Abstract
Although cerebral autoregulation (CA) appears well maintained during mild to moderate intensity dynamic exercise in young subjects, it is presently unclear how aging influences the regulation of cerebral blood flow during physical activity. Therefore, to address this question, middle cerebral artery blood velocity (MCAV), mean arterial pressure (MAP), and the partial pressure of arterial carbon dioxide (PaCO2) were assessed at rest and during steady-state cycling at 30% and 50% heart rate reserve (HRR) in 9 young (24 ± 3 yr; mean ± SD) and 10 older middle-aged (57 ± 7 yr) subjects. Transfer function analysis between changes in MAP and mean MCAV (MCAVmean) in the low-frequency (LF) range were used to assess dynamic CA. No age-group differences were found in PaCO2 at rest or during cycling. Exercise-induced increases in MAP were greater in older subjects, while changes in MCAVmean were similar between groups. The cerebral vascular conductance index (MCAVmean/MAP) was not different at rest (young 0.66 ± 0.04 cm·s−1·mmHg−1 vs. older 0.67 ± 0.03 cm·s−1·mmHg−1; mean ± SE) or during 30% HRR cycling between groups but was reduced in older subjects during 50% HRR cycling (young 0.67 ± 0.03 cm·s−1·mmHg−1 vs. older 0.56 ± 0.02 cm·s−1·mmHg−1; P < 0.05). LF transfer function gain and phase between MAP and MCAVmean was not different between groups at rest (LF gain: young 0.95 ± 0.05 cm·s−1·mmHg−1 vs. older 0.88 ± 0.06 cm·s−1·mmHg−1; P > 0.05) or during exercise (LF gain: young 0.80 ± 0.05 cm·s−1·mmHg−1 vs. older 0.72 ± 0.07 cm·s−1·mmHg−1 at 50% HRR; P > 0.05). We conclude that despite greater increases in MAP, the regulation of MCAVmean is well maintained during dynamic exercise in healthy older middle-aged subjects.
Keywords: cerebral blood flow, transfer function, leg cycling, blood pressure
cerebral autoregulation (CA) refers to the ability of the cerebral vasculature to maintain blood flow relatively constant over a wide range of perfusion pressures via changes in cerebrovascular resistance (44). Exercise presents a potential challenge to CA, not only due to rapid and robust fluctuations in arterial blood pressure but also due to increases in sympathetic nerve activity, cardiac output, and cerebral metabolism (26, 47, 50). Although CA appears well maintained during mild- to moderate-intensity dynamic exercise in young individuals (4, 38, 40), it is presently unclear how aging influences the regulation of cerebral blood flow during physical activity. This is an important question considering age-related alterations in peripheral circulatory control have been reported at rest (12, 33, 35) and during exercise (12, 45, 48). Indeed, exaggerated sympathetic vasoconstrictor tone and blunted vasodilator responsiveness have been demonstrated in dynamically exercising skeletal muscle of older individuals (12, 13, 31, 45). Whether these peripheral vascular changes with age are manifest in the cerebral circulation and alter CA is currently unknown. In addition, the resultant exaggerated pressor response to exercise may present an additional challenge to the regulation of cerebral blood flow during physical activity in older individuals (9, 14, 15).
Although age-related alterations in the control of cerebral blood flow have not always been found under resting conditions (6, 7, 34, 54), several studies have observed impairments in cerebral vascular function at rest in older compared with younger subjects (17, 24). Moreover, Heckmann and colleagues (21) recently suggested that cerebral autoregulatory mechanisms demonstrated a delayed responsiveness to exercise in older subjects. These authors noted that cerebrovascular resistance increased more slowly in older compared with younger subjects in response to increases in cerebral blood flow at the onset of a short bout (3 min) of supine leg cycling. Although this is suggestive of an age-related impairment in cerebral vascular control, a potential caveat is that both young and older subjects performed exercise at similar absolute workloads. Considering that peak work rate and aerobic capacity typically decrease with age (15), it is plausible that the older subjects were exercising at a greater relative intensity. Because cerebral blood flow responses to exercise have been shown to be proportional to exercise intensity (40), this may explain the greater increase in cerebral blood flow at exercise onset in the older subjects. In addition, since exercise was performed for only 3 min, it is probable that these results were not representative of steady-state exercise conditions.
Given this background, the present study was designed to investigate middle cerebral artery blood velocity (MCAV), mean arterial pressure (MAP), and the cerebral vascular conductance index at rest and during low- and moderate-intensity steady-state cycling in healthy young and older middle-aged subjects. In addition, dynamic CA was assessed using transfer function analysis between the changes in MAP and mean MCAV (MCAVmean) in the low-frequency range (4, 38–40). We tested the hypothesis that dynamic CA would be impaired in older subjects during dynamic exercise in association with greater increases in blood pressure compared with younger subjects.
METHODS
Nine young and ten older middle-aged subjects were recruited from the University of Missouri community and the surrounding area (Table 1). Both young and older subjects were recreationally active, but importantly none were competitive athletes. All experimental procedures and protocols conformed to the Declaration of Helsinki and were approved by the University of Missouri-Columbia Health Sciences Institutional Review Board and the Research and Development Committee of the Harry S. Truman Memorial Veterans Hospital. Each subject gave written informed consent. Before participation each subject completed a medical health history questionnaire, and a blood chemistry screening was performed after a 12-h overnight fast. No subjects had a history or symptoms of cardiovascular, pulmonary, metabolic, or neurological disease, and none were using prescribed or over-the-counter medications. Although hormonal status of the women participants was not directly assessed, all older women were postmenopausal and not taking any hormone replacement, and young women performed the main steady-state exercise protocol (described below) around the early follicular phase of the menstrual cycle, in which plasma estrogen and progesterone concentrations are generally low (20). Subjects were requested to abstain from caffeinated beverages for 12 h and strenuous physical activity and alcohol for at least 24 h before experimental sessions. On experimental days, the subjects arrived at the laboratory a minimum of 2 h following a light meal. All subjects were familiarized with the equipment and procedures before any experimental sessions.
Table 1.
