Abstract
Hormone therapy (HT) is a potential treatment to relieve symptoms of menopause and prevent the onset of disease such as osteoporosis in postmenopausal women. We evaluated changes in markers of exercise-induced skeletal muscle damage and inflammation [serum creatine kinase (CK), serum lactate dehydrogenase (LDH), and skeletal muscle mRNA expression of IL-6, IL-8, IL-15, and TNF-α] in postmenopausal women after a high-intensity resistance exercise bout. Fourteen postmenopausal women were divided into two groups: women not using HT (control; n = 6, 59 ± 4 yr, 63 ± 17 kg) and women using traditional HT (HT; n = 8, 59 ± 4 yr, 89 ± 24 kg). Both groups performed 10 sets of 10 maximal eccentric repetitions of single-leg extension on the Cybex dynamometer at 60°/s with 20-s rest periods between sets. Muscle biopsies of the vastus lateralis were obtained from the exercised leg at baseline and 4 h after the exercise bout. Gene expression was determined by RT-PCR for IL-6, IL-8, IL-15, and TNF-α. Blood draws were performed at baseline and 3 days after exercise to measure CK and LDH. Independent t-tests were performed to test group differences (control vs. HT). A probability level of P ≤ 0.05 was used to determine statistical significance. We observed significantly greater changes in mRNA expression of IL-6, IL-8, IL-15, and TNF-α (P ≤ 0.01) in the control group compared with the HT group after the exercise bout. CK and LDH levels were significantly greater after exercise (P ≤ 0.01) in the control group. Postmenopausal women not using HT experienced greater muscle damage after maximal eccentric exercise, indicating a possible protective effect of HT against exercise-induced skeletal muscle damage.
Keywords: creatine kinase, lactate dehydrogenase, tumor necrosis factor-α
hormone therapy (HT) is commonly used in postmenopausal women to treat symptoms of menopause. However, little is known of the effects of HT on skeletal muscle function. A protective effect of endogenous estrogen against exercise-induced skeletal muscle damage may exist, but the effects of HT on exercise-induced skeletal muscle damage following eccentric exercise have not been examined. Previous studies have indicated that estrogen protects skeletal muscle against damage caused by high-intensity resistance exercise (20, 40). In general, decreased levels of creatine kinase (CK) are present in women after eccentric and sustained exercise compared with men (41), indicating a potential protective role of estrogen against muscle damage. Furthermore, women have lower CK levels at rest (25) and after endurance exercise (34).
Minimal evidence exists to demonstrate a protective effect of HT against skeletal muscle damage. Estrogen therapy in postmenopausal women after intense aerobic exercise did not offer a protective effect against exercise-induced skeletal muscle damage (13). Alternatively, a study in young women reported that oral contraceptive use lowers CK levels after eccentric exercise (9). An explanation of potential mechanisms and more conclusive results will help to determine the effects of estrogen/HT on exercise-induced skeletal muscle damage.
High-intensity eccentric exercise is widely used as a method of promoting skeletal muscle damage and is marked by loss of muscular strength, muscle soreness, and elevated blood concentrations of muscle proteins such as CK (11). Activities that involve eccentric muscle actions appear to be particularly disruptive to muscle structure. Evidence of myofibrillar disruption, inability to buffer calcium and Z band streaming in sarcomere structure, occurs in both high-intensity eccentric and concentric muscle actions; however, greater disruptions occur during eccentric muscle action (11). Primary skeletal muscle damage promotes infiltration by inflammatory cells (30, 31) to produce an array of cytokines to regulate the inflammatory process, including IL-6, IL-8, IL-15, and TNF-α (2, 22, 24, 42). These muscle-derived cytokines, commonly referred to as myokines (30), can be directly assessed by sampling muscle tissue to ensure that skeletal muscle damage is present (46). A single bout of exercise promotes increased circulation of IL-6, IL-8, IL-15, and TNF-α (28–31). Serum CK and serum lactate dehydrogenase (LDH) are often analyzed to indirectly assess the degree of muscle damage. High CK and LDH levels are associated with exercise-induced skeletal muscle damage. Elevated levels of CK and LDH following resistance exercise are reported 3–7 days after the exercise bouts (4, 12), including eccentric resistance training. Therefore, by measuring CK and LDH levels we can indirectly assess the degree of skeletal muscle damage in postmenopausal women.
Examining the effects of eccentric resistance exercise on markers of skeletal muscle damage including CK, LDH, and mRNA expression of IL-6, IL-8, IL-15, and TNF-α in postmenopausal women using HT is unique in this population. The purpose of this investigation was to determine whether HT attenuates markers of exercise-induced skeletal muscle damage after maximal eccentric exercise in postmenopausal women. We hypothesized that in postmenopausal women, after a single bout of maximal eccentric exercise, HT attenuates skeletal muscle damage as reflected by decreased markers of exercise-induced muscle damage and inflammation.
METHODS
Study participants.
Fourteen healthy, untrained (no participation in consistent, structured resistance or cardiovascular training) postmenopausal women, 55–65 yr of age, participated in the study. Participants provided written informed consent to protocols approved by the University of Southern California Institutional Review Board (IRB). Participants must have not had spontaneous menstrual bleeding for at least 6 mo. Participants were assigned to either a control group (not taking any form of hormone therapy) or a hormone therapy (HT) group (taking some form of oral hormone therapy for at least 3 mo). Participants not using HT must have been free from drug therapy for at least 3 mo.
