Abstract
Polarized and pseudostratified primary airway epithelia present barriers that significantly reduce their transfection efficiency and the efficacy of RNA interference oligonucleotides. This creates an impediment in studies of the airway epithelium, diminishing the utility of loss-of-function as a research tool. Here we outline methods to introduce RNAi oligonucleotides into primary human and porcine airway epithelia grown at an air-liquid interface and difficult-to-transfect transformed epithelial cell lines grown on plastic. At the time of plating, we reverse transfect small-interfering RNA (siRNA), Dicer-substrate siRNA, or microRNA oligonucleotides into cells by use of lipid or peptide transfection reagents. Using this approach we achieve significant knockdown in vitro of hypoxanthine-guanine phosphoribosyltransferase, IL-8, and CFTR expression at the mRNA and protein levels in 1–3 days. We also attain significant reduction of secreted IL-8 in polarized primary pig airway epithelia 3 days posttransfection and inhibition of CFTR-mediated Cl− conductance in polarized air-liquid interface cultures of human airway epithelia 2 wk posttransfection. These results highlight an efficient means to deliver RNA interference reagents to airway epithelial cells and achieve significant knockdown of target gene expression and function. The ability to reliably conduct loss-of-function assays in polarized primary airway epithelia offers benefits to research in studies of epithelial cell homeostasis, candidate gene function, gene-based therapeutics, microRNA biology, and targeting the replication of respiratory viruses.
Keywords: primary airway epithelium, transfection, gene silencing, RNA interference, siRNA, air-liquid interface, porcine airway epithelial cells
small interfering RNAs (siRNA) mediate posttranscriptional inhibition of targeted genes, offering a novel approach to achieve specific silencing in the airway epithelium. These approaches have broad applications for studies of cell biology, disease pathogenesis, and therapeutics. Primary cultures of airway epithelia have emerged as a powerful model to study epithelial cell biology and the impact of diseases on tissue function (18, 22, 39). Cells grown at the air-liquid interface (ALI) form a polarized, pseudostratified columnar epithelium that closely resembles the morphology of the in vivo surface epithelium of the conducting airways (2, 18, 24, 33, 63, 64), providing an opportunity to study cell biology, disease pathogenesis, and treatments (66).
Histological studies have shown that ALI cultures of primary airways grown for 4 wk or more form a well-differentiated epithelium resembling the in vivo architecture (12, 56). However, another study has observed that airway epithelia differentiated and formed an apical surface covered by cilia within 10 days after seeding (66). Karp and coworkers (32) demonstrated that ALI cultures of primary airway epithelia develop transepithelial electrical resistance within 3 days after seeding and changed little over the next 11 days, indicating that the development of tight junctions and a barrier function occur before the development of a fully differentiated ciliated phenotype. Furthermore, they report that, at 14 days postseeding, 90% of the cells expressed markers that are produced only by mature columnar epithelia (32, 66). This suggests that a polarized phenotype with a clear distinction of apical and basolateral domains develops by 1 wk postseeding, before complete differentiation of the epithelium occurs. Culture methods and seeding/maintenance media compositions vary between laboratories, possibly accounting for contrasting reports on differentiation times. The culture methods used herein (32, 66) result in a differentiation time line as described in Fig. 1A. Primary airway epithelial cells cultured by these methods (32, 66) yield a ciliated, polarized, pseudostratified epithelium 14 days postseeding (No Treatment, Fig. 1B). These cultures are similar in morphology and bioelectric properties to 28-day ALI cultures (51). When transfected by methods described in this study, either with single- (NC ssRNA) or double-stranded (NC dsRNA, CFTR DsiRNA) oligonucleotides, ALI cultures of primary airway epithelia form a polarized, pseudostratified epithelium by 14 days postseeding (Fig. 1B). These cells are also morphologically similar to cells transfected with similar reagents at the time of seeding and maintained in ALI culture for 28 days (51).
Fig. 1.
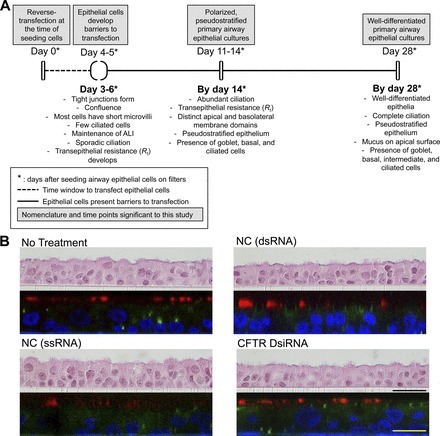
Primary airway epithelial cells form a polarized, pseudostratified epithelium 14 days postseeding. A: schematic highlighting time course and morphological characteristics of airway epithelial cell differentiation at the air-liquid interface (32, 66). B: primary airway epithelial cells from 2 different non-cystic fibrosis (CF) human donors were transfected with the mentioned oligonucleotides at a final concentration of 100 nM. At 14 days posttransfection, epithelial sheets on filters were either fixed, embedded in paraffin, sectioned, and stained with hematoxylin and eosin (H&E), or immunostained and visualized by confocal microscopy. Presented here are representative images of epithelial sheets on filters: vertical section images of H&E-stained epithelia (top panels), and X-Z section images of immunostained epithelia visualized by confocal microscopy (bottom panels). NC dsRNA, negative control, double-stranded oligonucleotide; NC ssRNA, negative control, single-stranded oligonucleotide; DsiRNA, dicer-substrate siRNA. Immunohistochemistry: green, zonula occludens-1; red, anti-β-tubulin (cilia); blue, ToPro3 (nuclear stain). N = 2 non-CF donors, 2 cultures per donor. Scale bars: black (25 μM); yellow (50 μM).
Through gain and loss of function experiments, investigators have the opportunity to investigate the role of specific gene products in biological processes. However, the pseudostratified epithelium presents significant barriers to the delivery of nucleic acid based reagents such as plasmids (17, 65), double-stranded oligonucleotides (9, 23, 36, 50), and single-stranded oligonucleotides (9, 23). It is evident that physical barriers, airway secretions, and host defense mechanisms (9, 17, 23, 49, 62) significantly hinder the delivery and efficacy of the siRNA reagents. The mucosal surface of the airways and the alveoli elicits immunogenic responses to RNA interference (RNAi) oligonucleotides that confound results (13, 31, 35, 45, 54), and some animal studies attempting to deliver siRNAs to the respiratory tract have reported little efficacy (23).
In a previous study, we reported that primary pig and human airway epithelia grown at the ALI develop barriers for the entry of dicer-substrate siRNA (DsiRNA) into cells by 5 days postseeding, thus prevent silencing of cellular targets (36). This presents a significant impediment to the use of polarized, pseudostratified primary airway epithelia as a model system. Although viral vectors offer one approach to overcome such limitations, we focus here on synthetic nucleic acid delivery. In some experimental settings delivery of oligonucleotide reagents is desirable, straightforward (RNAi reagents are introduced directly into the cytoplasm) and may avoid cellular responses to virus components.
Here we outline an efficient and readily applied technique to deliver oligonucleotide reagents to primary epithelia to achieve loss of gene function that persists into polarized, pseudostratified epithelia. This simple approach uses a technique termed reverse transfection, in which the reagent complexes are added to cells at the time of seeding. This method has wide applications for in vitro studies of epithelial cell function in cells from humans and animal models.
MATERIALS AND METHODS
Introduction of RNAi oligonucleotides into airway epithelial cells to mediate potent gene specific silencing.
