Abstract
The role of PKC and RhoA/ROCK pathways in the phasic activities in the rectal smooth muscles (RSM) in the basal state is not known. We examined this issue by determining the effects of PKC inhibitors (calphostin C and Gö-6850) and a ROCK inhibitor (Y-27632) on the slow-rate (∼3/min) and fast-rate (∼25/min) phasic activities. We also examined the corresponding signal transduction cascades and the PKC and ROCK enzymatic activities in the RSM in the basal state. PKC inhibition with calphostin C and Gö-6850 (10−5 M) caused a significant decrease (∼25%) in slow-rate (but not fast-rate) phasic activity (monitored by frequency and amplitude of contractions) of the RSM. Conversely, ROCK inhibition with Y-27632 (10−5 M) caused a significant decrease not only in slow-rate, but also fast-rate, phasic activity caused by ROCK inhibition in the RSM. Western blot analysis revealed that the PKC inhibition-induced decrease in RSM phasic activity was associated with decreases in PKCα translocation, phosphorylated (Thr38) PKC-potentiated inhibitor (CPI-17), and phosphorylated (Thr18/Ser19) 20-kDa myosin regulatory light chain. Conversely, decreases in the phasic activity in the RSM by ROCK inhibition were accompanied by the additional decrease in phosphorylated (Thr696) myosin phosphatase target subunit 1. Data show that while PKC and RhoA/ROCK pathways play a significant role in slow-rate high-amplitude spontaneous phasic activity, only the RhoA/ROCK pathway primarily mediates fast-rate low-amplitude phasic activity, in the RSM. Such knowledge is important in the understanding of the pathophysiology of large intestinal motility disorders. Relative contributions of the PKC vs. the RhoA/ROCK pathway in the phasic activity remain to be determined.
Keywords: smooth muscle, phasic activity, Rho kinase, protein kinase C, rectoanal incontinence
fecal incontinence affects ∼10% of the adult US population (45) and is multifactorial (2, 28, 43). These factors are impairment of basal internal anal sphincter (IAS) tone and external anal sphincter, puborectalis, and colorectal smooth muscle compliance. Recently, significant data in intact IAS of humans and animals demonstrated that the predominant molecular regulatory mechanism for IAS tone is the RhoA/ROCK pathway over the PKC pathway (26, 30, 32). However, little is known about the patterns of rectal smooth muscle (RSM) contractions and regulatory mechanisms of the RSM, especially in the basal state.
A good starting point would be analysis of the contractile patterns in the phasic activity in the RSM in the basal state (29, 30, 32). In the gastrointestinal tract smooth muscle, spontaneous contractions vary from 3 min−1 (in the stomach) to 8–12 min−1 (in the small and large intestine) (33). However, there is limited literature on the characterization of spontaneous phasic activity in the RSM. Such analysis is important to determine the molecular mechanisms controlling different patterns of the phasic activities. Such phasic activity, however, should not be confused with the initial peak contraction routinely investigated in response to different agonists and electrical field stimulation (EFS) (11, 16, 22, 39), shown to be mediated primarily via the Ca2+/calmodulin/myosin light chain (MLC) kinase (MLCK) pathway.
Knowledge of the regulatory control mechanisms for the phasic activity in the RSM is critical in understanding the large intestinal compliance factor for rectoanal incontinence (2, 28, 43) and a number of other gastrointestinal motility disorders (15), such as inflammatory bowel disease, Hirschsprung's disease, and constipation. Such phasic activity in the RSM in synchrony with colonic activity may participate in the colorectal excitatory reflex (27).
It is well known that contraction of smooth muscle is primarily mediated by phosphorylation of 20-kDa myosin regulatory light chain (MLC20), and the degree and duration of contraction depend on the balance between the activities of MLCK and MLC phosphatase (MLCP). Recent studies have shown that the small GTP-binding protein RhoA and associated kinase (ROCK) (29, 31, 39) and PKC (3, 23, 38, 44) play important roles in regulation of MLCP activity via Ca2+ sensitization and, consequently, in contraction of the smooth muscles. Accordingly, the RhoA/ROCK pathway causes inactivation of MLCP via phosphorylation of the noncatalytic subunit of MLCP, whereas PKC activation inhibits MLCP via phosphorylation of PKC-potentiated inhibitor (CPI-17), the endogenous inhibitory protein of the catalytic subunit of MLCP (20).
Examination of the effects of the selective PKC inhibitors calphostin C (13, 21, 36) and Gö-6850 (5, 42) and the ROCK inhibitor Y-27632 (9, 17, 31, 34) provides important tools to study the roles of PKC and RhoA/ROCK in the phasic activity of the RSM. Such functional studies will be combined in parallel with the enzymatic activity assays and the signal transduction studies [phosphorylated (Thr696) myosin phosphatase-targeting subunit (MYPT1), phosphorylated (Thr38) CPI-17, and phosphorylated (Thr18/Ser19) MLC20] leading to the eventual phasic activity in the RSM. There are no data that compare the roles of PKC vs. RhoA/ROCK in the phasic activities of the RSM.
Therefore, the purpose of the present investigation is to analyze the phasic motility pattern of the RSM and to investigate the roles of RhoA/ROCK and PKC pathways in the molecular control mechanisms.
MATERIALS AND METHODS
Tissue preparation.
As previously described (30), Sprague-Dawley rats (300–350 g body wt) were killed by decapitation. The anal canal with an adjacent region of the RSM was quickly removed and transferred to oxygenated (95% O2-5% CO2) Krebs physiological solution (KPS) of the following composition (in mM): 118.07 NaCl, 4.69 KCl, 2.52 CaCl2, 1.16 MgSO4 1.01 NaH2PO2, 25 NaHCO3, and 11.10 glucose (37°C). Extraneous adventitious blood vessels and skeletal muscle tissues connected to the anal canal were removed carefully by sharp dissection. The anal canal was then opened and pinned flat, with the mucosal side up, and mucosa was removed carefully by sharp dissection and divided into two distinct regions, the IAS and RSM. From the IAS and RSM, circular smooth muscle strips (1 × 7 mm) were prepared. The experimental protocols of the study were approved by the Institutional Animal Care and Use Committee of Thomas Jefferson University and were carried out in accordance with the recommendations of the American Association for the Accreditation of Laboratory Animal Care.
