Abstract
Vitamin D slows the progression of chronic kidney disease. Furthermore, activators of vitamin D receptors (VDR) have suppressant effects on the renin-angiotensin system, as well as anti-inflammatory and antifibrotic actions. This study aimed to evaluate the cytoprotective effects of paricalcitol, a VDR activator, at the mitochondrial level using an obstructive nephropathy model [unilateral ureteral obstruction (UUO)]. Rats subjected to UUO and controls were treated daily with vehicle or paricalcitol. The control group underwent a sham surgery. The treatment was done for 15 days (30 ng/kg). The following were determined: biochemical parameters; fibrosis; apoptosis; mitochondrial morphology; VDR, AT1 receptor, and NADPH oxidase 4 expression; and NADPH oxidase activity (in total and in mitochondrial fractions from the renal cortex). VDR activation prevented fibrosis (20 ± 5 vs. 60 ± 10%) and the number of TUNEL-positive apoptotic cells (10 ± 3 vs. 25 ± 4) in UUO. Biochemical, histological, and molecular studies suggest mitochondrial injury. Electron microscopy revealed in UUO electronically luminous material in the nucleus. Some mitochondria were increased in size and contained dilated crests and larger than normal spaces in their interiors. These changes were not present with paricalcitol treatment. Additionally, high AT1-receptor mRNA and NADPH activity was reverted in mitochondrial fractions from obstructed paricalcitol-treated animals (0.58 ± 0.06 vs. 0.95 ± 0.05 relative densitometry units and 9,000 ± 800 vs. 15,000 ± 1,000 relative fluorescence units·μg protein−1·min−1, respectively). These changes were consistent with an improvement in VDR expression (0.75 ± 0.05 vs. 0.35 ± 0.04 relative densitometry units). These results suggest that paricalcitol confers a protective effect and reveal, as well, a possible AT1 receptor-dependent protective effect that occurs at the mitochondrial level.
Keywords: vitamin D receptor, obstructive nephropathy, paricalcitol, mitochondria, AT1 receptors
typically, as in most forms of progressive kidney disease, the obstructive nephropathies display interstitial fibrosis and apoptosis. Indeed, urinary tract obstruction, a frequent congenital cause of renal failure in children and a major one in adults (20, 38), shares with other progressive kidney diseases a common pathogenic pathway that leads to progressive interstitial fibrosis, peritubular capillary loss with hypoxia, and the destruction of functioning nephrons. Consequently, damaged tubular cells, interstitial macrophages, and myofibroblasts produce cytokines and growth factors that promote an inflammatory state in the kidney, induce tubular cell apoptosis, and provoke the accumulation of the extracellular matrix (12). Experimental and clinical evidence indicate that vitamin D deficiency (characterized by dysfunction of tubular epithelial cells and/or loss of renal parenchyma) and angiotensin II (ANG II) upregulation play a pivotal role in the progression of renal disease associated with obstructive nephropathy (21, 43). The increasing levels of ANG II induce proinflammatory cytokines, NF-κB activation, adhesion molecules, chemokines, growth factors, and oxidative stress (12, 21, 24). In addition, accumulating data suggest that ANG II stimulates the intracellular formation of reactive oxygen species (ROS), such as the superoxide anion and hydrogen peroxide. ANG II activates several subunits of the membrane-bound multicomponent NAD(P)H oxidase and also increases ROS formation in the mitochondria (36). Furthermore, oxidative stress has been implicated as one of the major underlying mechanisms behind many acute and chronic renal diseases (31), and the overproduction of reactive oxygen intermediates has previously been identified as a key component of apoptotic pathways (9).
Conversely, active vitamin D slows the progression of chronic kidney disease (3). Of particular interest are the pleiotropic actions of vitamin D and its analogs, which are mediated by the vitamin D receptor (VDR), a ligand-dependent transcription factor that belongs to the steroid nuclear receptor gene family (8, 34). Furthermore, VDR activators have not only suppressant effects on the renin-angiotensin system (RAS), but also anti-inflammatory and antifibrotic ones (16, 17, 22). Thus paricalcitol (19-nor-1,25-dihydroxyvitamin D2) is able to ameliorate renal interstitial fibrosis in obstructive nephropathy (40). Moreover, combination therapy with paricalcitol and trandolapril demonstrated additive efficacy in retarding renal scar formation during obstructive nephropathy (39). Compared with wild-type mice, VDR-null mice developed more severe renal damage in the obstructed kidney, with marked tubular atrophy and interstitial fibrosis. Treatment with the angiotensin type 1 antagonist losartan eliminated the difference in obstruction-induced interstitial fibrosis between wild-type and VDR-null mice, signifying that ANG II contributes to the enhanced renal fibrosis observed in obstructed VDR-null kidneys (43).
Finally, the mitochondrial localization of VDR has been recently reported (37) and suggests the mitochondrial nongenomic activity of VDR. This effect could be associated with the RAS system, since kidney mitochondrial injury is attenuated by the AT1 receptor (AT1R) blockade in experimental models (5).
The present study was performed to evaluate the cytoprotective effects of paricalcitol at the mitochondrial level in an obstructive nephropathy animal model. In addition, we discuss the possibility that obstructive renal injury in mitochondria could be a consequence of the excessive response of ANG II AT1R.
Biochemical, histological, and molecular studies suggest the existence of mitochondrial injury during unilateral ureteral obstruction (UUO). Unprecedented, electron microscopy revealed ultra-structural changes, as well as mitochondria that were increased in size and that had dilated crests and spaces in their interiors. Also, high AT1 expression and NADPH activity in parallel with low VDR expression were reverted with paricalcitol treatment.
MATERIALS AND METHODS
All experimental procedures were previously approved by the Ethics Committee on Laboratory Animal Research at Cuyo University School of Medicine, Mendoza (IACUL A5780-01), Argentina, in accordance with the guidelines of the CEEA (Committee of Ethical Animal Experimentation of Argentina).
Surgical procedure and pharmacological treatment.
The urinary tracts (left kidney) of female Wistar-Kyoto rats (each animal weighing from 180 to 200 g) were surgically obstructed. Animals were first anesthetized with a ketamine-xylazine cocktail. Using an aseptic technique, a midline abdominal incision was performed; the left ureter was completely ligated with silk at the union between the pelvis and proximal ureter. The abdominal incision was closed with sutures; the rats were allowed to awaken and were given free access to water and food. In addition, a control group underwent a sham surgery. Last, a nephrectomy of the left kidney was performed 15 days after the operation on both the obstructed and the sham-operated animals. Rats were considered to have a successful ureteral obstruction when ureteral dilation was >2 mm (as determined using a millimetric eyepiece). Animals [control group: control kidney cortex (CC); and obstructed group: obstructed kidney cortex (OC)] were divided into two groups: untreated or vehicle treated (CC and OC) and paricalcitol treated (CC + Pari and OC + Pari). The paricalcitol treatment was done for 15 days (from surgery until nephrectomy), injecting 30 ng·kg−1·day−1 intraperitoneally. Finally, the animals were formed into four groups of 10 animals each.
