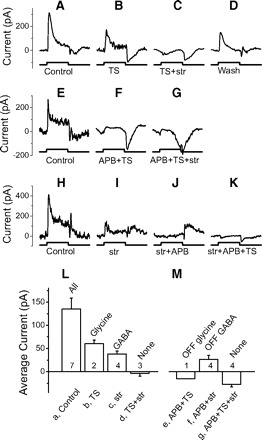
Off parasol cells receive on glycinergic and on and off GABAergic feedforward inhibition. A–K: inhibitory currents with different combinations of drugs in 3 off parasol cells. Each row represents recordings from 1 cell. Top 2 rows: isolating the glycinergic currents. Bottom row: isolating the GABAergic currents. Spots are 400 μm. L: average on currents; M: average off currents for each transmitter. The average results are only from the stimuli with 400 μm spots.
Morphological properties of four types of ganglion cells
The average dendritic diameters of the four cell types are on beta 210 ± 10 (SE) μm (n = 24), on parasol 349 ± 21 μm (n = 18), off beta 196 ± 19 μm (n = 9), and off parasol 310 ± 13 μm (n = 8). Their dendrites are stratified in 53.4 ± 2.2% (on beta, n = 7), 45.9 ± 6.8% (on parasol, n = 3), 34.0 ± 2.1% (off beta, n = 9), and 22.9 ± 1.9% (off parasol, n = 8) of the IPL. On beta and off beta cells show many small and short branches, swelling, and varicosities, and they are diffusely stratified. On parasol and off parasol cells show few short branches, mono- and narrowly stratified, with almost no overlap between branches. The dendritic morphology of each of the four types is shown in Fig. 1A and the stratification is shown in B. These ganglion cells are similar to on-G4 (on beta), off-G4 (off beta), G2 (on parasol), and G5 (off parasol) cells studied by Rockhill et al. (2002). These four types of ganglion cells are also compared with Rockhill et al. (2002) in our previous research (Roska et al. 2006). We believe that these cell types comprise most of the monostratified small and middle ganglion cells based on the morphological classification (Rockhill et al. 2002).
Physiological response properties: all four types had concentric antagonistic receptive fields
The time course of spiking, evoked by a 200 μm bright flash is shown in Fig. 2. Beta cells (Fig. 2, A and B) show more sustained responses while parasol cells (C and D) show more transient responses. Figure 2, E–H, shows that the response of all four types is suppressed with larger spots, indicating that they have antagonistic, concentric receptive fields. These cells are similar to the small or middle size brisk sustained (on and off beta) and brisk transient (on and off parasol) ganglion cells described earlier (Amthor et al. 1989b; Caldwell and Daw 1978a; Roska and Werblin 2001).
The classification of ganglion cells can be challenging. Ganglion cells can be grouped into two main categories based on morphology and function: One group includes ganglion cells with concentric receptive fields, such as alpha and beta cells. Another group includes nonconcentric organized ganglion cells, or cells with special functions, such as LED, DS cells, uniformity detector (Amthor et al. 1989a,b; Caldwell and Daw 1978a; Levick 1967). We studied four types of ganglion cell, but they comprise most of the small and medium size concentric ganglion cells. The only other concentric ganglion cell is the alpha cell, extensively studied elsewhere (Manookin et al. 2008; Munch et al. 2009; Zaghloul et al. 2007). Nonconcentric ganglion cells such as LED (van Wyk et al. 2006), DSGC (Fried et al. 2002, 2005; Lee and Zhou 2006), and G3 (Hoshi and Mills 2009) have also been characterized in other studies.
Feed-forward inhibition to on beta cells
Figure 3 shows that on beta cells receive both on and off glycinergic inhibition as well as on and off GABAergic inhibition. Figure 3, A–O, shows examples of inhibitory currents from four on beta cells perfused using different sequences of blockers. Each row represents recording from one cell, and drugs in perfusion are indicated below each trace. Figure 3, P and Q, shows the average currents generated in the presence of different drugs.
GLYCINERGIC CURRENTS.
On beta cells receive on-dominant inhibitory currents (Fig. 3, A, E, I, and L, 1st column, control responses). These currents show GABA insensitive components revealed in the presence of TPMPA and SR95531 (TS) in solution. GABA insensitive currents could be blocked by adding strychnine (Fig. 3C), and the on component of this current could be eliminated by adding APB (F), suggesting that on glycinergic amacrine cells provide inhibitory input to on beta cells. APB did not block the off component of the GABA insensitive current (Fig. 3F), but this off current was blocked by strychnine (G), suggesting that on beta cells also receive inputs from off glycinergic amacrine cells.
GABAERGIC CURRENTS.
On beta cells also receive strychnine insensitive currents (Fig. 3J), which could be blocked by TS. The on component of this current could be blocked by APB (Fig. 3M), indicating that on GABAergic amacrine cells provide input to on beta cells. APB didn't block the off component of the glycine insensitive current (Fig. 3M), but this off current could be blocked by TS (N), indicating off GABAergic amacrine cells also provide input to on beta cells.
With all the major inhibitory blockers in the perfusion, we measured some inward current at 0 mV as shown in Fig. 3N. This inward current is likely due to an incomplete space clamp of the dendritic regions and may be from off bipolar input. Although the cell was not fully space clamped, TS did block the off current in Fig. 3M, suggesting the presence of an off GABAergic current.
The average currents are shown in Fig. 3, P and Q. ANOVA test indicates there is difference among these seven currents (P < 0.0001). Currents b and c are significantly larger than current d (t-test, P < 0.0001 and P = 0.012) and current e and f are significantly larger than current g (t-test, P = 0.0002 and P = 0.04), indicating on beta cells receive glycinergic and GABAergic inhibition at both on and off.
Feed-forward inhibition to on parasol cells
Following the same procedures used for studying on beta cells, we found that on parasol cells receive on glycinergic inhibition (Fig. 4, A–D), on GABAergic inhibition (H–J) and off GABAergic inhibition (K–N). Unlike on beta cell, on parasol cell may not receive off glycinergic inhibition. This is explained in the following text.
