Abstract
Adult skeletal muscle in mammals is a stable tissue under normal circumstances but has remarkable ability to repair after injury. Skeletal muscle regeneration is a highly orchestrated process involving the activation of various cellular and molecular responses. As skeletal muscle stem cells, satellite cells play an indispensible role in this process. The self-renewing proliferation of satellite cells not only maintains the stem cell population but also provides numerous myogenic cells, which proliferate, differentiate, fuse, and lead to new myofiber formation and reconstitution of a functional contractile apparatus. The complex behavior of satellite cells during skeletal muscle regeneration is tightly regulated through the dynamic interplay between intrinsic factors within satellite cells and extrinsic factors constituting the muscle stem cell niche/microenvironment. For the last half century, the advance of molecular biology, cell biology, and genetics has greatly improved our understanding of skeletal muscle biology. Here, we review some recent advances, with focuses on functions of satellite cells and their niche during the process of skeletal muscle regeneration.
I. INTRODUCTION: SATELLITE CELLS AS ADULT STEM CELLS IN MUSCLE
Skeletal muscle is a form of striated muscle tissue, accounting for ∼40% of adult human body weight. Skeletal muscle is composed of multinucleated contractile muscle cells (also called myofibers). During development, myofibers are formed by fusion of mesoderm progenitors called myoblasts. In neonatal/juvenile stages, the number of myofibers remains constant, but each myofiber grows in size by fusion of satellite cells, a population of postnatal muscle stem cells. Adult mammalian skeletal muscle is stable under normal conditions, with only sporadic fusion of satellite cells to compensate for muscle turnover caused by daily wear and tear. However, skeletal muscle has a remarkable ability to regenerate after injury. Responding to injury, skeletal muscle undergoes a highly orchestrated degeneration and regenerative process that takes place at the tissue, cellular, and molecular levels. This results in the reformation of innervated, vascularized contractile muscle apparatuses. This regeneration process greatly relies on the dynamic interplay between satellite cells and their environment (stem cell niche). During the last half century, advances in molecular biology, cell biology, and genetics has greatly improved our understanding of skeletal muscle regeneration. In particular, extensive research on satellite cells and their niche has elucidated many cellular and molecular mechanisms that underlie skeletal muscle regeneration. These studies have contributed to the development of therapeutic strategies. These strategies serve to alleviate the physiological and pathological conditions associated with poor muscle regeneration observed in sarcopenia and muscular dystrophy.
Here, we concentrate on the functions of satellite cells and the regulation of their niche during the process of skeletal muscle regeneration. We first describe the current understanding of satellite cells with respect to their characteristics, heterogeneity, and embryonic origin. We then provide an integrated view of the roles played by satellite cells during muscle regeneration and normal postnatal muscle growth. We also discuss the contribution of several nonsatellite cell populations in muscle regeneration and their lineage relationships with satellite cells. Next, we focus on the satellite cell niche with emphasis on the regulatory mechanisms associated with each niche component. We further review the links between malfunction of satellite cells and their niche factors during aging. This review focuses on satellite cells and their niche in mammalian models, paying limited attention to the studies of satellite cell biology in other model organisms.
A. A Brief History of Satellite Cells
Half a century ago, Alexander Mauro observed a group of mononucleated cells at the periphery of adult skeletal muscle myofibers by electron microscopy (329). These cells were named satellite cells due to their sublaminar location and intimate association with the plasma membrane of myofibers.
The direct juxtaposition of satellite cells and myofibers immediately raised a hypothesis that these cells may be involved in skeletal muscle growth and regeneration (329). Indeed, experiments by [3H]thymidine labeling and electron microscopy demonstrated that satellite cells undergo mitosis, assume a cytoplasm-enriched morphology, and contribute to myofiber nuclei (355, 437). Later on, [3H]thymidine tracing experiments indicated that satellite cells are mitotically quiescent in adult muscle but can quickly enter the cell cycle following muscle injury (499). The same study also demonstrated that satellite cells give rise to proliferating myoblasts (myogenic progenitors cells), which were previously shown to form multinucleated myotubes in vitro (276, 499, 574). More definitive evidence came from in vitro cultures of individually dissected myofibers, whereby the behaviors of single myofibers and their resident satellite cells during regeneration can be tracked by phase-contrast microscopy (51, 277). It was observed that myofiber necrosis is accompanied by satellite cell outgrowth, clonal expansion, and later fusion to form functional regenerated myotubes. These experiments support the notion that it is the satellite cell, rather than the myonuclei, that contribute to postnatal muscle growth and repair.
The pivotal function of the satellite cell in muscle regeneration stimulated research to determine their regulatory mechanisms. This started with the finding that quiescent satellite cells on single myofibers were activated to enter the cell cycle by a mitogen originating from crushed skeletal muscles (53). In search of this mitogen, it was found that the proliferation and differentiation of satellite cell-derived myoblasts cultured in vitro respond to various growth factors in a dose-dependent manner (10, 11). Numerous studies further revealed that the proliferation and differentiation of satellite cells during muscle regeneration is profoundly influenced by innervation, the vasculature, hormones, nutrition, and the extent of tissue injury (121, 224, 250, 332, 360, 410). Interestingly, satellite cells in contact with the plasma membrane of intact myofibers were found to have reduced sensitivity to mitogen stimulation (50). All of these findings led to an intriguing notion that satellite cells reside in a special microenvironment in vivo, which profoundly affects their behavior.
By definition, stem cells found in adult tissues can both replicate themselves (self-renew) and give rise to functional progeny (differentiate). Although the ability of satellite cells to differentiate into myofibers was clearly proven, their ability to self-renew was once questioned. The first evidence of satellite cell self-renewal came from a single myofiber transplantation assay. It was found that as few as seven satellite cells, when transplanted into irradiated regeneration-insufficient mice along with their resident single myofiber, can give rise to hundreds of satellite cells and thousands of myonuclei. Most importantly, transplanted satellite cells on a single myofiber were able to support subsequent rounds of muscle regeneration (105). These observations demonstrate that satellite cells are bona fide muscle stem cells.
Asymmetric division is a common manner of stem cell self-renewal. Close examination of satellite cell divisions on single myofibers revealed that satellite cells can undergo both asymmetric division and symmetric division (280). The fashion of symmetric versus asymmetric division largely depends on the relative position of the daughter cells in relation to the myofiber. This finding strongly argues that satellite cell self-renewal is governed by the structure and signaling present in their immediate niche (280). Further investigation has revealed an important role for noncanonical Wnt signaling in the regulation of satellite cell self-renewal (293).
The aforementioned observations, together with those from other studies, formed the basis of our current view of satellite cells as muscle stem cells whose functions are dictated by their surrounding niche.
B. Identification and Isolation of Satellite Cells
Classically, satellite cells were identified based on a unique anatomical location: beneath the surrounding basal lamina and outside the myofiber plasma membrane. This anatomical location gives satellite cells a “wedged” appearance when viewd by electrictron microscropy (329). This technique also revealed other morphological characteristics of satellite cells: large nuclear-to-cytoplasmic ratio, few organelles, small nucleus, and condensed interphase chromatin. This morphology is in harmony with the notion that most satellite cells, in healthy unstressed muscles, are mitotically quiescent (in G0 phase) and transcriptionally inactive (472). In addition to electron microscopy, satellite cells can be identified by phase-contrast microscopy on single myofiber explants (53). Based on the same principle, the behaviors of satellite cells on single myofiber explants can be recorded by utilizing live cell imaging techniques (490). The identification of satellite cells by fluorescence microscopy relies on specific biomarkers (FIGURE 1). In adult skeletal muscle, all or most of satellite cells express the paired domain transcription factors Pax7 (478) and Pax3 (72), myogenic regulatory factor Myf5 (116), homeobox transcription factor Barx2 (336), cell adhesion protein M-cadherin (244), tyrosine receptor kinase c-Met (13), cell surface attachment receptor α7-intergin (73, 192), cluster of differentiation protein CD34 (37), transmembrane heparan sulfate proteoglycans syndecan-3 and syndecan-4 (113), chemokine receptor CXCR4 (429), caveolae-forming protein caveolin-1 (192, 552), calcitonin receptor (177), and nuclear envelope proteins lamin A/C and emerin (192). Of these, Pax7 is the canonical biomarker for satellite cells as it is specifically expressed in all quiescent and proliferating satellite cells (478) across multiple species, including human (334), monkey (334), mouse (478), pig (402), chick (216), salamander (353), frog (96), and zebrafish (219). Of note, some of the aforementioned satellite cell markers (e.g., α7-intergin, CD34) are also expressed on other cell types within skeletal muscle, and thus should not be utilized alone to unequivocally identify satellite cells. Satellite cells can be identified by fluorescence microscopy via combined immunofluorescence labeling of laminin and M-cadherin, which respectively label the basal lamina and plasma membrane of satellite cells contacting the myofibers (517). In vivo, satellite cell populations can be visualized with the aid of newly developed bioluminescence imaging techniques (454, 558).
Figure 1.
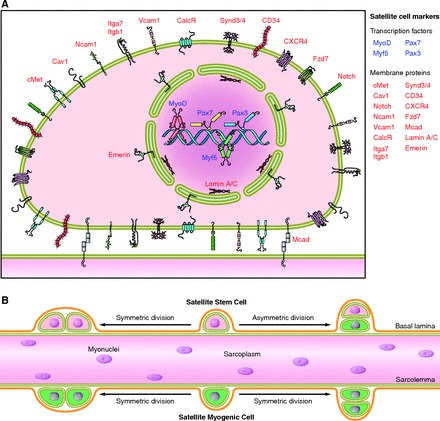
Characteristics of the satellite cell. A: numerous proteins are expressed in satellite cells and have been used as markers to distinguish between surrounding cell types within skeletal muscle. Due to heterogeneity in satellite cell populations, it is unlikely that all of these markers are expressed in a given satellite cell at a specific time. Nevertheless, this panel summarizes the cellular location of markers used to identify satellite cells. B: the satellite cell population is heterogeneous and can be classified in a hierarchical manner based on function and gene expression. Evidence from lineage tracing experiments identified a subpopulation of satellite cells having never expressed the myogenic transcription factor Myf5 (satellite stem cells) are placed hierarchically above satellite cells that have expressed Myf5 at some point during development (satellite myogenic cells). Satellite stem cells, upon asymmetric division (typically in a apical-basal orientation), will give rise to two daughter cells, only one of which has activated Myf5. Functional differences in regenerative potential exist between satellite stem cells and satellite myogenic cells. Following transplantation, satellite stem cells preferentially repopulate the satellite cell niche and contribute to long-term muscle regeneration. In contrast, satellite myogenic cells preferentially differentiate upon transplantation in vivo.
Multiple methodologies have been developed to isolate satellite cells from skeletal muscle. The choice of methods largely depends on the isolation scale and the subsequent experiment. In small scale, limited amounts of satellite cells can be released from single myofiber explants by physical trituration (446) or enzymatic digestion (107). In large scale, satellite cells can be isolated from skeletal muscles by fluorescent-activated cell sorting (FACS). In the latter method, single cells are released from muscle tissue chunks by enzymatic digestion, followed by immunofluorescence labeling of satellite cell-specific cell surface markers (positive selection) and definitive cell surface markers for nonsatellite cell populations (negative selection). As Pax7 is specifically expressed in satellite cells within skeletal muscle, satellite cells, in both quiescent and proliferating stages, can be FACS-sorted by fluorescent protein expression in tamoxifen-injected animals carrying both a Pax7CreER allele and a fluorescence Cre reporter allele (e.g., R26R-EYFP). In addition, two transgenic mouse strains, which carry fluorescence proteins driven by Nestin- or Pax7-regulatory elements, are also useful for isolating satellite cells by FACS (63, 127).
Protocols have been developed to isolate satellite cells in bulk utilizing their characteristic proliferation and adhesion characteristics under defined culturing conditions (144, 364, 425). It is noteworthy that the satellite cell progeny cultured on regular collagen-coated plastic dishes and under activation conditions, also called satellite cell-derived primary myoblasts, are molecularly and functionally distinct from the satellite cells freshly isolated from muscles (160, 177, 350). In addition, the gene expression profile of these cultured primary myoblasts differs from that of activated satellite cells in vivo (398). These essential differences are possibly due to the lack of a satellite cell niche in in vitro culturing conditions. One challenge in future studies is to understand the components and role of the satellite cell niche to better control and manipulate their quiescence, activation, proliferation, and differentiation.
C. Heterogeneity of Satellite Cell Population
Satellite cells were considered to be a homogeneous population of committed muscle progenitors (52). However, recent evidence demonstrated that the satellite cell population is heterogeneous and that satellite cells differ in their gene expression signatures, myogenic differentiation propensity, stemness, and lineage potential to assume nonmyogenic fates.
With regard to gene expression, it was first observed that only a subset of satellite cells expresses Pax3, a close paralog to Pax7 (350, 431). Although Pax3+ satellite cells tend to be associated with skeletal muscle of certain anatomical locations (e.g., diaphragm and truck muscles), the difference of Pax3 expression does not seem to correlate with their embryonic origins, metabolic fiber types, or types of innervating motor neurons. Examination of the expression of satellite cell markers CD34, M-cadherin, and Myf5 by immunofluorescence staining revealed that a subpopulation of satellite cells do not express these markers (37). Similarly, human satellite cells also manifest heterogeneity in their Pax7, neuronal cell adhesion molecule (NCAM), c-Met, and Dlk1 expression (311). By RT-qPCR, two recent studies showed that satellite cells from head or body muscles are distinct in their molecular signatures (225, 456). Notably, the heterogeneity of gene expression in single satellite cells was investigated in a recent study, wherein the expression of Pax7, Pax3, Myf5, and MyoD was interrogated by RT-qPCR for 40 individually FACS-isolated muscle stem cells (CD45−/CD11b−/CD31−/Sca-1−/α7-intergin+/CD34+) (454). All of these muscle stem cells express Pax7, indicating they are satellite cells. In addition to the varied expression of Pax3, it was also found that 25% of investigated satellite cells also express MyoD, a basic helix-loop-helix transcription factor critical for myogenic commitment and differentiation. A recent study discovered a small subpopulation of satellite cells, characterized by their surface markers Sca-1+/ABCG2+ (as compared with Sca-1−/ABCG2− for most satellite cells), is able to exclude Hoechst 33342 dye and thus belongs to the skeletal muscle side population (satellite-SP cells) (518). After transplantation into damaged muscle, satellite-SP cells can both fuse to regenerating myofibers and return to quiescence in the satellite cell niche. However, by lineage tracing, ABCG2+ cells (including satellite-SP cells) seem to only have minor contribution to myofiber regeneration (146).
Satellite cells are also heterogenic in their differentiation potential. Satellite cells on single myofibers isolated from various skeletal muscle sources were transplanted into irradiated muscles of mdx/nude mice, which underwent repeated regeneration and were depleted of endogenous satellite cells (105). It was observed that the number of regenerated myofibers contributed by tibialis anterior (TA) originated grafts was significantly less than that derived from either extensor digitorum longus (EDL) or soleus muscle. Although the potential contribution from donor myofibers cannot be precisely evaluated, this observation strongly suggests inherent differences in proliferation/differentiation potential of satellite cells and/or composition of satellite cell subpopulations from various muscles. In line with this view, continuous BrdU labeling of satellite cells in vivo revealed two satellite cell populations that are distinct in terms of their mitotic rates (471). It was found that the majority (∼80%) of satellite cells readily enter the cell cycle (responsive population), whereas the remaining 20% of satellite cells (reserve population) do this in a much slower manner. It has been proposed that the reserve population maintains in the quiescent state at the beginning of muscle growth/regeneration and only moves into this proliferative state in response to the need for extensive muscle growth/regeneration (471). In line with this view, a recent study traced satellite cell divisions by a fluorescent dye PKH26, which revealed the minority of PKH26high slow-dividing satellite cells retained long-term self-renewal ability (388). Notably, a recent study thoroughly compared the gene expression profiles and proliferation/differentiation potential of satellite cells isolated from limb and facial muscles of adult and aged mice (387). This study revealed broad satellite cell heterogeneity at both the population and single-cell levels. Despite their heterogeneous gene expression profiles, satellite cells isolated from limb (EDL) and facial (masseter) muscles are comparable in their ability to repair a limb (TA) muscle injury after transplantation. This finding suggests that although satellite cells from various muscles have distinct gene expression and behaviors in vitro, their regeneration potential in vivo might be largely determined by host stem cell niche and microenvironment.
Most importantly, recent studies revealed satellite cell heterogeneity in terms of their stemness and indicate that only a small percentage of satellite cells are true stem cells (109, 280, 489). By immunofluorescence staining of freshly isolated single myofibers from Myf5-nLacZ mice, our group demonstrated that 13% of quiescent satellite cells on EDL muscles are LacZ−, making them distinct from the LacZ+ satellite cells (280). As Myf5 is a myogenic regulatory factor, the absence of Myf5 expression suggests a less committed cell fate for those LacZ− satellite cells. Moreover, by the Cre-LoxP based lineage tracing technique, our group further discovered that 10% of satellite cells in Myf5Cre;R26R-YFP mice do not express YFP (Pax7+/YFP− satellite cells), indicating that this small percent of satellite cells have never expressed Myf5 as did the majority of satellite cells (Pax7+/YFP+). Remarkably, these two distinct satellite cell subpopulations also differ in terms of their regenerative potential: the Pax7+/YFP− satellite cells were able to reconstitute the stem cell niche and repaired muscles in a sustainable manner, whereas the Pax7+/YFP+ satellite cells directly underwent myogenic differentiation when transplanted in regenerating muscles of Pax7−/− mice. We further demonstrated that only Pax7+/YFP− satellite cells could undergo asymmetric cell divisions, giving rise to a Pax7+/YFP− satellite stem cell and a Pax7+/YFP+ satellite committed progenitor cell. These findings indicate that a hierarchical lineage progression from the Pax7+/YFP− (satellite stem cell) to the Pax7+/YFP+ (satellite committed progenitors) exists within the total satellite cell population. Consistent with this notion, multiple studies reported that only a subset of satellite cells undergo asymmetric division in vivo or in vitro (108, 109, 489). For example, Numb, an inhibitor of Notch signaling and a cell-fate determinant, was found to asymmetrically distribute in some but not all satellite cell divisions (108, 489). By pulse labeling or tandem pulse labeling of growing/regenerating muscles with halogenated thymidine analogs, it was further discovered that all “older” chromosomes (the template chromosome during DNA replication during S phase) cosegregate into the more stemlike daughter cell, whereas “younger” chromosomes are inherited by the more differentiated daughter cell during asymmetric divisions of satellite cells both in vivo and in vitro (109, 489). According to the “immortal DNA strand” hypothesis (75), this preferential retention of “older” chromosomes protects stem cells against the accumulation of mutations introduced during DNA replications (109, 489). Alternatively, it has been also proposed that the nonrandom segregation of sister chromatids, and hence their different epigenetic states, is essential to the gene expression patterns and cellular fates of satellite stem cells and satellite progenitor cells (289). In summary, the aforementioned findings support satellite cell heterogeneity whereby the existence of a hierarchy delineates a small population of true stem cells (satellite stem cells) from a more committed myogenic progenitor population of satellite cells. The satellite stem cells are less committed to the myogenic lineage and tend to retain older template DNA during division. Through asymmetric division, satellite stem cells self-renew to replenish the stem cell pool and produce more committed myogenic progenitors that participate in skeletal muscle growth and regeneration.
Satellite cells also exhibit heterogeneity in respect to their cell fate potential. Our group first revealed that satellite cells have an intrinsic potential to differentiate into multiple mesenchymal lineages (23). When cultured on solubilized basement membrane matrix (matrigel), satellite cells from single myofibers spontaneously differentiate into myocytes, adipocytes, and osteocytes. This finding indicates that satellite cells functionally resemble bone marrow-derived mesenchymal stem cells. This notion is substantiated by another study wherein satellite cells were found to assume the adipocyte lineage, which can be enhanced upon oxygen-rich culture conditions (120). By clonal analysis, it was found that myogenic and adipogenic satellite cells are two separate populations in the satellite cell compartment, although both populations express the myogenic marker Pax7 as well as the adipogenic markers peroxisome proliferator-activated receptors γ (PPARγ) and CCAAT/enhancer binding proteins (C/EBPs) (485). This similar molecular signature may reflect a common developmental origin of satellite cells and adipogenic progenitors in embryonic somites (discussed in sect. IIE). Satellite cell heterogeneity with regard to myogenic and nonmyogenic potential was thoroughly investigated by in vitro differentiation and in vivo transplant assays (486). In this study, myofiber-associated satellite cells were isolated from undamaged skeletal muscle by a two-step enzymatic digestion and sorted by FACS based on their differential expression of cell surface markers, CD45 and Sca-1. It was found that the vast majority of myogenic satellite cells are within the CD45−/Sca-1− population and exhibited no in vitro differentiation potential into fibroblasts or adipocytes. In contrast, the minor population of CD45−/Sca-1+ satellite cells can differentiate into both fibroblasts and adipocytes in culture, a similarity that is shared with CD45−/Sca-1+ mesenchymal stem cells found in multiple tissues (316, 401, 417, 441, 509, 519). Despite the plasticity of satellite cells in vitro, it is important to note that myogenesis is the predominant fate of satellite cells in vivo as fibrosis or adipose infiltration is not normally observed in young healthy muscle. Furthermore, recent studies indicate that intramuscular adipocytes and fibroblasts can also arise from fibrocyte/adipocyte progenitors (FAPs), which reside in the muscle interstitium (252, 541; and discussed in sect. IIIB1). Indeed, lineage tracing experiments indicate that adipocytes derived from myofibers isolated from MyoDiCre;R26R-EYFP mice have never transcribed MyoD (507). As the vast majority of adult satellite cells were permanently labeled with EYFP in this experiment, this finding argues that most satellite cells from myofiber cultures do not spontaneously differentiate into adipocytes. Further investigation with more definitive lineage tracing (e.g., Pax7CreER;R26R-EYFP) methods would clarify the exact contribution of satellite cells to other nonmyogenic lineages both in vitro and in vivo.
As discussed here, multiple lines of evidence demonstrate that satellite cells represent a heterogeneous population. However, our understanding of this heterogeneity is far from complete. First, although several markers can separate the total satellite cell population into functional subpopulations, it is still unknown whether these subpopulations are homogeneous in their function and gene expression. Further studies to identify additional satellite markers will potentially help distinguish the various satellite cell lineages. Moreover, future investigations should attempt to identify the intrinsic differences between satellite cell subpopulations at the molecular and functional levels during muscle regeneration. Such findings would elucidate regulatory mechanisms governing the transition between different satellite cell subpopulations and potentially distinct roles of satellite cell subpopulations during muscle regeneration. In addition, it would be of great importance to understand the dynamics of satellite cell heterogeneity in response to various environmental cues with regard to research in muscle regeneration and disease.
D. Variance of Satellite Cells Number and Location
In addition to the heterogeneity of satellite cells, the quantity of satellite cells differs between muscles, myofiber types, developmental stages, and species. In general, satellite cells account for 30–35% of the sublaminal nuclei on myofibers in early postnatal murine muscles, and this number declines to 2–7% in adult muscles (9, 227, 450, 469). In adults, the percentage of satellite cells in soleus muscle is generally two- to fourfold higher than that in tibialis anterior muscle or EDL muscle (190, 466, 500). Within the same muscle, the number of satellite cells found on slow muscle myofibers (type I) is generally higher than those on fast myofibers (type IIa and type IIb) (190, 324, 383). The biological meaning and the potential regulatory mechanisms underlying these phenomena are poorly understood. However, it is conceivable that these variances may reflect intrinsic heterogeneity of satellite cells on different myofibers and implicate a potential role of myofibers as a niche factor in regulating the homeostasis of their resident satellite cells.
Along a myofiber, the distribution of satellite cells is not random. It has been reported that the density of satellite cells is higher at the ends of the myofibers, where the longitudinal growth of skeletal muscles happens (14). A higher incidence of satellite cells has been observed at perisynaptic regions compared with that at extrasynaptic regions (190, 269, 567). Moreover, satellite cells have been observed in close proximity to capillaries (100, 466). In fact, 88% of satellite cells in adult human muscles were observed to be located within a 21-μm distance of a capillary (100). This tight association with capillaries seemed to be compromised by denervation (130). These observations indicate that homing of satellite cells is influenced by their niche, both by local motor neurons and blood vessels (discussed in sect. IIIB).
It is noteworthy that some reported variation in satellite cell numbers may be partially due to techniques or statistical analysis employed in satellite cell counting. For example, satellite cell counting based on immunofluorescence labeling of satellite cell specific markers relies on the comparable expression levels of these markers on all satellite cells under investigation. As such, special caution should be taken into account when interpreting data between independent experiments.
E. Origins of Adult Satellite Cells
1. Embryonic origins
By classic techniques of developmental biology, it has long been established that skeletal muscles within both the adult trunk and limbs develop from embryonic somites (462). However, the exact origin of adult satellite cells was obscure until recently.
Early experiments using a quail-chick chimera technique suggested a somitic origin of satellite cells in amniotes (18). Embryonic somites are segments of paraxial mesoderm formed on both sides of the body axis. In this experiment, somites from donor quail embryos were transplanted into host chick embryos. After embryonic development, the contribution of quail cells to the chick satellite cell compartment was determined using Feulgen staining, which distinguishes quail-specific interphase nuclei from those of chick. It was found that donor cells from quail somites integrated into the chick limb and contributed to both terminally differentiated muscle fibers and satellite cells. This finding indicated a common somitic origin for all myogenic cell lineages, including satellite cells. However, the progenitor cell types at the origin and the developmental route remain unknown.
Advances in mouse genetics, particularly the generation of Pax3 and Pax7 knock-in reporter alleles, allows precise tracing of Pax3/Pax7-expressing myogenic progenitor cells during muscle development in a temporal and spatial manner (265, 326, 350, 432, 433). These reporters together with labeling of cells by electroporation (40, 203), retrovirus (464), Cre-LoxP based lineage tracing (263, 300, 464), and traditional quail-chick transplantation (203, 464), jointly shed light on the embryonic origins of adult satellite cells. Accumulating evidence indicates that adult satellite cells originate from the dermomyotome (203, 265, 434, 464), an epithelial structure formed on the dorsal part of the somite. The dermomyotome contains multipotent progenitor cells, which eventually give rise to multiple adult tissues/cell types including dermal fibroblasts, endothelial cells, vascular smooth muscle, brown fat tissue, and all skeletal muscles of the trunk and limbs (71). The cell fate decisions largely depend on the relative position of these multipotent progenitor cells with respect to adjacent tissues such as the notochord, neural tube, dorsal ectoderm, and myotome (71).
Embryonic muscle development takes place in two successive stages. During the first stage, a group of postmitotic mononucleated myocytes, expressing Myf5 and Mrf4, migrate out from the border regions of the dermomyotome and form primitive muscles beneath the dermomyotome (204, 261). These primitive muscles constitute the primary myotome and are the source of fetal and adult trunk muscles. During the second stage of muscle growth, the central portion of the dermomyotome undergoes an epithelial-to-mesenchymal transition (EMT). During EMT, tightly packed epithelial cells tease apart and turn in a loose mesenchymal state prior to assuming different developmental fates: cells in the medial dermomyotome will develop into brown fat, dermis, and trunk muscle, while cells in the lateral dermomyotome will give rise to endothelia and limb muscles. The different cell fates assumed by the same group of progenitor cells are proposed to be due to asymmetric cell division (40, 101). EMT is accompanied by the extensive cell migration (203, 265, 434, 464). With the breakdown of dermomyotome, a group of proliferating progenitor cells, expressing both Pax3 and Pax7, migrate from the central region of the dermomyotome into the previously formed primary myotome. Upon arrival, some progenitor cells continue to proliferate and replenish the progenitor pool. These cells, which were absent from the primary myotome at earlier stages, count for the majority of all proliferating cells in embryonic/fetal trunk muscles (203, 434). Some of the proliferating Pax3+/Pax7+ progenitor cells persist into late fetal development stages and are enveloped beneath the basal lamina of developing myofibers (203, 434). These cells, which reside in the satellite cell compartment, are presumed to subsequently become the postnatal satellite cells in trunk muscles. Besides proliferation, progenitor cells also exit the cell cycle and begin differentiating into embryonic/fetal truck muscles. Cell cycle withdrawal is concomitant with the expression of the myogenic regulatory factors MyoD and Myf5 (453).
During EMT, another group of proliferating progenitor cells, expressing Pax3 (but not Pax7 in mouse), delaminate from the ventral-lateral border of the dermomyotome and migrate to the limb bud mesenchyme (265, 392, 464). These progenitor cells still maintain their multipotency as they give rise to the limb vascular, lymphatic endothelia, and limb muscles (226, 264). At E11.5 of mouse embryo development, some progenitor cells start to express Pax7 in the anterior limb buds (433). The expression of Pax7 specifies these cells to the myogenic lineage (242). Similar to the myotome-located progenitors, these Pax3+/Pax7+ progenitor cells in limb buds undergo proliferation/differentiation while a portion withdraw from cell cycle and become satellite cells.
All together, these observations indicate that Pax3+/Pax7+ embryonic progenitor cells are the major source of adult satellite cells in truck and limb muscles; it is, however, noteworthy that the aforementioned observations from lineage tracing and immunofluorescence labeling experiments cannot exclude the possibility that some adult satellite cells may originate from other sources during fetal and postnatal muscle development. For example, embryonic dorsal aorta explants, when cultured and disaggregated in vitro, can efficiently give rise to myogenic precursors (129). These myogenic precursors are similar to satellite cells in their gross morphology and expression of molecular markers. Given that adult satellite cells also express endothelial markers, it was proposed that some adult satellite cells originated from the embryonic dorsal aorta (129). However, recent studies revealed that both skeletal muscles and smooth muscles found in dorsal aorta are derived from the same Pax3+ cell population in the paraxial mesoderm (155). Thus it is also possible that the observed similarities are merely reminiscent of a common embryonic origin before myogenic specification.
Distinct from trunk and limb muscles, head muscles have multiple embryonic origins. The majority of head muscles, including branchiomeric muscles and most extraocular muscles, arise from the cranial paraxial mesoderm (CPM) (225, 540). Posterior neck muscles and tongue develop from occipital somites. Similar to progenitor cells migrating to limb buds, the progenitor cells for tongue delaminate from the ventral-lateral border of occipital somites. A small fraction of extraocular muscles also arise from the prechordal mesoderm (PM). On the basis of observations from lineage tracing experiments, it has been found that adult satellite cells of the various head muscles originate from their corresponding embryonic muscles and express distinct combinations of signature genes (225). Unlike trunk and limb progenitors, most progenitor cells for head muscles (except for tongue) express MesP1 rather than Pax3.
2. Alternative origins of adult satellite cells
Accumulating evidence indicates that some adult satellite cells may have alternative origins other than dermomyotome-derived Pax3+/Pax7+ progenitor cells.
First, multiple studies have demonstrated that several types of nonsatellite cells can reconstitute the satellite cell niche and turn into bona fide satellite cells (Pax7-expressing myogenic cells) after transplantation into regenerating skeletal muscles (for details, see sect. IID). It remains, however, largely unknown to what extent these cells contribute to the adult satellite cell pool and muscle development under physiological conditions. Notably, a recent study utilizing TN-APCreERT2 and VE-cadherinCreERT2 alleles showed that alkaline phosphatase (ALPL) expressing pericytes, but not VE-cadherin-expressing endothelial cells, can develop into postnatal satellite cells and participate in normal development of limb muscles (135).
It is noteworthy that some adult satellite cells in mammals may be derived from dedifferentiation of fetal/adult myofibers. It has long been established that the dedifferentiation of “terminally” differentiated multinucleated myofibers occurs in injured skeletal muscle of urodele amphibians, such as the newt (69). Nevertheless, it remains controversial as to whether mammalian myotubes in vitro or myofibers in vivo can undergo a similar dedifferentiation process. Studies utilizing immortalized murine myoblast cell lines (e.g., C2C12 or pmi28 cells) have shown that some myotubes formed by these cells can dedifferentiate into mononucleated myogenic cells in the presence of protein extract from regenerating newt muscles (331), bioactive compounds like myoseverin or its derivatives (149, 443), ciliary neurotrophic factor (CNTF) (95), or by genetic manipulations such as overexpression of Msx1 (379), Twist1 (232), Barx2 (335), or knockdown of Rb1 in conjunction with a deficiency for Cdkn2a (p16Ink4a) (395). By a novel fusion-dependent lineage tracing technique, two recent studies reported that differentiated myotubes formed by satellite cell-derived primary myoblasts (397) or muscle-derived cells (MDCs) (359) can dedifferentiate into Pax7-expressing mononucleated myogenic cells in vitro in response to an inhibitor cocktail (a tyrosine phosphatase inhibitor plus an apoptosis inhibitor) or within regenerating muscle in vivo. Although still not experimentally tested on myofibers formed during development, these intriguing observations jointly suggest that some adult satellite cells may be “recycled” from multinucleated myofibers in vivo during postnatal muscle growth or regeneration. Future studies may employ the fusion-dependent lineage tracing technique in chimeric mice to investigate the physiological relevance of this alternative source of satellite cells.
II. FUNCTIONS OF SATELLITE CELLS IN MUSCLE REGENERATION
Skeletal muscles consist of myofibers, neurons, vasculature networks, and connective tissues, of which the structural and functional element of skeletal muscle is the myofiber. Each myofiber is surrounded by the endomysium (also called the basement membrane or basal lamina). Bundles of myofibers are surrounded by the perimysium, while the entire muscle is contained within the epimysium. Each myofiber is anchored at its extremities to tendons or tendon-like fascia at the myotendinous junctions (MTJs) (531). Myofibers are composed of actin and myosin myofibrils repeated as a sarcomere, which is the basic functional unit of skeletal muscle. Responding to the signals from motor neurons, myofibers depolarize and release calcium from the sarcoplasmic reticulum (SR). This drives the movement of actin and myosin filaments relative to one another and leads to sarcomere shortening and muscle contraction.
Based on their physiological properties, skeletal muscle fibers can be grouped into a slow-contracting/fatigue-resistant type and a fast-contracting/fatigue-susceptible type. Myofibers also vary in terms of their myosin heavy chain (MyHC) isoforms (fast or slow) and metabolism types (oxidative or glycolytic). The choice of myosin gene expression is under the dynamic regulation of thyroid hormone and work load (reviewed in Ref. 28). Recent studies demonstrated that the specification of myosin expression is also regulated by intronic microRNAs within MyHC genes (545, 546).
