Abstract
Despite clinically available methods of diaphragm pacing, most patients with ventilator-dependent tetraplegia are still dependent on mechanical ventilation. Given the significant disadvantages of these devices, additional pacing options are needed. The objective of this study was to evaluate a novel and potentially more physiological method of inspiratory muscle activation, which involves the application of high-frequency (>200 Hz) stimulation to the ventral surface of the spinal cord in the high thoracic region. Studies were performed in 13 anesthetized dogs. High-frequency spinal cord stimulation (HF-SCS) results in the activation of both the diaphragm and inspiratory intercostal muscles, in concert, at physiological firing frequencies and the generation of large inspired volumes. Mean maximum firing frequencies of motor units in the parasternal (2nd interspace), the external intercostal (3rd interspace), and the diaphragm muscles were 10.6 ± 0.4, 11.7 ± 0.4, and 10.4 ± 0.3 Hz, respectively. These values were not significantly different from those occurring during spontaneous breathing at comparable inspired volumes. Maximum inspired volume was 0.93 ± 0.01 liter, which approximates the inspiratory capacity of these animals. Moreover, ventilation can be maintained on a chronic basis by this method (6 h) without evidence of system fatigue. Our results suggest that HF-SCS results in activation of spinal cord tracts that synapse with the inspiratory motoneuron pools, allowing processing of the stimulus and consequent physiological activation of the inspiratory muscles. HF-SCS has the potential to provide an effective method of inspiratory muscle pacing.
Keywords: diaphragm pacing, phrenic nerve pacing
diaphragm pacing (DP) is a clinically useful modality to provide artificial ventilatory support in patients with ventilator-dependent tetraplegia (14, 32, 34). DP has significant advantages compared with mechanical ventilation, including greater mobility, elimination of fear of ventilator disconnection, elimination of embarrassment and social stigma associated with connection to a respirator, better speech quality, and improved sense of well-being and overall health (4, 13, 32).
Current methods of DP involve the placement of electrodes on each of the phrenic nerves in the thorax (33) or in the body of the diaphragm in the vicinity of the phrenic nerve motor points (18, 19). With both methods, bilateral phrenic nerve stimulation is necessary to maintain adequate ventilation. Despite careful prescreening of patients to ensure phrenic nerve viability, however, many patients (∼50%) have inadequate inspired volume generation to maintain full-time ventilatory support (45, 53). There are also many patients who are ineligible for pacing due to inadequate phrenic nerve function. The vast majority of ventilator-dependent tetraplegic persons, therefore, still require the use of mechanical ventilation.
Several factors may account for inadequate inspired volume production during DP. First, peripheral nerve electrodes generally do not achieve complete diaphragm activation due to the high thresholds of some axons (29, 35, 41, 49). Second, this methodology results in a reversed recruitment order of motor units: large-diameter motor axons that innervate large, more fatigueable muscle fibers are activated initially, while smaller, fatigue-resistant muscle fibers are only recruited at higher stimulus levels, if at all (42). With reconditioning, during which DP is applied with low frequencies (<20 Hz), however, the diaphragm is converted from a normally mixed fiber population to one comprised predominantly of slow fibers that have relatively low maximum force-generating capacities (1, 33, 46, 47). Consequently, fiber-type conversion improves endurance but restricts maximum inspired volume generation. Finally, there is lack of coincident inspiratory intercostal/accessory (IC) muscle activation, a muscle group that is responsible for the generation of ∼35–40% of the vital capacity (2).
In this paper, we describe a new method of inspiratory muscle activation in which diaphragm activation in concert with inspiratory intercostal muscle activation can be achieved with upper thoracic spinal cord simulation (SCS) at high stimulus frequencies (>200 Hz). This is a unique method of inspiratory muscle activation since stimulation occurs via spinal pathways that synapse with inspiratory motoneurons, resulting in a more physiological recruitment pattern. High-frequency spinal cord stimulation (HF-SCS), therefore, may provide a more effective means of inspiratory muscle pacing.
MATERIALS AND METHODS
Studies were performed on 13 mongrel dogs (mean weight 16.5 ± 0.6 kg) with the approval of the Institutional Animal Care and Use Committee of Case Western Reserve University. Each animal was anesthetized initially with pentobarbital sodium (PB, 25 mg/kg) given intravenously. Additional doses of PB (1–2 mg/kg) were provided, as required.
A cuffed endotracheal tube (10-mm ID) was sutured into the trachea in the midcervical region. A catheter was placed in the femoral vein to administer fluids and supplemental anesthesia; a catheter was also placed in the femoral artery to monitor blood pressure (model PT300, Grass Instruments, Quincy, MA) and heart rate (P511 AC Amplifier, Grass Instruments). Body temperature was maintained with a heating blanket (Harvard Apparatus, Cambridge, MA) at 38 ± 0.5°C. End-tidal Pco2 was monitored at the trachea and oxygen saturation from the earlobe (Nellcor N-100, Hayward, CA). Airway pressure was measured at functional residual capacity (FRC) following airway occlusion with a pressure transducer (Validyne, MP45, Northridge, CA) connected to the airway opening. Tidal volume was recorded by electrical integration of the flow signal from a pneumotachograph (Series 3700, Hans Rudolph, Kansas City, MO).