Subject characteristics
| Young | Older | |
|---|---|---|
| Men/Women, n/n | 6/3 | 6/4 |
| Age, yr | 24±3 | 57±7* |
| Weight, kg | 73±10 | 75±15 |
| Height, cm | 174±6 | 172±8 |
| BMI, kg/m2 | 24±3 | 25±4 |
| Cholesterol, mg/dl | 146±35 | 195±25* |
| Triglycerides, mg/dl | 71±23 | 88±29 |
| LDL, mg/dl | 87±32 | 124±19* |
| HDL, mg/dl | 46±9 | 53±17 |
| Glucose, mg/dl | 84±6 | 102±12* |
| BUN, mg/dl | 13±3 | 16±4 |
| Na+, meq/l | 139±2 | 140±2 |
| K+, meq/l | 4.0±0.3 | 4.2±0.4 |
Values are means ± SD. BMI, body mass index; LDL, low-density lipoprotein; HDL, high-density lipoprotein; BUN, blood urea nitrogen.
Significantly different from young (P < 0.05).
Experimental Measurements
Heart rate (HR) was continuously monitored using a lead II electrocardiogram (ECG; Q710, Quinton Instruments, Bothell, WA). Beat-to-beat blood pressure was measured using finger photoplethysmography (Finometer, Finapres Medical Systems, Amsterdam, The Netherlands) obtained from the middle finger of the left hand, which was supported at the level of the right atrium on an adjustable padded bedside table. In addition, brachial artery blood pressure was measured at heart level in the right arm every minute using an automated sphygmomanometer equipped with a microphone for the detection of Korotkoff sounds (SunTech Medical Instruments, Raleigh, NC). Before recordings were started, diastolic blood pressure (DBP) of the Finometer was matched with DBP measurements obtained from the brachial artery. These methods of blood pressure measurement have been previously validated for use during dynamic exercise (2, 5, 43). The partial pressure of end-tidal carbon dioxide was obtained on a breath-by-breath basis using a metabolic measurement system (TrueOne 2400, ParvoMedics, Salt Lake City, UT) that was calibrated before each experimental session using known standard gases. An estimate of arterial carbon dioxide tension (PaCO2) was subsequently calculated using the equations of Jones et al. (28). Blood velocity in the right middle cerebral artery was measured using transcranial Doppler ultrasound (DWL, Sipplingen, Germany) with a 2-MHz probe placed over the temporal window and fixed with an adjustable headband and adhesive ultrasonic gel (Tensive, Parker Laboratories, Orange, NJ). The cerebral vascular conductance index (CVCi) was calculated as MCAVmean/MAP. Ratings of perceived exertion were obtained using the standard 6–20 Borg scale (3). All cardiovascular variables were sampled at 1,000 Hz and stored for off-line analysis (Powerlab, AD Instruments, Bella Vista, NSW, Australia).
Experimental Procedures
Incremental maximal exercise test.
To exclude the possibility of any exercise-induced arrhythmias or blood pressure abnormalities, and to ascertain peak HR for the determination of steady-state workloads, all subjects performed a continuous incremental maximal exercise test. Subjects were seated in a semirecumbent position on a medical exam table equipped with an electrically braked cycle ergometer with toe clips (Angio V2, Lode, Groningen, The Netherlands). Subjects were instrumented with a 12-lead ECG, and blood pressure was measured with the aforementioned automated sphygmomanometer. Following a 3-min warm up period of cycling at 60 rpm, the workload was increased by 25 W every minute. Peak responses were determined at the power output where the subject could no longer maintain a pedal frequency of 60 rpm despite strong verbal encouragement. All subjects gave a maximal rating of perceived exertion (i.e., 19–20) at exhaustion.
Cerebral vascular responses to steady-state exercise.
After ∼3–7 days from the incremental maximal exercise test, subjects returned to the laboratory to perform two bouts of cycling at steady-state HRs corresponding to 30% and 50% of HR reserve (HRR), representing low- and moderate-intensity exercise (1). Following instrumentation for the measurement of HR, arterial blood pressure, and cerebral blood flow velocity, subjects rested quietly for 15 min. For the assessment of PaCO2, subjects then respired through a low-resistance mouthpiece (model 2700, Hans Rudolph, Kansas City, MO) attached to the metabolic measurement system for 3 min while a nose clip was worn to prevent nasal breathing. For each exercise bout, subjects maintained a pedal frequency of 60 rpm, and the workload was gradually increased until the target HR was achieved (∼3–5 min), after which 15 min of steady-state cycling was performed. During the last 2 min of exercise, breath-by-breath samples were taken for the determination of PaCO2. Before the cessation of exercise, subjects were asked to provide a rating of perceived exertion. Each exercise bout was separated by a minimum of 30 min to allow full recovery and the reestablishment of baseline HR and MAP. The order of the low- and moderate-intensity trials was randomized. Throughout the test, subjects were reminded to keep their upper limbs relaxed to aid blood pressure measurements, and their head facing forward to prevent movement artifacts in the transcranial Doppler signal.
Transfer Function Analysis for Dynamic CA
Five-minute steady-state data segments at rest and during low- and moderate-intensity cycling were used for transfer function analysis to identify indexes of dynamic CA. Beat-to-beat values of MAP and MCAVmean were obtained by integrating analog signals within each cardiac cycle, then linearly interpolated and resampled at 2 Hz for spectral analysis (38, 40, 41, 55, 56). For an estimate of dynamic CA using the transfer function, the cross-spectrum between changes in MAP and MCAVmean were calculated and divided by the autospectrum of MAP. Transfer function gain measurements reflect the relative amplitude relationship between changes in MAP and MCAVmean over a particular frequency range and were used to quantify the ability of the cerebral vascular bed to buffer changes in cerebral blood velocity induced by transient alterations in blood pressure (55, 56). Transfer function phase measurements were used to determine the temporal relationship between changes in MAP and MCAVmean over a particular frequency range (55, 56).