Mammalian skeletal muscle during adulthood is a stable postmitotic tissue with infrequent turnover of myonuclei (467). Minor lesions inflicted by day-to-day wear and tear can be repaired without causing cell death, inflammatory responses, or histological changes. For instance, local plasma membrane damage caused by spontaneous eccentric muscle contractions can be efficiently repaired by recruiting intracellular vesicles to patch the damaged membrane (30, 508). This repair process involves dysferlin and caveolin-3 (30, 181), and mutations of these genes cause limb girdle muscular dystrophy 2B (LGMD-2B) (36, 314) and 1C (LGMD-1C) (344), respectively. In contrast, severe muscle injuries due to either traumatic lesions (e.g., extensive physical activity such as resistance training, or exposure to myotoxin) or genetic defects (e.g., muscular dystrophies) are accompanied by myofiber necrosis, inflammatory responses, activation of satellite cells, proliferation, and differentiation of satellite cell-derived myoblasts (FIGURE 2). This process, starting from myofiber necrosis and ending with new myofiber formation, is called muscle regeneration. It should be stressed that satellite cells play a pivotal role during muscle regeneration under either physiological conditions (e.g., extensive exercise) (400, 457) or pathological conditions (e.g., myotoxin induced injury) (301, 361, 457). This notion is clearly supported by the findings that ablation of the total satellite cell pool (all Pax7+ cells) in adulthood completely abolished muscle regeneration (301, 361, 457). It has been reported that several types of nonsatellite cells can undergo myogenic differentiation and contribute to muscle regeneration after transplantation into regenerating muscle (24, 210, 287, 345, 413). Nevertheless, the contribution of these cells to adult muscle regeneration seems to be negligible compared with satellite cells, implying the physiological relevance of nonsatellite cell-based myogenesis might depend on Pax7 expression and/or the existence of considerable numbers of satellite cells.
Figure 2.
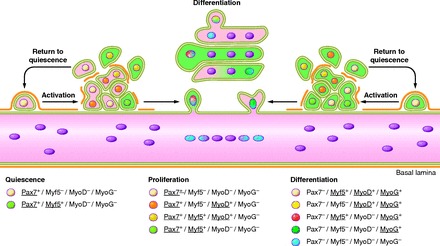
Satellite cell activation, differentiation, and fusion. The myogenic program is orchestrated by key transcription factors that dictate the progression from quiescence, activation, proliferation, and differentiation/self-renewal of satellite cells. This results in the transformation of individual satellite cells into a syncytial contractile myofiber. Initially satellite cells are mitotically quiescent (G0 phase) and reside in a sublaminar niche. Quiescent satellite cells are characterized by their expression of Pax7 and Myf5 but not MyoD or Myogenin. Damage to the environment surrounding satellite cells results in the deterioration of the basal lamina and their exit from the quiescent state (satellite cell activation). Proliferating satellite cells and their progeny are often referred to as myogenic precursor cells (MPC) or adult myoblasts. Adult myoblasts express the myogenic transcription factors MyoD and Myf5. Following proliferation, adult myoblasts begin differentiation by downregulating Pax7. The initiation of terminal differentiation and fusion begins with the expression of Myogenin, which in concert with MyoD will activate muscle specific structural and contractile genes. During regeneration, activated satellite cells have the capability to return to quiescence to maintain the satellite cell pool. This ability is critical for long-term muscle integrity.
In this section, we examine the extensive cellular and molecular dynamics during muscle regeneration, with emphasis on satellite cell. We also review the potential of nonsatellite cell lineages on muscle regeneration. At the end of this section, we briefly describe the function of satellite cells in normal postnatal muscle development, and compare and contrast this with muscle regeneration in adulthood.
A. An Introduction to Muscle Regeneration
Muscle regeneration occurs in three sequential but overlapping stages: 1) the inflammatory response; 2) the activation, differentiation, and fusion of satellite cells; and 3) the maturation and remodeling of newly formed myofibers.
Muscle degeneration begins with necrosis of damaged muscle fibers. This event is initiated by dissolution of the myofiber sarcolemma, which leads to increased myofiber permeability. Disruption of myofiber integrity is reflected by increased plasma levels of muscle proteins and microRNAs, such as creatine kinase (17) and miR-133a (292), which are usually restricted to the myofiber cytosol. Similarly, the compromised sarcolemmal integrity also allows the uptake of low-molecular-weight dyes, such as Evans blue or procion orange, by the damaged myofiber, which is a reliable indication of sarcolemmal damage associated with extensive exercise and muscle degenerative diseases (218, 328, 396). Moreover, myofiber necrosis is accompanied by increased calcium influx or calcium release from the SR, which in turn activates calcium-dependent proteolysis and drives tissue degeneration (reviewed in Refs. 7, 19, 39). In this process, calpain, a calcium-activated protease, has been shown to cleave myofibrillar and other cytoskeletal proteins (reviewed in Ref. 145). Myofiber necrosis also activates the complement cascade and induces inflammatory responses (389). Subsequent to inflammatory responses, chemotactic recruitment of circulating leukocytes occurs at local sites of damage (reviewed in Ref. 530). Neutrophils are the first inflammatory cells to infiltrate the damaged muscle, with a significant increase in their number being observed as early as 1–6 h after myotoxin- or exercise-induced muscle damage (168). Following neutrophil infiltration, two distinct subpopulations of macrophages sequentially invade the injured muscle and become the predominant inflammatory cells (91). The early invading macrophages, characterized by the surface markers CD68+/CD163−, reach their highest concentration in damaged muscle at ∼24 h after the onset of injury and thereafter rapidly decline. These CD68+/CD163− macrophages secrete proinflammatory cytokines, such as tumor necrosis factor-α (TNF-α) and interleukin (IL)-1, and are responsible for the phagocytosis of cellular debris. A second population of macrophages, characterized by the surface markers CD68−/CD163+, reach their peak at 2–4 days after injury. These macrophages secrete anti-inflammatory cytokines, such as IL-10, and persist in damaged muscle until the termination of inflammation. Notably, the CD68−/CD163+ macrophages also reportedly facilitate the proliferation and differentiation of satellite cells (80, 81, 303, 341, 503).
A highly orchestrated regeneration process follows muscle degeneration. A hallmark of this stage is extensive cell proliferation. Blocking cell proliferation by colchicine treatment (411) or irradiation (423) drastically reduced muscle regenerative capacity. Experiments by [3H]thymidine labeling have clearly demonstrated that the proliferation of satellite cells and their progeny provide a sufficient source of new myonuclei for muscle repair (498, 499, 554; reviewed in Ref. 79 and discussed in sect. IIB). It is commonly agreed that following proliferation, myogenic cells differentiate and fuse to existing damaged fibers or fuse with one another to form myofibers de novo. This process, in many but not all respects, recapitulates embryonic myogenesis.
Muscle regeneration can be characterized by a series of morphological characteristics based on histological and immunofluorescence staining. On muscle cross-sections, newly formed myofibers can be readily distinguished by their small caliber and centrally located myonuclei. These myofibers are often basophilic in the beginning of regeneration due to protein synthesis and the expression of embryonic/developmental forms of MyHC (217, 562). On muscle longitudinal sections and on isolated single myofibers, the centrally localized myonuclei were observed in discrete segments of regenerating myofibers or along the entire new myofiber, which suggests that cell fusion during regeneration happens in a focal, rather than diffuse, manner (58). Occasionally, concentrated regenerative processes may appear as local protrusions (also called budding) on myofibers. Muscle regeneration can often lead to architectural changes of the regenerated myofibers, which are presumably due to incomplete fusion of regenerating fibers within the same basal lamina (57, 58, 64, 465). Newly formed myotubes may not fuse to each other, resulting in clusters of small caliber myofibers within the same basal lamina. Alternatively, they may fuse only at one end, leading to the formation of forked (also called branching or splitting) myofibers. Myofiber branching was commonly observed in muscles from patients suffering neuromuscular diseases, in hypertrophied muscles, and in aging muscles, suggesting this phenotype may relate to abnormal muscle regenerative capacity (59, 90). Small regenerating myofibers may also form outside the basal lamina in the interstitium, due to migration of satellite cells or other types of myogenic cells. Finally, the reconstitution of myofiber integrity may be prevented by scar tissue that separates the two regenerative sites, leading to the formation of a new myotendinous junction.
At the end of muscle regeneration, newly formed myofibers increase in size, and myonuclei move to the periphery of the muscle fiber. Under normal conditions, the regenerated muscles are morphologically and functionally indistinguishable from undamaged muscles.
B. Satellite Cell Activation and Differentiation
In intact muscle, satellite cells are sublaminal and mitotically quiescent (G0 phase). Quiescent satellite cells are characterized by their expression of Pax7 but not MyoD or Myogenin (116). Examination of β-galactosidase activity in Myf5-LacZ mice indicated that the Myf5 locus is active in ∼90% of quiescent satellite cells, which suggests most satellite cells are committed to the myogenic lineage (37).
Upon exposure to signals from a damaged environment, satellite cells exit their quiescent state and start to proliferate (satellite cell activation). Proliferating satellite cells and their progeny are often referred to as myogenic precursor cells (MPC) or adult myoblasts. Satellite cell activation is governed by multiple niche factors and signaling pathways (discussed in detail in sect. IIIA). Satellite cell activation is not only restricted to the site of muscle damage. In fact, localized damage at one end of a muscle fiber leads to the activation of all satellite cells along the same myofiber and migration of these satellite cells to the regeneration site (473). Satellite cell activation is also accompanied by extensive cell mobility/migration. It has been observed that satellite cells can migrate between myofibers and even muscles across barriers of basal lamina and connective tissues during muscle development, growth, and regeneration (241, 251, 557). Recently, sialomucin CD34, whose expression is high on quiescent satellite cells but dramatically reduced during satellite cell activation, was demonstrated to act as an antiadhesive molecule to facilitate migration and promote the proliferation of satellite cells at very early stages of muscle regeneration (8). In addition, dynamic regulation of Eph receptors and ephrin ligands in activated satellite cells and regenerating myofibers have been shown to direct satellite cell migration (506).
Unlike quiescent satellite cells, myogenic precursor cells are characterized by the rapid expression of myogenic transcription factors MyoD (111, 114, 116, 175, 207, 497, 572, 585) and Myf5 (111, 116). Of note, the presence of MyoD, desmin, and Myogenin in satellite cells was observed as early as 12 h after injury, which is before any noticeable sign of satellite cell proliferation (426, 497). This early expression of MyoD is proposed to be associated with a subpopulation of committed satellite cells, which are poised to differentiate without proliferation (426). In contrast, the majority of satellite cells express either MyoD or Myf5 by 24 h following injury (111, 116, 585) and subsequently coexpress both factors by 48 h (111, 116). The ability of satellite cells to upregulate either MyoD or Myf5 suggests these two transcription factors may have different functions in adult myogenesis.
First, MyoD−/− mutant mice display markedly reduced muscle mass (338). This atrophy phenotype is reportedly due to delayed myogenic differentiation (564, 573). Similarly, muscle regeneration is also impaired in MyoD−/− mice, resulting in an increased number of myoblasts within the damaged area (338). These MyoD−/− myoblasts persist for extended periods of time, fail to differentiate, and do not fuse into myotubes. This is consistent with the notion that MyoD−/− myoblasts, when cultured in myogenic differentiation conditions, continue to proliferate and eventually give rise to a decreased number of differentiated mononucleated myocytes (114, 452, 573). Intriguingly, transplanted MyoD−/− myoblasts have been reported to survive and engraft into MyoD+/+ regenerating muscles with improved efficacy (compared with wild-type myoblasts). This phenotype is reportedly due to their increased stem cell characteristics and repressed apoptotic potential (22, 231). After regeneration, these transplanted MyoD−/− myoblasts not only give rise to myonuclei but also contribute to the satellite cell pool (22). On the other hand, ectopic expression of MyoD in NIH-3T3 and C3H10T1/2 fibroblasts is sufficient to activate the complete myogenic program in these cells (234). Taken together, these observations indicate that expression of MyoD is an important determinant of myogenic differentiation, and in the absence of MyoD, activated myoblasts have a propensity for proliferation and self-renewal (452). In contrast to the MyoD−/− mice, Myf5−/− mutant mice show a myofiber hypertrophy phenotype (187), and the proliferation of Myf5−/− myoblasts is compromised (187, 542). Together, these results implicate a distinct role for Myf5 in adult myoblast proliferation, while MyoD is essential for differentiation. Notably, the disparate functions of Myf5 and MyoD in adult muscle regeneration parallel the proposed roles for these transcription factors throughout the development of distinct myogenic lineages during embryogenesis (188, 213, 256–259; reviewed in Ref. 260). Together, the aforementioned observations suggest a hypothesis that satellite cells enter different myogenic programs depending on whether Myf5 or MyoD expression predominates (450). Predominance of MyoD expression would drive the program toward early differentiation, as exemplified by the behavior of Myf5−/− myoblasts (349). In contrast, predominance of Myf5 expression would direct the program into enhanced proliferation and delayed differentiation, as shown by the behavior of MyoD−/− myoblasts (452). Finally, myoblasts coexpressing both Myf5 and MyoD would exhibit the intermediate growth and differentiation propensities as shown by most of satellite cell-derived myoblasts. This hypothesis is consistent with the observation that MyoD and Myf5 have different expression profiles throughout the cell cycle. MyoD expression peaks in mid G1, whereas Myf5 expression is maximal at the G0 and G2 phases of the cell cycle (273). Therefore, disruptions to the MyoD/Myf5 ratio may determine the choice of myogenic programs. This hypothesis also explains the spectrum of proliferation and differentiation potential observed in different primary myoblast clones cultured in vitro.
Several studies have revealed that MyoD expression in proliferating myoblasts is positively regulated by serum response factor (SRF), which binds to the serum response element (SRE) within the MyoD regulatory region (186, 285). In proliferating myoblasts, SRF only drives low levels of MyoD expression (286), whose activity is inhibited by cyclin D1 induced cyclin dependent kinase 4 (Cdk4) (587). However, the induction of MEF2 expression prior to differentiation enables MEF2 to out-compete SRF for the SRE binding site and leads to high levels of MyoD expression and initiation of differentiation (286). This function of MEF2 is further regulated by a member of the myocardin family of transcription factors, MASTR, whose expression is upregulated in response to muscle injury (348).
Notably, our group recently revealed a pro-proliferation function of MyoD in myoblasts (191). We found that the γ isoform of p38 kinase (p38γ) phosphorylates MyoD, which negates the transcriptional activation potential of MyoD and leads to a repressive MyoD complex occupying the Myogenin promoter (191). This positive effect of p38γ on myoblast proliferation is also supported by the observation that Myogenin is prematurely expressed in p38γ-deficient muscle, which displays markedly reduced myoblast proliferation (191). These results also support the notion that the functional state of MyoD depends on cofactors present in the MyoD transcriptional complex.
Moreover, multiple studies demonstrated that MyoD expression does not always warrant myogenic commitment. Monitoring satellite cell lineage progression revealed that some Pax7+/MyoD+ proliferating myoblasts could retract back to a Pax7+/MyoD− state and eventually return to quiescence (127, 216, 584; discussed in sect. IIC). In addition, the reciprocal inhibition of Pax7 with MRFs (MyoD and Myogenin) has been revealed in C3H10T1/2 fibroblasts and MM14 myoblasts in vitro (385). It was found that Pax7 decreases MyoD transcription activity and stability, whereas Myogenin represses Pax7 transcription likely via the HMGB1-RAGE axis (385, 438). Based on these observations, it was proposed that the ratio of Pax7 and MyoD activities are critical for satellite cell fate determination (385). A high ratio of Pax7 to MyoD (as seen in quiescent satellite cells) keeps satellite cells in their quiescent state. An intermediate ratio of Pax7 to MyoD allows satellite cells to proliferate, but not differentiate. Satellite cells with a low Pax7-to-MyoD ratio begin to differentiate, and further reduction in Pax7 levels are observed following activation of Myogenin.
After limited rounds of proliferation, the majority of satellite cells enter the myogenic differentiation program and begin to fuse to damaged myofibers or fuse to each other to form new myofibers. The initiation of terminal differentiation starts with the expression of Myogenin and Myf6 (also called Mrf4) (114, 116, 207, 497, 572). The induction of Myogenin expression primarily depends on MyoD and is proposed to enhance expression of a subset of genes previously initiated by MyoD (83). Target genes of MyoD and Myogenin have been revealed by candidate approaches (405), ChIP-on-chip experiments (44, 56, 83), and more recently by ChIP-Seq analysis (84). These investigations jointly reveal a convoluted hierarchical gene expression circuitry centered on MyoD and its immediate downstream targets: Myogenin and Mef2 transcription factors (Mef2s). Based on the temporal expression pattern of MyoD, Myogenin and Mef2s, a feed-forward regulatory circuit is proposed. In this hypothesis, myogenic differentiation is an irreversible procedure and is driven by the sequential expression of key transcription factors (master regulators), which are destined to transduce gene expression signals to their target genes (45, 405). A large portion of target genes induced by MyoD, Myogenin, and Mef2s are muscle-specific structural and contractile genes, such as those encoding actins, myosins, and troponins. The expression of these genes is essential for the proper formation, morphology, and function of skeletal muscle and thus they are regulated by multiple mechanisms (41).
First, the transcriptional activities of MyoD, Myogenin, and Mef2s are regulated by posttranscriptional modifications (reviewed in Ref. 420). The α and β isoforms of p38 kinase (p38-α/β) have an important role in the expression of muscle-specific genes (405) and in muscle terminal differentiation (571). The function of p38-α/β is at least partially responsible for the phosphorylation of Mef2s as inhibition of p38-α/β disrupts the transcriptional activities of Mef2s. In contrast, the expression of constitutively active forms of p38-α/β promotes myogenesis (117, 221, 390, 420, 571, 589). p38-α/β activity stimulates the binding of MyoD and Mef2s to the promoters of muscle-specific genes, leading to the recruitment of chromatin remodeling complexes, and ultimately the RNA polymerase II holoenzyme (405, 424, 491). Similarly, the transcriptional activity of Myogenin is regulated by protein kinase A (PKA) (308) and protein kinase C (PKC)(309). Protein inhibitors of MRFs also control myogenic gene expression. The transcriptional activity of MRFs relies on heterodimerization with E proteins (ITF1, ITF2, E12, E47) (291, 362, 363). This heterodimerization is negatively regulated by a group of inhibition of DNA binding proteins (Ids: Id1, Id2, Id3, and Id4), which are also helix-loop-helix proteins but lack the basic DNA-binding domain (42, 43). Id proteins heterodimerize with E proteins and prevent their association with MRFs, thus abrogating myogenic gene expression (43). Similar results occur when Mist1 directly interacts with MyoD and prevents MyoD from binding E-boxes (298). MyoD activity can be further inhibited by the sequestration of E proteins, by Twist (504). Finally, the transcriptional activity of MyoD is also determined by specific cofactors present on the promoters of myogenic genes (reviewed in Ref. 450). In vitro, MyoD associates with histone acetyltransferases (HATs) p300 and p300/CBP (CREB-binding protein)-associated factor (PCAF) on E-box motifs of its target genes (419). This association is presumed to induce histone acetylation and transcriptional activation (45). MyoD also interacts with histone deactylases (HDACs), which negatively regulate the transcriptional activity of MyoD either directly (325) or in a Mef2-dependent manner (319).
Besides MRFs and their regulators, other factors have been shown to be involved in myogenic differentiation. MicroRNAs are 20–22nt noncoding small RNAs, which function to repress translation and reduce the stability of their target mRNAs. Recent studies demonstrated that MRFs, such as Myf5, MyoD, and Myogenin, activate the expression of a collection of myogenic microRNAs (e.g., miR-1, miR-133, miR-206 together called MyomiRs). These myogenic microRNAs, in conjunction with other microRNAs, modulate the expression levels of key myogenic transcription factors and regulators, such as Pax3 (119, 196, 231), Pax7 (94, 139), SRF (93), c-Met (526, 577), and Dek (98) during satellite cell activation/proliferation and differentiation (also reviewed in Refs. 374, 565). Furthermore, recent studies have demonstrated the requirement for caspase-3 and its activation of CAD (caspase-activated DNase) in initiating myoblasts differentiation in vitro (164, 290). Activation of CAD by caspase-3 induces double-stranded DNA breaks (DSBs) in the genome and the association of CAD with various target promoters. One such promoter is the cyclin-dependent kinase (CDK) inhibitor p21(Waf1/Cip1) which following binding by CAD is activated (290). MyoD also induces the expression of p21(Waf1/Cip1) (209, 215) and subsequent permanent cell cycle arrest. p21 is known to dephosphorylate the retinoblastoma protein (Rb) and cause the inactivation of the Rb-associated E2F family of transcription factors known to activate S-phase genes (reviewed in Ref. 559). Thus, through this pathway, MyoD induces permanent cell cycle withdrawal in myoblasts. Consistently, myoblasts in p21−/− mice are defective in cell cycle arrest and myotube formation, leading to increased apoptosis (555, 588). Similarly, Rb-deficient myoblasts cannot complete cell cycle withdrawal and arrest during both S and G2 phases of the cell cycle (378).
After exiting the cell cycle, myogenic cells undergo cell-to-cell fusion to repair damaged myofibers or form nascent multinucleated myofibers. The cellular events in this complex process have been extensively studied (274, 312, 418, 428, 553). Akin to embryonic myogenesis, de novo formation of myofibers during muscle regeneration happens in two stages. In the first stage, individual differentiated myoblasts fuse to one another and generate nascent myotubes with few nuclei. In the second phase, additional myoblasts incorporate into the nascent myotubes, forming a mature myofiber with increased size and expression of contractile proteins. In recent years, studies in myoblast fusion in vitro have identified a cadre of cell surface, extracellular, and intracellular molecules, which are important for these two stages of myogenic cell fusion (reviewed in Ref. 238). For example, cell membrane proteins β1-integrin (474), VLA-4 integrin (445), integrin receptor V-CAM (445), caveolin-3 (180), and transcription factor FKHR (Forkhead in human rhabdomyosarcoma, also called FOXO1a) have been shown to act in myoblast-to-myoblast fusion. Whereas the cytokine IL-4 (238) and calcium and calmodulin activated NFATC2 (nuclear factor of activated T cell isoforms C2) pathway (237) is critical for the fusion of myoblasts with nascent myotubes.
As discussed here, activation of satellite cells following muscle injury results in the expansion of the myogenic cell pool and leads to the initiation of the myogenic program. This program, orchestrated by key transcription factors, dictates the balance between proliferation and differentiation and drives the functional transformation from individual proliferating myogenic cells to a syncytial contractile myofiber. Although numerous studies have shed light on this complex process, there are still many interesting questions that remain to be answered. For example, what are the intrinsic and extrinsic mechanisms that determine the scale of satellite cell proliferation in vivo, which essentially generate sufficient but not excessive numbers of myogenic cells for muscle regeneration? Similarly, what mechanisms regulate the magnitude of muscle regeneration in response to variable levels of damage and eventually prevent muscle atrophy or hypertrophy? With the recent advances in high-throughput sequencing and system biology, is it possible to elucidate a core regulatory network of myogenic gene expression and employ such knowledge on directing myogenic determination and differentiation from multipotent cells? The answers to these questions will improve our understanding of muscle regeneration and facilitate future development of therapeutic approaches to cure muscle diseases.
C. Satellite Cell Self-Renewal
A hallmark of stem cells is the ability to self-renew. Stem cells can divide and self-renew in two fashions: asymmetric cell division and symmetric cell division. In asymmetric cell division, one parental stem cell gives rise to two functionally different daughter cells: one daughter stem cell and another daughter cell destined for differentiation. In symmetric cell division, one parental stem cell divides into two daughter stem cells of equal stemness. In either fashion, the number of stem cells is maintained at a constant level. Stem cell self-renewal by asymmetric cell division is exemplified by neuronal stem cells (590). Stem cell self-renewal by symmetric cell division is frequently observed in hematopoietic stem cells and mammalian male germline stem cells (spermatogonia) (570).
The self-renewing capability of satellite cells is clearly demonstrated by their remarkable ability to sustain the capacity of muscle to regenerate. For example, in a single myofiber transplantation experiment (105), 7–22 satellite cells together with their intact myofibers were transplanted into irradiated muscles of immunodeficient dystrophic (scid-mdx) mice. It was found that one grafted myofiber can give rise to over 100 new myofibers, which contain ∼25,000–30,000 differentiated myonuclei. In addition, the grafted satellite cells can undergo a 10-fold expansion via self-renewal. The expanded satellite cells are functional as they can be activated and support further rounds of muscle regeneration (105). Similarly, the self-renewing capability of satellite cells was further proven by single satellite cell transplantation experiments (454). In this study, single Lin−/α7-integrin+/CD34+ cells freshly isolated by FACS were transplanted into irradiated muscles of scid-mdx mice. It was observed that progeny from these single cells not only generated myofibers, but also migrated to the satellite cell niche and persisted in host muscles (454).
An intriguing question is how satellite cells renew themselves. By using the Cre-LoxP based permanent lineage tracing technique (Myf5Cre;R26R-loxP-stop-loxP-YFP), our group first demonstrated that satellite cells can undergo both asymmetric and symmetric divisions within their natural niche environment in mildly damaged EDL muscle (280). The choice of asymmetric versus symmetric division is largely correlated to the mitotic spindle orientation relative to the longitude axis of the myofiber. Asymmetric divisions are only observed for Pax7+/Myf5− satellite cells, which give rise to one satellite stem cell (Pax7+/Myf5−) and one satellite myogenic (Pax7+/Myf5+) cell. Asymmetric division predominantly happens when the mitotic spindle is perpendicular to the myofiber axis (apical-basal division) with the satellite stem cell (Pax7+/Myf5−) in close contact with the basal lamina (basal) and the Pax7+/Myf5+ satellite myogenic cell adjacent to the myofiber plasma membrane (apical). Occasionally, it was observed that Pax7 expression is dampened in the apical satellite cell, suggesting a progression towards terminal differentiation. Furthermore, the asymmetric nature of this kind of division is also underscored by the observation that the apical Pax7+/Myf5+ cells express higher levels of the Notch ligand Delta-1, whereas the basal Pax7+/Myf5− satellite cells express higher levels of the Notch-3 receptor. Symmetric divisions were observed for both Pax7+/Myf5− satellite cells and Pax7+/Myf5+ satellite cells and in either case the mitotic spindle was frequently parallel to the myofiber axis (planar division) and both daughter cells were in contact with the myofiber plasma membrane and the basal lamina.
When both satellite stem cells and satellite myogenic cells are freshly sorted and transplanted into Pax7−/− muscles lacking any functional endogenous satellite cell, both cell types can colonize the host muscle. However, only Pax7+/Myf5− satellite cells can efficiently reconstitute the satellite cell compartment (∼7-fold more efficient than Pax7+/Myf5+). These observations indicate that Pax7+/Myf5− satellite cells represent a bona fide stem cell population, which can undergo self-renewal by both asymmetric division and symmetric division (280).
Although asymmetric self-renewal may be sufficient under physiological conditions, satellite stem cells may favor symmetric self-renewal divisions to replenish and expand their population in response to acute need for a large number of satellite cells after injury or disease state (354). Indeed, it was observed that Pax7+/Myf5− satellite stem cells represent ∼10% of total satellite cells in intact muscles, whereas this percentage increases to ∼30% at 3 wk after injury (280). In search of intrinsic and extrinsic cues regulating Pax7+/Myf5− satellite stem cell self-renewal, our group further revealed that symmetric self-renewal is promoted by a niche factor, Wnt7a, during muscle regeneration (293; discussed in sect. IIIA1a). Therefore, the mode of satellite cell self-renewal is governed by the satellite cell niche.
With regards to activated satellite cells returning to quiescence, observations on the dynamic expression of Pax7, MyoD, and Myogenin in ex vivo cultured single myofibers suggest it is possible (127, 216, 584). After myofiber isolation (the isolation procedure per se is a form of injury to initiate muscle regeneration), satellite cells are activated and rapidly start to express MyoD (572, 584). It has been observed that almost all myoblasts express MyoD after ∼24 h of in vitro culture (584). While most of these proliferating Pax7+/MyoD+ myoblasts differentiate into Pax7−/MyoD+/Myogenin+ cells after 72 h of culture, some Pax7+/MyoD+ myoblasts (also called reserve cells) nonetheless have been observed to repress MyoD expression and maintain Pax7 expression and eventually withdraw from the cell cycle (216, 584). These Pax7+/MyoD− satellite cells acquired the quiescence marker Nestin, albeit at a much later (3–4 wk in culture) stage (127). These observations suggest that quiescent satellite cells renew by lineage regression from committed myogenic cells at least in vitro, and this renewal may be promoted by Pax7 (584). In addition, the capability of activated satellite cells to generate reserve cells may be an important mechanism to maintain the size of the satellite cell pool, as reduction of such capability was suggested to lead to decreased numbers of satellite cells with age (128). Intriguingly, this lineage regression has also been recently observed in vivo (482). Sprouty-1, a negative regulator of receptor tyrosine kinase (RTK) signaling, plays a critical role in satellite cell renewal (482). Sprouty-1 is expressed in quiescent but not proliferating satellite cells (177, 482). Upon injury, released fibroblast growth factor (FGF) and hepatocyte growth factor (HGF) act through the RTK/ERK pathway to stimulate satellite cell proliferation (482). Most satellite cells entered the cell cycle during regeneration as indicated by BrdU labeling. It was confirmed that some satellite cell-derived myogenic progenitors withdrew from the cell cycle, returned to the satellite cell compartment, and regained Sprouty-1 expression, indicative of satellite cell renewal in vivo. In the absence of Sprouty-1, it was observed that satellite cell-derived myogenic progenitors proliferate and differentiate normally, but markedly reduced numbers of satellite cells exist after regeneration. Further investigations revealed that Sprouty-1 is required only for a distinct subset of myogenic progenitors to return to their quiescence state (482). These results indicate that Sprouty-1 is essential for some satellite cells to regain quiescence following regeneration. The function of Sprouty-1 in this process is most likely due to its inhibitory effect on the ERK pathway and subsequent enhanced cell cycle withdrawal. It should be stressed that this renewal of satellite cells at a population level and the aforementioned self-renewal of satellite cells at a single-cell level are not mutually exclusive but rather represent two different perspectives of the same regeneration process. Future studies are needed to identify the essential differences between Sprouty-1-dependent and Sprouty-1-independent populations and understand whether these differences reflect any other functional distinction. In addition, it would be interesting to investigate the regulatory mechanisms governing Sprouty-1 expression, for example, whether the reappearance of Sprouty-1 is regulated by Pax7 or Wnt signaling.
D. Contributions and Therapeutic Potential of Nonsatellite Cells in Trauma-Induced Muscle Regeneration
1. Bone marrow stem cells
The myogenic potential of bone marrow cells was demonstrated by either intravenous injection of bone marrow cells into subjects following muscle injury or directly via intramuscular injection of whole bone marrow into injured muscles. Both of these methods led to the incorporation of bone marrow-derived cells into newly formed myofibers within regenerating muscles (54, 166, 210). Because of the extremely low efficacy of incorporation, doubts persist regarding the ability of bone marrow-derived cells to reconstitute the satellite cell niche (210). Nevertheless, lineage tracing experiments demonstrated that bone marrow cells, when systematically injected into irradiated mice, were able to reconstitute the satellite cell niche and expressed satellite cell markers, such as Myf5, c-Met, and α7-integrin (287). These bone marrow-derived satellite cells can undergo myogenic differentiation in vitro and give rise to myofibers when injected into damaged muscles (287). Adult bone marrow contains hematopoietic stem cells (HSC) and stromal cells (also called mesenchymal stem cells). Due to a lack of a standardized definition, conflicting results exist regarding the exact bone marrow-derived progenitor cell type(s) that contribute to muscle regeneration. Bone marrow-residing hematopoietic stem cells, isolated by cell surface markers c-Kit+/Sca-1+ or CD45+/Sca-1+, or CD45+/c-Kit+/Sca-1+ are able to incorporate into newly forming myofibers (1, 78, 112, 581), although it was unclear whether the progeny of these cells were able to occupy the satellite cell niche. It was also demonstrated that Mac-1low bone marrow SP cells, particularly those c-Kit+/CD11b− immature cells, were able to generate myofibers (147, 382). On the other hand, mesenchymal stem cells were able to incorporate into myofibers in injured muscles (140, 488) and gave rise to Pax7+ satellite cells, which can support multiple rounds of muscle regeneration (140). Interestingly, the high myofiber turnover rate induced by muscle stress and injury seems to facilitate bone marrow cell engraftment into regenerating muscles (1, 68, 287, 460, 487). These results further emphasize the importance of the microenvironment on satellite cell specification. In accordance with this, stromal cell-derived factor-1 (SDF-1)/chemokine receptor 4 (CXCR4) signaling and chemokine receptor 2 (CCR2) have been implicated in guiding bone marrow cells towards muscle injury sites (429, 510). However, it has also been reported that SDF-1/CXCR4 signaling is dispensable for the homing of CD45+ hematopoietic lineage cells to injured muscle, which are instead responsive to HGF/c-Met signaling (447). Overall, it is noteworthy that bone marrow transplantation has not been reported to robustly or therapeutically ameliorate any muscle disease. Future studies are required to define the subpopulation(s) of bone marrow cells, which have long-term myogenic potential in vivo especially in the context of muscle diseases. Also, it would be interesting to investigate whether bone marrow-derived cells are involved in postnatal muscle growth since satellite cells with similar markers have been identified in adult muscles.
2. Muscle side population cells
Similar to bone marrow, skeletal muscle contains a unique cell population termed muscle side population (SP) cells, which can be isolated based on their ability to efflux Hoechst 33342 dye (210, 245). The majority of muscle SP cells are characterized by CD45−/c-Kit−/Sca-1+/ABCG2+/Pax7−/Myf5−/Desmin− and reside in the skeletal muscle interstitium juxtaposed to blood vessels, which make them distinct from satellite cells and bone marrow-derived SP cells (24, 146, 210, 439, 464). Multiple lines of evidence indicate that muscle SP cells are heterogenic (24, 464). Initially, a minor population of CD45+ muscle SP cells was identified (24, 464, 478). These CD45+ muscle SP cells persist in Pax7−/− germline null mice and can efficiently differentiate into hematopoietic cells in culture (24, 478). In addition, CD45+ muscle SP cells also have myogenic potential when isolated from both wild-type and Pax7−/− mice. They can be induced to differentiate into myogenic cells following coculture with primary myoblasts or through the forced expression of Pax7 or MyoD (24, 477). In addition, lineage tracing experiments have revealed that only the main population of CD45− muscle SP cells arise from embryonic Pax3+ hypaxial somitic cells, suggesting the minor CD45+ SP cells might have distinct origins, possibly from endothelial, hematopoietic stem, or bone marrow cells (464). This is consistent with results from transcriptome analysis of muscle SP cells in resting and regenerating muscles, which indicate the increased expression of c-Kit and CD45 in the muscle SP population 5 days after injury (337). Furthermore, it was also demonstrated that somite-derived CD45− SP cells have more myogenic potential than CD45+ SP cells when cultured in vitro, suggesting that the developmental origin of SP cells affect their intrinsic myogenic capacity (464). Lastly, a recent study revealed that a subpopulation of satellite cells also have an SP phenotype (518). These SP cells that reside in the satellite cell compartment, termed satellite-SP cells, are characterized by CD45−/Sca-1+/ABCG2+/Pax7+/Syndecan-4+, which makes them distinct from the majority of CD45− SP cells in muscle. Following direct injection into regenerating muscles, the grafted muscle SP cells were found in myofibers and the satellite cell niche, which suggests that these cells can contribute to long-term muscle regeneration (24). Importantly, when introduced intravenously into mdx mice, muscle SP cells from wild-type mice incorporate into host myofibers and restore their dystrophin expression (27, 210), suggesting these cells are able to migrate to regenerating muscles through the bloodstream. Indeed, compared with satellite cells and their derivatives, muscle SP cells are much more efficient at engrafting into mdx hosts after systemic delivery (368). Interestingly, the systemic delivery of wild-type SP cells into mdx mice can also repopulate the bone marrow of an irradiated host, although at lower efficacy compared with bone marrow-derived SP cells (210).