A laminectomy was performed at the T4–T5 level for electrode placement. A four-plate electrode with 4-mm contact leads (model 3986A, Medtronic, Minneapolis, MN) was positioned under direct vision onto the ventral surface of the spinal cord and advanced 3 cm to the T2 level. We have ascertained based on many previous postmortem canine studies that this distance results in electrode placement between the T2 and T3 motor units. Based on preliminary trials, this location was found to result in maximal inspired volume generation. An indifferent ground electrode was implanted in the back musculature. A Grass square-wave pulse stimulator (model S88, Grass Instruments) equipped with a stimulus isolation unit (PSIU6, Grass Instruments) was used to provide electrical stimulation over a wide range of stimulus frequencies (0–1,000 Hz) and stimulus amplitudes (0–15 mA). Stimulus train duration was fixed at 1.2 s since a plateau in pressure and volume generation is generally achieved by this time and this duration is in the range of that applied during inspiratory muscle pacing.
Bipolar teflon-coated, stainless steel fine-wire electrodes, uninsulated at their terminal 5 mm, were used to assess multiunit inspiratory muscle EMG recordings of the parasternal (2nd interspace), dorsal portion of the external intercostal muscles (3rd interspace, posterior axillary line), and costal diaphragm (via the 7th interspace). Intercostal recordings were made from muscles over these regions of the rib cage since they are electrically active during resting breathing (10, 21, 22). Inspiratory muscle activation was further characterized by single motor unit (SMU) recordings. SMU recordings were made using teflon-coated stainless steel electrodes with an uninsulated portion of only ∼1 mm, to provide greater selectivity. EMG potentials were amplified (1,000–10,000 times) and filtered (50 Hz to 5.0 kHz) (model BMI-830, Charles Ward Enterprises, Ardmore, PA). All parameters were monitored and stored on an eight-channel data-acquisition recorder (model Dash 8X, AstroMed, West Warwick, RI) for subsequent analysis (AstroView X, Data Review Software, AstroMed).
To model patients with spinal cord injury, the cervical spinal cord was sectioned at the C2 level using watchmaker forceps. Complete section was verified by lifting a hook across the area of transaction.
Protocol 1 (n = 4).
Following C2 section, changes in airway pressure and inspired volume were assessed during SCS over a wide range of stimulus currents (0.3–6 mA, 0.2-ms pulse width). Our evaluation was limited to 6 mA since higher levels resulted in significant body movement and therefore have no practical application. At each level of stimulus current, the effects of changes in stimulus frequency were assessed at 50, 100, 200, 300, and 400 Hz.
Inspiratory capacity of each animal was obtained by passive inflation with a volume syringe to an airway pressure of +30 cmH2O.
Protocol 2 (n = 6).
EMG recording electrodes were positioned in the parasternal and external intercostal muscles and costal diaphragm to assess their pattern of activation. Multiunit and SMU measurements of each muscle were initially made during spontaneous breathing. Following C2 section, EMG measurements were also made during HF-SCS (300 Hz) with adjustments in stimulus amplitude to approximate the magnitude of inspired volume observed during spontaneous breathing. For SMU recordings, 4–10 sites were sampled from each muscle. At each site, one to four SMUs could be distinguished on the basis of their morphology. In two animals, the possibility that EMG activities may have resulted from cross-contamination from other muscles was examined. SMUs were recorded before and after section of the left internal intercostal nerve (2nd interspace), section of the left external intercostal nerve (3rd interspace), and left cervical phrenicotomy, in separate trials.
Protocol 3 (n = 3).
Following C2 section, the capacity of chronic stimulation to sustain artificial ventilation was evaluated by applying a fixed stimulus paradigm (300 Hz, 0.5 mA, 0.2-ms pulse width) over a prolonged time period. Based on preliminary trials, this paradigm generated inspired volumes in the physiological range; respiratory rate (stimulus trains/min) was adjusted to maintain end-tidal Pco2 between 35 and 40 mmHg over a 10- to 15-min period. Inspiratory time was fixed at 1.2 s. Continuous pacing was then maintained over an arbitrarily predetermined period of 5.5 h. During prolonged pacing, end-tidal Pco2, oxygen saturation, mean blood pressure, and heart rate were continuously monitored. Inspired volume, minute ventilation, and airway pressure generation were also monitored every 30 min. Forelimb and hindlimb muscle activation was also monitored by extensor carpi radialis and quadriceps EMG, respectively.
Following 5.5 h of chronic stimulation, stimulus parameters were increased for an additional 30 min in each animal to induce hyperventilation. This was performed as an additional test to assess the capacity of the system to sustain artificial ventilation.
Data analysis.
Curves were constructed relating stimulus current to inspired volume and airway pressure generation at fixed stimulus frequencies of 50, 100, 200, 300, and 400 Hz. SMU analysis included determination of mean peak discharge frequencies. Comparisons were made, where applicable, using repeated-measures ANOVA and post hoc Newman-Keuls tests. A P value < 0.05 was accepted as statistically significant. Data are reported as means ± SE.
RESULTS
Protocol 1.
The effects of SCS on inspired volume and negative airway pressure generation during stimulation at 50 Hz and 300 Hz at various stimulus amplitudes are shown for a representative animal in Fig. 1. At any given level of stimulus current, inspired volume and airway pressure generation were substantially larger at 300 Hz compared with 50 Hz. For example, with stimulation at 2 mA, inspired volume and negative airway pressure were 970 ml and 68 cmH2O compared with 360 ml and 18 cmH2O, with 300 and 50 Hz, respectively. Stimulation currents greater than 3 mA were associated with mild flexion of the forepaws; however, no visible movement was observed with lower stimulus currents.
Fig. 1.