The following protocol, briefly summarized in Fig. 2, was developed to transfect double-stranded oligonucleotides (siRNAs, DsiRNA, microRNA mimics), and single-stranded oligonucleotides (anti-microRNAs, antisense oligonucleotides) into human and pig primary airway epithelial cells. The protocol utilizes reverse transfection. In contrast to standard transfection, in which transfection complexes are added to preplated cells, reverse transfection is performed by adding the cells and the transfection complexes into the wells essentially at the same time. We have successfully used this protocol in difficult-to-transfect cell lines including CFBE41o− cells (37, 53), Calu-3 cells (51, 53), and T84 cells. Although not formally tested, we expect this approach to be applicable also to mouse epithelial cells and other difficult-to-transfect primary cells and cell lines. Two popular sizes of permeable supports for growing epithelial cells at the ALI have surface areas of 0.33 and 0.6 cm2. Owing to their size differences, we present two methods (methods 1 and 2, Fig. 2) that have been standardized by us to account for increased cell density and transfection volume. We also present methods (Fig. 2) validated for two separate transfection reagents (methods 1 and 2: Lipofectamine RNAiMAX; method 3: Transductin) (16), providing investigators with several options to adapt this protocol to their cell types of interest.
Fig. 2.
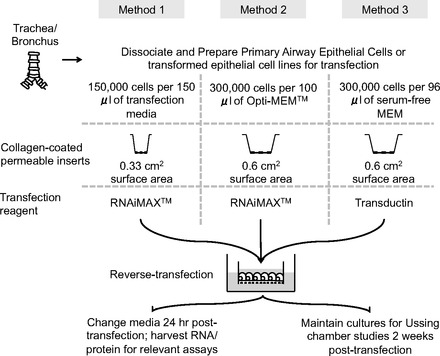
Schematic highlighting the protocol steps required to transfect primary airway epithelial cells with oligonucleotides.
Before transfection, we prepare the precoated inserts by adding 100 μl of collagen (human placental collagen Type VI, Sigma-Aldrich) to the apical surface of Transwell permeable supports (0.33-cm2 0.4-μm polyester membrane, Costar product no. 3470, Corning, Corning, NY). For larger Millicell cell culture inserts (0.6-cm2, 3.0 μm polycarbonate, Millipore polycarbonate filters, Millipore, Bedford, MA), we add 250 μl of collagen (human placental collagen Type VI, Sigma-Aldrich) to the apical surface (33). We then incubate the cultures for a minimum of 4 h at 37°C or store at room temperature until needed. Transfections can also be performed in the wells of 96- and 48-well plates with or without collagen coating. Collagen coating is not necessary for harvesting RNA or protein posttransfection. To prepare for transfection, cells growing on plastic are dissociated with 0.25% trypsin (Life Technologies) and washed with transfection media twice to remove antibiotics. Alternatively, airway epithelial cells from native tissue (trachea and bronchus) can be dissociated by pronase enzyme digestion (33).
Method 1: For 0.33-cm2 inserts or wells in a 96-well plate, wash cells twice in transfection media and resuspend at a density of 150,000 cells per 150 μl of transfection media. Transfection medium is prepared by using DMEM/F-12 medium supplemented with 10% FBS. Method 2: For 0.6-cm2 inserts or wells in a 48-well plate, wash cells twice in Opti-MEM (Life Technologies) and resuspend at a density of 300,000 cells per 100 μl of Opti-MEM. Method 3: When using the transfection reagent Transductin (16) for 0.6-cm2 inserts or wells in a 48-well plate, wash cells twice with serum-free MEM (Sigma-Aldrich) and resuspend at a density of 300,000 cells per 96 μl of serum-free MEM. To prepare inserts or wells in 96- or 48-well plates for transfection, aspirate collagen solution using a glass pipette and rinse each insert and/or well thoroughly using sterile PBS(−/−) (Life Technologies).
When preparing reagents for transfection, care is taken to either dispense the reagents individually into each insert/well or to use a polypropylene tube to make a master mix. The use of polypropylene tubes minimizes the loss of oligonucleotides from adherence to the walls of tubes.
Method 1: For the 0.33-cm2 inserts or wells in a 96-well plate, dilute the oligonucleotide (siRNA duplex, DsiRNA, microRNA) in 50 μl of Opti-MEM. The solution is gently mixed and set aside for 2–3 min. Optimize the oligonucleotide concentration by performing dose-response studies. To the above mix, add 1.0 μl of Lipofectamine RNAiMAX. Mix the resultant solution gently, dispense into insert/well, and set aside for 10–15 min. Next add 150,000 cells in 150 μl of transfection media and incubate for 6 h at 37°C in a 5% CO2 incubator. Method 2: For the 0.6-cm2 inserts or wells in a 48-well plate, dilute the oligonucleotide (siRNA duplex, DsiRNA, miRNA) in 25 μl of Opti-MEM. Optimize the oligonucleotide concentration by performing dose-response studies. Similarly, dilute 3 μl of Lipofectamine RNAiMAX in a separate tube in 25 μl of Opti-MEM. Next mix the contents of the two tubes and centrifuge briefly and allow to stand at room temperature for 20 min. Next add 50 μl of the oligonucleotide-RNAiMAX mixture to a single insert or well of the 48-well plate. Then add 300,000 cells suspended in 100 μl of Opti-MEM, and incubate for 24 h at 37°C in a 5% CO2 incubator. Method 3: The protocol provided is for 0.6-cm2 inserts or wells in a 48-well plate. Prepare and dilute the oligonucleotides and Transductin solutions on ice. Prepare a master mix in a polypropylene tube. Then dilute 4.5 μM of Transductin and oligonucleotide in PBS(−/−) in a total volume of 24 μl, vortex and centrifuge briefly, and incubate on ice for 30 min. Optimize the oligonucleotide concentration by performing dose-response studies. Then add 300,000 cells in 96 μl serum-free MEM to the oligonucleotide-Transductin mixture. Finally, add 120 μl of this mixture to the apical surface of the insert or the well, and incubate for 24 h at 37°C in a 5% CO2 incubator.
Posttransfection, the cells may be treated differently, depending on the purpose of the experiment. All transfection media are aspirated from the apical surface of the insert and 500 μl of maintenance media added to the basolateral side of the insert. Care is taken not to disturb the cells when removing media. For cells seeded on plastic or cells on inserts, transfection media are next replaced with complete media specific to the cell types. Cells growing on inserts formed tight junctions within 24–48 h, and the apical surface becomes dry within 2–3 days postseeding. If cultures prepared with primary airway epithelial cells or cell lines are to be maintained for long periods, the maintenance medium is replaced on the basolateral side every 5–6 days. Primary airway epithelial cells can be assayed in Ussing chamber studies at 14 days postseeding. We routinely isolate RNA from cells 24 h posttransfection, and protein for ELISA and immunoblots 72 h posttransfection.
RNA isolation.
Total RNA from primary airway epithelial cells (human and pig), Calu-3 cells, T84 cells, and PK1 cells was isolated by use of the mirVana miRNA isolation kit, TRIzol Reagent (Life Technologies, Carlsbad, CA) (52), or the SV96 Total RNA Isolation System (Promega, Madison, WI), according to the manufacturer's protocol. Total RNA was tested for quality on an Agilent model 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA). Only samples with an RNA integrity number over 7.0 were selected for downstream processing.
Primary human and pig airway epithelia.
Airway epithelia from human and pig trachea and primary bronchus removed from organs donated for research and were cultured at the ALI (33). These studies were approved by the Institutional Review Board and the Office of Animal Resources (Institutional Animal Care and Use Committee) of the University of Iowa. Airway epithelial cells were dissociated from native tissue by pronase enzyme digestion as previously reported (32). The culture medium consists of DMEM/F12 (Life Technologies), 2% Utroser G (Biosepra SA, Cedex, France), 100 U/ml pencillin, and 100 μg/ml streptomycin.
Cell lines.