Fig. 4.
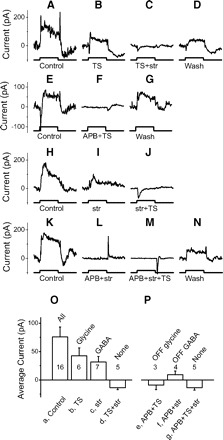
On parasol cells receive on glycinergic and on and off GABAergic feedforward inhibition. A–N: inhibitory currents with different combinations of drugs in 4 on parasol cells. Each row represents recordings from 1 cell. Top 2 rows: isolating the glycinergic currents. Bottom 2 rows: isolating the GABAergic currents. Spots are 400 μm (A–J) and 1,000 μm (K–N). O: average on currents; P: average off currents for each transmitter. The average results are only from the stimuli with 400 μm spots.
NO MEASURABLE OFF GLYCINERGIC INPUT TO ON PARASOL CELLS.
We measured little current in the presence of APB and TS (Fig. 4F), suggesting that on parasol cells receive little or no input from off glycinergic amacrine cells. If on parasol cell receives any off glycinergic input, it must be very weak because the average currents e are not significantly different from g in Fig. 4P (P = 0.59). We measured some inward currents because of inadequate space clamp (Fig. 4F), which may come from bipolar input. Furthermore, the current in Fig. 4F was not caused by bad seal (or leak) because the current returned to the control level after washing out blockers (G).
The average currents are shown in Fig. 4, O and P. ANOVA test indicates there is difference among these seven currents (P = 0.001). Currents b and c are significantly larger than current d (t-test, P = 0.006 and P = 0.004), indicating on parasol cells receive both on glycinergic and on GABAergic inhibition. Current e is not different with current g (t-test, P = 0.59), indicating on parasol cells don't receive off glycinergic inhibition. Current f is significantly different from current g (P = 0.018), indicating on parasol cells receive off GABAergic inhibition. To summarize, we show that on parasol cells receive on glycinergic inhibition and both on and off GABAergic inhibition, but receive no off glycinergic inhibition.
Feed-forward inhibition to off beta cells
Following the same procedures as used to study the on beta cells, we found that off beta cells receive on glycinergic inhibition (Fig. 5, A–C). Unlike on beta cell but similar as on parasol cell, there is no strong current remaining in the presence of APB and TS (Fig. 5E), suggesting that off beta cells receive little or no input from off glycinergic amacrine cells. Unlike on beta cell or on parasol cell, off beta cells receive very weak glycine insensitive current (Fig. 5, G and J), either in on or off. These weak residual currents decrease when ST is added to perfusion (Fig. 5, H and K), indicating off beta cells may receive weak on and off GABAergic currents.
Fig. 5.
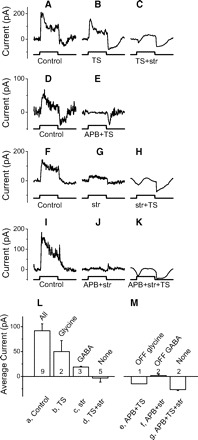
Off beta cells receive on glycinergic and very weak GABAergic feedforward inhibition. A–K: inhibitory currents with different combinations of drugs in 4 off beta cells. Each row represents recordings from 1 cell. Top 2 rows: isolating the glycinergic currents. Bottom 2 rows: isolating the GABAergic currents. Spots are 400 μm. L: average on currents; M: average off currents for each transmitter. The average results are only from the stimuli with 400 μm spots.
The average currents are shown in Fig. 5, L and M. ANOVA test indicates that there is a difference among these seven currents (P = 0.0003). Current b is significantly larger than current d (t-test, P = 0.03), although there are only two cases in current b. Moreover, we did have another three cases with strychnine in perfusion, and strychnine significantly reduced the control current (currents a and c are significantly different, t-test, P = 0.014). All of these five cases suggest that off beta cells receive on glycinergic inhibition. Current c isn't significantly different from current d (t-test, P = 0.10), indicating off beta cells may not receive on GABAergic currents. Current f is weak but significantly different with current g (P = 0.01), indicating off beta cells receive off GABAergic currents. We have only one case with APB and TS in perfusion (current e), and it isn't different from current g (t-test, P = 0.19). Together these measurements suggest that off beta cells receive dominant on glycinergic current. They also receive off GABAergic inhibition. On GABAergic inhibition is very weak and it isn't significant. Off beta cell may not receive off glycinergic inhibition.
Feed-forward inhibition to off parasol cells
Following the same procedures used for studying the on beta cells, we found that off parasol cells receive on glycinergic inhibition (Fig. 6, A–D), on and off GABAergic inhibition (H–K). Unlike the on beta cell but similar to on parasol and off beta cells, there is no strong current remaining in the presence of APB and TS (Fig. 6, E–G), suggesting that off parasol cells may not receive inputs from off glycinergic amacrine cells. Unlike the other three types of ganglion cell, we did not use GABA blockers to isolate the on glycine insensitive currents (shown in Fig. 6I). But because the dominant inhibitory currents to ganglion cells are mediated either by glycine or GABA, these glycine insensitive currents are likely GABAergic.
The average currents are shown in Fig. 6, L and M. ANOVA test indicates there is difference among these seven currents (P < 0.0001). Current b is significantly larger than current d (t-test, P = 0.008), although we only have two cases here. Moreover in another four cases, strychnine significantly reduced the control current (currents a and c are significantly different, P = 0.016). All of these six cases suggest that off parasol cells receive on glycinergic inhibition. Current c is significantly larger than current d (t-test, P = 0.007), and current f is significantly larger than current g (t-test, P = 0.002), indicating off parasol cells receive on and off GABAergic inhibition. We have only one case with APB and TS in perfusion (current e, and it isn't different with current g (t-test, P = 0.38). Thus off parasol cells appear to receive on glycinergic inhibition, and on and off GABAergic inhibition. They receive little or no off glycinergic inhibition.
Spatial properties of feedforward inhibition
SPATIAL PROPERTIES OF GLYCINERGIC INHIBITION.