Biochemical parameters.
The plasma concentrations of urea, calcium, phosphorus, and creatinine were determined using colorimetric assays (Sigma kits). Intact parathyroid hormone was determined by ELISA. All parameters were measured at the Mega Diagnostic Center of Mendoza (Argentina Biochemical Foundation).
Histological studies.
Renal cortex sections were fixed in 10% phosphate-buffered formalin (pH = 7.1) for 24–48 h before being embedded in paraffin and serially sectioned (5 mm) on a microtome (Leica). Paraffin sections were subjected to staining with Masson's trichrome stain. For this protocol, renal cortex sections were treated sequentially with Weigert's iron hematoxylin solution for 10 min, Biebrich scarlet-acid fuchsin for 2 min, phosphotungstic acid/phosphomolybdic acid for 10 min, and aniline blue for 5 min. Tissue was destained in 1% acetic acid for 2 min, dehydrated through graded ethanol to xylene, and mounted for examination by light microscopy (25).
Morphometric evaluation of interstitial fibrosis.
For all morphological evaluations, the observer was blinded to the origin of the histological sections. A standard point-counting method (15, 28) was used to quantitate the fibrosis of the renal interstitium. The fibrosis of the renal cortical interstitium was determined in sections by using the Masson's trichrome method to stain collagen fibers. Blue-stained interstitial fibrotic areas were assessed by using an image analyzer (Image-Pro Plus, Bethesda, MD). Ten consecutive fields were randomly selected in the renal cortex and were evaluated at ×400 on a 10 × 10 grid-imprinted reticule. All points not counted within tubular cells, lumen, glomeruli, or vascular space were considered interstitial. The number of grid points containing blue collagen staining in the interstitium was divided by the total number of points in the fields (1,000) to obtain the percentage of the fractional area of interstitial collagen deposition. Results were expressed as a percentage of the measured area.
Identification of cellular apoptosis: terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling technique.
After the digesting and quenching steps, equilibration buffer was applied directly to the sections, and working-strength TdT enzyme was then applied directly. A biotin-conjugated anti-digoxigenin antibody (Sigma) was used. Then the sections were incubated with biotinylated anti-mouse IgG (Dako, Carpinteria, CA) at 1:100 dilution for 45 min to perform reverse transcription and later with peroxidase-labeled streptavidin-biotin complex/horseradish peroxidase (Dako) at 1:100 dilution for 45 min for reverse transcription. After a brief wash, 3,3′-diaminobenzidine tetrahydrochloride (0.5 mg/ml)/H2O2 (0.01%), a chromogen substrate, was incorporated. For positive controls, sections from involuting prostates were used (n = 2). For the quantification of apoptotic epithelial cells in cross-sectioned cortex areas, 10 consecutive fields were randomly selected and were evaluated at ×400, on a 10×10 grid, using an image analyzer.
Electron microscopy.
Immediately on being separated from organs, tissue samples were fixed by immersion in a fixative solution (1:10). Fixative solution was obtained diluting one phosphate saline buffer (PBS) tablet, following the manufacturer's instructions, in 200 ml of double-distilled water and 2% glutaraldehyde (vol/vol), 2% of fresh p-formaldehyde (vol/vol), and 2% of picric acid as saturated solution. After 2 h at room temperature, the samples were reduced and placed in an OsO4 solution overnight at 4°C. The next day, the samples were dehydrated in alcohol-acetone grading up to 100% and embedded in Epon 812 (Sigma). Ultrathin sections were obtained with an Ultracut microtome (Leitz) and stained with lead citrate and uranyl using conventional staining methods. Observations were made and micrographs created using a Zeiss 900 microscope.
Mitochondria isolation from tissue.
All steps were carried out at 4°C. To ∼200 mg of tissue (renal cortex) were added 5 ml of mitochondrial isolation buffer (10 mM HEPES pH 7.4, 70 mM sucrose, 200 mM mannitol, 1 mM EDTA, protease inhibitor cocktail; Sigma, St. Louis, MO) (13). The tissue was homogenized with a Dounce glass homogenizer (Wheaton, catalog no. 357 544). The lysate was then subjected to centrifugation at 1,000 g for 10 min, yielding a nuclear pellet and postnuclear supernatant. The heavy mitochondrial fraction was obtained from the postnuclear supernatant after centrifugation at 3,000 g for 10 min. This pellet was resuspended, and the 3,000 g spin was repeated to obtain the final heavy mitochondrial pellet. The supernatant from the 3,000 g spins was then subjected to 15,000 g for 10 min. The resulting light mitochondrial pellet was resuspended, and sequential 3,000 and 15,000 g spins yielded the final light mitochondrial pellet. The purity of mitochondrial fractions was established as previously described (30), with minor modifications.
Reverse transcription-polymerase chain reaction and semiquantification of mRNA for VDR, AT1R, NADPH oxidase 4, and β-actin.
Total ribonucleic acid from cortical renal tissue and/or the mitochondrial fractions of the renal cortex were obtained by using Trizol reagent (Gibco BRL). One microgram of ribonucleic acid was denatured in the presence of 0.5 μg/50 μl oligo (dT)15 primer and 40 units recombinant ribonuclease inhibitor (Promega). Reverse transcription was performed in the presence of the mixture using 200 units of reverse transcriptase in reaction buffer, 0.5 mM of deoxyribonucleotides triphosphate each, and incubated for 60 min at 42°C. The complementary DNA (10 μl) was amplified by polymerase chain reaction under standard conditions. Each sample was measured for VDR, AT1R, NADPH oxidase 4 (NOX4), and β-actin (primers in Table 1, Integrated DNA Technologies). The VDR, AT1R, and NOX4 signals were standardized against the β-actin signal for each sample, and results were expressed as a ratio.
Table 1.
Sets of primers for the RT-PCR procedure
| Primer | Sequence | Annealing, °C | Predicted Product Size, bp |
|---|---|---|---|
| VDR | 62 | 227 | |
| Sense | 5′-GACTTTGACCGGAACGTGCG-3′ | ||
| Antisense | 5′-CATCATGCCGATGTCCACAC-3′ | ||
| AT1R | 55 | 385 | |
| Sense | 5′-GCACACTGGCAATGTAATGC-3′ | ||
| Antisense | 5′-GTTGAACAGAACAAGTGACC-3′ | ||
| NOX4 | 55 | 66 | |
| Sense | 5′-TCAACTGCAGCCTGATCCTTT-3′ | ||
| Antisense | 5′-TCTGTGATCCGCGAAGGTAAG-3′ | ||
| β-Actin | 65 | 201 | |
| Sense | 5′-TGGAGAAGAGCTATGAGCTGCCTG-3′ | ||
| Antisense | 5′-GTGCCACCAGACAGCACTGTGTTG-3′ |
Sense and antisense sequences, annealing temperatures, and predicted product size in bp are shown. VDR, vitamin D receptors; AT1R, AT1 receptor; NOX4, NADPH oxidase 4.
