Abstract
Bladder inflammation (cystitis) underlies numerous bladder pathologies and is elicited by a plethora of agents such as urinary tract infections, bladder outlet obstruction, chemotherapies, and catheters. Pattern recognition receptors [Toll-like receptors (TLRs) and Nod-like receptors (NLRs)] that recognize pathogen- and/or damage-associated molecular patterns (PAMPs and/or DAMPs, respectively) are key components of the innate immune system that coordinates the production (TLRs) and maturation (NLRs) of proinflammatory IL-1β. Despite multiple studies of TLRs in the bladder, none have investigated NLRs beyond one small survey. We now demonstrate that NLRP3 and NLRC4, and their binding partners apoptosis-associated speck-like protein containing a COOH-terminal caspase recruitment domain (ASC) and NLR family apoptosis inhibitory protein (NAIP), are expressed in the bladder and localized predominantly to the urothelia. Activated NLRs form inflammasomes that activate caspase-1. Placement of a NLRP3- or NLRC4-activating PAMP or NLRP3-activating DAMPs into the lumen of the bladder stimulated caspase-1 activity. To investigate inflammasomes in vivo, we induced cystitis with cyclophosphamide (CP, 150 mg/kg ip) in the presence or absence of the inflammasome inhibitor glyburide. Glyburide completely blocked CP-induced activation of caspase-1 and the production of IL-1β at 4 h. At 24 h, glyburide reduced two markers of inflammation by 30–50% and reversed much of the inflammatory morphology. Furthermore, glyburide reversed changes in bladder physiology (cystometry) induced by CP. In conclusion, NLRs/inflammasomes are present in the bladder urothelia and respond to DAMPs and PAMPs, whereas NLRP3 inhibition blocks bladder dysfunction in the CP model. The coordinated response of NLRs and TLRs in the urothelia represents a first-line innate defense that may provide an important target for pharmacological intervention.
Keywords: inflammasome, bladder, inflammation, cystitis, caspase-1
in the bladder, inflammation underlies, or is a contributing factor in, the majority of bladder pathologies, including voiding dysfunction, pelvic pain syndromes, and cancer. By far the most common bladder pathology is urinary tract infection, which is the second most common type of infection, behind only respiratory infections (i.e., the common cold). Urinary tract infections affect at least 60% of all women in their lifetime and account for between 8 and 10 million visits to outpatient clinics (and $3.5 billion) annually in the United States alone (21, 24). Copious studies have delineated the molecular interactions between the host and various pathogens that elicit this malady (for reviews, see Refs. 27, 38, and 57), but the bladder is also susceptible to sterile inflammation induced by numerous noninfectious agents such as bladder outlet obstruction, bladder stones, stents, and chemotherapies [with the best studied being cyclophosphamide (CP)]. Given the prevalence and healthcare costs of sterile and nonsterile bladder inflammation, there is considerable interest in understanding the common underlying mechanism. Work in other tissues has begun to cast light on the mechanisms of this innate response. Central to this work has been an understanding of pattern recognition receptors, specifically, Toll-like receptors (TLRs) and Nod-like receptors (NLRs) (for reviews, see Refs. 2, 12, 19, 56, and 62).
Cell membrane-bound TLRs and cytoplasmic NLRs coordinate to initiate an inflammatory response through the recognition of patterns found on pathogens [pathogen-associated molecular patterns (PAMPs)] or various molecules that represent damage to a given tissue [damage-associated molecular patterns (DAMPs)] (45, 72). PAMPs represent well-known immunogenic components of bacteria, such as lipopolysaccharide (LPS) and flagellin (45), whereas DAMPs such as ATP are often released from necrotic cells. Each TLR recognizes their specific ligand. For example, TLR4 recognizes LPS, whereas TLR5 binds to flagella. Binding of the ligand activates a constellation of effector pathways including NF-κB, which stimulates the transcription of pro-IL-1β (58).
Numerous NLRs are known or have been predicted, although few have been extensively studied (12, 43, 76). The best known is NLRP3 (NALP3), which responds to DAMPs such as ATP as well as the PAMP LPS. Activation of NLRP3 stimulates the binding of apoptosis-associated speck-like protein containing a COOH-terminal caspase recruitment domain (ASC) through homotypic binding of pyrin domains. ASC has a caspase recruitment domain and serves as an adaptor that binds NLRP3 to procaspase-1 to form a structure known as an inflammasome (43, 64), which has been referred to as the “central processing unit” of the inflammatory response (14). Formation of the inflammasome cleaves procaspase-1 to caspase-1, and the mature caspase-1 then processes pro-IL-1β, leading to the release of mature IL-1β by pyroptosis. Inflammasomes are also responsible for the cleavage and release of IL-18. IL-1β and IL-18 then act as proinflammatory cytokines, triggering the inflammatory response. Another well-known NLR, NLRC4, functions in an identical manner except that it contains a caspase recruitment domain and thus does not need an adaptor. However, its ligand specificity is determined by another protein, one of the NLR family apoptosis inhibitory proteins (NAIPs) (23, 54). For example, NAIP5 bridges flagella binding to NLRC4, whereas NAIP2 triggers activation by proteins that compose the bacterial type 3 secretion systems. The net result, release of mature IL-1β and IL-18, is the same.
TLRs have been extensively studied in the bladder (57, 68, 69), where they are thought to play important roles in sterile and infectious inflammation. Given the interdependency of TLR and NLR systems in other tissues, it is somewhat surprising that very little has been published regarding NLRs in the bladder. The only direct evidence that these receptors exist in the bladder is from a tissue survey by Kummer et al. in 2007 (42). Indirect evidence has been put forth by Martins et al. (50), who demonstrated that the well-known cystitis-inducing agent CP activated P2X7 channels, which are required for the activation of NLRP3 by DAMPs (16). In this report, we begin a more systematic exploration of the presence, activation, and importance of NLRs/inflammasomes in the bladder. We explored the presence and localization of NLRP3 and NLRC4, and their respective binding partners ASC and NAIP, using immunohistochemistry while examining their activation with relevant PAMPs and DAMPs. One well-established rodent cystitis model with relevant clinical implications is induced by CP.
CP is a nitrogen mustard alkylating agent that has been widely used for >50 yr as a chemotherapeutic agent to combat various neoplasms (25, 73). While highly effective, patients undergoing treatment with CP frequently experienced acute hemorrhagic cystitis (HC), a diffuse inflammation of the bladder leading to dysuria, hematuria, and hemorrhage (for reviews of HC, see Refs. 13 and 31). Treatment with this drug also led to physiological changes reminiscent of overactive bladder, which resulted in its adaptation as an animal model of this disorder (6, 22, 61). A metabolite of CP, acrolein, was found to be responsible for a large portion of the detrimental effects of this drug (8, 11). Consequently, oncologists now concomitantly administer 2-mercaptoethane sulfonate (Mesna), an orally available drug that accumulates in urine and acts as an acrolein chelator. This practice has reduced the incidence of acute HC arising from chemotherapy but not eliminated it (30). Indeed, in current practice, HC remains a clinically relevant, potentially devastating, and challenging entity for physicians to treat. It is particularly prevalent in leukemia and lymphoma patients undergoing CP conditioning in preparation for stem cell transplant (17, 28, 31, 47, 66). In this study, we used the CP-induced model of cystitis and the inflammasome inhibitor glyburide (Gly) (46) to assess the contribution of NLRP3 to bladder inflammation and the in vivo physiological bladder dysfunction caused by this drug. Excitingly, Gly is a well-tolerated Federal Drug Administration (FDA)-approved drug used in the treatment of diabetes. Its efficacy in combating CP-induced HC in rodent models suggests immediate translational relevance for treating a disorder in need of effective management.