Typical glycinergic amacrine cells have narrow dendritic fields (Menger et al. 1998). If bipolar cells are not strongly coupled, so that local activity is not diffused, glycinergic inhibition would drive ganglion cells from local regions within the ganglion cell dendritic fields. Figure 7 shows that activity peaked for stimulus spots of 300 μm for glycinergic inhibition. This suggests that these four types of ganglion cells receive local glycinergic inhibition confined to the dendritic area, consistent with the narrow morphology of glycinergic amacrine cells (Menger et al. 1998).
The results in Fig. 7 show that glycinergic activity is strong in regions corresponding to the dendritic area of ganglion cells, but these results do not preclude additional glycinergic input from surrounding regions. To test for broader glycinergic input, we used bright annular stimuli that fell outside the dendritic area. The outer diameter of the annuli was held at 1,000 μm, and we adjusted the inner diameter to encircle the dendritic region as shown in Fig. 8A. The results in Figs. 4–6 show that on parasol, off beta, and off parasol cells receive only on glycinergic but not off glycinergic inhibition. If the on glycinergic inhibition arose from the outside of dendritic area, the annulus would elicit an increase of inhibitory current. Instead we found that glycinergic inhibitory currents actually decreased in response to bright annuli as shown in Fig. 8, B–D. These annular stimuli enforce the notion that on glycinergic inhibition to on parasol, off beta and off parasol cells is confined to the dendritic field of these ganglion cells. The decrease in inhibition may reflect the action of the antagonistic surround of the bipolar cells that drive the glycinergic amacrine cells.
Fig. 8.
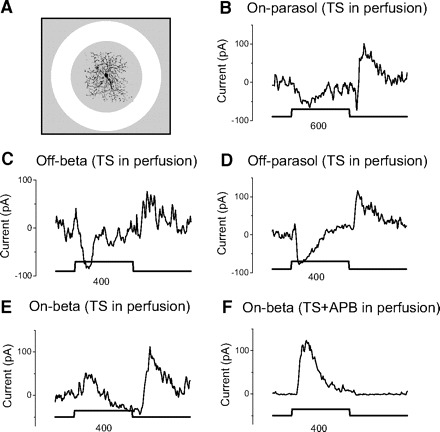
Bright annular stimuli show that all glycinergic components are elicited from within the dendritic area (center). A: the best annular stimulus to separate center and surround components. The outer diameter of annulus is always 1,000 μm, and the inner diameter is adjusted to encompass the dendrites, and shown below each trace in B–F. The diameters of dendrite of on beta, on parasol, off beta, and off parasol cells are 280, 460, 290, and 320 μm, respectively. Drugs in perfusion are shown above each inset. TS is always in the perfusion to block GABAergic currents and isolate the glycinergic currents. We did not add 2-amino-4-phosphonobutyric acid (APB) in perfusion for on parasol, off beta, and off parasol cells because from Figs. 4–6 we already knew these cells receive only on glycinergic inhibition. Square lines indicate the on (1.5 s) and off of annuli. Currents in E and F are from the same on beta cell.
On beta cells receive both on and off glycinergic inhibition, making the interpretation of the site of inhibition elicited by the annulus more difficult. In these cells inhibitory currents increased at both on and off of the annulus (Fig. 8E). But with APB added to block on activity, the off current disappeared (Fig. 8F) suggesting that this current was actually a surround-mediated on-like current from center. Conversely, the on current (in Fig. 8E) persisted when adding APB (F), indicating it was an off current from center. These results suggest that both the on and off glycinergic inhibition in on beta cells is elicited from regions corresponding to the dendritic area and not from the surround.
Because the dendritic branching of ganglion cells is often somewhat elongated, we were careful to use an annulus that was more than one but less than two times the measured receptive field diameter to assure that the annulus fell completely outside the dendrites of the recorded ganglion cell as shown in Fig. 8A.
SPATIAL PROPERTIES OF GABAERGIC INHIBITION.
The spatial properties of GABAergic inhibition are more complex than glycinergic inhibition, consisting of two temporally and spatially separable components. We designate the transient currents as those generated during the first 200 ms and the sustained currents as those generated from 400 to 1,000 ms after the initiation of the stimulus. These designations are based on the observation that most of the peaks of sustained GABAergic activity occur after 400 ms, whereas most of the peaks of transient GABAergic activity occur before 200 ms.
Figure 9A shows the on GABAergic currents in all four ganglion cell types elicited by different sized spots in the presence of strychnine. We measured both sustained (open arrows) and transient (filled arrows) GABAergic components (see methods). The largest transient currents are most evident with the largest spot, whereas the largest sustained currents are seen within 200 or 400 μm spots. Off GABAergic currents also show the same two components (Fig. 9B, with the presence of strychnine and APB). The on beta, on parasol, and off parasol cells show strong transient and sustained GABAergic currents. But the off beta cells receive only weak transient and sustained GABAergic currents. We emphasize that the off currents increased significantly after APB was added, comparing Fig. 9, A and B. It is very possible the on currents masked the off currents partially in Fig. 9A because we always use bright stimuli in our experiments and the time of stimulus is only 1.5 s. After we blocked the on current with APB, the stronger off currents were unmasked (Fig. 9B). We don't know whether dark spot stimuli could evoke stronger off currents in control because we always used bright spots. It is also possible that there is direct inhibition to off inputs from the on pathway, so that after using APB, the direct inhibition would decrease and off currents would become stronger.
Fig. 9.

Two components of GABAergic inhibitory currents are evoked by different stimulus spot sizes in the presence of strychnine. A: on GABAergic currents. B: off GABAergic currents and on currents are blocked with APB. Square lines indicate the 1.5 s flash of bright stimuli at on and off. Open arrow, peak of sustained current; filled arrow, peak of transient current. Spot sizes shown below the red lines are 200, 400, 600, and 1000 μm from left to right columns.