Measurement of isometric tension.
The smooth muscle strips were transferred to 2-ml muscle baths containing oxygenated KPS at 37°C. One end of the strips was anchored at the bottom of the muscle bath, and the other end was connected to a force transducer (FORT10, WPI, Sarasota, FL). Isometric tension was measured by the PowerLab 8SP data acquisition system (ADInstruments, Castle Hill, Australia) and recorded using Chart 4.1.2 (ADInstruments). Each smooth muscle strip was initially stretched to a tension of 1.0 g. The muscle strips were allowed to equilibrate for 1 h, during which they were washed with KPS every 20 min. The RSM developed spontaneous low- and high-frequency phasic activities superimposed on a low-grade tone, and such smooth muscles primarily responded by contraction to EFS (6). Depending on the experimental protocol, we examined the effects of different concentrations (10−8–10−4 M) of calphostin C, Gö-6850, and Y-27632 on the basal activity of the RSM.
PKC and ROCK activity.
PKC and ROCK activity were measured in tissue homogenates of the RSM before and after treatment with different concentrations of inhibitors at 0, 2, 4, 6, 8, and 10 min. The smooth muscle tissue strips were flash-frozen using Wollenberger tongs precooled in liquid N2 (30) and homogenized in ice-cold lysis buffer consisting of 50 mM Tris·HCl, pH 7.5, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, a protease inhibitor mixture, and Na3VO4, a phosphatase inhibitor (Pierce Biotechnology, Rockford, IL). The protein concentration in tissue lysates was determined using a BCA protein assay kit (Pierce Biotechnology). Kinase activity was measured using nonradioactive kinase assay kits (catalog no. EKS-420, Assay Designs for PKC; catalog no. STA-416, Cell Biolabs). RSM tissue lysates were titrated to determine the optimal protein concentration for the final activity assay; 5–40 μg were used for PKC activity assay, and 0.5–16 μg were used for ROCK activity assay. PKC and ROCK activities in tissue lysates were calculated on the basis of PKC and ROCK standard concentration curves using specific 450-nm absorbance according to the manufacturer's instructions. All samples were assayed in quadruplicate, and the ELISA was performed twice.
Tissue lysate preparation and Western blot analysis.
While the isometric tension was monitored, the RSM strips were quick-frozen before and after pretreatment with calphostin C, Gö-6850, and Y-27632. Once the response to these inhibitors reached a plateau, the tissue chambers were rapidly lowered, exposing the tissues, which were quickly snap-frozen, as described above. The tissue samples were cut into small pieces (∼1-mm cubes) and homogenized on ice in buffer (1% SDS, 1.0 mM Na3VO4, and 10 mM Tris, pH 7.4) using a volume equal to five times the tissue weight. The homogenates were centrifuged (14,000 rpm) for 5 min, and the supernatants were collected. The protein concentration in the supernatants was determined using a BCA protein assay reagent kit, with BSA used as a standard (Pierce Biotechnology) (19). Twenty micrograms of protein in 20 μl of lysates were mixed with 2× Laemmli sample buffer (with final concentrations of 62.5 mM Tris, 1% SDS, 15% glycerol, 0.005% bromophenol blue, and 2% β-mercaptoethanol) and placed in a boiling water bath for 5 min. Proteins in the samples were separated by SDS-polyacrylamide gel [7.5% gel for phosphorylated (Thr696) MYPT1 and 15% gel for phosphorylated (Thr18/Ser19) MLC20 and phosphorylated (Thr38) CPI-17]. The separated proteins were electrophoretically transferred using the iBlot dry blotting system (Invitrogen) onto a nitrocellulose membrane for phosphorylated (Thr696) MYPT1 or a polyvinylidene difluoride membrane for phosphorylated (Thr38) CPI-17 and phosphorylated (Thr18/Ser19) MLC20 at 25 V for 14 min at room temperature. To block nonspecific antibody binding, the membrane was soaked for 1 h at room temperature in LI-COR buffer (LI-COR Biosciences, Lincoln, NE). The membrane was then incubated with the specific primary antibodies [1:1,000 dilution for phosphorylated (Thr38) CPI-17, phosphorylated (Thr696) MYPT1, and phosphorylated (Thr18/Ser19) MLC20 and 1:20,000 dilution for α-actin] diluted in LI-COR buffer containing 0.1% Tween 20 for 1 h at room temperature. After they were washed three times for 10 min each with Tris-buffered saline-Tween 20, the membranes were incubated with the IRdye680- and IRdye800-conjugated secondary antibody (LI-COR Biosciences) in darkness [bovine anti-rabbit diluted 1:10,000 for ROCK-II, MYPT1, and phosphorylated (Thr696) MYPT1 and bovine anti-goat diluted 1:5,000 for phosphorylated (Thr38) CPI-17]. The membranes were washed three times for 10 min each with Tris-buffered saline-Tween 20 and finally kept in PBS on a shaker for 10 min at room temperature in darkness and scanned by an infrared scanner (LI-COR Biosciences).
Chemicals and drugs.
Calphostin C, Gö-6850, and Y-27632 were purchased from Biomol (Plymouth Meeting, PA). The following antibodies were used: α-actin and MLC20 antibodies (Sigma, St. Louis, MO); phosphorylated (Thr696) MYPT1, phosphorylated (Thr38) CPI-17, and phosphorylated (Thr18/Ser19) MLC20 antibodies (Santa Cruz Biotechnology, Santa Cruz, CA); and IRdye680- and IRdye800-conjugated mouse, goat, and rabbit secondary antibodies (LI-COR Biosciences).