MATERIALS AND METHODS
Animals and Pharmacological Treatments
Adult female Sprague-Dawley rats (175–199 g, Harlan, Prattville, AL) were used in all experiments. All experimental protocols were performed in accordance with guidelines set forth in the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were reviewed and approved by the Institutional Animal Care and Use Committee of the Medical University of South Carolina. Rats were housed in an Association for Assessment and Accreditation of Laboratory Animal Care-approved colony room maintained at constant temperature and humidity on a 12:12-h light-dark cycle and given ad libitum access to food and water.
For the instillation of PAMPs and DAMPs, rats were anesthetized with an intraperitoneal injection of ketamine hydrochloride (90 mg/kg) and xylazine (10 mg/kg). Their abdomens were opened, urine removed from the bladder through a 28-gauge needle, and each PAMP or DAMP was instilled in the lumen (300 μl) for 2 h. The needle was removed, and the abdominal flap was closed with a staple. One hour later, a half dose of ketamine-xylazine (45 and 5 mg/kg, respectively) was administered intraperitoneally. Two hours after PAMP/DAMP instillation, rats were euthanized and urothelia were isolated. The concentrations of PAMP/DAMPs were as follows: 100 μg/ml LPS (Esherichia coli 055:B5; Calbiochem-EMD Millipore, Billerica, MA), 10 mM ATP (Wako Chemicals USA, Richmond, VA), 200 μg/ml monosodium urate crystals (MSU; Enzo Life Sciences, Farmingdale, NY), and 12.5 μg/ml flagellin (Enzo Life Sciences).
For experiments involving Gly (glibenclamide; Enzo Life Sciences), rats were given Gly (2.5 mg/kg in 10% ethanol in PBS po) or vehicle one evening (5 PM) and again in the morning 16 h later (9 AM). Four hours later (1 PM), CP (150 mg/kg) or saline was administered intraperitoneally, and, 4 h thereafter, animals were euthanized for measurements of caspase-1 activity and urinary cytokine levels. For experiments of inflammation and urodynamics at 24 h, Gly or vehicle was readministered 4 and 20 h after CP (5 PM and 9 AM). Twenty-four hours after CP injection (1 PM), rats were euthanized for caspase-1 analysis, injected with Evans blue dye, or subjected to urodynamics as described below.
Histology and Immunocytochemistry
For immunocytochemical analysis, bladders were isolated and immersed overnight in 10% neutral buffered formalin (4°C). Thereafter, bladders were transferred to 70% ethanol and maintained at 4°C until embedded in paraffin blocks (<1 wk) using standard techniques. Sections (5 μm) were cut and stained with hematoxylin and eosin using routine methodological techniques or stained with the Vectastain ABC staining kit (Vector Labs, Burlingame, CA). For immunocytochemistry, sections were deparaffinized and hydrated through a graded alcohol series and subjected to antigen retrieval for 30 min with citrate-based antigen unmasking solution (Vector Labs) using the manufacturer's recommended protocol. Endogenous peroxidase was then quenched (10 min in 3% H2O2), and sections were blocked using normal goat serum (30 min). Sections were then incubated with primary antibodies (1:50 dilution) against NLRP3, ASC, NLRC4, NAIP, or their isotype controls for 1 h. Anti-NLRP3 (SC-66846, rabbit polyclonal), anti-ASC (SC-22514, rabbit polyclonal), anti-NAIP (SC-11064, goat polyclonal) were all obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-NLRC4 (AVARP00019, rabbit polyclonal) was obtained from Aviva Systems Biology (San Diego, CA). Isotype controls are from Santa Cruz Biotechnology (SC-2027, rabbit) and Invitrogen (02-6202, goat). Sections were then washed and incubated (30 min) with biotinylated secondary antibody provided with the Vectastain ABC staining kits (Vector Labs). Slides were washed, incubated with the Vectastain ABC reagent (30 min), washed again, and then incubated in 3,3′-diaminobenzidine peroxidase substrate solution until signals developed. Slides were then washed, counterstained briefly with hematoxylin, coverslipped, and analyzed by light microscopy.
Caspase-1 Assay
At the indicated times, animals were given an intraperitoneal injection of ketamine hydrochloride (90 mg/kg) and xylazine (10 mg/kg). The abdominal cavity was then opened, and the bladder was exposed. Urine was removed by needle puncture. Bladders were then removed, weighed, and placed in ice-cold PBS. Animals were euthanized by thoracotomy. Using techniques similar to what have been reported elsewhere by us and others (26, 33, 51), urothelia were obtained by scraping. Briefly, the bladder was opened from the sphincter to the dome, pinned down, and gently scraped with a size 11 scalpel blade, with the blade twisted to lift cells. Cells were placed in ice-cold PBS, pelleted (800 g, 10 min), resuspended in 25 μl of 10 mM MgCl2 and 0.25% Igepal CA-630, mixed with an equal volume of 40 mM HEPES (pH 7.4), 20 mM NaCl, 2 mM EDTA, and 20% glycerol, and stored at −20°C until analyzed.
Caspase-1 activity was measured using a fluorometric assay modified from previous studies (20, 29, 34). Briefly, cytoplasmic extracts were thawed, and protein concentrations were assessed by Bradford assay (7). Extract (20 μl) was then combined with 50 μl assay buffer [25 mm HEPES, 5% sucrose, 0.05% CHAPS (pH 7.5), 10 μl of 100 mM DTT, and 20 μl of 1 mM substrate N-acetyl-Tyr-Val-Ala-Asp-7-amino-4-trifluoromethylcoumarin (Ac-DEVD-AFC)] in blacked-walled 96-well plates. Plates were then loaded onto a plate reader, fluorescence (excitation: 400 nm and emission: 505 nm) was measured every 30 s for 15 min, and the slope was determined. A standard curve of fluorescence versus free AFC (free AFC was obtained from Sigma) was then used to calculate the specific activity of caspase-1.
Urine Chemokine Analysis
To obtain urine following the indicated treatments, rats were anesthetized with an intraperitoneal injection of ketamine hydrochloride (90 mg/kg) and xylazine (10 mg/kg), the bladder was externalized, and urine was recovered by needle puncture. Urine was stored at −70°C until analyzed by mutiplex assays for levels of Il-1β and IL-18. Briefly, urine was diluted (typically 1:2) on a 96-well filter plate and analyzed in duplicate on the BioPlex platform (BioRad, Luminex Technology, Hercules, CA) as previously described (36, 37) Concentrations (in pg/ml) were then extrapolated from a seven-point standard curve for each cytokine using a five-parameter logistic fit using Bioplex software. Creatinine levels were assayed (typically with a 1:5 dilution) using a urinary creatinine kit obtained from Cayman Chemical (Ann Arbor, MI) and the manufacturer's recommended procedures. Concentrations were extrapolated from an eight-point standard curve, and cytokine levels were then adjusted for creatinine levels and reported as picograms of cytokine per milligram of creatinine.
Evans Blue Extravasation
Evans blue extravasation was measured as previously described (35). Briefly, rats were injected with 25 mg/kg Evans blue dye (intravenously in 200 μl). One hour later, bladders were dissected, weighed, placed in formamide (1 ml), and incubated in the dark at 56°C overnight. The absorbance of the formamide was measured (620 nm) and compared with a standard curve. Results were calculated as nanograms of Evans blue per milligram of tissue (49, 63).
Urodynamics
Catheter implantation.