Calu-3 cells (derived from human bronchial submucosal glands) (26) and T84 cells (derived from human colonic adenocarcinoma cell line) (14, 42) are well-characterized epithelial cell lines that form adherent epithelial sheets and are extensively used to study electrolyte transport and its regulation by hormones and neurotransmitters. PK1 cells are an adherent epithelial cell line derived from the kidney of a normal, healthy male pig (46). Culture conditions for these cells are as follows: Calu-3: MEM (Life Technologies), 10% FBS (Atlanta Biologicals, Miami, FL) and 1% Pen Strep (Life Technologies); CFBE41o− (37, 53): Advanced DMEM (GIBCO), 1% l-glutamine (GIBCO), 10% FBS (Atlanta Biologicals), and 1% Pen Strep (GIBCO); T84: DMEM/F12, 5% FBS, and 1% Pen Strep; PK1: Medium 199 (Life Technologies) and 3% FBS.
Oligonucleotide reagents.
The DsiRNAs were designed (34, 55), synthesized, and validated (4, 10) by Integrated DNA Technologies (Coralville, IA). All accompanying control sequences (Scr) were also generated by Integrated DNA Technologies.
SS = Sense strand, AS = Antisense strand. All DNA bases are in bold. hsCFTR DsiRNA: SS-5′ pGGAAGAAUUCUAUUCUCAAUCCAAT, AS-3′ UUCCUUCUUAAGAUAAGAGUUAGGUUA; ssHPRT DsiRNA against pig HPRT: SS-5′ pCCAGUAAAGUUAUCACAUGUUCUAG, AS-3′ GUGGUCAUUUCAAUAGUGUACAAGAUC; hsHPRT DsiRNA against human HPRT: SS-5′ pGCCAGACUUUGUUGGAUUUGAAATT, AS-3′ UUCGGUCUGAAACAACCUAAACUUUAA; ssIL8 1098 DsiRNA: SS-5′ pGGCAAAUUGUUAAACGAACAGAAUTA, AS-3′ AACCGUUUAACAAUUUGCUUGUCUUAU; ssIL8 1466 DsiRNA: SS 5′ pUGAGUGUAACUAUAGAACAUUUACA, AS-3′ ACACUCACAUUGAUAUCUUGUAAAUGU; Scr (Negative control for DsiRNAs): SS-5′ pCGUUAAUCGCGUAUAAUACGCGUAT, AS-3′ CAGCAAUUAGCGCAUAUUAUGCGCAUA.
RNAi reagents.
Lipofectamine RNAiMAX was purchased from Invitrogen (Life Technologies). Transductin (Integrated DNA Technologies) is a peptide-based transduction delivery method for siRNAs, developed by Dr. Steven Dowdy (16). It consists of a small fusion protein comprised of multiple peptide transduction domains connected to a double-stranded RNA binding domain (PTD-DRBD). The fusion protein can be purified from bacteria expressing PTD-DRBD from a modified pTAT vector (available from Dr. Dowdy's laboratory). The detailed protocol for purification of the protein is available (16).
Quantitative RT-PCR.
Total RNA (250 ng) was reverse transcribed using oligo(dT) (Roche Biochemicals, Indianapolis, IN) and random hexamers (Life Technologies) and Superscript II (Life Technologies) according to manufacturer's instructions. One-fifteenth of the cDNA was then amplified and analyzed by TaqMan assay in the 7900HT Real-Time PCR system (Applied Biosystems, Foster City, CA) by using synthesized primer-probe pairs (Integrated DNA Technologies) and Immolase DNA polymerase (Bioline, Taunton, MA). The reaction mix was contained in a total volume of 10 μl and the reaction condition was an initial cycle of 95°C for 10 min, then 40 cycles of 95°C for 15 s and 60°C for 1 min. All data were normalized to the internal standard, RPL4 mRNA for pig airway samples and SFRS9 mRNA for human airway samples. Absolute quantification of a mRNA target sequence within an unknown sample was determined by reference to a standard curve. All results of the samples were presented as remaining target mRNA level compared with the mRNA level in control samples (scrambled control, Scr), which was normalized to 100%. All experiments were performed in quadruplicate. Quantitative PCR assays used: CFTR: Forward CAACATCTAGTGAGCAGTCAGG, Reverse CCCAGGTAAGGGATGTATTGTG, Probe/56-FAM/TCCAGATCCTGGAAATCAGGGTTAGT/3IABkFQ/; ssHPRT: Forward GGTCAAGCAGCATAATCCAA, Reverse GGCATAGCCTACCACAAAC, Probe/56-FAM/CAAGGTTGCAAGCTTGCTGGTGAA/3IABkFQ/; hsHPRT: Forward GACTTTGCTTTCCTTGGTCAG, Reverse GGCTTATATCCAACACTTCGTGGG, Probe/56-FAM/ATGGTCAAGGTCGCAAGCTTGCTGGT/3IABkFQ/; ssIL8: Forward GCTGGTCAGACATAGGGTT, Reverse GTATAGAACAACGTGCATGGG, Probe/56-FAM/CCAGAGAAATCACAGGATGCCCAGTT/3IABkFQ/; hsSFRS9: Forward TGTGCAGAAGGATGGAGT, Reverse CTGGTGCTTCTCTCAGGATA, Probe/56-FAM/TGGAATATGCCCTGCGTAAACTGGA/3IABkFQ/; ssRPL4: Forward AACCAAGGAGGCTGTTCT, Reverse GGCCGACGGTTTCTCATTT, Probe/56-FAM/GCTTCTGAAGAAGCTTAAGGCCTGGA/3IABkFQ/.
Electrophysiology studies.
Transepithelial Cl− current measurements were made in Ussing chambers at ∼2 wk postseeding (30). Briefly, primary cultures were mounted in a modified Ussing chamber (Physiologic Instruments chambers). Transepithelial Cl− current was measured under short-circuit conditions. Cultures were incubated overnight with 10 μM forskolin and 100 μM 3-isobutyl-1-methylxanthine (IBMX). After measuring baseline current, the transepithelial current response to sequential apical addition of 100 μM amiloride (Amil), 100 μM 4,4′-diisothiocyanoto-stilbene-2,2′-disulfonic acid (DIDS), 4.8 mM [Cl−], 10 μM forskolin and 100 μM (IBMX), and 100 μM GlyH-101 was measured. Studies were conducted with a Cl− concentration gradient containing 135 mM NaCl, 1.2 mM MgCl2, 1.2 mM CaCl2, 2.4 mM K2PO4, 0.6 mM KH2PO4, 5 mM dextrose, and 5 mM HEPES (pH 7.4) on the basolateral surface, and gluconate substituted for Cl− on the apical side.
Immunoblotting.
Cell lines or primary cultures were washed with PBS and lysed in freshly prepared lysis buffer [1% Triton, 25 mM Tris pH 7.4, 150 mM NaCl, protease inhibitors (cOmplete, mini, EDTA-free, Roche)] for 30 min at 4°C. The lysates were centrifuged at 20,000 relative centrifugal force for 20 min at 4°C, and the supernatant was quantified by BCA protein assay kit (Thermo Fisher Scientific, Rockford, IL). Protein [20 μg (Calu-3) or 50 μg (human primary airway epithelial cultures) per lane] was separated on a 7% SDS-PAGE gel for Western blot analysis. Antibodies were procured for CFTR [R-769 (1:2,000), Cystic Fibrosis Foundation Therapeutics, Bethesda, MD] and α-tubulin (1:10,000, Sigma-Aldrich, St. Louis, MO). Protein abundance was quantified by densitometry using an AlphaInnotechFluorochem Imager (AlphaInnotech, Randburg, South Africa). For CFTR, bands B and C were quantified separately. All band densities were normalized to α-tubulin.
IL-8 protein abundance.