From the temporal sequences, the frequency-domain transforms were computed with a fast Fourier transformation algorithm. The transfer function H(f) between the two signals was calculated as H(f) = Sxy(f)/Sxx(f), where Sxx(f) is the autospectrum of the input signal and Sxy(f) is the cross-spectrum between the two signals. The transfer function magnitude |H(f)| and phase spectrum |Φ(f)| were obtained from the real [HR(f)] and imaginary [HI(f)] components of the complex transfer function:
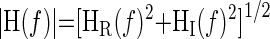 |
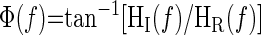 |
Additionally, the transfer function H(f) was normalized to the mean values of input (x) and output (y) variables as H′(f) = Sxy(f)x/Sxx(f)y, and the normalized gain was calculated as 20 log|H′(f)| to express values in decibels.
The squared coherence function [MSC(f)] was estimated as:
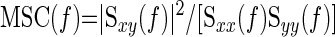 |
where Syy(f) is the autospectrum of MCAVmean. The squared coherence function reflects the fraction of output power that can be linearly related to the input power at each frequency. Similarly to a correlation coefficient, it varies between 0 and 1, with a coherence of >0.5 taken as suggestive of a stable relationship between two oscillations reflecting the statistical reliability of transfer function analysis between input and output.
Spectral power of MAP and MCAVmean, and transfer function gain, phase, and coherence were calculated in the very low (VLF; 0.02–0.07 Hz), low (LF; 0.07–0.20 Hz) and high (HF; 0.20–0.30 Hz) frequency ranges. The arterial blood pressure fluctuations in the HF range, such as those induced by respiration, are transferred to MCAVmean, whereas arterial blood pressure fluctuations in the VLF and LF ranges are independent of the respiratory frequency and are dampened by autoregulatory mechanisms (11). Thus the dynamic buffering capacity of the cerebral vasculature is dependent on the frequency of the fluctuations in perfusion pressure. As such, we used the VLF and LF ranges of each variable to identify dynamic CA at rest and during exercise (27, 40, 41, 56).
Statistical Analysis
Statistical comparisons of physiological variables were made utilizing a repeated-measures two-way ANOVA. A Student-Newman-Keuls test was employed post hoc to investigate significant main effects and interactions of group (young vs. older) and condition (rest vs. low exercise vs. moderate exercise). Statistical significance was set at P < 0.05. Analyses were conducted using SigmaStat (Jandel Scientific Software, SPSS, Chicago, IL) for Windows.
RESULTS
Subject Characteristics
The mean age difference between young and older subjects was 33 yr. There were no significant age-group differences in body weight, body mass index, triglycerides, high-density lipoprotein (HDL), blood urea nitrogen, or electrolytes (Table 1). Total cholesterol, low-density lipoprotein (LDL), and glucose tended to be higher in the older subjects; however, values were not substantially greater than the upper limit for healthy individuals. All subjects had normal resting and maximal exercise ECGs, and, as expected, the peak HR response to the incremental exercise test was significantly higher in younger subjects (young 185 ± 3 beats/min vs. older 160 ± 4 beats/min; mean ± SE; P < 0.05).
Steady-State Cardiovascular Measures
All measurements were performed in the semirecumbent position. At rest there were no significant age-group differences in HR, systolic blood pressure (SBP), MAP, MCAVmean, CVCi, or PaCO2 (P > 0.05); however, resting DBP was significantly higher in the older compared with younger subjects (P < 0.01; Table 2). During both low- and moderate-intensity steady-state leg cycling, the SBP and MAP responses were greater in older subjects (P < 0.01; Table 2 and Fig. 1A). Likewise, DBP was significantly greater in older individuals during exercise (P < 0.01). Overall, DBP remained the same or slightly increased in the older group during low- and moderate-intensity cycling, whereas it progressively decreased from rest in the younger group. Indeed, during moderate-intensity cycling, DBP was significantly lower than rest in the younger subjects (P < 0.05; Table 2). Pulse pressure was significantly increased during low- and moderate-intensity exercise, and no age-group differences were found (Table 2 and Fig. 1B). For the group, MCAVmean increased slightly but significantly during low- and moderate-intensity cycling (P < 0.05), but no differences were observed between older and younger subjects (P > 0.05; Table 2 and Fig. 1C). CVCi was not altered from rest in the young individuals during either low- or moderate-intensity cycling (P > 0.05). Similarly, no changes in CVCi were observed in the older individuals during low-intensity exercise. However, a significant reduction in CVCi was observed during moderate-intensity exercise in the older subjects (P < 0.01), leading to a significant age-group difference (Fig. 1D). No differences in PaCO2 were found between the young and older subjects; however, PaCO2 was slightly but significantly reduced from rest during moderate-intensity exercise (P < 0.05; Table 2). The RPE values obtained during low-intensity (young 9 ± 1 vs. older 9 ± 1; mean ± SE; P > 0.05) and moderate-intensity cycling (young 13 ± 1 vs. older 13 ± 1; P > 0.05) were similar in young and older subjects (P > 0.05).
Table 2.
Steady-state physiological measurements at rest and during low- and moderate-intensity leg cycling in young and older subjects
| SBP, mmHg | DBP, mmHg | MAP, mmHg | Pulse Pressure, mmHg | Heart Rate, beats/min | MCAVmean, cm/s | CVCi, cm·s−1·mmHg−1 | PaCO2, mmHg | |
|---|---|---|---|---|---|---|---|---|
| Rest | ||||||||
| Young | 120±3 | 74±1 | 90±1 | 45±3 | 65±3 | 60±4 | 0.66±0.04 | 44±1 |
| Older | 123±3 | 81±2* | 95±2 | 46±6 | 61±3 | 64±2 | 0.67±0.03 | 43±1 |
| Low Ex | ||||||||
| Young | 137±5 | 70±1 | 92±2 | 67±5 | 96±3† | 64±3 | 0.70±0.04 | 44±2 |
| Older | 151±4*† | 82±2* | 105±2*† | 69±4 | 90±3† | 68±2 | 0.65±0.02 | 43±1 |
| Moderate Ex | ||||||||
| Young | 160±4†‡ | 68±3† | 99±2†‡ | 92±5 | 123±3†‡ | 66±2 | 0.67±0.03 | 43±1 |
| Older | 182±4*†‡ | 86±3* | 118±2*†‡ | 96±4 | 112±3*†‡ | 65±2 | 0.56±0.02*†‡ | 41±1 |
| P value | ||||||||
| Group | 0.012 | <0.001 | <0.001 | 0.870 | 0.097 | 0.478 | 0.180 | 0.355 |
| Condition | <0.001 | 0.664 | <0.001 | <0.001 | <0.001 | 0.021 | 0.001 | 0.003 |
| Interaction | 0.002 | 0.006 | <0.001 | 0.290 | 0.005 | 0.244 | 0.001 | 0.942 |
Values are means ± SE. SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure; MCAVmean, middle cerebral artery mean blood velocity; CVCi, cerebral vascular conductance index; PaCO2, estimated arterial carbon dioxide tension; Ex, exercise.
