Abstract
Infant suckling is a complex behavior that includes cycles of rhythmic sucking as well as intermittent swallows. This behavior has three cycle types: 1) suck cycles, when milk is obtained from the teat and moved posteriorly into the valleculae in the oropharynx; 2) suck-swallow cycles, which include both a rhythmic suck and a pharyngeal swallow, where milk is moved out of the valleculae, past the larynx, and into the esophagus; and 3) postswallow suck cycles, immediately following the suck-swallow cycles. Because muscles controlling these behaviors are active in all three types of cycles, we tested the hypothesis that different patterns of electromyographic (EMG) activity in the mylohyoid, hyoglossus, stylohyoid, and thyrohyoid muscles of the pig characterized each cycle type. Anterior mylohyoid EMG activity occurred regularly in every cycle and was used as a cycle marker. Thyrohyoid activity, indicating the pharyngeal swallow, was immediately preceded by increased stylohyoid and hyoglossus activity; it divided the suck-swallow cycle into two phases. Timed from the onset of the suck-swallow cycle, the first phase had a relatively fixed duration while the duration of the second phase, timed from the thyrohyoid, varied directly with cycle duration. In short-duration cycles, the second phase could have a zero duration so that thyrohyoid activity extended into the postswallow cycle. In such cycles, all swallowing activity that occurred after the thyrohyoid EMG and was associated with bolus passage through the pharynx fell into the postswallow cycle. These data suggest that while the activity of some muscles, innervated by trigeminal and cervical plexus nerves, may be time locked to the cycle onset in swallowing, the cycle period itself is not. The postswallow cycle consequently contains variable amounts of pharyngeal swallowing EMG activity. The results exemplify the complexity of the relationship between rhythmic sucking and the swallow.
Keywords: electromyographic, motor activity, dysphagia, swallowing
in the pig, suckling consists of two different motor activities (13, 42). First, there are continuing cyclical oral movements that transport milk from the teat through the oral cavity, so that it accumulates in the valleculae/oropharynx, before any swallow. Individual cycles of this type are called “sucks” even though the behavior includes more than the simple acquisition of milk from the teat. Second, swallowing occurs as an intermittent activity (commonly after 1–4 suck cycles) in which the accumulated milk is emptied from the valleculae into the esophagus; this activity approximates to the second or pharyngeal stage of the human swallow. The pharyngeal swallow is likely to be an expression of activity in central pattern generating circuits (18) although it is frequently referred to as a “reflex.” It can be elicited as a motor response of limited variability following specific sensory triggers, particularly fluid accumulation in the valleculae (13, 32, 37, 41). In the conscious suckling animal, the pharyngeal swallow always appears as an additional event occurring within what is otherwise a suck cycle.
There is a kernel of electromyographic (EMG) activity that characterizes the pharyngeal swallow, whether it occurs as an isolated reflex in a decerebrate animal (41) or as a component of ongoing cyclical oral activity (13, 43). While some aspects of the pattern of EMG activity of the pharyngeal swallow change when it occurs in the context of rhythmic oral activity, i.e., in suck/swallow cycles, the relative timings of the EMG activities in hyoglossus, stylohyoid, and thyrohyoid (utilized in this study) are unchanged (13, 43).
The timing of all individual cycle types can be separately defined by the onset of mylohyoid EMG activity (16, 30, 48, 52). Swallowing cycles are usually longer than preceding suck, lick, or lap cycles, presumably because of the addition, within the cycle, of the components of EMG activity that represent the pharyngeal swallow (20, 34, 45).
In the intact piglet, the duration of the pharyngeal swallow, defined as the time taken for a bolus of milk to pass from the valleculae to the esophagus, approaches the duration of a single suck cycle (4). However, the movement of the bolus out of the valleculae starts about halfway through the associated suck-swallow cycle (42). This suggests that components of the swallowing process extend into the immediate postswallow cycle as also suggested by Newman et al. (35) for the human infant. Because of this, an analysis of the characteristics of the different suckling cycles needed to include the additional category of “immediate postswallow cycle.” Although the patterns of EMG activity, during suckling and drinking, have previously been investigated in intact animals (23, 42, 44), no statistically valid comparisons have been made among the EMG activities recorded from different muscles in suck, suck/swallow, and immediate postswallow cycles.
The aim of this study was to test the hypothesis that each of the three cycle types had identifiable characteristics with respect to cycle duration, to intracycle timing of EMG activity, or to EMG amplitude. However, EMG records from conventional intramuscular bipolar wire electrodes represent a small sample of the overall activity generated in a large pool of motor units, not all of which necessarily subserve the same motor function. The envelope of electrical activity, recorded by a single electrode, consequently cannot be uncritically accepted as an indicator of the activity of the muscle as a whole. Thus it is possible for a single named muscle to have significantly different EMG patterns for different functions (26, 41, 46, 50). This implies that at least dual electrodes are required in each muscle to record representative activity reliably; this procedure has been adopted in the current study.
Furthermore, we wished to examine the exact temporal relationship of the pharyngeal swallow to the suck-swallow cycle within which it was generated, to test the assumption that there was a fixed phase relationship of the pharyngeal swallow to the cycle period in which it occurred (42). The alternative hypothesis was that swallows were inserted into individual suck-swallow cycles with variable timing as suggested by a rabbit study (48). The possibility of variable timing of the pharyngeal swallow is of interest because, although variable delay of some swallows has been considered to be part of normal variation in adult humans (27, 29), delayed pharyngeal swallow onset in children is reported to be associated with dysphagia, with penetration of the larynx, and with tracheal aspiration (24). In the context of rhythmic suckling, potential variations in swallow timing are best assessed statistically from large data samples using EMG markers for swallowing and for rhythmic intraoral transport.