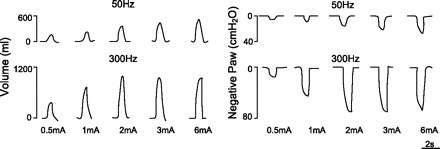
Comparison of 50- and 300-Hz stimulation on inspired volume and negative airway pressure generation (Paw) in a representative animal. For any given stimulus amplitude, these parameters were significantly higher at 300 Hz.
Mean inspired volume and negative airway pressure generation during electrical stimulation over a wide range of stimulus frequencies and amplitudes are presented in Fig. 2. There were progressive increases in the magnitude of inspired volume and airway pressure generation with increasing stimulus frequency from 50 to 300 Hz. With conventional stimulus frequencies (50 Hz), inspired volumes and airway pressures were substantially smaller than those achieved with higher frequencies (P < 0.05 compared with 100 Hz and P < 0.001 compared with higher stimulus frequencies). Values at 200 Hz were also significantly smaller than those at 300 Hz (P < 0.01). Values at 400 Hz, however, were not significantly different from those obtained at 300 Hz. In addition, maximum values of inspired volume and airway pressure were achieved at much lower levels of current during high- compared with low-frequency stimulation (i.e., leftward shift of these curves with increasing stimulus frequency). Mean maximum inspired volume and negative airway pressure were 0.93 ± 0.01 liter and 67 ± 4 cmH2O, respectively (300 Hz, 3 mA). The inspiratory capacity of these animals was 1.04 ± 0.02 liter, which was significantly larger than maximum values achieved with HF-SCS (P < 0.05).
Fig. 2.
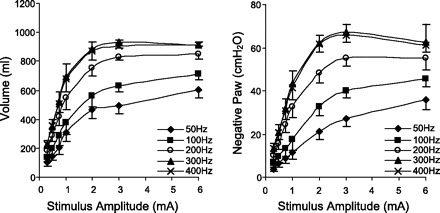
Relationships between stimulus amplitude and inspired volume (left) and negative Paw generation (right) at different stimulus frequencies. With increasing stimulus frequencies between 50 and 300 Hz, there were significant increases in the magnitude of these parameters. The 300- and 400-Hz curves, however, were not statistically different.
Protocol 2.
Multiunit EMGs of the parasternal and external intercostal muscles and costal diaphragm are shown for one animal in Fig. 3. EMGs obtained during spontaneous breathing are shown in the left panel and those obtained at a comparable inspired volume during HF-SCS are shown in the right panel. As with spontaneous breathing, HF-SCS generated an asynchronous EMG pattern (i.e., the time when each spike is fired by one motoneuron is not correlated with the time when the spikes of the other motoneurons are discharged) in each of these inspiratory muscles. In contrast, direct phrenic nerve stimulation, as with all motor nerve stimulation techniques, results in synchronous activation of all axons, as reflected in the single action potential following each stimulus artifact spike.
Fig. 3.
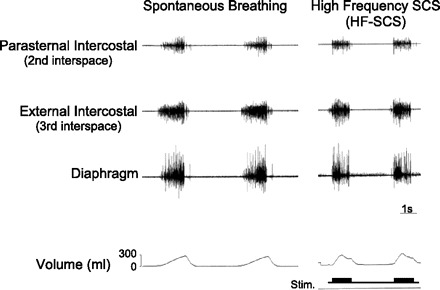
Multiunit EMGs of the parasternal intercostal muscle, external intercostal muscle, and diaphragm during spontaneous breathing (left) and during HF-SCS (right) at comparable inspired volumes. As with spontaneous breathing, HF-SCS results in an asynchronous EMG pattern. This pattern is in marked contrast to the synchronous EMG pattern observed during phrenic nerve stimulation.
Specific characterization of single motor units in the diaphragm is shown for one animal during HF-SCS in Fig. 4. In response to HF-SCS (300 Hz, 0.5 mA), three separate motor units are readily identified. Each of the three SMUs is shown superimposed in the right portion of Fig. 4, confirming their similar morphology. In this instance, firing frequencies ranged between 7.8 and 14.9 Hz. A representative example of SMU recordings from the parasternal and external intercostal muscles and diaphragm during spontaneous breathing and HF-SCS (300 Hz, 0.5 mA) is shown in Fig. 5. The raw tracings are displayed above a plot of instantaneous firing frequencies of a SMU from each muscle. Superimpositions of the SMU recordings are also provided. The responses observed during HF-SCS appear qualitatively similar to those occurring during spontaneous breathing. Firing frequencies gradually increase during the breath until a plateau is reached in the mid to latter portion of the breath. Similar response patterns during HF-SCS were observed in each animal.
Fig. 4.
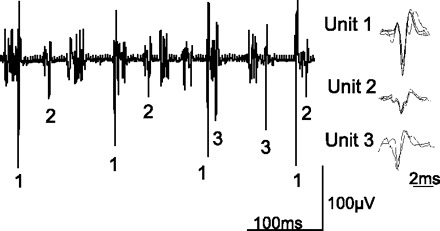
Single motor unit (SMU) recordings from the diaphragm during HF-SCS in one animal. Three separate motor units are readily identified. Each of the three SMUs is shown superimposed at right, confirming their similar morphology. See text for further explanation.
Fig. 5.