Muscle SP cells constitute a distinct cell population based on their unique properties. However, it is still unclear whether the SP phenotype per se is of any importance to the muscle regeneration process. Although muscle SP cells out-perform the main population in muscle regeneration, future studies are needed to carefully compare muscle SP cells with other defined myogenic precursors, such as non-SP satellite cells, based on their myogenic potential.
3. PW1+ interstitial cells
It has been reported that some interstitial cells, characterized by their expression of PW1 (also called peg3), might be involved in perinatal skeletal muscle growth (345). Intriguingly, a recent study from the same group reported that adult stem cells/progenitors residing in multiple tissues/organs can be directly identified and isolated from PW1-reporter mice (46). PW1 is encoded by a ∼8.5 kb long, intronless transcript, which is strongly transcribed in skeletal muscle in both embryonic and postnatal stages (435). Within skeletal muscle, PW1 expression was detected in both satellite cells and a group of Sca-1+/CD34+/Pax7− interstitial cells (PIC) (351, 376, 476). In transgenic mice with a dominant negative form of PW1 (ΔPW1) driven by a Myogenin promoter, embryonic and fetal muscle development is normal but postnatal muscle growth is severely impaired with a profound phenotype, to some extent, reminiscent to that of Pax7−/− germline mutant mice (376, 478). Compared with wild-type mice, the number of quiescent satellite cells is markedly reduced in postnatal muscle of ΔPW1 mice (376). In contrast, the number of Pax7−/PW1+ interstitial cells (PIC) is significantly increased within postnatal muscles from Pax7−/− mice (345), which is accompanied by progressive loss of Pax7+ satellite cells (345, 380, 478). Interestingly, PICs have myogenic potential when cultured in vitro (345). This myogenic potential is enhanced by coculture with satellite cells and is compromised in PICs isolated from Pax7−/− mice (345). Importantly, when transplanted into injured muscles, FACS isolated Sca-1+/CD34+/CD45− PICs give rise to both interstitial cells and Pax7+ satellite cells, whereas Sca-1−/CD34+/CD45− cells (enriched for satellite cells) only derive into Pax7+ satellite cells (345). Although not directly assessed by lineage tracing, these observations together support a hypothesis that PICs may contribute to the satellite cell population during postnatal muscle growth and during adult muscle regeneration (345). In addition, Pax7 expression is presumably essential for the myogenic specification of PICs as PICs isolated from Pax7−/− mice cannot give rise to myogenic cells in vitro (345). Of note, Pax3 lineage tracing experiments further indicated that PICs do not arise from embryonic Pax3+ myogenic progenitor cells, indicating PICs and satellite cells are derived from distinct lineages (345).
These intriguing observations also raised several issues regarding the identity and lineage progression of the Sca-1+/CD34+/CD45−/(>50% PW1+) interstitial cells. A recent study indicated that FAPs isolated from adult muscle are characterized by similar markers Sca-1+/CD34+/CD45− (also CD31−) but only give rise to adipogenic and fibrogenic lineages in vitro (252 and discussed in sect. IIIB1a). Coculture with myogenic cells cannot promote the myogenic potential of FAPs (252). Accordingly, FAPs also do not assume the myogenic lineage in vivo when transplanted into either uninjured or injured muscles. These discrepant observations on PICs and FAPs lead to two possibilities: 1) FAPs are depleted of CD31+ cells and thus the myogenic potential resides in these CD31+ cells; or more likely 2) PICs and FAPs represent the same cell population but in young and adult stages, respectively. Notably, muscle SP cells (Sca-1+/ABCG2+) are also CD34+ and thus could be originating from the same lineage as FAPs and PICs (226). Future studies may provide direct answers in terms of the lineage origin and hierarchy of these cell types. Future investigations should also focus on the mechanisms that regulate the myogenic potential of these interstitial cells.
4. Muscle-derived stem cells
A series of studies have demonstrated that a distinct cell population, termed muscle-derived stem cells (MDSCs), can be isolated from skeletal muscles based on their weak adhesion characteristics and long-term proliferation behaviors in culture (247, 294, 421, 422). Further characterization of MDSCs indicated that these cells are CD45−/M-Cadherin−/CD34−/Flk-1+/Sca-1+/Desmin+, which makes them distinct from satellite cells and hematopoietic cells but more similar to muscle SP cells (294, 421). MDSCs and satellite cell-derived myoblasts in culture appear to represent two distinct populations as evidenced by the finding that MDSCs can be isolated from Pax7−/− germline null mice (318). Like muscle SP cells, the myogenic differentiation of MDSCs depends on Pax7 (318). A unique characteristic of MDSCs is their multipotency (294, 421, 536). It has been reported that MDSCs can differentiate into myogenic, adipogenic, osteogenic, chondrogenic, and hematopoietic lineages (reviewed in Ref. 404). After intra-arterial transplantation, MDSCs contribute to regenerated myofibers, incorporate into the satellite cell niche, and also give rise to endothelial and neural cells (421). Interestingly, like muscle SP cells, MDSCs can also repopulate the hematopoietic lineage in irradiated murine hosts, and the reconstituted bone marrow can in turn contribute to muscle regeneration (82). In addition, MDSCs are more efficient than myoblasts at forming dystrophin+ myofibers when directly transplanted into mdx mice (243). Due to the prolonged isolation procedure of MDSCs and the accompanied dynamics of gene expression, it is difficult to trace the origin of MDSCs in vivo based on cell surface markers. Future investigation by candidate lineage tracing and clonal analysis may help to elucidate the lineage relationship between MDSC and other myogenic cells.
5. Mesoangioblasts
Mesoangioblasts arise from the embryonic dorsal aorta and are characterized by CD34+/c-Kit+/Flk-1+/NKX2.5−/Myf5−/Oct4− expression (129, 343). Mesoangioblasts are highly proliferative when cultured in vitro, and long-term cultured mesoangioblasts are multipotent, being able to give rise to multiple mesodermal lineages, such as bone, cartilage, smooth, cardiac, and skeletal muscle following transplantation (343). Although the contribution of mesoangioblasts to normal muscle development is controversial, multiple studies have demonstrated that mesoangioblasts can be employed to facilitate muscle regeneration, particularly for dystrophic muscles. First, it was found that α-sarcoglycan−/− mesoangioblasts can be transduced in vitro with α-sarcoglycan expressing lentiviral vector and robustly generate α-sarcoglycan+ myofibers following transplantation into α-sarcoglycan−/− mice (a model for limb girdle muscular dystrophy) via intra-artery injection (458). The high efficiency of normal myofiber reconstitution observed in this study suggests that intrinsic properties of mesoangioblasts may help them travel to regenerating muscles via the bloodstream. In addition, mesoangioblasts can release immunosuppressive and tolerogenic molecules, which supposedly help mesoangioblasts incorporate into allogeneic dystrophic hosts (211). The myogenic potential of mesoangioblasts isolated from human patient biopsies suffering from dermatomyositis (DM), polymyositis (PM) and inclusion-body myositis (IBM) were compared (352). It was observed that mesoangioblasts from the IBM patients could not differentiate into myofibers when cultured in vitro or xenotransplanted into injured mouse muscles. Interestingly, this defect can be rescued by either transient overexpressing of MyoD or knockdown of a MyoD inhibitor protein, bHLH-B3, which suggests that the myogenic program of mesoangioblasts is MyoD-dependent (352). Several investigations demonstrated that factors involved in normal muscle regeneration also facilitate mesoangioblast-based muscle regeneration. For example, HMGB1, SDF-1, CXCR4, and α4-integrin have been shown to enhance the migration of mesoangioblasts from blood vessels toward injured muscles (182, 399). Similarly, pretreatment of mesoangioblasts with nitric oxide before transplantation also stimulated α-sarcoglycan expression in mesoangioblast-derived myofibers (475). Taken together, investigations on mesoangioblasts have revealed great therapeutic potential for the amelioration of human dystrophic diseases. Future studies may focus on identifying the adult counterparts of these cells and characterizing their myogenic potentials.
6. Pericytes
Pericytes (also called Rouget cells or Mural cells) are contractile connective tissue cells residing beneath the microvascular basement membrane. Pericytes originate from the embryonic sclerotome and are believed to regulate the blood flow in capillaries (415, 284, 406). As a multipotent stem cell population, pericytes can differentiate into adipocytes (161), chondrocytes (161), and osteoblasts (142) in vitro. Two recent studies indicated that pericytes, which do not express Pax7, Myf5, or MyoD, can differentiate into skeletal muscles both in vitro and in vivo (135, 136), suggesting pericyte-mediated myogenesis may follow a myogenic differentiation program distinct from that of satellite cells. FACS sorted human pericytes display the following cell surface marker combination: CD45−/CD34−/CD56−/CD144−/CD146+/PDGFR-β1+/NG2 proteoglycan+, which makes them distinct from embryonic mesoangioblasts, hematopoietic cells, or satellite cells (136). Intriguingly, cells carrying the same surface marks can be isolated from various tissues, including pancreas, adipose, and placenta and differentiate into skeletal muscle cells when cultured in vitro, irrespective of their origins (118). The fact that pericytes also display cell surface markers characteristic of mesenchymal stem cells (CD10+/CD13+/CD44+/CD73+/CD90+) raised the hypothesis that pericytes may represent a significant portion of mesenchymal stem cells in the adult (85, 118, 163). Xenographic transplantation of human pericytes into combined immune deficient-X-linked, mouse muscular dystrophy (scid-mdx) mice through the femoral artery gave rise to numerous myofibers expressing human dystrophin (136). Interestingly, a small portion (3–5%) of transplanted pericytes incorporated beneath the basal lamina of myofibers, indicating that pericytes are able to occupy the satellite cell niche in dystrophic muscle. Importantly, pericyte transplantation significantly improved the physiological performance of treated dystrophic muscles, indicating that pericyte-derived myofibers are functional. Similar promising results were achieved in a prototype experiment, wherein pericytes isolated from Duchenne patients were transduced in vitro with a human mini-dystrophin expressing lentiviral vector and transplanted into scid-mdx mice (136). The myogenic potential, together with their abilities to be cultured in vitro and penetrate the blood vessel wall, makes pericytes a promising candidate for future cell-based therapies to treat muscular dystrophy. Future research may investigate whether transplanted pericytes possess sufficient long-term myogenic capability in dystrophic muscles.
7. CD133+ cells
Recent studies demonstrated that a fraction of CD133+ (also called AC133) mononucleated cells in adult peripheral blood have myogenic potential (535). Freshly isolated human CD133+ cells can undergo myogenic differentiation when cocultured with myogenic cells or exposed to Wnt-producing cells in vitro (535). Importantly, when xenotransplanted via intra-arterial or intramuscular injection, these CD133+ cells can fuse with regenerating muscles in scid-mdx mice and improve the force generation of treated muscles. Moreover, donor human CD133+ cells, expressing satellite cell markers M-Cadherin and Myf5, were detected in the satellite cell compartment after transplantation, indicating they can reconstitute the satellite cell niche (535). A small subpopulation of CD133+/ CD34+ double positive cells can be identified in the blood and skeletal muscle interstitium (233). Similar to blood-derived CD133+ cells, human muscle-derived CD133+ cells manifested remarkable myogenic differentiation capacity in regenerating muscle (372). As CD133+ cells can be readily isolated from the blood, manipulated in vitro and delivered through the circulation, a couple of studies have explored their application to treating Duchenne muscular dystrophy (DMD). In one of these studies, CD133+ cells isolated from human dystrophic muscles were genetically engineered ex vivo to restore dystrophin expression and subsequently delivered into scid-mdx mice (41). Treated dystrophic muscles were improved in terms of muscle morphology, function, and dystrophin expression, although the long-term effect of this treatment has not been documented so far. The safety of this CD133+ cell therapy (without dystrophin correction) was later confirmed in human DMD patients by autologous transplantation (534). Taken together, CD133+ cells are one of the most promising candidates for cell-based therapy of DMD. Future studies should focus on whether these cells can survive long term and support repeated bouts of regeneration in DMD patients.
8. Embryonic stem cells and induced pluripotent stem cells
Embryonic stem (ES) cells are pluripotent stem cells, which can differentiate into all three germ layers and hence have been extensively studied for cell-based therapies. In an early study, mouse ES cells cocultured with muscle cells were transplanted into irradiated mdx mice by intramuscular injection (47). Donor-derived myofibers were occasionally found on the surface of the host muscles. The low efficacy of engraftment in this study suggested that selective markers might be necessary to enrich myogenic precursors from ES cell cultures. In a later study, a subpopulation of CD73+/N-CAM+ human ES cells, which are enriched for mesenchymal precursors, were injected into regenerating muscle of scid mice (31). It was observed that human donor cells gave rise to myoblasts and established prolonged engraftment; however, the overall efficiency was not compared with other myogenic cells, such as satellite cells. Recently, several strategies have been established to achieve persistent engraftment and avoid teratoma formation. In one study, Pax3 was transiently expressed to induce paraxial mesoderm formation during embryoid body differentiation, and PDGFR-α, a paraxial mesoderm marker, was subsequently used to isolate a homogeneous population of proliferating myogenic progenitors (124). The transplantation of this population into mdx mice resulted in improved muscle function, and teratoma formation was prevented. In another study, PDGFR-α+ cells were directly isolated from ES cells in an earlier stage without Pax3 induction (455). It was found that these early paraxial mesodermal cells, when transplanted into injured muscles, could give rise to bona fide satellite cells that can further differentiate into functional myofibers. Similarly, transient expression of Pax7 seems to facilitate the myogenic specification of ES cells (125). Recently, it was shown that SM/C-2.6+ cells isolated from cultured embryoid bodies can differentiate into skeletal muscle myofibers both in vitro and in vivo (88). When transplanted into injured muscles, these cells can incorporate into the satellite cell compartment and express satellite cell markers, such as Pax7 and M-cadherin. Importantly, these ES-derived satellite cells undergo extensive self-renewal during subsequent muscle injury and can be further transplanted with high engraftment efficiency (88).
Over the last five years, various types of differentiated somatic cells have been successfully induced into pluripotent stem cells (iPS cells) via a process involving transcription factor based reprogramming (371, 384, 515, 516, 582). Given their adult somatic origin and pluripotent nature, iPS cells hold tremendous potential for cell-based therapy. In an attempt to apply iPS cells to muscle regeneration, a recent study demonstrated that SM/C-2.6+ cells isolated from mouse iPS cells have comparable regenerative potential to those from mES cells (346). In addition, conditional expression of Pax7 in human iPS cells was successful in inducing large quantities of myogenic progenitors, which were able to efficiently engraft and produce abundant human-derived dystrophin+ myofibers in dystrophic mouse muscle (123). Notably, a new strategy of iPS cell-based therapy to treat DMD was reported recently (267). In this study, iPS cells were derived from mdx mice or a human DMD patient, and the genetic deficiency of dystrophin in these cells was corrected by transferring a human artificial chromosome (HAC) containing a complete genomic dystrophin sequence via microcell-mediated chromosome transfer (MMCT). These genetically corrected iPS cells can differentiate into muscle-like tissues and can be used to generate chimeric mice, wherein tissue-specific expression of dystrophin was observed (267). These inspiring results warrant future investigations on regulatory mechanisms governing the myogenic specification of ES and iPS cells.
E. Satellite Cells in Perinatal/Juvenile Muscle Growth
In neonatal mouse muscle, satellite cells account for ∼30–35% of the sublaminal nuclei on myofibers (9, 227, 471). This proportion of satellite cells decreases while the number of myonuclei increases over postnatal growth (150). Experiments by [3H]thymidine labeling indicate that satellite cells are proliferative in growing muscle, give rise to myonuclei, and continue to fuse with myofibers (355, 470, 481). The cell cycle time of juvenile satellite cells in growing rat muscles was determined to be ∼32 h, with 14 h within the S phase (471). This high proliferation rate of juvenile satellite cells is likely due to a large requirement for muscle growth at this stage. Like their adult counterparts, juvenile satellite cells are also likely heterogeneous. By continuous BrdU labeling and tandem BrdU/[3H]thymidine labeling, two subpopulations of satellite cells in growing muscle have been identified (471). A fast-cycling satellite cell population, accounting for ∼80% of the cells, was readily labeled over the first 5 days of continuous BrdU infusion. In contrast, slow-dividing satellite cells incorporated BrdU at a much slower rate, and some of them were not labeled with BrdU even after an additional 9 days. Moreover, only a small portion of the satellite cells labeled with BrdU during the first 5 days could be labeled with [3H]thymidine during a second 5-day continuous infusion. This observation suggests that 1) the majority of fast-cycling satellite cells only undergo a limited number of mitotic divisions prior to fusion, and 2) the number of fast-cycling satellite cells in growing muscle are maintained by the slow-cycling satellite cells through asymmetric divisions (471). Interestingly, the functional difference and lineage relationship between these two satellite cell populations during postnatal muscle growth largely resemble that of satellite cells and myoblasts during adult muscle regeneration. This analogy may reflect a common scheme of myogenesis in both postnatal muscle growth and adult muscle regeneration.
Despite their similarities, studies have revealed distinct genetic requirements for both juvenile and adult satellite cell populations. Pax7 is required for the maintenance of a functional myogenic cell population in the perinatal/juvenile stage, as muscle growth and regeneration are severely impaired in Pax7 germline null mutants during this period (279, 393, 478). In contrast, Pax7 seems to be dispensable for adult muscle regeneration in Pax7 conditional knockout mutants, wherein Pax7 expression is ablated only in the adult stage (299). In conditional Pax7 knockout mice, satellite cells, expressing M-cadherin but not Pax7, were observed in the satellite cell compartment in adult muscle after one round of regeneration, and these cells can support a second round of regeneration (299). The regenerative capacity of Pax7− satellite cells was not due to a compensatory mechanism by Pax3, as muscle regeneration is not impaired by simultaneous deletion of both Pax7 and Pax3 in adulthood. It is well known that both juvenile and adult satellite cells express Pax7 (478). The apparent distinct requirement for Pax7 in juvenile but not adult muscle regeneration may suggest that Pax7 is only necessary for the initial establishment of perinatal satellite cells and/or de novo myogenic specification of other nonsatellite cell lineages, which presumably contribute to the total satellite cell pool during postnatal growth. In contrast, the maintenance of satellite cell establishment might be Pax7 independent and the rare occasion of de novo myogenic specification from other nonsatellite cell lineages may be negligible in adulthood. In this sense, the sustained expression of Pax7 in adult satellite cells may be due to sustained interactions with their niche, rather than a necessity for Pax7 function. In support of this hypothesis, genetically marked hematopoietic stem cells (bone marrow derived CD45+/c-Kit+/Sca-1+/Lin− cells) have been shown to express Pax7 once they are present in the satellite cell niche (148, 581).
III. ANATOMIC AND FUNCTIONAL DIMENSIONS OF THE SATELLITE CELL NICHE
Based on the stem cell niche concept, behaviors of tissue-specific stem cells are determined by structural and biochemical cues emanating from the surrounding microenvironment (380). In recent years, adult stem cells and their corresponding niches were identified in numerous tissues including bone marrow (566), brain (322), testis (240), liver (528), intestine (55), heart (302), white fat (441, 519), and skin (174). Similar to these adult stem cells, satellite cells are also present in a highly specified niche, which consists of the extracellular matrix (ECM), vascular and neural networks, different types of surrounding cells, and various diffusible molecules (FIGURE 3). Furthermore, satellite cells, as one of the niche components, also influence each other by means of cell-cell interaction and autocrine or paracrine signals. The dynamic interactions between satellite cells and their niche specifically regulate satellite cell quiescence, self-renewal, proliferation, and differentiation. In this fashion, muscle atrophy or excessive growth is prevented, and the satellite cell pool is maintained during regeneration. Understanding the interactions between satellite cells and their niche is of paramount importance for the development of therapies to treat both age-related skeletal muscle atrophy (sarcopenia) and skeletal muscle diseases. In the following section, we will define the scope of the satellite cell niche and discuss how these niche factors affect satellite cells during muscle regeneration.
Figure 3.
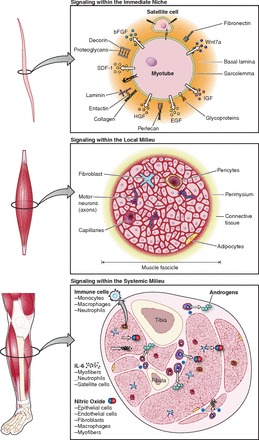
The satellite cell niche. A: satellite cells reside between the basal lamina and the sarcolemma of adult skeletal myofibers and as such are influenced by structural and biochemical cues emanating from this microenvironment. A complex set of diffusible molecules (e.g., Wnt, IGF, and FGF) are exchanged between the satellite cell and the myofiber to maintain quiescence or promote activation. In addition, numerous extracellular matrix components and cellular receptors are present either on the surface of the sarcolemma, satellite cell, or contained within the basal lamina. These components comprise the immediate niche of the satellite cell and dictate rapid changes in the satellite cell state. B: the muscle fascicle defines the extremities of the local milieu an environment that is more diverse compared with the immediate niche due to the heterogeneity of cell types and signaling factors. The local milieu surrounding the satellite cell is made up of other myofibers in addition to interstitial cells, capillaries, and neuromuscular junctions. Each of these cell types influences the surrounding environment and thereby affects the state of the satellite cell. C: the systemic milieu contains the greatest diversity with which the satellite cell is influenced. Exposure of satellite cells to the host's immune system, and circulating hormones along with the skeleton and surrounding skeletal muscles present the broadest environment for the satellite cell. Changes that occur in the systemic milieu affect the satellite cell gradually. Prolonged exposure of signals from the systemic milieu plays an important role in the ability of satellite cells to participate in multiple rounds of regeneration.
A. The Immediate Niche
1. Satellite cell: regulatory signaling pathways
A) WNT SIGNALING.
Wnt signaling controls diverse biological processes, such as cell proliferation, cell fate determination, cell adhesion, cell polarity, and morphology. Wnt signaling is activated by binding of extracellular Wnt family glycoproteins with Frizzled receptors and low-density lipoprotein receptor-related protein (LRP5, LRP6) pairs. The Frizzled receptor can initiate two distinct signaling pathways: the Wnt/β-catenin and Wnt/PCP pathways. Wnt/β-catenin pathway is characterized by the regulation of β-catenin stabilization and its entry into the nucleus. The Wnt/PCP pathway is involved in planar cell polarization (PCP) and establishment of polarized cellular structures (183).
In the Wnt/β-catenin pathway, the level and subcellular localization of β-catenin determine the ultimate output. Normal levels of β-catenin dimerize and associate with both transmembrane cadherins and cytoskeleton-bound α-catenin, which promotes cell adhesion and controls cell shape. The normal levels of cytoplasmic β-catenin are maintained by constitutive degradation of excess β-catenin by GSK3-β. In the cytoplasm, GSK3-β and β-catenin complex with Axin and adenomatous polyposis coli (APC), leading to β-catenin phosphorylation by GSK3-β. Phosphorylated β-catenin is bound by the β-TrCP component of the ubiquitin ligase complex and degraded by the proteosome. GSK3-β is constitutively active but can be inhibited by Dishevelled (Dvl), which is activated by Wnts binding to a Frizzled and LRP receptor pair. Thus, when Wnt signaling is active, elevated levels of cytoplasmic β-catenin enter the nucleus and interact with Tcf/Lef transcription factors to regulate gene expression (373).
Accumulating evidence indicates that Wnt/β-catenin signaling is involved in satellite cell function during muscle regeneration, although its defined roles remain controversial. First, Brack et al. (65) suggested that Wnt signaling promotes myogenic commitment and terminal differentiation in adult myogenesis. By examining the effects of exogenous Wnt3a and sFRP3 as activator and inhibitor of Wnt signaling, respectively, it was found that regenerating muscles in vivo and myoblasts derived from cultured single myofibers are responsive to Wnt signaling only at a late stage of differentiation. Activation of Wnt/β-catenin signaling increased both Desmin expression in myogenic cells in vitro and the size of newly formed myofibers in vivo, whereas inhibition of Wnt signaling caused the opposite effects. Interestingly, Notch signaling seems to antagonize the effects of Wnt signaling at early stages of myoblast proliferation. This counteracting effect is reflected by the levels of the active form of GSK3-β, which are high in the early Notch activating stage and low in later Wnt activating stage. It was proposed that low activity of Wnt signaling in early proliferation allows the expansion of enough myoblasts by Notch signaling for later differentiation (65). Notably, although this study reported that the mRNA levels of Wnt ligands and Frizzled receptors within isolated myofibers increase in late culture, it is still possible that Wnt ligands may originate from other sources in vivo, such as the ECM, and may function through preexisting Frizzled receptors on satellite cells.
Perez-Ruiz et al. (407) proposed that β-catenin promotes self-renewal of satellite cells and prevents them from immediate myogenic differentiation. By retrovirus-based overexpression and RNAi knockdown of proliferating Pax7+/MyoD+ satellite cells on single myofibers, it was found that constitutive expression of stabilized β-catenin, or inhibition of degradation by GSK3β leads to downregulation of MyoD and Myogenin expression. In contrast, when β-catenin was downregulated by RNAi or when its target genes were repressed by the dominant-negative β-catenin-ERD, fewer Pax7+/MyoD− myoblasts were observed. As β-catenin levels determined the observed cellular changes, it was proposed that the Wnt/β-catenin pathway is involved (407). It should be stressed that although MyoD expression can be regulated by directly changing β-catenin levels, the physiological relevance of this phenomenon still largely depends on the availability of Wnt ligands and expression of Frizzled receptors on satellite cells. In addition, a recent study revealed that β-catenin directly interacts with MyoD to enhance MyoD binding to E-box elements to initiate the myogenic program (271). However, in vivo studies are required to further confirm and elaborate on the mechanism whereby β-catenin interacts with MyoD.
Otto et al. (391) proposed that Wnt ligands are important regulators of proliferation for satellite cells during adult muscle regeneration. mRNAs for Wnt ligands (Wnt1, Wnt3a, Wnt5a, Wnt11) were expressed in single myofibers (together with satellite cells) upon 2 days of culture in vitro. These data along with reporter assays indicated that Wnt signaling is active at this point. It was found that exogenous Wnt1, Wnt3a, and Wnt5a induced satellite cell proliferation, whereas exogenous Wnt4 and Wnt6, whose expression is absent from regenerating muscle, inhibited the proliferation. This is consistent with the inhibitory effect of ECGC, a potent Wnt signaling inhibitor, on satellite cell proliferation (391). Importantly, the expression and subcellular location of the active form of β-catenin (Act-β-Cat) was carefully examined by immunofluorescence staining in this study. Nuclear Act-β-Cat was observed only after MyoD expression and was absent in Myogenin+ cells, which suggests Wnt signaling is inactive either before satellite cell activation or after differentiation determination. This temporal progression of Wnt signaling was further confirmed in mouse regenerating muscles and human dystrophic muscle biopsies. Thus Otto et al. (391) concluded that activating Wnt ligands function to promote satellite cell proliferation during muscle regeneration, whereas inhibitory Wnt ligands antagonize the proliferation by secluding β-catenin to the cell membrane in quiescent satellite cells. Perplexingly, the pro-proliferation function of Wnt3a signaling revealed by this study seems to oppose the pro-differentiation function reported by Brack et al. (65). This might be due to different sources of Wnt ligands, either from cultured Wnt-expressing cells or from recombinant protein, which in turn affect Wnt concentration and effective time.
In addition to β-catenin-dependent gene activation, recent studies indicate that the binding of Wnt ligands with Frizzled/LRP receptors can also lead to a transduction cascade to establish PCP, which is referred to as the Wnt/PCP pathway (reviewed in Ref. 492). In this signaling pathway, signaling from Wnt ligand/receptor binding activates Rho/Rac small GTPase and Jun NH2-terminal kinase (JNK) via Dishevelled. Dishevelled activates Rac, which further activates JNK to assist in the subsequent regulation of cytoskeleton organization and gene expression. The Wnt/PCP signaling pathway plays an important role in the development of anterior-posterior extension of the neural tube and the establishment of the aligned cellular structure in epithelium.
Emerging evidence reveals that satellite cells are under the regulation of Wnt/PCP signaling pathway. Our group demonstrated that quiescent satellite cells can be separated into two functional subpopulations based on their Myf5 expression: Pax7+/Myf5− satellite stem cells and Pax7+/Myf5+ satellite progenitor cells (280). Of interest, we determined that mRNAs of the Frizzled7 receptor are preferentially present in Pax7+/Myf5− quiescent satellite stem cells (293). However, upon injury, Frizzled7 is ubiquitously expressed in all satellite cells and their proliferating progeny. In a frozen injury model, Wnt1, Wnt2, Wnt5b, Wnt8b, Wnt10a, Wnt16a, Frizzled receptors, and sFRP inhibitors were increased in muscles 3 days postinjury (acute phase of regeneration). In contrast, an increase of Wnt7a and Wnt10a was detected in muscles 6 days postinjury, when satellite cells returned to the sublaminar position. It was determined that Wnt7a acts through the Frizzled7 receptor to specifically activate the Wnt/PCP pathway and induce cell polarity both ex vivo and in vitro. Activation of the Wnt/PCP pathway by recombinant Wnt7a simulated the symmetrical division of satellite cells and hence expanded the satellite stem cell population on single myofiber explants. Knockdown of Wnt/PCP downstream component, Vangl2, by RNAi caused the opposite effects, confirming the involvement of Wnt/PCP pathway in this process. In vivo, Wnt7a injection into regenerating muscles remarkably improved muscle regeneration as evidenced by increased muscle mass and myofiber size. We postulated that the activation of Wnt/PCP pathway promotes the planar alignment of two daughter cells along the myofiber during satellite cell division. This alignment and the induced cell polarity may facilitate the attachment of both daughter cells to the basal lamina and hence maintain their stem cell fate (293). Although it is still unknown, one possible source of endogenous Wnt7a is the regenerative myofiber, given its close proximity to satellite cells and perfect alignment to the basal lamina (65, 413). Intriguingly, a recent study revealed synergistic functions of Wnt/β-catenin and Wnt/PCP pathways in oriented elongation of myocytes during embryonic muscle patterning (205). Thus it is enticing to further speculate that the activated Wnt/β-catenin pathway in regenerating myofibers may induce Wnt7a secretion and activation of the Wnt/PCP pathway in adjacent satellite stem cells, which in turn would direct their symmetric division and replenish the satellite cell pool.
Wnt signaling is also implicated in satellite cell-related transdifferentiation. Our group reported that muscle residing CD45+/Sca1+ cells increased in number and underwent myogenic differentiation in injured muscles but not in intact muscles (413). CD45+/Sca1+ cells express Frizzled receptors and upregulated β-catenin during regeneration, suggesting the Wnt/β-catenin pathway is required for the myogenic commitment of these cells. Indeed, the myogenic commitment of CD45+/Sca1+ cells can be induced by lithium treatment, or coculture with myoblasts or Wnt secreting cells. Daily injections of Wnt signaling inhibitors, sFRPs, severely reduced the myogenic recruitment of CD45+/Sca1+ cells during regeneration. These data further suggest that Wnt signaling is required for muscle regeneration and may increase the myogenic potential of CD45+/Sca1+ cells (413). Similarly, it was reported that β-catenin is required and sufficient for myogenic commitment of P19 embryonic carcinoma cells (408). Of note, a recent study showed that Wnt signaling is important for transdifferentiation of satellite cells into fibrogenic cells (66). Activated satellite cells from old mice have higher Wnt signaling activity and a greater tendency to assume fibrogenic fate in vitro, compared with those from young mice. Exposure of activated satellite cells from old mice to young serum or pairing with young mice in a heterochronic parabiotic system reversed the Wnt signaling activity and associated fibrogenic tendency, suggesting they are not intrinsic. Conversely, activated satellite cells from young mice exposed to aged serum or linked parabiotically activated Wnt signaling and became fibrogenic. These observations indicated that the fibrogenic transdifferentiation of satellite cells is influenced by an unknown circulating factor (66). As the effect of this factor can be neutralized by a recombinant secreted form of Frizzled, it was believed that an increase in Wnt ligands in old mice may account for the fibrogenic transformation observed (66). Notably, an alternative possibility is that certain Wnt inhibitors are decreased in old mice. This possibility is supported by a recent finding that a Wnt binding protein, Klotho, is a circulating hormone and can affect tissue aging (281, 313). Wnt signaling also controls the balance between myogenic and adipogenic potentials of myoblasts in vitro. First, downregulation of Wnt/β-catenin signaling mediated by Wnt10b was implicated in the increase of adipogenic differentiation of aged myoblasts (527). Similarly, it was observed that in Wnt10b−/− mice, the adipogenic potential of myoblasts increased and excessive lipid accumulated in regenerating myofibers (549). A recent mechanistic study indicated that Wnt10b activated the Wnt/β-catenin pathway and that SREBP-1c-mediated lipogenesis and insulin resistance reciprocally counteract each other and thus determine the myogenic and adipogenic fate of myoblasts (2). The above-mentioned experiments serve to demonstrate the complexities involved in Wnt regulation of myogenesis. Future studies focused on in vivo regulation of a myogenic to adipogenic switch will elaborate on the physiological relevance of the above experimental evidence.
B) NOTCH SIGNALING.
Notch signaling regulates cell proliferation, differentiation, and cell fate determination (21). The activation of Notch signaling pathway is initiated by the binding of Delta and Jagged family of ligands to Notch transmembrane receptors, the result of which induces sequential enzymatic cleavage of the Notch receptor and release of an active truncated form of Notch, termed the Notch intracellular domain (NICD). Upon enzymatic cleavage, the NICD translocates from the cytoplasm to the nucleus and activates the CSL (CBF1, Suppressor of Hairless and Lag-1) family of transcription factors, leading to target gene expression.
Notch signaling plays key roles in skeletal muscle regeneration (reviewed in Ref. 320). Upon muscle injury, the Notch ligand Delta is quickly upregulated in both activated satellite cells and in myofibers (108). The upregulation of Delta in satellite cells is accompanied by the appearance of the NICD, indicative of activated Notch signaling. Activation of Notch signaling stimulates the proliferation of satellite cells and their progeny and thus leads to the expansion of proliferating myoblasts. Indeed, inhibition of Notch signaling abolishes satellite cell activation and impairs muscle regeneration (108). Reversely, constitutive Notch activation in satellite cells from Pax7-CreER;ROSANotch resulted in increased Pax7+ satellite cells and impaired muscle regeneration (560). In aged muscles, the expression of Notch in satellite cells remains unchanged (107). However, the induction of Delta in myofibers is no longer responsive to muscle injury, which results in reduced satellite cell proliferation and impaired muscle regeneration (107).
At the onset of myogenic differentiation, however, the inactivation of Notch signaling in myoblasts is required for their fusion (108). Two mechanisms exist to downregulate Notch signaling. The first involves Numb, a Notch signaling inhibitor, which is asymmetrically partitioned during satellite cell/myoblast division (108, 489). The daughter cell inherits Numb and activates the expression of Desmin leading to differentiation (108). Numb inhibits Notch signaling by inducing ubiquitination of the NICD, which abolishes its activity (21). Second, the activation of Wnt signaling in differentiating myoblasts antagonizes Notch signaling and facilitates terminal differentiation (65). This is supported by observations that activation of Notch signaling is associated with phosphorylation of tyrosine 216 of GSK-3b, whereas Notch inhibitor treatment or activation of Wnt signaling in myoblasts coincided with dephosphorylation of this residue (65). Although the precise mechanism remains ambiguous, the crosslink between Wnt signaling and Notch signaling at Dishevelled and Presenilin has been reported (131). In addition, β-catenin has been reported to associate with MyoD and facilitate the transcription activity of MyoD (271). Thus it would be interesting to investigate whether MyoD/β-catenin can drive the expression of Notch signaling inhibitors, such as Numb, Itch, or the newly reported bHLH transcription factor Stra13 (511).