Suprapubic catheters were implanted into rats as previously described (35). Briefly, animals were injected intraperitoneally with ketamine hydrochloride (90 mg/kg) and xylazine (10 mg/kg) and subsequently given carprofen (2.5 mg/kg sc) after induction. The bladder was then exposed, and a polyethylene (PE-50) catheter with a flared end inserted into a hole created in the dome of the bladder by a scalpel. The catheter was then secured in place with a purse string suture, and the other end of the catheter was externalized at the back of the neck. The rat was fitted with a SAI Quick Connect Harness from Strategic Applications (PHM-119-1, SAI Infusion Technologies, Lake Villa, IL), and the catheter was plugged with a leur lock connector and stopper. The rat was given >72 h to recover.
Cystometry.
After the indicated treatments, the SAI Quick Connect harness was removed, and each rat was placed into a restrainer (modified from item HLD-RM, Kent Scientific, Torrington, CT) with a small hole near the rear, which was fitted with a funnel. The urethra was positioned above the funnel, and the entire restrainer placed inside a Small Animal Cystometry Lab Station (Catamount Research and Development, St. Albans, VT). The catheter was attached to an infusion pump and in-line pressure transducer, and sterile isotonic saline (0.9% NaCl) was continuously infused into the bladder at a rate of 80 μl/min. An analytic balance beneath the wire-bottom animal cage measured the amount of urine voided during cystometry. Bladder pressure and infused and voided bladder volumes were continuously measured using MED-CMG software provided by Catamount. A single cystometrogram (CMG) is defined as the simultaneous recording of these three parameters during a single filling-voiding cycle. The infusion was continued for 60–120 min until at least three CMGs were measured. Data were analyzed using CMG Analysis software (version 1.06, Catamount).
For every CMG, voiding pressure is measured as the peak intravesical pressure during voiding, threshold pressure is the intravesical pressure just before the initiation of micturition, void volume is the amount of urine voided, the intercontraction interval (ICI) is the time between successive micturitions, nonvoiding contractions (NVCs) are defined as rises in intravesical pressure that exceed 7 mmHg but are not associated with voiding of urine, and NVC amplitude is the peak pressure associated with a given NVC.
Statistical Analysis
Comparisons between experimental groups in caspase-1, IL-1β, IL-18, bladder weight, and Evans blue dye extravasation experiments were performed using ANOVA. Since comparisons in urodynamic parameters (i.e., voiding pressure, threshold pressure, void volume, ICI, and NVCs) involved repeated (correlated) measurements within rats over time, general linear mixed models were used. General linear mixed models are ideal for clustered data analysis, as they account for between- and within-subject variation (52). All statistical analyses were conducted using SAS (version 9.3, Cary, NC).
RESULTS
NLRP3 and NLRC4 Inflammasomes Are Present in the Bladder Epithelium
The presence of NLRP3 and NLRC4 inflammasomes in the bladder was assessed by immunocytochemistry. As shown in Fig. 1, top (×20 digital micrographs scaled to fit the figure frame; 9.3% of the original size), NLRP3 and its adaptor protein ASC were present in the bladder and localized predominately to urothelia (arrows). This was also seen with NLRC4 and its binding protein NAIP. Virtually no expression of NLRP3 or ASC was found in the smooth muscle (detrusor) layer, although some staining was apparent with NLRC4 and NAIP (arrowheads), particularly in vascular elements (oval regions). The areas indicated by rectangles were cropped from the original micrographs and are presented in full scale in Fig. 1, middle. These images suggest that staining for these molecules is primarily cytoplasmic. No staining was apparent with isotype antibody controls (presented as scaled micrographs; Fig. 1, bottom).
Fig. 1.
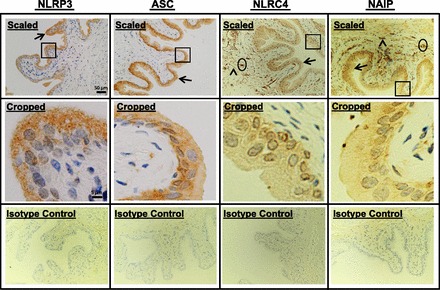
Detection of inflammasomes in the bladder by immunocytochemistry. Bladders from untreated rats were fixed, sectioned (5 μm), stained with the indicated antibodies, and counterstained with hematoxylin. Digital micrographs (2,560 × 1,920 pixels) were then taken at ×20 magnification. Scaled images (top) represent the entire micrograph scaled to 9.3% of the original size. Arrows indicate staining in urothelia, arrowheads indicate staining in the detrusor, and oval regions represent areas of staining in vascular elements. Areas encompassed by rectangles were cropped out of the full-sized micrographs and presented full scale in cropped images (middle). Isotype control images (bottom) were stained with the respective isotype antibodies as described in materials and methods. Micrographs were reduced to 9.3% of the original size to accurately serve as controls for the scaled images. All micrographs are from a representative experiment repeated three independent times. NLR, Nod-like receptor; ASC, apoptosis-associated speck-like protein containing a COOH-terminal caspase recruitment domain; NAIP, NLR family apoptosis inhibitory protein.
NLRP3 and NLRC4 Are Activated by PAMPs and DAMPs
To determine if NLRP3 and NLRC4 inflammasomes could be activated by relevant stimuli, a variety of DAMPs and PAMPs were placed in the lumen of the bladder and allowed to remain indwelling for 2 h. Afterward, caspase-1 activity in urothelia was assessed as described in materials and methods. As shown in Fig. 2, LPS (a NLRP3-activating PAMP), ATP and MSU (NLRP3-activating DAMPs), and flagellin (a NLRC4-activating PAMP) all stimulated caspase-1-specific activity to a statistically significant degree compared with control (P < 0.05 for all).
Fig. 2.
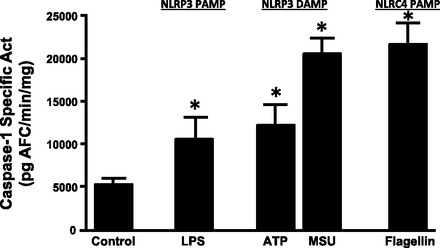
Caspase-1 activity in urothelia after intravesicular challenge with pathogen- and/or damage-associated molecular patterns (PAMPs and/or DAMPs, respectively). Bladders in unconscious rats were drained with a 28-gauge needle, and each DAMP or PAMP was instilled in the lumen (300 μl) for 2 h. Urothelial lysates were then assessed for caspase-1 activity. Treatments were as follows: lipopolysaccharide (LPS; 100 μg/ml), ATP (10 mM), monosodium urate crystals (MSU; 200 μg/ml), and flagellin (12.5 μg/ml). Bars are means ± SEM; n = 23 for control, 12 for LPS, 13 for ATP, 4 for MSU, and 6 for flagellin treatment. *Significant (P < 0.05) differences from control as assessed by ANOVA modeling.
Gly Blocks CP Induction of Caspase-1 Activity and Release of IL-1β and IL-18
CP treatment is widely used to induce cystitis in rodent models, where a single intraperitoneal injection can induce profound symptoms within 24 h (1, 40). The main activating agent has been shown to be the metabolite acrolein, which induces necrosis and apoptosis in urothelia (39, 41, 60, 65), resulting in sterile inflammation. Because necrosis releases DAMPs, CP would be predicted to activate the NLRP3 inflammasome. To assess this possibility, rats were treated with the NLRP3 inhibitor Gly (or vehicle) and CP (or saline) according to the dosing regimen shown in Fig. 3, and a variety of end points were measured. Briefly, rats were given Gly (2.5 mg/kg po) or vehicle (1 ml of 10% ethanol in PBS) 20 h and again 4 h before the intraperitoneal injection of CP (150 mg/kg) or saline (3.75 ml/kg). Four hours later, end points related to inflammasome activation (caspase-1 and urinary IL-1β and Il-18 levels) were measured. This time point was chosen based on the study by Smaldone et al. (67), which indicated that IL-1β and Il-18 levels were maximal at this time after CP injection and decreased thereafter. This transient rise is consistent with these cytokines functioning as proinflammatory cytokines and working to initiate the inflammatory reaction. As shown in Fig. 4A, CP treatment stimulated more than a twofold increase (P < 0.001) in caspase-1 activity after 4 h. Pretreatment with Gly alone had no effect on basal levels but completely blocked the activation of caspase-1 by CP (P < 0.001). A similar response was seen with urinary IL-1β, in which CP stimulated an increase (P < 0.02), and this was blocked by Gly treatment (P < 0.02). Interestingly, Gly alone reduced basal IL-1β levels, but the amount was not significant (P < 0.078). IL-18 showed a high constitutive production that was not significantly enhanced by CP (P < 0.87). While Gly did reduce IL-18 production in both saline- and CP-treated groups, the variability prevented these changes from reaching significance (P < 0.11 and P < 0.17 vs. vehicle controls and P < 0.06 and P < 0.10 vs. CP treatment, respectively).