Figure 10 shows the average normalized activities versus the size of stimulus. Red and black curves represent sustained and transient GABAergic currents. In most of these eight insets, sustained GABAergic currents are elicited over a narrow region spanning the dendritic field: sustained activity doesn't increase for larger spots. But large spots covering regions far from the dendritic field evoke greater transient activity. This suggests that wide field amacrine cells carry transient GABAergic inhibition from the surround, while local GABAergic amacrine cells carry more locally elicited sustained inhibition to these ganglion cells.
Ganglion cells receive both on and off as well as center and surround GABAergic inhibition, making it difficult to separate components in the presence of strychnine alone. But when we eliminated on inhibition with APB, we measured two distinct components of off GABAergic inhibition in response to annular stimuli: one from the surround, the other from the center. Four examples are shown in Fig. 11. The outer diameters of annuli are always 1,000 μm. The inner diameters vary to fit the best request as we described in Fig. 8 and are shown below each trace. Because the on system has been blocked, all the currents elicited here are from off system. Filled arrows indicate the off currents from center (dendritic area) because they appear when annuli are on, while open arrows indicate the off currents from surround because they appear when annuli are off. Thus on beta, on parasol, and off parasol cells receive both sustained center and transient surround off GABAergic inhibition. Again, off beta cells show very minimal GABAergic currents that are hard to measure.
Fig. 11.
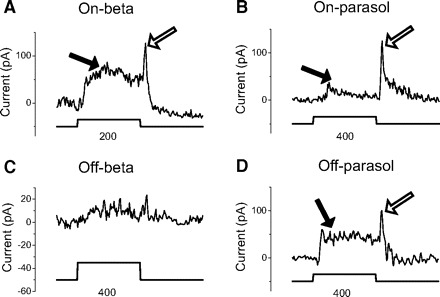
A sustained center and transient surround GABAergic inputs to ganglion cells. Bright annular stimuli clearly show 2 GABAergic components. One is from center and another is from surround. Strychnine and APB are always in perfusion to isolate the off GABAergic currents and eliminate the glycinergic currents and on GABAergic currents. Annulus outer diameter is 1,000 μm and inside diameter is either 400 or 200 μm as indicated. The diameters of the dendrites of on beta, on parasol, off beta, and off parasol cells are 190, 370, 230, and 320 μm. Stimulus trace, indicates the on (1.5 s) and off of bright annuli. Open arrows, sustained center currents; filled arrows, transient surround currents.
Temporal properties of feedforward inhibition
GLYCINERGIC, SUSTAINED-GABAERGIC AND TRANSIENT-GABAERGIC CURRENTS ARE TEMPORALLY SEPARABLE.
The average peak latencies of the different inhibitory components in the four types of ganglion cells are shown in Fig. 12. The peak latency of transient GABAergic current always has a shorter latency than that of sustained GABAergic current by definition. But the peak latency of glycinergic current, although it is sustained, may be shorter or longer. The peak latencies of transient GABAergic current are always <200 ms, and in most cases, are <150 ms. The peak latencies of sustained GABAergic current are >400 ms in most cases. An exception is the latency of the on sustained GABAergic current to off parasol cells, which is <400 ms. Glycinergic currents show intermediate peak latencies between 200 and 400 ms except the peak latency of off glycinergic current to on beta cells, which is longer and similar to that of sustained GABAergic current. We show only the glycinergic current in off beta cells but not GABAergic currents.
We have already measured the amplitude of GABAergic currents from off beta cells in Fig. 10 when we studied their spatial properties. The average current amplitude could be measured at latencies from 0 to 200 ms or from 400 to 1,000 ms. But it is difficult to calculate the latency when the currents are small.
To obtain a more accurate measure of latency, we merged the peak latencies from transient GABAergic inhibition in all the types of ganglion cells and in both on and off stimuli and showed the distribution by the black histogram in Fig. 12E. The average peak latency of transient GABAergic inhibition is 131 ± 7 ms (n = 27). Similarly, the distributions of peak latencies of glycinergic and sustained GABAergic inhibition are shown by gray and hatched histograms in Fig. 12E. The average peak latencies of glycinergic and sustained GABAergic inhibition are 349 ± 24 ms (n = 32) and 454 ± 23 ms (n = 27). Solid lines are the curve fitting of normal distribution. ANOVA test indicates there is a significant difference among these three groups of latency (P < 0.0001). The peak latency of transient GABAergic inhibition is significantly different with that of glycinergic and sustained GABAergic inhibition (t-test, P < 0.0001, P < 0.0001); the peak latency of glycinergic inhibition is significantly different with that of sustained GABAergic inhibition (t-test, P = 0.003). Thus it is likely that glycinergic, sustained GABAergic, and transient GABAergic inhibition are carried by different amacrine cell populations and provide different functions because each has a different receptive field dimension correlated with a corresponding latency of inhibition.
Correlating transmitter content with physiology and morphology
It would be useful to know which morphological amacrine cell types are responsible for each of the three major inhibitory inputs to the ganglion cells. This requires the correlation of morphology, physiology, and transmitter content in each amacrine cell population. No previous study has yet established these three-way correlations. We were able to correlate the morphology of 21 amacrine cells with their transmitter content with immuno staining (see methods). Nine cells contain glycine with average dendritic diameter of 158 ± 50 (SE) μm, and 12 cells contain GABA with average dendritic diameter of 492 ± 152 μm. This small number of successful measurements cannot unequivocally establish the correlations, but our results are consistent with earlier results in this paper and with many earlier partial studies correlating morphology with transmitter content (Bloomfield and Volgyi 2007; Menger et al. 1998).
Three examples of these correlations are shown correlating immuno staining with morphology and physiology in Fig. 13. The first column shows the narrow dendrites of a glycinergic-stained amacrine cell that correlates with the receptive field of excitatory input of 200–300 μm. The middle column shows an example of a GABA-stained amacrine cell with narrow dendrites that correlates with the narrow receptive field ∼200 μm. The right column shows a GABA-containing amacrine cell with broad dendritic spread that correlates with the very wide excitatory receptive field of ∼500 μm with no apparent inhibitory surround. These examples may be the representative of three main groups of amacrine cells that provide most of the inhibitory inputs (glycinergic, sustained GABAergic, and transient GABAergic) to ganglion cells.