Significantly different from young (P < 0.05).
Significantly different from rest (P < 0.05).
Significantly different from Low Ex (P < 0.05).
Fig. 1.
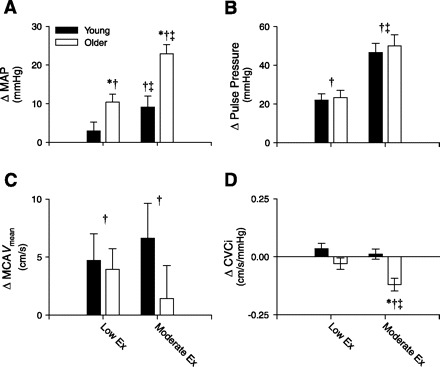
Summary data showing the changes (Δ) from rest in mean arterial pressure (MAP; A), pulse pressure (B), middle cerebral artery mean blood velocity (MCAVmean; C), and the cerebral vascular conductance index (CVCi; D) during low-intensity (Low Ex) and moderate-intensity (Moderate Ex) leg cycling in young and older subjects. *Significantly different from young (P < 0.05). †Significantly different from rest (P < 0.05). ‡Significantly different from Low Ex (P < 0.05). Values are means ± SE.
Spectral Analysis and Transfer Function Analysis
No significant age-group differences were found in the VLF and LF MCAVmean power spectral density (PSD) at rest or during exercise (P > 0.05; Table 3). Similarly, VLF and LF MAP PSD were not different between young and older subjects. However, the VLF MAP PSD exhibited a condition effect with a significant difference between the low- and moderate-intensity exercise bouts (Table 3). The VLF and LF transfer function gain between MAP and MCAVmean were not significantly different between the young and older subjects (P > 0.05; Fig. 2). The LF transfer function gain decreased from rest during both low- and moderate-intensity cycling (P < 0.05), whereas the VLF transfer function gain was only different from rest during moderate exercise (Fig. 2). Overall, similar age-group and condition effects were found for the normalized VLF and LF transfer function gains (Table 3). An age-group difference was found in the VLF coherence between MAP and MCAVmean with younger subjects exhibiting lower values under all conditions (Fig. 2). In contrast, the LF coherence was not different between young and older subjects at any time point studied (P > 0.05), but progressively decreased from rest to low-intensity (P < 0.01) and from low- to moderate-intensity exercise (P < 0.05; Fig. 2). However, LF coherence values remained above 0.5 under all conditions. The VLF and LF phase between MAP and MCAVmean was not significantly different between the young and older subjects (P > 0.05) or between rest and exercise (P > 0.05; Fig. 2).
Table 3.
Spectral power, and normalized transfer function gain in the LF (0.07–0.2 Hz) and VLF (0.02–0.07 Hz) ranges at rest and during low- and moderate-intensity leg cycling in young and older subjects
| LF MAP PSD, mmHg2 | LF MCAVmean PSD, cm2/s2 | LF nGain, db | VLF MAP PSD, mmHg2 | VLF MCAVmean SD, cm2/s2 | VLF nGain, db | |
|---|---|---|---|---|---|---|
| Rest | ||||||
| Young | 3.12±0.56 | 3.02±0.71 | 2.75±0.46 | 4.48±0.63 | 3.18±0.70 | −2.64±1.23 |
| Older | 3.39±0.89 | 2.23±0.52 | 1.80±0.51 | 8.80±1.21 | 4.74±1.32 | −2.82±1.21 |
| Low Ex | ||||||
| Young | 3.34±0.72 | 2.27±0.41 | 0.52±0.58 | 4.05±0.65 | 2.96±0.67 | −3.54±1.65 |
| Older | 3.29±0.90 | 1.85±0.40 | 0.72±0.52 | 5.43±1.15 | 2.33±0.42 | −3.54±0.91 |
| Moderate Ex | ||||||
| Young | 3.26±0.47 | 2.31±0.19 | 0.72±0.68 | 7.00±1.24 | 3.69±0.85 | −3.95±1.34 |
| Older | 3.48±0.87 | 1.85±0.29 | 1.51±0.75 | 8.55±2.10 | 2.52±0.57 | −4.36±1.06 |
| P value | ||||||
| Group | 0.845 | 0.167 | 0.986 | 0.096 | 0.928 | 0.943 |
| Condition | 0.987 | 0.339 | 0.005 | 0.024 | 0.138 | <0.001 |
| Interaction | 0.969 | 0.987 | 0.208 | 0.309 | 0.102 | 0.994 |
Values are means ± SE. LF, low frequency; VLF, very low frequency; PSD, power spectral density; nGain, normalized gain.
Fig. 2.
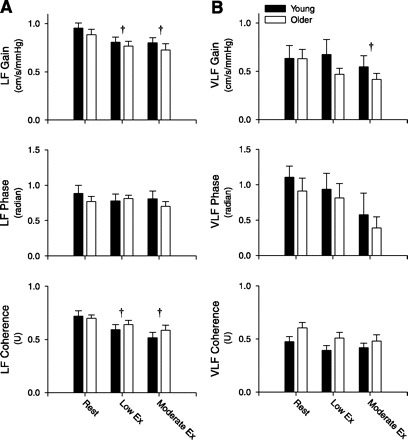
Summary data for the transfer function gain, phase, and coherence between MAP and MCAVmean in the low-frequency (LF; A) and very low-frequency (VLF; B) ranges at rest and during low- and moderate-intensity leg cycling in young and older subjects. †Significantly different from rest (P < 0.05). Values are means ± SE.