As both Notch receptors and their ligands are transmembrane proteins, Notch signaling is an important signaling pathway mediating cell-cell communications. Our group recently demonstrated that Megf10, a multiple EGF repeat-containing membrane protein, may function similar to Delta and Jagged in Notch signaling activation (235). Notch-mediated cell-cell communications, presumably conveyed between “bumping” satellite cells or between satellite cells and the myofiber, may serve as a controlling mechanism for satellite cell quiescence and the number of satellite cells on a single myofiber under physiological conditions. In line with this view, double knockout mice of Hey1 and HeyL, two downstream target genes of Notch signaling, showed remarkably reduced number of satellite cells, which is likely due to failure of satellite cells in keeping quiescence in adult muscle (176).
Three Notch receptors are abundantly expressed on satellite cells: Notch1, Notch2, and Notch3. A recent study suggests that Notch3 may act as a Notch1 repressor by activating Nrarp, a negative-feedback regulator of Notch signaling (272). In line with this view, Notch3-deficient mice showed markedly increased numbers of sublaminar quiescent satellite cells and muscle hyperplasia after repetitive muscle injuries, which are phenotypically opposite to those from Notch1 mutants.
In addition to its functions during muscle regeneration, Notch signaling is also involved in the maintenance of cell quiescence in resting health muscle. It was found that genetic abrogation of Rbpj, the main effector for canonical Notch signaling, specifically in satellite cells results in the loss of their quiescent state (356). In Pax7-CT2;Rbpjflox/− mice, Tamoxifen induction and Rbpj depletion in satellite cells gradually decreased the number of Pax7+ satellite cells, which is accompanied with spontaneous myogenic differentiation and failure of muscle regeneration upon injury. Interestingly, the spontaneous myogenic differentiation of the majority of mutant satellite cells in this model seems to start with G0 (quiescent) satellite cells without entry into S phase in vivo.
The above findings support the notion that Notch signaling is critical for satellite cell activation and proliferation. As Notch signaling can be activated by cell-to-cell contact, it is very intriguing to envision that communal satellite cells existing on the same myofiber can signal each other. This community niche effect may serve to determine the number of quiescent satellite cells on a myofiber and/or control the size of the satellite cell pool during regeneration. In this respect, recent studies have revealed a close relationship between Notch signaling and Hippo (Salvador/Warts/Hippo) signaling pathways in determining cell proliferation and organ size in Drosophila (77, 339, 412). It would be interesting to investigate the interactions of these two pathways in satellite cell activation and proliferation.
C) SPHINGOLIPID SIGNALING.
Sphingolipids are a large group of naturally occurring glycolipids, characterized by their sphingoid backbone. Although originally recognized as inert precursors and intermediate products in lipid metabolism, sphingolipids, particularly ceramide, ceramide-1-phosphate, sphingosine, and sphingosine-1-phosphate (S1P), have been recently revealed as important bioactive signaling molecules in regulating cell proliferation, migration, death, and senescence (reviewed in Ref. 223). Sphingomyelin is the most abundant component in the sphingolipid pathway and also serves as a precursor of ceramide, sphingosine, and S1P. It was found that sphingomyelin is enriched in plasma membrane of quiescent satellite cells, yet it markedly diminishes after satellite cell activation and reappears in some Pax7+/MyoD− satellite cells returning to the quiescent state (369). The dynamics of sphingomyelin are connected with the biosynthesis of mitogenic S1P, which promotes satellite cells to enter the cell cycle on ex vivo cultured myofibers and improves muscle regeneration (370, 461). On the other hand, pharmacological inhibition of sphingomyelin-to-S1P processing reduced the number of activated satellite cells and perturbed muscle regeneration. This action of S1P on satellite cell activation and muscle regeneration is in line with the proliferative, proinflammatory (through COX-2) and antiapoptotic (through inhibition of caspase-3 and BAX) functions of S1P-mediated pathways in general. Accumulating evidence indicates that S1P, being one of the more soluble sphingolipids, acts through S1P receptors (S1PRs; a group of high-affinity G protein-coupled receptors) on satellite cells in an autocrine/paracrine fashion (76, 122). In addition, abnormal modulation of S1P activity is also involved in the pathology of muscular dystrophy (as exemplified in mdx mice) (317).
Our current understanding of the functions of bioactive sphingolipids on satellite cells is far from complete. For example, the role of ceramide, another central player of the sphingolipid signaling pathway, on satellite cell activation/proliferation/differentiation during muscle regeneration is vastly unknown. Moreover, whether S1P may bind to other intracellular proteins and exert S1PR-independent actions remains to be investigated.
2. The myofiber niche
The myofibers are the primary component of the satellite cell niche due to their direct contact with satellite cells. The overarching effect provided by the myofiber for satellite cells was revealed through selective killing of myofibers with Marcaine while leaving the basal lamina intact. This resulted in greater numbers of proliferating satellite cells compared with viable myofibers (50). This suggested that the myofiber emanates a “quiescent” signal either by its physical association or by releasing chemical compounds (50, 53). In accordance with this hypothesis, recent studies have revealed numerous satellite cell regulatory factors that are presented on myofibers or secreted by myofibers. First, myofibers secrete SDF-1, which serves as ligand to the cell surface receptor CXCR4 on satellite cells (429, 486). SDF-1/CXCR4 signaling has been shown to stimulate satellite cell migration (429). In addition, it has been found that the transmembrane Notch ligand Delta is upregulated on myofibers after injury, which can activate the Notch signaling cascade in satellite cells and hence induce their proliferation (107). Such an activation signal from myofibers is probably amplified by the expression of both Notch receptors and Delta ligands on satellite cells, functioning in autocrine and juxtacrine fashion (108, 280).
In aged muscle, both the caliber and the number of myofibers decline (202), although the direct effect of these morphological changes is not known. Interestingly, the presence of Delta ligands is not sufficiently expressed on aged myofibers, an indication of diminished Notch signaling possibly compromising satellite cell activation and muscle regeneration (107).
3. ECM and associated factors
The basal lamina of the myofiber is composed of a network of ECM that directly contacts the satellite cell and separates it from the muscle interstitium. The basal lamina of skeletal muscle is composed of type IV collagen, laminin, entactin, fibronectin, perlecan, and decorin glycoproteins along with other proteoglycans (459). These ECM molecules are mainly synthesized and excreted by interstitial fibroblasts but can also be produced and remodeled by myoblasts during muscle development and regeneration (278). Satellite cells reside between the basal lamina, composed primarily of a type IV collagen network, and the apical sarcolemma covered in laminin (568). The basal lamina presents a large number of binding sites for α7/β1-integrins; sites that anchor the actin cytoskeleton of satellite cells to the ECM (501; reviewed in Ref. 330). This physical tethering is critical for satellite cell activation as it allows the transduction of extracellular mechanical force into intracellular chemical signals (62; reviewed in Ref. 73). In addition, muscle specific laminin-2 (α2/β1/γ1 subunits) and laminin-4 (α2/β2/γ1) are also associated with myofibers though α7/β1-integrins and dystroglycan on the surface of myofibers (208; reviewed in Ref. 459). Proteoglycans reside on the surface of satellite cells and function as receptors to bind a suite of secreted, yet inactive growth factor precursors. These precursors, including HGF (521), basic fibroblast growth factor (bFGF) (141), epidermal growth factor (EGF) (193), insulin-like growth factor isoforms (IGF-I, IGF-II) (323) and various Wnt glycoproteins (65, 293), originate from either satellite cells, myofibers, interstitial cells, or serum. In resting muscle, the sequestering of inactive growth factors exists as a local reservoir, to facilitate a rapid response to muscle injury. These growth factors can be swiftly rendered active by proteolytic enzymes present in serum or interstitium, such as thrombin, serine proteases, and matrix metalloproteinases (MMPs). This highly coordinated process serves to stimulate satellite cell survival, activation, and proliferation upon muscle injury (249, 288, 386, 521, 576).
A) HGF/SCATTER FACTOR.
HGF is a heterodimer of its α and β subunits, both of which are processed from a single-chain inactive precursor (pro-HGF). HGF is critical for cell growth, migration, and organ morphogenesis through its mitogen, motogen, and morphogen activities (reviewed in Refs. 381, 586). In skeletal muscle, HGF functions in the early phase of muscle regeneration and is one of the key activators of satellite cells (342, 521). In intact muscle, low levels of HGF mRNA can be detected in satellite cells but not in fibroblasts, suggesting an autocrine function of HGF (13, 484, 521). However, the majority of active and inactive forms of HGF are sequestered by heparan sulfate proteoglycans (HSPGs) within the basal lamina (13, 484, 520, 521). Upon injury, the transcription of HGF is increased in proportion to the degree of injury, and an active form of HGF is released from the ECM without the need of proteolytic cleavage of pro-HGF (484, 520, 521, 525, 569). In vitro and in vivo data have revealed that release of nitric oxide synthase (NOS) from the basal lamina after myofiber stretch or damage leads to the production of nitric oxide (NO), which in turn activates MMPs and release HGF from local HSPGs (522, 523, 576). Alternatively, the observed rapid upregulation of HGF following muscle injury is due to release of HGF from intact organs, such as the spleen (513).
Released HGF and newly synthesized HGF act directly on satellite cells and myoblasts through the cell surface receptor c-Met found on both quiescent and activated satellite cells (13, 116, 179, 521). Multiple studies have demonstrated that the activation of HGF/c-Met pathway stimulates quiescent satellite cells to enter the cell cycle, increases myoblasts proliferation, and inhibits myogenic differentiation (13, 179, 342, 521). Fittingly, HGF isolated from crushed muscle extracts induces quiescent satellite cell activation, and an anti-HGF antibody abolished this activity (521). Furthermore, direct injection of HGF into injured muscle blocked the regeneration process but increased myoblast numbers (342, 521). Forced expression of a constitutive active form of c-Met in C2C12 myoblasts resulted in the inhibition of differentiation (15). HGF inhibits myoblast differentiation by coordinately repressing transcriptional activity of MRF/E-protein complexes, increasing MyoD inhibitor Twist expression, as well as decreasing p27kip1 expression (179, 306). The dual function of HGF in promoting proliferation and inhibiting differentiation involves sustained activation of MAPK/Erk signaling through binding of Grb2 to phosphorylated c-Met (214, 304, 305). Moreover, HGF promotes satellite cell migration to the site of injury as demonstrated by the in vitro chemotactic activity of this factor on satellite cells and C2C12 myoblasts (49, 512, 551). The function of HGF in muscle regeneration is important during the early phase of repair because over time levels of HGF decline and exogenous HGF does not affect muscle regeneration when administrated at later stages (342, 521). Therefore, these results demonstrate that HGF is a satellite cell activator, playing a critical role in the early stage of muscle regeneration. The activation of satellite cells by HGF is due to both transcription activation of HGF in satellite cells and release of HGF from the ECM by MMPs, which coordinately function in an autocrine and paracrine fashion to induce the proliferation of satellite cells.
B) FIBROBLAST GROWTH FACTORS.
Fibroblast growth factors (FGFs) are a large family of mitogens, involved in cell growth, survival, migration, and embryonic development. Numerous FGFs have been found in skeletal muscle in vivo and upon culture and differentiation of myoblasts in vitro (12, 199, 222, 266). Inhibition of endogenous FGF-1 by siRNAs led to myogenic differentiation of myoblasts in vitro, while exogenous FGF-1, -2, -4, and -6 robustly stimulate the proliferation of rat primary myoblasts in vitro (266, 483). Of the particular FGF family members, FGF-6 is of particular interest given its muscle specific expression and upregulation during muscle regeneration (134, 172). A study in FGF-6−/− mutant mice revealed severe muscle regeneration defects characterized by a reduction in MyoD− and Myogenin-expressing cells along with increased collagen deposition and fibrosis (172). FGF-2 is also likely involved in myoblast proliferation during muscle regeneration as endogenous FGF-2 was found in the basal lamina (113). Moreover, injecting a neutralizing antibody of FGF-2 at the time of injury (297) or injecting exogenous FGF-2 into mdx mice (296) caused a delay in myoblast proliferation and increased myoblast proliferation, respectively. In addition, FGFs also facilitate muscle regeneration through their well-known function in angiogenesis (295).
FGF signaling is mediated by virtue of four transmembrane tyrosine kinase receptors known as FGFR1–4, which differ in their specificity of ligand binding. Of these, FGFR1 and FGFR4 are the most prominent receptors in rat satellite cells in vitro (172). In vitro, FGF-1 can induce the expression of FGFR1, and this induction effect can be further augmented by HGF (483). Stable overexpression of FGFR1 increases myoblast proliferation and delays their differentiation, whereas overexpression of a dominant negative form of FGFR1 leads to the opposite effects (463). In skeletal muscle, dimerization of FGFs with FGFRs leads to tyrosine phosphorylation of FGFR and activates the downstream Ras/MAPK pathway. This is supported by the finding that constitutively expressed active RAS in myoblasts induces proliferation and blocks differentiation even in the absence of exogenous FGFs (162). In addition, inhibition of mitogen-activated protein kinase kinase (MAPKK) activity completely abolishes the FGF2-dependent inhibition of myogenic differentiation in vitro (561). Therefore, activation of the Ras/MAPK signaling pathway seems to play a central role in FGF/FGFR-mediated pro-proliferation and anti-differentiation effects in skeletal muscle cells.
In addition to the canonical FGF receptors, HSPGs also serve as low-affinity FGF receptors (reviewed in Ref. 427). Although HSPGs are ubiquitously present on most mammalian cells, both quiescent and activated satellite cells specifically express syndecan-3 and syndecan-4 (113). Further investigations revealed that syndecan-3−/− and syndecan-4−/− mutant mice display distinct panels of phenotypes, implicating separate roles of these two HSPGs in satellite cell function (115). It is believed that HSPGs interact with both FGFs and FGF receptors to promote ligand recognition (236). In accordance with this, satellite cell activation and proliferation is impaired and MAPK signaling is completely abolished in myoblasts derived from syndecan-4−/− mutant mice (115). In contrast, hyperplasia of satellite cells and myonuclei and overactivation of MAPK signaling were observed in syndecan-3-mutant mice, which suggests that an inhibitory mechanism is missing (115). In light of this, the downregulation of syndecan-4 mRNA was observed in turkey satellite cells in response to exogenous FGF-2 (548).
C) IGF.
IGF-I and IGF-II play important roles in the regulation of satellite cell activity (reviewed in Ref. 409). IGF-I has pleiotropic functions including anti-inflammation, cell migration, and stimulation of both proliferation and differentiation in satellite cells. In contrast, IGF-II is believed to only promote myogenic differentiation (11, 103, 151, 169, 170, 547). Elevation of IGF-I signaling within muscle cells in vitro results in muscle hypertrophy with increased DNA and protein content in muscle (6, 34, 87, 103, 365). The hypertrophic effects of IGF-I are attributed to both the activation of satellite cell proliferation, which gives rise to more myonuclei, and protein synthesis, which increases the cytoplasmic-to-DNA volume ratio (32, 35, 366). IGF-II expression and secretion are elevated prior to myoblast differentiation (171), and knock-down of either IGF-I or IGF-II in vitro results in impaired myogenic differentiated (158, 171). Consistently, IGF2−/− mutant mice are ∼40% lighter compared with the weight of wild-type mice at birth, and this defect persists postnatally (133).
IGF can function in endocrine, autocrine, and paracrine fashions to promote satellite cell proliferation and differentiation, but all of these are mediated by IGF-I binding to the IGF-I receptor (IGF1R), which is a ligand-activated receptor tyrosine kinase. In contrast to IGF-I-induced muscle hypertrophy, IGF1R−/− mutant mice display a dystrophic phenotype and usually die at birth due to weak respiratory muscles (315). Activation of IGF1R in satellite cells induces the expression of MRFs (170) and initiates intracellular signaling cascades involving both mitogenic and myogenic responses (110, 367). Two primary signaling pathways are activated by IGF1R. In the first pathway, activated IGF1R recruits and phosphorylates insulin receptor substrate proteins (IRSs), leading to the activation of phosphatidylinositol 3-kinase (PI3K). Activation of PI3K is involved in multiple cellular processes including an anti-apoptotic effect mediated by activation of the AKT pathway (502), modulation of intracellular calcium levels by the inositol phosphate cascade (156), and promoting protein translation (5). Although evidence supports that IGF-I-dependent satellite cell proliferation is mediated by the AKT/mTOR pathway (220), the activation of PI3K/AKT and p38-MAPK pathways are also involved in myoblast differentiation (197, 539). In the second pathway, IGF1R activates the Ras/Raf/extracellular response kinases (ERKs) cascade, which in turn activates other protein kinases and several transcription factors (110, 347). The activation of the Ras/Raf/ERK pathway is also required for satellite cell proliferation (110). In addition to the aforementioned pathways, accumulating evidence indicates that the calcium/calcineurin pathway is also involved in IGF-I signaling-dependent muscle differentiation and hypertrophy (137, 173, 366, 479). Specifically, it has been revealed that GATA-2-mediated myofiber hypertrophy depends on IGF-I stimulation of the calcineurin-mediated signaling pathway (366). Further evidence of the pleiotropic functions of IGF-I involves a role in anti-inflammatation demonstrated by a reduction in chronic inflammation upon IGF-Iϵ treatment during muscle regeneration (357).
Activated satellite cells and cultured myoblasts express insulin-like growth factor binding proteins (IGFBPs), which are a family of secreted proteins that specifically bind IGFs and function as carriers during circulation and regulate IGF turnover, transport, and half-life (reviewed in Ref. 253). Of the five IGFBPs, IGFBP-5 plays a critical role in muscle growth and differentiation. IGFBP-5 expression is induced during differentiation and precedes that of IGF-II in cultured myoblasts (246, 436). It was found that IGFBP-5 functions to promote myogenic differentiation by switching on an IGF-II-mediated positive-feedback loop (436). Knockdown of IGFBP-5 impairs myogenesis and suppresses IGF-II expression (436).
Notably, recent studies demonstrated that alternative splicing of IGF-I pre-mRNA gives rise to a unique peptide, termed mechano-growth factor (MGF) (194). MGF promotes myoblast proliferation in vitro (26, 578). However, the existence of MGF and its functions in vivo remain controversial (discussed in Ref. 327).
D) MMPS.
Matrix metalloproteinases (MMPs) are a family of zinc-dependent enzymes that can degrade components of the extracellular matrix such as collagens, elastin, fibronectin, laminin, and proteoglycans. In skeletal muscle, MMPs play an important role in satellite cell activation, migration, and differentiation during muscle regeneration (reviewed in Ref. 86). Of more than 20 MMPs, MMP-2, -3, -7, and -9 were found in skeletal muscle. In particular, MMP-2 and MMP-9, which can degrade denatured collagen types (gelatins) and heparan sulfate proteoglycans, have critical functions on ECM remodeling in skeletal muscle regeneration. Upon injury, MMP-2 is secreted by satellite cells and regenerating myofibers, and its activity is elevated in both degenerative and regenerating stages (167, 178, 270). In contrast, MMP-9 is generated by leukocytes and macrophages, and its activity drastically decreases after the degenerative stage (270). In the degeneration stage, activated MMP-2 and MMP-9 degrade collagen IV in the muscle ECM, which allows satellite cells to migrate across the basement membrane to the site of injury (377). In the regeneration stage, it is believed that MMP-2 is involved in remodeling and maintenance of the ECM. Recent studies indicate that NO upregulates enzymatic activity and protein expression levels of MMP-2 (270) and MMP-9, and this activation of MMP-2 is required for the release of HGF from the ECM upon satellite cell activation (575, 576).
E) ECM STIFFNESS.
Recent studies reveal a previously unappreciated role for the stiffness of the ECM in regulating satellite cell proliferation and differentiation. Resting healthy skeletal muscles and cultured myotubes possess a similar elastic stiffness (elastic modulus ∼12 kPa) (106, 152), whereas aged (184, 444) and dystrophic (152) skeletal muscles are apparently stiffer (elastic modulus ∼418 kPa). These changes of stiffness are presumably due to increased extracellular matrix deposition, in particular collagen deposition by fibroblasts, a result of repeated muscle regeneration. The significance of changes in stiffness was evaluated by assessing the proliferation and differentiation ability of myoblasts on various in vitro culturing surfaces. Primary myoblast proliferation on polyacrylamide gels cross-linked with entactin, type IV collagen, and laminin is optimal at an elastic modulus equal to 21 kPa, while it is impaired on softer (3 kPa) or stiffer (80 kPa) culturing substrates (61). Similarly, C2C12 myoblasts optimally differentiate on collagen-coated polyacrylamide gels that mimic the physiological elasticity of skeletal muscle (12 kPa), whereas either softer (5 kPa) or stiffer (420 kPa) gels greatly compromise their differentiation efficiency (152). Although the stiffness of the ECM surrounding the satellite cell niche has not been directly measured yet, these in vitro data draw attention to a causal relationship between biophysical stimuli and satellite cell function in vivo.
B. The Microenvironment Beyond the Immediate Niche
1. Local milieu
In addition to the immediate niche surrounding satellite cells, local interstitial cells, motor neurons, blood vessels, and their associated secretable factors reside within skeletal muscle and have the potential to regulate satellite cell function and affect muscle regeneration.
A) INTERSTITIAL CELLS.
Interstitial cells are the major component of the stromal tissue between the basal lamina and epimysial sheath surrounding skeletal muscle. Fibroblasts comprise a major component of the interstitial stromal cell population (51). Fibroblasts secrete growth factors, such as FGFs, and also contribute to the skeletal muscle ECM by depositing collagen, laminin, fibronectin, HSPGs, tenascin, and NCAM (185). Increased levels of fat and connective tissue (fibrosis), evidence for increased numbers of adipocytes and fibroblasts, respectively, are well documented for several physiological and pathological conditions, such as aging, obesity, and muscular dystrophy (38, 66, 195, 198, 200). It is believed that satellite cells may account for the increase in adipocytes and fibroblasts due to transdifferentiation along alternative mesenchymal lineages. This notion is supported by the observations that myoblasts can undergo adipogenic differentiation in vitro (239, 310, 485, 579) and satellite cells on single myofibers can give rise to both adipocytes and fibroblasts in vitro (23, 66). Recently, two studies revealed that these intramuscular adipocytes and fibrocytes can arise from muscle residing fibrocyte/adipocyte progenitors (FAPs) (252, 541).
In one study, a population of SM/C-2.6−/PDGFR-α+ cells is able to differentiate into adipocytes in culture (541). In vivo, these cells localize to the interstitial space between myofibers and muscle bundles. After transplantation into glycerol-injected fatty degenerating muscle, SM/C-2.6−/PDGFR-α+ cells can differentiate into adipocytes, a result that is not observed in normal regenerating muscle (541). In another study, a Sca-1+/ CD34− or α7-integrin−/CD45−/CD31− cell population was isolated and found to spontaneously form both adipocytes and ER-TR7+ fibroblasts in culture (252). Although the origin of these cells was not directly investigated, it was further demonstrated that these Sca-1+/α7-integrin− cells also express PDGFR-α, and count for the vast majority of PDGFR-α+ cells in skeletal muscle (252). This suggests strong similarities exist between the cell populations used in both studies. Consequently, Sca-1+/α7-integrin− cells also only give rise to adipocytes in degenerating muscle in vivo, in contrast to their limited adipogenic potential in intact muscle and in injured regenerating muscle. Moreover, a single Sca-1+/α7-integrin− cell was able to differentiate into both adipocytes and fibroblasts in vitro, indicating that these cells are bipotent and thus termed FAPs (252).
Both studies clearly demonstrate that FAPs do not have myogenic potential either in vitro or in vivo, which makes them distinct from satellite cells and other muscle-residing myogenic cells (252, 541). The distinct adipogenic potentials of FAPs in degenerating and regenerating muscles suggest that the differentiation of FAPs is regulated by their local microenvironment. Indeed, both groups demonstrated that FAPs proliferated and transiently increased in number in regenerating muscle without differentiating into adipocytes or fibroblasts (252, 541). Moreover, when adipogenic FAPs isolated from degenerating muscle were transplanted into regenerating muscle, the regenerative microenvironment was sufficient to inhibit adipogenic differentiation (541). These observations indicate that regenerating muscle provides a specialized microenvironment, which favors myogenic differentiation and maintains FAPs in their undifferentiated state. Importantly, the undifferentiated FAPs in such a microenvironment may facilitate the myogenic differentiation of satellite cells, which is supported by the observation that, in co-culture conditions, FAPs increase the commitment of myoblasts to terminal differentiation (252). IL-6 signaling was implicated in this process as evidenced by induced IL-6 expression in FAPs in response to muscle regeneration (252).
In summary, these intriguing results support the hypothesis that skeletal muscle provides a balanced environment for coexistence of both myogenic precursors (myoblasts) and adipogenic/fibrogenic precursors (FAPs) during muscle regeneration. Regeneration is supported by both satellite cells and FAPs, whereas in degenerating muscle, this balance is disrupted and FAPs differentiate into adipocytes (and presumably also fibroblasts). These findings also raise several interesting questions including: How do satellite cells respond to increased adipocytes and fibroblasts in the aforementioned physiological and pathological conditions? Recent observations regarding the effect of fibrosis on the stiffness of the ECM raise the question as to whether increased depositions from fibroblasts or adipocytes can alter myofiber stiffness and therefore affect the physiological roles attributed to satellite cells (152; discussed in sect. IIIA). Finally, it would be interesting to investigate the embryonic origin and postnatal lineage progression of FAPs, particularly their lineage relationship to the CD45−/Sca-1+ mesenchymal stem cells found in multiple tissues (316, 401, 417, 441, 509, 519). Further study is required to understand whether other regulatory factors are also involved in the determination of FAP differentiation and whether these factors change in response to various physiological and pathological conditions.
In addition to FAPs, recent studies identified telocytes (TCs, formerly called interstitial Cajal-like cells or ICLCs) as a new type of cells within muscle interstitium, which are in close vicinity of satellite cells, myofibers, nerve endings, and blood vessels (414). Unlike satellite cells and fibroblasts, skeletal muscle TCs express the cell surface marker c-kit. Transmission electron microscopy (TEM) examination of TCs in muscle and studies on TCs from other tissues suggest TCs transmit intercellular signaling by shedding/intaking microvesicles (also called exosomes), which are enriched for proteins and RNAs (230, 414, 543). Although their exact role in muscle regeneration is still unknown, the finding that these cells secrete VEGF suggests telocytes are an important player in promoting satellite cells self-renewal, facilitating vasculogenesis, and preventing fibrosis (132; further discussed in sect. IIIB1c).
Another cellular component of skeletal muscle interstitium is the SP, a population of cells characterized by their expression of Abcg2 and hence the ability to efflux Hoechst dye. A recent study indicated that endothelial cells and interstitial cells constitute the major fraction of skeletal muscle SP (146). Ablation of Abcg2 impairs skeletal muscle regeneration. In addition, a small percentage of these SP cells have myogenic potential and may contribute to myogenic regeneration in injured muscle (discussed in sect. IID).
B) MOTOR NEURONS.
It is well known that denervation results in progressive skeletal muscle atrophy. During acute denervation of muscle, the percentage of satellite cells increases during the first week, indicating a proliferation phase similar to muscle injury (468). However, long-term denervation results in a drastic decline in satellite cell numbers (442, 550), which is at least partially due to decreased mitotic capability and increased apoptosis of satellite cells. Experiments illustrate that an absence of PCNA (proliferating cell nuclear antigen)-positive satellite cell can be found on myofibers denervated for more than 1 wk. This implies that long-term denervation may cause satellite cells to lose their capability to enter the mitotic cell cycle (282). Second, satellite cells from muscle denervated for 6 and 10 wk displayed a twofold increase of apoptosis, compared with control cells from normal innervated muscle (248). Although the underlying mechanism of a neural influence on satellite cell behavior is still elusive, neurotrophins such as nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF) are involved.
NGF is expressed in developing muscles (563), and its expression is downregulated after birth (153). The expression of NGF reappears in adult muscle under several pathological conditions such as muscular dystrophy (537) and amyotrophic lateral sclerosis (283), wherein expression of NGF was found in regenerating myofibers and connective tissue fibroblasts within the damaged muscles (99, 537), suggesting that it may be involved in muscle regeneration (340). Indeed, ectopic expression of NGF neutralizing antibodies in vivo causes muscle dystrophy (448). Within skeletal muscle, NGF seems to bind to its low-affinity receptor p75NTR (138), which is expressed in satellite cells (358) and human primary myoblasts and myotubes (33). The expression of both NGF and p75NTR is upregulated during primary myoblast differentiation in vitro, and they function to stimulate morphological changes involved in myoblast fusion (138).
Similar to NGF, developing muscles express high levels of BDNF, whereas in adult myofibers it is not detectable (201, 358). BDNF is present in most adult satellite cells, and its expression is correlated with Pax3 expression (358). In cultured primary myoblasts, the expression of BDNF is decreased dramatically during myogenic differentiation; in concordance, a reduction in endogenous BDNF leads to early myogenic differentiation, while addition of exogenous BDNF can reverse this phenotype (358). In vivo, BDNF−/−satellite cells are decreased and hence compromise muscle regeneration (102). These observations suggest that the function of BDNF in adult skeletal muscle is to maintain satellite cells in their quiescent state and prevent their myogenic differentiation (358). This is consistent with the observation that neural electrical activity stimulates the activation of satellite cells (reviewed in Ref. 470) and represses the expression of BDNF (358). Since the TrkB receptor of BDNF was not detected in skeletal muscle, BDNF signaling is proposed to occur via the p75NTR in a similar fashion to NGF.
Taken together, these data indicate that neurotrophic factors function in an autocrine fashion to regulate satellite cell behavior and muscle regeneration. Thus it is conceivable that systematic release of neurotrophic factors after denervation may also exert similar regulatory roles on satellite cell activation and differentiation. In addition, denervation can also cause secondary effects on satellite cells by directly influencing the physiological properties of myofibers (229). Moreover, it has been recently found that satellite cells may control myofiber innervation during muscle regeneration by secreting semaphorin 3A, a well-known factor involved in axon guidance and growth (524). This finding further emphasizes the notion that proper muscle regeneration relies on the dynamic interaction of satellite cells with their niche.
In aged muscle, examination of neuromuscular junctions by EM revealed decreases in nerve terminal area, mitochondria and synaptic vesicles, along with occasional denervated postsynaptic regions (159). These changes, in addition to progressive myofiber atrophy, may potentially disturb signals between myofibers and satellite cells.
C) VASCULATURE.
Skeletal muscle is nourished by the microvascular network. The importance of this niche component is reflected by the fact that most satellite cells are closely associated with capillaries in intact adult muscle (100, 465). It is also well known that angiogenesis and myogenesis proceed at the same time during muscle regeneration (321). Vascular endothelial growth factor (VEGF) plays an important role in satellite cell function and muscle regeneration (416). It has been reported that overexpression of VEGF by adenovirus can stimulate satellite cell proliferation and improve myofiber regeneration in vivo (20). A recent study revealed that in coculture, endothelial cells promote myoblasts proliferation by secreting a panel of growth factors, such as IGF-I, HGF, FGF, platelet-derived growth factor-BB (PDGF-BB), and VEGF (100). In addition, it was found that periendothelial cells (smooth muscle cells and endomysial fibroblasts) promote a subset of myoblasts (presumably reserve cells) to return to the quiescent state (100). This procedure mimics the self-renewal of satellite cells during muscle regeneration. Indeed, recent studies demonstrated that the Tie-2 receptor is preferentially expressed by quiescent satellite cells and angiopoietin-1/Tie-2 signaling acts through ERK1/2 pathway to promote the reentry to quiescence and thus self-renewal of a subset of satellite cells (3, 100). Based on these observations, it was proposed that during muscle regeneration, while vessels are not stabilized, endothelial cells and myogenic precursors interact with each other to promote both myogenesis and angiogenesis. Once homeostasis of muscle is achieved, the proximity of satellite cells and periendothelial cells permits angiopoietin-1, which is secreted by periendothelial cells, to bind Tie-2 receptors on satellite cells to simultaneously stabilize vessels and promote satellite cell quiescence (4, 100). In addition to endothelial cells, satellite cells and myoblasts, differentiating myofibers also generate VEGF within skeletal muscle (70, 92, 189). VEGF can function in an autocrine fashion through VEGF receptors present on various myogenic cells to stimulate migration, prevent apoptosis, and promote differentiation (92, 189). In addition, VEGF stimulation can reportedly activate the Akt pathway and promote myofiber hypertrophy (70, 514).
In aged muscle, it was observed that while myofibers lose their close contact with the microvascular network, there is a corresponding VEGF level decrease (451). In addition, endothelial nitric oxide synthase (eNOS) secreted by endothelial cells is also decreased in aged muscle (580; reviewed in Ref. 67).
Taken together, recent studies have begun to unveil interactions between satellite cells and cells within the microvasculature, experiments that imply a potentially profound functional role necessitating the close proximity of satellite cells to the microvasculature. Future studies are needed to further elucidate these interactions, particularly those of periendothelial cells, during muscle regeneration.
2. Systemic milieu
A) IMMUNE CELLS.
Only a small number of immune cells reside within intact skeletal muscle. During the early stages of muscle injury, immune cells are recruited and play important roles in the regeneration process (reviewed in Ref. 532). Upon injury, immune cells rapidly infiltrate the muscle to remove necrotic tissue and secrete soluble factors that serve to activate satellite cells (reviewed in Ref. 530). As such, immune cells constitute a transient local environment for satellite cells. Depletion of macrophages and monocytes impairs subsequent muscle regeneration (206). Satellite cells and immune cells attract one another through chemokines (chemoattraction) (440). Satellite cells have been demonstrated to secrete a panel of pro-inflammatory cytokines, such as IL-1, IL-6, and TNF-α, to facilitate immune cell infiltration and function (reviewed in Ref. 92). In turn, immune cells secrete a wealth of diffusible factors, such as growth factors, IL-6, globular adiponectin, ECM components, and ECM remodeling MMPs. These diffusible factors generate ECM chemoattractive fragments, help satellite cells escape from the basal lamina of myofibers, and promote satellite cell proliferation. In addition, cell-to-cell contact between immune cells and satellite cells protects satellite cells from apoptosis (503).
Aged muscle displays a functional deficit in the immune cells surrounding the satellite cell niche. In vitro studies indicate that the capabilities of free radical generation, phagocytosis, and chemotaxis are decreased in aged immune cells (25). The perturbation between satellite cells and immune cells impedes satellite cell activation and migration.
B) IL-6.