NADPH activity assay.
NADPH oxidase is the most efficient ROS-producing system in this as well as in other models of RAS stimulation. Cellular injury from oxidative stress occurs when ROS accumulate in excess in the host's defense mechanisms. NADPH oxidase activity is highly involved in apoptosis and fibrosis induction because it produces anion superoxide.
NADPH oxidase activity was measured in the renal cortex and in the mitochondrial fractions of the renal cortex using the Luminol (5-amino-2,3-dihydro-1,4-phthalazine, Sigma-Aldrich) technique. Samples were homogenized and centrifuged at 6,000 rpm for 30 min. The supernatant was separated and again centrifuged, this time at 19,500 rpm, and the protein concentration of the membrane fraction lysate was quantified by the Lowry assay using bovine serum albumin as a standard. A sample (40 μl) of the membrane fraction resuspended in lysis buffer was rapidly read in the spectrofluorometer (FluoroCount; AF10001, Cambers) to establish the basal value of each sample. Then, 2 μl of β-NADH (Sigma) 0.1 mmol/l and 2 μl of Luminol 5 μmol/l in dimethyl sulfoxide were incorporated and read for 10 min (360-nm excitation and 460-nm emissions). The values were expressed as relative fluorescence units (RFU) per microgram of protein and per minute of incubation.
Statistical analysis.
The results were assessed by one-way ANOVA for comparisons among groups. Differences among groups were determined by Bonferroni posttest. A P < 0.05 was considered to be significant. Results are given as means ± SE. Statistical tests were performed by using GraphPad Instat version 3.00 for Windows 95 (GraphPad Software, La Jolla, CA).
RESULTS
Serum chemistries.
Table 2 shows the biochemical parameters measured in the serum of the test rats. No differences between groups in any of the assessed parameters were shown, suggesting that, in the UUO model, one intact kidney is sufficient for sustaining renal function.
Table 2.
Biochemical parameters in UUO: paricalcitol's effect
| Control | Control + Pari | UUO | UUO + Pari | |
|---|---|---|---|---|
| Urea, mg/dl | 40 ± 5 | 38 ± 4 | 47 ± 4 | 41 ± 3 |
| Creatinine, mg/dl | 0.70 ± 0.02 | 0.68 ± 0.03 | 0.75 ± 0.05 | 0.72 ± 0.03 |
| Calcium, mg/dl | 6 ± 0.03 | 5.95 ± 0.05 | 6.20 ± 0.15 | 6.10 ± 0.10 |
| Phosphorus, mg/dl | 7 ± 0.20 | 6.97 ± 0.30 | 7.50 ± 0.30 | 7.20 ± 0.10 |
| Ca × P product, mg2/dl2 | 42 ± 1.50 | 41.47 ± 2 | 46.50 ± 3 | 43.92 ± 2 |
| PTH, pg/ml | 41 ± 5 | 40 ± 4 | 48 ± 4 | 43 ± 3 |
Values are means ± SE; n = 10. Urea, creatinine, calcium, phosphorus (mg/dl), calcium × phosphorus (Ca × P) product (mg2/dl2), and parathyroid hormone (PTH) in pg/ml were measured in the serum of rats. Pari, paricalcitol; UUO, unilateral ureteral obstruction. No significant changes were found in any of the groups evaluated (P = nonsignificant).
Paricalcitol's effect on interstitial fibrosis, apoptosis, and ultrastructural mitochondrial changes during UUO.
Figure 1A shows the degree of tubulointerstitial fibrosis (after 15 days of UUO) in cortical sections of rat kidneys that were subjected to treatment with either vehicle or with paricalcitol.
Fig. 1.
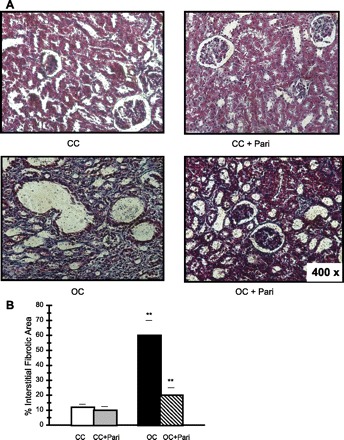
Masson's Trichrome-stained sections of rat kidney cortexes. A: control kidney cortex from vehicle-treated rats (CC), control kidney cortex from paricalcitol-treated rats (CC + Pari), obstructed kidney cortex from vehicle-treated rats (OC), and obstructed kidney cortex from paricalcitol-treated rats (OC + Pari). Magnification ×400. B: representative graphics of Masson's trichrome quantification. The interstitial fibrotic area of the obstructed kidneys revealed a significant expansion of the interstitial space compared with the cortical areas of the control group (OC vs. CC). Values are means ± SE; n = 10 for all groups. **P < 0.01. Conversely, in a group of rats given paricalcitol after unilateral obstruction, the expansion of the interstitial space was significantly decreased (OC + Pari vs. OC). **P < 0.01. No differences were observed in the cortexes of CC + Pari animals related to those from CC animals. P = nonsignificant (NS).
In comparison to those of the controls, kidneys subjected to UUO showed higher collagen accumulation in the expanded interstitium, along with cellular interstitial infiltrates in the cortex. Kidneys from rats treated with paricalcitol for 15 days after the obstruction surgery had less interstitial collagen deposition compared with what was seen in kidneys from obstructed vehicle-treated animals.
The interstitial fibrotic area of the OC revealed a fivefold expansion of the interstitial space compared with that of the CC (60 ± 10 vs. 12 ± 2.0%; P < 0.01, n = 10). In the OC + Pari group of rats, the expansion of the interstitial space was significantly decreased (20 ± 5 vs. 60 ± 10%; P < 0.01, n = 10). No differences were observed in the interstitial fibrotic areas from the CC compared with CC + Pari [10 ± 2.5 vs. 12 ± 2%; P = nonsignificant (NS), n = 10] (Fig. 1B).
Figure 2 shows an increased number of terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL)-positive apoptotic cells in tubular epithelial cells from the OC compared with those of the CC (25 ± 4 vs. 5 ± 2; P < 0.001, n = 10). Conversely, the number of TUNEL-positive apoptotic cells in OC + Pari was significantly lower than the number found in the OC (10 ± 3 vs. 25 ± 4; P < 0.01, n = 10).
Fig. 2.