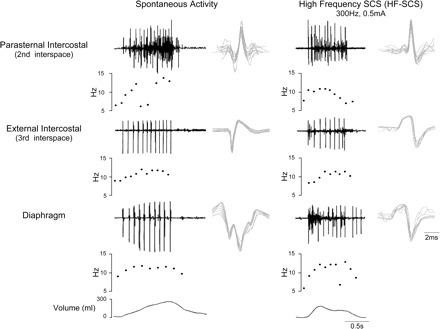
SMU activity recorded from the parasternal intercostal muscle, external intercostal muscle, and diaphragm during spontaneous breathing (left) and during HF-SCS (right) in one animal. EMG, instantaneous motor unit discharge frequency, and volume are plotted. See text for further explanation.
During spontaneous breathing, 31, 34, and 42 single motor units were evaluated for the parasternal and external intercostal muscles and diaphragm, respectively. Mean firing frequencies were 12.3 ± 0.6, 12.1 ± 0.5, and 10.8 ± 0.3 Hz, respectively. During HF-SCS, 56, 58, and 80 motor units were evaluated for the parasternal and external intercostal muscles and diaphragm, respectively. Mean firing frequencies were 10.6 ± 0.4, 11.7 ± 0.4, and 10.4 ± 0.3 Hz, respectively [nonsignificant (NS) for each comparison during spontaneous breathing vs. HF-SCS]. Mean inspired volumes during spontaneous breathing and HF-SCS were not significantly different at 0.29 ± 0.03 and 0.30 ± 0.02 liter, respectively.
The effects of internal and external intercostal nerve section and left cervical phrenicotomy on the SMU activity of each respective muscle are shown for one animal in Fig. 6. Following nerve section of each respective inspiratory muscle, EMG activities were eliminated, confirming the absence of cross-contamination from other muscles.
Fig. 6.
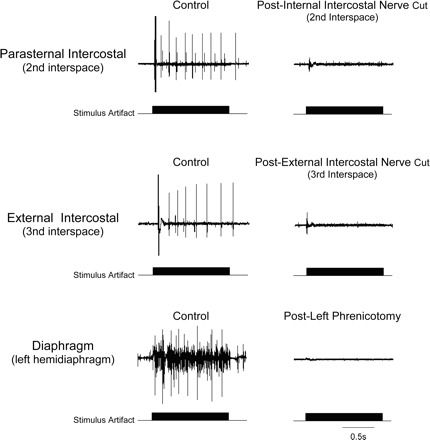
Effects of left internal (top) and external (middle) nerve section and left cervical phrenicotomy (bottom) on the SMU activities of each representative muscle. See text for further explanation.
Protocol 3.
The mean effects of chronic HF-SCS applied over a 6-h period on airway pressure, inspired volume, end-tidal Pco2, and oxygen saturation are presented in Fig. 7. Stimulus parameters and train rate (breaths/min) were set at the same values (300 Hz, 0.5 mA, 0.2 ms, 7/min) in each animal. Throughout the initial 5.5 h of chronic stimulation, airway pressure generation and inspired volume production remained unchanged. Mean airway pressure and inspired volume were 29 ± 1 cmH2O and 0.45 ± 0.07 liter during the first hour, and 26 ± 1 cmH2O and 0.46 ± 0.12 liter during the final hour, respectively (NS for each). Mean end-tidal Pco2 and oxygen saturation during chronic stimulation are provided in Table 1. As shown, there were no significant changes in these values over the period of stimulation. Importantly, progressive elevations in Pco2 did not occur in any animal. Finally, mean blood pressure and heart rate remained stable throughout the period of stimulation at 95 ± 9 mmHg and 107 ± 7 min−1 during the first hour and 88 ± 5 mmHg and 105 ± 5 min−1 during the final hour of stimulation (NS for each, Fig. 7). In each animal, the increases in stimulus parameters during the final 30 min of pacing resulted in a sustained fall in end-tidal Pco2 to 26 ± 1 mmHg.
Fig. 7.
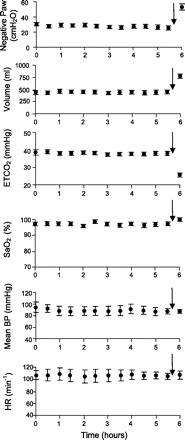
Negative airway pressure generation (Paw) (following airway occlusion), inspired volume, end-tidal Pco2, oxygen saturation (SaO2), mean blood pressure (BP), and heart rate (HR) are plotted every 30 min during continuous inspiratory muscle pacing with HF-SCS. Results are means ± SE (n = 3). Airway pressure and inspired volume generation remained constant throughout the initial 5.5 h of stimulation. End-tidal Pco2 and oxygen saturation were also maintained between 35 and 40 mmHg and 95 and 99%, respectively. Mean blood pressure and heart rate also remained stable throughout this period. After 5.5 h of continuous pacing, stimulus parameters were increased, resulting in an increase in inspired volume and consequent fall in Pco2, which was sustained over a 30-min period.
Table 1.
Ventilation parameters monitored during 6 h of continuous HF-SCS
| Time, hour | ETCO2, mmHg | SaO2, % | ||
|---|---|---|---|---|
| Stimulus parameters: 300 Hz, 0.5 mA, 0.2-s pulse width | ||||
| 0 | 38.7±1.7 | 97.5±0.5 | ||
| 2 | 38.3±0.9 | 96.0±1.0 | ||
| 4 | 38.0±0.6 | 97.5±0.5 | ||
| 5.5 | 37.7±0.7 | 97.5±0.5 | ||
| Stimulus parameters: 300 Hz, 1.5 mA, 0.2-s pulse width | ||||
| 6 | 25.7±0.7 | 100±0.0 | ||
Values are means ± SE. HF-SCS, high-frequency spinal cord stimulation; ETCO2, end-tidal Pco2; SaO2, oxygen saturation.