DISCUSSION
We isolated three types of feed-forward inhibitory inputs that were measured in each of the four types of ganglion cells studied here. They are local glycinergic, local sustained GABAergic, and broad transient GABAergic inhibition. Each ganglion cell type receives a different relative contribution of these three inhibitory components. Two of these inhibitory components, glycinergic inhibition and sustained GABAergic inhibition, were elicited over narrow regions roughly coincident with the dendritic fields of ganglion cells. The third type, transient GABAergic inhibition, was driven from regions peripheral to the dendritic area. Each inhibitory component correlated with a distinct characteristic response latency: Broad transient GABAergic inhibition had the shortest latency, glycinergic inhibition had an intermediate latency, and sustained GABAergic inhibition had the longest latency (Fig. 12). We suggest these three inhibitory components form basic building blocks for the small and middle, monostratified narrow field concentric ganglion cells we studied here. These components may also form the major inhibitory components of other ganglion cell types as well.
We summarize the components of the feedforward inhibitory currents in Table 1. From this table we can see that all the glycinergic currents are local, no matter of they are on or off. GABAergic currents can be either local or broad, but local GABAergic currents are only sustained, and broad GABAergic currents are only transient. No broad sustained or local transient GABAergic currents were found. Only on beta cell gets off glycinergic current and off beta cell gets very weak GABAergic current.
Table 1.
Summary of different forms of feedforward inhibition to the ganglion cell studied here
| Temporal |
||||||
|---|---|---|---|---|---|---|
| Transmitters | Sign | Spatial | on Beta | on Parasol | off Beta | off Parasol |
| Glycinc | on | Local | Sustained | Sustained | Sustained | Sustained |
| off | Local | Sustained | None | None | None | |
| GABA | on | Local | Sustained | Sustained | Weak | Sustained |
| Broad | Transient | Transient | Weak | Transient | ||
| off | Local | Sustained | Sustained | Weak | Sustained | |
| Broad | Transient | Transient | Weak | Transient | ||
Summary of 6 different forms of feedforward inhibition to the 4 types of ganglion cell studied here. First row indicates the types of ganglion cell. Second row indicates the categories of inhibition. Local, inhibitory input is located within the ganglion cell dendritic field. Broad, inhibitory input is elicited from outside of dendritic field. Sustained sustained current >400 ms, tran., transient current <200 ms. None, no significant current was measured. Weak, inhibitory current is <20 pA.
Functional role for each feedforward inhibitory component
BROAD TRANSIENT GABAERGIC INHIBITION MAY MEDIATE SACCADIC SUPPRESSION BUT NOT EDGE ENHANCEMENT.
Very broad, short latency GABAergic inhibition has been associated with saccadic suppression (Roska and Werblin 2003) as well as objective motion sensitive (OMS) response (Baccus et al. 2008; Olveczky et al. 2003). This function may be carried by amacrine cells similar to the polyaxonal amacrine cells, a large group of neurons with very broad dendritic fields that appear to propagate information over longer lateral distances and can be blocked by TTX and SR (Roska and Werblin 2003; Taylor 1999; Volgyi et al. 2002). This TTX sensitive GABAergic inhibition probably does not participate the traditional surround because in light adapted retina, blocking action potentials with TTX in rabbit retina didn't affect the size of the receptive-field center or block surround responses in ganglion cells (Bloomfield 1996). Thus the wide field TTX sensitive amacrine cell activity probably contributes more to global than to local events. Sustained amacrine cells are not likely to participate in OMS response because their activity is not correlated with the excitatory activity of motion-sensitive ganglion cells (Olveczky et al. 2003).
NARROW FIELD GABAERGIC INHIBITION IS A LIKELY CANDIDATE FOR MEDIATING EDGE ENHANCEMENT.
Although a broad range of surround dimensions will work, the optimal dimension for an edge-enhancing inhibitory surround is close to the dimension of the excitatory center (Marr 1982). The narrow field GABAergic inhibition is a good candidate for edge enhancement because it has the appropriate dimensions, sign, and time course. Evidence suggests that the sustained local GABA system may provide concentric receptive field surround inhibition, and is stronger when the nearby surround was stimulated (Cook and McReynolds 1998a). Spike recording in cat ganglion cells also revealed a disinhibitory area surrounding the inhibitory area: a large and broad stimulus actually increases the spike rate (Ikeda and Wright 1972). So the optimal strength of surround inhibition is constrained by an outer more peripheral inhibition. The broad field GABAergic amacrine cell is less optimal for edge enhancement. Its dimension could be more than twice the size of the ganglion cell dendritic field, and its response is transient, and even faster than excitation (Roska and Werblin 2003; Roska et al. 2006).
Similar GABAergic inhibitory components have been reported in salamander. Two functionally and anatomically distinct types of lateral inhibition were found in mudpuppy retina to contribute the receptive field organization of ganglion cells: sustained lateral inhibition and transient lateral inhibition (Belgum et al. 1987; Cook and McReynolds 1998a). These forms of inhibition were mediated by GABA and consisted of two components: a TTX-insensitive inhibition elicited by local illumination and a TTX-sensitive inhibition elicited by more distant illumination (Cook and McReynolds 1998b). Furthermore, sustained amacrine cells in mudpuppy and salamander retina extend only locally (Werblin et al. 1988; Yang et al. 1991). Experiments in rabbit also showed that lateral inhibition at the level of the ganglion cell is preferentially mediated through GABAergic amacrine cells (Flores-Herr et al. 2001). Considering that the transient TTX-sensitive inhibition is mediated by wide-field amacrine cells with axon (Schwartz 1973; Taylor 1999; Thibos and Werblin 1978a,b; Werblin 1972; Werblin and Copenhagen 1974), the spatial and temporal properties of GABAergic lateral inhibition by earlier work are very similar as the two GABAergic components we found here.
GLYCINERGIC CIRCUITRY MEDIATES CROSSOVER INHIBITION AND CONTROLS TIMING.