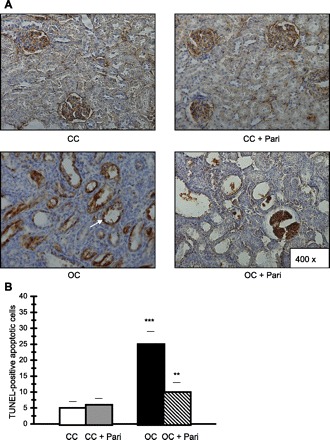
Histological sections of the cortexes of kidneys following unilateral obstruction for 15 days. Paricalcitol's cytoprotective effect is shown. A: localization of apoptotic nuclei by terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) technique. Apoptotic nuclei appear as heavy brown-stained nuclei in tubule epithelial cells (arrow). Fifteen-day-old kidney cortexes are from CC and CC + Pari rats. Apoptotic cells are rarely seen in tubule epithelial cells. Fifteen days after ipsilateral obstruction in the renal cortex (OC), apoptotic nuclei appear as heavy brown-stained nuclei in dilated collecting ducts and in lesser proportions in proximal tubules. There is a slight increase in apoptotic cells in epithelium from dilated collecting ducts and proximal tubules in the obstructed kidney cortexes of OC + Pari rats. B: quantification of apoptotic epithelial cells in cross-sectioned cortex areas. There was an increased number of TUNEL-positive nuclei in tubular epithelial cells in obstructed cortex from OC animals compared with CC. Values are means ± SE; n = 10. ***P < 0.001. Conversely, in obstructed cortexes from OC + Pari animals, the number of TUNEL-positive apoptotic cells was significantly lower than what was found in the vehicle-treated animals (OC). **P < 0.01.
Figure 3 shows the results of the electron microscopy study. Small cortical areas of renal tissues were preserved in both conditions, protected–paricalcitol–or not (A and C). Within these areas, some cortical tubules show typical cylindrical cells with microvilli and abundant mitochondria. These characteristics were better preserved in the OC + Pari group compared with what was found in animals with obstructed renal conditions alone (compare Fig. 3, C and D, with A and B). But neither condition was observed in the renal cortexes of the control animals, regardless of whether or not they had received paricalcitol treatment (Fig. 3, E and F). Nuclear material appeared electron lucent (n, Fig. 3C) and some mitochondria presented the space between mitochondrial cristae dilated (arrows, Fig. 3D) in obstructed, nontreated animals.
Fig. 3.
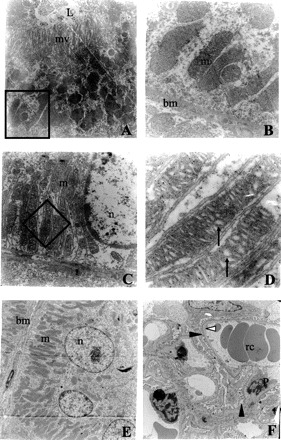
Electron microscopy study of the cortex of the kidney following unilateral obstruction for 15 days. Effects of paricalcitol at the mitochondrial level are shown. A and B: electron microscopy obtained from the cortical cortex of an obstructed kidney treated with paricalcitol. C and D: the same experimental conditions as A and B, but without treatment. E and F: a normal kidney from a rat treated with paricalcitol. The areas delimited in A and C by the empty square are magnified in the right column (B and D, respectively). Note that the nuclear material (n; C) appears luminescent, and mitochondria are present in the space between the dilated mitochondrial crestae (arrows; D) in nontreated tubules. Presumably, both pictures correspond to convoluted distal tubules, but, because of the specific experimental conditions, most of the ultrastructural characteristics were modified. Convoluted distal tubules (E) or glomeruli (F) showing normal ultrastructure under paricalcitol treatment were included for comparison. Normal capillary vessels with red cells (rc) surrounded with pedicels (black arrowhead) from podocytes (P) and preserved filtrating membrane (white arrowhead) could be observed, indicating a normal renal glomeruli (F). Magnification is ×8,000 (A), ×60,000 (B), ×10,000 (C), ×72,000 (D), and ×3,000 (E and F). L, lumen; m, mitochondria; mv, microvilli; bm, basal membrane.
Electron microscopy revealed, in obstructed, nontreated animals, electronically luminous nuclear material in the nucleus. We also noted that the mitochondria were increased in size, with dilated crests and spaces in their interiors. These mitochondrial changes were not present in the paricalcitol-treated animals.
Paricalcitol's effect on VDR, AT1R, NOX4 expression, and NADPH activity in total fractions from renal cortexes during UUO.
We found lower VDR mRNA expression in the OC obstructed cortexes of nontreated rats (OC) compared with such expression in the CC [0.20 ± 0.05 vs. 0.60 ± 0.05 relative densitometry units (RDU); P < 0.01, n = 10] as well as higher AT1R mRNA expression (as ascertained by reverse transcription-polymerase chain reaction) (1.60 ± 0.05 vs. 1.00 ± 0.09 RDU; P < 0.01, n = 10), respectively. To the contrary, in the OC + Pari vs. the OC, increased VDR mRNA expression (0.50 ± 0.04 vs. 0.20 ± 0.05 RDU; P < 0.01, n = 10) and decreased AT1R expression at the mRNA level (1.20 ± 0.03 vs. 1.60 ± 0.05 RDU; P < 0.001, n = 10) were demonstrated (Fig. 4).
Fig. 4.
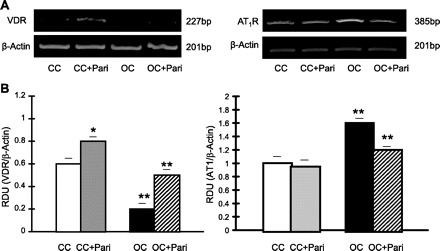
Paricalcitol's effect on vitamin D receptors (VDR) and AT1 receptor (AT1R) expression in the renal cortex during unilateral ureteral obstruction (UUO). A: representative gels of VDR (227 bp) and AT1R (385 bp) mRNA in the cortexes of OC and CC rats (paricalcitol treated and nonparicalcitol treated) are shown. The corresponding housekeeping β-actin is included below. B: graphical representation of VDR/β-actin mRNA ratio shows lower mRNA expression in OC vs. CC, as well as a higher AT1R-to-β-actin mRNA ratio. **P < 0.01 for both. Conversely, after paricalcitol treatment, higher VDR mRNA expression and lower AT1R mRNA expression were found in OC + Pari vs. OC. **P < 0.01 for both. Interestingly, after paricalcitol treatment, higher VDR mRNA expression was also found in CC + Pari vs. CC. *P < 0.05. Values are means ± SE; n = 10. RDU, relative densitometry units.