MATERIALS AND METHODS
Animals and surgical procedures.
All experiments that generated data for this paper were approved by the Harvard University Institutional Animal Care and Use Committee (IACUC) (23–05) and used previously described procedures (13, 40, 41, 43). Videoradiographic and EMG data were collected in a series of feeding sessions using seven infant minipigs between 20 and 30 days of age (preweaning). Under general anesthesia (2–5% isofluorane in oxygen administered by face mask) and aseptic conditions, the supra- and infrahyoid muscles were exposed via a midline submandibular incision and individually identified in accordance with an atlas of pig anatomy (36). Bipolar wire electrodes were implanted into the hyoglossus (close to its origin on the hyoid), into the stylohyoid, and into thyrohyoid in duplicate, as previously described (41, 42). Patch electrodes (28) were sewn, in triplicate, onto the surface of the mylohyoid muscle at varying anteroposterior positions along a line halfway between the midline and the mandibular attachment of the muscle, i.e., neither overlying the geniohyoid nor close to the anterior digastric (13). In all cases, each of the multiple bipolar electrodes was located in or on each muscle so as to be laterally displaced from their neighboring electrodes, relative to the long axes of the muscle fibers. All electrodes were preconnected to a microconnector; the electrode wires were led out through the midline incision. The microconnectors were then attached to a short cable with a standard 25-pin D-connector. While still under anesthesia, this cable and the microconnectors were enclosed in a Vetwrap bandage around the thorax and throat to prevent the animals from rubbing the connectors directly against anything. After the surgical procedures and postoperative medication, the animals were left to recover for a minimum of 4 h before food was offered and any swallows were recorded. Animals were alert, standing, and indicating they were hungry 2–3 h postsurgery.
The experimental period lasted 2–3 days after which the animals were killed following IACUC standards. A postmortem was then performed to confirm the position and state of the electrodes.
Experimental feeding methodology and data recording.
From their time of arrival at the Harvard University vivarium, animals were fed infant pig formula from a standard baby bottle, fitted with a special pig nipple (Nasco, Fort Atkinson, WI). The bottle was fixed at the end wall of a narrow Plexiglas box that housed the animal. Purely as a check on the oral behaviors, the movements of suckling (suck cycles interspersed with suck/swallow and postswallow cycles) and the movements of the bolus containing barium were also recorded in the lateral (sagittal) plane, using digital videoradiography (Siemens Tridoros 150G3 cineradiographic apparatus with a Sony DCR-VX1000 digital video camera).
The cable from the electrodes was attached directly to an MA-300 EMG System (Motion Lab Systems). EMG signals from the selected muscles were amplified (×400–10,000) using with a bandpass of 20 Hz to 2 kHz plus a 60-Hz notch filter. The EMG signals were then recorded on a TEAC RD-145T digital data recorder together with the synchronization signals from the X-ray apparatus; an overall digitization rate of 6 kHz was used.
Recording protocol.
EMG data were recorded while the pig suckled milk containing barium, from a bottle with a veterinary teat. The subsequent procedure for data selection was based on the EMG records being free of artifacts and obvious noise; the later stages of irregular feeding, when the animal was approaching satiation, were excluded. The methods used to record and to carry out the initial processing of EMG activity, detailed below, were identical to those previously used to record swallowing activity in suckling pigs (13, 41, 42).
Data analysis.
Previous experience with bipolar wire electrodes (1-mm bare tips separated by 2 mm) has indicated that the detection of EMG signals is restricted to a small volume of each muscle (41). The amplified and bandpass filtered EMG activities were processed by rectification and 10 ms constant time reset integration (42) followed, if necessary, by computation of a statistically defined noise threshold that allowed background activity to be rejected (39). These processes were combined in a computer program that also presented a display of the quantified EMG signals and allowed sections of the data to be indexed for further analysis. This indexing defined individual cycles using the onset of anterior mylohyoid EMG activity as the marker for the timing of successive rhythmic oral cycles (16, 30, 48, 52). The sample in this study included the activity in a total of 1,615 cycles from seven animals.
This first step divided up the processed EMG data into individual cycles of activity that had varying durations and varying peak amplitudes. In the first stage of statistical analysis, the cycle durations (to the nearest 10 ms because of reset integration) and the peak EMG amplitudes (recorded by each electrode in each cycle) were stored as unscaled data. In the second stage of analysis (described in detail below), the data were further processed to arrive at median cycle profiles. In a third stage (also described in detail below), the timing of peak thyrohyoid activity was determined within each suck/swallow cycle.
Cycles were differentiated into suck and suck/swallow cycles on the basis of the amplitude of the thyrohyoid EMG activity. This was confirmed by examining the precisely synchronized fluoroscopic films in which sucks and swallows were easily differentiated by the movement of milk containing barium. The difficulty of using purely kinematic data to determine the timing of the swallow, or any other oropharyngeal event, was that, with videoradiography at 30 frames/s, individual data points represented events only every 33.3 ms, and only a limited number of the recorded frames would be available in true lateral projection because of animal behavior. In contrast, the EMG data were recorded at 6 kHz, and, even after subsequent reset integration, the time units were only 10 ms in duration. All of these data were independent of head position or movement. The finer time gradient of the EMG data permitted a more detailed analysis with higher temporal resolution than was possible with the kinematic data.