Fig. 3.
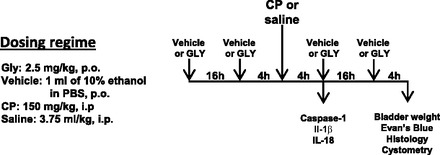
Dosing regimen used to study the effects of glyburide (Gly) on various parameters in the cyclophosphamide (CP)-induced model of cystitis. This regimen was used for the results shown in Figs. 4–8 and Table 1. Vehicle, Gly, and CP were administered at the indicated doses, and animals were harvested for various analyses where indicated.
Fig. 4.
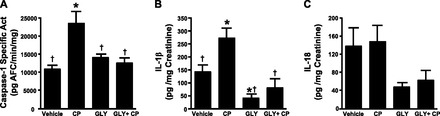
Effects of Gly on inflammasome activation in urothelia and urinary cytokine levels. Rats were subjected to the treatment regimen shown in Fig. 3. At the end of treatment, urine was collected, and urothelia were isolated and assayed as described in materials and methods. A: caspase-1 activity in urothelia after the indicated treatments. Bars are means ± SE; n = 5 for each group. *Significant (P < 0.001) difference from control as assessed by ANOVA modeling and Fisher's least-significant-difference (LSD) test; †significant (P < 0.002) difference from vehicle + CP treatment. B: IL-1β levels in urine after the indicated treatments normalized to creatinine. Bars are means ± SE; n = 3 for vehicle, 5 for CP, 3 for Gly, and 3 for Gly + CP treatment. *Significant (P < 0.02) difference from control as assessed by ANOVA modeling and Fisher's LSD test; †significant (P < 0.02) difference from vehicle + CP treatment. C: IL-18 levels in urine after the indicated treatments normalized to creatinine. Bars are means ± SE. No significant differences were detected.
Gly Reduces Bladder Inflammation
To assess the effects of Gly on the resulting inflammation and bladder function, a second set of animals was given additional Gly 4 and 16 h after CP, as shown in Fig. 3. Twenty-four hours after CP, animals were assessed for bladder weight, Evan's blue extravasation, histological changes, and urodynamics. Twenty-four hours is a common time point used in studies of CP-induced inflammation with a single bolus injection. Moreover, the longer time period is necessary for the accumulating inflammation to exert demonstrable effects on bladder physiology (i.e., urodynamics). Figure 5 shows the resulting bladder weights, a common marker of bladder inflammation and a marker often used in the CP model (1, 9, 55). As shown in Fig. 4, CP treatment induced a greater than twofold increase (P < 0.0001) in bladder weight, whereas Gly had no effect (P = 0.90). However, Gly reduced the gain in wet weight stimulated by CP by 29% (P = 0.01).
Fig. 5.
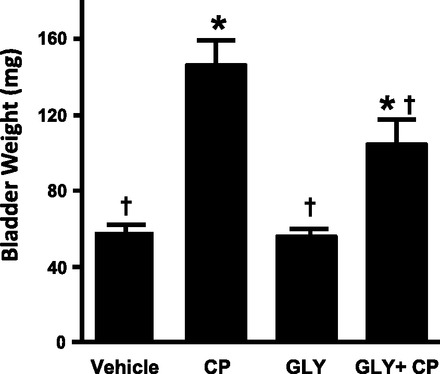
Effect of Gly on CP-induced bladder weight changes. Rats were subjected to the treatment regimen shown in Fig. 3. At the end of treatment, bladders were harvested, cleaned of all fat, and weighed. Bars are means ± SE; n = 4 for vehicle, 8 for CP, 4 for Gly, and 7 for Gly + CP treatment. *Significant (P < 0.05) differences from control as assessed by ANOVA modeling; †significant difference from vehicle + CP treatment.
To confirm this result using a more direct measurement of inflammation, we examined the extravasation of Evan's blue dye into bladder tissue in the experimental groups shown in Fig. 3. As shown morphologically in Fig. 6A and quantitatively in Fig. 6B, very little Evans blue dye moved into bladder tissue in vehicle- and Gly only-treated samples. In contrast, CP induced a nearly 100-fold influx of dye (P < 0.001), with 50% of this influx being blocked by Gly treatment (P = 0.02).
Fig. 6.
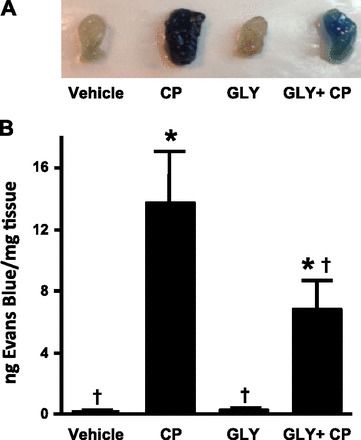
Effect of Gly on CP-induced Evan's blue dye extravasation. Rats were subjected to the treatment regimen shown in Fig. 3. At the end of the experiment, rats were injected (intravenously) with 25 mg/kg Evans blue. One hour later, rats were euthanized and their bladders were harvested A: photograph of a representative bladder from each of the indicated treatment groups. B: Evans blue extracted from the bladders treated in the indicated ways. Bladders were weighed and incubated in formamide at 56°C overnight. Absorbance at 620 nm was analyzed, and measurements (in ng) of Evan's blue were determined from a standard curve. Bars are means ± SE; n = 4 for vehicle, 4 for CP, 4 for Gly, and 7 for Gly + CP treatment. *Significant (P < 0.05) differences from control as assessed by ANOVA modeling; †significant difference from vehicle + CP treatment.
Finally, we directly examined inflammation via histology with hematoxylin and eosin-stained sections. As shown in Fig. 7, histology of the bladder mucosa is normal after vehicle treatment and becomes markedly edematous with urothelial mucosal ulceration (large arrow) and a brisk neutrophilic inflammatory cell infiltrate (small arrows) after CP treatment. Changes were most severe in the dome (data not shown). Bladders of rats treated with Gly were histologically identical to those of vehicle-treated rats. Histological sections from rats treated with both CP and Gly demonstrated an improvement in the overall bladder architecture, displaying variable and focal improvement with a subjective decrease in edematous changes and areas of decreased neutrophilic infiltrate.
Fig. 7.
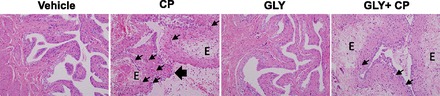
Effect of Gly on CP-induced histological changes. Rats were subjected to the treatment regimen shown in Fig. 3. At the end of the experiment, rats were euthanized, and their bladders were removed, fixed, sectioned, and then stained with hematoxylin and eosin as described in materials and methods. E, edema. The large arrow indicates urothelial ulceration (sloughing), and the small arrow indicates neutrophils. Micrographs are from a representative experiment repeated four independent times.