Figure 5 shows that there is higher NOX4 mRNA expression in the OC compared with that of the CC (0.80 ± 0.04 vs. 0.60 ± 0.03 RDU; P < 0.01, n = 10), whereas in the OC + Pari vs. the OC, decreased NOX4 mRNA expression (0.40 ± 0.05 vs. 0.80 ± 0.04 RDU; P < 0.01, n = 10) was demonstrated (Fig. 5, A and B). Parallel to this, NADPH oxidase activity was significantly enhanced in OC membrane fractions (28,000 ± 2,000 vs. 15,000 ± 1,500 RFU·μg protein−1·min−1, OC vs. CC; P < 0.01, n = 10). Contrarily, in OC + Pari membrane fractions, lower NADPH oxidase activity was shown compared with that of the OC (17,000 ± 1,500 vs. 28,000 ± 2,000 RFU·μg protein−1·min−1, OC vs. CC; P < 0.01, n = 10) (Fig. 5C).
Fig. 5.
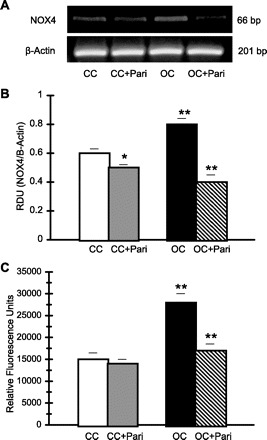
Effect of paricalcitol on NADPH oxidase 4 (NOX4) expression and NADPH activity in the renal cortex during UUO. A: representative gel of NOX4 (66 bp) mRNA in the cortexes of OC and CC rats (paricalcitol treated and nonparicalcitol treated) are shown. The corresponding housekeeping β-actin is included below. B: graphical representation of NOX4-to-β-actin mRNA ratio shows higher mRNA expression in the OC (15 days) vs. CC. **P < 0.01. Conversely, after paricalcitol treatment, lower NOX4 mRNA expression was demonstrated in OC + Pari vs. OC. **P < 0.01. Interestingly, after paricalcitol treatment, lower NOX4 mRNA expression was also found in CC + Pari vs. CC. *P < 0.05. Values are means ± SE; n = 10. C: NADPH oxidase activity increased in OC (15 days) vs. CC. **P < 0.01. After paricalcitol treatment, lower NADPH oxidase activity was shown in the cortexes of the obstructed kidneys (15 days) of rats compared with those from nontreated rats. **P < 0.01. Values are means ± SE; n = 10.
No differences were observed in NADPH oxidase activity when we compared the CC + Pari membrane fractions relation to the CC membrane fractions (14,000 ± 1,000 vs. 15,000 ± 1,500 RFU·μg protein−1·min−1; P = NS, n = 10) (Fig. 5C). However, lower NOX4 mRNA expression in the CC + Pari compared with those of CC (0.50 ± 0.02 vs. 0.60 ± 0.03 RDU; P < 0.05, n = 10) was demonstrated (Fig. 5, A and B).
Paricalcitol's effect on VDR, AT1R, NOX4 expression, and NADPH activity in mitochondrial fractions from renal cortexes during UUO.
In Fig. 6, it can be seen that VDR mRNA expression in mitochondrial fractions from OC is very low compared with such expression in mitochondrial fractions from CC (0.35 ± 0.04 vs. 0.80 ± 0.05 RDU; P < 0.01, n = 10). In addition, the increased AT1R mRNA expression in mitochondrial fractions from OC compared with such expression in mitochondrial fractions from CC (0.95 ± 0.05 vs. 0.70 ± 0.04 RDU; P < 0.01, n = 10), is shown, whereas in mitochondrial fractions from OC + Pari vs. mitochondrial fractions from the OC, increased VDR mRNA expression (0.75 ± 0.05 vs. 0.35 ± 0.04 RDU; P < 0.01, n = 10) and decreased AT1R expression at the mRNA level (0.58 ± 0.06 vs. 0.95 ± 0.05 RDU; P < 0.01, n = 10) are demonstrated (Fig. 6). Next, the decreased AT1R mRNA expression in mitochondrial fractions from CC + Pari compared with that in mitochondrial fractions from CC (0.50 ± 0.05 vs. 0.70 ± 0.04 RDU; P < 0.05, n = 10) is shown.
Fig. 6.
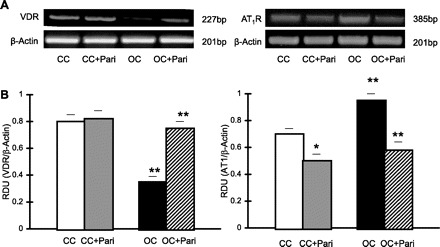
Effect of paricalcitol on VDR and AT1R expression in mitochondrial fractions from renal cortex during UUO. A: representative gels of VDR (227 bp) and AT1R (385 bp) mRNA in mitochondrial fractions from OC and CC rats (paricalcitol treated and nonparicalcitol treated) are shown. The corresponding housekeeping β-actin is included below. B: graphical representation of VDR-to-β-actin mRNA ratio shows lower mRNA expression in OC (15 days) vs. CC, as well as a higher AT1R-to-β-actin mRNA ratio. **P < 0.01 for both. Conversely, after paricalcitol treatment, higher VDR mRNA expression and lower AT1R mRNA expression were found in OC + Pari vs. OC. **P < 0.01 for both. On the other hand, after paricalcitol treatment, lower AT1R mRNA expression was also found in CC + Pari vs. CC. *P < 0.05. Values are means ± SE; n = 10.
Finally, Fig. 7 shows the elevated NOX4 mRNA expression in mitochondrial fractions from OC compared with that in mitochondrial fractions from CC (0.90 ± 0.08 vs. 0.40 ± 0.05 RDU; P < 0.01, n = 10). In mitochondrial fractions from OC + Pari vs. mitochondrial fractions from OC, decreased NOX4 mRNA expression (0.39 ± 0.04 vs. 0.90 ± 0.08 RDU; P < 0.01, n = 10) is demonstrated (Fig. 7, A and B). Correspondingly, NADPH oxidase activity was significantly enhanced in mitochondrial fractions from OC (15,000 ± 1,000 vs. 7,000 ± 500 RFU·μg protein−1·min−1, OC vs. CC; P < 0.01, n = 10). It is interesting to note that, in mitochondrial fractions from OC + Pari, lower NADPH oxidase activity compared with that found in the mitochondrial fractions from the OC (9,000 ± 800 vs. 15,000 ± 1,000 RFU·μg protein−1·min−1; P < 0.01, n = 10) was found (Fig. 7C).
Fig. 7.