The thyrohyoid muscle is recognized as important for laryngeal elevation in swallowing, and EMG activity in this muscle has previously been found to be an indicator of the pharyngeal component of the swallow (10, 30, 31, 38). Cycles containing the onset of high-amplitude thyrohyoid EMG activity were consequently classed as suck/swallow cycles with, in each case, the immediately following cycle designated a “postswallow” cycle. Cycles without obvious thyrohyoid activity (in which milk was transferred and accumulated in the valleculae) (13) were classed as suck cycles.
In the first stage of analysis, these unscaled EMG data were used to define anterior mylohyoid EMG onsets and so determine the cycle durations; the peak amplitudes of EMG activity were then determined in the individual muscles within each cycle. These data were then processed to obtain average cycle durations and median cyclical peak EMG amplitudes for the named muscles, quite separately from the data processing in the second stage of analysis.
The second stage of analysis required the data to be both amplitude and time scaled to extract common phase-related events. Because the dominant effect on signal amplitude detected by intramuscular wire electrodes is the distance of the electrode from the nearest active muscle fiber (1, 8), amplitudes were equalized across all electrodes and across all cycles by normalizing the peak amplitudes detected by each electrode in each cycle and scaling all other values proportionately. The cycle durations in all indexed segments of data were also normalized. This process produced, for each electrode, cycles of EMG activity, each of which had an identical peak amplitude of 100 units and a cycle duration of 100 units (41, 42). In this study, two consecutive suck cycles were processed together for ease of comparison as were pairs of consecutive suck/swallow and postswallow cycles. The data matrix for each muscle was summarized for each animal by finding the median (50th percentile) level of activity separately in each of the corresponding 100 time bins of the cycles in the analysis period (or 200 bins in the case of 2 consecutive or doublet cycles).
To visualize the major amplitude differences between the three cycle types, two separate patterns of activity were compared. The first pattern was generated from the median profiles of the EMG activity of sets of two successive suck cycles processed as doublets. The second was generated from the median profiles of doublets consisting of a suck/swallow cycle and the immediate postswallow cycle. The peak activities in both doublets were scaled respectively to the previously calculated median amplitudes for suck and for swallow cycles obtained from the amplitudes of the unscaled EMG data recorded by the same electrode (see first stage of analysis).
Because the amplitudes of the EMG signals detected by each electrode were highly dependent upon the proximity of the nearest active muscle fibers, the raw EMG amplitudes in different cycle types could only be reliably compared on an individual electrode basis. This meant that comparisons across muscles and across animals could only be made using an internal scaling function for the activities recorded by each electrode. The reference measure chosen was the median of the cyclical peak EMG amplitudes detected by the same electrode in pure sucking cycles, which were the most numerous cycles. The medians of the cyclical peak EMG amplitudes in suck-swallow cycles and in the postswallow cycles recorded from the same electrode were then expressed relative to the median amplitude in the suck cycles. These ratio data were log transformed so that the median of the peak amplitudes of the suck cycles had an amplitude of 100 arbitrary units. Because the EMG activities from all electrode sites were individually scaled relative to their specific suck cycle reference levels, the process allowed comparisons to be made between the EMG activities recorded by different electrodes.
The absolute time relationship between thyrohyoid EMG activity (as an indicator of the pharyngeal swallow) and mylohyoid activity (as an indicator of the beginning and end of the suck/swallow cycle) was obtained by dividing the interval between the onset of the successive mylohyoid bursts into two phases with respect to the peak of thyrohyoid EMG activity.
Data analysis.
Several statistical analyses were used for different portions of the data. The differences in cycle durations were tested using a mixed-model ANOVA with cycle type as a fixed factor and animal as a random factor. Differences in scaled peak amplitude were also tested using a mixed-model ANOVA, with cycle type and muscle as fixed factors, and animal as a random factor.
Peak thyrohyoid activity divided suck-swallow cycles into two phases. To examine the relationship between the duration of each of the two phases and the total length of that cycle, we plotted phase length against the total length of the parent cycle. A linear regression line was then fitted to the data of each phase. The slope coefficients (Pearson) from each animal were determined separately for each animal. A slope not significantly different from zero indicated that the phase length was constant and independent of cycle length. A significant, nonzero coefficient indicated that slope varied as a function of cycle length. In all cases, we used P < 0.05 as the level of significance.
RESULTS
EMG signals recorded from the electrodes in the mylohyoid, hyoglossus, stylohyoid, and thyrohyoid muscles allowed individual suckling cycles and the associated swallowing events to be identified. Cycles without significant thyrohyoid activity were identified as suck cycles and those with significant thyrohyoid activity were identified as suck/swallow cycles (Fig. 1). The cycle immediately following an identified suck/swallow cycle was classed as a postswallow cycle, as explained in the Introduction. This overall cycle classification was confirmed in representative periods of suckling by referring to videoradiographic recordings obtained simultaneously with the EMG data.
Fig. 1.
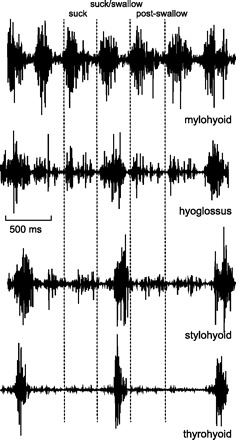
Electromyographic (EMG) activity in 4 hyoid related muscles during suckling on an artificial teat. The onset of each burst of mylohyoid EMG (vertical dotted lines), recorded from an anterior mylohyoid site, was used as the marker for each suckling cycle. Swallow cycles were identified by thyrohyoid activity, and confirmed on synchronous videofluorography.
Thyrohyoid activity, which marked each swallow, usually peaked in the interval between mylohyoid bursts, i.e., within the cycles defined by mylohyoid activity (Fig. 1). However, particularly in the case of short-duration cycles, the peak of thyrohyoid activity could be attained at the start of the following cycle, i.e., in the immediate postswallow cycle (Fig. 2).