Data analysis.
All the calculations for force experiments were done using GraphPad Prism 5.3. Values are means ± SE. Relative densities of Western blots were calculated using ImageJ software (National Institutes of Health) and normalized to 1. One-way ANOVA followed by a Bonferroni's post hoc test was used (P < 0.05) to calculate statistical significance.
RESULTS
Inhibition of PKC activity by calphostin C.
PKC activity data revealed that, in the basal state, maximal PKC activity in RSM and IAS tissues was observed with 30 μg of the tissue lysates (n = 4; Fig. 1A). The PKC inhibitor calphostin C (10−5 M) caused a time-dependent decrease in PKC activity that was nearly obliterated within 8 min of administration (Fig. 1B). The specificity of the effect of calphostin C was further evident from the data that this inhibitor, in contrast to the ROCK inhibitor Y-27632, caused a significant and concentration-dependent decrease in PKC activity in tissue lysates (P < 0.05, n = 4; Fig. 1C). Figure 1D shows the basal values of PKC activity and their decreases following 8-min applications of 10−8–10−4 M calphostin C; maximal inhibition was achieved in the presence of 10−5 M calphostin C.
Fig. 1.
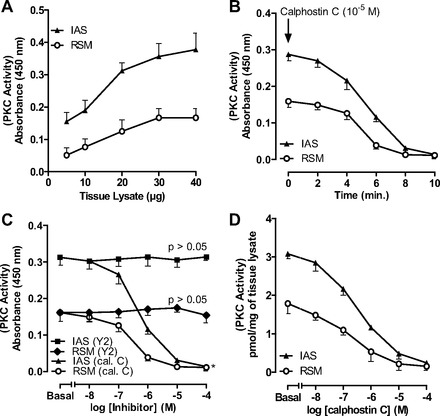
PKC activity in rectal smooth muscle (RSM) and internal anal sphincter (IAS) smooth muscle tissue lysates. A: PKC activity assay shows 30 μg of RSM and IAS lysate protein to be most optimal. B: time course for absolute decrease in basal PKC activity in 30 μg of tissue lysates over 10 min following pretreatment of tissues with calphostin C (10−5 M). C: decrease in PKC activity in 30 μg of tissue lysates before (basal) and after 10−8–10−4 M calphostin C (cal. C) and Y-27632 (Y2). D: absolute decrease in PKC activity in RSM after treatment with calphostin C.
Comparison of ROCK activity levels in the IAS vs. RSM: influence of calphostin C vs. Y-27632.
Parallel data with ROCK activity in the RSM and IAS showed that the optimal concentration of the tissue lysates was 8 μg (Fig. 2A) and that ROCK activity was significantly higher in the IAS than RSM (3.67 ± 0.24 vs. 1.8 ± 0.33 pmol/mg, P < 0.05, n = 4; Fig. 2, A and C). The incubation required for maximal inhibition by Y-27632 was determined to be 8 min (Fig. 2B). Data further revealed the specificity of the ROCK inhibitor (P < 0.05, n = 4; Fig. 2C), as calphostin C had no significant effect (P > 0.05; Fig. 2C).
Fig. 2.
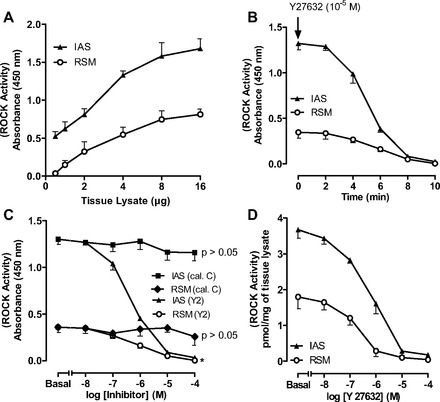
ROCK activity in RSM and IAS smooth muscle tissue lysates. A: standard curve for ROCK activity assay in 0.5–16 μg of tissue lysates. B: time course for absolute decrease in basal ROCK activity in 8 μg of RSM and IAS tissue lysates over 10 min. C: decrease in ROCK activity in RSM vs. IAS before (basal) and after 10−8–10−4 M calphostin C or Y-27632. As expected, ROCK activity is higher in IAS than RSM. D: absolute values for concentration-dependent decrease in ROCK activity by Y-27632 (10−8–10−4 M) in IAS and RSM.
Influence of PKC and ROCK inhibitors on rate and amplitude of slow-rate phasic activity in the RSM.
Frequency of contraction data in the IAS and RSM was analyzed using built-in software for the rate analysis (PowerLab, ADInstruments). Two main types of phasic activity were recorded in the RSM: slow-rate (2.7 ± 0.09 min−1; Fig. 3A) and fast-rate (25 ± 1.4 min−1; Fig. 4A). A typical RSM force recording (Fig. 3B, inset) shows that PKC inhibition with calphostin C or Gö-6850 caused significant inhibition of frequency (absolute and %maximal; P < 0.05, n = 5; Fig. 3, A and B) and amplitude (P < 0.05, n = 5; Fig. 3, C and D) of slow-rate contractions in the RSM. Interestingly, Gö-6850 was more effective than calphostin C. Y-27632 also caused a concentration-dependent decrease in the rate and amplitude of slow-rate phasic activity of the RSM and was more effective than calphostin C and Gö-6850 (P < 0.01, n = 5; Fig. 3). The maximal effective concentration of Gö-6850 (10−5 M) caused a decrease in the rate and amplitude of 15% and 28%, respectively; in the case of Y-27632, these values were 40% and 53%, respectively.
Fig. 3.