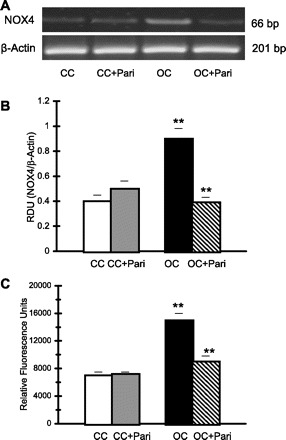
Paricalcitol's effect on NOX4 expression and NADPH activity in mitochondrial fractions from renal cortex during UUO. A: representative gel of NOX4 (66 bp) mRNA in mitochondrial fractions from OC and CC rats (paricalcitol treated and nonparicalcitol treated) are shown. The corresponding housekeeping β-actin is included below. B: graphical representation of NOX4-to-β-actin mRNA ratio shows higher mRNA expression in OC (15 days) vs. CC. **P < 0.01. To the contrary, after paricalcitol treatment, lower NOX4 mRNA expression was demonstrated in OC + Pari vs. OC. **P < 0.01. No change was noted after paricalcitol treatment in CC + Pari vs. CC. P = NS. Values are means ± SE; n = 10. C: NADPH oxidase activity was increased in mitochondrial fractions from OC (15 days) vs. CC. **P < 0.01. However, after paricalcitol treatment, lower NADPH oxidase activity was shown in the mitochondrial fractions of obstructed cortexes (15 days) in the kidneys of rats compared with the obstructed cortexes of kidneys from nontreated rats. **P < 0.01. Values are means ± SE; n = 10.
No differences were observed in NADPH oxidase activity when we compared mitochondrial fractions from the CC + Pari to mitochondrial fractions from the CC (7,200 ± 300 vs. 7,000 ± 500 RFU·μg protein−1·min−1; P = NS, n = 10) (Fig. 7C). Similarly, no differences were found in NOX4 mRNA expression when we compared mitochondrial fractions from CC + Pari to CC (0.50 ± 0.06 vs. 0.40 ± 0.05 RDU; P < 0.05, n = 10) (Fig. 7, A and B).
DISCUSSION
The present study aimed to evaluate the cytoprotective effects of paricalcitol, a VDR activator, at the mitochondrial level using an obstructive nephropathy model. In addition and unprecedented, we discuss the possibility that obstructive renal injury in mitochondria could be a consequence of the excessive response of ANG II AT1R.
Renal mitochondrial damage is a key component in obstructive nephropathy (26) and can be evaluated by the UUO model, since this model is a well-described form of renal fibrosis/apoptosis and as such is also considered a model of chronic kidney disease (32).
In 1979, Ichikawa and Brenner (18) were the first to suggest that ANG II causes kidney damage during obstructive nephropathy. More recently, Tan and colleagues (39) indicated that paricalcitol had renal protective effects, comparable to that of trandolapril, in reducing interstitial fibrosis and inflammation. Combination therapy had additive efficacy in retarding renal scar formation during obstructive nephropathy. Furthermore, experimental and clinical evidence propose that vitamin D deficiency, as well as ANG II upregulation, play a pivotal role in the progression of renal disease associated with obstructive nephropathy (10, 14, 21, 24, 33, 40, 41), and that VDR attenuates obstructive renal injury at least in part by suppressing the RAS (43). The pleiotropic actions of vitamin D and its analogs are mediated by a specific VDR, a ligand-dependent transcription factor that belongs to the steroid/nuclear receptor gene family (8, 34). In agreement, we find significantly higher AT1R expression and lower VDR expression in the renal cortex, resulting from UUO, and these key molecular expressions are supported by the apoptosis and fibrosis findings in obstructed kidneys. By means of ANG II, RAS stimulates the intracellular formation of such ROS as superoxide anion and hydrogen peroxide (12, 21, 24). More specifically, ANG II activates several subunits of the membrane-bound multicomponent NAD(P)H oxidase and also increases ROS formation in the mitochondria (36). Moreover, ANG II upregulation stimulates NOX4-derived ROS via AT1R (11), and NOX4 is the major NADPH oxidase isoform expressed in renal cells (27). In this regard, the high NADPH oxidase activity related to higher NOX4 expression in the obstructed renal cortex during UUO was established by us. Also, and unprecedented, electron microscopy of obstructed nontreated animals revealed that mitochondria were of an increased size and contained dilated cristae and larger than normal spaces in their interiors. Therefore, our experimental model establishes a link between apoptosis and fibrosis induction and mitochondrial injury (probably modulated by AT1R exaltation). High AT1R expression associated with low VDR expression could favor the activity of NADPH oxidase, which causes oxidative damage in the obstructed renal cortex. The possible opposing causal relationship between AT1R and VDR expression during UUO was established when paricalcitol administration reversed key molecular and enzymatic parameters and/or improved apoptosis, fibrosis, and mitochondrial damage. In support of this, the VDR agonists decrease oxidative stress and proinflammatory cytokines and prevent RAS activation (including the AT1R) (42). Furthermore, paricalcitol inhibited renin transcription, countering the compensatory ACEI-induced rise in renin, thus achieving a more complete blockade of the RAS (22). Vitamin D analogs may enhance the renoprotective effects of RAS inhibitors. This suggestion was strengthened by Husain and colleagues (17), who demonstrated that paricalcitol and enalapril combination therapy affords greater protection against aortic inflammatory and oxidative injury in atherosclerosis. Moreover, combination therapy with paricalcitol and trandolapril demonstrated additive efficacy in retarding renal scar formation during obstructive nephropathy (39). Therefore, results from our laboratory and those of other authors suggest an interesting opposing relationship between VDR and AT1R in this CKD model.
Finally, in view of the fact that mitochondria are energy-producing organelles that conduct other key cellular tasks, mitochondrial damage may impair various aspects of tissue function, and therefore dysfunctional mitochondria seem to contribute to the pathophysiology of renal disease (6).
Mitochondrial localization of VDR has been recently reported (37) and suggests the mitochondrial nongenomic activity of VDR. In view of this, we propose that the mitochondrial nongenomic activity of VDR could be associated with RAS system (AT1R) counterbalance, since kidney mitochondrial injury is attenuated by AT1R blockade (5) and since, as well, VDR reduces obstructive renal injury by suppressing RAS (43).
We believe that our study is the first to show AT1R mRNA expression in mitochondrial fractions from the obstructed cortexes of nontreated rats, as well as decreased VDR mRNA expression in mitochondrial fractions from the same group was shown. Additionally, elevated NOX4 mRNA expression and concomitant high NADPH oxidase activity were established. Oxidative stress may downregulate the VDR in mitochondrial fractions from obstructed renal cortexes as a consequence of vitamin D metabolism modulation (4). Earlier, upregulated NOX4 mitochondrial expression by cardiac stress and aging was demonstrated (2), and more specifically, increased NADHP oxidase activity and apoptosis induction regulated by the mitochondrial signal pathway during neonatal UUO were verified in our laboratory (35). Furthermore, increased oxidative stress resulted in reduced total antioxidant activity, and enhanced NADPH oxidase activity was demonstrated (23). The switching on of apoptotic signals from UUO is amplified in the mitochondria by releasing cytochrome c to activate the caspase cascade. Activation of transcription factors targeting downstream genes, some of which are apoptosis-related genes, can play a critical role in promoting apoptosis. In this regard, an expanding role for the vitamin D3 in control of proliferation, differentiation, and apoptosis has been reviewed (29), as well as has 1,25(OH)2D3 reduced apoptosis induction by inhibited mitochondrial cytochrome c release and minimized c-Jun-NH2-terminal kinase activation and interleukin-6 production (19).