Porcine IL-8 protein abundance was measured 72 h after reverse transfection of pig airway epithelial cells with IL-8 DsiRNA. Basolateral media in the control and sample wells were replaced 24 h and 48 h posttransfection with serum-free DMEM/F12 media. Porcine IL-8 was measured in basolateral media using the standard sandwich ELISA (porcine CXCL8/IL-8 DouSet, catalog no. DY535, R&D Systems, Minneapolis, MN) according to the manufacturer's protocol. A 96-well plate was coated with 2 μg/ml mouse anti-porcine IL-8 capture antibody, sealed, and allowed to incubate overnight at room temperature. The plate was then aspirated, washed with a wash buffer (0.05% Tween 20 in PBS, pH 7.4) and then blocked with 1% BSA for 2 h. The plate was washed again and porcine IL-8 standards (0–8,000 pg/ml) and samples were added to the plate in duplicate. The plate was incubated for 2 h at room temperature. Following incubation, the plate was washed three times in wash buffer and a biotinylated goat anti-porcine IL-8 detection antibody was added at a concentration of 70 ng/ml and incubated for 1 h at room temperature. Following the incubation in the detection antibody, the signal was increased with the addition of avidin-horseradish peroxidase. A substrate solution containing chromagen tetramethylbenzidine was added for signal development. Reaction was stopped with the addition of 2 N H2SO4. The optical density was determined by reading the plate on a microplate reader (Molecular Devices, Sunnyvale, CA) set to 450 nm. A standard curve was generated by using a four-parameter logistic curve fit and sample IL-8 concentrations were extrapolated from this curve.
Flow cytometry.
Four hours posttransfection, primary human and pig airway epithelia were suspended in 300 μl of Accumax (Millipore), achieving a cell density of ∼50,000 cells/100 μl. Cells were pipetted repeatedly to ensure single cell suspension. We used flow cytometry to determine the percentage of transfected cells. Samples were read on a Becton Dickinson LSR II (BD Biosciences, San Jose, CA). The live cell population was selected following gating with propidium iodide. Positively transfected live cells were gated using control cells and cells transfected with Cy3-tagged RNAi following excitation via exposure to a 561-nm laser. Data were analyzed using FlowJo software. The control treatments received the Cy3-tagged RNAi reagents in the absence of transfection reagents (RNAiMAX, Transductin).
Immunohistochemistry.
Epithelial sheets on filters were fixed with 2% paraformaldehyde, washed with 1× PBS, and permeabilized in SuperBlock Blocking Buffer (Thermo Scientific) containing 0.2% Triton X-100. Cells were then blocked in SuperBlock and incubated in mouse anti-zonula occludens-1 antibody (1:100 dilution, Life Technologies) overnight at 4°C. Cells were washed in 1× PBS, incubated with goat anti-mouse Alexa 488, washed again, and counterstained with anti-β-tubulin Cy3 (cilia; Sigma) and ToPro3 (nuclear stain; Life Technologies). Immunostaining was visualized by confocal microscopy (Bio-Rad Multi-Photon Confocal).
Histochemistry.
Epithelial sheets on filters were fixed with Zn formalin, embedded in paraffin, sectioned at 5-μm thickness, and stained with hematoxylin (Leica Biosystems) and eosin (Sigma) stain. Sections were visualized by light microscopy.
Statistical analysis.
Data are presented as a means ± standard error of individual data points and median. Statistical significance between groups was determined by Student's t-test and the Wilcoxon signed-rank test. A P value <0.05 was considered significant.
RESULTS
Transfection efficiency in primary human and pig airway epithelia.
Transfection efficiency was assessed by flow cytometry in primary human and pig airway epithelial cells transfected with Cy-3 labeled scrambled siRNAs 4 h posttransfection. Using reverse transfection, we achieved greater than 80% transfection efficiency in human airway epithelia with RNAiMAX and Transductin (Fig. 3A). Similar levels of transfection efficiency were achieved in pig airway epithelia (Fig. 3A).
Fig. 3.
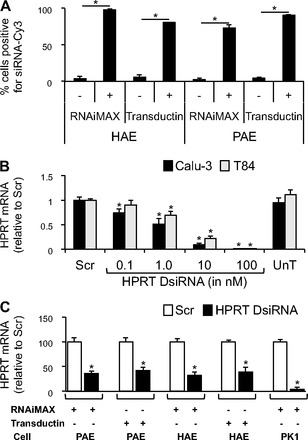
Silencing of hypoxanthine-guanine phosphoribosyltransferase (HPRT) in primary airway epithelia, Calu-3 cells, T84 cells, and PK1 cells. A: transfection efficiency of Cy-3-labeled siRNAs in primary human (HAE, 3 donors, 3 replicates each, n = 9) and pig (PAE, 2 donors, 4 replicates each, n = 8) airway epithelial cells. Data presented as a percentage of positive cells by flow cytometry, performed 4 h posttransfection. Control samples received no transfection reagent. B: HPRT mRNA abundance by quantitative RT-PCR (RT-qPCR) in Calu-3 (n = 8) and T84 (n = 8) cells 24 h posttransfection. Scr at 100 nM. C: HPRT mRNA abundance by RT-qPCR 24 h posttransfection in primary pig airway epithelia (3 donors, 3 replicates each, n = 9; DsiRNAs at 100 nM), primary human airway epithelia (3 donors, 3 replicates each, n = 9; DsiRNAs at 100 nM), and PK1 cells (n = 9; DsiRNAs at 1 nM). All cells were reverse transfected with DsiRNAs in formulation with RNAiMAX or Transductin. UnT, Untransfected; Scr, Scrambled. Error bars indicate means ± SE; statistical significance was determined by Student's t-test (assuming unequal variance) and Wilcoxon signed-rank test; *P < 0.05 by both analyses.
Silencing of HPRT in primary airway epithelia, Calu-3 cells, T84 cells, and PK1 cells.
Hypoxanthine-guanine phosphoribosyltransferase (HPRT), a ubiquitously expressed transcript, plays a central role in generating purine nucleotides through the purine salvage pathway (57). Owing to its ubiquitous expression and average expression levels, HPRT is a good control mRNA against which silencing efficiency can be examined at 24–48 h posttransfection. Calu-3 and T84 cells were reverse transfected in a 96-well plate with HPRT DsiRNAs complexed with Lipofectamine RNAiMAX. We observed a dose-dependent knockdown of HPRT mRNA levels in response to increasing concentrations of the DsiRNA (Fig. 3B). Primary airway epithelia harvested from human and pig airways were reverse transfected on 0.6-cm2 semipermeable membrane filters, and PK1 cells were reverse transfected in the wells of a 48-well plate. Primary human and pig airway epithelia exhibited greater than 60% knockdown of HPRT mRNA levels at 24 h posttransfection (Fig. 3C). PK1 cells showed greater than 95% knockdown of HPRT mRNA levels (Fig. 3C).
Silencing of IL-8 in pig primary airway epithelia.
IL-8 is an important proinflammatory cytokine in many lung diseases (25) and tracks with cystic fibrosis lung disease severity (6, 7, 28). Thus reducing IL-8 levels might reduce neutrophilic inflammation. IL-8 has been a target for gene silencing in previous studies (15, 58), but it is difficult to introduce RNAi oligonucleotides into polarized, pseudostratified airway epithelial cells (36). This creates a hurdle when gene function is studied several days posttransfection. Primary pig airway epithelia were reverse transfected on 0.6-cm2 semipermeable membrane filters, and PK1 cells were reverse transfected in wells of a 48-well plate with two separate DsiRNAs against IL-8 (termed 1098 or 1466), complexed either with RNAiMAX or Transductin. Significant reduction in IL-8 mRNA abundance was observed with both DsiRNAs in both cell types (Fig. 4A). To test whether IL-8 knockdown reduced IL-8 secretion, primary pig airway epithelia were reverse transfected on 0.6-cm2 semipermeable membrane filters with two separate DsiRNAs against IL-8, complexed with either RNAiMAX or Transductin. A significant drop in IL-8 protein secretion was observed 72 h posttransfection with both DsiRNAs (Fig. 4B).
Fig. 4.