IL-6 is a ubiquitous pleiotropic cytokine, which can be produced by many cell types, such as hematopoietic cells, fibroblasts, and vascular endothelial cells. Although IL-6 is primarily produced following an immune response, convincing evidence demonstrates that IL-6 is also involved in satellite cell-mediated muscle hypertrophy and regeneration. Chronic systematic elevation of IL-6 leads to muscle atrophy in aging and disease states (29, 212; reviewed in Ref. 154). Likewise, transgenic mice overexpressing IL-6 display a muscle wasting (cachexia) phenotype (538). It is also well documented that exercise causes an increase in IL-6 levels within muscle (533; reviewed in Ref. 403). During regeneration, satellite cells, myofibers, and neutrophils are the principal sources of IL-6, the expression of which is regulated by calcineurin-NFAT, NF-κB, AP-1, IL-1, and NO signaling pathways (74, 333, 583; reviewed in Ref. 403).
The IL-6 receptor (IL-6Rα) is expressed on the myofiber sarcolemma and is responsive to exercise (268). Muscle-derived IL-6 can act in an autocrine fashion by binding to the IL-6Rα on the sarcolemma and activate the JAK/STAT3 signaling pathway (480; reviewed in Refs. 403, 430). Binding of IL-6 to the receptor IL-6Rα leads to JAK2 phosphorylation and subsequent STAT3 phosphorylation. Phosphorylated STAT3 dimerizes and translocates to the nucleus where it acts as a transcription factor for target gene activation.
It has been shown that IL-6 can also induce satellite cell proliferation (80). Recently, it was demonstrated that IL-6 is involved in satellite cell-mediated muscle hypertrophy (480). Although early studies demonstrated that muscle regeneration in IL-6−/− null mice was comparable to that of wild type (556), a recent study reported a blunted hypertrophic response and less contribution of satellite cell myonuclei to regenerating myofibers (480). Furthermore, it was shown that the proliferation of satellite cells from IL-6−/− mice was impaired both in vivo and in vitro, which is due to the attenuation of STAT3 signaling (480).
C) ANDROGENS.
The musculature of men and woman differs in mass, fiber size, and various other physiological properties. These differences are in part due to the anabolic actions of circulating androgens, e.g., testosterone, on muscle size and muscle strength (reviewed in Refs. 97, 228). Androgens bind to androgen receptors (AR) and lead to the dimerization and nuclear translocation of AR. In the nucleus, AR homodimers bind to the DNA motifs or androgen response elements (AREs) and activate transcriptional target genes.
Satellite cells express androgen receptors and are receptive to androgen signaling (143); however, the endogenous androgens acting on satellite cells have yet to be determined. Exogenous testosterone increases satellite cell numbers by promoting satellite cell activation and proliferation (255, 494). This function is predicted to involve the induction of Notch signaling in satellite cells (494). Furthermore, androgen administration causes increased myonuclei and muscle hypertrophy in a dose-dependent manner (254, 493, 495). Multiple mechanisms have been implicated in androgen-induced muscle hypertrophy. First, androgen administration has been shown to elevate local IGF-I levels (165, 307) through a postulated induction of IGF1R expression on satellite cells (529). Androgen administration can also elevate intracellular calcium and inositol 1,4,5-trisphosphate (IP3) levels, which induces phosphorylation of ERK1/2 and contributes to affect the IGF pathway (157). Additionally, androgen may promote muscle hypertrophy by counteracting the catabolic effect of glucocorticoid hormones and promote the myogenic differentiation (48, 496).
During aging, systemic levels of androgens decline, which is concomitant with a loss in muscle mass and a gain in fat mass (228, 544).
D) NITRIC OXIDE.
NO is a diffusible small molecule, which can freely pass through cell membranes. It can be produced by nitric oxide synthase (NOS) in diverse cell types, including epithelial cells, endothelial cells, fibroblasts, hepatocytes, macrophages, and skeletal muscle cells (60). Currently three NOS isoforms exist: endothelial NOS (eNOS), neuronal NOS (nNOS), and inducible NOS (iNOS) (394).
NO has profound functions in intact muscle as well as during muscle regeneration (reviewed in Ref. 505). In skeletal muscle, nNOS is constitutively expressed within the sarcolemma of myofibers (275). Loss of nNOS from the sarcolemma (e.g., as a result of a dystrophin deficiency) leads to inflammation and myofiber lysis, implicating a protective function for NO in skeletal muscle (89). iNOS levels are extremely low in intact muscle, yet drastically induced by macrophages and myofibers in response to muscle injury (449; reviewed in Ref. 262). In the degenerating phase, NO, generated by macrophages and myofibers, promotes the lysis of necrotic myofibers by macrophages while reducing other inflammation-induced damage (375; reviewed in Ref. 505). During regeneration, NO induces satellite cell activation, as evidenced by the inhibitory effect of l-arginine methyl ester (l-NAME), a NOS inhibitor, on satellite cell activation (16). NOS inhibition prevents the binding of HGF to the c-Met receptor on satellite cells, whereas treatment with l-arginine, the NOS substrate, resulted in an increase in satellite cell activation. Accordingly, iNOS−/− mice display delayed satellite cell activation following muscle injury, although injured muscles were still able to regenerate (16). The function of NO-dependent satellite cell activation stems from the ability of NO to promote the release of HGF from the ECM (575, 576). MMP-2 was demonstrated to be involved in this process (575). In addition, it was also suggested that NO may regulate satellite cell migration (104) and prevent fibrosis by inhibiting transforming growth factor-β signaling during muscle regeneration (126).
IV. CONCLUDING REMARKS AND PERSPECTIVES
Compelling evidence indicates that adult satellite cells represent a heterogeneous population of stem cells and committed myogenic progenitors in skeletal muscle. At the population level, the stem cell characteristics of satellite cells are well represented by their remarkable and sustained ability to support skeletal muscle regeneration. At the individual level, satellite cells vary in their self-renewal, proliferation, and myogenic differentiation potential. These intrinsic differences of satellite cells likely reflect the distinct needs of 1) maintaining a sustainable reservoir of stem cells, 2) producing sufficient numbers of myogenic cells, and 3) generating, during the course of muscle regeneration, functional contractile muscular structures. Throughout regeneration, the hierarchical and collaborative behaviors of satellite cells are influenced by changes in their niche. These changes include diverse molecules, cells, and structures that constitute a dynamic niche continually influencing the multiple tasks set forth for satellite cells.
Satellite cells are an ideal cellular model system to study adult stem cells and tissue regeneration. Extensive studies over the last three decades have accumulated much knowledge and insights. Satellite cells are currently not applicable to regenerative medicine due to difficulties in their isolation and loss of stemness ex vivo. However, satellite cell-based cell therapy would greatly benefit from future studies in lineage progression to better delineate satellite cell hierarchy along with physiologically relevant interactions between satellite cells and other myogenic cells. Moreover, such issues may be resolved following the maturation of techniques involving the generation of iPS or other types of ES-like multipotent cells. If so, the broad interest of future studies should focus on identification of the intrinsic and extrinsic regulatory mechanisms that govern satellite cell commitment and differentiation throughout the muscle regeneration. Future advances in system biology and bioengineering may hopefully harness these previous achievements towards developing therapeutic approaches for sarcopenia and muscle dystrophic diseases.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
We credit L. Bucklin for the human leg anatomy image used in FIGURE 3 and Alexander Grayston for critical review of the manuscript.
Address for reprint requests and other correspondence: M. A. Rudnicki, Ottawa Hospital Research Institute, 501 Smyth Rd., Ottawa, Ontario, Canada K1H 8L6 (e-mail: mrudnicki@ohri.ca).
REFERENCES
- 1. Abedi M, Greer DA, Colvin GA, Demers DA, Dooner MS, Harpel JA, Weier HU, Lambert JF, Quesenberry PJ. Robust conversion of marrow cells to skeletal muscle with formation of marrow-derived muscle cell colonies: a multifactorial process. Exp Hematol 32: 426–434, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Abiola M, Favier M, Christodoulou-Vafeiadou E, Pichard AL, Martelly I, Guillet-Deniau I. Activation of Wnt/beta-catenin signaling increases insulin sensitivity through a reciprocal regulation of Wnt10b and SREBP-1c in skeletal muscle cells. PLoS One 4: e8509, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Abou-Khalil R, Le Grand F, Pallafacchina G, Valable S, Authier FJ, Rudnicki MA, Gherardi RK, Germain S, Chretien F, Sotiropoulos A, Lafuste P, Montarras D, Chazaud B. Autocrine and paracrine angiopoietin 1/Tie-2 signaling promotes muscle satellite cell self-renewal. Cell Stem Cell 5: 298–309, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Abou-Khalil R, Mounier R, Chazaud B. Regulation of myogenic stem cell behavior by vessel cells: the “menage a trois” of satellite cells, periendothelial cells and endothelial cells. Cell Cycle 9: 892–896, 2010 [DOI] [PubMed] [Google Scholar]
- 5. Adams GR. Autocrine and/or paracrine insulin-like growth factor-I activity in skeletal muscle. Clin Orthop Relat Res S188–196, 2002 [DOI] [PubMed] [Google Scholar]
- 6. Adams GR, McCue SA. Localized infusion of IGF-I results in skeletal muscle hypertrophy in rats. J Appl Physiol 84: 1716–1722, 1998 [DOI] [PubMed] [Google Scholar]
- 7. Alderton JM, Steinhardt RA. How calcium influx through calcium leak channels is responsible for the elevated levels of calcium-dependent proteolysis in dystrophic myotubes. Trends Cardiovasc Med 10: 268–272, 2000 [DOI] [PubMed] [Google Scholar]
- 8. Alfaro LA, Dick SA, Siegel AL, Anonuevo AS, McNagny KM, Megeney LA, Cornelison DD, Rossi FM. CD34 promotes satellite cell motility and entry into proliferation to facilitate efficient skeletal muscle regeneration. Stem Cells 29: 2030–2041, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Allbrook DB, Han MF, Hellmuth AE. Population of muscle satellite cells in relation to age and mitotic activity. Pathology 3: 223–243, 1971 [DOI] [PubMed] [Google Scholar]
- 10. Allen RE, Boxhorn LK. Inhibition of skeletal muscle satellite cell differentiation by transforming growth factor-beta. J Cell Physiol 133: 567–572, 1987 [DOI] [PubMed] [Google Scholar]
- 11. Allen RE, Boxhorn LK. Regulation of skeletal muscle satellite cell proliferation and differentiation by transforming growth factor-beta, insulin-like growth factor I, and fibroblast growth factor. J Cell Physiol 138: 311–315, 1989 [DOI] [PubMed] [Google Scholar]
- 12. Allen RE, Dodson MV, Luiten LS. Regulation of skeletal muscle satellite cell proliferation by bovine pituitary fibroblast growth factor. Exp Cell Res 152: 154–160, 1984 [DOI] [PubMed] [Google Scholar]
- 13. Allen RE, Sheehan SM, Taylor RG, Kendall TL, Rice GM. Hepatocyte growth factor activates quiescent skeletal muscle satellite cells in vitro. J Cell Physiol 165: 307–312, 1995 [DOI] [PubMed] [Google Scholar]
- 14. Allouh MZ, Yablonka-Reuveni Z, Rosser BW. Pax7 reveals a greater frequency and concentration of satellite cells at the ends of growing skeletal muscle fibers. J Histochem Cytochem 56: 77–87, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Anastasi S, Giordano S, Sthandier O, Gambarotta G, Maione R, Comoglio P, Amati P. A natural hepatocyte growth factor/scatter factor autocrine loop in myoblast cells and the effect of the constitutive Met kinase activation on myogenic differentiation. J Cell Biol 137: 1057–1068, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Anderson JE. A role for nitric oxide in muscle repair: nitric oxide-mediated activation of muscle satellite cells. Mol Biol Cell 11: 1859–1874, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Angelini C, Di Mauro S, Margreth A. Relationship of serum enzyme changes to muscle damage in vitamin E deficiency of the rabbit. Sperimentale 118: 349–369, 1968 [PubMed] [Google Scholar]
- 18. Armand O, Boutineau AM, Mauger A, Pautou MP, Kieny M. Origin of satellite cells in avian skeletal muscles. Arch Anat Microsc Morphol Exp 72: 163–181, 1983 [PubMed] [Google Scholar]
- 19. Armstrong RB. Initial events in exercise-induced muscular injury. Med Sci Sports Exercise 22: 429–435, 1990 [PubMed] [Google Scholar]
- 20. Arsic N, Zacchigna S, Zentilin L, Ramirez-Correa G, Pattarini L, Salvi A, Sinagra G, Giacca M. Vascular endothelial growth factor stimulates skeletal muscle regeneration in vivo. Mol Ther 10: 844–854, 2004 [DOI] [PubMed] [Google Scholar]
- 21. Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science 284: 770–776, 1999 [DOI] [PubMed] [Google Scholar]
- 22. Asakura A, Hirai H, Kablar B, Morita S, Ishibashi J, Piras BA, Christ AJ, Verma M, Vineretsky KA, Rudnicki MA. Increased survival of muscle stem cells lacking the MyoD gene after transplantation into regenerating skeletal muscle. Proc Natl Acad Sci USA 104: 16552–16557, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Asakura A, Komaki M, Rudnicki M. Muscle satellite cells are multipotential stem cells that exhibit myogenic, osteogenic, adipogenic differentiation. Differentiation 68: 245–253, 2001 [DOI] [PubMed] [Google Scholar]
- 24. Asakura A, Seale P, Girgis-Gabardo A, Rudnicki MA. Myogenic specification of side population cells in skeletal muscle. J Cell Biol 159: 123–134, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ashcroft GS, Mills SJ, Ashworth JJ. Ageing and wound healing. Biogerontology 3: 337–345, 2002 [DOI] [PubMed] [Google Scholar]
- 26. Ates K, Yang SY, Orrell RW, Sinanan AC, Simons P, Solomon A, Beech S, Goldspink G, Lewis MP. The IGF-I splice variant MGF increases progenitor cells in ALS, dystrophic, and normal muscle. FEBS Lett 581: 2727–2732, 2007 [DOI] [PubMed] [Google Scholar]
- 27. Bachrach E, Li S, Perez AL, Schienda J, Liadaki K, Volinski J, Flint A, Chamberlain J, Kunkel LM. Systemic delivery of human microdystrophin to regenerating mouse dystrophic muscle by muscle progenitor cells. Proc Natl Acad Sci USA 101: 3581–3586, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Baldwin KM, Haddad F. Effects of different activity and inactivity paradigms on myosin heavy chain gene expression in striated muscle. J Appl Physiol 90: 345–357, 2001 [DOI] [PubMed] [Google Scholar]
- 29. Baltgalvis KA, Berger FG, Pena MM, Davis JM, Muga SJ, Carson JA. Interleukin-6 and cachexia in ApcMin/+ mice. Am J Physiol Regul Integr Comp Physiol 294: R393–R401, 2008 [DOI] [PubMed] [Google Scholar]
- 30. Bansal D, Miyake K, Vogel SS, Groh S, Chen CC, Williamson R, McNeil PL, Campbell KP. Defective membrane repair in dysferlin-deficient muscular dystrophy. Nature 423: 168–172, 2003 [DOI] [PubMed] [Google Scholar]
- 31. Barberi T, Bradbury M, Dincer Z, Panagiotakos G, Socci ND, Studer L. Derivation of engraftable skeletal myoblasts from human embryonic stem cells. Nat Med 13: 642–648, 2007 [DOI] [PubMed] [Google Scholar]
- 32. Bark TH, McNurlan MA, Lang CH, Garlick PJ. Increased protein synthesis after acute IGF-I or insulin infusion is localized to muscle in mice. Am J Physiol Endocrinol Metab 275: E118–E123, 1998 [DOI] [PubMed] [Google Scholar]
- 33. Baron P, Scarpini E, Meola G, Santilli I, Conti G, Pleasure D, Scarlato G. Expression of the low-affinity NGF receptor during human muscle development, regeneration, and in tissue culture. Muscle Nerve 17: 276–284, 1994 [DOI] [PubMed] [Google Scholar]
- 34. Barton-Davis ER, Shoturma DI, Musaro A, Rosenthal N, Sweeney HL. Viral mediated expression of insulin-like growth factor I blocks the aging-related loss of skeletal muscle function. Proc Natl Acad Sci USA 95: 15603–15607, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Barton-Davis ER, Shoturma DI, Sweeney HL. Contribution of satellite cells to IGF-I induced hypertrophy of skeletal muscle. Acta Physiol Scand 167: 301–305, 1999 [DOI] [PubMed] [Google Scholar]
- 36. Bashir R, Britton S, Strachan T, Keers S, Vafiadaki E, Lako M, Richard I, Marchand S, Bourg N, Argov Z, Sadeh M, Mahjneh I, Marconi G, Passos-Bueno MR, Moreira Ede S, Zatz M, Beckmann JS, Bushby K. A gene related to Caenorhabditis elegans spermatogenesis factor fer-1 is mutated in limb-girdle muscular dystrophy type 2B. Nat Genet 20: 37–42, 1998 [DOI] [PubMed] [Google Scholar]
- 37. Beauchamp JR, Heslop L, Yu DS, Tajbakhsh S, Kelly RG, Wernig A, Buckingham ME, Partridge TA, Zammit PS. Expression of CD34 and Myf5 defines the majority of quiescent adult skeletal muscle satellite cells. J Cell Biol 151: 1221–1234, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Beggs ML, Nagarajan R, Taylor-Jones JM, Nolen G, Macnicol M, Peterson CA. Alterations in the TGFbeta signaling pathway in myogenic progenitors with age. Aging Cell 3: 353–361, 2004 [DOI] [PubMed] [Google Scholar]
- 39. Belcastro AN, Shewchuk LD, Raj DA. Exercise-induced muscle injury: a calpain hypothesis. Mol Cell Biochem 179: 135–145, 1998 [DOI] [PubMed] [Google Scholar]
- 40. Ben-Yair R, Kalcheim C. Lineage analysis of the avian dermomyotome sheet reveals the existence of single cells with both dermal and muscle progenitor fates. Development 132: 689–701, 2005 [DOI] [PubMed] [Google Scholar]
- 41. Benchaouir R, Meregalli M, Farini A, D'Antona G, Belicchi M, Goyenvalle A, Battistelli M, Bresolin N, Bottinelli R, Garcia L, Torrente Y. Restoration of human dystrophin following transplantation of exon-skipping-engineered DMD patient stem cells into dystrophic mice. Cell Stem Cell 1: 646–657, 2007 [DOI] [PubMed] [Google Scholar]
- 42. Benezra R, Davis RL, Lassar A, Tapscott S, Thayer M, Lockshon D, Weintraub H. Id: a negative regulator of helix-loop-helix DNA binding proteins. Control of terminal myogenic differentiation. Ann NY Acad Sci 599: 1–11, 1990 [DOI] [PubMed] [Google Scholar]
- 43. Benezra R, Davis RL, Lockshon D, Turner DL, Weintraub H. The protein Id: a negative regulator of helix-loop-helix DNA binding proteins. Cell 61: 49–59, 1990 [DOI] [PubMed] [Google Scholar]
- 44. Bergstrom DA, Penn BH, Strand A, Perry RL, Rudnicki MA, Tapscott SJ. Promoter-specific regulation of MyoD binding and signal transduction cooperate to pattern gene expression. Mol Cell 9: 587–600, 2002 [DOI] [PubMed] [Google Scholar]
- 45. Berkes CA, Tapscott SJ. MyoD and the transcriptional control of myogenesis. Semin Cell Dev Biol 16: 585–595, 2005 [DOI] [PubMed] [Google Scholar]
- 46. Besson V, Smeriglio P, Wegener A, Relaix F, Nait Oumesmar B, Sassoon DA, Marazzi G. PW1 gene/paternally expressed gene 3 (PW1/Peg3) identifies multiple adult stem and progenitor cell populations. Proc Natl Acad Sci USA 108: 11470–11475, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bhagavati S, Xu W. Generation of skeletal muscle from transplanted embryonic stem cells in dystrophic mice. Biochem Biophys Res Commun 333: 644–649, 2005 [DOI] [PubMed] [Google Scholar]
- 48. Bhasin S, Taylor WE, Singh R, Artaza J, Sinha-Hikim I, Jasuja R, Choi H, Gonzalez-Cadavid NF. The mechanisms of androgen effects on body composition: mesenchymal pluripotent cell as the target of androgen action. J Gerontol A Biol Sci Med Sci 58: M1103–M1110, 2003 [DOI] [PubMed] [Google Scholar]
- 49. Bischoff R. Chemotaxis of skeletal muscle satellite cells. Dev Dyn 208: 505–515, 1997 [DOI] [PubMed] [Google Scholar]
- 50. Bischoff R. Interaction between satellite cells and skeletal muscle fibers. Development 109: 943–952, 1990 [DOI] [PubMed] [Google Scholar]
- 51. Bischoff R. Regeneration of single skeletal muscle fibers in vitro. Anat Rec 182: 215–235, 1975 [DOI] [PubMed] [Google Scholar]
- 52. Bischoff R. The satellite cell and muscle regeneration. Myology 97–118, 1994 [Google Scholar]
- 53. Bischoff R. A satellite cell mitogen from crushed adult muscle. Dev Biol 115: 140–147, 1986 [DOI] [PubMed] [Google Scholar]
- 54. Bittner RE, Schofer C, Weipoltshammer K, Ivanova S, Streubel B, Hauser E, Freilinger M, Hoger H, Elbe-Burger A, Wachtler F. Recruitment of bone-marrow-derived cells by skeletal and cardiac muscle in adult dystrophic mdx mice. Anat Embryol 199: 391–396, 1999 [DOI] [PubMed] [Google Scholar]
- 55. Bjerknes M, Cheng H. Gastrointestinal stem cells. II. Intestinal stem cells. Am J Physiol Gastrointest Liver Physiol 289: G381–G387, 2005 [DOI] [PubMed] [Google Scholar]
- 56. Blais A, Tsikitis M, Acosta-Alvear D, Sharan R, Kluger Y, Dynlacht BD. An initial blueprint for myogenic differentiation. Genes Dev 19: 553–569, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Blaivas M, Carlson BM. Muscle fiber branching–difference between grafts in old and young rats. Mech Ageing Dev 60: 43–53, 1991 [DOI] [PubMed] [Google Scholar]
- 58. Blaveri K, Heslop L, Yu DS, Rosenblatt JD, Gross JG, Partridge TA, Morgan JE. Patterns of repair of dystrophic mouse muscle: studies on isolated fibers. Dev Dyn 216: 244–256, 1999 [DOI] [PubMed] [Google Scholar]
- 59. Bockhold KJ, Rosenblatt JD, Partridge TA. Aging normal and dystrophic mouse muscle: analysis of myogenicity in cultures of living single fibers. Muscle Nerve 21: 173–183, 1998 [DOI] [PubMed] [Google Scholar]
- 60. Bogdan C. Nitric oxide and the regulation of gene expression. Trends Cell Biol 11: 66–75, 2001 [DOI] [PubMed] [Google Scholar]
- 61. Boonen KJ, Rosaria-Chak KY, Baaijens FP, van der Schaft DW, Post MJ. Essential environmental cues from the satellite cell niche: optimizing proliferation and differentiation. Am J Physiol Cell Physiol 296: C1338–C1345, 2009 [DOI] [PubMed] [Google Scholar]
- 62. Boppart MD, Burkin DJ, Kaufman SJ. Alpha7beta1-integrin regulates mechanotransduction and prevents skeletal muscle injury. Am J Physiol Cell Physiol 290: C1660–C1665, 2006 [DOI] [PubMed] [Google Scholar]
- 63. Bosnakovski D, Xu Z, Li W, Thet S, Cleaver O, Perlingeiro RC, Kyba M. Prospective isolation of skeletal muscle stem cells with a Pax7 reporter. Stem Cells 26: 3194–3204, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Bourke DL, Ontell M. Branched myofibers in long-term whole muscle transplants: a quantitative study. Anat Rec 209: 281–288, 1984 [DOI] [PubMed] [Google Scholar]
- 65. Brack AS, Conboy IM, Conboy MJ, Shen J, Rando TA. A temporal switch from notch to Wnt signaling in muscle stem cells is necessary for normal adult myogenesis. Cell Stem Cell 2: 50–59, 2008 [DOI] [PubMed] [Google Scholar]
- 66. Brack AS, Conboy MJ, Roy S, Lee M, Kuo CJ, Keller C, Rando TA. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science 317: 807–810, 2007 [DOI] [PubMed] [Google Scholar]
- 67. Brandes RP, Fleming I, Busse R. Endothelial aging. Cardiovasc Res 66: 286–294, 2005 [DOI] [PubMed] [Google Scholar]
- 68. Brazelton TR, Nystrom M, Blau HM. Significant differences among skeletal muscles in the incorporation of bone marrow-derived cells. Dev Biol 262: 64–74, 2003 [DOI] [PubMed] [Google Scholar]
- 69. Brockes JP, Kumar A. Plasticity and reprogramming of differentiated cells in amphibian regeneration. Nat Rev Mol Cell Biol 3: 566–574, 2002 [DOI] [PubMed] [Google Scholar]
- 70. Bryan BA, Walshe TE, Mitchell DC, Havumaki JS, Saint-Geniez M, Maharaj AS, Maldonado AE, D'Amore PA. Coordinated vascular endothelial growth factor expression and signaling during skeletal myogenic differentiation. Mol Biol Cell 19: 994–1006, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Buckingham M. Myogenic progenitor cells and skeletal myogenesis in vertebrates. Curr Opin Genet Dev 16: 525–532, 2006 [DOI] [PubMed] [Google Scholar]
- 72. Buckingham M, Bajard L, Chang T, Daubas P, Hadchouel J, Meilhac S, Montarras D, Rocancourt D, Relaix F. The formation of skeletal muscle: from somite to limb. J Anat 202: 59–68, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Burkin DJ, Kaufman SJ. The alpha7beta1 integrin in muscle development and disease. Cell Tissue Res 296: 183–190, 1999 [DOI] [PubMed] [Google Scholar]
- 74. Cahill CM, Rogers JT. Interleukin (IL) 1beta induction of IL-6 is mediated by a novel phosphatidylinositol 3-kinase-dependent AKT/IkappaB kinase alpha pathway targeting activator protein-1. J Biol Chem 283: 25900–25912, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Cairns J. Mutation selection and the natural history of cancer. Nature 255: 197–200, 1975 [DOI] [PubMed] [Google Scholar]
- 76. Calise S, Blescia S, Cencetti F, Bernacchioni C, Donati C, Bruni P. Sphingosine 1-phosphate stimulates proliferation and migration of satellite cells: role of S1P receptors. Biochim Biophys Acta 1823: 439–450, 2012 [DOI] [PubMed] [Google Scholar]
- 77. Camargo FD, Gokhale S, Johnnidis JB, Fu D, Bell GW, Jaenisch R, Brummelkamp TR. YAP1 increases organ size and expands undifferentiated progenitor cells. Curr Biol 17: 2054–2060, 2007 [DOI] [PubMed] [Google Scholar]
- 78. Camargo FD, Green R, Capetanaki Y, Jackson KA, Goodell MA. Single hematopoietic stem cells generate skeletal muscle through myeloid intermediates. Nat Med 9: 1520–1527, 2003 [DOI] [PubMed] [Google Scholar]
- 79. Campion DR. The muscle satellite cell: a review. Int Rev Cytol 87: 225–251, 1984 [DOI] [PubMed] [Google Scholar]
- 80. Cantini M, Carraro U. Macrophage-released factor stimulates selectively myogenic cells in primary muscle culture. J Neuropathol Exp Neurol 54: 121–128, 1995 [DOI] [PubMed] [Google Scholar]
- 81. Cantini M, Giurisato E, Radu C, Tiozzo S, Pampinella F, Senigaglia D, Zaniolo G, Mazzoleni F, Vitiello L. Macrophage-secreted myogenic factors: a promising tool for greatly enhancing the proliferative capacity of myoblasts in vitro and in vivo. Neurol Sci 23: 189–194, 2002 [DOI] [PubMed] [Google Scholar]
- 82. Cao B, Zheng B, Jankowski RJ, Kimura S, Ikezawa M, Deasy B, Cummins J, Epperly M, Qu-Petersen Z, Huard J. Muscle stem cells differentiate into haematopoietic lineages but retain myogenic potential. Nat Cell Biol 5: 640–646, 2003 [DOI] [PubMed] [Google Scholar]
- 83. Cao Y, Kumar RM, Penn BH, Berkes CA, Kooperberg C, Boyer LA, Young RA, Tapscott SJ. Global and gene-specific analyses show distinct roles for Myod and Myog at a common set of promoters. EMBO J 25: 502–511, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Cao Y, Yao Z, Sarkar D, Lawrence M, Sanchez GJ, Parker MH, MacQuarrie KL, Davison J, Morgan MT, Ruzzo WL, Gentleman RC, Tapscott SJ. Genome-wide MyoD binding in skeletal muscle cells: a potential for broad cellular reprogramming. Dev Cell 18: 662–674, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Caplan AI. All MSCs are pericytes? Cell Stem Cell 3: 229–230, 2008 [DOI] [PubMed] [Google Scholar]
- 86. Carmeli E, Moas M, Reznick AZ, Coleman R. Matrix metalloproteinases and skeletal muscle: a brief review. Muscle Nerve 29: 191–197, 2004 [DOI] [PubMed] [Google Scholar]
- 87. Chakravarthy MV, Davis BS, Booth FW. IGF-I restores satellite cell proliferative potential in immobilized old skeletal muscle. J Appl Physiol 89: 1365–1379, 2000 [DOI] [PubMed] [Google Scholar]
- 88. Chang H, Yoshimoto M, Umeda K, Iwasa T, Mizuno Y, Fukada S, Yamamoto H, Motohashi N, Miyagoe-Suzuki Y, Takeda S, Heike T, Nakahata T. Generation of transplantable, functional satellite-like cells from mouse embryonic stem cells. FASEB J 23: 1907–1919, 2009 [DOI] [PubMed] [Google Scholar]
- 89. Chao DS, Gorospe JR, Brenman JE, Rafael JA, Peters MF, Froehner SC, Hoffman EP, Chamberlain JS, Bredt DS. Selective loss of sarcolemmal nitric oxide synthase in Becker muscular dystrophy. J Exp Med 184: 609–618, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Charge SB, Brack AS, Hughes SM. Aging-related satellite cell differentiation defect occurs prematurely after Ski-induced muscle hypertrophy. Am J Physiol Cell Physiol 283: C1228–C1241, 2002 [DOI] [PubMed] [Google Scholar]
- 91. Chazaud B, Brigitte M, Yacoub-Youssef H, Arnold L, Gherardi R, Sonnet C, Lafuste P, Chretien F. Dual and beneficial roles of macrophages during skeletal muscle regeneration. Exerc Sport Sci Rev 37: 18–22, 2009 [DOI] [PubMed] [Google Scholar]
- 92. Chazaud B, Sonnet C, Lafuste P, Bassez G, Rimaniol AC, Poron F, Authier FJ, Dreyfus PA, Gherardi RK. Satellite cells attract monocytes and use macrophages as a support to escape apoptosis and enhance muscle growth. J Cell Biol 163: 1133–1143, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Chen JF, Mandel EM, Thomson JM, Wu Q, Callis TE, Hammond SM, Conlon FL, Wang DZ. The role of microRNA-1 and microRNA-133 in skeletal muscle proliferation and differentiation. Nature Genet 38: 228–233, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Chen JF, Tao Y, Li J, Deng Z, Yan Z, Xiao X, Wang DZ. microRNA-1 and microRNA-206 regulate skeletal muscle satellite cell proliferation and differentiation by repressing Pax7. J Cell Biol 190: 867–879, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Chen X, Mao Z, Liu S, Liu H, Wang X, Wu H, Wu Y, Zhao T, Fan W, Li Y, Yew DT, Kindler PM, Li L, He Q, Qian L, Fan M. Dedifferentiation of adult human myoblasts induced by ciliary neurotrophic factor in vitro. Mol Biol Cell 16: 3140–3151, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Chen Y, Lin G, Slack JM. Control of muscle regeneration in the Xenopus tadpole tail by Pax7. Development 133: 2303–2313, 2006 [DOI] [PubMed] [Google Scholar]
- 97. Chen Y, Zajac JD, MacLean HE. Androgen regulation of satellite cell function. J Endocrinol 186: 21–31, 2005 [DOI] [PubMed] [Google Scholar]
- 98. Cheung TH, Quach NL, Charville GW, Liu L, Park L, Edalati A, Yoo B, Hoang P, Rando TA. Maintenance of muscle stem-cell quiescence by microRNA-489. Nature 482: 524–528, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Chevrel G, Hohlfeld R, Sendtner M. The role of neurotrophins in muscle under physiological and pathological conditions. Muscle Nerve 33: 462–476, 2006 [DOI] [PubMed] [Google Scholar]
- 100. Christov C, Chretien F, Abou-Khalil R, Bassez G, Vallet G, Authier FJ, Bassaglia Y, Shinin V, Tajbakhsh S, Chazaud B, Gherardi RK. Muscle satellite cells and endothelial cells: close neighbors and privileged partners. Mol Biol Cell 18: 1397–1409, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Cinnamon Y, Ben-Yair R, Kalcheim C. Differential effects of N-cadherin-mediated adhesion on the development of myotomal waves. Development 133: 1101–1112, 2006 [DOI] [PubMed] [Google Scholar]
- 102. Clow C, Jasmin BJ. Skeletal muscle-derived BDNF regulates satellite cell differentiation and muscle regeneration. Mol Biol Cell 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Coleman ME, DeMayo F, Yin KC, Lee HM, Geske R, Montgomery C, Schwartz RJ. Myogenic vector expression of insulin-like growth factor I stimulates muscle cell differentiation and myofiber hypertrophy in transgenic mice. J Biol Chem 270: 12109–12116, 1995 [DOI] [PubMed] [Google Scholar]
- 104. Collins-Hooper H, Woolley TE, Dyson L, Patel A, Potter P, Baker RE, Gaffney EA, Maini PK, Dash PR, Patel K. Age-related changes in speed and mechanism of adult skeletal muscle stem cell migration. Stem Cells 30: 1182–1195, 2012 [DOI] [PubMed] [Google Scholar]
- 105. Collins CA, Olsen I, Zammit PS, Heslop L, Petrie A, Partridge TA, Morgan JE. Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell 122: 289–301, 2005 [DOI] [PubMed] [Google Scholar]
- 106. Collinsworth AM, Zhang S, Kraus WE, Truskey GA. Apparent elastic modulus and hysteresis of skeletal muscle cells throughout differentiation. Am J Physiol Cell Physiol 283: C1219–C1227, 2002 [DOI] [PubMed] [Google Scholar]