During continuous pacing, there was no limb motion, and extensor carpi radialis and quadriceps EMGs were electrically silent.
DISCUSSION
This study represents the first demonstration of physiological activation of the inspiratory muscles utilizing electrical stimulation techniques. This is indicated by two critical observations. First, HF-SCS results in an asynchronous pattern of both diaphragm and inspiratory intercostal muscle activation, which resembles that observed during spontaneous breathing. This pattern stands in marked contrast to the synchronous activation of all axons, as reflected in the single action potential following each stimulus spike, as seen with direct phrenic nerve stimulation, ventral root stimulation of the intercostal muscles, and all other motor nerve stimulation techniques. Second, analysis of single motor units indicates that the firing frequency during HF-SCS is not significantly different from that observed during spontaneous breathing. Moreover, in studies on normal human subjects, other investigators have reported similar values of 10.8 ± 0.3, 11.9 ± 0.4, and 12.6 ± 0.5 Hz for the parasternal and external intercostal muscles and diaphragm, respectively (50). In previous studies in cats, investigators have also found similar firing frequency for the diaphragm (39, 52).
This study also demonstrates that HF-SCS, with a single electrode positioned on the ventral surface of the upper thoracic spinal cord, at high stimulus frequencies and low stimulus currents, results in near-maximum diaphragm and intercostal muscle activation. This is evidenced by the fact that 1) the magnitude of inspired volume achieved by this method approximates the inspiratory capacity, and 2) maximum bilateral phrenic nerve stimulation and combined intercostal and phrenic nerve stimulation result in negative airway pressures of ∼44 and ∼68 cmH2O, respectively (23), whereas HF-SCS resulted in a mean airway pressure of 67 cmH2O. Taken together, these results indicate that HF-SCS has the potential to provide a much more natural and effective method of inspiratory muscle pacing.
Importantly, the results of this investigation also demonstrate that artificial ventilation can be maintained for prolonged periods by HF-SCS. Based on the stability of inspired volume over several hours, and the fact that ventilation could be increased at the end of the trial resulting in a reduction in end-tidal Pco2 into the 25- to 30-mmHg range, our results further suggest that chronic pacing was accomplished without significant system fatigue.
Mechanism of inspiratory muscle activation via HF-SCS.
In contrast to the pattern of motor unit recruitment observed during DP, spontaneous breathing is characterized by an orderly recruitment of diaphragm motor units, which has been shown to follow Henneman's size principle (3, 11, 26, 37, 38, 40, 51). According to this principle, smaller motoneurons that have lower membrane surface area and higher input resistance are more excitable. Consequently, smaller motoneurons have a lower recruitment threshold than larger ones and are recruited first. In support of this principle, studies have shown that motor units with slower axonal conduction velocities, which are known to correlate with smaller motoneuron size, are recruited first during inspiratory efforts (12, 51). Based on this thesis, motoneuron activation and therefore motor unit recruitment proceed in an orderly fashion from slow to fast units. Consequently, slow fibers, which are fatigue resistant and generate relatively low-twitch and maximum forces, are recruited first. This is followed by the progressive recruitment of faster fibers, which are more fatigueable, but capable of generating much larger forces (11, 30, 43). Implicit in this concept is the fact that control of motoneuron recruitment pattern resides at the level of the motoneuron pools and does not require input from bulbospinal premotoneurons (11, 12, 43).
With regard to HF-SCS, the observation that the applied stimulus currents result in a physiological pattern of activation indicates that the mechanism of inspiratory muscle activation entails stimulation of spinal cord tracts that synapse with the diaphragm and inspiratory intercostal motoneuron pools. In contrast to virtually all functional electrical stimulation applications (24, 25, 31, 44), this method allows for processing of the stimulus within the motoneuron pools resulting in a more physiological recruitment pattern. The mechanism of pathway activation arises from the fact that motoneurons, previously thought of as passive integrators of synaptic input, possess intrinsic membrane properties (as described above) that allow processing of afferent signals (3, 6). It is important to mention, however, that the precise pattern of motor unit recruitment was not investigated in the present study. Further investigations will be necessary, therefore, to assess this parameter.
While the specific spinal cord tracts mediating this response are not known, Decima and von Euler (7) and Decima et al. (8, 9) have described afferents from the lower thoracic intercostal and abdominal muscles (T9–T13) that reflexly facilitate phrenic motoneuron activity via spinal cord pathways (intercostal to phrenic reflex). Afferents from the inspiratory and expiratory intercostal muscles monosynaptically excite the homonymous motoneurons of the same spinal segment and also motoneurons of adjacent segments (intercostal to intercostal and intercostal to phrenic facilitatory reflexes) (27, 28). We speculate that activation of the inspiratory motoneuron pools by HF-SCS may be mediated by these same excitatory tracts.
Comparison to current methods of DP.
There are a number of factors that may contribute to reduced inspired volume production and consequent inability to maintain satisfactory full-time ventilatory support with current methods of DP. First, with inspiratory muscle pacing and virtually all other functional electrical stimulation methods to restore muscular function, electrodes are positioned near motor roots (24, 25), on peripheral nerves (31), or at functional motor points (44). This methodology results in synchronous activation of motor axons within the field of stimulation (36) and a reversed order of motor unit recruitment. In general, large-diameter motor axons, which innervate large, more fatigueable motor units, are more easily excited by imposed electric fields. Smaller, fatigue-resistant motor units require higher stimulus levels and may not be fully activated, resulting in incomplete axonal stimulation and therefore incomplete muscle activation (42). Assuming that HF-SCS results in an orderly pattern of motor unit recruitment, the early engagement of the weakest and least fatigueable motor units with progression to stronger and more fatigueable units during inspiration (rather than synchronous activation of all motor units) serves to maximize the range of force modulation and thereby inspired volume generation.