In our present study, apoptosis induction and mitochondrial morphological changes were not present in the cortexes of the obstructed kidneys of paricalcitol-treated animals. Moreover, higher AT1R, NOX4 mRNA, and NADPH activities were reverted in mitochondrial fractions from the cortexes of the obstructed kidneys of paricalcitol-treated animals. These changes were consistent with an improvement in VDR expression. Previously, Crivello (4) demonstrated (in bovine proximal tubule cells) that elevated oxidative stress acts to limit the expression of mitochondrial vitamin D3 1α- and 24-hydroxylase activities. In addition, treatment with paricalcitol, enalapril, or the combination of the two protected uremic rats from cardiac oxidative stress by inhibiting cardiac NADPH oxidase activity (16). We also found that renal mitochondrial dysfunction in spontaneously hypertensive rats is attenuated by losartan treatment (7). Therefore, mitochondrial VDR could be associated with mitochondrial RAS system counterbalance, although mitochondrial RAS has not previously been described. However, a discovery of enormous scientific significance shows a functional mitochondrial angiotensin system coupled with mitochondrial nitric oxide production (1).
The analysis of our results, taken together with the knowledge of other investigators, allows us to suggest that the activator of VDR, paricalcitol, has a cytoprotective role, revealing for the first time a possible AT1R-dependent effect at the mitochondrial level in an obstructive nephropathy model.
Perspectives.
It is very well known that ANG II is a proinflammatory hormone, and that it increases mitochondrial oxidative stress regulating apoptosis. However, VDR stimulation has the opposite effect. The new aspect presented in this paper is the idea of the existence of RAS and VDR at the mitochondrial level, and that their regulation of oxidative stress directly affects the inflammatory process and, subsequently, apoptosis. This concept could have an interesting impact on the treatment of renal and other pathologies.
GRANTS
This work was supported by a grant (awarded to Walter Manucha) from the Research and Technology Council of Cuyo University (SeCyT), Mendoza, Argentina/N: 882/07.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
I.M.G., L.A., L.J.M., M.W.F., and M.N.M. performed experiments; I.M.G., L.A., M.W.F., L.F., and W.M. analyzed data; I.M.G., L.A., L.J.M., M.W.F., L.F., and W.M. interpreted results of experiments; I.M.G., L.A., and L.J.M. prepared figures; L.A. and L.J.M. drafted manuscript; M.N.M., L.F., and W.M. conception and design of research; L.F. and W.M. edited and revised manuscript; L.F. and W.M. approved final version of manuscript.
ACKNOWLEDGMENTS
Thanks go to Bob Ritchie of the RCMI Publications Office (5G12RR003050) for help in preparing this manuscript.
REFERENCES
- 1. Abadir PM, Foster DB, Crow M, Cooke CA, Rucker JJ, Jain A, Smith BJ, Burks TN, Cohn RD, Fedarko NS, Carey RM, O'Rourke B, Walston JD. Identification and characterization of a functional mitochondrial angiotensin system. Proc Natl Acad Sci USA 108: 14849–14854, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ago T, Matsushima S, Kuroda J, Zablocki D, Kitazono T, Sadoshima J. The NADPH oxidase Nox4 and aging in the heart. Aging (Albany NY) 2: 1012–1016, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Barreto FC, de Oliveira RA, Oliveira RB, Jorgetti V. Pharmacotherapy of chronic kidney disease and mineral bone disorder. Expert Opin Pharmacother 12: 2627–2640, 2011 [DOI] [PubMed] [Google Scholar]
- 4. Crivello JF. Oxidative stress limits vitamin D metabolism by bovine proximal tubule cells in vitro. Arch Biochem Biophys 262: 471–480, 1988 [DOI] [PubMed] [Google Scholar]
- 5. de Cavanagh EM, Ferder L, Toblli JE, Piotrkowski B, Stella I, Fraga CG, Inserra F. Renal mitochondrial impairment is attenuated by AT1 blockade in experimental Type I diabetes. Am J Physiol Heart Circ Physiol 294: H456–H465, 2008 [DOI] [PubMed] [Google Scholar]
- 6. de Cavanagh EM, Inserra F, Ferder M, Ferder L. From mitochondria to disease: role of the renin-angiotensin system. Am J Nephrol 27: 545–553, 2007 [DOI] [PubMed] [Google Scholar]
- 7. de Cavanagh EM, Toblli JE, Ferder L, Piotrkowski B, Stella I, Inserra F. Renal mitochondrial dysfunction in spontaneously hypertensive rats is attenuated by losartan but not by amlodipine. Am J Physiol Regul Integr Comp Physiol 290: R1616–R1625, 2006 [DOI] [PubMed] [Google Scholar]
- 8. Dusso AS, Brown AJ. Mechanism of vitamin D action and its regulation. Am J Kidney Dis 32, Suppl 2: S13–S24, 1998 [DOI] [PubMed] [Google Scholar]
- 9. Franklin RA, Rodriguez-Mora OG, Lahair MM, McCubrey JA. Activation of the calcium/calmodulin-dependent protein kinases as a consequence of oxidative stress. Antioxid Redox Signal 8: 1807–1817, 2006 [DOI] [PubMed] [Google Scholar]
- 10. Gonzalez EA, Sachdeva A, Oliver DA, Martin KJ. Vitamin D insufficiency and deficiency in chronic kidney disease. A single center observational study. Am J Nephrol 24: 503–510, 2004 [DOI] [PubMed] [Google Scholar]
- 11. Gorin Y, Ricono JM, Wagner B, Kim NH, Bhandari B, Choudhury GG, Abboud HE. Angiotensin II-induced ERK1/ERK2 activation and protein synthesis are redox-dependent in glomerular mesangial cells. Biochem J 381: 231–239, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Grande MT, Pérez-Barriocanal F, López-Novoa JM. Role of inflammation in tubulo-interstitial damage associated to obstructive nephropathy. J Inflamm 7: 19, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Greenawalt JW. The isolation of outer and inner mitochondrial membranes. Methods Enzymol 31: 310–323, 1974 [DOI] [PubMed] [Google Scholar]
- 14. Holick MF. Vitamin D for health and in chronic kidney disease. Semin Dial 18: 266–275, 2005 [DOI] [PubMed] [Google Scholar]