We recorded glycinergic inhibition in four target cell types. They are on glycinergic inhibition to off ganglion cells (in off beta and off parasol cells), off glycinergic inhibition to on ganglion cells (in on beta cell), and on glyciergic inhibition to on ganglion cells (in on beta and on parasol cells). Glycinergic inhibition typically carries inhibition from on to off or from off to on pathways as crossover inhibition (Hsueh et al. 2008; Menger et al. 1998; Molnar and Werblin 2007; Weiss et al. 2008). Unlike wide field GABAergic inhibition, glycinergic inhibition is likely carried by large populations of small-field amacrine cells with bushy dendritic trees terminating at different levels within the inner plexiform layer (Menger et al. 1998), and this inhibition seldom extends outside the ganglion cell receptive field. Some well studied glycinergic amacrine cells have very narrow dendritic field (<100 μm) in both cat, rabbit, and rat, such as AII in rabbit (Strettoi et al. 1992; Volgyi et al. 2002); AII, A8, A3, and A4 in cat (Kolb et al. 1981; Marc 1989), and small cells in rat (Menger et al. 1998).
Glycinergic inhibition is unlikely to have a spatial influence on ganglion cell activity. In rabbit ganglion cells strychnine didn't substantially affect the center surround balance (Caldwell and Daw 1978b). Certain wide field phenomena, such as the shift effect, show considerable sensitivity to GABA antagonists such as picrotoxin but are unaffected by strychnine (Frishman and Linsenmeier 1982). However, glycinergic amacrine cells receive input from bipolar cells that inherit surround inhibition, generated at either the outer or inner plexiform layer. These glycinergic amacrine cells themselves can carry antagonistic surround properties from bipolar cells to other amacrine and ganglion cells. For the crossover pathways, this amacrine cell-mediated surround will augment other inhibitory surround inputs.
We also measured on inhibition to on ganglion cells. This local glycinergic inhibition may control timing, as suggested in LED, that receives on and off glycinergic inhibition (van Wyk et al. 2006). It was also suggested that in cat the predominant role of the glycinergic A4/A8/AII system is less spatial than temporal (Marc 1989).
Some earlier reported forms of inhibition are similar to the local forms of on and off glycinergic inhibition we measured here. Study in guinea pigs showed that off alpha cells receive on glycinergic inhibition, and this glycinergic inhibitory current can be evoked only within the central region of the receptive field (Manookin et al. 2008). Studies in transgenetic mice showed that alpha-like off cells receive on glycinergic inhibition (Munch et al. 2009). Studies in mouse showed that on sustained cells got both on and off inhibition, whereas off sustained and off transient cells only got on inhibition, and the inhibition in off sustained cells was from on glycinergic amacrine cells (van Wyk et al. 2009). All of these inhibitory currents decreased with full field stimuli (van Wyk et al. 2009), indicating they may be generated only locally. Taken together, it appears that, glycinergic inhibition is generally elicited within to the dendritic field area regardless of the dendritic spread of ganglion cell and is involved more in temporal than spatial processing.
Possible morphological candidates for amacrine cells mediating the three inhibitory components
Amacrine cells in rabbit can be segregated into three groups based on the dendritic size (MacNeil et al. 1999), small, middle, and large amacrine cells. These dimensions correlate with the local glycinergic, local sustained GABAergic and broad transient GABAergic forms of inhibition. The small group includes cells with dendritic fields <100 μm. The middle group includes cells with dendritic fields between 150 and 300 μm. The large group includes cells with dendritic fields >200 μm. Most of cells in small and middle groups are multi- or broadly stratified, suggesting that they carry information between on and off sublayers. Neurons in large group with very broad dendritic fields appear to propagate information over longer distances. Moreover studies in rat showed that all the small amacrine cells use glycine as the transmitter (Menger et al. 1998), whereas all the large amacrine cells use GABA as the transmitter. Taken together, it is likely that the narrow crossover and noncrossover inhibition is carried by the small amacrine cell group, the sustained GABAergic inhibition (or local GABAergic) is carried by some the middle group, and the transient GABAergic inhibition (or broad GABAergic) is carried by some amacrine cells in the large group.
Although our immunohistochemical study in amacrine cells was limited (Fig. 13), we extracted three examples: a glycinergic amacrine cell with narrow dendrite and narrow physiological dimensions, a GABAergic amacrine cell with narrow dendrite and narrow physiological dimensions; and a GABAergic amacrine cell with broad dendrite and broad physiological dimensions. These three types are consistent with our suggestion that these three types embody the three major functional groups in amacrine cells.
Could transient and sustained GABA inhibition originate in a single cell type?
To generate two different GABAergic inhibitory kinetics from one amacrine cell type, GABA release from this amacrine cell must have two different kinetics or there must be two kinetically separable GABA receptors at the ganglion cell. Under either condition, we would have measured both transient and sustained narrow and broad GABAergic inhibition. However, we measured either narrow sustained GABAergic inhibition or broad transient GABAergic inhibition. We never measured broad sustained or narrow transient GABAergic inhibition. So it is more likely that there exist two different types of GABAergic amacrine cells providing narrow sustained or broad transient inhibition.
Why we limited our study to these four ganglion cell types
Ganglion cells are generally classified either by morphology or function. Early physiological studies indicated that ganglion cells in rabbit could be put into two groups, those with concentric receptive fields and others with complex receptive field (Amthor et al. 1989a,b; Caldwell and Daw 1978a; Levick 1965). Many of the functions and circuits of complex ganglion cells are well studied, such as direction selective cells (Fried et al. 2005; Lee and Zhou 2006), local edge detectors (van Wyk et al. 2006), and on DS cells (Ackert et al. 2009; Sun et al. 2006). The functions and circuits in ganglion cells with large concentric receptive field (alpha like cells) have also been studied (Manookin et al. 2008; Munch et al. 2009; Zaghloul et al. 2007). But the circuits of small and medium size ganglion cells with concentric receptive field have not been fully evaluated.