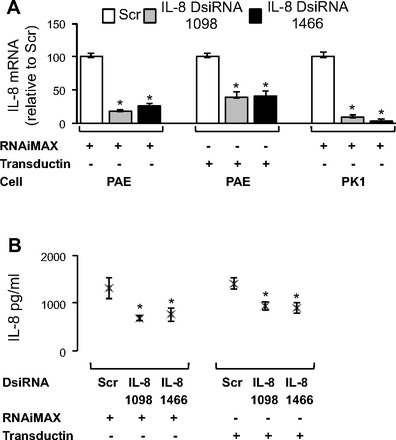
Silencing of IL-8 in primary airway epithelia and PK1 cells. A: IL-8 mRNA abundance by RT-qPCR 24 h posttransfection in primary pig airway epithelia (3 donors, 3 replicates each, n = 9; DsiRNAs at 100 nM), and PK1 cells (n = 9; DsiRNAs at 1 nM). B: secreted IL-8 abundance by ELISA 72 h posttransfection in primary pig airway epithelia (3 donors, 3 replicates each, n = 9; DsiRNAs at 100 nM). All cells were reverse transfected with DsiRNAs in formulation with RNAiMAX or Transductin. 1098 and 1466 are 2 separate DsiRNAs against IL-8. Error bars indicate means ± SE; statistical significance was determined by Student's t-test (assuming unequal variance) and Wilcoxon signed-rank test; *P < 0.05 by both statistical analyses.
Silencing of CFTR in human primary airway epithelia and Calu-3 cells.
Targeting CFTR for silencing in primary human airway epithelia and Calu-3 cells allowed us to test the utility of this method in studying long-term knockdown. We measured the effects of DsiRNAs on endogenous CFTR levels in human primary airway epithelial cells that express CFTR at very low levels (60) and Calu-3 epithelial cells that express CFTR abundantly (26). We note that, in contrast to expression of recombinant CFTR, endogenous CFTR expression in Calu-3 cells and in primary airway epithelia produces primarily the band C form of the protein and little band B (48, 61). Primary airway epithelia or Calu-3 cells were reverse transfected in wells of a 96-well plate with DsiRNAs complexed with RNAiMAX. This caused a significant dose-dependent decrease in CFTR mRNA and protein levels in both airway epithelial cells (Fig. 5, A and B) and Calu-3 cells (Fig. 5, C and D). Since CFTR creates an anion permeability, its function can be assayed by measuring transepithelial electrical conductance in polarized airway epithelial cells and Calu-3 cells. Calu-3 cells grown at the ALI form a polarized epithelium with stable transepithelial resistance measurements within 14 days postseeding (21). Since ALI cultures of primary airway epithelia are also polarized and pseudostratified within 14 days (66), we chose to measure transepithelial electrical conductance in oligonucleotide-transfected cells at 14 days posttransfection. Primary airway epithelia or Calu-3 cells were reverse transfected in 0.33-cm2 0.4-μm polyester membrane inserts with DsiRNAs complexed with RNAiMAX. Two weeks posttransfection, a significant reduction in CFTR-mediated Cl− conductance and current was observed in both polarized primary airway epithelial cells (Fig. 6, A–C), and polarized Calu-3 cells (Fig. 6, D–F).
Fig. 5.
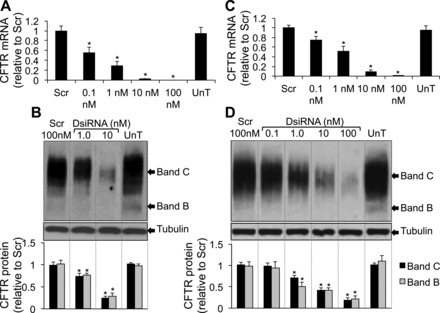
Silencing of CFTR mRNA and protein expression. A: CFTR mRNA abundance by RT-qPCR in primary human airway epithelial cells 24 h posttransfection (3 donors, 4 replicates each, n = 12). B: representative immunoblot depicting CFTR protein abundance in primary human airway epithelial cells 72 h posttransfection. Densitometry and relative fold change of CFTR protein abundance in primary airway epithelia (3 donors, 2 replicates each, n = 6). C: CFTR mRNA abundance by RT-qPCR in Calu-3 cells 24 h posttransfection (n = 8). D: representative immunoblot depicting CFTR protein abundance in Calu-3 cells 72 h posttransfection. Densitometry and relative fold change of CFTR protein abundance in Calu-3 cells (n = 8). All cells were reverse transfected with DsiRNAs in formulation with RNAiMAX. Lane representing UnT (untransfected) condition reassembled in B and D. Error bars indicate means ± SE; statistical significance was determined by Student's t-test (assuming unequal variance) and Wilcoxon signed-rank test; *P < 0.05 by both statistical analyses.
Fig. 6.
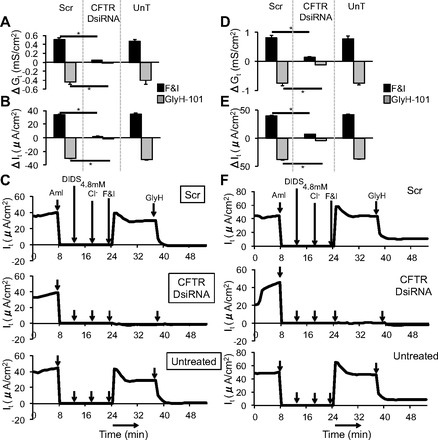
Silencing of CFTR-mediated transepithelial electrical conductance. Changes in (A and D) conductance (Gt) and (B and E) transepithelial current (It) measured in polarized primary human airway epithelial cultures (A and B) (3 donors, 6 replicates each, n = 18) and polarized Calu-3 cultures (D and E) (n = 8) 14 days posttransfection. C and F: representative tracings of It responses measured 14 days posttransfection after sequential apical application of indicated reagents in polarized primary human airway epithelial cultures (C) and polarized Calu-3 cultures (F). Arrows indicate time of addition of reagents. Aml, amiloride; DIDS, 4,4′-diisothiocyano-2,2′-stilbenedisulfonic acid; F&I, forskolin and 1-methyl-3-(2-methylpropyl)-7H-purine-2,6-dione; GlyH, CFTR inhibitor GlyH-101. All cells were reverse transfected with DsiRNAs (100 nM) in formulation with RNAiMAX. Error bars indicate means ± SE; statistical significance was determined by Student's t-test (assuming unequal variance) and Wilcoxon signed-rank test; *P < 0.05 by both statistical analyses.
DISCUSSION
The manipulation of gene expression in primary airway epithelia provides a powerful tool to investigate gene function and to experimentally perturb a model epithelium.
The use of RNAi to modify gene expression in ALI cultures provides researchers the opportunity to study the function of candidate genes in disease pathogenesis, to develop reagents to inhibit replication or shedding of respiratory viruses (1, 5, 13, 20, 41, 59), and to investigate novel gene targets for therapeutic rescue of protein maturation in diseases like cystic fibrosis (ΔF508-CFTR) (29, 43, 47, 53). Although there has been much progress in siRNA design to avoid immunogenicity, toxicity, and off-target effects (4, 8, 11, 17, 19, 23, 36, 50, 62), the use of loss of function through RNAi has been limited in polarized, pseudostratified ALI epithelial cultures because of inefficiencies in delivery and the presence of barriers for entry of RNAi reagents (4, 8, 11, 17, 19, 23, 36, 50, 62). To overcome this limitation we developed and applied a simple approach that exploits the susceptibility of cells to transfection at the time of plating. Through this method we achieved efficient gene silencing in polarized, pseudostratified epithelia and in cell lines.