Participants were recruited to the HT group if they were using one of the following types of oral HT for at least 3 mo: Premarin (0.3-, 0.45-, 0.625-mg tablet/day), Prempro (0.3-, 0.45-, 0.625-mg tablet/day), Estrace (0.5-, 1.0-mg tablet/day), FemHT (0.5-, 1.0-mg tablet/day), or Menest (0.3-, 0.625-mg tablet/day). Premarin, Menest, and Estrace are synthetic forms of HT containing estrogen only. Prempro and FemHT are synthetic forms of HT containing estrogen and progestin. Oral forms and doses were chosen based on popularity of usage and common doses prescribed as determined by a university gynecologist. Because of the minimal research in HT use and myogenic factors, there was no justification to limit recruitment of HT users to either estrogen alone or estrogen/progestin forms. Additionally, we did not expect the different types of HT to affect the outcomes of the study.
Screening of participants was performed with clinical history, physical exam, and laboratory tests, including complete blood count with differential, coagulation profile, fasting blood glucose, liver and kidney function tests, thyroid-stimulating hormone (TSH), lipid profile, urinalysis, ECG, and chest X-ray. Exclusion criteria included routine use of anti-inflammatory medications, cancer within the last 5 years, diabetes or fasting blood sugar >126 mg/dl, active cirrhosis or hepatitis, uncontrolled hypothyroidism or hyperthyroidism, lung disease limiting or requiring O2, creatinine clearance <30 ml/min, alanine aminotransferase (ALT) > 1.5× upper limit of normal (ULN), cardiovascular abnormalities (myocardial infarction, heart failure, active angina, or ischemia within the last 6 mo), musculoskeletal disease or injury (rheumatoid arthritis, neuropathy, back injury, etc.) that would prevent resistance training, use of testosterone or other anabolic therapies, and use of heparin or coumadin within the last 6 mo.
Experimental design.
The study included five visits: preentry 1 (health screening), preentry 2, visit 3, visit 4, and visit 5. All participants were familiarized with the procedures and equipment and had anthropometric measures made before the acute exercise bout (preentry visit 2). Dietary analysis and physical activity history were assessed (preentry visit 1). The control and HT groups performed 10 sets of 10 repetitions of maximal eccentric knee extensions (visit 4). Muscle biopsies were obtained at baseline (visit 3) and 4 h after exercise (visit 4). Participants fasted at least 6 h before each biopsy.
Anthropometric and body composition testing.
Total body mass, fat mass, fat-free mass, percent fat, and bone mineral density (BMD) were determined with total body dual-energy X-ray absorptiometry (DEXA; model DPX-IQ 2288 with Smart Scan version 4.7e; Lunar Radiation, Madison, WI) during preentry visit 2. Quality assurance was performed with a single acrylic block three times per week to confirm the accuracy and precision of the DEXA system. The same experienced investigator was responsible for performing and analyzing all scans. The coefficient of variation for lean and fat mass for repeated measures in our laboratory is <1%.
Physical activity assessment.
All potential participants were prescreened over the phone by the principal investigator to ensure that they were not participating in regular strenuous physical activity. Physical activity levels were assessed for each participant during preentry visit 1 with the Entry Questionnaire and Physical Activity Scale (1).
Dietary analysis.
The participants completed a 3-day dietary food record provided by the University of Southern California (USC) General Clinical Research Center (GCRC) Bionutrition Department. The participants were instructed to record their dietary intake over the course of 2 weekdays and 1 weekend day. Participants discussed their completed food record with a registered dietician from the Bionutrition Department to determine accuracy in portion size estimates and detail of food intake. Analysis of the dietary record was performed by the registered dietician with Nutritionist Pro (Nutrition Analysis Software version 1.3, Jones and Bartlett Publishers, Sudbury, MA).
Strength testing.
Strength testing was preceded by 5 min of warm-up on a cycle ergometer. Strength testing was performed with the Cybex Norm dynamometer (Cybex International Ronkonkoma, NY). Peak concentric and eccentric torque of the knee extensors were performed with the dominant leg using the Cybex Norm dynamometer at 60°/s. The Cybex was calibrated immediately before the exercise bout. Participants were positioned on the Cybex by visually aligning the placement of the lateral femoral condyle with the axis of rotation for the Cybex. A strap was placed distally on the dominant leg at the level of the load cell. The load cell was positioned 3 cm proximal to the talocural joint. During the maximal loading, a shoulder harness, a hip restraint, and a thigh strap (exercised leg) were used to limit excessive movement and secure the participant to the device. The settings were recorded to ensure exact placement for each exercise bout.
The tester determined the participants' peak eccentric and concentric torque. This was determined to assess whether the participants were generating maximal effort during each set of the acute training bout. Participants received detailed instructions on the eccentric and concentric leg extension exercises and performed no more than five repetitions of each exercise to achieve peak torque. Strength testing was performed once because of recent indications that repeat strength testing may not be necessary to accurately assess maximal strength (39). Participants recorded and orally confirmed a modified Borg soreness scale (5) 2–3 days after the strength testing.