DISCUSSION
The present study is the first to examine the regulation of cerebral blood flow during steady-state dynamic exercise in healthy older middle-aged subjects. First, we found that despite greater increases in blood pressure during exercise in older individuals, no differences in MCAVmean were observed between young and older subjects. Second, no significant age-group differences were found in the VLF or LF transfer function gain or phase between MAP and MCAVmean during low- or moderate-intensity cycling, indicating that the ability of the cerebral vasculature to respond to spontaneous fluctuations in MAP (i.e., dynamic CA) was preserved with age during exercise. Collectively, these data suggest that the regulation of MCAVmean is well maintained during dynamic exercise in healthy older middle-aged subjects.
Cerebral blood flow is influenced by neurogenic, neurohumoral, endothelial, as well as metabolic factors (26, 47, 50). As such, exercise presents a potential challenge to CA, not only due to the rapid and robust fluctuations in arterial blood pressure but also due to increases in sympathetic nerve activity, cardiac output, and cerebral metabolism (26, 47, 50). In the present study, we hypothesized that this may be particularly true in older individuals, considering previous reports of age-related alterations in peripheral circulatory control during exercise (12, 45). Indeed, aging-induced impairments in metabolic vasodilatation and exaggerated sympathetic vasoconstriction have been reported in the vasculature of dynamically exercising skeletal muscle and are believed to contribute to age-related reductions in exercising muscle blood flow (12, 13, 31, 45, 48). In addition, exaggerated pressor responses to exercise may present an additional challenge to the regulation of cerebral blood flow during physical activity in older individuals (9, 12, 14, 15). However, despite greater exercise-induced increases in blood pressure in the older subjects, our results indicate that during low- and moderate-intensity steady-state cycling, MCAVmean responses were similar in young and older individuals. In this regard, the slight but significant increases in MCAVmean observed during steady-state cycling are in agreement with previous studies in young subjects and likely are requisite to meet the demands of exercise-induced increases in cerebral metabolism (10, 23, 30, 40, 41, 50, 53). Thus, in contrast to age-related impairments in peripheral blood flow, the regulation of cerebral blood flow appears to be well maintained during dynamic exercise with age.
Of note, we found that the cerebral vascular conductance index was significantly reduced in older subjects during the moderate-intensity cycling bout, suggesting that cerebral vasoconstriction occurred. Although the cause for this decrease in cerebral vascular conductance is unclear, we suggest that this response may be normal and necessary to offset the greater blood pressure response to exercise (ΔMAP from rest: young 9 ± 3 mmHg vs. older 23 ± 2 mmHg; P < 0.05). In this regard, a comparison of the moderate-intensity cycling bout in the young to the low-intensity cycling bout in the older subjects, in which MAP responses to exercise were closely matched (ΔMAP from rest: young 9 ± 3 mmHg vs. older 10 ± 2 mmHg; P < 0.05), indicates very similar cerebral vascular conductance responses (see Table 2). Thus we interpret the greater decrease in conductance in the older subjects during moderate exercise as a normal CA response. However, other factors cannot be completely discounted, including an enhanced activation of sympathetic outflow directed to the cerebral vasculature (8). At present, sympathetic neural control of the cerebral circulation is controversial; however, it is known that cerebral arteries are richly innervated with sympathetic nerve fibers (36, 37), and a direct effect of sympathetic activation on cerebral blood flow has been reported in several disease states (25, 29). Considering previous reports have suggested an exaggerated exercise-induced sympathoexcitation in older individuals (46, 52), it is plausible that an exaggerated sympathetically mediated cerebral vasoconstriction occurs in older individuals at moderate to high exercise intensities (8). In addition, although no age-group effect was found, the lower PaCO2 during moderate-intensity exercise in older subjects may have contributed to the significant decrease in cerebral vascular conductance from rest. Further studies in this area are warranted.
Along with consideration of static CA, we also assessed dynamic measures of CA at rest and during exercise. Indeed, recent studies have emphasized the importance of evaluating dynamic CA using frequency-domain analysis in order to more fully examine the ability of the cerebral vasculature to rapidly respond to changes in perfusion pressure (18, 42, 55, 56). However, we found no significant age-group differences in either the VLF or LF transfer function gain between MAP and MCAVmean during low- or moderate-intensity cycling. These data indicate that the ability of the cerebral vasculature to respond to spontaneous fluctuations in MAP was preserved in healthy older subjects during exercise. Thus similar to the static measurements made, no age-related alterations in dynamic CA was found. These findings are in agreement with previous studies reporting that dynamic CA was maintained in healthy older subjects at rest and during various laboratory stressors (6, 7, 34, 54). In contrast, the only other paper to examine aging effects on cerebral vascular responses during exercise indicated that cerebral autoregulatory mechanisms demonstrated a delayed responsiveness in older subjects (21). These authors found a delayed increase in cerebrovascular resistance in older individuals in response to increases in cerebral blood flow at the onset of a short bout (3 min) of supine leg cycling. The reason for the conflicting findings between these previous data and the present results is unclear; however, differences in exercise workloads between young and older subjects (absolute vs. relative) and time points studied (onset vs. steady state) likely contributed. In this regard, the findings of the present study indicate that compared with younger subjects exercising at the same relative intensities, the regulation of cerebral blood flow is well maintained during steady-state dynamic exercise in healthy older middle-aged subjects.
In the present study, in addition to the LF range between MAP and MCAVmean, we also utilized the VLF range to more completely assess dynamic CA. Although the majority of studies have focused on the LF range (4, 27, 34, 40, 41), recent studies suggest that CA may be more active in the VLF range than in the LF range (32, 56). Thus we considered the possibility that changes in the VLF range may be important when moving from rest to exercise and possibly contribute to age-related changes in cerebral blood flow control. However, similar to the LF range, no age-group differences were found in the VLF gain or phase between MAP and MCAVmean. Interestingly, the VLF and LF transfer function gains decreased from rest to exercise in both the young and older subjects. These findings are indicative of improved dynamic CA and suggest that during low- to moderate-intensity dynamic exercise, for any given change in blood pressure, smaller oscillations in MCAVmean occur, an effect that appears unaltered with age. Although it remains to be determined if CA is more active in the VLF or in the LF range in humans, the relatively consistent responses between the VLF and LF ranges at rest and during exercise clearly indicate a preserved dynamic CA with aging.