Fig. 2.
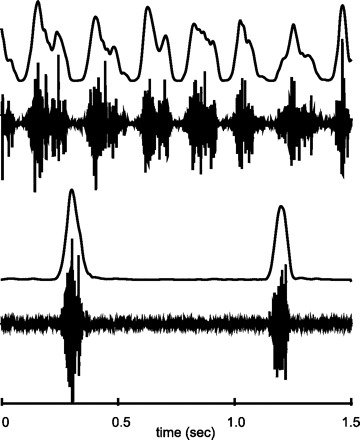
Raw EMG data and the profile of the processed EMG signals for 7 suck and for 2 suck-swallow cycles in mylohyoid and thyrohyoid. The 2nd burst of thyrohyoid activity peaked early in the “postswallow” cycle. For display purposes, the processed signals have been linearly interpolated to match the number of sample points in the raw EMG.
Across all the animals in the study, the mean duration of the suck cycles preceding a swallow was 240.3 ms (SD=40.0; n = 864; range 130–480 ms), the mean of the immediate postswallow cycles was 239.2 ms (SD=47.5; n = 363; range 110–360 ms) and of swallow cycles was 262.6 ms (SD=48.4; n = 388; range 140–490 ms). These durations of pure suck and of postswallow cycles did not differ from each other (P = 0.290), but both were shorter than the suck-swallow cycles (P < 0 .001). The relative durations of each cycle type did, however, vary among the animals (P < 0.001), and individual suck-swallow cycles could be longer than the immediately preceeding suck cycles by anything from 0 to 70 ms.
The number of suck cycles preceding each suck-swallow cycle also varied among the animals. In four animals, each suck-swallow was preceded by two to seven cycles, but, in two animals, each suck-swallow cycle was often preceded by only one suck cycle. In one animal, swallowing periodically occurred in a series of suck/swallow and postswallow cycles with no intervening pure suck cycles.
Comparisons between the different amplitude signals recorded by different electrodes in different cycle types was made possible by scaling their EMG activities to the medians of the amplitudes of the EMG peaks in pure suck cycles (see materials and methods). Scaled EMG activity was of greatest amplitude in each muscle during suck-swallow cycles (P < 0.001; Fig. 3). The difference between scaled EMG amplitude in pure suck and in postswallow cycles was not significant (P = 0.95) except in the case of the thyrohyoid muscle (P < 0.001). In that case, a significant difference arose because the high-amplitude, swallow-related, burst of EMG activity of the thyrohyoid frequently extended into the postswallow cycle and, periodically, only reached its peak amplitude within that postswallow cycle.
Fig. 3.
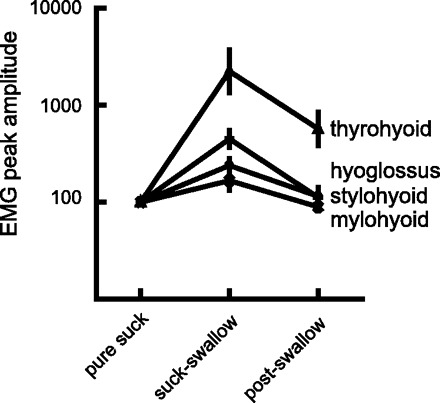
The ratios of the amplitudes of peak EMG activity in suck-swallow and postswallow cycles shown relative to the median peaks in suck cycles. Note that the mylohyoid data contain values from both anterior and posterior sites on the mylohyoid. The suck cycle amplitudes were designated an arbitrary 100 units. Note that the vertical axis is a log scale. The base data were the rectified, constant time (10 ms) reset EMG.
Two separate patterns of EMG activity were recorded from the mylohyoid during swallowing. The first, in anterior mylohyoid, was a simple repetition of the activity recorded during sucking (Fig. 4, A and B). The second pattern was a higher amplitude, and slightly delayed signal seen only in swallowing (Fig. 4C); it was found more frequently at posterior mylohyoid sites. Although there was variation with cycle type, no variation with electrode site was found in the thyrohyoid or stylohyoid muscles. In the restricted region near the hyoid attachment of hyoglossus, some temporal variation in hyoglossus EMG activity was found, but no systematic differences in signal with electrode site were evident. The processed thyrohyoid EMG activity in a suck-swallow cycle had a simple single peaked form. The timings of these bursts of thyrohyoid activity did not initially appear to have a fixed proportional relationship to the cycle defined by mylohyoid EMG activity (Fig. 2). However, when adequate numbers (over 20) of suck/swallow cycles were divided into two phases defined by the peak of the thyrohyoid activity, a clearer pattern emerged. The duration of the first phase, extending from the onset of mylohyoid activity to the peak of thyrohyoid activity, was largely independent of cycle duration (Fig. 5A and Table 1), although there was also obvious random variation around the regression line. However, the duration of the period from the peak of thyrohyoid activity to the onset of the next mylohyoid burst was a direct and linear function of cycle duration (P < 0.001) (Fig. 5A and Table 1). When peak stylohyoid activity was used to divide up the suck/swallow cycle, there was again an early, relatively stable duration phase and a later, clearly labile phase that reflected cycle duration, i.e., the relationship between the phase durations and cycle duration echoed that obtained with thyrohyoid division of the same cycles in the same animal (Fig. 5, A and B).
Fig. 4.