We previously reported that polarized, pseudostratified primary epithelia derived from human or porcine trachea and bronchi could not be accessed for efficient RNAi by using oligonucleotides (36, 53). Others have noted similar inefficiencies in the delivery of oligonucleotides to well-differentiated epithelia in vitro and in vivo (23, 44, 50). Our previous work indicates that the process of differentiation associated with the formation of a polarized, pseudostratified epithelium results in a highly resistant epithelial barrier within 5–6 days of plating; this prevents the entry of oligonucleotides regardless of how they are formulated (36, 53).
The method of oligonucleotide delivery described here provides a means to ask experimental questions in a relevant model system. The knockdown of HPRT, IL-8, and CFTR expression and function in polarized cultures of primary human and pig airway epithelial cells both highlights the utility of the method to achieve targeted gene knockdown in pseudostratified epithelia and emphasizes the ease with which loss-of-function studies can be carried out to address broader questions dependent on protein function of the targeted gene. Of note, the extent of target mRNA knockdown is inversely proportional to target abundance and turnover (3), and the rate of oligonucleotide digestion by nucleases (38). Target knockdown is also influenced by the oligonucleotide sequence, concentration, chemical modifications, and rate of cell division. In our studies we transfected cells at maximal seeding density (maximum number of cells capable of forming a confluent sheet in the surface area transfected) to ensure minimal loss of RNAi reagents due to cell division. We tested a panel of 10 DsiRNAs against each gene before selecting the sequence that gave the highest knockdown with low off-target silencing. We also focused on DsiRNAs because they have been shown to evoke potent RNAi (10, 34). Furthermore, the use of chemically modified oligonucleotides confers nuclease resistance and increases specificity (4, 10, 19, 27, 40), two factors critical to achieving long-term (at least 2–3 wk duration) knockdown of target genes observed in our studies. We encourage readers to consider these aspects of oligonucleotide design and target mRNA properties when assessing knockdown. Finally, we also validated the utility of this protocol to deliver microRNA mimics and anti-microRNAs (51, 53).
We demonstrated this strategy to be effective in primary human and porcine airway epithelial cells, Calu-3 cells, T84 cells, and CFBE cells, both here and in a previously published work (51, 53). Furthermore, we observed persistent knockdown of SIN3A and CFTR mRNA and protein expression 14 days posttransfection (53). This knockdown correlated with changes in CFTR-mediated Cl− conductance 2 wk posttransfection in polarized ALI cultures (53). This method does not adversely affect cell viability. Measurement of lactate dehydrogenase release by polarized Calu-3 cells 4, 8, 12, and 16 days posttransfection showed no significant difference between untreated and DsiRNA transfected cells (53). We note that transfection of primary cells with single- or double-stranded oligonucleotides did not appreciably change the morphology of epithelia studied 2 wk later (Fig. 1B) and 4 wk later (51). This finding, coupled with the knowledge that the bioelectric properties of polarized primary epithelia and Calu-3 cells remained intact 2 wk and 4 wk posttransfection (51, 53), suggests that this method is well tolerated and nontoxic.
In conclusion, this method provides a novel avenue for researchers to circumvent the barrier properties of polarized, pseudostratified primary airway epithelia. By transfecting poorly differentiated cells, investigators can conduct experiments and address questions that apply to polarized, pseudostratified cells, while maintaining knockdown of targeted genes. Although research is underway to understand the barrier properties of well-differentiated airway epithelia and exploit that knowledge to develop successful strategies for gene silencing (36), the approach here outlined provides a practical solution for performing loss-of-function studies in polarized, pseudostratified epithelia.
DISCLOSURES
No conflicts of interest, financial or otherwise are declared by the author(s).
AUTHOR CONTRIBUTIONS
S.R., S.K., B.L.D., and P.B.M. conception and design of research; S.R., S.K., A.M.J., C.W.-L., and M.A.B. performed experiments; S.R., S.K., C.W.-L., B.L.D., and P.B.M. analyzed data; S.R., S.K., and P.B.M. interpreted results of experiments; S.R., S.K., and C.W.-L. prepared figures; S.R., S.K., B.L.D., and P.B.M. drafted manuscript; S.R., S.K., A.M.J., M.A.B., B.L.D., and P.B.M. edited and revised manuscript; S.R., S.K., A.M.J., C.W.-L., M.A.B., B.L.D., and P.B.M. approved final version of manuscript.
REFERENCES
- 1. Alvarez R, Elbashir S, Borland T, Toudjarska I, Hadwiger P, John M, Roehl I, Morskaya SS, Martinello R, Kahn J, Van Ranst M, Tripp RA, DeVincenzo JP, Pandey R, Maier M, Nechev L, Manoharan M, Kotelianski V, Meyers R. RNA interference-mediated silencing of the respiratory syncytial virus nucleocapsid defines a potent antiviral strategy. Antimicrob Agents Chemother 53: 3952–3962, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Antunes MB, Woodworth BA, Bhargave G, Xiong G, Aguilar JL, Ratner AJ, Kreindler JL, Rubenstein RC, Cohen NA. Murine nasal septa for respiratory epithelial air-liquid interface cultures. Biotechniques 43: 195–196, 2007 [DOI] [PubMed] [Google Scholar]
- 3. Arvey A, Larsson E, Sander C, Leslie CS, Marks DS. Target mRNA abundance dilutes microRNA and siRNA activity. Mol Syst Biol 6: 363, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Behlke MA. Chemical modification of siRNAs for in vivo use. Oligonucleotides 18: 305–319, 2008 [DOI] [PubMed] [Google Scholar]
- 5. Bitko V, Musiyenko A, Shulyayeva O, Barik S. Inhibition of respiratory viruses by nasally administered siRNA. Nature Med 11: 50–55, 2005 [DOI] [PubMed] [Google Scholar]
- 6. Bonfield TL, Konstan MW, Berger M. Altered respiratory epithelial cell cytokine production in cystic fibrosis. J Allergy Clin Immunol 104: 72–78, 1999 [DOI] [PubMed] [Google Scholar]
- 7. Bonfield TL, Panuska JR, Konstan MW, Hilliard KA, Hilliard JB, Ghnaim H, Berger M. Inflammatory cytokines in cystic fibrosis lungs. Am J Respir Crit Care Med 152: 2111–2118, 1995 [DOI] [PubMed] [Google Scholar]
- 8. Boudreau RL, Spengler RM, Hylock RH, Kusenda BJ, Davis HA, Eichmann DA, Davidson BL. siSPOTR: a tool for designing highly specific and potent siRNAs for human and mouse. Nucleic Acids Res 41: e9, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Caci E, Melani R, Pedemonte N, Yueksekdag G, Ravazzolo R, Rosenecker J, Galietta LJ, Zegarra-Moran O. Epithelial sodium channel inhibition in primary human bronchial epithelia by transfected siRNA. Am J Respir Cell Mol Biol 40: 211–216, 2009 [DOI] [PubMed] [Google Scholar]