It should be noted that the coherence values for the VLF range tended to be lower than those for the LF range, particularly in the younger subjects. Although these lower values are consistent with previous studies (27, 41, 54–56), the reason for this finding is unclear. Overall, the coherence analysis and interpretation should be considered. Coherence functions have primarily been used to indicate the strength of the linear relationship between MAP and MCAVmean and validate the measurements of transfer function being made (4, 27, 34, 38, 40, 41, 54–56). Thus, when coherence is high (>0.5), MAP and MCAVmean vary closely together and the transfer function gain can be used to evaluate the effectiveness of CA. Indeed, in the present study, LF coherence values remained above 0.5 under all conditions in both young and older subjects. However, other ideas have recently evolved with regard to the coherence function, particularly in the VLF range. It has been suggested that at these very low frequencies (<0.07 Hz) the fluctuations in MCAVmean are more independent of changes in arterial pressure (low coherence) because the cerebral vasculature can effectively buffer such slow changes in blood pressure, and thus fluctuations in MCAVmean can occur independently of blood pressure (27, 56). In fact, some researchers have proposed that a low coherence value is suggestive of an effective CA (27). However, the usage of the coherence in this way requires further validation, and therefore, as in previous studies (4, 27, 34, 38, 40, 41), we have relied on the LF transfer function gain as our primary index of dynamic CA.
Several potential limitations in the design and interpretation of the present investigation should be considered. First, it is realized that changes in MCAVmean are only proportional to changes in cerebral blood flow if middle cerebral artery diameter remains unchanged. Although we cannot completely rule out that changes in vessel diameter influenced the MCAVmean measurements, previous studies in humans directly measuring middle cerebral artery diameter have demonstrated that the diameter remains relatively constant under a variety of experimental conditions and to various stimuli (19, 49). Furthermore, during dynamic exercise it has been demonstrated that MCAVmean increases in parallel with the inflow of the ipsilateral internal carotid artery (22) and with cerebral blood flow determined using the “initial slope index” of the 133Xenon clearance method, which is considered to represent the average cerebral blood flow (30). Indeed, a recent review of the literature reported that the diameter of large cerebral arteries does not change significantly during exercise and that the regulation of cerebral blood flow takes place in the smaller arteries (50). Thus we would contend that the changes in MCAVmean reported in the present investigation likely reflect changes in cerebral blood flow (50).
Another potential limitation of the present study is the small sample size and the possibility that sex differences and in turn hormone status may have influenced our results as both men and women were included. Although the number of subjects is similar to previous studies that employed a within-between subject design to examine the influence of aging, we cannot completely discount the potential that differences may have been detected with a larger number of subjects (i.e., type II error). In addition, since all older women were postmenopausal and not taking any hormone replacement and young women completed the steady-state exercise protocol around the early follicular phase of the menstrual cycle in which plasma estrogen and progesterone concentrations are generally low (20), further studies are needed to examine the influence of sex hormones on CA in women.
The mean age of the older subjects in the present study (57 ± 7 yr) was more indicative of a middle-aged group. Although we did not observe any differences in CA, this age range is similar to that of previous investigations reporting age-related alterations in peripheral blood flow control as well as changes in cardiovascular responses to exercise (13–15, 17, 35, 51, 54). Thus we believe the results of the present study provide novel insight into the regulation of cerebral blood flow during dynamic exercise as one advances in age. However, we would caution readers in extrapolating our findings to subjects over 70 yr of age. In addition, the results of the present study are specific to steady-state dynamic exercise at low and moderate relative intensities, and thus the potential for age-related differences at higher exercise intensities or when comparing at absolute workloads cannot be dismissed. Nonetheless, the intensities studied were chosen because they are an integral part of the recommended exercise prescription for healthy older adults (16).
In summary, we found that despite greater increases in blood pressure during exercise in older individuals, no differences in MCAVmean were observed between young and older subjects. Furthermore, we observed no significant age-group differences in dynamic CA during low- or moderate-intensity steady-state dynamic exercise performed at an equivalent relative intensity in young and older individuals. Thus the ability of the cerebral vasculature to respond to spontaneous fluctuations in MAP was preserved with age during exercise. Collectively, these data suggest that the regulation of MCAVmean is well maintained during dynamic exercise in healthy older middle-aged subjects.
GRANTS
This research is the result of work supported with resources and the use of facilities at the Harry S. Truman Memorial Veterans Hospital in Columbia, MO, by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-076636 to P. J. Fadel, by an American College of Sports Medicine Visiting Scholar Award to S. Ogoh, and by an American Heart Association Postdoctoral Fellowship Award to J. P. Fisher.
Acknowledgments
We appreciate the time and effort expended by all the volunteer subjects.