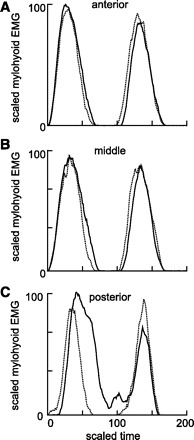
Processed mylohyoid EMG activity of pairs of consecutive suck cycles (dotted lines) and of pairs of successive suck-swallow cycles with postswallow cycles (solid line). The data are the median profiles of activity, derived from a single sequence of suckling in a single animal (suck cycle doublets: n = 28; suck-swallow with postswallow cycles: n = 7). The EMG activity was recorded from three different equidistant and successively anteroposterior sites on the mylohyoid (A–C).
Fig. 5.
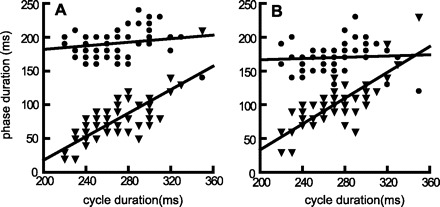
A: the durations of the early phases (mylohyoid-thyrohyoid,= circles) and of the late phases (thyrohyoid-mylohyoid, = triangles) of suck-swallow cycles plotted against total cycle duration. B: similar data, but using peak stylohyoid activity to define the early and late phases. Data from a single suckling period (68 swallows) in one animal.
Table 1.
Regressions of the duration of early and late components of the swallow against suck-swallow cycle duration
| Phase 1 Mylo1 |
Phase 2 Mylo2 |
||||
|---|---|---|---|---|---|
| Pig No. | Slope | r2 | Slope | r2 | No. of Swallows |
| Thyhy | |||||
| 1 | 0.083 | 0.02 | 0.917 | 0.69 | 105 |
| 2 | 0.092 | 0.02 | 0.908 | 0.66 | 46 |
| 3 | 0.112 | 0.12 | 0.744 | 0.32 | 68 |
| 4 | −0.022 | 0.01 | 1.022 | 0.91 | 35 |
| Styhy | |||||
| 3 | 0.046 | 0.004 | 0.954 | 0.612 | 68 |
| 5 | 0.053 | 0.003 | 0.947 | 0.470 | 22 |
Mylo1, activity at beginning of a suck-swallow cycle; Mylo2, activity at the beginning of the subsequent postswallow cycle: Thyhy, thyrohyoid; Styhy, stylohyoid. All phase 1 regression lines had a nonsignificant slope. All phase 2 regression lines had a statistically significant slope (P < 0.01).
In only one animal was there sufficient amplitude of the stylohyoid EMG during suck cycles to permit reliable division of those cycles into two phases and so allow an analysis similar to that of the suck-swallow cycles. In the data from 70 suck cycles, the regression line for the first phase had a low slope (0.16; r2 = 0.06, P = 0.62) while the second phase had a steep slope (0.84; r2 = 0.65, P < 0.001).
DISCUSSION
Traditionally, the swallow has been treated as an independent self-standing reflex event (6, 32) although it is increasingly being considered in its normal biological context of continuing oral activity both in humans (3, 5, 47) and in animals (20, 30, 45), The current study first used regularly occurring events in the cyclical EMG activities of suckling to define three different cycle types. Second, the study proceeded with particular reference to variation in the pattern of EMG activity in the two cycle types (suck-swallow and postswallow) that were closely associated with pharyngeal swallows.
Differences in duration and EMG pattern among three cycle types.
The durations of both suck and postswallow cycles were similar (240 ms), whereas the suck-swallow cycle durations were, on average, 22 ms longer. Similarly, the amplitudes of the scaled EMG activities (see materials and methods) in suck-swallow cycles were always of higher amplitude than in either the suck or the postswallow cycles (Fig. 3). Thyrohyoid EMG activity deviated from this pattern, particularly when the suck-swallow cycle duration was shorter than the interval between mylohyoid and thyrohyoid activity. In that case, the thyrohyoid burst extended into the following postswallow cycle, and this artificially inflated the calculated values for the amplitude of EMG activity arising in that cycle (Fig. 3). Because thyrohyoid activity is a marker for the early part of the pharyngeal swallow (12, 16), if the suck-swallow cycle is short, the later components of the pharyngeal swallow must extend into the following postswallow cycle. Consequently, the postswallow cycle is not wholly equivalent to a pure suck cycle despite the other similarities referred to above.
The clear differences among EMG signals recorded at different sites on the mylohyoid muscle during suck-swallow cycles (Fig. 4, A-C) suggested functional compartmentalization and/or different task units (7) within the mylohyoid, as in other oral muscles (26, 46, 50). EMG activity in the mylohyoid has traditionally been, and continues to be considered, a marker of the swallow (6, 19, 21, 22) although earlier EMG studies in humans using intramuscular electrodes (25, 33, 51) have described mylohyoid EMG activity as occurring in cyclical oral activity, independent of any swallowing, as well as in association with swallowing. In this study, anterior mylohyoid EMG activity signaled the onset of a cycle of oral activity irrespective of the cycle type, as is true in other nonprimate species (16, 48, 52).
In the posterior mylohyoid, the presence of the higher-amplitude delayed EMG activity during suck-swallow cycles suggested that additional sets of motor units were recruited that were specific to these cycles. Separate functional subgroups of mylohyoid fibers, specifically activated in a swallow, exist in both the decerebrate pig (41) and the intact rabbit (31). The differences in activation pattern between anterior and posterior mylohyoid may also be linked to biomechanical differences. The anterior mylohyoid fibers end in the midline raphe while the more vertically oriented posterior fibers attach to the hyoid and have the potential to be more involved in hyolaryngeal elevation during swallowing (31, 49).
Temporal relationship between rhythmic oral activity and the pharyngeal swallow.