- 107. Conboy IM, Conboy MJ, Smythe GM, Rando TA. Notch-mediated restoration of regenerative potential to aged muscle. Science 302: 1575–1577, 2003 [DOI] [PubMed] [Google Scholar]
- 108. Conboy IM, Rando TA. The regulation of Notch signaling controls satellite cell activation and cell fate determination in postnatal myogenesis. Dev Cell 3: 397–409, 2002 [DOI] [PubMed] [Google Scholar]
- 109. Conboy MJ, Karasov AO, Rando TA. High incidence of non-random template strand segregation and asymmetric fate determination in dividing stem cells and their progeny. PLoS Biol 5: e102, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Coolican SA, Samuel DS, Ewton DZ, McWade FJ, Florini JR. The mitogenic and myogenic actions of insulin-like growth factors utilize distinct signaling pathways. J Biol Chem 272: 6653–6662, 1997 [DOI] [PubMed] [Google Scholar]
- 111. Cooper RN, Tajbakhsh S, Mouly V, Cossu G, Buckingham M, Butler-Browne GS. In vivo satellite cell activation via Myf5 and MyoD in regenerating mouse skeletal muscle. J Cell Sci 112: 2895–2901, 1999 [DOI] [PubMed] [Google Scholar]
- 112. Corbel SY, Lee A, Yi L, Duenas J, Brazelton TR, Blau HM, Rossi FM. Contribution of hematopoietic stem cells to skeletal muscle. Nat Med 9: 1528–1532, 2003 [DOI] [PubMed] [Google Scholar]
- 113. Cornelison DD, Filla MS, Stanley HM, Rapraeger AC, Olwin BB. Syndecan-3 and syndecan-4 specifically mark skeletal muscle satellite cells and are implicated in satellite cell maintenance and muscle regeneration. Dev Biol 239: 79–94, 2001 [DOI] [PubMed] [Google Scholar]
- 114. Cornelison DD, Olwin BB, Rudnicki MA, Wold BJ. MyoD(−/−) satellite cells in single-fiber culture are differentiation defective and MRF4 deficient. Dev Biol 224: 122–137, 2000 [DOI] [PubMed] [Google Scholar]
- 115. Cornelison DD, Wilcox-Adelman SA, Goetinck PF, Rauvala H, Rapraeger AC, Olwin BB. Essential and separable roles for Syndecan-3 and Syndecan-4 in skeletal muscle development and regeneration. Genes Dev 18: 2231–2236, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Cornelison DD, Wold BJ. Single-cell analysis of regulatory gene expression in quiescent and activated mouse skeletal muscle satellite cells. Dev Biol 191: 270–283, 1997 [DOI] [PubMed] [Google Scholar]
- 117. Cox DM, Du M, Marback M, Yang EC, Chan J, Siu KW, McDermott JC. Phosphorylation motifs regulating the stability and function of myocyte enhancer factor 2A. J Biol Chem 278: 15297–15303, 2003 [DOI] [PubMed] [Google Scholar]
- 118. Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, Norotte C, Teng PN, Traas J, Schugar R, Deasy BM, Badylak S, Buhring HJ, Giacobino JP, Lazzari L, Huard J, Peault B. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 3: 301–313, 2008 [DOI] [PubMed] [Google Scholar]
- 119. Crist CG, Montarras D, Pallafacchina G, Rocancourt D, Cumano A, Conway SJ, Buckingham M. Muscle stem cell behavior is modified by microRNA-27 regulation of Pax3 expression. Proc Natl Acad Sci USA 106: 13383–13387, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Csete M, Walikonis J, Slawny N, Wei Y, Korsnes S, Doyle JC, Wold B. Oxygen-mediated regulation of skeletal muscle satellite cell proliferation and adipogenesis in culture. J Cell Physiol 189: 189–196, 2001 [DOI] [PubMed] [Google Scholar]
- 121. d'Albis A, Lenfant-Guyot M, Janmot C, Chanoine C, Weinman J, Gallien CL. Regulation by thyroid hormones of terminal differentiation in the skeletal dorsal muscle. I. Neonate mouse. Dev Biol 123: 25–32, 1987 [DOI] [PubMed] [Google Scholar]
- 122. Danieli-Betto D, Peron S, Germinario E, Zanin M, Sorci G, Franzoso S, Sandona D, Betto R. Sphingosine 1-phosphate signaling is involved in skeletal muscle regeneration. Am J Physiol Cell Physiol 298: C550–C558, 2010 [DOI] [PubMed] [Google Scholar]
- 123. Darabi R, Arpke RW, Irion S, Dimos JT, Grskovic M, Kyba M, Perlingeiro RC. Human ES- and iPS-derived myogenic progenitors restore dystrophin and improve contractility upon transplantation in dystrophic mice. Cell Stem Cell 10: 610–619, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Darabi R, Gehlbach K, Bachoo RM, Kamath S, Osawa M, Kamm KE, Kyba M, Perlingeiro RC. Functional skeletal muscle regeneration from differentiating embryonic stem cells. Nat Med 14: 134–143, 2008 [DOI] [PubMed] [Google Scholar]
- 125. Darabi R, Santos FN, Filareto A, Pan W, Koene R, Rudnicki MA, Kyba M, Perlingeiro RC. Assessment of the myogenic stem cell compartment following transplantation of Pax3/Pax7-induced embryonic stem cell-derived progenitors. Stem Cells 29: 777–790, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Darmani H, Crossan J, McLellan SD, Meek D, Adam C. Expression of nitric oxide synthase and transforming growth factor-beta in crush-injured tendon and synovium. Mediators Inflamm 13: 299–305, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Day K, Shefer G, Richardson JB, Enikolopov G, Yablonka-Reuveni Z. Nestin-GFP reporter expression defines the quiescent state of skeletal muscle satellite cells. Dev Biol 304: 246–259, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Day K, Shefer G, Shearer A, Yablonka-Reuveni Z. The depletion of skeletal muscle satellite cells with age is concomitant with reduced capacity of single progenitors to produce reserve progeny. Dev Biol 340: 330–343, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. De Angelis L, Berghella L, Coletta M, Lattanzi L, Zanchi M, Cusella-De Angelis MG, Ponzetto C, Cossu G. Skeletal myogenic progenitors originating from embryonic dorsal aorta coexpress endothelial and myogenic markers and contribute to postnatal muscle growth and regeneration. J Cell Biol 147: 869–878, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. De Castro Rodrigues A, Andreo JC, de Mattos Rodrigues SP. Myonuclei and satellite cells in denervated rat muscles: an electron microscopy study. Microsurgery 26: 396–398, 2006 [DOI] [PubMed] [Google Scholar]
- 131. De Strooper B, Annaert W. Where Notch and Wnt signaling meet. The presenilin hub. J Cell Biol 152: F17–20, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Deasy BM, Feduska JM, Payne TR, Li Y, Ambrosio F, Huard J. Effect of VEGF on the regenerative capacity of muscle stem cells in dystrophic skeletal muscle. Mol Ther J Am Soc Gene Ther 17: 1788–1798, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. DeChiara TM, Efstratiadis A, Robertson EJ. A growth-deficiency phenotype in heterozygous mice carrying an insulin-like growth factor II gene disrupted by targeting. Nature 345: 78–80, 1990 [DOI] [PubMed] [Google Scholar]
- 134. deLapeyriere O, Ollendorff V, Planche J, Ott MO, Pizette S, Coulier F, Birnbaum D. Expression of the Fgf6 gene is restricted to developing skeletal muscle in the mouse embryo. Development 118: 601–611, 1993 [DOI] [PubMed] [Google Scholar]
- 135. Dellavalle A, Maroli G, Covarello D, Azzoni E, Innocenzi A, Perani L, Antonini S, Sambasivan R, Brunelli S, Tajbakhsh S, Cossu G. Pericytes resident in postnatal skeletal muscle differentiate into muscle fibres and generate satellite cells. Nature Commun 2: 499, 2011 [DOI] [PubMed] [Google Scholar]
- 136. Dellavalle A, Sampaolesi M, Tonlorenzi R, Tagliafico E, Sacchetti B, Perani L, Innocenzi A, Galvez BG, Messina G, Morosetti R, Li S, Belicchi M, Peretti G, Chamberlain JS, Wright WE, Torrente Y, Ferrari S, Bianco P, Cossu G. Pericytes of human skeletal muscle are myogenic precursors distinct from satellite cells. Nat Cell Biol 9: 255–267, 2007 [DOI] [PubMed] [Google Scholar]
- 137. Delling U, Tureckova J, Lim HW, De Windt LJ, Rotwein P, Molkentin JD. A calcineurin-NFATc3-dependent pathway regulates skeletal muscle differentiation and slow myosin heavy-chain expression. Mol Cell Biol 20: 6600–6611, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Deponti D, Buono R, Catanzaro G, De Palma C, Longhi R, Meneveri R, Bresolin N, Bassi MT, Cossu G, Clementi E, Brunelli S. The low-affinity receptor for neurotrophins p75NTR plays a key role for satellite cell function in muscle repair acting via RhoA. Mol Biol Cell 20: 3620–3627, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Dey BK, Gagan J, Dutta A. miR-206 and -486 induce myoblast differentiation by downregulating Pax7. Mol Cell Biol 31: 203–214, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Dezawa M, Ishikawa H, Itokazu Y, Yoshihara T, Hoshino M, Takeda S, Ide C, Nabeshima Y. Bone marrow stromal cells generate muscle cells and repair muscle degeneration. Science 309: 314–317, 2005 [DOI] [PubMed] [Google Scholar]
- 141. DiMario J, Buffinger N, Yamada S, Strohman RC. Fibroblast growth factor in the extracellular matrix of dystrophic (mdx) mouse muscle. Science 244: 688–690, 1989 [DOI] [PubMed] [Google Scholar]
- 142. Doherty MJ, Ashton BA, Walsh S, Beresford JN, Grant ME, Canfield AE. Vascular pericytes express osteogenic potential in vitro and in vivo. J Bone Miner Res 13: 828–838, 1998 [DOI] [PubMed] [Google Scholar]
- 143. Doumit ME, Cook DR, Merkel RA. Testosterone up-regulates androgen receptors and decreases differentiation of porcine myogenic satellite cells in vitro. Endocrinology 137: 1385–1394, 1996 [DOI] [PubMed] [Google Scholar]
- 144. Doumit ME, Merkel RA. Conditions for isolation and culture of porcine myogenic satellite cells. Tissue Cell 24: 253–262, 1992 [DOI] [PubMed] [Google Scholar]
- 145. Dourdin N, Balcerzak D, Brustis JJ, Poussard S, Cottin P, Ducastaing A. Potential m-calpain substrates during myoblast fusion. Exp Cell Res 246: 433–442, 1999 [DOI] [PubMed] [Google Scholar]
- 146. Doyle MJ, Zhou S, Tanaka KK, Pisconti A, Farina NH, Sorrentino BP, Olwin BB. Abcg2 labels multiple cell types in skeletal muscle and participates in muscle regeneration. J Cell Biol 195: 147–163, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Doyonnas R, LaBarge MA, Sacco A, Charlton C, Blau HM. Hematopoietic contribution to skeletal muscle regeneration by myelomonocytic precursors. Proc Natl Acad Sci USA 101: 13507–13512, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Dreyfus PA, Chretien F, Chazaud B, Kirova Y, Caramelle P, Garcia L, Butler-Browne G, Gherardi RK. Adult bone marrow-derived stem cells in muscle connective tissue and satellite cell niches. Am J Pathol 164: 773–779, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Duckmanton A, Kumar A, Chang YT, Brockes JP. A single-cell analysis of myogenic dedifferentiation induced by small molecules. Chem Biol 12: 1117–1126, 2005 [DOI] [PubMed] [Google Scholar]
- 150. Enesco M, Puddy D. Increase in the number of nuclei and weight in skeletal muscle of rats of various ages. Am J Anat 114: 235–244, 1964 [DOI] [PubMed] [Google Scholar]
- 151. Engert JC, Berglund EB, Rosenthal N. Proliferation precedes differentiation in IGF-I-stimulated myogenesis. J Cell Biol 135: 431–440, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Engler AJ, Griffin MA, Sen S, Bonnemann CG, Sweeney HL, Discher DE. Myotubes differentiate optimally on substrates with tissue-like stiffness: pathological implications for soft or stiff microenvironments. J Cell Biol 166: 877–887, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Ernfors P, Wetmore C, Eriksdotter-Nilsson M, Bygdeman M, Stromberg I, Olson L, Persson H. The nerve growth factor receptor gene is expressed in both neuronal and non-neuronal tissues in the human fetus. Int J Dev Neurosci 9: 57–66, 1991 [DOI] [PubMed] [Google Scholar]
- 154. Ershler WB, Keller ET. Age-associated increased interleukin-6 gene expression, late-life diseases, and frailty. Annu Rev Med 51: 245–270, 2000 [DOI] [PubMed] [Google Scholar]
- 155. Esner M, Meilhac SM, Relaix F, Nicolas JF, Cossu G, Buckingham ME. Smooth muscle of the dorsal aorta shares a common clonal origin with skeletal muscle of the myotome. Development 133: 737–749, 2006 [DOI] [PubMed] [Google Scholar]
- 156. Espinosa A, Estrada M, Jaimovich E. IGF-I and insulin induce different intracellular calcium signals in skeletal muscle cells. J Endocrinol 182: 339–352, 2004 [DOI] [PubMed] [Google Scholar]
- 157. Estrada M, Espinosa A, Muller M, Jaimovich E. Testosterone stimulates intracellular calcium release and mitogen-activated protein kinases via a G protein-coupled receptor in skeletal muscle cells. Endocrinology 144: 3586–3597, 2003 [DOI] [PubMed] [Google Scholar]
- 158. Ewton DZ, Falen SL, Florini JR. The type II insulin-like growth factor (IGF) receptor has low affinity for IGF-I analogs: pleiotypic actions of IGFs on myoblasts are apparently mediated by the type I receptor. Endocrinology 120: 115–123, 1987 [DOI] [PubMed] [Google Scholar]
- 159. Fahim MA, Robbins N. Ultrastructural studies of young and old mouse neuromuscular junctions. J Neurocytol 11: 641–656, 1982 [DOI] [PubMed] [Google Scholar]
- 160. Fan Y, Maley M, Beilharz M, Grounds M. Rapid death of injected myoblasts in myoblast transfer therapy. Muscle Nerve 19: 853–860, 1996 [DOI] [PubMed] [Google Scholar]
- 161. Farrington-Rock C, Crofts NJ, Doherty MJ, Ashton BA, Griffin-Jones C, Canfield AE. Chondrogenic and adipogenic potential of microvascular pericytes. Circulation 110: 2226–2232, 2004 [DOI] [PubMed] [Google Scholar]
- 162. Fedorov YV, Rosenthal RS, Olwin BB. Oncogenic Ras-induced proliferation requires autocrine fibroblast growth factor 2 signaling in skeletal muscle cells. J Cell Biol 152: 1301–1305, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Feng J, Mantesso A, De Bari C, Nishiyama A, Sharpe PT. Dual origin of mesenchymal stem cells contributing to organ growth and repair. Proc Natl Acad Sci USA 108: 6503–6508, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Fernando P, Kelly JF, Balazsi K, Slack RS, Megeney LA. Caspase 3 activity is required for skeletal muscle differentiation. Proc Natl Acad Sci USA 99: 11025–11030, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Ferrando AA, Sheffield-Moore M, Yeckel CW, Gilkison C, Jiang J, Achacosa A, Lieberman SA, Tipton K, Wolfe RR, Urban RJ. Testosterone administration to older men improves muscle function: molecular and physiological mechanisms. Am J Physiol Endocrinol Metab 282: E601–E607, 2002 [DOI] [PubMed] [Google Scholar]
- 166. Ferrari G, Cusella-De Angelis G, Coletta M, Paolucci E, Stornaiuolo A, Cossu G, Mavilio F. Muscle regeneration by bone marrow-derived myogenic progenitors. Science 279: 1528–1530, 1998 [DOI] [PubMed] [Google Scholar]
- 167. Ferre PJ, Liaubet L, Concordet D, SanCristobal M, Uro-Coste E, Tosser-Klopp G, Bonnet A, Toutain PL, Hatey F, Lefebvre HP. Longitudinal analysis of gene expression in porcine skeletal muscle after post-injection local injury. Pharm Res 24: 1480–1489, 2007 [DOI] [PubMed] [Google Scholar]
- 168. Fielding RA, Manfredi TJ, Ding W, Fiatarone MA, Evans WJ, Cannon JG. Acute phase response in exercise. III. Neutrophil and IL-1 beta accumulation in skeletal muscle. Am J Physiol Regul Integr Comp Physiol 265: R166–R172, 1993 [DOI] [PubMed] [Google Scholar]
- 169. Florini JR, Ewton DZ, Coolican SA. Growth hormone and the insulin-like growth factor system in myogenesis. Endocr Rev 17: 481–517, 1996 [DOI] [PubMed] [Google Scholar]
- 170. Florini JR, Ewton DZ, Roof SL. Insulin-like growth factor-I stimulates terminal myogenic differentiation by induction of myogenin gene expression. Mol Endocrinol 5: 718–724, 1991 [DOI] [PubMed] [Google Scholar]
- 171. Florini JR, Magri KA, Ewton DZ, James PL, Grindstaff K, Rotwein PS. “Spontaneous” differentiation of skeletal myoblasts is dependent upon autocrine secretion of insulin-like growth factor-II. J Biol Chem 266: 15917–15923, 1991 [PubMed] [Google Scholar]
- 172. Floss T, Arnold HH, Braun T. A role for FGF-6 in skeletal muscle regeneration. Genes Dev 11: 2040–2051, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Friday BB, Horsley V, Pavlath GK. Calcineurin activity is required for the initiation of skeletal muscle differentiation. J Cell Biol 149: 657–666, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Fuchs E. Finding one's niche in the skin. Cell Stem Cell 4: 499–502, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Fuchtbauer EM, Westphal H. MyoD and myogenin are coexpressed in regenerating skeletal muscle of the mouse. Dev Dyn 193: 34–39, 1992 [DOI] [PubMed] [Google Scholar]
- 176. Fukada S. Molecular regulation of muscle stem cells by “quiescence genes.” Yakugaku zasshi J Pharmaceutical Soc Japan 131: 1329–1332, 2011 [DOI] [PubMed] [Google Scholar]
- 177. Fukada S, Uezumi A, Ikemoto M, Masuda S, Segawa M, Tanimura N, Yamamoto H, Miyagoe-Suzuki Y, Takeda S. Molecular signature of quiescent satellite cells in adult skeletal muscle. Stem Cells 25: 2448–2459, 2007 [DOI] [PubMed] [Google Scholar]
- 178. Fukushima K, Nakamura A, Ueda H, Yuasa K, Yoshida K, Takeda S, Ikeda S. Activation and localization of matrix metalloproteinase-2 and -9 in the skeletal muscle of the muscular dystrophy dog (CXMDJ). BMC Musculoskelet Disord 8: 54, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Gal-Levi R, Leshem Y, Aoki S, Nakamura T, Halevy O. Hepatocyte growth factor plays a dual role in regulating skeletal muscle satellite cell proliferation and differentiation. Biochim Biophys Acta 1402: 39–51, 1998 [DOI] [PubMed] [Google Scholar]
- 180. Galbiati F, Volonte D, Engelman JA, Scherer PE, Lisanti MP. Targeted down-regulation of caveolin-3 is sufficient to inhibit myotube formation in differentiating C2C12 myoblasts. Transient activation of p38 mitogen-activated protein kinase is required for induction of caveolin-3 expression and subsequent myotube formation. J Biol Chem 274: 30315–30321, 1999 [DOI] [PubMed] [Google Scholar]
- 181. Galbiati F, Volonte D, Minetti C, Chu JB, Lisanti MP. Phenotypic behavior of caveolin-3 mutations that cause autosomal dominant limb girdle muscular dystrophy (LGMD-1C). Retention of LGMD-1C caveolin-3 mutants within the Golgi complex. J Biol Chem 274: 25632–25641, 1999 [DOI] [PubMed] [Google Scholar]
- 182. Galvez BG, Sampaolesi M, Brunelli S, Covarello D, Gavina M, Rossi B, Constantin G, Torrente Y, Cossu G. Complete repair of dystrophic skeletal muscle by mesoangioblasts with enhanced migration ability. J Cell Biol 174: 231–243, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Gao C, Chen YG. Dishevelled: the hub of Wnt signaling. Cell Signal 22: 717–727, 2010 [DOI] [PubMed] [Google Scholar]
- 184. Gao Y, Kostrominova TY, Faulkner JA, Wineman AS. Age-related changes in the mechanical properties of the epimysium in skeletal muscles of rats. J Biomech 41: 465–469, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Gatchalian CL, Schachner M, Sanes JR. Fibroblasts that proliferate near denervated synaptic sites in skeletal muscle synthesize the adhesive molecules tenascin(J1), N-CAM, fibronectin, and a heparan sulfate proteoglycan. J Cell Biol 108: 1873–1890, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Gauthier-Rouviere C, Vandromme M, Tuil D, Lautredou N, Morris M, Soulez M, Kahn A, Fernandez A, Lamb N. Expression and activity of serum response factor is required for expression of the muscle-determining factor MyoD in both dividing and differentiating mouse C2C12 myoblasts. Mol Biol Cell 7: 719–729, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Gayraud-Morel B, Chretien F, Flamant P, Gomes D, Zammit PS, Tajbakhsh S. A role for the myogenic determination gene Myf5 in adult regenerative myogenesis. Dev Biol 312: 13–28, 2007 [DOI] [PubMed] [Google Scholar]
- 188. Gensch N, Borchardt T, Schneider A, Riethmacher D, Braun T. Different autonomous myogenic cell populations revealed by ablation of Myf5-expressing cells during mouse embryogenesis. Development 135: 1597–1604, 2008 [DOI] [PubMed] [Google Scholar]
- 189. Germani A, Di Carlo A, Mangoni A, Straino S, Giacinti C, Turrini P, Biglioli P, Capogrossi MC. Vascular endothelial growth factor modulates skeletal myoblast function. Am J Pathol 163: 1417–1428, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Gibson MC, Schultz E. The distribution of satellite cells and their relationship to specific fiber types in soleus and extensor digitorum longus muscles. Anat Rec 202: 329–337, 1982 [DOI] [PubMed] [Google Scholar]
- 191. Gillespie MA, Le Grand F, Scime A, Kuang S, von Maltzahn J, Seale V, Cuenda A, Ranish JA, Rudnicki MA. p38-γ-dependent gene silencing restricts entry into the myogenic differentiation program. J Cell Biol 187: 991–1005, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Gnocchi VF, White RB, Ono Y, Ellis JA, Zammit PS. Further characterisation of the molecular signature of quiescent and activated mouse muscle satellite cells. PLoS One 4: e5205, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Golding JP, Calderbank E, Partridge TA, Beauchamp JR. Skeletal muscle stem cells express anti-apoptotic ErbB receptors during activation from quiescence. Exp Cell Res 313: 341–356, 2007 [DOI] [PubMed] [Google Scholar]
- 194. Goldspink G. Research on mechano growth factor: its potential for optimising physical training as well as misuse in doping. Br J Sports Med 39: 787–788, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Goldspink G, Fernandes K, Williams PE, Wells DJ. Age-related changes in collagen gene expression in the muscles of mdx dystrophic and normal mice. Neuromuscul Disord 4: 183–191, 1994 [DOI] [PubMed] [Google Scholar]
- 196. Goljanek-Whysall K, Sweetman D, Abu-Elmagd M, Chapnik E, Dalmay T, Hornstein E, Munsterberg A. MicroRNA regulation of the paired-box transcription factor Pax3 confers robustness to developmental timing of myogenesis. Proc Natl Acad Sci USA 108: 11936–11941, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Gonzalez I, Tripathi G, Carter EJ, Cobb LJ, Salih DA, Lovett FA, Holding C, Pell JM. Akt2, a novel functional link between p38 mitogen-activated protein kinase and phosphatidylinositol 3-kinase pathways in myogenesis. Mol Cell Biol 24: 3607–3622, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Goodpaster BH, Wolf D. Skeletal muscle lipid accumulation in obesity, insulin resistance, and type 2 diabetes. Pediatr Diabetes 5: 219–226, 2004 [DOI] [PubMed] [Google Scholar]
- 199. Grass S, Arnold HH, Braun T. Alterations in somite patterning of Myf-5-deficient mice: a possible role for FGF-4 and FGF-6. Development 122: 141–150, 1996 [DOI] [PubMed] [Google Scholar]
- 200. Greco AV, Mingrone G, Giancaterini A, Manco M, Morroni M, Cinti S, Granzotto M, Vettor R, Camastra S, Ferrannini E. Insulin resistance in morbid obesity: reversal with intramyocellular fat depletion. Diabetes 51: 144–151, 2002 [DOI] [PubMed] [Google Scholar]
- 201. Griesbeck O, Parsadanian AS, Sendtner M, Thoenen H. Expression of neurotrophins in skeletal muscle: quantitative comparison and significance for motoneuron survival and maintenance of function. J Neurosci Res 42: 21–33, 1995 [DOI] [PubMed] [Google Scholar]
- 202. Grimby G, Saltin B. The ageing muscle. Clin Physiol 3: 209–218, 1983 [DOI] [PubMed] [Google Scholar]
- 203. Gros J, Manceau M, Thome V, Marcelle C. A common somitic origin for embryonic muscle progenitors and satellite cells. Nature 435: 954–958, 2005 [DOI] [PubMed] [Google Scholar]
- 204. Gros J, Scaal M, Marcelle C. A two-step mechanism for myotome formation in chick. Dev Cell 6: 875–882, 2004 [DOI] [PubMed] [Google Scholar]
- 205. Gros J, Serralbo O, Marcelle C. WNT11 acts as a directional cue to organize the elongation of early muscle fibres. Nature 457: 589–593, 2009 [DOI] [PubMed] [Google Scholar]
- 206. Grounds MD. Phagocytosis of necrotic muscle in muscle isografts is influenced by the strain, age, and sex of host mice. J Pathol 153: 71–82, 1987 [DOI] [PubMed] [Google Scholar]
- 207. Grounds MD, Garrett KL, Lai MC, Wright WE, Beilharz MW. Identification of skeletal muscle precursor cells in vivo by use of MyoD1 and myogenin probes. Cell Tissue Res 267: 99–104, 1992 [DOI] [PubMed] [Google Scholar]
- 208. Gullberg D, Tiger CF, Velling T. Laminins during muscle development and in muscular dystrophies. Cell Mol Life Sci 56: 442–460, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Guo K, Wang J, Andres V, Smith RC, Walsh K. MyoD-induced expression of p21 inhibits cyclin-dependent kinase activity upon myocyte terminal differentiation. Mol Cell Biol 15: 3823–3829, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Gussoni E, Soneoka Y, Strickland CD, Buzney EA, Khan MK, Flint AF, Kunkel LM, Mulligan RC. Dystrophin expression in the mdx mouse restored by stem cell transplantation. Nature 401: 390–394, 1999 [DOI] [PubMed] [Google Scholar]
- 211. Guttinger M, Tafi E, Battaglia M, Coletta M, Cossu G. Allogeneic mesoangioblasts give rise to alpha-sarcoglycan expressing fibers when transplanted into dystrophic mice. Exp Cell Res 312: 3872–3879, 2006 [DOI] [PubMed] [Google Scholar]
- 212. Haddad F, Zaldivar F, Cooper DM, Adams GR. IL-6-induced skeletal muscle atrophy. J Appl Physiol 98: 911–917, 2005 [DOI] [PubMed] [Google Scholar]
- 213. Haldar M, Karan G, Tvrdik P, Capecchi MR. Two cell lineages, myf5 and myf5-independent, participate in mouse skeletal myogenesis. Dev Cell 14: 437–445, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Halevy O, Cantley LC. Differential regulation of the phosphoinositide 3-kinase and MAP kinase pathways by hepatocyte growth factor vs. insulin-like growth factor-I in myogenic cells. Exp Cell Res 297: 224–234, 2004 [DOI] [PubMed] [Google Scholar]
- 215. Halevy O, Novitch BG, Spicer DB, Skapek SX, Rhee J, Hannon GJ, Beach D, Lassar AB. Correlation of terminal cell cycle arrest of skeletal muscle with induction of p21 by MyoD. Science 267: 1018–1021, 1995 [DOI] [PubMed] [Google Scholar]
- 216. Halevy O, Piestun Y, Allouh MZ, Rosser BW, Rinkevich Y, Reshef R, Rozenboim I, Wleklinski-Lee M, Yablonka-Reuveni Z. Pattern of Pax7 expression during myogenesis in the posthatch chicken establishes a model for satellite cell differentiation and renewal. Dev Dyn 231: 489–502, 2004 [DOI] [PubMed] [Google Scholar]
- 217. Hall-Craggs EC, Seyan HS. Histochemical changes in innervated and denervated skeletal muscle fibers following treatment with bupivacaine (marcain). Exp Neurol 46: 345–354, 1975 [DOI] [PubMed] [Google Scholar]
- 218. Hamer PW, McGeachie JM, Davies MJ, Grounds MD. Evans Blue Dye as an in vivo marker of myofibre damage: optimising parameters for detecting initial myofibre membrane permeability. J Anat 200: 69–79, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Hammond CL, Hinits Y, Osborn DP, Minchin JE, Tettamanti G, Hughes SM. Signals and myogenic regulatory factors restrict pax3 and pax7 expression to dermomyotome-like tissue in zebrafish. Dev Biol 302: 504–521, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Han B, Tong J, Zhu MJ, Ma C, Du M. Insulin-like growth factor-1 (IGF-1) and leucine activate pig myogenic satellite cells through mammalian target of rapamycin (mTOR) pathway. Mol Reprod Dev 75: 810–817, 2008 [DOI] [PubMed] [Google Scholar]
- 221. Han J, Jiang Y, Li Z, Kravchenko VV, Ulevitch RJ. Activation of the transcription factor MEF2C by the MAP kinase p38 in inflammation. Nature 386: 296–299, 1997 [DOI] [PubMed] [Google Scholar]
- 222. Hannon K, Kudla AJ, McAvoy MJ, Clase KL, Olwin BB. Differentially expressed fibroblast growth factors regulate skeletal muscle development through autocrine and paracrine mechanisms. J Cell Biol 132: 1151–1159, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Hannun YA, Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol 9: 139–150, 2008 [DOI] [PubMed] [Google Scholar]
- 224. Hansen-Smith FM, Picou D, Golden MH. Muscle satellite cells in malnourished and nutritionally rehabilitated children. J Neurol Sci 41: 207–221, 1979 [DOI] [PubMed] [Google Scholar]
- 225. Harel I, Nathan E, Tirosh-Finkel L, Zigdon H, Guimaraes-Camboa N, Evans SM, Tzahor E. Distinct origins and genetic programs of head muscle satellite cells. Dev Cell 16: 822–832, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. He L, Papoutsi M, Huang R, Tomarev SI, Christ B, Kurz H, Wilting J. Three different fates of cells migrating from somites into the limb bud. Anat Embryol 207: 29–34, 2003 [DOI] [PubMed] [Google Scholar]
- 227. Hellmuth AE, Allbrook DB. Muscle satellite cell numbers during the postnatal period. J Anat 110: 503, 1971 [PubMed] [Google Scholar]
- 228. Herbst KL, Bhasin S. Testosterone action on skeletal muscle. Curr Opin Clin Nutr Metab Care 7: 271–277, 2004 [DOI] [PubMed] [Google Scholar]
- 229. Hermanson JW, Moschella MC, Ontell M. Effect of neonatal denervation-reinnervation on the functional capacity of a 129ReJ dy/dy murine dystrophic muscle. Exp Neurol 102: 210–216, 1988 [DOI] [PubMed] [Google Scholar]
- 230. Hinescu ME, Gherghiceanu M, Suciu L, Popescu LM. Telocytes in pleura: two- and three-dimensional imaging by transmission electron microscopy. Cell Tissue Res 343: 389–397, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Hirai H, Verma M, Watanabe S, Tastad C, Asakura Y, Asakura A. MyoD regulates apoptosis of myoblasts through microRNA-mediated down-regulation of Pax3. J Cell Biol 191: 347–365, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Hjiantoniou E, Anayasa M, Nicolaou P, Bantounas I, Saito M, Iseki S, Uney JB, Phylactou LA. Twist induces reversal of myotube formation. Differ Res Biol Diversity 76: 182–192, 2008 [DOI] [PubMed] [Google Scholar]
- 233. Hollemann D, Budka H, Loscher WN, Yanagida G, Fischer MB, Wanschitz JV. Endothelial and myogenic differentiation of hematopoietic progenitor cells in inflammatory myopathies. J Neuropathol Exp Neurol 67: 711–719, 2008 [DOI] [PubMed] [Google Scholar]
- 234. Hollenberg SM, Cheng PF, Weintraub H. Use of a conditional MyoD transcription factor in studies of MyoD trans-activation and muscle determination. Proc Natl Acad Sci USA 90: 8028–8032, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Holterman CE, Le Grand F, Kuang S, Seale P, Rudnicki MA. Megf10 regulates the progression of the satellite cell myogenic program. J Cell Biol 179: 911–922, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Horowitz A, Tkachenko E, Simons M. Fibroblast growth factor-specific modulation of cellular response by syndecan-4. J Cell Biol 157: 715–725, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Horsley V, Friday BB, Matteson S, Kegley KM, Gephart J, Pavlath GK. Regulation of the growth of multinucleated muscle cells by an NFATC2-dependent pathway. J Cell Biol 153: 329–338, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Horsley V, Pavlath GK. Forming a multinucleated cell: molecules that regulate myoblast fusion. Cells Tissues Organs 176: 67–78, 2004 [DOI] [PubMed] [Google Scholar]
- 239. Hu E, Tontonoz P, Spiegelman BM. Transdifferentiation of myoblasts by the adipogenic transcription factors PPAR gamma and C/EBP alpha. Proc Natl Acad Sci USA 92: 9856–9860, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Huckins C. The spermatogonial stem cell population in adult rats. I. Their morphology, proliferation and maturation. Anat Rec 169: 533–557, 1971 [DOI] [PubMed] [Google Scholar]
- 241. Hughes SM, Blau HM. Migration of myoblasts across basal lamina during skeletal muscle development. Nature 345: 350–353, 1990 [DOI] [PubMed] [Google Scholar]
- 242. Hutcheson DA, Zhao J, Merrell A, Haldar M, Kardon G. Embryonic and fetal limb myogenic cells are derived from developmentally distinct progenitors and have different requirements for beta-catenin. Genes Dev 23: 997–1013, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Ikezawa M, Cao B, Qu Z, Peng H, Xiao X, Pruchnic R, Kimura S, Miike T, Huard J. Dystrophin delivery in dystrophin-deficient DMDmdx skeletal muscle by isogenic muscle-derived stem cell transplantation. Hum Gene Ther 14: 1535–1546, 2003 [DOI] [PubMed] [Google Scholar]
- 244. Irintchev A, Zeschnigk M, Starzinski-Powitz A, Wernig A. Expression pattern of M-cadherin in normal, denervated, and regenerating mouse muscles. Dev Dyn 199: 326–337, 1994 [DOI] [PubMed] [Google Scholar]