Acute eccentric resistance training bout.
The participants completed one acute bout of single-leg knee extension of maximal eccentric isokinetic loading on the dominant leg with the Cybex Norm dynamometer (Cybex International) at 60°/s during visit 4. The Cybex was calibrated immediately before the exercise bout. Participants were positioned on the Cybex with the settings recorded from the strength testing visit. The exercise protocol consisted of 10 sets of 10 maximal eccentric repetitions, with the participants performing the eccentric component while the investigator performed the concentric component by moving the limb back to the starting position (15° degrees of knee flexion). Each repetition was separated by the time it took the testing investigator to manually return the lever arm back to 15° degrees of knee flexion (i.e., starting position). Each of the 10 sets was separated by 20 s. Biofeedback was provided on the computer monitor, and verbal encouragement was provided by the tester during each maximal contraction. The training bout occurred ∼2 wk after strength testing and 1 wk after the baseline muscle biopsy to allow recovery from soreness. Participants recorded and orally confirmed a modified Borg soreness scale (5) 2–3 days after the training bout.
Blood draws and assays.
Venous blood was collected from the antecubital vein by standard sterile procedures at the USC GCRC and was analyzed by the Los Angeles County-USC Clinical Laboratory for chemistries and blood counts. Two blood draws were performed. Blood draw 1 was performed during preentry visit 1, and blood draw 2 was performed during visit 5. Participants fasted for 6–8 h before each blood draw. CK and LDH were measured with immunoassays in the USC Core Laboratory with an automated immunoassay analyzer (Immulite 1000, Siemans Healthcare Diagnostics, Deerfield, IL).
Muscle biopsies.
Percutaneous muscle biopsy samples (150–200 mg) were obtained from the vastus lateralis of the exercised leg 1 wk before the acute exercise bout (visit 3) and 4 h after exercise (visit 4). Four hours after exercise is an appropriate time point to assess changes in mRNA expression stimulated by exercise (22, 48). Biopsy specimens were collected under sterile conditions and local anesthesia (1% lidocaine) with a 5-mm Stille biopsy needle (Micrins Surgical, Lake Forest, IL) from the midportion of the vastus lateralis muscle. The postexercise biopsy was performed at a distance of 2–4 cm proximal to the first site. Muscle tissue samples were immediately flash-frozen in liquid nitrogen and stored at −80°C until being processed for analysis.
RNA extraction and cDNA synthesis.
Total RNA was isolated after homogenization (Kinematica Polytron PT1200C,) of 30–40 mg of muscle tissue with a monophasic solution including guanidine isothiocyanate-containing lysis buffer and β-mercaptoethanol. The concentration and purity of the RNA were determined with a UV spectrophotometer (NanoDrop ND-1000, Thermo Scientific, Waltham, MA) by measuring absorbance at 260 nm and 280 nm. Five hundred nanograms of total skeletal muscle RNA was reverse transcribed to synthesize cDNA with Taqman reverse transcription reagents according to the manufacturer's instructions (Applied Biosystems, Branchburg, NJ). RNA was reverse transcribed into cDNA with the following temperature/time protocol: 25°C for 10 min, 48°C for 30 min, 95°C for 5 min, and 4°C infinite (Mycycler, Bio-Rad, Hercules, CA).
Oligonucleotide primers for PCR.
Oligonucleotide primers were used to amplify the mRNA expression of IL-6, IL-8, IL-15, and TNF-α. Primer sequences were designed with the Primer3 program (35). The primer sequences for the specific target mRNAs and melt curve temperatures are shown in Table 1. Melt curves were determined for each primer set to ensure amplification of pure PCR products. GAPDH was used as an internal control for detecting changes in mRNA expression with quantitative real-time PCR (qRT-PCR), which was previously used as an internal control to examine myogenic mRNA expression after resistance exercise (3, 18, 21, 23, 32, 44, 45).
Table 1.
Primer sequences used for qRT-PCR
| PCR Primer Sequence 5′→3′ | Melt Curve, °C | Average CT | |
|---|---|---|---|
| IL-6 | F: CTA TGA ACT CCT TCT CCA CAA CGC CTT | 82 | 21.3 |
| R: GGG GCG GCT ACA TCT TTG GAA TCT T | |||
| IL-8 | F: GCT CTG TGT GAA GGT GCA GTT TTG CCA A | 82 | 20.5 |
| R: GGC GCA GTG TGG TCC ACT CTC AAT | |||
| IL-15 | F: CCG TGG CTT TGA GTA ATG AGA ATT TCG AA | 79 | 20.9 |
| R: CCT GCA CTG AAA CAG CCC AAA ATG AA | |||
| TNF-α | F: CCC AGG CAG TCA GAT CAT CTT CTC GAA | 85 | 19.6 |
| R: CTG GTT ATC TCT CAG CTC CAC GCC ATT | |||
| GAPDH | F: AGC CAC ATC GCT CAG ACA | 79 | 18.3 |
| R: GCC CAA TAC GAC CAA ATC C |
qRT-PCR, quantitative real-time PCR; F, forward; R, reverse; CT, cycle threshold.
Quantitative real-time PCR.