The relationship between the pharyngeal swallows and the cycles of oral activity into which the swallows are inserted is complex (13, 43) and incompletely understood. The earlier assumption was that the pharyngeal swallow was inserted in the slow opening phase of a cycle of movement (17, 34, 45), at a specific locus. However, a rabbit study (48) has implied that the pharyngeal swallow can be inserted into cycles of oral movement at different temporal loci. Similarly, if one looks at only a limited number of cycles from our data, the high-amplitude burst of thyrohyoid EMG activity can appear to have been randomly timed within the cycle defined by the mylohyoid EMG bursts (Fig. 2). However, when a large number of suck-swallow cycles was considered and each suck-swallow cycle was divided into two phases by peak thyrohyoid EMG (Fig. 5A) or stylohyoid (Fig. 5B) activity, there was clear evidence that, on average, the early phase of the cycle was stable in duration while the later phase had a duration that was linearly related to cycle duration. In each phase, there was also an additional random timing component that could have had methodological or unrecognized biological origins.
The above description of a two-phase division of the suck-swallow cycle is similar to the limited data presented in this paper on suck cycle division and also to the general characteristics of other rhythmic movements in mammals. The central pattern generators that produce several different rhythmic reciprocating mammalian movements, such as locomotion or scratching, characteristically have the same two main phases, one of which has a relatively stable duration while the other changes as a function of (or dominates) cycle duration (15). This asymmetry in cycle generation exists even in the absence of sensory feedback (9, 14).
Relationship of pharyngeal swallow to timing of postswallow cycle.
The early phase of suck-swallow cycles, timed from mylohyoid onset (Fig. 5) to thyrohyoid peak, had a relatively constant duration. This supported the earlier proposition that the pharyngeal swallow had a fixed time relationship to the onset of suck-swallow cycles but, in contrast, the relationship of that marker to the termination of the suck-swallow cycle was highly variable. If the suck-swallow cycle ended before thyrohyoid activity had peaked, that cycle could contain only the swallow-related hyoglossal, stylohyoid, omohyoid, and posterior mylohyoid EMG activities that precede the thyrohyoid peak (13, 41). In such a case, the postswallow cycle contained the components of the pharyngeal swallow that followed thyrohyoid activity, primarily genioglossus, geniohyoid, inferior pharyngeal constrictor, cricothyroid, sternothyroid, and sternohyoid activity (13, 41). Conversely, longer-duration suck-swallow cycles contained more or all of that activity. This situation has major implications for studies attempting to extract the average cyclical pattern of EMG activity from a series of swallow-related cycles. It is essential to take account of what activities are contained within, or excluded from, the time frame of each of those cycles.
The data support the view that the changing relationship between the sequential components (13, 43) of the pharyngeal swallows and the ends of suck-swallow cycles is primarily a function of the changing duration of the rhythmic oral cycle. It is known that the durations of all cycles gradually increase over the medium term (over roughly 30–50 s of continuous suckling), which may well reflect central or peripheral aspects of fatigue. Within that longer trend, there are also successive 3- to 5-s subsections, each of which contains about three to five swallows and starts with a temporary decrease in cycle duration followed by a relatively rapid increase (11). In the infant pig, the biological significance of such changes in cycle durations for the efficient performance of swallowing is uncertain but, in humans, shortening the duration of drinking cycles is associated with reduced hyoid excursion and reduced swallowed volume (2). If a similar mechanism operated in the infant pig, then the rapid suckling at the start of a feeding session would be associated with a limitation on volume per swallow while the converse would apply subsequently as the suckling rate fell. One could speculate that such a mechanism would limit and stabilize the liquid load on the pharynx over wide ranges of suckling frequencies.
In conclusion, anterior mylohyoid EMG activity was a marker for the start of individual cycles in pig suckling. The longer duration of suck-swallow cycles, compared with pure suck or with immediate postswallow cycles, may be explained by the insertion of components of the pharyngeal swallow into the cycle.
The amplitudes of EMG activity in hyoglossus, stylohyoid, posterior mylohyoid, and particularly in thyrohyoid were greater in suck-swallow cycles. The timing of peak thyrohyoid activity was stable relative to the anterior mylohyoid activity at the onset of suck-swallow cycles. Conversely, the delay between the thyrohyoid peak and the anterior mylohyoid activity marking the onset of the following cycle was a linear function of suck-swallow cycle duration. Preliminary evidence was found for similar stable and labile phase divisions in suck cycles.
As suck-swallow cycles became shorter, the stable latency thyrohyoid activity occupied a relatively later position in that cycle and could extend into the postswallow cycle. In this situation, all components of the pharyngeal swallow occurring after the thyrohyoid burst were either partly or wholly contained within the postswallow cycle.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: A.J.T., A.W.C., and R.Z.G. conception and design of research; A.J.T., A.W.C., and R.Z.G. performed experiments; A.J.T. and R.Z.G. analyzed data; A.J.T., A.W.C., and R.Z.G. interpreted results of experiments; A.J.T. and R.Z.G. drafted manuscript; A.J.T., A.W.C., and R.Z.G. edited and revised manuscript; A.J.T., A.W.C., and R.Z.G. approved final version of manuscript; R.Z.G. prepared figures.