- 245. Jackson KA, Mi T, Goodell MA. Hematopoietic potential of stem cells isolated from murine skeletal muscle. Proc Natl Acad Sci USA 96: 14482–14486, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. James PL, Jones SB, Busby WH, Jr, Clemmons DR, Rotwein P. A highly conserved insulin-like growth factor-binding protein (IGFBP-5) is expressed during myoblast differentiation. J Biol Chem 268: 22305–22312, 1993 [PubMed] [Google Scholar]
- 247. Jankowski RJ, Huard J. Establishing reliable criteria for isolating myogenic cell fractions with stem cell properties and enhanced regenerative capacity. Blood Cells Mol Dis 32: 24–33, 2004 [DOI] [PubMed] [Google Scholar]
- 248. Jejurikar SS, Marcelo CL, Kuzon WM., Jr Skeletal muscle denervation increases satellite cell susceptibility to apoptosis. Plast Reconstr Surg 110: 160–168, 2002 [DOI] [PubMed] [Google Scholar]
- 249. Jenniskens GJ, Veerkamp JH, van Kuppevelt TH. Heparan sulfates in skeletal muscle development and physiology. J Cell Physiol 206: 283–294, 2006 [DOI] [PubMed] [Google Scholar]
- 250. Jirmanova I, Thesleff S. Ultrastructural study of experimental muscle degeneration and regeneration in the adult rat. Z Zellforsch Mikrosk Anat 131: 77–97, 1972 [DOI] [PubMed] [Google Scholar]
- 251. Jockusch H, Voigt S. Migration of adult myogenic precursor cells as revealed by GFP/nLacZ labelling of mouse transplantation chimeras. J Cell Sci 116: 1611–1616, 2003 [DOI] [PubMed] [Google Scholar]
- 252. Joe AW, Yi L, Natarajan A, Le Grand F, So L, Wang J, Rudnicki MA, Rossi FM. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol 12: 153–163, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Jones JI, Clemmons DR. Insulin-like growth factors and their binding proteins: biological actions. Endocr Rev 16: 3–34, 1995 [DOI] [PubMed] [Google Scholar]
- 254. Joubert Y, Tobin C. Satellite cell proliferation and increase in the number of myonuclei induced by testosterone in the levator ani muscle of the adult female rat. Dev Biol 131: 550–557, 1989 [DOI] [PubMed] [Google Scholar]
- 255. Joubert Y, Tobin C. Testosterone treatment results in quiescent satellite cells being activated and recruited into cell cycle in rat levator ani muscle. Dev Biol 169: 286–294, 1995 [DOI] [PubMed] [Google Scholar]
- 256. Kablar B, Asakura A, Krastel K, Ying C, May LL, Goldhamer DJ, Rudnicki MA. MyoD and Myf-5 define the specification of musculature of distinct embryonic origin. Biochem Cell Biol 76: 1079–1091, 1998 [PubMed] [Google Scholar]
- 257. Kablar B, Krastel K, Tajbakhsh S, Rudnicki MA. Myf5 and MyoD activation define independent myogenic compartments during embryonic development. Dev Biol 258: 307–318, 2003 [DOI] [PubMed] [Google Scholar]
- 258. Kablar B, Krastel K, Ying C, Asakura A, Tapscott SJ, Rudnicki MA. MyoD and Myf-5 differentially regulate the development of limb versus trunk skeletal muscle. Development 124: 4729–4738, 1997 [DOI] [PubMed] [Google Scholar]
- 259. Kablar B, Krastel K, Ying C, Tapscott SJ, Goldhamer DJ, Rudnicki MA. Myogenic determination occurs independently in somites and limb buds. Dev Biol 206: 219–231, 1999 [DOI] [PubMed] [Google Scholar]
- 260. Kablar B, Rudnicki MA. Skeletal muscle development in the mouse embryo. Histol Histopathol 15: 649–656, 2000 [DOI] [PubMed] [Google Scholar]
- 261. Kalcheim C, Ben-Yair R. Cell rearrangements during development of the somite and its derivatives. Curr Opin Genet Dev 15: 371–380, 2005 [DOI] [PubMed] [Google Scholar]
- 262. Kaminski HJ, Andrade FH. Nitric oxide: biologic effects on muscle and role in muscle diseases. Neuromuscul Disord 11: 517–524, 2001 [DOI] [PubMed] [Google Scholar]
- 263. Kanisicak O, Mendez JJ, Yamamoto S, Yamamoto M, Goldhamer DJ. Progenitors of skeletal muscle satellite cells express the muscle determination gene, MyoD. Dev Biol 332: 131–141, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Kardon G, Campbell JK, Tabin CJ. Local extrinsic signals determine muscle and endothelial cell fate and patterning in the vertebrate limb. Dev Cell 3: 533–545, 2002 [DOI] [PubMed] [Google Scholar]
- 265. Kassar-Duchossoy L, Giacone E, Gayraud-Morel B, Jory A, Gomes D, Tajbakhsh S. Pax3/Pax7 mark a novel population of primitive myogenic cells during development. Genes Dev 19: 1426–1431, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266. Kastner S, Elias MC, Rivera AJ, Yablonka-Reuveni Z. Gene expression patterns of the fibroblast growth factors and their receptors during myogenesis of rat satellite cells. J Histochem Cytochem 48: 1079–1096, 2000 [DOI] [PubMed] [Google Scholar]
- 267. Kazuki Y, Hiratsuka M, Takiguchi M, Osaki M, Kajitani N, Hoshiya H, Hiramatsu K, Yoshino T, Kazuki K, Ishihara C, Takehara S, Higaki K, Nakagawa M, Takahashi K, Yamanaka S, Oshimura M. Complete genetic correction of ips cells from Duchenne muscular dystrophy. Mol Ther 18: 386–393, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Keller P, Penkowa M, Keller C, Steensberg A, Fischer CP, Giralt M, Hidalgo J, Pedersen BK. Interleukin-6 receptor expression in contracting human skeletal muscle: regulating role of IL-6. FASEB J 19: 1181–1183, 2005 [DOI] [PubMed] [Google Scholar]
- 269. Kelly AM. Perisynaptic satellite cells in the developing and mature rat soleus muscle. Anat Rec 190: 891–903, 1978 [DOI] [PubMed] [Google Scholar]
- 270. Kherif S, Lafuma C, Dehaupas M, Lachkar S, Fournier JG, Verdiere-Sahuque M, Fardeau M, Alameddine HS. Expression of matrix metalloproteinases 2 and 9 in regenerating skeletal muscle: a study in experimentally injured and mdx muscles. Dev Biol 205: 158–170, 1999 [DOI] [PubMed] [Google Scholar]
- 271. Kim CH, Neiswender H, Baik EJ, Xiong WC, Mei L. Beta-catenin interacts with MyoD and regulates its transcription activity. Mol Cell Biol 28: 2941–2951, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272. Kitamoto T, Hanaoka K. Notch3 null mutation in mice causes muscle hyperplasia by repetitive muscle regeneration. Stem Cells 28: 2205–2216, 2010 [DOI] [PubMed] [Google Scholar]
- 273. Kitzmann M, Carnac G, Vandromme M, Primig M, Lamb NJ, Fernandez A. The muscle regulatory factors MyoD and myf-5 undergo distinct cell cycle-specific expression in muscle cells. J Cell Biol 142: 1447–1459, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274. Knudsen KA, Horwitz AF. Tandem events in myoblast fusion. Dev Biol 58: 328–338, 1977 [DOI] [PubMed] [Google Scholar]
- 275. Kobzik L, Reid MB, Bredt DS, Stamler JS. Nitric oxide in skeletal muscle. Nature 372: 546–548, 1994 [DOI] [PubMed] [Google Scholar]
- 276. Konigsberg IR. Clonal analysis of myogenesis. Science 140: 1273–1284, 1963 [DOI] [PubMed] [Google Scholar]
- 277. Konigsberg UR, Lipton BH, Konigsberg IR. The regenerative response of single mature muscle fibers isolated in vitro. Dev Biol 45: 260–275, 1975 [DOI] [PubMed] [Google Scholar]
- 278. Kovanen V. Intramuscular extracellular matrix: complex environment of muscle cells. Exerc Sport Sci Rev 30: 20–25, 2002 [DOI] [PubMed] [Google Scholar]
- 279. Kuang S, Charge SB, Seale P, Huh M, Rudnicki MA. Distinct roles for Pax7 and Pax3 in adult regenerative myogenesis. J Cell Biol 172: 103–113, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280. Kuang S, Kuroda K, Le Grand F, Rudnicki MA. Asymmetric self-renewal and commitment of satellite stem cells in muscle. Cell 129: 999–1010, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281. Kurosu H, Ogawa Y, Miyoshi M, Yamamoto M, Nandi A, Rosenblatt KP, Baum MG, Schiavi S, Hu MC, Moe OW, Kuro-o M. Regulation of fibroblast growth factor-23 signaling by klotho. J Biol Chem 281: 6120–6123, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282. Kuschel R, Yablonka-Reuveni Z, Bornemann A. Satellite cells on isolated myofibers from normal and denervated adult rat muscle. J Histochem Cytochem 47: 1375–1384, 1999 [DOI] [PubMed] [Google Scholar]
- 283. Kust BM, Copray JC, Brouwer N, Troost D, Boddeke HW. Elevated levels of neurotrophins in human biceps brachii tissue of amyotrophic lateral sclerosis. Exp Neurol 177: 419–427, 2002 [DOI] [PubMed] [Google Scholar]
- 284. Kutcher ME, Herman IM. The pericyte: cellular regulator of microvascular blood flow. Microvasc Res 77: 235–246, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285. L'Honore A, Lamb NJ, Vandromme M, Turowski P, Carnac G, Fernandez A. MyoD distal regulatory region contains an SRF binding CArG element required for MyoD expression in skeletal myoblasts and during muscle regeneration. Mol Biol Cell 14: 2151–2162, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286. L'Honore A, Rana V, Arsic N, Franckhauser C, Lamb NJ, Fernandez A. Identification of a new hybrid serum response factor and myocyte enhancer factor 2-binding element in MyoD enhancer required for MyoD expression during myogenesis. Mol Biol Cell 18: 1992–2001, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287. LaBarge MA, Blau HM. Biological progression from adult bone marrow to mononucleate muscle stem cell to multinucleate muscle fiber in response to injury. Cell 111: 589–601, 2002 [DOI] [PubMed] [Google Scholar]
- 288. Langsdorf A, Do AT, Kusche-Gullberg M, Emerson CP, Jr, Ai X. Sulfs are regulators of growth factor signaling for satellite cell differentiation and muscle regeneration. Dev Biol 311: 464–477, 2007 [DOI] [PubMed] [Google Scholar]
- 289. Lansdorp PM. Immortal strands? Give me a break. Cell 129: 1244–1247, 2007 [DOI] [PubMed] [Google Scholar]
- 290. Larsen BD, Rampalli S, Burns LE, Brunette S, Dilworth FJ, Megeney LA. Caspase 3/caspase-activated DNase promote cell differentiation by inducing DNA strand breaks. Proc Natl Acad Sci USA 107: 4230–4235, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291. Lassar AB, Davis RL, Wright WE, Kadesch T, Murre C, Voronova A, Baltimore D, Weintraub H. Functional activity of myogenic HLH proteins requires hetero-oligomerization with E12/E47-like proteins in vivo. Cell 66: 305–315, 1991 [DOI] [PubMed] [Google Scholar]
- 292. Laterza OF, Lim L, Garrett-Engele PW, Vlasakova K, Muniappa N, Tanaka WK, Johnson JM, Sina JF, Fare TL, Sistare FD, Glaab WE. Plasma MicroRNAs as sensitive and specific biomarkers of tissue injury. Clin Chem 55: 1977–1983, 2009 [DOI] [PubMed] [Google Scholar]
- 293. Le Grand F, Jones AE, Seale V, Scime A, Rudnicki MA. Wnt7a activates the planar cell polarity pathway to drive the symmetric expansion of satellite stem cells. Cell Stem Cell 4: 535–547, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294. Lee JY, Qu-Petersen Z, Cao B, Kimura S, Jankowski R, Cummins J, Usas A, Gates C, Robbins P, Wernig A, Huard J. Clonal isolation of muscle-derived cells capable of enhancing muscle regeneration and bone healing. J Cell Biol 150: 1085–1100, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295. Lefaucheur JP, Gjata B, Lafont H, Sebille A. Angiogenic and inflammatory responses following skeletal muscle injury are altered by immune neutralization of endogenous basic fibroblast growth factor, insulin-like growth factor-1 and transforming growth factor-beta 1. J Neuroimmunol 70: 37–44, 1996 [DOI] [PubMed] [Google Scholar]
- 296. Lefaucheur JP, Sebille A. Basic fibroblast growth factor promotes in vivo muscle regeneration in murine muscular dystrophy. Neurosci Lett 202: 121–124, 1995 [DOI] [PubMed] [Google Scholar]
- 297. Lefaucheur JP, Sebille A. Muscle regeneration following injury can be modified in vivo by immune neutralization of basic fibroblast growth factor, transforming growth factor beta 1 or insulin-like growth factor I. J Neuroimmunol 57: 85–91, 1995 [DOI] [PubMed] [Google Scholar]
- 298. Lemercier C, To RQ, Carrasco RA, Konieczny SF. The basic helix-loop-helix transcription factor Mist1 functions as a transcriptional repressor of myoD. EMBO J 17: 1412–1422, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299. Lepper C, Conway SJ, Fan CM. Adult satellite cells and embryonic muscle progenitors have distinct genetic requirements. Nature 460: 627–631, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300. Lepper C, Fan CM. Inducible lineage tracing of Pax7-descendant cells reveals embryonic origin of adult satellite cells. Genesis 48: 424–436, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301. Lepper C, Partridge TA, Fan CM. An absolute requirement for Pax7-positive satellite cells in acute injury-induced skeletal muscle regeneration. Development 138: 3639–3646, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302. Leri A, Kajstura J, Anversa P, Frishman WH. Myocardial regeneration and stem cell repair. Curr Probl Cardiol 33: 91–153, 2008 [DOI] [PubMed] [Google Scholar]
- 303. Lescaudron L, Peltekian E, Fontaine-Perus J, Paulin D, Zampieri M, Garcia L, Parrish E. Blood borne macrophages are essential for the triggering of muscle regeneration following muscle transplant. Neuromuscul Disord 9: 72–80, 1999 [DOI] [PubMed] [Google Scholar]
- 304. Leshem Y, Gitelman I, Ponzetto C, Halevy O. Preferential binding of Grb2 or phosphatidylinositol 3-kinase to the met receptor has opposite effects on HGF-induced myoblast proliferation. Exp Cell Res 274: 288–298, 2002 [DOI] [PubMed] [Google Scholar]
- 305. Leshem Y, Halevy O. Phosphorylation of pRb is required for HGF-induced muscle cell proliferation and is p27kip1-dependent. J Cell Physiol 191: 173–182, 2002 [DOI] [PubMed] [Google Scholar]
- 306. Leshem Y, Spicer DB, Gal-Levi R, Halevy O. Hepatocyte growth factor (HGF) inhibits skeletal muscle cell differentiation: a role for the bHLH protein twist and the cdk inhibitor p27. J Cell Physiol 184: 101–109, 2000 [DOI] [PubMed] [Google Scholar]
- 307. Lewis MI, Horvitz GD, Clemmons DR, Fournier M. Role of IGF-I and IGF-binding proteins within diaphragm muscle in modulating the effects of nandrolone. Am J Physiol Endocrinol Metab 282: E483–E490, 2002 [DOI] [PubMed] [Google Scholar]
- 308. Li L, Heller-Harrison R, Czech M, Olson EN. Cyclic AMP-dependent protein kinase inhibits the activity of myogenic helix-loop-helix proteins. Mol Cell Biol 12: 4478–4485, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309. Li L, Zhou J, James G, Heller-Harrison R, Czech MP, Olson EN. FGF inactivates myogenic helix-loop-helix proteins through phosphorylation of a conserved protein kinase C site in their DNA-binding domains. Cell 71: 1181–1194, 1992 [DOI] [PubMed] [Google Scholar]
- 310. Li Y, Foster W, Deasy BM, Chan Y, Prisk V, Tang Y, Cummins J, Huard J. Transforming growth factor-beta1 induces the differentiation of myogenic cells into fibrotic cells in injured skeletal muscle: a key event in muscle fibrogenesis. Am J Pathol 164: 1007–1019, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311. Lindstrom M, Pedrosa-Domellof F, Thornell LE. Satellite cell heterogeneity with respect to expression of MyoD, myogenin, Dlk1 and c-Met in human skeletal muscle: application to a cohort of power lifters and sedentary men. Histochem Cell Biol 134: 371–385, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312. Lipton BH, Konigsberg IR. A fine-structural analysis of the fusion of myogenic cells. J Cell Biol 53: 348–364, 1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313. Liu H, Fergusson MM, Castilho RM, Liu J, Cao L, Chen J, Malide D, Rovira II, Schimel D, Kuo CJ, Gutkind JS, Hwang PM, Finkel T. Augmented Wnt signaling in a mammalian model of accelerated aging. Science 317: 803–806, 2007 [DOI] [PubMed] [Google Scholar]
- 314. Liu J, Aoki M, Illa I, Wu C, Fardeau M, Angelini C, Serrano C, Urtizberea JA, Hentati F, Hamida MB, Bohlega S, Culper EJ, Amato AA, Bossie K, Oeltjen J, Bejaoui K, McKenna-Yasek D, Hosler BA, Schurr E, Arahata K, de Jong PJ, Brown RH., Jr Dysferlin, a novel skeletal muscle gene, is mutated in Miyoshi myopathy and limb girdle muscular dystrophy. Nat Genet 20: 31–36, 1998 [DOI] [PubMed] [Google Scholar]
- 315. Liu JP, Baker J, Perkins AS, Robertson EJ, Efstratiadis A. Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r). Cell 75: 59–72, 1993 [PubMed] [Google Scholar]
- 316. Liu Y, Gao L, Zuba-Surma EK, Peng X, Kucia M, Ratajczak MZ, Wang W, Enzmann V, Kaplan HJ, Dean DC. Identification of small Sca-1(+), Lin(−), and CD45(−) multipotential cells in the neonatal murine retina. Exp Hematol 37: 1096–1107, 2009 [DOI] [PubMed] [Google Scholar]
- 317. Loh KC, Leong WI, Carlson ME, Oskouian B, Kumar A, Fyrst H, Zhang M, Proia RL, Hoffman EP, Saba JD. Sphingosine-1-phosphate enhances satellite cell activation in dystrophic muscles through a S1PR2/STAT3 signaling pathway. PLoS One 7: e37218, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318. Lu A, Cummins JH, Pollett JB, Cao B, Sun B, Rudnicki MA, Huard J. Isolation of myogenic progenitor populations from Pax7-deficient skeletal muscle based on adhesion characteristics. Gene Ther 15: 1116–1125, 2008 [DOI] [PubMed] [Google Scholar]
- 319. Lu J, McKinsey TA, Zhang CL, Olson EN. Regulation of skeletal myogenesis by association of the MEF2 transcription factor with class II histone deacetylases. Mol Cell 6: 233–244, 2000 [DOI] [PubMed] [Google Scholar]
- 320. Luo D, Renault VM, Rando TA. The regulation of Notch signaling in muscle stem cell activation and postnatal myogenesis. Semin Cell Dev Biol 16: 612–622, 2005 [DOI] [PubMed] [Google Scholar]
- 321. Luque E, Pena J, Martin P, Jimena I, Vaamonde R. Capillary supply during development of individual regenerating muscle fibers. Anat Histol Embryol 24: 87–89, 1995 [DOI] [PubMed] [Google Scholar]
- 322. Ma DK, Bonaguidi MA, Ming GL, Song H. Adult neural stem cells in the mammalian central nervous system. Cell Res 19: 672–682, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323. Machida S, Booth FW. Insulin-like growth factor 1 and muscle growth: implication for satellite cell proliferation. Proc Nutr Soc 63: 337–340, 2004 [DOI] [PubMed] [Google Scholar]
- 324. Mackey AL, Kjaer M, Charifi N, Henriksson J, Bojsen-Moller J, Holm L, Kadi F. Assessment of satellite cell number and activity status in human skeletal muscle biopsies. Muscle Nerve 40: 455–465, 2009 [DOI] [PubMed] [Google Scholar]
- 325. Mal A, Sturniolo M, Schiltz RL, Ghosh MK, Harter ML. A role for histone deacetylase HDAC1 in modulating the transcriptional activity of MyoD: inhibition of the myogenic program. EMBO J 20: 1739–1753, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326. Mansouri A, Stoykova A, Torres M, Gruss P. Dysgenesis of cephalic neural crest derivatives in Pax7−/− mutant mice. Development 122: 831–838, 1996 [DOI] [PubMed] [Google Scholar]
- 327. Matheny RW, Jr, Nindl BC, Adamo ML. Minireview: Mechano-growth factor: a putative product of IGF-I gene expression involved in tissue repair and regeneration. Endocrinology 151: 865–875, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328. Matsuda R, Nishikawa A, Tanaka H. Visualization of dystrophic muscle fibers in mdx mouse by vital staining with Evans blue: evidence of apoptosis in dystrophin-deficient muscle. J Biochem 118: 959–964, 1995 [DOI] [PubMed] [Google Scholar]
- 329. Mauro A. Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol 9: 493–495, 1961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330. Mayer U. Integrins: redundant or important players in skeletal muscle? J Biol Chem 278: 14587–14590, 2003 [DOI] [PubMed] [Google Scholar]
- 331. McGann CJ, Odelberg SJ, Keating MT. Mammalian myotube dedifferentiation induced by newt regeneration extract. Proc Natl Acad Sci USA 98: 13699–13704, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332. McGeachie JK, Grounds MD. Initiation and duration of muscle precursor replication after mild and severe injury to skeletal muscle of mice. An autoradiographic study. Cell Tissue Res 248: 125–130, 1987 [DOI] [PubMed] [Google Scholar]
- 333. McKay BR, De Lisio M, Johnston AP, O'Reilly CE, Phillips SM, Tarnopolsky MA, Parise G. Association of interleukin-6 signalling with the muscle stem cell response following muscle-lengthening contractions in humans. PLoS One 4: e6027, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334. McLoon LK, Wirtschafter J. Activated satellite cells in extraocular muscles of normal adult monkeys and humans. Invest Ophthalmol Vis Sci 44: 1927–1932, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335. Meech R, Gomez M, Woolley C, Barro M, Hulin JA, Walcott EC, Delgado J, Makarenkova HP. The homeobox transcription factor Barx2 regulates plasticity of young primary myofibers. PLoS One 5: e11612, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336. Meech R, Gonzalez KN, Barro M, Gromova A, Zhuang L, Hulin JA, Makarenkova HP. Barx2 is expressed in satellite cells and is required for normal muscle growth and regeneration. Stem Cells 30: 253–265, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337. Meeson AP, Hawke TJ, Graham S, Jiang N, Elterman J, Hutcheson K, Dimaio JM, Gallardo TD, Garry DJ. Cellular and molecular regulation of skeletal muscle side population cells. Stem Cells 22: 1305–1320, 2004 [DOI] [PubMed] [Google Scholar]
- 338. Megeney LA, Kablar B, Garrett K, Anderson JE, Rudnicki MA. MyoD is required for myogenic stem cell function in adult skeletal muscle. Genes Dev 10: 1173–1183, 1996 [DOI] [PubMed] [Google Scholar]
- 339. Meignin C, Alvarez-Garcia I, Davis I, Palacios IM. The salvador-warts-hippo pathway is required for epithelial proliferation and axis specification in Drosophila. Curr Biol 17: 1871–1878, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340. Menetrey J, Kasemkijwattana C, Day CS, Bosch P, Vogt M, Fu FH, Moreland MS, Huard J. Growth factors improve muscle healing in vivo. J Bone Joint Surg Br 82: 131–137, 2000 [DOI] [PubMed] [Google Scholar]
- 341. Merly F, Lescaudron L, Rouaud T, Crossin F, Gardahaut MF. Macrophages enhance muscle satellite cell proliferation and delay their differentiation. Muscle Nerve 22: 724–732, 1999 [DOI] [PubMed] [Google Scholar]
- 342. Miller KJ, Thaloor D, Matteson S, Pavlath GK. Hepatocyte growth factor affects satellite cell activation and differentiation in regenerating skeletal muscle. Am J Physiol Cell Physiol 278: C174–C181, 2000 [DOI] [PubMed] [Google Scholar]
- 343. Minasi MG, Riminucci M, De Angelis L, Borello U, Berarducci B, Innocenzi A, Caprioli A, Sirabella D, Baiocchi M, De Maria R, Boratto R, Jaffredo T, Broccoli V, Bianco P, Cossu G. The meso-angioblast: a multipotent, self-renewing cell that originates from the dorsal aorta and differentiates into most mesodermal tissues. Development 129: 2773–2783, 2002 [DOI] [PubMed] [Google Scholar]
- 344. Minetti C, Sotgia F, Bruno C, Scartezzini P, Broda P, Bado M, Masetti E, Mazzocco M, Egeo A, Donati MA, Volonte D, Galbiati F, Cordone G, Bricarelli FD, Lisanti MP, Zara F. Mutations in the caveolin-3 gene cause autosomal dominant limb-girdle muscular dystrophy. Nat Genet 18: 365–368, 1998 [DOI] [PubMed] [Google Scholar]
- 345. Mitchell KJ, Pannerec A, Cadot B, Parlakian A, Besson V, Gomes ER, Marazzi G, Sassoon DA. Identification and characterization of a non-satellite cell muscle resident progenitor during postnatal development. Nat Cell Biol 12: 257–266, 2010 [DOI] [PubMed] [Google Scholar]
- 346. Mizuno Y, Chang H, Umeda K, Niwa A, Iwasa T, Awaya T, Fukada SI, Yamamoto H, Yamanaka S, Nakahata T, Heike T. Generation of skeletal muscle stem/progenitor cells from murine induced pluripotent stem cells. FASEB J 2010 [DOI] [PubMed] [Google Scholar]
- 347. Moelling K, Schad K, Bosse M, Zimmermann S, Schweneker M. Regulation of Raf-Akt Cross-talk. J Biol Chem 277: 31099–31106, 2002 [DOI] [PubMed] [Google Scholar]
- 348. Mokalled MH, Johnson AN, Creemers EE, Olson EN. MASTR directs MyoD-dependent satellite cell differentiation during skeletal muscle regeneration. Genes Dev 26: 190–202, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349. Montarras D, Lindon C, Pinset C, Domeyne P. Cultured myf5 null and myoD null muscle precursor cells display distinct growth defects. Biol Cell 92: 565–572, 2000 [DOI] [PubMed] [Google Scholar]
- 350. Montarras D, Morgan J, Collins C, Relaix F, Zaffran S, Cumano A, Partridge T, Buckingham M. Direct isolation of satellite cells for skeletal muscle regeneration. Science 309: 2064–2067, 2005 [DOI] [PubMed] [Google Scholar]
- 351. Moresi V, Pristera A, Scicchitano BM, Molinaro M, Teodori L, Sassoon D, Adamo S, Coletti D. Tumor necrosis factor-alpha inhibition of skeletal muscle regeneration is mediated by a caspase-dependent stem cell response. Stem Cells 26: 997–1008, 2008 [DOI] [PubMed] [Google Scholar]
- 352. Morosetti R, Mirabella M, Gliubizzi C, Broccolini A, De Angelis L, Tagliafico E, Sampaolesi M, Gidaro T, Papacci M, Roncaglia E, Rutella S, Ferrari S, Tonali PA, Ricci E, Cossu G. MyoD expression restores defective myogenic differentiation of human mesoangioblasts from inclusion-body myositis muscle. Proc Natl Acad Sci USA 103: 16995–17000, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353. Morrison JI, Loof S, He P, Simon A. Salamander limb regeneration involves the activation of a multipotent skeletal muscle satellite cell population. J Cell Biol 172: 433–440, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354. Morrison SJ, Kimble J. Asymmetric and symmetric stem-cell divisions in development and cancer. Nature 441: 1068–1074, 2006 [DOI] [PubMed] [Google Scholar]
- 355. Moss FP, Leblond CP. Nature of dividing nuclei in skeletal muscle of growing rats. J Cell Biol 44: 459–462, 1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356. Mourikis P, Sambasivan R, Castel D, Rocheteau P, Bizzarro V, Tajbakhsh S. A critical requirement for notch signaling in maintenance of the quiescent skeletal muscle stem cell state. Stem Cells 30: 243–252, 2012 [DOI] [PubMed] [Google Scholar]
- 357. Mourkioti F, Rosenthal N. IGF-1, inflammation and stem cells: interactions during muscle regeneration. Trends Immunol 26: 535–542, 2005 [DOI] [PubMed] [Google Scholar]
- 358. Mousavi K, Jasmin BJ. BDNF is expressed in skeletal muscle satellite cells and inhibits myogenic differentiation. J Neurosci 26: 5739–5749, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359. Mu X, Peng H, Pan H, Huard J, Li Y. Study of muscle cell dedifferentiation after skeletal muscle injury of mice with a Cre-Lox system. PLoS One 6: e16699, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360. Mulvaney DR, Marple DN, Merkel RA. Proliferation of skeletal muscle satellite cells after castration and administration of testosterone propionate. Proc Soc Exp Biol Med 188: 40–45, 1988 [DOI] [PubMed] [Google Scholar]
- 361. Murphy MM, Lawson JA, Mathew SJ, Hutcheson DA, Kardon G. Satellite cells, connective tissue fibroblasts and their interactions are crucial for muscle regeneration. Development 138: 3625–3637, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362. Murre C, McCaw PS, Baltimore D. A new DNA binding and dimerization motif in immunoglobulin enhancer binding, daughterless, MyoD, and myc proteins. Cell 56: 777–783, 1989 [DOI] [PubMed] [Google Scholar]
- 363. Murre C, McCaw PS, Vaessin H, Caudy M, Jan LY, Jan YN, Cabrera CV, Buskin JN, Hauschka SD, Lassar AB. Interactions between heterologous helix-loop-helix proteins generate complexes that bind specifically to a common DNA sequence. Cell 58: 537–544, 1989 [DOI] [PubMed] [Google Scholar]
- 364. Musaro A, Barberi L. Isolation and culture of mouse satellite cells. Methods Mol Biol 633: 101–111, 2010 [DOI] [PubMed] [Google Scholar]
- 365. Musaro A, McCullagh K, Paul A, Houghton L, Dobrowolny G, Molinaro M, Barton ER, Sweeney HL, Rosenthal N. Localized Igf-1 transgene expression sustains hypertrophy and regeneration in senescent skeletal muscle. Nat Genet 27: 195–200, 2001 [DOI] [PubMed] [Google Scholar]
- 366. Musaro A, McCullagh KJ, Naya FJ, Olson EN, Rosenthal N. IGF-1 induces skeletal myocyte hypertrophy through calcineurin in association with GATA-2 and NF-ATc1. Nature 400: 581–585, 1999 [DOI] [PubMed] [Google Scholar]
- 367. Musaro A, Rosenthal N. Maturation of the myogenic program is induced by postmitotic expression of insulin-like growth factor I. Mol Cell Biol 19: 3115–3124, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368. Muskiewicz KR, Frank NY, Flint AF, Gussoni E. Myogenic potential of muscle side and main population cells after intravenous injection into sub-lethally irradiated mdx mice. J Histochem Cytochem 53: 861–873, 2005 [DOI] [PubMed] [Google Scholar]
- 369. Nagata Y, Kobayashi H, Umeda M, Ohta N, Kawashima S, Zammit PS, Matsuda R. Sphingomyelin levels in the plasma membrane correlate with the activation state of muscle satellite cells. J Histochem Cytochem 54: 375–384, 2006 [DOI] [PubMed] [Google Scholar]
- 370. Nagata Y, Partridge TA, Matsuda R, Zammit PS. Entry of muscle satellite cells into the cell cycle requires sphingolipid signaling. J Cell Biol 174: 245–253, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371. Nakagawa M, Koyanagi M, Tanabe K, Takahashi K, Ichisaka T, Aoi T, Okita K, Mochiduki Y, Takizawa N, Yamanaka S. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat Biotechnol 26: 101–106, 2008 [DOI] [PubMed] [Google Scholar]
- 372. Negroni E, Riederer I, Chaouch S, Belicchi M, Razini P, Di Santo J, Torrente Y, Butler-Browne GS, Mouly V. In vivo myogenic potential of human CD133+ muscle-derived stem cells: a quantitative study. Mol Ther 17: 1771–1778, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373. Nelson WJ, Nusse R. Convergence of Wnt, beta-catenin, and cadherin pathways. Science 303: 1483–1487, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374. Nguyen HT, Frasch M. MicroRNAs in muscle differentiation: lessons from Drosophila and beyond. Curr Opin Genet Dev 16: 533–539, 2006 [DOI] [PubMed] [Google Scholar]
- 375. Nguyen HX, Tidball JG. Interactions between neutrophils and macrophages promote macrophage killing of rat muscle cells in vitro. J Physiol 547: 125–132, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376. Nicolas N, Marazzi G, Kelley K, Sassoon D. Embryonic deregulation of muscle stress signaling pathways leads to altered postnatal stem cell behavior and a failure in postnatal muscle growth. Dev Biol 281: 171–183, 2005 [DOI] [PubMed] [Google Scholar]
- 377. Nishimura T, Nakamura K, Kishioka Y, Kato-Mori Y, Wakamatsu J, Hattori A. Inhibition of matrix metalloproteinases suppresses the migration of skeletal muscle cells. J Muscle Res Cell Motil 29: 37–44, 2008 [DOI] [PubMed] [Google Scholar]
- 378. Novitch BG, Mulligan GJ, Jacks T, Lassar AB. Skeletal muscle cells lacking the retinoblastoma protein display defects in muscle gene expression and accumulate in S and G2 phases of the cell cycle. J Cell Biol 135: 441–456, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379. Odelberg SJ, Kollhoff A, Keating MT. Dedifferentiation of mammalian myotubes induced by msx1. Cell 103: 1099–1109, 2000 [DOI] [PubMed] [Google Scholar]
- 380. Ohlstein B, Kai T, Decotto E, Spradling A. The stem cell niche: theme and variations. Curr Opin Cell Biol 16: 693–699, 2004 [DOI] [PubMed] [Google Scholar]
- 381. Ohnishi T, Daikuhara Y. Hepatocyte growth factor/scatter factor in development, inflammation and carcinogenesis: its expression and role in oral tissues. Arch Oral Biol 48: 797–804, 2003 [DOI] [PubMed] [Google Scholar]
- 382. Ojima K, Uezumi A, Miyoshi H, Masuda S, Morita Y, Fukase A, Hattori A, Nakauchi H, Miyagoe-Suzuki Y, Takeda S. Mac-1(low) early myeloid cells in the bone marrow-derived SP fraction migrate into injured skeletal muscle and participate in muscle regeneration. Biochem Biophys Res Commun 321: 1050–1061, 2004 [DOI] [PubMed] [Google Scholar]
- 383. Okada S, Nonaka I, Chou SM. Muscle fiber type differentiation and satellite cell populations in normally grown and neonatally denervated muscles in the rat. Acta Neuropathol 65: 90–98, 1984 [DOI] [PubMed] [Google Scholar]
- 384. Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature 448: 313–317, 2007 [DOI] [PubMed] [Google Scholar]