Present address for J. P. Fisher: School of Sport and Exercise Sciences, Univ. of Birmingham, Edgbaston, Birmingham B15 2TT, UK.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.American College of Sports Medicine. American College of Sports Medicine Position Stand. The recommended quantity and quality of exercise for developing and maintaining cardiorespiratory and muscular fitness, and flexibility in healthy adults. Med Sci Sports Exerc 30: 975–991, 1998 [DOI] [PubMed] [Google Scholar]
- 2.Bogert LW, van Lieshout JJ. Non-invasive pulsatile arterial pressure and stroke volume changes from the human finger. Exp Physiol 90: 437–446, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc 14: 377–381, 1982 [PubMed] [Google Scholar]
- 4.Brys M, Brown CM, Marthol H, Franta R, Hilz MJ. Dynamic cerebral autoregulation remains stable during physical challenge in healthy persons. Am J Physiol Heart Circ Physiol 285: H1048–H1054, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Cameron JD, Stevenson I, Reed E, McGrath BP, Dart AM, Kingwell BA. Accuracy of automated auscultatory blood pressure measurement during supine exercise and treadmill stress electrocardiogram-testing. Blood Press Monit 9: 269–275, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Carey BJ, Eames PJ, Blake MJ, Panerai RB, Potter JF. Dynamic cerebral autoregulation is unaffected by aging. Stroke 31: 2895–2900, 2000 [DOI] [PubMed] [Google Scholar]
- 7.Carey BJ, Panerai RB, Potter JF. Effect of aging on dynamic cerebral autoregulation during head-up tilt. Stroke 34: 1871–1875, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Cassaglia PA, Griffiths RI, Walker AM. Sympathetic nerve activity in the superior cervical ganglia increases in response to imposed increases in arterial pressure. Am J Physiol Regul Integr Comp Physiol 294: R1255–R1261, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Daida H, Allison TG, Squires RW, Miller TD, Gau GT. Peak exercise blood pressure stratified by age and gender in apparently healthy subjects. Mayo Clin Proc 71: 445–452, 1996 [DOI] [PubMed] [Google Scholar]
- 10.Dalsgaard MK, Quistorff B, Danielsen ER, Selmer C, Vogelsang T, Secher NH. A reduced cerebral metabolic ratio in exercise reflects metabolism and not accumulation of lactate within the human brain. J Physiol 554: 571–578, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diehl RR, Linden D, Lucke D, Berlit P. Phase relationship between cerebral blood flow velocity and blood pressure. A clinical test of autoregulation. Stroke 26: 1801–1804, 1995 [DOI] [PubMed] [Google Scholar]
- 12.Dinenno FA, Joyner MJ. Alpha-adrenergic control of skeletal muscle circulation at rest and during exercise in aging humans. Microcirculation 13: 329–341, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Fadel PJ, Wang Z, Watanabe H, Arbique D, Vongpatanasin W, Thomas GD. Augmented sympathetic vasoconstriction in exercising forearms of postmenopausal women is reversed by oestrogen therapy. J Physiol 561: 893–901, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fisher JP, Ogoh S, Ahmed A, Aro MR, Gute D, Fadel PJ. Influence of age on cardiac baroreflex function during dynamic exercise in humans. Am J Physiol Heart Circ Physiol 293: H777–H783, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Fleg JL, O'Connor F, Gerstenblith G, Becker LC, Clulow J, Schulman SP, Lakatta EG. Impact of age on the cardiovascular response to dynamic upright exercise in healthy men and women. J Appl Physiol 78: 890–900, 1995 [DOI] [PubMed] [Google Scholar]
- 16.Fletcher GF, Balady G, Blair SN, Blumenthal J, Caspersen C, Chaitman B, Epstein S, Sivarajan Froelicher ES, Froelicher VF, Pina IL, Pollock ML. Statement on exercise: benefits and recommendations for physical activity programs for all Americans. A statement for health professionals by the Committee on Exercise and Cardiac Rehabilitation of the Council on Clinical Cardiology, American Heart Association. Circulation 94: 857–862, 1996 [DOI] [PubMed] [Google Scholar]
- 17.Fu CH, Yang CC, Kuo TB. Age-related changes in cerebral hemodynamics and their correlations with cardiac autonomic functions. Neurol Res 28: 871–876, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Giller CA. The frequency-dependent behavior of cerebral autoregulation. Neurosurgery 27: 362–368, 1990 [DOI] [PubMed] [Google Scholar]
- 19.Giller CA, Bowman G, Dyer H, Mootz L, Krippner W. Cerebral arterial diameters during changes in blood pressure and carbon dioxide during craniotomy. Neurosurgery 32: 737–741; discussion 741–742, 1993 [PubMed] [Google Scholar]
- 20.Guyton AC, Hall JE. Textbook of Medical Physiology. Philadelphia, PA: Elsevier Saunders, 2005
- 21.Heckmann JG, Brown CM, Cheregi M, Hilz MJ, Neundorfer B. Delayed cerebrovascular autoregulatory response to ergometer exercise in normotensive elderly humans. Cerebrovasc Dis 16: 423–429, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Hellstrom G, Fischer-Colbrie W, Wahlgren NG, Jogestrand T. Carotid artery blood flow and middle cerebral artery blood flow velocity during physical exercise. J Appl Physiol 81: 413–418, 1996 [DOI] [PubMed] [Google Scholar]
- 23.Hellstrom G, Wahlgren NG. Physical exercise increases middle cerebral artery blood flow velocity. Neurosurg Rev 16: 151–156, 1993 [DOI] [PubMed] [Google Scholar]
- 24.Hoffman WE, Albrecht RF, Miletich DJ. The influence of aging and hypertension on cerebral autoregulation. Brain Res 214: 196–199, 1981 [DOI] [PubMed] [Google Scholar]
- 25.Ide K, Boushel R, Sorensen HM, Fernandes A, Cai Y, Pott F, Secher NH. Middle cerebral artery blood velocity during exercise with beta-1 adrenergic and unilateral stellate ganglion blockade in humans. Acta Physiol Scand 170: 33–38, 2000 [DOI] [PubMed] [Google Scholar]
- 26.Ide K, Secher NH. Cerebral blood flow and metabolism during exercise. Prog Neurobiol 61: 397–414, 2000 [DOI] [PubMed] [Google Scholar]