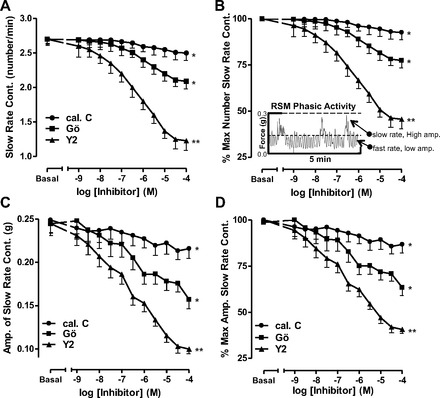
Effect of calphostin C, Gö-6850 (Gö), and Y-27632 (10−8–10−4 M) on slow-rate phasic activity in RSM. A: absolute change in rate of contraction (Cont) with different inhibitors. B: percent maximal decrease. Inset: RSM force recording. Dashed lines in the inset represent cutoff filters for the high- and low-amplitude phasic activities. C and D: corresponding changes in amplitudes (Amp) as absolute decrease and percent maximal decrease in slow-rate phasic activity in RSM. Data show significant decrease in rate, as well as amplitude, following PKC inhibitors (calphostin C and Gö-6850, *P < 0.05, n = 5) and even further significantly higher with the ROCK inhibitor Y-27632 (**P < 0.01, n = 5).
Fig. 4.
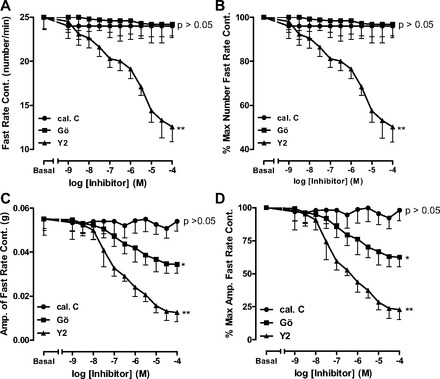
Significant decrease in fast-rate phasic activity in RSM in terms of rate (A and B) and amplitude (C and D), following pretreatment of the smooth muscle with different concentrations of the ROCK inhibitor Y-27632 (**P < 0.01, n = 5). Note that the PKC inhibitor calphostin C has no effect on rate or amplitude (P > 0.05, n = 5), while Gö-6850 also has no significant effect on rate (P > 0.05, n = 5) but causes a small, but significant, decrease in amplitude (*P < 0.05).
Influence of PKC and ROCK inhibitors on rate and amplitude of fast-rate phasic activity in the RSM.
In contrast to the slow-rate phasic activity, the fast-rate phasic activity frequency in the RSM was not affected by calphostin C or Gö-6850 (P > 005, n = 5; Fig. 4, A and B). Interestingly, unlike calphostin C, Gö-6850 caused a small, but significant, decrease in amplitude of the fast-rate phasic activity (P < 0.05; Fig. 4, C and D). In sharp contrast, Y-27632 caused a substantial, significant, and concentration-dependent decrease in rate and amplitude of the fast-rate phasic activity in the RSM (n = 5, P < 0.01; Fig. 4).
As shown in Figs. 5 and 6, rate and amplitude of the slow- and fast-rate phasic activity in the RSM were almost abolished by 0 Ca2+. Additionally, the data summarize the effects of maximally inhibitory concentrations of calphostin C, Gö-6850, Y-27632, and Y-27632 + Gö-6850. The data show that, in inhibiting the slow-rate, as well the fast-rate, contraction in terms of rate and amplitude, Y-27632 caused significantly greater inhibition than calphostin C or Gö-6850 (P < 0.05). The data further reveal a further significant decrease in the amplitude of the slow-rate (n = 5, P < 0.05; Fig. 5), but not fast-rate (n = 5, P > 0.05; Fig. 6), phasic activity in the RSM by Y-27632 + Gö-6850 compared with either inhibitor alone. These data suggest a role of PKC and RhoA/ROCK pathways in the slow rate of spontaneous activity and that the fast rate of activity in the RSM is primarily mediated by the RhoA/ROCK pathway.
Fig. 5.
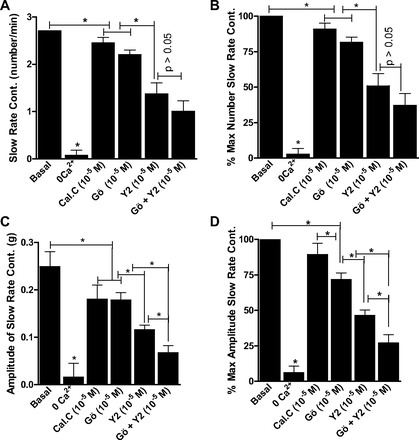
Effects of calphostin C, Gö-6850, and Y-27632 in maximally effective concentrations (10−5 M), as well as Gö-6850 + Y-27632. Note significant decrease in slow-rate phasic activity in RSM in rate (A and B) and amplitude (C and D; *P < 0.05, n = 5). However, inhibition of amplitude of slow-rate phasic activity in RSM is significantly greater in the presence of Gö-6850 + Y-27632 than Gö-6850 or Y-27632 alone (C and D; *P < 0.05). Slow-rate phasic activity in RSM is nearly abolished by 0 Ca2+ (*P < 0.05, n = 5).
Fig. 6.
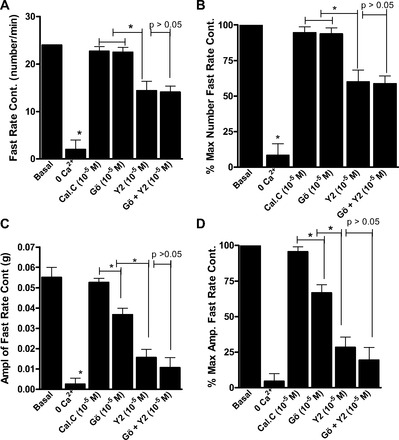
Effects of calphostin C, Gö-6850, Y-27632, and Gö-6850 + Y-27632 in maximally effective concentrations (10−5 M) on fast-rate phasic activity in RSM. Although the ROCK inhibitor Y-27632 causes a significant decrease in rate (A and B) and amplitude (C and D; *P < 0.05, n = 5), PKC inhibitors have no significant effect on fast-rate phasic activity in RSM (A–D). Gö-6850 + Y-27632 does not produce a further significant decrease in RSM activity compared with Y-27632 alone (P > 0.05). Note that 0 Ca2+ nearly abolishes fast-rate phasic activity in RSM (*P < 0.05, n = 5).