- 10. Collingwood MA, Rose SD, Huang L, Hillier C, Amarzguioui M, Wiiger MT, Soifer HS, Rossi JJ, Behlke MA. Chemical modification patterns compatible with high potency dicer-substrate small interfering RNAs. Oligonucleotides 18: 187–200, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Davidson BL, McCray PB., Jr Current prospects for RNA interference-based therapies. Nat Rev Genet 12: 329–340, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. de Jong PM, van Sterkenburg MA, Hesseling SC, Kempenaar JA, Mulder AA, Mommaas AM, Dijkman JH, Ponec M. Ciliogenesis in human bronchial epithelial cells cultured at the air-liquid interface. Am J Respir Cell Mol Biol 10: 271–277, 1994 [DOI] [PubMed] [Google Scholar]
- 13. DeVincenzo J, Lambkin-Williams R, Wilkinson T, Cehelsky J, Nochur S, Walsh E, Meyers R, Gollob J, Vaishnaw A. A randomized, double-blind, placebo-controlled study of an RNAi-based therapy directed against respiratory syncytial virus. Proc Natl Acad Sci USA 107: 8800–8805, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dharmsathaphorn K, McRoberts JA, Mandel KG, Tisdale LD, Masui H. A human colonic tumor cell line that maintains vectorial electrolyte transport. Am J Physiol Gastrointest Liver Physiol 246: G204–G208, 1984 [DOI] [PubMed] [Google Scholar]
- 15. Durcan N, Murphy C, Cryan SA. Inhalable siRNA: potential as a therapeutic agent in the lungs. Mol Pharm 5: 559–566, 2008 [DOI] [PubMed] [Google Scholar]
- 16. Eguchi A, Meade BR, Chang YC, Fredrickson CT, Willert K, Puri N, Dowdy SF. Efficient siRNA delivery into primary cells by a peptide transduction domain-dsRNA binding domain fusion protein. Nat Biotechnol 27: 567–571, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ferrari S, Geddes DM, Alton EW. Barriers to and new approaches for gene therapy and gene delivery in cystic fibrosis. Adv Drug Deliv Rev 54: 1373–1393, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fulcher ML, Gabriel S, Burns KA, Yankaskas JR, Randell SH. Well-differentiated human airway epithelial cell cultures. Methods Mol Med 107: 183–206, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Ge Q, Dallas A, Ilves H, Shorenstein J, Behlke MA, Johnston BH. Effects of chemical modification on the potency, serum stability, and immunostimulatory properties of short shRNAs. RNA 16: 118–130, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ge Q, Filip L, Bai A, Nguyen T, Eisen HN, Chen J. Inhibition of influenza virus production in virus-infected mice by RNA interference. Proc Natl Acad Sci USA 101: 8676–8681, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Grainger CI, Greenwell LL, Lockley DJ, Martin GP, Forbes B. Culture of Calu-3 cells at the air interface provides a representative model of the airway epithelial barrier. Pharm Res 23: 1482–1490, 2006 [DOI] [PubMed] [Google Scholar]
- 22. Gray TE, Guzman K, Davis CW, Abdullah LH, Nettesheim P. Mucociliary differentiation of serially passaged normal human tracheobronchial epithelial cells. Am J Respir Cell Mol Biol 14: 104–112, 1996 [DOI] [PubMed] [Google Scholar]
- 23. Griesenbach U, Kitson C, Escudero Garcia S, Farley R, Singh C, Somerton L, Painter H, Smith RL, Gill DR, Hyde SC, Chow YH, Hu J, Gray M, Edbrooke M, Ogilvie V, MacGregor G, Scheule RK, Cheng SH, Caplen NJ, Alton EW. Inefficient cationic lipid-mediated siRNA and antisense oligonucleotide transfer to airway epithelial cells in vivo. Respir Res 7: 26, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gruenert DC, Finkbeiner WE, Widdicombe JH. Culture and transformation of human airway epithelial cells. Am J Physiol Lung Cell Mol Physiol 268: L347–L360, 1995 [DOI] [PubMed] [Google Scholar]
- 25. Harada A, Sekido N, Akahoshi T, Wada T, Mukaida N, Matsushima K. Essential involvement of interleukin-8 (IL-8) in acute inflammation. J Leukoc Biol 56: 559–564, 1994 [PubMed] [Google Scholar]
- 26. Haws C, Finkbeiner WE, Widdicombe JH, Wine JJ. CFTR in Calu-3 human airway cells: channel properties and role in cAMP-activated Cl− conductance. Am J Physiol Lung Cell Mol Physiol 266: L502–L512, 1994 [DOI] [PubMed] [Google Scholar]
- 27. Hefner E, Clark K, Whitman C, Behlke MA, Rose SD, Peek AS, Rubio T. Increased potency and longevity of gene silencing using validated Dicer substrates. J Biomol Tech 19: 231–237, 2008 [PMC free article] [PubMed] [Google Scholar]
- 28. Hillian AD, Londono D, Dunn JM, Goddard KA, Pace RG, Knowles MR, Drumm ML. Modulation of cystic fibrosis lung disease by variants in interleukin-8. Genes Immun 9: 501–508, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hutt DM, Herman D, Rodrigues AP, Noel S, Pilewski JM, Matteson J, Hoch B, Kellner W, Kelly JW, Schmidt A, Thomas PJ, Matsumura Y, Skach WR, Gentzsch M, Riordan JR, Sorscher EJ, Okiyoneda T, Yates JR, 3rd, Lukacs GL, Frizzell RA, Manning G, Gottesfeld JM, Balch WE. Reduced histone deacetylase 7 activity restores function to misfolded CFTR in cystic fibrosis. Nat Chem Biol 6: 25–33, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Itani OA, Chen JH, Karp PH, Ernst S, Keshavjee S, Parekh K, Klesney-Tait J, Zabner J, Welsh MJ. Human cystic fibrosis airway epithelia have reduced Cl− conductance but not increased Na+ conductance. Proc Natl Acad Sci USA 108: 10260–10265, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Judge AD, Robbins M, Tavakoli I, Levi J, Hu L, Fronda A, Ambegia E, McClintock K, MacLachlan I. Confirming the RNAi-mediated mechanism of action of siRNA-based cancer therapeutics in mice. J Clin Invest 119: 661–673, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Karp PH, Moninger TO, Weber SP, Nesselhauf TS, Launspach JL, Zabner J, Welsh MJ. An in vitro model of differentiated human airway epithelia. Methods for establishing primary cultures. Methods Mol Biol 188: 115–137, 2002 [DOI] [PubMed] [Google Scholar]
- 33. Karp PH, Moninger TO, Weber SP, Nesselhauf TS, Launspach JL, Zabner J, Welsh MJ. An in vitro model of differentiated human airway epithelia. Methods for establishing primary cultures. Methods Mol Biol 188: 115–137, 2002 [DOI] [PubMed] [Google Scholar]
- 34. Kim DH, Behlke MA, Rose SD, Chang MS, Choi S, Rossi JJ. Synthetic dsRNA Dicer substrates enhance RNAi potency and efficacy. Nat Biotechnol 23: 222–226, 2005 [DOI] [PubMed] [Google Scholar]
- 35. Kleinman ME, Yamada K, Takeda A, Chandrasekaran V, Nozaki M, Baffi JZ, Albuquerque RJ, Yamasaki S, Itaya M, Pan Y, Appukuttan B, Gibbs D, Yang Z, Kariko K, Ambati BK, Wilgus TA, DiPietro LA, Sakurai E, Zhang K, Smith JR, Taylor EW, Ambati J. Sequence- and target-independent angiogenesis suppression by siRNA via TLR3. Nature 452: 591–597, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Krishnamurthy S, Behlke MA, Ramachandran S, Salem AK, McCray PB, Jr, Davidson BL. Manipulation of cell physiology enables gene silencing in well-differentiated airway epithelia. Mol Ther Nucleic Acids 1: e41, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kunzelmann K, Schwiebert EM, Zeitlin PL, Kuo W-L, Stanton BA, Gruenert DC. An immortalized cystic fibrosis tracheal epithelial cell line homozygous for the ΔF508 CFTR mutation. Am J Respir Cell Mol Biol 8: 522–529, 1993. [DOI] [PubMed] [Google Scholar]