A qRT-PCR method was applied to determine relative expression levels of mRNAs for IL-6, IL-8, IL-15, and TNF-α. A total of 10 ng of cDNA was added to each of the 20-μl PCR reactions for IL-6, IL-8, IL-15, TNF-α, and GAPDH. Specifically, each PCR reaction contained the following mixture: 10 μl of 2.5× iQSYBRgreen supermix (Bio-Rad), 7 μl of RNase-free water, 10 ng of cDNA, and 10 pmol of each primer (forward and reverse) of interest. All samples were run in quadruplicate. Each PCR reaction was amplified with the Bio-Rad iCycler iQ thermal cycler (Bio-Rad). Thermal cycling conditions were specified by the manufacturer.
mRNA quantification.
All data were determined by normalizing the cDNA measured in eight replicates (4 replicates repeated) for each participant sample to GAPDH (internal control) and then averaging the data to account for the change in mRNA expression as a result of the exercise stimulus. The data were then normalized to biopsy 1 (preexercise) for each participant to determine the fold change of mRNA expression after exercising. All participant samples were then averaged to determine the average mRNA expression before and after exercise for each group.
To test PCR efficiency, serial dilutions (1, 0.5, 0.250, 0.125, 0.062, 0.031) of cDNA for GAPDH and each gene of interest (GOI) were amplified by RT-PCR using gene-specific primers. Efficiency [efficiency = 10(1/slope)−1] was calculated with the slope of the standard curve for each respective gene (17). The PCR efficiencies are 99% for GAPDH; 97% for IL-6, IL-8, and TNF-α; and 98% for IL-15. mRNA expression was evaluated by a relative quantification method (48). Briefly, this method is based on the fact that the difference in threshold cycles (ΔCT) between the GOI and the internal control genes (ICG) is proportional to the relative expression level of the GOI. The data were analyzed by using the 2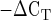 method (36) to compare relative mRNA expression (arbitrary units) between preexercise and postexercise biopsies, referred to as the fold change in mRNA expression.
method (36) to compare relative mRNA expression (arbitrary units) between preexercise and postexercise biopsies, referred to as the fold change in mRNA expression.
Statistical methods.
Statistical analyses were performed with SPSS version 16.0 (SPSS, Chicago, IL). Descriptive statistics and Pearson correlations were used to analyze the control and HT groups. Independent t-tests were performed to test group differences (control vs. HT). A probability level of P ≤ 0.05 was used to determine statistical significance. Group differences in mRNA expression were determined by repeated-measures analysis of covariance (ANCOVA) with weight, body mass index (BMI), BMD, bone mineral content (BMC), percent fat, fat mass, lean mass, protein intake, and carbohydrate intake as covariates. A priori analysis determined that a sample size of six participants in each group would have 80% power to detect a difference in means for MyoD mRNA expression of 2.0, assuming that the common standard deviation is 1.0 with a two-group t-test, with an effect size of 2.0 using a 0.05 two-sided significance level (32). We chose to determine our power analysis on the basis of changes in mRNA expression of MyoD based on MyoD mRNA expression previously examined in older women (32).
RESULTS
Baseline characteristics of the study participants are displayed in Table 2. The study results indicated that weight and BMI were significantly greater in the HT group compared with the control group (P < 0.05). There were no statistically significant differences between groups with dietary intake and muscle strength. One-repetition maximum (1-RM) strength testing values and peak torque generated during the acute training bout were not significantly different between the groups. There were no significant group differences in perceived muscle soreness. Average ratings of perceived muscle soreness for both groups were 5 (strong perceived soreness) out of 10 for the 1-RM testing and 8 (very strong perceived soreness) out of 10 for the acute exercise bout.
Table 2.
Participant characteristics
| Control (n = 6) | HT (n = 8) | |
|---|---|---|
| Age, yr | 59.2±4.2 | 58.5±3.7 |
| Height, cm | 160.4±9.0 | 163.8±3.0 |
| Weight, kg | 63.1±17.4 | 89.5±23.7* |
| BMI, kg/cm2 | 35.4±11.5 | 47.2±11.9 |
| Percent fat | 34.1±13.6 | 45.1±7.9 |
| LBM, kg | 41.6±4.3 | 49.1±5.0* |
| Estrogen, pg/ml | 54.7±5.3 | 392.6±81.0* |
| Eccentric peak torque, Nm | 108.7±52.9 | 132.7±58.6 |
| Concentric peak torque, Nm | 75.3±19.9 | 84.4±28.7 |
| Peak torque†, Nm | 101.3±17.7 | 118.3±32.4 |
Data are means ± SD for n subjects. HT, hormone therapy; BMI, body mass index; LBM, lean body mass.
Significantly different from control group, P < 0.05;
measured during acute exercise.
Although there were group differences in weight and lean mass, our results from ANCOVA indicated that participant characteristics were not significant covariates. Therefore, the data presented reflect results from independent t-tests for all outcome variables: CK, LDH, IL-6, IL-8, IL-15, and TNF-α. Pre- and postexercise values for serum CK and LDH are displayed in Fig. 1. There were no significant differences in CK and LDH levels at rest between the two groups (P > 0.05). After exercise, CK and LDH significantly increased in the control group compared with baseline levels (P < 0.001). After exercise, CK and LDH were not significantly elevated in the HT group compared with baseline levels (P > 0.001). CK and LDH were significantly different in the two groups after exercise (P < 0.001). The study results show significant increases from baseline in mRNA expression of TNF-α, IL-6, IL-8, and IL-15 in both the control and HT groups after exercise (P < 0.001, Fig. 2). TNF-α, IL-6, IL-8, and IL-15 mRNA expression were significantly greater in the control group compared with the HT group (P < 0.01).