Effect of the PKC activator phorbol 12,13-dibutyrate before and after calphostin C in the RSM.
As shown in Fig. 7, the significant and concentration-dependent increase in RSM contraction by phorbol 12,13-dibutyrate (PDBu) was blocked by the PKC inhibitor calphostin C (n = 7, P < 0.05). These data suggest that the PDBu-stimulated increase in the phasic activity of the RSM is mediated by PKC.
Fig. 7.
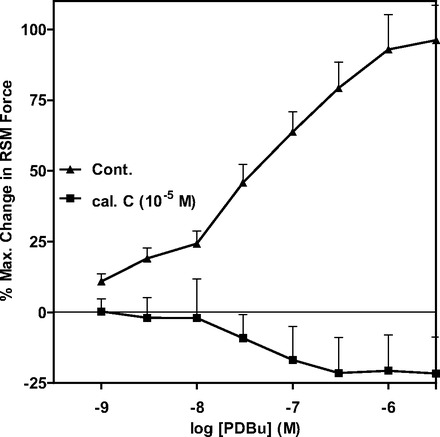
The PKC activator phorbol 12,13-dibutyrate (PDBu) causes concentration-dependent contraction in RSM that is significantly inhibited by calphostin C.
Effect of PKC vs. ROCK inhibition on PKC translocation.
Western blot data show higher basal levels of PKCα in the particulate than cytosolic fraction of the RSM (P < 0.05, n = 4; Fig. 8). This trend was significantly reversed by the PKC inhibitors, and not by Y-27632 (P > 0.05). These data further show the selective effects of calphostin C and Gö-6850 on PKC activation.
Fig. 8.
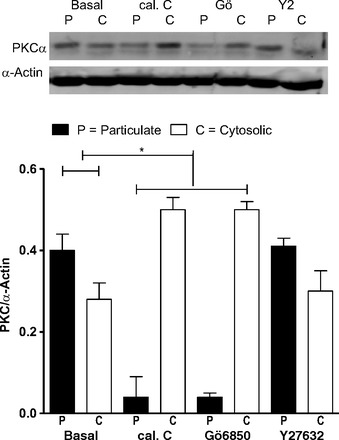
Western blot analysis of PKCα levels in RSM [in particulate or membrane (P) and cytosolic (C) fractions] in the basal state and following pretreatment with calphostin C, Gö-6850, and Y-27632 (all 10−5 M). Note significant (*P < 0.05) translocation of PKCα from membrane to cytosol after treatment with calphostin C and Gö-6850, but not Y-27632.
Effect of calphostin C and Y-27632 on phosphorylated (Thr38) CPI-17, phosphorylated (Thr696) MYPT1, and phosphorylated (Thr18/Ser19) MLC20.
Phosphorylated (Thr38) CPI-17 and phosphorylated (Thr18/Ser19) MLC20 in the RSM were decreased significantly by calphostin C and Gö-6850 and further decreased significantly by Y-27632 (P < 0.05; Fig. 9, A and C). In contrast, however, the basal levels of phosphorylated (Thr696) MYPT1 were also significantly decreased by Y-27632 (P < 0.05; Fig. 9). These changes in the signal transduction cascade following pretreatment with the PKC- and ROCK-selective inhibitors suggest convergence of PKC and RhoA/ROCK on phosphorylated (Thr38) CPI-17 and phosphorylated (Thr18/Ser19) MLC20 in the spontaneous phasic activity in the RSM.
Fig. 9.
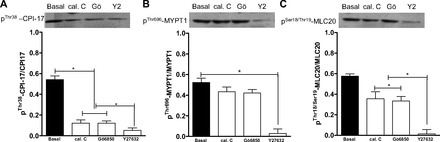
Western blot analysis showing significant (*P < 0.05) decreases in levels of phosphorylated (Thr38) PKC-potentiated inhibitor (CPI-17; A) and phosphorylated (Thr18/Ser19) 20-kDa myosin regulatory light chain (MLC20; C; *P < 0.05), but not phosphorylated (Thr696) myosin phosphatase-targeting subunit 1 (MYPT1; B; P > 0.05), in RSM following pretreatment with calphostin C and Gö-6850 (10−5 M). Y-27632 causes a significant decrease in levels of phosphorylated (Thr38) CPI-17 and phosphorylated (Thr18/Ser19) MLC20, as well as phosphorylated (Thr696) MYPT1 (A–C; *P < 0.05).
DISCUSSION
These studies show, for the first time, that the spontaneous phasic activity in the RSM involves PKC and RhoA/ROCK pathways. [In contrast to the phasic activity in RSM, a number of studies have shown that basal tone in the lower esophageal sphincter and IAS in humans and animals is mediated primarily via the RhoA/ROCK pathway (25, 30, 32, 37).] The present data also show selectivity of the effects of PKC and ROCK inhibitors on the respective pathways. Additionally, these studies suggest that PKC activation via phosphorylated CPI-17/phosphorylated MLC20 and RhoA/ROCK activation via phosphorylated CPI-17/phosphorylated MYPT1/phosphorylated MLC20 is the primary mechanism for the phasic activity in the RSM. A direct link, however, between these pathways and the subsequent signal transduction remains to be established.
The present studies suggest that the RSM serves as an important model for examination of molecular control mechanisms for spontaneous phasic activity in the gastrointestinal tract. On the basis of the automated analysis, spontaneous phasic activity in the RSM was broadly classified as slow (∼3 min−1) and fast (∼25 min−1). Furthermore, the slow-rate phasic activity has high amplitude (∼250 mg), and the high-rate phasic activity has low amplitude (∼55 mg). The diverse rates of the phasic activity in the RSM have been previously reported in the large intestine (33, 40, 41) and have been grouped as second and minute rhythms in the smooth muscles (10).