- 27.Iwasaki K, Levine BD, Zhang R, Zuckerman JH, Pawelczyk JA, Diedrich A, Ertl AC, Cox JF, Cooke WH, Giller CA, Ray CA, Lane LD, Buckey JC Jr, Baisch FJ, Eckberg DL, Robertson D, Biaggioni I, Blomqvist CG. Human cerebral autoregulation before, during and after spaceflight. J Physiol 579: 799–810, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jones NL, Robertson DG, Kane JW. Difference between end-tidal and arterial Pco2 in exercise. J Appl Physiol 47: 954–960, 1979 [DOI] [PubMed] [Google Scholar]
- 29.Jordan J, Shannon JR, Black BK, Paranjape SY, Barwise J, Robertson D. Raised cerebrovascular resistance in idiopathic orthostatic intolerance: evidence for sympathetic vasoconstriction. Hypertension 32: 699–704, 1998 [DOI] [PubMed] [Google Scholar]
- 30.Jorgensen LG, Perko G, Secher NH. Regional cerebral artery mean flow velocity and blood flow during dynamic exercise in humans. J Appl Physiol 73: 1825–1830, 1992 [DOI] [PubMed] [Google Scholar]
- 31.Koch DW, Leuenberger UA, Proctor DN. Augmented leg vasoconstriction in dynamically exercising older men during acute sympathetic stimulation. J Physiol 551: 337–344, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kolb B, Rotella DL, Stauss HM. Frequency response characteristics of cerebral blood flow autoregulation in rats. Am J Physiol Heart Circ Physiol 292: H432–H438, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Lakatta EG. Cardiovascular regulatory mechanisms in advanced age. Physiol Rev 73: 413–467, 1993 [DOI] [PubMed] [Google Scholar]
- 34.Lipsitz LA, Mukai S, Hamner J, Gagnon M, Babikian V. Dynamic regulation of middle cerebral artery blood flow velocity in aging and hypertension. Stroke 31: 1897–1903, 2000 [DOI] [PubMed] [Google Scholar]
- 35.Najjar SS, Scuteri A, Lakatta EG. Arterial aging: is it an immutable cardiovascular risk factor? Hypertension 46: 454–462, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Nelson E, Rennels M. Innervation of intracranial arteries. Brain 93: 475–490, 1970 [DOI] [PubMed] [Google Scholar]
- 37.Nielsen KC, Owman C. Adrenergic innervation of pial arteries related to the circle of Willis in the cat. Brain Res 6: 773–776, 1967 [DOI] [PubMed] [Google Scholar]
- 38.Ogoh S, Brothers RM, Barnes Q, Eubank WL, Hawkins MN, Purkayastha S, AOY, Raven PB. The effect of changes in cardiac output on middle cerebral artery mean blood velocity at rest and during exercise. J Physiol 569: 697–704, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ogoh S, Dalsgaard MK, Yoshiga CC, Dawson EA, Keller DM, Raven PB, Secher NH. Dynamic cerebral autoregulation during exhaustive exercise in humans. Am J Physiol Heart Circ Physiol 288: H1461–H1467, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Ogoh S, Fadel PJ, Zhang R, Selmer C, Jans O, Secher NH, Raven PB. Middle cerebral artery flow velocity and pulse pressure during dynamic exercise in humans. Am J Physiol Heart Circ Physiol 288: H1526–H1531, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Ogoh S, Fisher JP, Purkayastha S, Dawson EA, Fadel PJ, White MJ, Zhang R, Secher NH, Raven PB. Regulation of middle cerebral artery blood velocity during recovery from dynamic exercise in humans. J Appl Physiol 102: 713–721, 2007 [DOI] [PubMed] [Google Scholar]
- 42.Panerai RB, Dawson SL, Potter JF. Linear and nonlinear analysis of human dynamic cerebral autoregulation. Am J Physiol Heart Circ Physiol 277: H1089–H1099, 1999 [DOI] [PubMed] [Google Scholar]
- 43.Parati G, Casadei R, Groppelli A, Di Rienzo M, Mancia G. Comparison of finger and intra-arterial blood pressure monitoring at rest and during laboratory testing. Hypertension 13: 647–655, 1989 [DOI] [PubMed] [Google Scholar]
- 44.Paulson OB, Strandgaard S, Edvinsson L. Cerebral autoregulation. Cerebrovasc Brain Metab Rev 2: 161–192, 1990 [PubMed] [Google Scholar]
- 45.Proctor DN, Parker BA. Vasodilation and vascular control in contracting muscle of the aging human. Microcirculation 13: 315–327, 2006 [DOI] [PubMed] [Google Scholar]
- 46.Proctor DN, Shen PH, Dietz NM, Eickhoff TJ, Lawler LA, Ebersold EJ, Loeffler DL, Joyner MJ. Reduced leg blood flow during dynamic exercise in older endurance-trained men. J Appl Physiol 85: 68–75, 1998 [DOI] [PubMed] [Google Scholar]
- 47.Querido JS, Sheel AW. Regulation of cerebral blood flow during exercise. Sports Med 37: 765–782, 2007 [DOI] [PubMed] [Google Scholar]
- 48.Schrage WG, Eisenach JH, Joyner MJ. Ageing reduces nitric-oxide- and prostaglandin-mediated vasodilatation in exercising humans. J Physiol 579: 227–236, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schreiber SJ, Gottschalk S, Weih M, Villringer A, Valdueza JM. Assessment of blood flow velocity and diameter of the middle cerebral artery during the acetazolamide provocation test by use of transcranial Doppler sonography and MR imaging. Am J Neuroradiol 21: 1207–1211, 2000 [PMC free article] [PubMed] [Google Scholar]
- 50.Secher NH, Seifert T, Van Lieshout JJ. Cerebral blood flow and metabolism during exercise: implications for fatigue. J Appl Physiol 104: 306–314, 2008 [DOI] [PubMed] [Google Scholar]
- 51.Smith EG, Voyles WF, Kirby BS, Markwald RR, Dinenno FA. Ageing and leg postjunctional alpha-adrenergic vasoconstrictor responsiveness in healthy men. J Physiol 582: 63–71, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Taylor JA, Hand GA, Johnson DG, Seals DR. Augmented forearm vasoconstriction during dynamic exercise in healthy older men. Circulation 86: 1789–1799, 1992 [DOI] [PubMed] [Google Scholar]
- 53.Thomas SN, Schroeder T, Secher NH, Mitchell JH. Cerebral blood flow during submaximal and maximal dynamic exercise in humans. J Appl Physiol 67: 744–748, 1989 [DOI] [PubMed] [Google Scholar]
- 54.Yam AT, Lang EW, Lagopoulos J, Yip K, Griffith J, Mudaliar Y, Dorsch NW. Cerebral autoregulation and ageing. J Clin Neurosci 12: 643–646, 2005 [DOI] [PubMed] [Google Scholar]
- 55.Zhang R, Zuckerman JH, Giller CA, Levine BD. Transfer function analysis of dynamic cerebral autoregulation in humans. Am J Physiol Heart Circ Physiol 274: H233–H241, 1998 [DOI] [PubMed] [Google Scholar]
- 56.Zhang R, Zuckerman JH, Iwasaki K, Wilson TE, Crandall CG, Levine BD. Autonomic neural control of dynamic cerebral autoregulation in humans. Circulation 106: 1814–1820, 2002 [DOI] [PubMed] [Google Scholar]