Our on beta and off beta cells are very similar to on and off G4 cells, and our on parasol and off parasol cells are very similar as G2 and G5 cells of Rockhill et al. (2002). They comprise all the monostratified small and middle ganglion cells. Other two types of ganglion cells with small and middle dendritic field are bistratified (G1 or LED and G3), which have been studied before (Hoshi and Mills 2009; van Wyk et al. 2006). All the types we studied here are brisk ganglion cells, and they constitute a large population compared with sluggish cells (Amthor et al. 1989a; Roska et al. 2006) in rabbit. Although we studied only four types of ganglion cells here, they represent the majority of concentric brisk ganglion cells with monostratified small and middle dendritic arborization.
DISCLOSURES
No conflicts of interest are declared by the authors.
REFERENCES
- Ackert JM, Farajian R, Volgyi B, Bloomfield SA. GABA blockade unmasks an OFF response in ON direction selective ganglion cells in the mammalian retina. J Physiol 587: 4481–4495, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amthor FR, Takahashi ES, Oyster CW. Morphologies of rabbit retinal ganglion cells with complex receptive fields. J Comp Neurol 280: 97–121, 1989a. [DOI] [PubMed] [Google Scholar]
- Amthor FR, Takahashi ES, Oyster CW. Morphologies of rabbit retinal ganglion cells with concentric receptive fields. J Comp Neurol 280: 72–96, 1989b. [DOI] [PubMed] [Google Scholar]
- Baccus SA, Olveczky BP, Manu M, Meister M. A retinal circuit that computes object motion. J Neurosci 28: 6807–6817, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badea TC, Nathans J. Quantitative analysis of neuronal morphologies in the mouse retina visualized by using a genetically directed reporter. J Comp Neurol 480: 331–351, 2004. [DOI] [PubMed] [Google Scholar]
- Belgum JH, Dvorak DR, McReynolds JS, Miyachi E. Push-pull effect of surround illumination on excitatory and inhibitory inputs to mudpuppy retinal ganglion cells. J Physiol 388: 233–243, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomfield SA. Effect of spike blockade on the receptive-field size of amacrine and ganglion cells in the rabbit retina. J Neurophysiol 75: 1878–1893, 1996. [DOI] [PubMed] [Google Scholar]
- Bloomfield SA, Volgyi B. Response properties of a unique subtype of wide-field amacrine cell in the rabbit retina. Vis Neurosci 24: 459–469, 2007. [DOI] [PubMed] [Google Scholar]
- Caldwell JH, Daw NW. New properties of rabbit retinal ganglion cells. J Physiol 276: 257–276, 1978a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell JH, Daw NW. Effects of picrotoxin and strychnine on rabbit retinal ganglion cells: changes in centre surround receptive fields. J Physiol 276: 299–310, 1978b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook PB, McReynolds JS. Modulation of sustained and transient lateral inhibitory mechanisms in the mudpuppy retina during light adaptation. J Neurophysiol 79: 197–204, 1998a. [DOI] [PubMed] [Google Scholar]
- Cook PB, McReynolds JS. Lateral inhibition in the inner retina is important for spatial tuning of ganglion cells. Nat Neurosci 1: 714–719, 1998b. [DOI] [PubMed] [Google Scholar]
- Euler T, Detwiler PB, Denk W. Directionally selective calcium signals in dendrites of starburst amacrine cells. Nature 418: 845–852, 2002. [DOI] [PubMed] [Google Scholar]
- Euler T, Masland RH. Light-evoked responses of bipolar cells in a mammalian retina. J Neurophysiol 83: 1817–1829, 2000. [DOI] [PubMed] [Google Scholar]
- Flores-Herr N, Protti DA, Wassle H. Synaptic currents generating the inhibitory surround of ganglion cells in the mammalian retina. J Neurosci 21: 4852–4863, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried SI, Munch TA, Werblin FS. Mechanisms and circuitry underlying directional selectivity in the retina. Nature 420: 411–414, 2002. [DOI] [PubMed] [Google Scholar]
- Fried SI, Munch TA, Werblin FS. Directional selectivity is formed at multiple levels by laterally offset inhibition in the rabbit retina. Neuron 46: 117–127, 2005. [DOI] [PubMed] [Google Scholar]
- Frishman LJ, Linsenmeier RA. Effects of picrotoxin and strychnine on non-linear responses of Y-type cat retinal ganglion cells. J Physiol 324: 347–363, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh KK, Bujan S, Haverkamp S, Feigenspan A, Wassle H. Types of bipolar cells in the mouse retina. J Comp Neurol 469: 70–82, 2004. [DOI] [PubMed] [Google Scholar]
- Hoshi H, Mills SL. Components and properties of the G3 ganglion cell circuit in the rabbit retina. J Comp Neurol 513: 69–82, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsueh HA, Molnar A, Werblin FS. Amacrine-to-amacrine cell inhibition in the rabbit retina. J Neurophysiol 100: 2077–2088, 2008. [DOI] [PubMed] [Google Scholar]
- Ichinose T, Lukasiewicz PD. Inner and outer retinal pathways both contribute to surround inhibition of salamander ganglion cells. J Physiol 565: 517–535, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda H, Wright MJ. The outer disinhibitory surround of the retinal ganglion cell receptive field. J Physiol 226: 511–544, 1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb H, Nelson R, Mariani A. Amacrine cells, bipolar cells and ganglion cells of the cat retina: a Golgi study. Vision Res 21: 1081–1114, 1981. [DOI] [PubMed] [Google Scholar]
- Lee S, Zhou ZJ. The synaptic mechanism of direction selectivity in distal processes of starburst amacrine cells. Neuron 51: 787–799, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levick WR. Receptive fields of rabbit retinal ganglion cells. Am J Optom Arch Am Acad Optom 42: 337–343, 1965. [DOI] [PubMed] [Google Scholar]