The evidence that PKC and RhoA/ROCK pathways play a major role in the slow-rate spontaneous phasic activity is based on the significant decrease in the rate and amplitude of such phasic activity following PKC and ROCK inhibition. The PKC- and RhoA/ROCK-mediated signal transduction associated with the slow-rate phasic activity in the RSM is mediated by phosphorylated CPI-17/phosphorylated MLC20 and phosphorylated CPI-17/phosphorylated MYPT1/phosphorylated MLC20, respectively. This is evident by the simultaneous decrease in membrane translocation of PKCα and ROCK II (data not shown) and the corresponding decreases in phosphorylated CPI-17/phosphorylated MLC20 and phosphorylated CPI-17/phosphorylated MYPT1/phosphorylated MLC20, respectively. The nature of this signal transduction cascade involving PKC and RhoA/ROCK in the mediation of RSM contractility is in agreement with earlier data that showed that RhoA/ROCK-mediated Ca2+ sensitization responsible for the smooth muscle contraction occurs via phosphorylated MYPT1, as well as phosphorylated CPI-17 (20, 23, 29, 39). On the other hand, smooth muscle contraction induced by the PKC pathway is associated with phosphorylation of CPI-17, an endogenous inhibitor of MLCP (8, 23). The role of PKC in the stimulated phasic activity in the RSM is shown by an increase in the contractile activity of the RSM by PDBu that is inhibited by calphostin C. Interestingly, in opossum IAS smooth muscle, PDBu causes relaxation (4), instead of contraction, as observed here in rat RSM.
The combined inhibition of PKC and RhoA/ROCK pathways causes further inhibition of the phasic activity, which is more than the sum of individual inhibitions. The data suggest that these pathways, in major part, work in parallel, and since the predominant pathway is RhoA/ROCK, it remains to be determined whether part of the PKC role is mediated via RhoA/ROCK activation, as suggested previously (18, 24). Furthermore, it has been shown that agonist-mediated phasic contraction of the smooth muscle may not only be tissue-specific, but also species-specific (35), as carbachol-mediated contraction was significantly blocked by the ROCK inhibitor Y-27632 in rat, but not guinea pig, urinary bladder smooth muscle.
Residual phasic activity following the combined inhibition of these pathways suggests that a portion of RSM phasic activity may be independent of PKC and RhoA/ROCK. This may not be explained on the basis of insufficient PKC and RhoA/ROCK inhibition, because these inhibitors in the concentrations used nearly obliterated their respective enzymatic activities. Additionally, similar data were obtained with the combination of PKC and ROCK inhibitors.
The role of PKC mediation in the slow-rate phasic activity in the RSM is supported by differential signal transduction studies as follows. In contrast to the basal tone in human and cat lower esophageal sphincter, ACh- and EFS-induced phasic contraction in the esophageal body is primarily mediated by PKC (12, 25, 37). Similar conclusions were drawn by the inhibition of carbachol-induced contraction of urinary bladder by PKC inhibitor (7). Additionally, a number of studies have shown that agonist-induced smooth muscle contraction involves not only PKC, but also RhoA/ROCK, pathways (1, 23, 35). The present data suggest that, in the RSM, the RhoA/ROCK pathway plays a dominant role in the slow-rate, as well as the fast-rate, phasic activity, as shown by a dramatic decrease in the rate and amplitude of these contractile activities following selective ROCK inhibition.
These data lead to the speculation that PKC and RhoA/ROCK pathways work cooperatively in the slow-rate phasic activity in the RSM, while the RhoA/ROCK pathway is critical in the fast-rate phasic activity. The critical role of the RhoA/ROCK pathway in the fast-rate low-amplitude phasic activity in the RSM is shown by selective and substantial inhibition of such spontaneous phasic activity by ROCK, but not by PKC. Such data are in agreement with recent studies in human myometrium, where it was shown that certain phasic activity is mediated primarily via the RhoA/ROCK pathway (14).
In conclusion, the spontaneous phasic activity in the RSM occurs in two forms: slow-rate high-amplitude and fast-rate low-amplitude. The slow-rate phasic activity is mediated cooperatively via the PKC and RhoA/ROCK pathways, whereas the fast-rate phasic activity is mediated primarily via the RhoA/ROCK pathway. Relative contributions of these pathways and the possibility of interaction between the two remain to be determined. Systematic investigation of the molecular control mechanisms responsible for the rate and amplitude of the spontaneous phasic activity in the RSM provides an important model for the pathophysiological changes in gastrointestinal smooth muscle without the need for an agonist.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant RO1 DK-035385 and an institutional grant from Thomas Jefferson University.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.S. performed the experiments; J.S. analyzed the data; J.S. and S.R. interpreted the results of the experiments; J.S. prepared the figures; J.S. and S.R. drafted the manuscript; S.R. is responsible for conception and design of the research; S.R. edited and revised the manuscript; S.R. approved the final version of the manuscript.
ACKNOWLEDGMENTS
We thank Dr. C. Vijay Krishna for valuable comments and suggestions.