Second, while maximum activation is usually achieved with 50-Hz stimulation during DP, stimulus frequencies of 20 Hz or less are generally applied, as chronic stimulation at higher frequencies may result in myopathic changes and muscle fatigue (5, 31–33). While low-frequency stimulation increases the endurance characteristics of electrically stimulated muscles by conversion from a mixed-fiber population to a uniform population of type I fibers, it also significantly reduces fiber diameter and maximum force generation and, in turn, maximum inspired volume generation (48). Since HF-SCS results in the activation of the inspiratory muscles at physiological firing frequencies, fiber type conversion may not occur, resulting in preserved histological characteristics and mechanical function.
Finally, there is lack of coincident inspiratory intercostal/accessory activation during DP, a muscle group that is responsible for the generation of 35–40% of the inspiratory capacity (2, 10). The lack of coincident intercostal activation also prevents the synergistic interaction of combined intercostal and diaphragm activation to generate inspired volume and airway pressure (23). While the relative degree of diaphragm and intercostal activation resulting from HF-SCS is unknown, the EMG data of the present study suggest that both muscle groups are activated in synchrony.
Study limitations.
We have previously demonstrated that upper thoracic SCS, on the ventral surface of the spinal cord at the T2 level, with conventional stimulus frequencies results in activation of the inspiratory intercostal muscles and the production of large inspired volumes (15–17). While intercostal pacing alone is not sufficient (24), combined intercostal and unilateral diaphragm pacing is successful in maintaining full-time ventilatory support (25). Intercostal pacing, however, results in nonspecific stimulation of the upper thoracic ventral roots and therefore some undesirable upper extremity movement (24, 25). Based on the results of this study, chronic HF-SCS does not result in significant upper extremity muscle activation. This is not surprising since HF-SCS does not depend on ventral root stimulation and results in maximum inspired volumes with lower stimulus currents. It is possible, therefore, that the potential clinical application of HF-SCS will not be associated with movement side effects.
The degree of expiratory muscle activation resulting from HF-SCS is unknown. It is possible that this technique also results in activation of expiratory motoneuron pools, as well. While this is possible, it would appear that this is a negligible effect since HF-SCS results in inspired volumes that approximate the inspiratory capacity. In addition, the expiratory intercostal muscles of the upper rib cage are very thin and generate negligible opposing positive pressure (20).
In summary, HF-SCS is a unique method of inspiratory muscle activation that results in physiological activation of the inspiratory muscles. It is possible that this technique may provide ventilator-dependent tetraplegic persons a more natural and effective method of inspiratory muscle pacing. Intact phrenic nerve function is necessary to activate the diaphragm with HF-SCS. However, since HF-SCS also results in activation of the inspiratory intercostal muscles, it is possible that this technique may also be successful in maintaining ventilatory support in tetraplegic persons with suboptimal phrenic nerve function.
GRANTS
This study received funding from The MetroHealth Foundation.
DISCLOSURES
A. F. DiMarco is a founder of and has a significant financial interest in Synapse BioMedical, , a manufacturer of diaphragm pacing systems and holds patents for spinal cord stimulation to restore cough and respiration. A patent concerning high-frequency spinal cord stimulation is pending.
Acknowledgments
We thank Dana Hromyak for invaluable technical assistance.
REFERENCES
- 1.Acker MA, Mannion JD, Brown WE, Salmons S, Henriksson J, Bitto T, Gale DR, Hammond R, Stephenson LW. Canine diaphragm muscle after 1 yr of continuous electrical stimulation: its potential as a myocardial substitute. J Appl Physiol 62: 1264–1270, 1987 [DOI] [PubMed] [Google Scholar]
- 2.Agostoni E, Mognoni P, Torri G, Agostoni AF. Static features of the passive rib cage and abdomen-diaphragm. J Appl Physiol 20: 1187–1193, 1965 [Google Scholar]
- 3.Berger AJ. Phrenic motoneurons in the cat: subpopulations and nature of respiratory drive potentials. J Neurophysiol 42: 76–90, 1979 [DOI] [PubMed] [Google Scholar]
- 4.Brown R, DiMarco AF, Hoit JD, Garshick E. Respiratory dysfunction and management in spinal cord injury. Respir Care 51: 853–870, 2006 [PMC free article] [PubMed] [Google Scholar]
- 5.Ciesielski TE, Fukuda Y, Glenn WW, Gorfien J, Jeffery K, Hogan JF. Response of the diaphragm muscle to electrical stimulation of the phrenic nerve. A histochemical and ultrastructural study. J Neurosurg 58: 92–100, 1983 [DOI] [PubMed] [Google Scholar]
- 6.Collins DF, Burke D, Gandevia SC. Large involuntary forces consistent with plateau-like behavior of human motoneurons. J Neurosci 21: 4059–4065, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Decima EE, von Euler C. Excitability of phrenic motoneurones to afferent input from lower intercostal nerves in the spinal cat. Acta Physiol Scand 75: 580–591, 1969 [DOI] [PubMed] [Google Scholar]
- 8.Decima EE, von Euler C, Thoden U. Intercostal-to-phrenic reflexes in the spinal cat. Acta Physiol Scand 75: 568–579, 1969 [PubMed] [Google Scholar]
- 9.Decima EE, von Euler C, Thoden U. Spinal intercostal-phrenic reflexes. Nature 214: 312–313, 1967 [DOI] [PubMed] [Google Scholar]
- 10.De Troyer A, Kirkwood PA, Wilson TA. Respiratory action of the intercostal muscles. Physiol Rev 85: 717–756, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Dick TE, Kong JJ, Berger AJ. Recruitment order of diaphragmatic motor units obeys Henneman's size principle. In: Respiratory Muscles and Their Neuromotor Control, edited by Sieck GC, Gandevia SC, Cameron WE. New York: Liss, 1987, p. 239–247.