REFERENCES
- 1. Buchthal F, Schmalbruch H. Motor unit of mammalian muscle. Physiol Rev 60: 901–942, 1980 [DOI] [PubMed] [Google Scholar]
- 2. Chi-Fishman G, Sonies BC. Kinematic strategies for hyoid movement in rapid sequential swallowing. J Speech Lang Hear Res 45: 457–468, 2002 [DOI] [PubMed] [Google Scholar]
- 3. Chi-Fishman G, Sonies BC. Motor strategy in rapid sequential swallowing: new insights. J Speech Language Hearing Res 43: 1481–1492, 2000 [DOI] [PubMed] [Google Scholar]
- 4. Crompton AW, German RZ, Thexton AJ. Mechanisms of swallowing and airway protection in infant mammals (Sus domesticus and Macaca fascicularis). J Zool 241: 89–102, 1997 [Google Scholar]
- 5. Daniels SK, Foundas AL. Swallowing physiology of sequential straw drinking. Dysphagia 16: 176–182, 2001 [DOI] [PubMed] [Google Scholar]
- 6. Doty RW, Bosma JF. An electromyographic analysis of reflex deglutition. J Neurophysiol 19: 44–60, 1956 [DOI] [PubMed] [Google Scholar]
- 7. English AW, Wolf SL, Segal RL. Compartmentalization of muscles and their motor nuclei: the partitioning hypothesis. Phys Ther 73: 857–867, 1993 [DOI] [PubMed] [Google Scholar]
- 8. Ertas M, Stalberg E, Falck B. Can the size principle be detected in conventional EMG recordings? Muscle Nerve 18: 453–459, 1995 [DOI] [PubMed] [Google Scholar]
- 9. Frigon A, Gossard JP. Asymmetric control of cycle period by the spinal locomotor rhythm generator in the adult cat. J Physiol 587: 4617–4628, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fukushima Si Shingai T, Kitagawa Ji Takahashi Y, Taguchi Y, Noda T, Yamada Y. Role of the pharyngeal branch of the vagus nerve in laryngeal elevation. Dysphagia 18: 58–63, 2003 [DOI] [PubMed] [Google Scholar]
- 11. German RZ, Crompton AW, Hertweck DW, Thexton AJ. Determinants of rhythm and rate in suckling. J Exp Zool 278: 1–8, 1997 [DOI] [PubMed] [Google Scholar]
- 12. German RZ, Crompton AW, Levitch LC, Thexton AJ. The mechanism of suckling in two species of infant mammal: miniature pigs and long-tailed macaques. J Exp Zool 261: 322–330, 1992 [DOI] [PubMed] [Google Scholar]
- 13. German RZ, Crompton AW, Thexton AJ. Integration of the reflex pharyngeal swallow into rhythmic oral activity in a neurologically intact pig model. J Neurophysiol 102: 1017–1025, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gossard JP, Sirois J, Noue P, Cote MP, Menard A, Leblond H, Frigon A. Chapter 2–the spinal generation of phases and cycle duration. Prog Brain Res 188: 15–29, 2011 [DOI] [PubMed] [Google Scholar]
- 15. Grillner S. Biological pattern generation: the cellular and computational logic of networks in motion. Neuron 52: 751–766, 2006 [DOI] [PubMed] [Google Scholar]
- 16. Harding R, Titchen DA. Oesophageal and diaphragmatic activity during sucking in lambs. J Physiol 321: 317–329, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hiiemae KM, Crompton AW. Mastication, food transport and swallowing. In: Functional Vertebrate Morphology, edited by Hildebrand M, Bramble D, Liem K, Wake D. Cambridge, UK: Harvard Univ Press, 1985, p. 262–290 [Google Scholar]
- 18. Jean A. Brain stem control of swallowing: neuronal network and cellular mechanisms. Physiol Rev 81: 929–969, 2001 [DOI] [PubMed] [Google Scholar]
- 19. Jean A. Brainstem organization of the swallowing network. Brain Behav Evol 25: 109–116, 1984 [DOI] [PubMed] [Google Scholar]
- 20. Kaplan JM, Grill HJ. Swallowing during ongoing fluid ingestion in the rat. Brain Res 499: 63–80, 1989 [DOI] [PubMed] [Google Scholar]
- 21. Kijima H, Shingai T, Takahashi Y, Kajii Y, Fukushima Si Taguchi Y, Noda T, Yamada Y. Nitric oxide modulates elicitation of reflex swallowing from the pharynx. Am J Physiol Regul Integr Comp Physiol 291: R651–R656, 2006 [DOI] [PubMed] [Google Scholar]
- 22. Kitagawa J, Nakagawa K, Hasegawa M, Iwakami T, Shingai T, Yamada Y, Iwata K. Facilitation of reflex swallowing from the pharynx and larynx. J Oral Sci 51: 167–171, 2009 [DOI] [PubMed] [Google Scholar]
- 23. Lang IM, Dana N, Medda BK, Shaker R. Mechanisms of airway protection during retching, vomiting and swallowing. Am J Physiol Gastrointest Liver Physiol 283: G529–G536, 2002 [DOI] [PubMed] [Google Scholar]
- 24. Lefton-Greif MA, Carroll JL, Loughlin GM. Long-term follow-up of oropharyngeal dysphagia in children without apparent risk factors. Pediatr Pulmonol 41: 1040–1048, 2006 [DOI] [PubMed] [Google Scholar]
- 25. Lehr RP, Blanton PL, Biggs NL. An electromyographic study of the mylohyoid muscle. Anatomic Rec 169: 651–660, 1971 [DOI] [PubMed] [Google Scholar]
- 26. Lev-Tov A, Tal M, Lavy R. Diverse firing properties of single motor units in the inner and outer portions of the guinea pig anterior digastric muscle. Arch Oral Biol 38: 169–178, 1993 [DOI] [PubMed] [Google Scholar]