REFERENCES
- 1. Bayguinov O, Dwyer L, Kim H, Marklew A, Sanders KM, Koh SD. Contribution of Rho-kinase to membrane excitability of murine colonic smooth muscle. Br J Pharmacol 163: 638–648, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bharucha AE. Outcome measures for fecal incontinence: anorectal structure and function. Gastroenterology 126: S90–S98, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Cao W, Sohn UD, Bitar KN, Biancani P, Harnett KM. MAPK mediates PKC-dependent contractions of cat esophageal and lower esophageal sphincter circular smooth muscle. Am J Physiol Gastrointest Liver Physiol 285: G86–G95, 2003 [DOI] [PubMed] [Google Scholar]
- 4. Chakder S, Sarma D, Rattan S. Mechanism of internal anal sphincter smooth muscle relaxation by phorbol 12,13-dibutyrate. Am J Physiol Gastrointest Liver Physiol 280: G1341–G1350, 2001 [DOI] [PubMed] [Google Scholar]
- 5. Davis PD, Elliott LH, Harris W, Hill CH, Hurst SA, Keech E, Kumar MK, Lawton G, Nixon JS, Wilkinson SE. Inhibitors of protein kinase C. 2. Substituted bisindolylmaleimides with improved potency and selectivity. J Med Chem 35: 994–1001, 1992 [DOI] [PubMed] [Google Scholar]
- 6. De Godoy MA, Rattan S. Role of phospholipase A2 (group I secreted) in the genesis of basal tone in the internal anal sphincter smooth muscle. Am J Physiol Gastrointest Liver Physiol 293: G979–G986, 2007 [DOI] [PubMed] [Google Scholar]
- 7. Ekman M, Andersson KE, Arner A. Receptor-induced phasic activity of newborn mouse bladders is inhibited by protein kinase C and involves T-type Ca2+ channels. BJU Int 104: 690–697, 2009 [DOI] [PubMed] [Google Scholar]
- 8. Eto M. Regulation of cellular protein phosphatase-1 (PP1) by phosphorylation of the CPI-17 family, C-kinase-activated PP1 inhibitors. J Biol Chem 284: 35273–35277, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fu XH, Gong MC, Jia TP, Somlyo AV, Somlyo AP. The effects of the Rho-kinase inhibitor Y-27632 on arachidonic acid-, GTPγs-, and phorbol ester-induced Ca2+-sensitization of smooth muscle. FEBS Lett 440: 183–187, 1998 [DOI] [PubMed] [Google Scholar]
- 10. Golenhofen K, Mandrek K. Phasic and tonic contraction processes in the gastrointestinal tract. Dig Dis Sci 9: 341–346, 1991 [DOI] [PubMed] [Google Scholar]
- 11. Gong MC, Cohen P, Kitazawa T, Ikebe M, Masuo M, Somlyo AP, Somlyo AV. Myosin light chain phosphatase activities and the effects of phosphatase inhibitors in tonic and phasic smooth muscle. J Biol Chem 267: 14662–14668, 1992 [PubMed] [Google Scholar]
- 12. Harnett KM, Cao W, Biancani P. Signal-transduction pathways that regulate smooth muscle function. I. Signal transduction in phasic (esophageal) and tonic (gastroesophageal sphincter) smooth muscles. Am J Physiol Gastrointest Liver Physiol 288: G407–G416, 2005 [DOI] [PubMed] [Google Scholar]
- 13. Hartzell HC, Rinderknecht A. Calphostin C, a widely used protein kinase C inhibitor, directly and potently blocks L-type Ca2+ channels. Am J Physiol Cell Physiol 270: C1293–C1299, 1996 [DOI] [PubMed] [Google Scholar]
- 14. Hudson CA, Heesom KJ, Lopez BA. Phasic contractions of isolated human myometrium are associated with Rho-kinase (ROCK)-dependent phosphorylation of myosin phosphatase-targeting subunit (MYPT1). Mol Hum Reprod 18: 265–279, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ihara E, Akiho H, Nakamura K, Turner SR, MacDonald JA. MAPKs represent novel therapeutic targets for gastrointestinal motility disorders. World J Gastroenterol 2: 19–25, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ihara E, Chappellaz M, Turner SR, MacDonald JA. The contribution of protein kinase C and CPI-17 signaling pathways to hypercontractility in murine experimental colitis. Neurogastroenterol Motil 24: e15–e26, 2012 [DOI] [PubMed] [Google Scholar]
- 17. Ishizaki T, Uehata M, Tamechika I, Keel J, Nonomura K, Maekawa M. Pharmacological properties of Y-27632, a specific inhibitor of Rho-associated kinases. Mol Pharmacol 57: 976–983, 2000 [PubMed] [Google Scholar]
- 18. Kandabashi T, Shimokawa H, Miyata K, Kunihiro I, Eto Y, Morishige K, Matsumoto Y, Obara K, Nakayama K, Takahashi S, Takeshita A. Evidence for protein kinase C-mediated activation of Rho-kinase in a porcine model of coronary artery spasm. Arterioscler Thromb Vasc Biol 23: 2209–2214, 2003 [DOI] [PubMed] [Google Scholar]
- 19. Kessler R, Fanestil D. Interference by lipids in the determination of protein using bicinchoninic acid. Anal Biochem 159: 138–142, 1986 [DOI] [PubMed] [Google Scholar]
- 20. Kitazawa T, Eto M, Woodsome TP, Khalequzzaman M. Phosphorylation of the myosin phosphatase targeting subunit and CPI-17 during Ca2+ sensitization in rabbit smooth muscle. J Physiol 546: 879–889, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kobayashi E, Nakano H, Morimoto M, Tamaoki T. Calphostin C (UCN-1028C), a novel microbial compound, is a highly potent and specific inhibitor of protein kinase C. Biochem Biophys Res Commun 15: 548–553, 1989 [DOI] [PubMed] [Google Scholar]
- 22. Murthy KS. Signaling for contraction and relaxation in smooth muscle of the gut. Annu Rev Physiol 68: 345–374, 2006 [DOI] [PubMed] [Google Scholar]