Fig. 1.
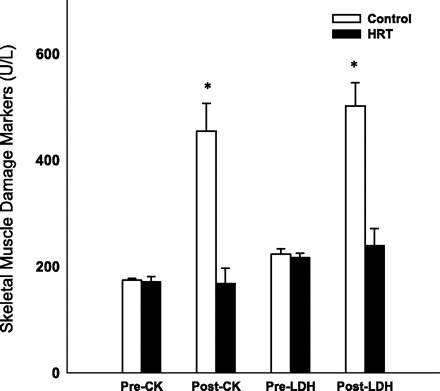
Changes in creatine kinase (CK) and lactate dehydrogenase (LDH) following exercise. Pre-, before exercise; Post-, after exercise. *Significantly different from baseline and hormone therapy (HRT) group, P < 0.05.
Fig. 2.
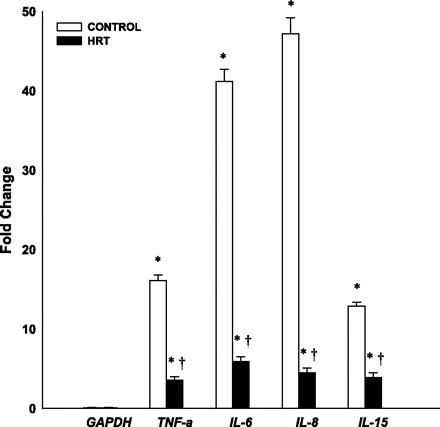
Cytokine mRNA expression at baseline and 4 h after exercise, normalized to GAPDH. Values are means ± SE. *Significantly different from baseline, P < 0.01; †significantly different from control group, P < 0.01.
DISCUSSION
We measured changes in markers of exercise-induced skeletal muscle damage including serum CK and LDH and mRNA expression of IL-6, IL-8, IL-15, and TNF-α in skeletal muscle following a maximal bout of eccentric exercise in postmenopausal women to determine whether HT provides a protective effect on exercise-induced skeletal muscle damage. In support of our hypothesis, after a maximal bout of eccentric resistance exercise HT attenuated skeletal muscle damage as indicated by decreased levels of serum CK and LDH and mRNA expression of IL-6, IL-8, IL-15, and TNF-α compared with postmenopausal women not using HT. Our study is the first investigation to examine exercise-induced skeletal muscle damage in postmenopausal women using HT by directly assessing mRNA expression in skeletal muscle tissue.
A potential protective role of estrogen against muscle damage has been proposed (20) and supported by the findings in our study with decreased levels of serum CK and LDH and mRNA expression of IL-6, IL-8, IL-15, and TNF-α in postmenopausal women using HT. This protective role of estrogen may be the result of an interaction of three processes: 1) high antioxidant capacity, 2) membrane stabilizing properties, and 3) gene regulation (20). The effects of synthetic estrogens or HT on skeletal muscle damage have received minimal attention. Dobridge and Hackney demonstrated that postmenopausal women taking HT had elevated CK levels similar to those not taking HT after eccentric exercise, indicating a lack of a protective effect of estrogen on muscle damage (13).
Our study is not in accordance with Dobridge and Hackney (13) because our findings demonstrated significantly elevated levels of serum CK and LDH in the control group, where extremely low levels of estrogen were present. The HT group did not have elevated levels of CK and LDH, indicating the presence of a potential protective effect of estrogen against muscle damage. Since the HT group had greater lean mass than the control group, it may be possible that the HT group was stronger and the exercise protocol was not sufficient to induce muscle damage. However, as shown by the 1-RM data and peak torque produced during the exercise bout, the two groups were similar in strength and reported the same degree of muscle soreness. A potential limitation to our study was the discrepancy in body mass between groups, and future studies may need to consider matching the groups for body mass.
Dobridge and Hackney (13) investigated estrogen use in nine postmenopausal women who performed a 30-min eccentric bout of treadmill running with a negative 10% grade at 70% maximal heart rate while taking HT and again after a 45-day washout period. There are two concerns with this study design, including an inadequate washout period and the use of an exercise protocol that may not be sufficient to induce muscle damage. The protocol employed in our study, 100 maximal eccentric knee extensions, has been used previously by our laboratory to successfully promote muscle damage (14, 19), unlike the protocol used by Dobridge and Hackney (13). Our study provides the first evidence of estrogen's protective effect on muscle damage in two different groups of postmenopausal women with and without HT use.