Gly Blocks the Negative In Vivo Effects of CP in the Bladder
To assess the contribution of inflammasomes to the in vivo bladder dysfunction associated with CP-induced cystitis, we performed cystometry on 24 h-treated animals from each of the four experimental groups shown in Fig. 3. Figure 8 shows representative micturition cycles from each of these groups, and Table 1 shows all relevant urodynamic parameters for the four groups and the between-group comparisons as assessed using general linear mixed models. As shown in Table 1, CP treatment significantly increased the voiding pressure and significantly reduced the void volume and ICI of these animals. Gly alone significantly increased the mean void volume and ICI. Gly completely prevented the decreases induced by CP. In fact, somewhat surprisingly, the combination of Gly + CP actually increased voiding pressure, void volume, and ICI over controls. Threshold pressure and number of NVCs per filling-voiding cycle were not significantly changed by any of the treatments.
Fig. 8.
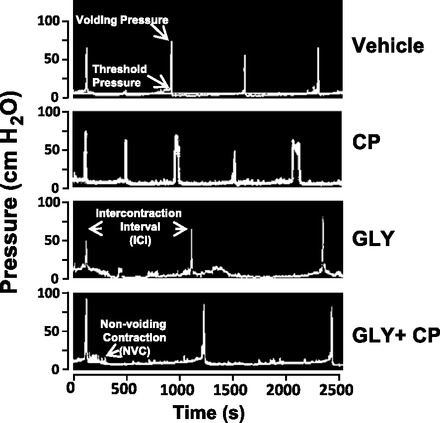
Effect of Gly on CP-induced urodynamics. Rats with suprapubic catheters implanted were subjected to the treatment regimen shown in Fig. 3A. At the end of the experiment, urodynamics were performed as described in materials and methods. A: representative pressure tracings from the four treatment groups. Voiding pressure, threshold pressure, intercontraction interval (ICI), and one nonvoiding contraction (NVC) are indicated. Not shown is a tracing from the scale indicating changes in weight coinciding with micturition, which is indicative of void volume.
Table 1.
Effect of glyburide on CP-induced urodynamic parameters
| Experimental Group | Voiding Pressure, cmH2O | Threshold Pressure, cmH2O | Void Volume, ml | Intercontraction Interval, s | Number of Nonvoiding Contractions per Cystometrogram |
|---|---|---|---|---|---|
| Vehicle | 59.7 ± 3.5 | 18.5 ± 2.2 | 0.90 ± 0.08 | 679 ± 50 | 1.61 ± 0.41 |
| Cyclophosphamide | 68.7 ± 3.9* | 19.8 ± 2.4 | 0.68 ± 0.09* | 506 ± 56* | 1.46 ± 0.64 |
| Glyburide | 64.3 ± 4.5 | 21.8 ± 2.7 | 1.28 ± 0.10* | 862 ± 66* | 2.33 ± 0.88 |
| Glyburide + cyclophosphamide | 74.7 ± 4.1* | 21.0 ± 2.5 | 1.21 ± 0.09* | 897 ± 60* | 2.28 ± 0.68 |
Values are means ± SE; n = 18 for vehicle, 7 for cyclophosphamide, 4 for glyburide, and 7 for glyburide + cyclophosphamide treatment. Rats with suprapubic catheters implanted were subjected to the treatment regimen shown in Fig. 3. At the end of the experiment, urodynamics were performed as described in materials and methods. For each rat in each group, three to eight filling-voiding cycles were analyzed. Each parameter was quantitated for each cycle, and the mean was determined. The mean of each parameter from each rat was averaged with the means from the other rats in the group, and this (the mean of the means) is shown here.
Significant differences from control as assessed with a general linear mixed model (P < 0.05).
DISCUSSION
Cystitis is a critical problem in urology, but understanding its underlying mechanisms is hampered by the heterogeneity of agents that induce the same physiological response patterns. Such agents necessarily engage a myriad of initiating pathways, all of which provide interesting and useful targets for pharmaceutical intervention in individual maladies. The identification of pathways common to all inflammatory stimuli would allow an interventional strategy that does not require the identification and understanding of each potential causative agent. As discussed above, the innate immune system is uniquely geared to respond to numerous insults and infectious agents by engaging a common set of pattern recognition receptors that recognize a multitude of DAMPs and PAMPs to initiate an inflammatory response. Central to the innate immune system is the production of proinflammatory cytokines such as IL-1β and IL-18. Studies have now clearly delineated converging pathways for the production of IL-1β whereby TLRs engage DAMPs and PAMPs and then signal through the NF-κB pathway to upregulate pro-IL-1β (priming). Engagement of DAMPs and PAMPs by NLRs results in the formation of inflammasomes and activation of caspase-1, which cleaves the pro-IL-1β to IL-β, stimulating its release through pyroptosis. Despite considerable work on TLRs in the bladder, virtually nothing is known about NLRs and inflammasomes in this important barrier tissue. Consequently, in this study, we showed the presence and activation of two of the best understood NLRs in bladder epithelia.
Inflammasome activation in various tissues often arises from resident or invading immune cells, although these structures have been found in epithelial cells such as the lung (75). Since the bladder epithelium represents a direct anatomic barrier to bacteria and irritative chemicals collected for excretion, it would seem likely that NLRs are present in this layer. Indeed, immunocytochemistry clearly demonstrated the presence and near exclusive localization of these structures to the urothelium. The presence of NLRP3 in this tissue is critical, for it is the only NLR thus far shown to respond to DAMPs. Consequently, it is likely to mediate sterile inflammation in the bladder and the effects of many nonbacterial cystitis-inducing agents including CP, as used in this study. The presence of NLRC4 is also significant as one of the ligands for this NLR is flagellin (23, 54), a well-known virulence factor found in the flagella of common uropathogens (74). We also showed the presence of ASC, which serves as an adaptor between NLRP3 (through its PYD domain) and caspase-1 (through its caspase recruitment domain). In contrast, NLRC4 has an endogenous caspase recruitment domain. In this NLR, NAIP proteins are thought to impart ligand specificity to NLRC4 activation with NAIP5 mediating the interaction with flagella (23) and NAIP2 facilitating the interaction with the type 3 secretion system present on many pathogenic bacteria (10, 15, 32, 54). Unfortunately, antibodies to individual rodent NAIPs are not available, although the presence of NAIP5 can be inferred by the response of urothelia to flagellin in this investigation. The presence of NAIP2 can also be predicted by studies implicating type 3 secretion systems in the virulence of uropathogenic bacteria (15), although additional studies will be necessary to demonstrate this.
The presence of inflammasome components in the urothelium suggest that they serve in a defensive capacity against attack from pathogens within the lumen of the bladder. Activation of inflammasomes was, indeed, demonstrated by the instillation of DAMPs and PAMPs well known to activate inflammasomes in other tissues. For example, inflammasomes were activated by intravesicular instillation of LPS, a well-known contributor to uropathogenic virulence (18, 57, 71) and an activator of the NLRP3 inflammasome (12, 43, 44, 64). We also stimulated caspase-1 activity by two NLRP3-activating DAMPs, ATP and MSU. While both ATP and MSU crystals are prototypical NLRP3 DAMPs, MSU is particularly relevant in the bladder as a significant number of bladder stones are composed of this material (53). In addition, flagellin, an NLRC4 ligand (23, 54) and uropathogenic virulence factor (74), also activated caspase-1 when placed in the lumen. Together, these results demonstrate inflammasome activation by their cognate PAMPs and DAMPs that are known to be present in the bladder lumen in pathological conditions. To demonstrate the importance of these structures to bladder inflammation, it was critical to block them in vivo in a common animal model of cystitis. Gly is a FDA-approved drug used in the treatment of type II diabetes. An inhibitor of the ATP-sensitive K+ channel, Gly has been shown to block inflammasome activation upstream of NLRP3 and downstream of the P2X7 ion channel (46).