- 27. Linden P, Tippett D, Johnston J, Siebens A, French J. Bolus position at swallow onset in normal adults: preliminary observations. Dysphagia 4: 146–150, 1989 [DOI] [PubMed] [Google Scholar]
- 28. Loeb G, Gans C. Electromyography for experimentalists. Chicago, IL: Univ of Chicago Press, 1986, p. P119–120 [Google Scholar]
- 29. Martin-Harris B, Brodsky MB, Michel Y, Lee FS, Walters B. Delayed initiation of the pharyngeal swallow: normal variability in adult swallows. J Speech Language Hearing Res 50: 585–594, 2007 [DOI] [PubMed] [Google Scholar]
- 30. McFarland DH, Lund JP. An investigation of the coupling between respiration, mastication and swallowing in the awake rabbit. J Neurophysiol 69: 95–108, 1993 [DOI] [PubMed] [Google Scholar]
- 31. Meng Y, Uchida K, Sato T, Yamamura K, Yamada Y. Difference in the burst patterns of digastric and mylohyoid activities during feeding in the freely behaving rabbit. Dysphagia 14: 78–84, 1999 [DOI] [PubMed] [Google Scholar]
- 32. Miller RF, Sherrington CS. Some observations on the bucco-pharyngeal stage of reflex deglutition in the cat. Q J Exp Physiol 9: 147–186, 1916 [Google Scholar]
- 33. Moller E. The chewing apparatus. An electromyographic study of the action of the muscles of mastication and its correlation to facial morphology. Acta Physiol Scand Suppl 280: 1–229, 1966 [PubMed] [Google Scholar]
- 34. Naganuma K, Inoue M, Yamamura K, Hanada K, Yamada Y. Tongue and jaw muscle activities during chewing and swallowing in freely behaving rabbits. Brain Res 915: 185–194, 2001 [DOI] [PubMed] [Google Scholar]
- 35. Newman LA, Cleveland RH, Blickman JG, Hillman RE, Jaramillo D. Videofluoroscopic analysis of the infant swallow. Invest Radiol 26: 870–873, 1991 [DOI] [PubMed] [Google Scholar]
- 36. Sack WO. Pig Anatomy and Atlas. Ithaca, NY: 1982 [Google Scholar]
- 37. Storey AT. Laryngeal initiation of swallowing. Exp Neurol 20: 359–365, 1968 [DOI] [PubMed] [Google Scholar]
- 38. Takagi M, Noda T, Yamada Y. Comparison of SLN-evoked swallows during rest and chewing in the freely behaving rabbit. Brain Res 956: 74–80, 2002 [DOI] [PubMed] [Google Scholar]
- 39. Thexton AJ. A randomisation method for discriminating between signal and noise recordings of rhythmic electromyographic activity. J Neurosci Methods 66: 93–98, 1996 [DOI] [PubMed] [Google Scholar]
- 40. Thexton AJ, Crompton AW. Control of swallowing. In: Frontiers of Oral Biology, edited by LInden R. Basel, Switzerland: Karger, 1998, p. 168–222 [Google Scholar]
- 41. Thexton AJ, Crompton AW, German RZ. Electromyographic activity during the reflex pharyngeal swallow in the pig: Doty and Bosma (1956) revisited. J Appl Physiol 102: 587–600, 2007 [DOI] [PubMed] [Google Scholar]
- 42. Thexton AJ, Crompton AW, German RZ. Transition from suckling to drinking at weaning: a kinematic and electromyographic study in miniature pigs. J Exp Zool 280: 327–343, 1998 [DOI] [PubMed] [Google Scholar]
- 43. Thexton AJ, Crompton AW, Owerkowicz T, German RZ. Impact of rhythmic oral activity on the timing of muscle activation in the swallow of the decerebrate pig. J Neurophysiol 101: 1386–1393, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Thexton AJ, German RZ. Synchronization of Electromyographic activity in oral musculature during suckling and drinking. Bull Mus Comp Zool 156: 249–256, 2001 [Google Scholar]
- 45. Thexton AJ, McGarrick JD. Tongue movement of the cat during lapping. Arch Oral Biol 33: 331–339, 1988 [DOI] [PubMed] [Google Scholar]
- 46. Tsuruyama K, Scott G, Widmer CG, Lund JP. Evidence for functional partitioning of the rabbit digastric muscle. Cell Tissue Organ 170: 170–182, 2002 [DOI] [PubMed] [Google Scholar]
- 47. Tsushima C, Saitoh E, Baba M, Yokoyama M, Fujii W, Okada S, Uematsu H. Hyoid movement and laryngeal penetration during sequential swallowing. J Med Dental Sci 56: 113–121, 2009 [PubMed] [Google Scholar]
- 48. Uchida K, Yamada Y, Sato T. The coordination of rhythmical drinking behavior with swallowing in rabbits. Physiol Behav 55: 795–801, 1994 [DOI] [PubMed] [Google Scholar]
- 49. van Eijden TMGJ, Koolstra JH. A model for mylohyoid muscle mechanics. J Biomechan 31: 1017–1024, 1998 [DOI] [PubMed] [Google Scholar]
- 50. van Lunteren E, Dick TE. Heterogeneity within geniohyoid motor unit subpopulations in firing patterns during breathing. Respir Physiol 124: 23–33, 2000 [DOI] [PubMed] [Google Scholar]
- 51. Vitti M, Basmajian JV. Integrated actions of masticatory muscles: simultaneous EMG from eight intramuscular electrodes. Anat Rec 187: 173–189, 1977 [DOI] [PubMed] [Google Scholar]
- 52. Weijs WA, Dantuma R. Electromyography and mechanics of mastication in the albino rat. J Morphol 146: 1–33, 1975 [DOI] [PubMed] [Google Scholar]