- Levick WR. Receptive fields and trigger features of ganglion cells in the visual streak of the rabbits retina. J Physiol 188: 285–307, 1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNeil MA, Heussy JK, Dacheux RF, Raviola E, Masland RH. The shapes and numbers of amacrine cells: matching of photofilled with Golgi-stained cells in the rabbit retina and comparison with other mammalian species. J Comp Neurol 413: 305–326, 1999. [PubMed] [Google Scholar]
- MacNeil MA, Heussy JK, Dacheux RF, Raviola E, Masland RH. The population of bipolar cells in the rabbit retina. J Comp Neurol 472: 73–86, 2004. [DOI] [PubMed] [Google Scholar]
- MacNeil MA, Masland RH. Extreme diversity among amacrine cells: implications for function. Neuron 20: 971–982, 1998. [DOI] [PubMed] [Google Scholar]
- Manookin MB, Beaudoin DL, Ernst ZR, Flagel LJ, Demb JB. Disinhibition combines with excitation to extend the operating range of the OFF visual pathway in daylight. J Neurosci 28: 4136–4150, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marc RE. The role of glycine in the mammalian retina. In: Progress in Retinal Research, edited by Osborne N, Chader J. New York: Pergamon, 1989, p 67–107 [Google Scholar]
- Marr D. Vision: A Computational Investigation into the Human Representation and Processing of Visual Information. New York: Freeman,1982. [Google Scholar]
- Masland RH. Neuronal diversity in the retina. Curr Opin Neurobiol 11: 431–436, 2001. [DOI] [PubMed] [Google Scholar]
- McMahon MJ, Packer OS, Dacey DM. The classical receptive field surround of primate parasol ganglion cells is mediated primarily by a non-GABAergic pathway. J Neurosci 24: 3736–3745, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menger N, Pow DV, Wassle H. Glycinergic amacrine cells of the rat retina. J Comp Neurol 401: 34–46, 1998. [DOI] [PubMed] [Google Scholar]
- Molnar A, Werblin F. Inhibitory feedback shapes bipolar cell responses in the rabbit retina. J Neurophysiol 98: 3423–3435, 2007. [DOI] [PubMed] [Google Scholar]
- Munch TA, da Silveira RA, Siegert S, Viney TJ, Awatramani GB, Roska B. Approach sensitivity in the retina processed by a multifunctional neural circuit. Nat Neurosci 12: 1308–1316, 2009. [DOI] [PubMed] [Google Scholar]
- Olveczky BP, Baccus SA, Meister M. Segregation of object and background motion in the retina. Nature 423: 401–408, 2003. [DOI] [PubMed] [Google Scholar]
- Rockhill RL, Daly FJ, MacNeil MA, Brown SP, Masland RH. The diversity of ganglion cells in a mammalian retina. J Neurosci 22: 3831–3843, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roska B, Molnar A, Werblin FS. Parallel processing in retinal ganglion cells: how integration of space-time patterns of excitation and inhibition form the spiking output. J Neurophysiol 95: 3810–3822, 2006. [DOI] [PubMed] [Google Scholar]
- Roska B, Werblin F. Vertical interactions across ten parallel, stacked representations in the mammalian retina. Nature 410: 583–587, 2001. [DOI] [PubMed] [Google Scholar]
- Roska B, Werblin F. Rapid global shifts in natural scenes block spiking in specific ganglion cell types. Nat Neurosci 6: 600–608, 2003. [DOI] [PubMed] [Google Scholar]
- Schwartz EA. Organization of on-off cells in the retina of the turtle. J Physiol 230: 1–14, 1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strettoi E, Raviola E, Dacheux RF. Synaptic connections of the narrow-field, bistratified rod amacrine cell (AII) in the rabbit retina. J Comp Neurol 325: 152–168, 1992. [DOI] [PubMed] [Google Scholar]
- Sun W, Deng Q, Levick WR, He S. ON direction-selective ganglion cells in the mouse retina. J Physiol 576: 197–202, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor WR. TTX attenuates surround inhibition in rabbit retinal ganglion cells. Vis Neurosci 16: 285–290, 1999. [DOI] [PubMed] [Google Scholar]
- Thibos LN, Werblin FS. The response properties of the steady antagonistic surround in the mudpuppy retina. J Physiol 278: 79–99, 1978a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibos LN, Werblin FS. The properties of surround antagonism elicited by spinning windmill patterns in the mudpuppy retina. J Physiol 278: 101–116, 1978b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wyk M, Taylor WR, Vaney DI. Local edge detectors: a substrate for fine spatial vision at low temporal frequencies in rabbit retina. J Neurosci 26: 13250–13263, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wyk M, Wassle H, Taylor WR. Receptive field properties of ON- and OFF-ganglion cells in the mouse retina. Vis Neurosci 26: 297–308, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volgyi B, Xin D, Bloomfield SA. Feedback inhibition in the inner plexiform layer underlies the surround-mediated responses of AII amacrine cells in the mammalian retina. J Physiol 539: 603–614, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss J, O'Sullivan GA, Heinze L, Chen HX, Betz H, Wassle H. Glycinergic input of small-field amacrine cells in the retinas of wildtype and glycine receptor deficient mice. Mol Cell Neurosci 37: 40–55, 2008. [DOI] [PubMed] [Google Scholar]
- Werblin FS. Lateral interactions at inner plexiform layer of vertebrate retina: antagonistic responses to change. Science 175: 1008–1010, 1972. [DOI] [PubMed] [Google Scholar]
- Werblin FS, Copenhagen DR. Control of retinal sensitivity. III. Lateral interactions at the inner plexiform layer. J Gen Physiol 63: 88–110, 1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werblin F, Maguire G, Lukasiewicz P, Eliasof S, Wu SM. Neural interactions mediating the detection of motion in the retina of the tiger salamander. Vis Neurosci 1: 317–329, 1988. [DOI] [PubMed] [Google Scholar]
- Yang CY, Lukasiewicz P, Maguire G, Werblin FS, Yazulla S. Amacrine cells in the tiger salamander retina: morphology, physiology, and neurotransmitter identification. J Comp Neurol 312: 19–32, 1991. [DOI] [PubMed] [Google Scholar]
- Zaghloul KA, Manookin MB, Borghuis BG, Boahen K, Demb JB. Functional circuitry for peripheral suppression in mammalian Y-type retinal ganglion cells. J Neurophysiol 97: 4327–4340, 2007. [DOI] [PubMed] [Google Scholar]