We chose to examine the effects of this drug in the CP-induced model of HC, which is a well-established rodent cystitis model with relevant clinical implications. Most of the negative effects of CP treatment in the bladder have been attributed to the metabolite acrolein. The inclusion of Mesna in the treatment regime to bind acrolein in the urine has drastically reduced the appearance of HC but not prevented it completely, particularly in leukemia and lymphoma patients undergoing CP conditioning before stem cell transplant. In the present study, we administered Gly at doses similar to a previously published study (59) twice before CP administration and examined inflammasome activity 4 h after CP, a time period previously shown to coincide with the maximal production of IL-1β and IL-18 (67). Analysis of caspase-1, IL-1β, and IL-18 levels confirmed complete inactivation of the inflammasome in this model.
The success of this drug in blocking inflammasome activation allowed us to subsequently assess the contribution of the inflammasome/IL-1β/IL-18 pathway to inflammation and physiological dysfunction in the bladder and to dissect its influence from other systems implicated in this model [such as TNF-α and IL-6 (48, 67)]. Thus, after blockade of the inflammasome pathway, the remaining inflammation and bladder dysfunction in a given model must be attributable to additional pathways. To do this, animals were provided additional Gly after CP (to insure continued inactivation) and assessed 24 h later, a time period often used in studies of CP-induced inflammation. The extended period is also necessary for the accumulating inflammation to exert demonstrable effects on bladder physiology (i.e., urodynamics). Analysis of bladder weight and Evans blue extravasation showed that inflammasomes are responsible for approximately half of the changes due to CP-induced inflammation in this tissue. Consistent with that, histological examination demonstrated an improvement in the overall bladder architecture with Gly treatment compared with CP only-treated samples, although the improvement was not complete. The lack of a complete block to inflammation is not surprising given that other studies have implicated additional proinflammatory cytokines in the initiation of cystitis, especially TNF-α and IL-6 (48, 67). Thus, the results indicate that inflammasomes play a critical, but not exclusive, role in inducing inflammation in the bladder in response to CP.
Humans suffering from cystitis complain not only of pain but also from urinary urgency, increased frequency, and urinary incontinence. We, therefore, sought to explore the contribution of inflammasomes and the IL-1β pathway to the in vivo bladder dysfunction associated with cystitis. Urodynamics revealed that Gly completely blocked the reduction in void volume and ICI stimulated by CP, although it did not affect the increase in voiding pressure. The totality of the void volume/ICI response was somewhat surprising given that significant inflammation (bladder weight, Evans blue extravasation, and histological changes) remained after our dosing regimen. The results suggest that inflammasomes and/or the IL-1β pathway are central to bladder filling dysregulation (or the sensing of fullness) caused by CP. Interestingly, Gly alone increased void volume and ICI, which suggests that basal inflammasome/IL-1β levels in the bladder contribute to normal bladder function. While the mechanism underlying this remains to be explored, IL-1β has been shown to directly bind and stimulate the bladder detrusor muscle (3–5), clearly indicating cross-talk between these two tissues that could conceivably regulate volume sensing. Moreover, synergistic interaction of IL-1β with other cytokines directly on the detrusor has been proposed to be involved in orchestrating the local inflammatory response in interstitial cystitis (4). Conversely, IL-1β could be acting directly on sensory nerves (70).
Beyond Kummer et al.'s original detection of NLRP3 in the bladder (42), this study is the first to identify two of the best-studied inflammasomes, NLRP3 and NLRC4, in the bladder. We localized them primarily to the bladder epithelium and demonstrated that they can be activated in vivo by relevant PAMPs and DAMPs placed in the lumen of the bladder. Importantly, in vivo bladder inflammation in the CP-induced model of HC was reduced, whereas physiological dysfunction was ameliorated, by the inflammasome inhibitor Gly, suggesting that inflammasomes are important mediators of bladder inflammation and represent prospective targets for pharmacological intervention. Moreover, the efficacy of Gly in this model shows the immediate translation potential of this well-tolerated FDA-approved drug in treating what is often a challenging disorder for physicians. Its use in other models/incidences of cystitis remains to be explored.
GRANTS
This work was supported by National Center for Research Resources Grants UL1-RR-029882 and UL1-TR-000062.
DISCLAIMER
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: F.M.H. and J.T.P. conception and design of research; F.M.H., N.P.V., J.G.K., J.D.P.-T., D.W.L., B.E.S., and L.S.S. performed experiments; F.M.H., D.W.L., P.J.N., and J.T.P. analyzed data; F.M.H. and J.T.P. interpreted results of experiments; F.M.H. prepared figures; F.M.H., P.J.N., and J.T.P. drafted manuscript; F.M.H., P.J.N., and J.T.P. edited and revised manuscript; F.M.H., P.J.N., and J.T.P. approved final version of manuscript.
REFERENCES
- 1.Auge C, Chene G, Dubourdeau M, Desoubzdanne D, Corman B, Palea S, Lluel P, Vergnolle N, Coelho AM. Relevance of the cyclophosphamide-induced cystitis model for pharmacological studies targeting inflammation and pain of the bladder. Eur J Pharmacol 707: 32–40, 2013 [DOI] [PubMed] [Google Scholar]
- 2.Biondo C, Mancuso G, Beninati C, Iaria C, Romeo O, Cascio A, Teti G. The role of endosomal toll-like receptors in bacterial recognition. Eur Rev Med Pharmacol Sci 16: 1506–1512, 2012 [PubMed] [Google Scholar]
- 3.Bouchelouche K, Alvarez S, Andersen L, Nordling J, Horn T, Bouchelouche P. Monocyte chemoattractant protein-1 production by human detrusor smooth muscle cells. J Urol 171: 462–466, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Bouchelouche K, Alvarez S, Horn T, Nordling J, Bouchelouche P. Human detrusor smooth muscle cells release interleukin-6, interleukin-8, and RANTES in response to proinflammatory cytokines interleukin-1beta and tumor necrosis factor-α. Urology 67: 214–219, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Bouchelouche K, Andresen L, Alvarez S, Nordling J, Nielsen OH, Bouchelouche P. Interleukin-4 and -13 induce the expression and release of monocyte chemoattractant protein 1, interleukin-6 and stem cell factor from human detrusor smooth muscle cells: synergy with interleukin-1β and tumor necrosis factor-α. J Urol 175: 760–765, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Boudes M, Uvin P, Kerselaers S, Vennekens R, Voets T, De Ridder D. Functional characterization of a chronic cyclophosphamide-induced overactive bladder model in mice. Neurourol Urodyn 30: 1659–1665, 2011 [DOI] [PubMed] [Google Scholar]