- 23. Murthy KS, Zhou H, Grider JR, Brautigan DL, Eto M, Makhlouf GM. Differential signalling by muscarinic receptors in smooth muscle: M2-mediated inactivation of myosin light chain kinase via Gi3, Cdc42/Rac1 and p21-activated kinase 1 pathway, and M3-mediated MLC20 (20 kDa regulatory light chain myosin II) phosphorylation via Rho-associated kinase/myosin phosphatase targeting subunit and protein kinase C/CPI-17 pathway. Biochem J 374: 145–155, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Niiro N, Koga Y, Ikebe M. Agonist-induced changes in the phosphorylation of the myosin-binding subunit of myosin light chain phosphatase and CPI-17, two regulatory factors of myosin light chain phosphatase, in smooth muscle. Biochem J 369: 117–128, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Park SY, Shim JH, Kim M, Sun YH, Kwak HS, Yan X, Choi BC, Im C, Sim SS, Jeong JH, Kim IK, Min YS, Sohn UD. MLCK and PKC involvements via Gi and RhoA protein in contraction by the electrical field stimulation in feline esophageal smooth muscle. Korean J Physiol Pharmacol 14: 29–35, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Patel CA, Rattan S. Spontaneously tonic smooth muscle has characteristically higher levels of RhoA/ROK compared with the phasic smooth muscle. Am J Physiol Gastrointest Liver Physiol 291: G830–G837, 2006 [DOI] [PubMed] [Google Scholar]
- 27. Radomirov R, Ivancheva C, Brading AF, Itzev D, Rakovska A, Negrev N. Ascending and descending reflex motor activity of recto-anal region-cholinergic and nitrergic implications in a rat model. Brain Res Bull 79: 147–155, 2009 [DOI] [PubMed] [Google Scholar]
- 28. Rao SS. Fecal incontinence. In: Sleisenger and Fordtran's Gastrointestinal and Liver Disease, edited by Feldman M, Friedman LS, Brandt LJ. Philadelphia, PA: Saunders Elsevier, 2010 [Google Scholar]
- 29. Rattan S, Benjamin P, Maxwell PJ., 4th RhoA/ROCK-kinase: pathophysiologic and therapeutic implications in gastrointestinal smooth muscle tone and relaxation. Gastroenterology 138: 13–18, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rattan S, De Godoy MA, Patel CA. Rho kinase as a novel molecular therapeutic target for hypertensive internal anal sphincter. Gastroenterology 131: 108–116, 2006 [DOI] [PubMed] [Google Scholar]
- 31. Rattan S, Patel CA. Selectivity of ROCK inhibitors in the spontaneously tonic smooth muscle. Am J Physiol Gastrointest Liver Physiol 294: G687–G693, 2008 [DOI] [PubMed] [Google Scholar]
- 32. Rattan S, Singh J. RhoA/ROCK pathway is the major molecular determinant of basal tone in intact human internal anal sphincter. Am J Physiol Gastrointest Liver Physiol 302: G664–G675, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sarna SK. In vivo myoelectric activity: methods, analysis, and interpretation. In: Handbook of Physiology. The Gastrointestinal System. Motility and Circulation. Bethesda, MD: Am. Physiol. Soc., 1989, sect. 6, vol. I, part 2 [Google Scholar]
- 34. Schaafsma D, Bos ST, Zuidhof AB, Zaagsma J, Meurs H. The inhaled Rho kinase inhibitor Y-27632 protects against allergen-induced acute bronchoconstriction, airway hyperresponsiveness, and inflammation. Am J Physiol Lung Cell Mol Physiol 295: L214–L219, 2008 [DOI] [PubMed] [Google Scholar]
- 35. Shabir S, Borisova L, Wray S, Burdyga T. Rho-kinase inhibition and electromechanical coupling in rat and guinea-pig ureter smooth muscle: Ca2+-dependent and -independent mechanisms. J Physiol 560: 839–855, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shimamoto H, Shimamoto Y, Kwan CY, Daniel EE. Participation of protein kinase C in endothelin-1-induced contraction in rat aorta: studies with a new tool, calphostin C. Br J Pharmacol 107: 282–287, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sims SM, Chrones T, Preiksaitis HG. Calcium sensitization in human esophageal muscle: role for RhoA kinase in maintenance of lower esophageal sphincter tone. J Pharmacol Exp Ther 327: 178–186, 2008 [DOI] [PubMed] [Google Scholar]
- 38. Sohn UD, Zoukhri D, Dartt D, Sergheraert C, Harnett KM, Behar J, Biancani P. Different protein kinase C isozymes mediate lower esophageal sphincter tone and phasic contraction of esophageal circular smooth muscle. Mol Pharmacol 51: 462–470, 1997 [PubMed] [Google Scholar]
- 39. Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev 83: 1325–1358, 2003 [DOI] [PubMed] [Google Scholar]
- 40. Takeuchi T, Niioka S, Kishi M, Ishii T, Nishio H, Hata F, Takewaki T, Takatsuji K. Nonadrenergic, noncholinergic relaxation mediated by nitric oxide with concomitant change in Ca2+ level in rectal circular muscle of rats. Eur J Pharmacol 353: 67–74, 1998 [DOI] [PubMed] [Google Scholar]
- 41. Terauchi A, Kobayashi D, Mashimo H. Distinct roles of constitutive nitric oxide synthases and interstitial cells of Cajal in rectoanal relaxation. Am J Physiol Gastrointest Liver Physiol 289: G291–G299, 2005 [DOI] [PubMed] [Google Scholar]
- 42. Toullec D, Pianetti P, Coste H, Bellevergue P, Grand-Perret T, Ajakane M, Baudet V, Boissin P, Boursier E, Loriolle F, Duhame L, Charon D, Kirilovsky J. The bisindolylmaleimide GF109203X is a potent and selective inhibitor of protein kinase C. J Biol Chem 24: 15771–15781, 1991 [PubMed] [Google Scholar]
- 43. Wald A. Clinical practice. Fecal incontinence in adults. N Engl J Med 356: 1648–1655, 2007 [DOI] [PubMed] [Google Scholar]
- 44. Walsh MP. The involvement of protein kinase C in myosin phosphorylation and force development in rat tail arterial smooth muscle. Biochem J 352: 573–582, 2000 [PMC free article] [PubMed] [Google Scholar]
- 45. Whitehead WE, Borrud L, Goode PS, Meikle S, Mueller ER, Tuteja A, Weidner A, Weinstein M, Ye W. Fecal incontinence in US adults: epidemiology and risk factors. Gastroenterology 137: 512–517, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]