Additionally, we measured markers of skeletal muscle damage directly from muscle tissue by examining mRNA expression of TNF-α, IL-6, IL-8, and IL-15 to accurately assess the effects of HT on skeletal muscle tissue. Cytokines released from muscle including IL-6, IL-8, IL-15, and TNF-α, more recently termed myokines, appear in skeletal muscle before inflammatory cells arrive (30). IL-6 mRNA expression increases after muscle contraction irrespective of glycogen depletion (22, 29, 37), as further indicated by our study, specifically to a greater degree in postmenopausal women not using HT. IL-6 may play a metabolic role, influencing glycogen levels in muscle (15). Specifically, IL-6 may increase glucose uptake by increasing GLUT4 translocation (8), and this relationship may be enhanced after eccentric exercise in postmenopausal women not using HT. The role of IL-8 in skeletal muscle after exercise remains unclear, yet it may play an angiogenic role by interacting with the chemokine receptor CXCR2 in capillaries to increase capillarization (6). Several studies have shown that IL-8 immediately increases after resistance exercise (2, 22, 28), similar to our study. The effect of eccentric resistance exercise on IL-8 mRNA expression was enhanced in postmenopausal women not using HT, which may lead to greater angiogenic effects compared with postmenopausal women using HT.
IL-15 blunts proteolysis and apoptosis in skeletal muscle in animal and human models (7, 16). Limited data exist on IL-15 activity in response to exercise. Nieman et al. (27) failed to observe any change in IL-15 mRNA expression from skeletal muscle tissue immediately after resistance exercise (10). However, our study demonstrated increased mRNA expression of IL-15 4 h after exercise consisting of 10 sets of 10 maximal repetitions of eccentric knee extension contractions in both study groups. This supports our contention that we employed an appropriate protocol to induce skeletal muscle damage following an acute high-intensity resistance exercise bout. Nieman et al. (27) utilized a resistance exercise protocol of moderate intensity, compared with our protocol, that included 4 sets of 10 repetitions at 40–60% of the subject's 1-RM. Our exercise protocol was extremely strenuous on the quadriceps muscle group; therefore, muscle biopsies of the vastus lateralis were appropriate to demonstrate change in mRNA expression. The exercise protocol by Nieman et al. (27) involved both upper and lower body exercises and may not have provided a great enough stimulus to the vastus lateralis, also the sampling site for their muscle biopsies. However, a potential limitation of our exercise protocol was the use of exclusively maximal eccentric actions that are not traditionally performed, unlike the protocol used by Nieman et al. (27).
TNF-α is associated with trauma to the muscle and leads to muscle catabolism in human models (33). TNF-α mRNA modestly elevates with resistance exercise (22, 27, 38) and plays a role in initiating the breakdown and removal of damaged muscle fragments (43). Strenuous physical activity, including eccentric contractions, induces an inflammatory response that elevates TNF-α in skeletal muscle tissue (22, 26, 47). The increased levels of TNF-α mRNA expression following exercise in the postmenopausal women not using HT provides direct evidence that skeletal muscle damage occurred in response to the eccentric exercise bout. The increased mRNA expression of TNF-α supports our additional myokine findings that HT attenuates exercise-induced skeletal muscle damage in postmenopausal women.
Our findings demonstrate that HT use in postmenopausal women attenuated exercise-induced skeletal muscle damage measured in both serum and mRNA expression of skeletal muscle tissue. However, this study further emphasizes the need for additional research to determine specific benefits and/or risks of decreased markers of skeletal muscle damage as a result of HT use. The attenuation of skeletal muscle damage may play an inhibitory role in the natural inflammatory and immune response involved in the repair process following damage to skeletal muscle. This would delay the repair process and increase the time for return to normal function. The attenuation of skeletal muscle damage with HT use affected myokines normally involved in the inflammatory process, as indicated by the decreased levels of mRNA expression of TNF-α, IL-6, IL-8, and IL-15. These myokines serve other important functions in skeletal muscle including glucose uptake, angiogenesis, and myogenesis, which may be negatively affected by attenuation from HT use. Conversely, HT use may protect skeletal muscle from degeneration to prevent sarcopenia, which may minimize strength loss, risk of falls, and incidence of fractures. Long-term intervention studies focusing on HT use and resistance exercise are necessary to examine extended benefits on muscle growth and degeneration.
In conclusion, we determined that a single bout of maximal eccentric resistance exercise induced skeletal muscle damage in postmenopausal women not using HT and to a lesser extent in women using HT, as indicated by elevated serum levels of CK and LDH and mRNA expression of TNF-α, IL-6, IL-8, and IL-15. Postmenopausal women using HT experienced decreased elevation of markers of skeletal muscle damage, emphasizing a potential protective effect of HT against exercise-induced muscle damage. Overall, our study presents unique findings that provide insight into the effects of eccentric resistance exercise and HT use on exercise-induced skeletal muscle damage in postmenopausal women.