- 7.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254, 1976 [DOI] [PubMed] [Google Scholar]
- 8.Brock N, Pohl J, Stekar J. Studies on the urotoxicity of oxazaphosphorine cytostatics and its prevention–I. Experimental studies on the urotoxicity of alkylating compounds. Eur J Cancer 17: 595–607, 1981 [DOI] [PubMed] [Google Scholar]
- 9.Chung CW, Zhang QL, Qiao LY. Endogenous nerve growth factor regulates collagen expression and bladder hypertrophy through Akt and MAPK pathways during cystitis. J Biol Chem 285: 4206–4212, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clements A, Young JC, Constantinou N, Frankel G. Infection strategies of enteric pathogenic Escherichia coli. Gut Microbes 3: 71–87, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Colvin OM. An overview of cyclophosphamide development and clinical applications. Curr Pharm Des 5: 555–560, 1999 [PubMed] [Google Scholar]
- 12.Davis BK, Wen H, Ting JP. The inflammasome NLRs in immunity, inflammation, and associated diseases. Annu Rev Immunol 29: 707–735, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Decker DB, Karam JA, Wilcox DT. Pediatric hemorrhagic cystitis. J Pediatr Urol 5: 254–264, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Di Virgilio F. The therapeutic potential of modifying inflammasomes and NOD-like receptors. Pharmacol Rev 65: 872–905, 2013 [DOI] [PubMed] [Google Scholar]
- 15.Diaz MR, King JM, Yahr TL. Intrinsic and extrinsic regulation of type III secretion gene expression in Pseudomonas aeruginosa. Front Microbiol 2: 89, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol 27: 519–550, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Dropulic LK, Jones RJ. Polyomavirus BK infection in blood and marrow transplant recipients. Bone Marrow Transplant 41: 11–18, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ejrnaes K. Bacterial characteristics of importance for recurrent urinary tract infections caused by Escherichia coli. Dan Med Bull 58: B4187, 2011 [PubMed] [Google Scholar]
- 19.Elinav E, Strowig T, Henao-Mejia J, Flavell RA. Regulation of the antimicrobial response by NLR proteins. Immunity 34: 665–679, 2011 [DOI] [PubMed] [Google Scholar]
- 20.Fernandes-Alnemri T, Litwack G, Alnemri ES. Mch2, a new member of the apoptotic Ced-3/Ice cysteine protease gene family. Cancer Res 55: 2737–2742, 1995 [PubMed] [Google Scholar]
- 21.Foxman B, Barlow R, D'Arcy H, Gillespie B, Sobel JD. Urinary tract infection: self-reported incidence and associated costs. Ann Epidemiol 10: 509–515, 2000 [DOI] [PubMed] [Google Scholar]
- 22.Geppetti P, Nassini R, Materazzi S, Benemei S. The concept of neurogenic inflammation. BJU Int 101, Suppl 3: 2–6, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Gong YN, Shao F. Sensing bacterial infections by NAIP receptors in NLRC4 inflammasome activation. Protein Cell 3: 98–105, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Griebling TL. Urinary tract infection in women. In: Urologic Diseases in America, edited by Litwin MS, Saigal CS. Washington, DC: National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, U.S. Government Printing Office, 2000, p. 587–619 [Google Scholar]
- 25.Gross R, Wulf G. Klinische und experimentelle Erfahrungen mit zyk lischen und nichtzyklischen Phosphamidestern des N-Losl in der Chemotherapie von Tumoren. Strahlentherapie 41: 361–367, 1959 [Google Scholar]
- 26.Haefliger JA, Tissieres P, Tawadros T, Formenton A, Beny JL, Nicod P, Frey P, Meda P. Connexins 43 and 26 are differentially increased after rat bladder outlet obstruction. Exp Cell Res 274: 216–225, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Hannan TJ, Totsika M, Mansfield KJ, Moore KH, Schembri MA, Hultgren SJ. Host-pathogen checkpoints and population bottlenecks in persistent and intracellular uropathogenic Escherichia coli bladder infection. FEMS Microbiol Rev 36: 616–648, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harkensee C, Vasdev N, Gennery AR, Willetts IE, Taylor C. Prevention and management of BK-virus associated haemorrhagic cystitis in children following haematopoietic stem cell transplantation–a systematic review and evidence-based guidance for clinical management. Br J Haematol 142: 717–731, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Hasegawa J, Kamada S, Kamiike W, Shimizu S, Imazu T, Matsuda H, Tsujimoto Y. Involvement of CPP32/Yama(-like) proteases in Fas-mediated apoptosis. Cancer Res 56: 1713–1718, 1996 [PubMed] [Google Scholar]
- 30.Haselberger MB, Schwinghammer TL. Efficacy of mesna for prevention of hemorrhagic cystitis after high-dose cyclophosphamide therapy. Ann Pharmacother 29: 918–921, 1995 [DOI] [PubMed] [Google Scholar]
- 31.Hu RQ, Mehter H, Nadasdy T, Satoskar A, Spetie DN, Rovin BH, Hebert L. Severe hemorrhagic cystitis associated with prolonged oral cyclophosphamide therapy: case report and literature review. Rheumatol Int 28: 1161–1164, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Hueck CJ. Type III protein secretion systems in bacterial pathogens of animals and plants. Microbiol Mol Biol Rev 62: 379–433, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hughes FM, Corn A, Nimmich A, Pratt-Thomas J, Purves J. Cyclophosphamide induces an early wave of acrolein-independent apoptosis in the urothelium. Adv Biosci Biotechnol 4: 9–14, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hughes FM, Jr, Bortner CD, Purdy GD, Cidlowski JA. Intracellular K+ suppresses the activation of apoptosis in lymphocytes. J Biol Chem 272: 30567–30576, 1997 [DOI] [PubMed] [Google Scholar]
- 35.Hughes FM, Jr, McKeithan P, Ellett J, Armeson KE, Purves JT. Simvastatin suppresses cyclophosphamide-induced changes in urodynamics and bladder inflammation. Urology 81: e209–e214, 2013 [DOI] [PubMed] [Google Scholar]
- 36.Jenkins DD, Lee T, Chiuzan C, Perkel JK, Rollins LG, Wagner CL, Katikaneni LP, Bass WT, Kaufman DA, Horgan MJ, Laungani S, Givelichian LM, Sankaran K, Yager JY, Martin R. Altered circulating leukocytes and their chemokines in a clinical trial of therapeutic hypothermia for neonatal hypoxic ischemic encephalopathy. Pediatr Crit Care Med 14: 786–795, 2013 [DOI] [PubMed] [Google Scholar]
- 37.Jenkins DD, Rollins LG, Perkel JK, Wagner CL, Katikaneni LP, Bass WT, Kaufman DA, Horgan MJ, Languani S, Givelichian L, Sankaran K, Yager JY, Martin RH. Serum cytokines in a clinical trial of hypothermia for neonatal hypoxic-ischemic encephalopathy. J Cereb Blood Flow Metab 32: 1888–1896, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jorgensen I, Seed PC. How to make it in the urinary tract: a tutorial by Escherichia coli. PLoS Pathog 8: e1002907, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kehrer JP, Biswal SS. The molecular effects of acrolein. Toxicol Sci 57: 6–15, 2000 [DOI] [PubMed] [Google Scholar]
- 40.Klinger MB, Dattilio A, Vizzard MA. Expression of cyclooxygenase-2 in urinary bladder in rats with cyclophosphamide-induced cystitis. Am J Physiol Regul Integr Comp Physiol 293: R677–R685, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Korkmaz A, Topal T, Oter S. Pathophysiological aspects of cyclophosphamide and ifosfamide induced hemorrhagic cystitis; implication of reactive oxygen and nitrogen species as well as PARP activation. Cell Biol Toxicol 23: 303–312, 2007 [DOI] [PubMed] [Google Scholar]