- 15. Hruska KA, Guo G, Wozniak M, et al. Osteogenic protein-1 prevents renal fibrogenesis associated with ureteral obstruction. Am J Physiol Renal Physiol 279: F130–F143, 2000 [DOI] [PubMed] [Google Scholar]
- 16. Husain K, Ferder L, Mizobuchi M, Finch J, Slatopolsky E. Combination therapy with paricalcitol and enalapril ameliorates cardiac oxidative injury in uremic rats. Am J Nephrol 29: 465–472, 2009 [DOI] [PubMed] [Google Scholar]
- 17. Husain K, Suarez E, Isidro A, Ferder L. Effects of paricalcitol and enalapril on atherosclerotic injury in mouse aortas. Am J Nephrol 32: 296–304, 2010 [DOI] [PubMed] [Google Scholar]
- 18. Ichikawa I, Brenner BM. Local intrarenal vasoconstrictor-vasodilator interactions in mild partial ureteral obstruction. Am J Physiol Renal Fluid Electrolyte Physiol 236: F131–F140, 1979 [DOI] [PubMed] [Google Scholar]
- 19. Kim R, Tanabe K, Emi M, Uchida Y, Inoue H, Toge T. Inducing cancer cell death by targeting transcription factors. Anticancer Drugs 14: 3–11, 2003 [DOI] [PubMed] [Google Scholar]
- 20. Klahr S, Morrissey J. Obstructive nephropathy and renal fibrosis. Am J Physiol Renal Physiol 283: F861–F875, 2002 [DOI] [PubMed] [Google Scholar]
- 21. Klahr S. Urinary tract obstruction. Semin Nephrol 21: 133–145, 2001 [DOI] [PubMed] [Google Scholar]
- 22. Li M, Batuman V. Vitamin D: a new hope for chronic kidney disease? Kidney Int 76: 1219–1221, 2009 [DOI] [PubMed] [Google Scholar]
- 23. Manucha W, Carrizo L, Ruete C, Molina H, Vallés P. Angiotensin II type I antagonist on oxidative stress and heat shock protein 70 (HSP 70) expression in obstructive nephropathy. Cell Mol Biol (Noisy-le-grand) 51: 547–555, 2005 [PubMed] [Google Scholar]
- 24. Manucha W. Biochemical-molecular markers in unilateral ureteral obstruction. Biocell 31: 1–12, 2007 [PubMed] [Google Scholar]
- 25. Masson PJ. AFIP modification. J Techn Methods 12: 75–90, 1929 [Google Scholar]
- 26. Møller JC, Jørgensen TM, Mortensen J. Proximal tubular atrophy: qualitative and quantitative structural changes in chronic obstructive nephropathy in the pig. Cell Tissue Res 244: 479–491, 1986 [DOI] [PubMed] [Google Scholar]
- 27. Montezano AC, Burger D, Ceravolo GS, Yusuf H, Montero M, Touyz RM. Novel Nox homologues in the vasculature: focusing on Nox4 and Nox5. Clin Sci (Lond) 120: 131–141, 2011 [DOI] [PubMed] [Google Scholar]
- 28. Morrissey J, Hruska K, Guo G, Wang S, Chen Q, Klahr S. Bone morphometric protein-7 improves renal fibrosis and accelerates the return of renal function. J Am Soc Nephrol 13: S14–S21, 2002 [PubMed] [Google Scholar]
- 29. Narvaez CJ, Zinser G, Welsh J. Functions of 1alpha,25-dihydroxyvitamin D(3) in mammary gland: from normal development to breast cancer. Steroids 66: 301–308, 2001 [DOI] [PubMed] [Google Scholar]
- 30. O'Beirne GB, Williams DC. The subcellular location in rat kidney of the peripheral benzodiazepine acceptor. Eur J Biochem 175: 413–421, 1988 [DOI] [PubMed] [Google Scholar]
- 31. Pedzik A, Paradowski M, Rysz J. [Oxidative stress in nephrology]. Pol Merkur Lekarski 28: 56–60, 2010 [PubMed] [Google Scholar]
- 32. Puri TS, Shakaib MI, Chang A, Mathew L, Olayinka O, Minto AW, Sarav M, Hack BK, Quigg RJ. Chronic kidney disease induced in mice by reversible unilateral ureteral obstruction is dependent on genetic background. Am J Physiol Renal Physiol 298: F1024–F1032, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Querings K, Girndt M, Geisel J, Georg T, Tilgen W, Reichrath J. 25-Hydroxyvitamin D deficiency in renal transplant recipients. J Clin Endocrinol Metab 91: 526–529, 2006 [DOI] [PubMed] [Google Scholar]
- 34. Rachez C, Freedman LP. Mechanisms of gene regulation by vitamin D(3) receptor. A network of coactivator interactions. Gene 246: 9–21, 2000 [DOI] [PubMed] [Google Scholar]
- 35. Rinaldi Tosi ME, Bocanegra V, Manucha W, Gil Lorenzo A, Vallés PG. The Nrf2-Keap1 cellular defense pathway and heat shock protein 70 (Hsp70) response. Role in protection against oxidative stress in early neonatal unilateral ureteral obstruction (UUO). Cell Stress Chaperones 16: 57–68, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sachse A, Wolf G. Angiotensin II-induced reactive oxygen species and the kidney. J Am Soc Nephrol 18: 2439–2446, 2007 [DOI] [PubMed] [Google Scholar]
- 37. Silvagno F, De Vivo E, Attanasio A, Gallo V, Mazzucco G, Pescarmona G. Mitochondrial localization of vitamin D receptor in human platelets and differentiated megakaryocytes. PLos One 5: e8670, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Smith JM, Stablein DM, Munoz R, Hebert D, McDonald RA. Contributions of the Transplant Registry: The 2006 Annual Report of the North American Pediatric Renal Trials and Collaborative Studies (NAPRTCS). Pediatr Transplant 11: 366–373, 2007 [DOI] [PubMed] [Google Scholar]
- 39. Tan X, He W, Liu Y. Combination therapy with paricalcitol and trandolapril reduces renal fibrosis in obstructive nephropathy. Kidney Int 76: 1248–1257, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tan X, Li Y, Liu Y. Paricalcitol attenuates renal interstitial fibrosis in obstructive nephropathy. J Am Soc Nephrol 17: 3382–3393, 2006 [DOI] [PubMed] [Google Scholar]
- 41. Tan X, Wen X, Liu Y. Paricalcitol inhibits renal inflammation by promoting vitamin D receptor-mediated sequestration of NF-kappaB signaling. J Am Soc Nephrol 19: 1741–1752, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wang XX, Jiang T, Shen Y, Santamaria H, Solis N, Arbeeny C, Levi M. Vitamin D receptor agonist doxercalciferol modulates dietary fat-induced renal disease and renal lipid metabolism. Am J Physiol Renal Physiol 300: F801–F810, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhang Y, Kong J, Deb DK, Chang A, Li YC. Vitamin D receptor attenuates renal fibrosis by suppressing the renin-angiotensin system. J Am Soc Nephrol 21: 966–973, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]