- 38. Layzer JM, McCaffrey AP, Tanner AK, Huang Z, Kay MA, Sullenger BA. In vivo activity of nuclease-resistant siRNAs. RNA 10: 766–771, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lechner JF, LaVeck MA. A serum-free method for culturing normal human bronchial epithelial cells at clonal density. J Tissue Cult Methods 9: 43–48, 1985 [Google Scholar]
- 40. Lennox KA, Behlke MA. Chemical modification and design of anti-miRNA oligonucleotides. Gene Ther 18: 1111–1120, 2011 [DOI] [PubMed] [Google Scholar]
- 41. Li BJ, Tang Q, Cheng D, Qin C, Xie FY, Wei Q, Xu J, Liu Y, Zheng BJ, Woodle MC, Zhong N, Lu PY. Using siRNA in prophylactic and therapeutic regimens against SARS coronavirus in Rhesus macaque. Nat Med 11: 944–951, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Madara JL, Stafford J, Dharmsathaphorn K, Carlson S. Structural analysis of a human intestinal epithelial cell line. Gastroenterology 92: 1133–1145, 1987 [DOI] [PubMed] [Google Scholar]
- 43. Meacham GC, Patterson C, Zhang W, Younger JM, Cyr DM. The Hsc70 co-chaperone CHIP targets immature CFTR for proteasomal degradation. Nat Cell Biol 3: 100–105, 2001 [DOI] [PubMed] [Google Scholar]
- 44. Merkel OM, Beyerle A, Librizzi D, Pfestroff A, Behr TM, Sproat B, Barth PJ, Kissel T. Nonviral siRNA delivery to the lung: investigation of PEG-PEI polyplexes and their in vivo performance. Mol Pharm 6: 1246–1260, 2009 [DOI] [PubMed] [Google Scholar]
- 45. Moschos SA, Frick M, Taylor B, Turnpenny P, Graves H, Spink KG, Brady K, Lamb D, Collins D, Rockel TD, Weber M, Lazari O, Perez-Tosar L, Fancy SA, Lapthorn C, Green MX, Evans S, Selby M, Jones G, Jones L, Kearney S, Mechiche H, Gikunju D, Subramanian R, Uhlmann E, Jurk M, Vollmer J, Ciaramella G, Yeadon M. Uptake, efficacy, and systemic distribution of naked, inhaled short interfering RNA (siRNA) and locked nucleic acid (LNA) antisense. Mol Ther 19: 2163–2168, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nielsen R, Birn H, Moestrup SK, Nielsen M, Verroust P, Christensen EI. Characterization of a kidney proximal tubule cell line, LLC-PK1, expressing endocytotic active megalin. J Am Soc Nephrol 9: 1767–1776, 1998 [DOI] [PubMed] [Google Scholar]
- 47. Okiyoneda T, Barriere H, Bagdany M, Rabeh WM, Du K, Hohfeld J, Young JC, Lukacs GL. Peripheral protein quality control removes unfolded CFTR from the plasma membrane. Science 329: 805–810, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ostedgaard LS, Meyerholz DK, Chen JH, Pezzulo AA, Karp PH, Rokhlina T, Ernst SE, Hanfland RA, Reznikov LR, Ludwig PS, Rogan MP, Davis GJ, Dohrn CL, Wohlford-Lenane C, Taft PJ, Rector MV, Hornick E, Nassar BS, Samuel M, Zhang Y, Richter SS, Uc A, Shilyansky J, Prather RS, McCray PB, Jr, Zabner J, Welsh MJ, Stoltz DA. The ΔF508 mutation causes CFTR misprocessing and cystic fibrosis-like disease in pigs. Sci Transl Med 3: 74ra24, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Parker D, Prince A. Innate immunity in the respiratory epithelium. Am J Respir Cell Mol Biol 45: 189–201, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Platz J, Pinkenburg O, Beisswenger C, Puchner A, Damm T, Bals R. Application of small interfering RNA (siRNA) for modulation of airway epithelial gene expression. Oligonucleotides 15: 132–138, 2005 [DOI] [PubMed] [Google Scholar]
- 51. Ramachandran S, Karp PH, Osterhaus SR, Jiang P, Wohlford-Lenane C, Lennox KA, Jacobi AM, Parekh K, Rose SD, Behlke MA, Xing Y, Welsh MJ, McCray PB., Jr Post-transcriptional regulation of CFTR expression and function by microRNAs. Am J Respir Cell Mol Biol 2013 May 6. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ramachandran S, Clarke LA, Scheetz TE, Amaral MD, McCray PB., Jr Microarray mRNA expression profiling to study cystic fibrosis. Methods Mol Biol 742: 193–212, 2011 [DOI] [PubMed] [Google Scholar]
- 53. Ramachandran S, Karp PH, Jiang P, Ostedgaard LS, Walz AE, Fisher JT, Keshavjee S, Lennox KA, Jacobi AM, Rose SD, Behlke MA, Welsh MJ, Xing Y, McCray PB., Jr A microRNA network regulates expression and biosynthesis of wild-type and ΔF508 mutant cystic fibrosis transmembrane conductance regulator. Proc Natl Acad Sci USA 109: 13362–13367, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Robbins M, Judge A, Ambegia E, Choi C, Yaworski E, Palmer L, McClintock K, MacLachlan I. Misinterpreting the therapeutic effects of small interfering RNA caused by immune stimulation. Hum Gene Ther 19: 991–999, 2008 [DOI] [PubMed] [Google Scholar]
- 55. Rose SD, Kim DH, Amarzguioui M, Heidel JD, Collingwood MA, Davis ME, Rossi JJ, Behlke MA. Functional polarity is introduced by Dicer processing of short substrate RNAs. Nucleic Acids Res 33: 4140–4156, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ross AJ, Dailey LA, Brighton LE, Devlin RB. Transcriptional profiling of mucociliary differentiation in human airway epithelial cells. Am J Respir Cell Mol Biol 37: 169–185, 2007 [DOI] [PubMed] [Google Scholar]
- 57. Stout JT, Caskey CT. HPRT: gene structure, expression, and mutation. Annu Rev Genet 19: 127–148, 1985 [DOI] [PubMed] [Google Scholar]
- 58. Thomas M, Lu JJ, Chen J, Klibanov AM. Non-viral siRNA delivery to the lung. Adv Drug Deliv Rev 59: 124–133, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Tompkins SM, Lo CY, Tumpey TM, Epstein SL. Protection against lethal influenza virus challenge by RNA interference in vivo. Proc Natl Acad Sci USA 101: 8682–8686, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Trapnell BC, Chu CS, Paakko PK, Banks TC, Yoshimura K, Ferrans VJ, Chernick MS, Crystal RG. Expression of cystic fibrosis transmembrane conductance regulator gene in the respiratory tract of normal individuals and individuals with cystic fibrosis. Proc Natl Acad Sci USA 88: 6565–6569, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Varga K, Jurkuvenaite A, Wakefield J, Hong JS, Guimbellot JS, Venglarik CJ, Niraj A, Mazur M, Sorscher EJ, Collawn JF, Bebok Z. Efficient intracellular processing of the endogenous cystic fibrosis transmembrane conductance regulator in epithelial cell lines. J Biol Chem 279: 22578–22584, 2004 [DOI] [PubMed] [Google Scholar]
- 62. White AF, Ponnazhagan S. Airway epithelium directed gene therapy for cystic fibrosis. Med Chem 2: 499–503, 2006 [DOI] [PubMed] [Google Scholar]
- 63. Woodworth BA, Antunes MB, Bhargave G, Palmer JN, Cohen NA. Murine tracheal and nasal septal epithelium for air-liquid interface cultures: a comparative study. Am J Rhinol 21: 533–537, 2007 [DOI] [PubMed] [Google Scholar]
- 64. Yamaya M, Finkbeiner WE, Chun SY, Widdicombe JH. Differentiated structure and function of cultures from human tracheal epithelium. Am J Physiol Lung Cell Mol Physiol 262: L713–L724, 1992 [DOI] [PubMed] [Google Scholar]
- 65. Zabner J, Fasbender AJ, Moninger T, Poellinger KA, Welsh MJ. Cellular and molecular barriers to gene transfer by a cationic lipid. J Biol Chem 270: 18997–19007, 1995 [DOI] [PubMed] [Google Scholar]
- 66. Zabner J, Zeiher BG, Friedman E, Welsh MJ. Adenovirus-mediated gene transfer to ciliated airway epithelia requires prolonged incubation time. J Virol 70: 6994–7003, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]