- 42.Kummer JA, Broekhuizen R, Everett H, Agostini L, Kuijk L, Martinon F, van Bruggen R, Tschopp J. Inflammasome components NALP 1 and 3 show distinct but separate expression profiles in human tissues suggesting a site-specific role in the inflammatory response. J Histochem Cytochem 55: 443–452, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Lamkanfi M, Dixit VM. The inflammasomes. PLoS Pathog 5: e1000510, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lamkanfi M, Dixit VM. Inflammasomes and their roles in health and disease. Annu Rev Cell Dev Biol 28: 137–161, 2012 [DOI] [PubMed] [Google Scholar]
- 45.Lamkanfi M, Dixit VM. Modulation of inflammasome pathways by bacterial and viral pathogens. J Immunol 187: 597–602, 2011 [DOI] [PubMed] [Google Scholar]
- 46.Lamkanfi M, Mueller JL, Vitari AC, Misaghi S, Fedorova A, Deshayes K, Lee WP, Hoffman HM, Dixit VM. Glyburide inhibits the cryopyrin/Nalp3 inflammasome. J Cell Biol 187: 61–70, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Leung AY, Yuen KY, Kwong YL. Polyoma BK virus and haemorrhagic cystitis in haematopoietic stem cell transplantation: a changing paradigm. Bone Marrow Transplant 36: 929–937, 2005 [DOI] [PubMed] [Google Scholar]
- 48.Malley SE, Vizzard MA. Changes in urinary bladder cytokine mRNA and protein after cyclophosphamide-induced cystitis. Physiol Genomics 9: 5–13, 2002 [DOI] [PubMed] [Google Scholar]
- 49.Martin Y, Avendaño C, Piedras MJ, Krzyzanowska A. Evaluation of Evans blue extravasation as a measure of peripheral inflammation. Protocol Exchange; 10.1038/protex.2010.209 [DOI] [Google Scholar]
- 50.Martins JP, Silva RB, Coutinho-Silva R, Takiya CM, Battastini AM, Morrone FB, Campos MM. The role of P2X7 purinergic receptors in inflammatory and nociceptive changes accompanying cyclophosphamide-induced haemorrhagic cystitis in mice. Br J Pharmacol 165: 183–196, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Masui T, Mann AM, Macatee TL, Okamura T, Garland EM, Smith RA, Cohen SM. Absence of ras oncogene activation in rat urinary bladder carcinomas induced by N-methyl-N-nitrosourea or N-butyl-N-(4-hydroxybutyl)nitrosamine. Carcinogenesis 13: 2281–2285, 1992 [DOI] [PubMed] [Google Scholar]
- 52.McCulloch C, Searle SR. Generalized, Linear, and Mixed Models. New York: John Wiley & Sons, 2001 [Google Scholar]
- 53.Miano R, Germani S, Vespasiani G. Stones and urinary tract infections. Urol Int 79, Suppl 1: 32–36, 2007 [DOI] [PubMed] [Google Scholar]
- 54.Miao EA, Mao DP, Yudkovsky N, Bonneau R, Lorang CG, Warren SE, Leaf IA, Aderem A. Innate immune detection of the type III secretion apparatus through the NLRC4 inflammasome. Proc Natl Acad Sci USA 107: 3076–3080, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Miki T, Matsunami M, Nakamura S, Okada H, Matsuya H, Kawabata A. ONO-8130, a selective prostanoid EP1 receptor antagonist, relieves bladder pain in mice with cyclophosphamide-induced cystitis. Pain 152: 1373–1381, 2011 [DOI] [PubMed] [Google Scholar]
- 56.Moghimpour Bijani F, Vallejo JG, Rezaei N. Toll-like receptor signaling pathways in cardiovascular diseases: challenges and opportunities. Int Rev Immunol 31: 379–395, 2012 [DOI] [PubMed] [Google Scholar]
- 57.Nielubowicz GR, Mobley HL. Host-pathogen interactions in urinary tract infection. Nat Rev Urol 7: 430–441, 2010 [DOI] [PubMed] [Google Scholar]
- 58.O'Neill LA. Signal transduction pathways activated by the IL-1 receptor/toll-like receptor superfamily. Curr Top Microbiol Immunol 270: 47–61, 2002 [PubMed] [Google Scholar]
- 59.Ohmasa F, Saito M, Oiwa H, Tsounapi P, Shomori K, Kitatani K, Dimitriadis F, Kinoshita Y, Satoh K. Pharmacological preconditioning of ATP-sensitive potassium channel openers on acute urinary retention-induced bladder dysfunction in the rat. BJU Int 110: E245–252, 2012 [DOI] [PubMed] [Google Scholar]
- 60.Ohtani T, Nakamura T, Toda K, Furukawa F. Cyclophosphamide enhances TNF-α-induced apoptotic cell death in murine vascular endothelial cell. FEBS Lett 580: 1597–1600, 2006 [DOI] [PubMed] [Google Scholar]
- 61.Parsons BA, Drake MJ. Animal models in overactive bladder research. Handb Exp Pharmacol 15–43, 2011 [DOI] [PubMed] [Google Scholar]
- 62.Petrasek J, Csak T, Szabo G. Toll-like receptors in liver disease. Adv Clin Chem 59: 155–201, 2013 [DOI] [PubMed] [Google Scholar]
- 63.Saitoh C, Yokoyama H, Chancellor MB, de Groat WC, Yoshimura N. Comparison of voiding function and nociceptive behavior in two rat models of cystitis induced by cyclophosphamide or acetone. Neurourol Urodyn 29: 501–505, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schroder K, Tschopp J. The inflammasomes. Cell 140: 821–832, 2010 [DOI] [PubMed] [Google Scholar]
- 65.Schwartz PS, Waxman DJ. Cyclophosphamide induces caspase 9-dependent apoptosis in 9L tumor cells. Mol Pharmacol 60: 1268–1279, 2001 [DOI] [PubMed] [Google Scholar]
- 66.Silva Lde P, Patah PA, Saliba RM, Szewczyk NA, Gilman L, Neumann J, Han XY, Tarrand J, Ribeiro R, Gulbis A, Shpall EJ, Jones R, Popat U, Walker JA, Petropoulos D, Chiattone A, Stewart J, El-Zimaity M, Anderlini P, Giralt S, Champlin RE, de Lima M. Hemorrhagic cystitis after allogeneic hematopoietic stem cell transplants is the complex result of BK virus infection, preparative regimen intensity and donor type. Haematologica 95: 1183–1190, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Smaldone MC, Vodovotz Y, Tyagi V, Barclay D, Philips BJ, Yoshimura N, Chancellor MB, Tyagi P. Multiplex analysis of urinary cytokine levels in rat model of cyclophosphamide-induced cystitis. Urology 73: 421–426, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Song J, Abraham SN. Innate and adaptive immune responses in the urinary tract. Eur J Clin Invest 38, Suppl 2: 21–28, 2008 [DOI] [PubMed] [Google Scholar]
- 69.Song J, Abraham SN. TLR-mediated immune responses in the urinary tract. Curr Opin Microbiol 11: 66–73, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stemkowski PL, Smith PA. Sensory neurons, ion channels, inflammation and the onset of neuropathic pain. Can J Neurol Sci 39: 416–435, 2012 [DOI] [PubMed] [Google Scholar]
- 71.Svanborg C, Godaly G. Bacterial virulence in urinary tract infection. Infect Dis Clin North Am 11: 513–529, 1997 [DOI] [PubMed] [Google Scholar]
- 72.Tang D, Kang R, Coyne CB, Zeh HJ, Lotze MT. PAMPs and DAMPs: signal 0s that spur autophagy and immunity. Immunol Rev 249: 158–175, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wilmanns H. Chemotherapie maligner Tumoren Erste Erfahrungen mit Endoxan. In: Asta-Forschung und Therapie. Brackwede, Germany: Asta-Werke, 1958 [Google Scholar]
- 74.Wright KJ, Seed PC, Hultgren SJ. Uropathogenic Escherichia coli flagella aid in efficient urinary tract colonization. Infect Immun 73: 7657–7668, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yeretssian G. Effector functions of NLRs in the intestine: innate sensing, cell death, and disease. Immunol Res 54: 25–36, 2012 [DOI] [PubMed] [Google Scholar]
- 76.Zambetti LP, Laudisi F, Licandro G, Ricciardi-Castagnoli P, Mortellaro A. The rhapsody of NLRPs: master players of inflammation…and a lot more. Immunol Res 53: 78–90, 2012 [DOI] [PubMed] [Google Scholar]