- 12.Dick TE, Kong FJ, Berger AJ. Correlation of recruitment order with axonal conduction velocity of supraspinally driven diaphragmatic motor units. J Neurophysiol 57: 245–259, 1987 [DOI] [PubMed] [Google Scholar]
- 13.DiMarco AF. Diaphragmatic pacing. In: Principles and Practices of Mechanical Ventilation (2nd ed.), edited by Tobin MJ. New York: McGraw-Hill, 2006
- 14.DiMarco AF. Neural prostheses in the respiratory system. J Rehabil Res Dev 38: 601–607, 2001 [PubMed] [Google Scholar]
- 15.DiMarco AF. Restoration of respiratory muscle function following spinal cord injury. Review of electrical and magnetic stimulation techniques. Respir Physiol Neurobiol 147: 273–287, 2005 [DOI] [PubMed] [Google Scholar]
- 16.DiMarco AF, Altose MD, Cropp A, Durand D. Activation of the inspiratory intercostal muscles by electrical stimulation of the spinal cord. Am Rev Respir Dis 136: 1385–1390, 1987 [DOI] [PubMed] [Google Scholar]
- 17.DiMarco AF, Budzinska K, Supinski GS. Artificial ventilation by means of electrical activation of the intercostal/accessory muscles alone. Am Rev Respir Dis 139: 961–967, 1989 [DOI] [PubMed] [Google Scholar]
- 18.DiMarco AF, Onders RP, Ignagni A, Kowalski KE, Mortimer JT. Phrenic nerve pacing via intramuscular diaphragm electrodes in tetraplegic subjects. Chest 127: 671–678, 2005 [DOI] [PubMed] [Google Scholar]
- 19.DiMarco AF, Onders RP, Kowalski KE, Miller ME, Ferek S, Mortimer JT. Phrenic nerve pacing in a tetraplegic patient via intramuscular diaphragm electrodes. Am J Respir Crit Care Med 166: 1604–1606, 2002 [DOI] [PubMed] [Google Scholar]
- 20.DiMarco AF, Romaniuk JR, Kowalski KE, Supinski G. Mechanical contribution of expiratory muscles to pressure generation during spinal cord stimulation. J Appl Physiol 87: 1433–1439, 1999 [DOI] [PubMed] [Google Scholar]
- 21.DiMarco AF, Romaniuk JR, Supinski GS. Action of the intercostal muscles on the rib cage. Respir Physiol 82: 295–306, 1990 [DOI] [PubMed] [Google Scholar]
- 22.DiMarco AF, Romaniuk JR, Supinski GS. Parasternal and external intercostal responses to various respiratory maneuvers. J Appl Physiol 73: 979–986, 1992 [DOI] [PubMed] [Google Scholar]
- 23.DiMarco AF, Supinski GS, Budzinska K. Inspiratory muscle interaction in the generation of changes in airway pressure. J Appl Physiol 66: 2573–2578, 1989 [DOI] [PubMed] [Google Scholar]
- 24.DiMarco AF, Supinski GS, Petro JA, Takaoka Y. Evaluation of intercostal muscle pacing to provide artificial ventilation in quadriplegics. Am J Resir Crit Care Med 150: 934–940, 1994 [DOI] [PubMed] [Google Scholar]
- 25.DiMarco AF, Takaoka Y, Kowalski KE. Combined intercostal and diaphragm pacing to provide artificial ventilation in patients with tetraplegia. Arch Phys Med Rehabil 86: 1200–1207, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Donnelly DF, Cohen MI, Sica AL, Zhang H. Responses of early and late onset phrenic motoneurons to lung inflation. Respir Physiol 61: 69–83, 1985 [DOI] [PubMed] [Google Scholar]
- 27.Downman CB. Skeletal muscle reflexes of splanchnic and intercostal nerve origin in acute spinal and decerebrate cats. J Neurophysiol 18: 217–235, 1955 [DOI] [PubMed] [Google Scholar]
- 28.Downman CB, Hussain A. Spinal tracts and supraspinal centres influencing visceromotor and allied reflexes in cats. J Physiol 141: 489–499, 1958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Enoka RM. Activation order of motor axons in electrically evoked contractions. Muscle Nerve 25: 763–764, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Fournier M, Sieck GC. Mechanical properties of muscle units in the cat diaphragm. J Neurophysiol 59: 1055–1066, 1988 [DOI] [PubMed] [Google Scholar]
- 31.Glenn WW. The treatment of respiratory paralysis by diaphragm pacing. Ann Thorac Surg 30: 106–109, 1980 [DOI] [PubMed] [Google Scholar]
- 32.Glenn WW, Hogan JF, Loke JS, Ciesielski TE, Phelps ML, Rowedder R. Ventilatory support by pacing of the conditioned diaphragm in quadriplegia. N Engl J Med 310: 1150–1155, 1984 [DOI] [PubMed] [Google Scholar]