REFERENCES
- 1.Aadahl M, Jorgensen T. Validation of a new self-report instrument for measuring physical activity. Med Sci Sports Exerc 35: 1196–1202, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Akerstrom T, Steensberg A, Keller P, Keller C, Penkowa M, Pedersen BK. Exercise induces interleukin-8 expression in human skeletal muscle. J Physiol 563: 507–516, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 3.Barber RD, Harmer DW, Coleman RA, Clark BJ. GAPDH as a housekeeping gene: analysis of GAPDH mRNA expression in a panel of 72 human tissues. Physiol Genomics 21: 389–395, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Beck TW, Housh TJ, Johnson GO, Schmidt RJ, Housh DJ, Coburn JW, Malek MH, Mielke M. Effects of a protease supplement on eccentric exercise-induced markers of delayed-onset muscle soreness and muscle damage. J Strength Cond Res 21: 661–667, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc 14: 377–381, 1982 [PubMed] [Google Scholar]
- 6.Brodal P, Ingjer F, Hermansen L. Capillary supply of skeletal muscle fibers in untrained and endurance-trained men. Am J Physiol Heart Circ Physiol 232: H705–H712, 1977 [DOI] [PubMed] [Google Scholar]
- 7.Busquets S, Figueras MT, Meijsing S, Carbo N, Quinn LS, Almendro V, Argiles JM, Lopez-Soriano FJ. Interleukin-15 decreases proteolysis in skeletal muscle: a direct effect. Int J Mol Med 16: 471–476, 2005 [PubMed] [Google Scholar]
- 8.Carey AL, Steinberg GR, Macaulay SL, Thomas WG, Holmes AG, Ramm G, Prelovsek O, Hohnen-Behrens C, Watt MJ, James DE, Kemp BE, Pedersen BK, Febbraio MA. Interleukin-6 increases insulin-stimulated glucose disposal in humans and glucose uptake and fatty acid oxidation in vitro via AMP-activated protein kinase. Diabetes 55: 2688–2697, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Carter A, Dobridge J, Hackney AC. Influence of estrogen on markers of muscle tissue damage following eccentric exercise. Fiziol Cheloveka 27: 133–137, 2001 [PubMed] [Google Scholar]
- 10.Chan MH, Carey AL, Watt MJ, Febbraio MA. Cytokine gene expression in human skeletal muscle during concentric contraction: evidence that IL-8, like IL-6, is influenced by glycogen availability. Am J Physiol Regul Integr Comp Physiol 287: R322–R327, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Clarkson PM, Hubal MJ. Exercise-induced muscle damage in humans. Am J Phys Med Rehabil 81: S52–S69, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Costa A, Dalloul H, Hegyesi H, Apor P, Csende Z, Racz L, Vaczi M, Tihanyi J. Impact of repeated bouts of eccentric exercise on myogenic gene expression. Eur J Appl Physiol 101: 427–436, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Dobridge JD, Hackney AC. The effects of estrogen on indices of skeletal muscle tissue damage after eccentric exercise in postmenopausal women. Fiziol Cheloveka 30: 98–102, 2004 [PubMed] [Google Scholar]
- 14.Dreyer HC, Blanco CE, Sattler FR, Schroeder ET, Wiswell RA. Satellite cell numbers in young and older men 24 hours after eccentric exercise. Muscle Nerve 33: 242–253, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Febbraio MA, Pedersen BK. Muscle-derived interleukin-6: mechanisms for activation and possible biological roles. FASEB J 16: 1335–1347, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Figueras M, Busquets S, Carbo N, Barreiro E, Almendro V, Argiles JM, Lopez-Soriano FJ. Interleukin-15 is able to suppress the increased DNA fragmentation associated with muscle wasting in tumour-bearing rats. FEBS Lett 569: 201–206, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Ginzinger DG. Gene quantification using real-time quantitative PCR: an emerging technology hits the mainstream. Exp Hematol 30: 503–512, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Jemiolo B, Trappe S. Single muscle fiber gene expression in human skeletal muscle: validation of internal control with exercise. Biochem Biophys Res Commun 320: 1043–1050, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Jensky NE, Sims JK, Rice JC, Dreyer HC, Schroeder ET. The influence of eccentric exercise on mRNA expression of skeletal muscle regulators. Eur J Appl Physiol 101: 473–480, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Kendall B, Eston R. Exercise-induced muscle damage and the potential protective role of estrogen. Sports Med 32: 103–123, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Kubica N, Kimball SR, Jefferson LS, Farrell PA. Alterations in the expression of mRNAs and proteins that code for species relevant to eIF2B activity after an acute bout of resistance exercise. J Appl Physiol 96: 679–687, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Louis E, Raue U, Yang Y, Jemiolo B, Trappe S. Time course of proteolytic, cytokine, and myostatin gene expression after acute exercise in human skeletal muscle. J Appl Physiol 103: 1744–1751, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Mahoney DJ, Carey K, Fu MH, Snow R, Cameron-Smith D, Parise G, Tarnopolsky MA. Real-time RT-PCR analysis of housekeeping genes in human skeletal muscle following acute exercise. Physiol Genomics 18: 226–231, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Malm C. Exercise-induced muscle damage and inflammation: fact or fiction? Acta Physiol Scand 171: 233–239, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Meltzer HY. Factors affecting serum creatine phosphokinase levels in the general population: the role of race, activity and age. Clin Chim Acta 33: 165–172, 1971 [DOI] [PubMed] [Google Scholar]