- 33.Glenn WW, Hogan JF, Phelps ML. Ventilatory support of the quadriplegic patient with respiratory paralysis by diaphragm pacing. Surg Clin North Am 60: 1055–1078, 1980 [DOI] [PubMed] [Google Scholar]
- 34.Glenn WW, Phelps ML, Elefteriades JA, Dentz B, Hogan JF. Twenty years of experience in phrenic nerve stimulation to pace the diaphragm. Pacing Clin Electrophysiol 9: 780–784, 1986 [DOI] [PubMed] [Google Scholar]
- 35.Grandjean PA, Mortimer JT. Recruitment properties of monopolar and bipolar epimysial electrodes. Ann Biomed Eng 14: 53–66, 1986 [DOI] [PubMed] [Google Scholar]
- 36.Gregory CM, Bickel CS. Recruitment patterns in human skeletal muscle during electrical stimulation. Phys Ther 85: 358–364, 2005 [PubMed] [Google Scholar]
- 37.Henneman E, Somjen G, Carpenter DO. Functional significance of cell size in spinal motoneurons. J Neurophysiol 28: 560–580, 1965 [DOI] [PubMed] [Google Scholar]
- 38.Hilaire G, Monteau R, Khatib M. Determination of recruitment order of phrenic motoneurons. In: Respiratory Muscles and Their Neuromotor Control, edited by Sieck GC, Gandevia SC, Cameron WE. New York: Liss, 1987, p. 249–261.
- 39.Iscoe S, Dankoff J, Migicovsky R, Polosa C. Recruitment and discharge frequency of phrenic motoneurones during inspiration. Respir Physiol 26: 113–128, 1976 [DOI] [PubMed] [Google Scholar]
- 40.Jodkowski JS, Viana F, Dick TE. Electrical properties of phrenic motoneurons in the cat: correlation with inspiratory drive. J Neurophysiol 58: 105–124, 1987 [DOI] [PubMed] [Google Scholar]
- 41.Lertmanorat Z, Durand DM. Extracellular voltage profile for reversing the recruitment order of peripheral nerve stimulation: a simulation study. J Neural Eng 1: 202–211, 2004 [DOI] [PubMed] [Google Scholar]
- 42.Levy M, Mizrahi J, Susak Z. Recruitment, force and fatigue characteristics of quadriceps muscles of paraplegics isometrically activated by surface functional electrical stimulation. J Biomed Eng 12: 150–156, 1990 [DOI] [PubMed] [Google Scholar]
- 43.Mantilla CB, Sieck GC. Invited review: mechanisms underlying motor unit plasticity in the respiratory system. J Appl Physiol 94: 1230–1241, 2003 [DOI] [PubMed] [Google Scholar]
- 44.Marsolais EB, Kobetic R. Functional walking in paralyzed patients by means of electrical stimulation. Clin Orthop Relat Res 175: 30–36, 1983 [PubMed] [Google Scholar]
- 45.Onders RP, Elmo MJ, Ignagni AR. Diaphragm pacing stimulation system for tetraplegia in individuals injured during childhood or adolescence. J Spinal Cord Med 30: S25–S29, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peterson DK, Nochomovitz M, DiMarco AF, Mortimer JT. Intramuscular electrical activation of the phrenic nerve. IEEE Trans Biomed Eng 33: 342–351, 1986 [DOI] [PubMed] [Google Scholar]
- 47.Peterson DK, Nochomovitz ML, Stellato TA, Mortimer JT. Long-term intramuscular electrical activation of the phrenic nerve: efficacy as a ventilatory prosthesis. IEEE Trans Biomed Eng 41: 1127–1135, 1994 [DOI] [PubMed] [Google Scholar]
- 48.Pette D, Vrbová G. What does chronic electrical stimulation teach us about muscle plasticity? Muscle Nerve 22: 666–677, 1999 [DOI] [PubMed] [Google Scholar]
- 49.Prochazka A. Comparison of natural and artificial control of movement. IEEE Trans Rehabil Eng 1: 7–17, 1993 [Google Scholar]
- 50.Saboisky JP, Gorman RB, De Troyer A, Gandevia SC, Butler JE. Differential activation among five human inspiratory motoneuron pools during tidal breathing. J Appl Physiol 102: 772–780, 2007 [DOI] [PubMed] [Google Scholar]
- 51.Sieck GC. Diaphragm muscle: structural and functional organization. Clin Chest Med 9: 195–210, 1988 [PubMed] [Google Scholar]
- 52.Sieck GC, Trelease RB, Harper RM. Sleep influences on diaphragmatic motor unit discharge. Exp Neurol 85: 316–335, 1984 [DOI] [PubMed] [Google Scholar]
- 53.Weese-Mayer DE, Silvestri JM, Kenny AS, Ilbawi MN, Hauptman SA, Lipton JW, Talonen PP, Garcia HG, Watt JW, Exner G, Baer GA, Elefteriades JA, Peruzzi WT, Alex CG, Harlid R, Vincken W, Davis GM, Decramer M, Kuenzle C, Saeterhaug A, Schöber JG. Diaphragm pacing with a quadripolar phrenic nerve electrode: an international study. Pacing Clin Electrophysiol 19: 1311–1319, 1996 [DOI] [PubMed] [Google Scholar]