- 26.Miles JL, Huber K, Thompson NM, Davison M, Breier BH. Moderate daily exercise activates metabolic flexibility to prevent prenatally induced obesity. Endocrinology 150: 179–186, 2009 [DOI] [PubMed] [Google Scholar]
- 27.Nieman DC, Davis JM, Brown VA, Henson DA, Dumke CL, Utter AC, Vinci DM, Downs MF, Smith JC, Carson J, Brown A, McAnulty SR, McAnulty LS. Influence of carbohydrate ingestion on immune changes after 2 h of intensive resistance training. J Appl Physiol 96: 1292–1298, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Nieman DC, Davis JM, Henson DA, Walberg-Rankin J, Shute M, Dumke CL, Utter AC, Vinci DM, Carson JA, Brown A, Lee WJ, McAnulty SR, McAnulty LS. Carbohydrate ingestion influences skeletal muscle cytokine mRNA and plasma cytokine levels after a 3-h run. J Appl Physiol 94: 1917–1925, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Ostrowski K, Rohde T, Zacho M, Asp S, Pedersen BK. Evidence that interleukin-6 is produced in human skeletal muscle during prolonged running. J Physiol 508: 949–953, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pedersen BK, Akerstrom TC, Nielsen AR, Fischer CP. Role of myokines in exercise and metabolism. J Appl Physiol 103: 1093–1098, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Petersen AM, Pedersen BK. The anti-inflammatory effect of exercise. J Appl Physiol 98: 1154–1162, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Raue U, Slivka D, Jemiolo B, Hollon C, Trappe S. Myogenic gene expression at rest and after a bout of resistance exercise in young (18–30 yr) and old (80–89 yr) women. J Appl Physiol 101: 53–59, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Reid MB, Li YP. Cytokines and oxidative signalling in skeletal muscle. Acta Physiol Scand 171: 225–232, 2001 [DOI] [PubMed] [Google Scholar]
- 34.Rogers MA, Stull GA, Apple FS. Creatine kinase isoenzyme activities in men and women following a marathon race. Med Sci Sports Exerc 17: 679–682, 1985 [DOI] [PubMed] [Google Scholar]
- 35.Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol 132: 365–386, 2000 [DOI] [PubMed] [Google Scholar]
- 36.Schmittgen TD, Zakrajsek BA. Effect of experimental treatment on housekeeping gene expression: validation by real-time, quantitative RT-PCR. J Biochem Biophys Methods 46: 69–81, 2000 [DOI] [PubMed] [Google Scholar]
- 37.Steensberg A, Febbraio MA, Osada T, Schjerling P, van Hall G, Saltin B, Pedersen BK. Interleukin-6 production in contracting human skeletal muscle is influenced by pre-exercise muscle glycogen content. J Physiol 537: 633–639, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Steensberg A, Keller C, Starkie RL, Osada T, Febbraio MA, Pedersen BK. IL-6 and TNF-alpha expression in, and release from, contracting human skeletal muscle. Am J Physiol Endocrinol Metab 283: E1272–E1278, 2002 [DOI] [PubMed] [Google Scholar]
- 39.Symons TB, Vandervoort AA, Rice CL, Overend TJ, Marsh GD. Effects of maximal isometric and isokinetic resistance training on strength and functional mobility in older adults. J Gerontol A Biol Sci Med Sci 60: 777–781, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Taaffe DR, Sipila S, Cheng S, Puolakka J, Toivanen J, Suominen H. The effect of hormone replacement therapy and/or exercise on skeletal muscle attenuation in postmenopausal women: a yearlong intervention. Clin Physiol Funct Imaging 25: 297–304, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Thompson HS, Scordilis SP, De Souza MJ. Serum creatine kinase activity varies with ovulatory status in regularly exercising, premenopausal women. Horm Res 65: 151–158, 2006 [DOI] [PubMed] [Google Scholar]
- 42.Tidball JG. Inflammatory cell response to acute muscle injury. Med Sci Sports Exerc 27: 1022–1032, 1995 [DOI] [PubMed] [Google Scholar]
- 43.Tidball JG. Inflammatory processes in muscle injury and repair. Am J Physiol Regul Integr Comp Physiol 288: R345–R353, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Touchberry CD, Wacker MJ, Richmond SR, Whitman SA, Godard MP. Age-related changes in relative expression of real-time PCR housekeeping genes in human skeletal muscle. J Biomol Tech 17: 157–162, 2006 [PMC free article] [PubMed] [Google Scholar]
- 45.Tricarico C, Pinzani P, Bianchi S, Paglierani M, Distante V, Pazzagli M, Bustin SA, Orlando C. Quantitative real-time reverse transcription polymerase chain reaction: normalization to rRNA or single housekeeping genes is inappropriate for human tissue biopsies. Anal Biochem 309: 293–300, 2002 [DOI] [PubMed] [Google Scholar]
- 46.Warren GL, Lowe DA, Armstrong RB. Measurement tools used in the study of eccentric contraction-induced injury. Sports Med 27: 43–59, 1999 [DOI] [PubMed] [Google Scholar]
- 47.Yamin C, Duarte JA, Oliveira JM, Amir O, Sagiv M, Eynon N, Amir RE. IL6 (-174) and TNFA (-308) promoter polymorphisms are associated with systemic creatine kinase response to eccentric exercise. Eur J Appl Physiol 104: 579–586, 2008 [DOI] [PubMed] [Google Scholar]
- 48.Yang Y, Creer A, Jemiolo B, Trappe S. Time course of myogenic and metabolic gene expression in response to acute exercise in human skeletal muscle. J Appl Physiol 98: 1745–1752, 2005 [DOI] [PubMed] [Google Scholar]