- 385. Olguin HC, Yang Z, Tapscott SJ, Olwin BB. Reciprocal inhibition between Pax7 and muscle regulatory factors modulates myogenic cell fate determination. J Cell Biol 177: 769–779, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386. Olwin BB, Rapraeger A. Repression of myogenic differentiation by aFGF, bFGF, and K-FGF is dependent on cellular heparan sulfate. J Cell Biol 118: 631–639, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387. Ono Y, Boldrin L, Knopp P, Morgan JE, Zammit PS. Muscle satellite cells are a functionally heterogeneous population in both somite-derived and branchiomeric muscles. Dev Biol 337: 29–41, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388. Ono Y, Masuda S, Nam HS, Benezra R, Miyagoe-Suzuki Y, Takeda S. Slow-dividing satellite cells retain long-term self-renewal ability in adult muscle. J Cell Sci 125: 1309–1317, 2012 [DOI] [PubMed] [Google Scholar]
- 389. Orimo S, Hiyamuta E, Arahata K, Sugita H. Analysis of inflammatory cells and complement C3 in bupivacaine-induced myonecrosis. Muscle Nerve 14: 515–520, 1991 [DOI] [PubMed] [Google Scholar]
- 390. Ornatsky OI, Cox DM, Tangirala P, Andreucci JJ, Quinn ZA, Wrana JL, Prywes R, Yu YT, McDermott JC. Post-translational control of the MEF2A transcriptional regulatory protein. Nucleic Acids Res 27: 2646–2654, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391. Otto A, Schmidt C, Luke G, Allen S, Valasek P, Muntoni F, Lawrence-Watt D, Patel K. Canonical Wnt signalling induces satellite-cell proliferation during adult skeletal muscle regeneration. J Cell Sci 121: 2939–2950, 2008 [DOI] [PubMed] [Google Scholar]
- 392. Otto A, Schmidt C, Patel K. Pax3 and Pax7 expression and regulation in the avian embryo. Anat Embryol 211: 293–310, 2006 [DOI] [PubMed] [Google Scholar]
- 393. Oustanina S, Hause G, Braun T. Pax7 directs postnatal renewal and propagation of myogenic satellite cells but not their specification. EMBO J 23: 3430–3439, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394. Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev 87: 315–424, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395. Pajcini KV, Corbel SY, Sage J, Pomerantz JH, Blau HM. Transient inactivation of Rb and ARF yields regenerative cells from postmitotic mammalian muscle. Cell Stem Cell 7: 198–213, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396. Palacio J, Galdiz JB, Alvarez FJ, Orozco-Levi M, Lloreta J, Gea J. Procion orange tracer dye technique vs. identification of intrafibrillar fibronectin in the assessment of sarcolemmal damage. Eur J Clin Invest 32: 443–447, 2002 [DOI] [PubMed] [Google Scholar]
- 397. Paliwal P, Conboy IM. Inhibitors of tyrosine phosphatases and apoptosis reprogram lineage-marked differentiated muscle to myogenic progenitor cells. Chem Biol 18: 1153–1166, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398. Pallafacchina G, Francois S, Regnault B, Czarny B, Dive V, Cumano A, Montarras D, Buckingham M. An adult tissue-specific stem cell in its niche: a gene profiling analysis of in vivo quiescent and activated muscle satellite cells. Stem Cell Res 4: 77–91, 2010 [DOI] [PubMed] [Google Scholar]
- 399. Palumbo R, Sampaolesi M, De Marchis F, Tonlorenzi R, Colombetti S, Mondino A, Cossu G, Bianchi ME. Extracellular HMGB1, a signal of tissue damage, induces mesoangioblast migration and proliferation. J Cell Biol 164: 441–449, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400. Parise G, McKinnell IW, Rudnicki MA. Muscle satellite cell and atypical myogenic progenitor response following exercise. Muscle Nerve 37: 611–619, 2008 [DOI] [PubMed] [Google Scholar]
- 401. Pasquinelli G, Pacilli A, Alviano F, Foroni L, Ricci F, Valente S, Orrico C, Lanzoni G, Buzzi M, Luigi Tazzari P, Pagliaro P, Stella A, Paolo Bagnara G. Multidistrict human mesenchymal vascular cells: pluripotency and stemness characteristics. Cytotherapy 12: 275–287, 2010 [DOI] [PubMed] [Google Scholar]
- 402. Patruno M, Caliaro F, Martinello T, Mascarello F. Expression of the paired box domain Pax7 protein in myogenic cells isolated from the porcine semitendinosus muscle after birth. Tissue Cell 40: 1–6, 2008 [DOI] [PubMed] [Google Scholar]
- 403. Pedersen BK, Febbraio MA. Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiol Rev 88: 1379–1406, 2008 [DOI] [PubMed] [Google Scholar]
- 404. Peng H, Huard J. Muscle-derived stem cells for musculoskeletal tissue regeneration and repair. Transpl Immunol 12: 311–319, 2004 [DOI] [PubMed] [Google Scholar]
- 405. Penn BH, Bergstrom DA, Dilworth FJ, Bengal E, Tapscott SJ. A MyoD-generated feed-forward circuit temporally patterns gene expression during skeletal muscle differentiation. Genes Dev 18: 2348–2353, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406. Peppiatt CM, Howarth C, Mobbs P, Attwell D. Bidirectional control of CNS capillary diameter by pericytes. Nature 443: 700–704, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407. Perez-Ruiz A, Ono Y, Gnocchi VF, Zammit PS. beta-Catenin promotes self-renewal of skeletal-muscle satellite cells. J Cell Sci 121: 1373–1382, 2008 [DOI] [PubMed] [Google Scholar]
- 408. Petropoulos H, Skerjanc IS. Beta-catenin is essential and sufficient for skeletal myogenesis in P19 cells. J Biol Chem 277: 15393–15399, 2002 [DOI] [PubMed] [Google Scholar]
- 409. Philippou A, Halapas A, Maridaki M, Koutsilieris M. Type I insulin-like growth factor receptor signaling in skeletal muscle regeneration and hypertrophy. J Musculoskelet Neuronal Interact 7: 208–218, 2007 [PubMed] [Google Scholar]
- 410. Phillips WD, Bennett MR. Elimination of distributed synaptic acetylcholine receptor clusters on developing avian fast-twitch muscle fibres accompanies loss of polyneuronal innervation. J Neurocytol 16: 785–797, 1987 [DOI] [PubMed] [Google Scholar]
- 411. Pietsch J. The effects of colchicine on regeneration of mouse skeletal muscle. Anat Rec 167–172, 1961. 13969756 [Google Scholar]
- 412. Polesello C, Tapon N. Salvador-warts-hippo signaling promotes Drosophila posterior follicle cell maturation downstream of notch. Curr Biol 17: 1864–1870, 2007 [DOI] [PubMed] [Google Scholar]
- 413. Polesskaya A, Seale P, Rudnicki MA. Wnt signaling induces the myogenic specification of resident CD45+ adult stem cells during muscle regeneration. Cell 113: 841–852, 2003 [DOI] [PubMed] [Google Scholar]
- 414. Popescu LM, Manole E, Serboiu CS, Manole CG, Suciu LC, Gherghiceanu M, Popescu BO. Identification of telocytes in skeletal muscle interstitium: implication for muscle regeneration. J Cell Mol Med 15: 1379–1392, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415. Pouget C, Pottin K, Jaffredo T. Sclerotomal origin of vascular smooth muscle cells and pericytes in the embryo. Dev Biol 315: 437–447, 2008 [DOI] [PubMed] [Google Scholar]
- 416. Prior BM, Lloyd PG, Yang HT, Terjung RL. Exercise-induced vascular remodeling. Exerc Sport Sci Rev 31: 26–33, 2003 [DOI] [PubMed] [Google Scholar]
- 417. Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science 276: 71–74, 1997 [DOI] [PubMed] [Google Scholar]
- 418. Przybylski RJ, Blumberg JM. Ultrastructural aspects of myogenesis in the chick. Lab Invest 15: 836–863, 1966 [PubMed] [Google Scholar]
- 419. Puri PL, Avantaggiati ML, Balsano C, Sang N, Graessmann A, Giordano A, Levrero M. p300 is required for MyoD-dependent cell cycle arrest and muscle-specific gene transcription. EMBO J 16: 369–383, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420. Puri PL, Sartorelli V. Regulation of muscle regulatory factors by DNA-binding, interacting proteins, and post-transcriptional modifications. J Cell Physiol 185: 155–173, 2000 [DOI] [PubMed] [Google Scholar]
- 421. Qu-Petersen Z, Deasy B, Jankowski R, Ikezawa M, Cummins J, Pruchnic R, Mytinger J, Cao B, Gates C, Wernig A, Huard J. Identification of a novel population of muscle stem cells in mice: potential for muscle regeneration. J Cell Biol 157: 851–864, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422. Qu Z, Balkir L, van Deutekom JC, Robbins PD, Pruchnic R, Huard J. Development of approaches to improve cell survival in myoblast transfer therapy. J Cell Biol 142: 1257–1267, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423. Quinlan JG, Lyden SP, Cambier DM, Johnson SR, Michaels SE, Denman DL. Radiation inhibition of mdx mouse muscle regeneration: dose and age factors. Muscle Nerve 18: 201–206, 1995 [DOI] [PubMed] [Google Scholar]
- 424. Rampalli S, Li L, Mak E, Ge K, Brand M, Tapscott SJ, Dilworth FJ. p38 MAPK signaling regulates recruitment of Ash2L-containing methyltransferase complexes to specific genes during differentiation. Nat Struct Mol Biol 14: 1150–1156, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425. Rando TA, Blau HM. Primary mouse myoblast purification, characterization, and transplantation for cell-mediated gene therapy. J Cell Biol 125: 1275–1287, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426. Rantanen J, Hurme T, Lukka R, Heino J, Kalimo H. Satellite cell proliferation and the expression of myogenin and desmin in regenerating skeletal muscle: evidence for two different populations of satellite cells. Lab Invest 72: 341–347, 1995 [PubMed] [Google Scholar]
- 427. Rapraeger AC. Syndecan-regulated receptor signaling. J Cell Biol 149: 995–998, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428. Rash JE, Fambrough D. Ultrastructural and electrophysiological correlates of cell coupling and cytoplasmic fusion during myogenesis in vitro. Dev Biol 30: 166–186, 1973 [DOI] [PubMed] [Google Scholar]
- 429. Ratajczak MZ, Majka M, Kucia M, Drukala J, Pietrzkowski Z, Peiper S, Janowska-Wieczorek A. Expression of functional CXCR4 by muscle satellite cells and secretion of SDF-1 by muscle-derived fibroblasts is associated with the presence of both muscle progenitors in bone marrow and hematopoietic stem/progenitor cells in muscles. Stem Cells 21: 363–371, 2003 [DOI] [PubMed] [Google Scholar]
- 430. Rawlings JS, Rosler KM, Harrison DA. The JAK/STAT signaling pathway. J Cell Sci 117: 1281–1283, 2004 [DOI] [PubMed] [Google Scholar]
- 431. Relaix F, Montarras D, Zaffran S, Gayraud-Morel B, Rocancourt D, Tajbakhsh S, Mansouri A, Cumano A, Buckingham M. Pax3 and Pax7 have distinct and overlapping functions in adult muscle progenitor cells. J Cell Biol 172: 91–102, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432. Relaix F, Polimeni M, Rocancourt D, Ponzetto C, Schafer BW, Buckingham M. The transcriptional activator PAX3-FKHR rescues the defects of Pax3 mutant mice but induces a myogenic gain-of-function phenotype with ligand-independent activation of Met signaling in vivo. Genes Dev 17: 2950–2965, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433. Relaix F, Rocancourt D, Mansouri A, Buckingham M. Divergent functions of murine Pax3 and Pax7 in limb muscle development. Genes Dev 18: 1088–1105, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434. Relaix F, Rocancourt D, Mansouri A, Buckingham M. A Pax3/Pax7-dependent population of skeletal muscle progenitor cells. Nature 435: 948–953, 2005 [DOI] [PubMed] [Google Scholar]
- 435. Relaix F, Weng X, Marazzi G, Yang E, Copeland N, Jenkins N, Spence SE, Sassoon D. Pw1, a novel zinc finger gene implicated in the myogenic and neuronal lineages. Dev Biol 177: 383–396, 1996 [DOI] [PubMed] [Google Scholar]
- 436. Ren H, Yin P, Duan C. IGFBP-5 regulates muscle cell differentiation by binding to IGF-II and switching on the IGF-II auto-regulation loop. J Cell Biol 182: 979–991, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437. Reznik M. Thymidine-3H uptake by satellite cells of regenerating skeletal muscle. J Cell Biol 40: 568–571, 1969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438. Riuzzi F, Sorci G, Sagheddu R, Donato R. HMGB1-RAGE regulates muscle satellite cell homeostasis through p38-MAPK- and myogenin-dependent repression of Pax7 transcription. J Cell Sci 125: 1440–1454, 2012 [DOI] [PubMed] [Google Scholar]
- 439. Rivier F, Alkan O, Flint AF, Muskiewicz K, Allen PD, Leboulch P, Gussoni E. Role of bone marrow cell trafficking in replenishing skeletal muscle SP and MP cell populations. J Cell Sci 117: 1979–1988, 2004 [DOI] [PubMed] [Google Scholar]
- 440. Robertson TA, Maley MA, Grounds MD, Papadimitriou JM. The role of macrophages in skeletal muscle regeneration with particular reference to chemotaxis. Exp Cell Res 207: 321–331, 1993 [DOI] [PubMed] [Google Scholar]
- 441. Rodeheffer MS, Birsoy K, Friedman JM. Identification of white adipocyte progenitor cells in vivo. Cell 135: 240–249, 2008 [DOI] [PubMed] [Google Scholar]
- 442. Rodrigues Ade C, Schmalbruch H. Satellite cells and myonuclei in long-term denervated rat muscles. Anat Rec 243: 430–437, 1995 [DOI] [PubMed] [Google Scholar]
- 443. Rosania GR, Chang YT, Perez O, Sutherlin D, Dong H, Lockhart DJ, Schultz PG. Myoseverin, a microtubule-binding molecule with novel cellular effects. Nature Biotechnol 18: 304–308, 2000 [DOI] [PubMed] [Google Scholar]
- 444. Rosant C, Nagel MD, Perot C. Aging affects passive stiffness and spindle function of the rat soleus muscle. Exp Gerontol 42: 301–308, 2007 [DOI] [PubMed] [Google Scholar]
- 445. Rosen GD, Sanes JR, LaChance R, Cunningham JM, Roman J, Dean DC. Roles for the integrin VLA-4 and its counter receptor VCAM-1 in myogenesis. Cell 69: 1107–1119, 1992 [DOI] [PubMed] [Google Scholar]
- 446. Rosenblatt JD, Lunt AI, Parry DJ, Partridge TA. Culturing satellite cells from living single muscle fiber explants. In Vitro Cell Dev Biol Anim 31: 773–779, 1995 [DOI] [PubMed] [Google Scholar]
- 447. Rosu-Myles M, Stewart E, Trowbridge J, Ito CY, Zandstra P, Bhatia M. A unique population of bone marrow cells migrates to skeletal muscle via hepatocyte growth factor/c-met axis. J Cell Sci 118: 4343–4352, 2005 [DOI] [PubMed] [Google Scholar]
- 448. Ruberti F, Capsoni S, Comparini A, Di Daniel E, Franzot J, Gonfloni S, Rossi G, Berardi N, Cattaneo A. Phenotypic knockout of nerve growth factor in adult transgenic mice reveals severe deficits in basal forebrain cholinergic neurons, cell death in the spleen, and skeletal muscle dystrophy. J Neurosci 20: 2589–2601, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449. Rubinstein I, Abassi Z, Coleman R, Milman F, Winaver J, Better OS. Involvement of nitric oxide system in experimental muscle crush injury. J Clin Invest 101: 1325–1333, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450. Rudnicki MA, Le Grand F, McKinnell I, Kuang S. The molecular regulation of muscle stem cell function. Cold Spring Harb Symp Quant Biol 73: 323–331, 2008 [DOI] [PubMed] [Google Scholar]
- 451. Ryan NA, Zwetsloot KA, Westerkamp LM, Hickner RC, Pofahl WE, Gavin TP. Lower skeletal muscle capillarization and VEGF expression in aged vs. young men. J Appl Physiol 100: 178–185, 2006 [DOI] [PubMed] [Google Scholar]
- 452. Sabourin LA, Girgis-Gabardo A, Seale P, Asakura A, Rudnicki MA. Reduced differentiation potential of primary MyoD−/− myogenic cells derived from adult skeletal muscle. J Cell Biol 144: 631–643, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453. Sabourin LA, Rudnicki MA. The molecular regulation of myogenesis. Clin Genet 57: 16–25, 2000 [DOI] [PubMed] [Google Scholar]
- 454. Sacco A, Doyonnas R, Kraft P, Vitorovic S, Blau HM. Self-renewal and expansion of single transplanted muscle stem cells. Nature 456: 502–506, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455. Sakurai H, Okawa Y, Inami Y, Nishio N, Isobe K. Paraxial mesodermal progenitors derived from mouse embryonic stem cells contribute to muscle regeneration via differentiation into muscle satellite cells. Stem Cells 26: 1865–1873, 2008 [DOI] [PubMed] [Google Scholar]
- 456. Sambasivan R, Gayraud-Morel B, Dumas G, Cimper C, Paisant S, Kelly RG, Tajbakhsh S. Distinct regulatory cascades govern extraocular and pharyngeal arch muscle progenitor cell fates. Dev Cell 16: 810–821, 2009 [DOI] [PubMed] [Google Scholar]
- 457. Sambasivan R, Yao R, Kissenpfennig A, Van Wittenberghe L, Paldi A, Gayraud-Morel B, Guenou H, Malissen B, Tajbakhsh S, Galy A. Pax7-expressing satellite cells are indispensable for adult skeletal muscle regeneration. Development 138: 3647–3656, 2011 [DOI] [PubMed] [Google Scholar]
- 458. Sampaolesi M, Torrente Y, Innocenzi A, Tonlorenzi R, D'Antona G, Pellegrino MA, Barresi R, Bresolin N, De Angelis MG, Campbell KP, Bottinelli R, Cossu G. Cell therapy of alpha-sarcoglycan null dystrophic mice through intra-arterial delivery of mesoangioblasts. Science 301: 487–492, 2003 [DOI] [PubMed] [Google Scholar]
- 459. Sanes JR. The basement membrane/basal lamina of skeletal muscle. J Biol Chem 278: 12601–12604, 2003 [DOI] [PubMed] [Google Scholar]
- 460. Santa Maria L, Rojas CV, Minguell JJ. Signals from damaged but not undamaged skeletal muscle induce myogenic differentiation of rat bone-marrow-derived mesenchymal stem cells. Exp Cell Res 300: 418–426, 2004 [DOI] [PubMed] [Google Scholar]
- 461. Sassoli C, Formigli L, Bini F, Tani A, Squecco R, Battistini C, Zecchi-Orlandini S, Francini F, Meacci E. Effects of S1P on skeletal muscle repair/regeneration during eccentric contraction. J Cell Mol Med 15: 2498–2511, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462. Scaal M, Christ B. Formation and differentiation of the avian dermomyotome. Anat Embryol 208: 411–424, 2004 [DOI] [PubMed] [Google Scholar]
- 463. Scata KA, Bernard DW, Fox J, Swain JL. FGF receptor availability regulates skeletal myogenesis. Exp Cell Res 250: 10–21, 1999 [DOI] [PubMed] [Google Scholar]
- 464. Schienda J, Engleka KA, Jun S, Hansen MS, Epstein JA, Tabin CJ, Kunkel LM, Kardon G. Somitic origin of limb muscle satellite and side population cells. Proc Natl Acad Sci USA 103: 945–950, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465. Schmalbruch H. The morphology of regeneration of skeletal muscles in the rat. Tissue Cell 8: 673–692, 1976 [DOI] [PubMed] [Google Scholar]
- 466. Schmalbruch H, Hellhammer U. The number of nuclei in adult rat muscles with special reference to satellite cells. Anat Rec 189: 169–175, 1977 [DOI] [PubMed] [Google Scholar]
- 467. Schmalbruch H, Lewis DM. Dynamics of nuclei of muscle fibers and connective tissue cells in normal and denervated rat muscles. Muscle Nerve 23: 617–626, 2000 [DOI] [PubMed] [Google Scholar]
- 468. Schultz E. Changes in the satellite cells of growing muscle following denervation. Anat Rec 190: 299–311, 1978 [DOI] [PubMed] [Google Scholar]
- 469. Schultz E. A quantitative study of the satellite cell population in postnatal mouse lumbrical muscle. Anat Rec 180: 589–595, 1974 [DOI] [PubMed] [Google Scholar]
- 470. Schultz E. Satellite cell behavior during skeletal muscle growth and regeneration. Med Sci Sports Exerc 21: S181–186, 1989 [PubMed] [Google Scholar]
- 471. Schultz E. Satellite cell proliferative compartments in growing skeletal muscles. Dev Biol 175: 84–94, 1996 [DOI] [PubMed] [Google Scholar]
- 472. Schultz E, Gibson MC, Champion T. Satellite cells are mitotically quiescent in mature mouse muscle: an EM and radioautographic study. J Exp Zool 206: 451–456, 1978 [DOI] [PubMed] [Google Scholar]
- 473. Schultz E, Jaryszak DL, Valliere CR. Response of satellite cells to focal skeletal muscle injury. Muscle Nerve 8: 217–222, 1985 [DOI] [PubMed] [Google Scholar]
- 474. Schwander M, Leu M, Stumm M, Dorchies OM, Ruegg UT, Schittny J, Muller U. Beta1 integrins regulate myoblast fusion and sarcomere assembly. Dev Cell 4: 673–685, 2003 [DOI] [PubMed] [Google Scholar]
- 475. Sciorati C, Galvez BG, Brunelli S, Tagliafico E, Ferrari S, Cossu G, Clementi E. Ex vivo treatment with nitric oxide increases mesoangioblast therapeutic efficacy in muscular dystrophy. J Cell Sci 119: 5114–5123, 2006 [DOI] [PubMed] [Google Scholar]
- 476. Seale P, Ishibashi J, Holterman C, Rudnicki MA. Muscle satellite cell-specific genes identified by genetic profiling of MyoD-deficient myogenic cell. Dev Biol 275: 287–300, 2004 [DOI] [PubMed] [Google Scholar]
- 477. Seale P, Ishibashi J, Scime A, Rudnicki MA. Pax7 is necessary and sufficient for the myogenic specification of CD45+:Sca1+ stem cells from injured muscle. PLoS Biol 2: E130, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 478. Seale P, Sabourin LA, Girgis-Gabardo A, Mansouri A, Gruss P, Rudnicki MA. Pax7 is required for the specification of myogenic satellite cells. Cell 102: 777–786, 2000 [DOI] [PubMed] [Google Scholar]
- 479. Semsarian C, Wu MJ, Ju YK, Marciniec T, Yeoh T, Allen DG, Harvey RP, Graham RM. Skeletal muscle hypertrophy is mediated by a Ca2+-dependent calcineurin signalling pathway. Nature 400: 576–581, 1999 [DOI] [PubMed] [Google Scholar]
- 480. Serrano AL, Baeza-Raja B, Perdiguero E, Jardi M, Munoz-Canoves P. Interleukin-6 is an essential regulator of satellite cell-mediated skeletal muscle hypertrophy. Cell Metab 7: 33–44, 2008 [DOI] [PubMed] [Google Scholar]
- 481. Shafiq SA, Gorycki MA, Mauro A. Mitosis during postnatal growth in skeletal and cardiac muscle of the rat. J Anat 103: 135–141, 1968 [PMC free article] [PubMed] [Google Scholar]
- 482. Shea KL, Xiang W, LaPorta VS, Licht JD, Keller C, Basson MA, Brack AS. Sprouty1 regulates reversible quiescence of a self-renewing adult muscle stem cell pool during regeneration. Cell Stem Cell 6: 117–129, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 483. Sheehan SM, Allen RE. Skeletal muscle satellite cell proliferation in response to members of the fibroblast growth factor family and hepatocyte growth factor. J Cell Physiol 181: 499–506, 1999 [DOI] [PubMed] [Google Scholar]
- 484. Sheehan SM, Tatsumi R, Temm-Grove CJ, Allen RE. HGF is an autocrine growth factor for skeletal muscle satellite cells in vitro. Muscle Nerve 23: 239–245, 2000 [DOI] [PubMed] [Google Scholar]
- 485. Shefer G, Wleklinski-Lee M, Yablonka-Reuveni Z. Skeletal muscle satellite cells can spontaneously enter an alternative mesenchymal pathway. J Cell Sci 117: 5393–5404, 2004 [DOI] [PubMed] [Google Scholar]
- 486. Sherwood RI, Christensen JL, Conboy IM, Conboy MJ, Rando TA, Weissman IL, Wagers AJ. Isolation of adult mouse myogenic progenitors: functional heterogeneity of cells within and engrafting skeletal muscle. Cell 119: 543–554, 2004 [DOI] [PubMed] [Google Scholar]
- 487. Sherwood RI, Christensen JL, Weissman IL, Wagers AJ. Determinants of skeletal muscle contributions from circulating cells, bone marrow cells, and hematopoietic stem cells. Stem Cells 22: 1292–1304, 2004 [DOI] [PubMed] [Google Scholar]
- 488. Shi D, Reinecke H, Murry CE, Torok-Storb B. Myogenic fusion of human bone marrow stromal cells, but not hematopoietic cells. Blood 104: 290–294, 2004 [DOI] [PubMed] [Google Scholar]
- 489. Shinin V, Gayraud-Morel B, Gomes D, Tajbakhsh S. Asymmetric division and cosegregation of template DNA strands in adult muscle satellite cells. Nat Cell Biol 8: 677–687, 2006 [DOI] [PubMed] [Google Scholar]
- 490. Siegel AL, Atchison K, Fisher KE, Davis GE, Cornelison DD. 3D timelapse analysis of muscle satellite cell motility. Stem Cells 27: 2527–2538, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 491. Simone C, Forcales SV, Hill DA, Imbalzano AN, Latella L, Puri PL. p38 pathway targets SWI-SNF chromatin-remodeling complex to muscle-specific loci. Nat Genet 36: 738–743, 2004 [DOI] [PubMed] [Google Scholar]
- 492. Simons M, Mlodzik M. Planar cell polarity signaling: from fly development to human disease. Annu Rev Genet 42: 517–540, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 493. Sinha-Hikim I, Artaza J, Woodhouse L, Gonzalez-Cadavid N, Singh AB, Lee MI, Storer TW, Casaburi R, Shen R, Bhasin S. Testosterone-induced increase in muscle size in healthy young men is associated with muscle fiber hypertrophy. Am J Physiol Endocrinol Metab 283: E154–E164, 2002 [DOI] [PubMed] [Google Scholar]
- 494. Sinha-Hikim I, Cornford M, Gaytan H, Lee ML, Bhasin S. Effects of testosterone supplementation on skeletal muscle fiber hypertrophy and satellite cells in community-dwelling older men. J Clin Endocrinol Metab 91: 3024–3033, 2006 [DOI] [PubMed] [Google Scholar]
- 495. Sinha-Hikim I, Roth SM, Lee MI, Bhasin S. Testosterone-induced muscle hypertrophy is associated with an increase in satellite cell number in healthy, young men. Am J Physiol Endocrinol Metab 285: E197–E205, 2003 [DOI] [PubMed] [Google Scholar]
- 496. Sinha-Hikim I, Taylor WE, Gonzalez-Cadavid NF, Zheng W, Bhasin S. Androgen receptor in human skeletal muscle and cultured muscle satellite cells: up-regulation by androgen treatment. J Clin Endocrinol Metab 89: 5245–5255, 2004 [DOI] [PubMed] [Google Scholar]
- 497. Smith CK, 2nd, Janney MJ, Allen RE. Temporal expression of myogenic regulatory genes during activation, proliferation, differentiation of rat skeletal muscle satellite cells. J Cell Physiol 159: 379–385, 1994 [DOI] [PubMed] [Google Scholar]
- 498. Snow MH. An autoradiographic study of satellite cell differentiation into regenerating myotubes following transplantation of muscles in young rats. Cell Tissue Res 186: 535–540, 1978 [DOI] [PubMed] [Google Scholar]
- 499. Snow MH. Myogenic cell formation in regenerating rat skeletal muscle injured by mincing. II. An autoradiographic study. Anat Rec 188: 201–217, 1977 [DOI] [PubMed] [Google Scholar]
- 500. Snow MH. A quantitative ultrastructural analysis of satellite cells in denervated fast and slow muscles of the mouse. Anat Rec 207: 593–604, 1983 [DOI] [PubMed] [Google Scholar]
- 501. Song WK, Wang W, Foster RF, Bielser DA, Kaufman SJ. H36-alpha 7 is a novel integrin alpha chain that is developmentally regulated during skeletal myogenesis. J Cell Biol 117: 643–657, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 502. Song YH, Li Y, Du J, Mitch WE, Rosenthal N, Delafontaine P. Muscle-specific expression of IGF-1 blocks angiotensin II-induced skeletal muscle wasting. J Clin Invest 115: 451–458, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 503. Sonnet C, Lafuste P, Arnold L, Brigitte M, Poron F, Authier FJ, Chretien F, Gherardi RK, Chazaud B. Human macrophages rescue myoblasts and myotubes from apoptosis through a set of adhesion molecular systems. J Cell Sci 119: 2497–2507, 2006 [DOI] [PubMed] [Google Scholar]
- 504. Spicer DB, Rhee J, Cheung WL, Lassar AB. Inhibition of myogenic bHLH and MEF2 transcription factors by the bHLH protein Twist. Science 272: 1476–1480, 1996 [DOI] [PubMed] [Google Scholar]
- 505. Stamler JS, Meissner G. Physiology of nitric oxide in skeletal muscle. Physiol Rev 81: 209–237, 2001 [DOI] [PubMed] [Google Scholar]
- 506. Stark DA, Karvas RM, Siegel AL, Cornelison DD. Eph/ephrin interactions modulate muscle satellite cell motility and patterning. Development 138: 5279–5289, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 507. Starkey JD, Yamamoto M, Yamamoto S, Goldhamer DJ. Skeletal muscle satellite cells are committed to myogenesis and do not spontaneously adopt nonmyogenic fates. J Histochem Cytochem 59: 33–46, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 508. Steinhardt RA, Bi G, Alderton JM. Cell membrane resealing by a vesicular mechanism similar to neurotransmitter release. Science 263: 390–393, 1994 [DOI] [PubMed] [Google Scholar]
- 509. Summer R, Fitzsimmons K, Dwyer D, Murphy J, Fine A. Isolation of an adult mouse lung mesenchymal progenitor cell population. Am J Respir Cell Mol Biol 37: 152–159, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 510. Sun D, Martinez CO, Ochoa O, Ruiz-Willhite L, Bonilla JR, Centonze VE, Waite LL, Michalek JE, McManus LM, Shireman PK. Bone marrow-derived cell regulation of skeletal muscle regeneration. FASEB J 23: 382–395, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 511. Sun H, Li L, Vercherat C, Gulbagci NT, Acharjee S, Li J, Chung TK, Thin TH, Taneja R. Stra13 regulates satellite cell activation by antagonizing Notch signaling. J Cell Biol 177: 647–657, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 512. Suzuki J, Yamazaki Y, Li G, Kaziro Y, Koide H. Involvement of Ras and Ral in chemotactic migration of skeletal myoblasts. Mol Cell Biol 20: 4658–4665, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 513. Suzuki S, Yamanouchi K, Soeta C, Katakai Y, Harada R, Naito K, Tojo H. Skeletal muscle injury induces hepatocyte growth factor expression in spleen. Biochem Biophys Res Commun 292: 709–714, 2002 [DOI] [PubMed] [Google Scholar]
- 514. Takahashi A, Kureishi Y, Yang J, Luo Z, Guo K, Mukhopadhyay D, Ivashchenko Y, Branellec D, Walsh K. Myogenic Akt signaling regulates blood vessel recruitment during myofiber growth. Mol Cell Biol 22: 4803–4814, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 515. Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131: 861–872, 2007 [DOI] [PubMed] [Google Scholar]
- 516. Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126: 663–676, 2006 [DOI] [PubMed] [Google Scholar]
- 517. Tamaki T, Akatsuka A, Yoshimura S, Roy RR, Edgerton VR. New fiber formation in the interstitial spaces of rat skeletal muscle during postnatal growth. J Histochem Cytochem 50: 1097–1111, 2002 [DOI] [PubMed] [Google Scholar]
- 518. Tanaka KK, Hall JK, Troy AA, Cornelison DD, Majka SM, Olwin BB. Syndecan-4-expressing muscle progenitor cells in the SP engraft as satellite cells during muscle regeneration. Cell Stem Cell 4: 217–225, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 519. Tang W, Zeve D, Suh JM, Bosnakovski D, Kyba M, Hammer RE, Tallquist MD, Graff JM. White fat progenitor cells reside in the adipose vasculature. Science 322: 583–586, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 520. Tatsumi R, Allen RE. Active hepatocyte growth factor is present in skeletal muscle extracellular matrix. Muscle Nerve 30: 654–658, 2004 [DOI] [PubMed] [Google Scholar]
- 521. Tatsumi R, Anderson JE, Nevoret CJ, Halevy O, Allen RE. HGF/SF is present in normal adult skeletal muscle and is capable of activating satellite cells. Dev Biol 194: 114–128, 1998 [DOI] [PubMed] [Google Scholar]
- 522. Tatsumi R, Hattori A, Ikeuchi Y, Anderson JE, Allen RE. Release of hepatocyte growth factor from mechanically stretched skeletal muscle satellite cells and role of pH and nitric oxide. Mol Biol Cell 13: 2909–2918, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 523. Tatsumi R, Liu X, Pulido A, Morales M, Sakata T, Dial S, Hattori A, Ikeuchi Y, Allen RE. Satellite cell activation in stretched skeletal muscle and the role of nitric oxide and hepatocyte growth factor. Am J Physiol Cell Physiol 290: C1487–C1494, 2006 [DOI] [PubMed] [Google Scholar]
- 524. Tatsumi R, Sankoda Y, Anderson JE, Sato Y, Mizunoya W, Shimizu N, Suzuki T, Yamada M, Rhoads RP, Jr, Ikeuchi Y, Allen RE. Possible implication of satellite cells in regenerative motoneuritogenesis: HGF upregulates neural chemorepellent Sema3A during myogenic differentiation. Am J Physiol Cell Physiol 297: C238–C252, 2009 [DOI] [PubMed] [Google Scholar]
- 525. Tatsumi R, Sheehan SM, Iwasaki H, Hattori A, Allen RE. Mechanical stretch induces activation of skeletal muscle satellite cells in vitro. Exp Cell Res 267: 107–114, 2001 [DOI] [PubMed] [Google Scholar]
- 526. Taulli R, Bersani F, Foglizzo V, Linari A, Vigna E, Ladanyi M, Tuschl T, Ponzetto C. The muscle-specific microRNA miR-206 blocks human rhabdomyosarcoma growth in xenotransplanted mice by promoting myogenic differentiation. J Clin Invest 119: 2366–2378, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 527. Taylor-Jones JM, McGehee RE, Rando TA, Lecka-Czernik B, Lipschitz DA, Peterson CA. Activation of an adipogenic program in adult myoblasts with age. Mech Ageing Dev 123: 649–661, 2002 [DOI] [PubMed] [Google Scholar]
- 528. Theise ND. Gastrointestinal stem cells. III. Emergent themes of liver stem cell biology: niche, quiescence, self-renewal, and plasticity. Am J Physiol Gastrointest Liver Physiol 290: G189–G193, 2006 [DOI] [PubMed] [Google Scholar]
- 529. Thompson SH, Boxhorn LK, Kong WY, Allen RE. Trenbolone alters the responsiveness of skeletal muscle satellite cells to fibroblast growth factor and insulin-like growth factor I. Endocrinology 124: 2110–2117, 1989 [DOI] [PubMed] [Google Scholar]
- 530. Tidball JG. Inflammatory cell response to acute muscle injury. Med Sci Sports Exerc 27: 1022–1032, 1995 [DOI] [PubMed] [Google Scholar]
- 531. Tidball JG, Daniel TL. Myotendinous junctions of tonic muscle cells: structure and loading. Cell Tissue Res 245: 315–322, 1986 [DOI] [PubMed] [Google Scholar]
- 532. Tidball JG, Villalta SA. Regulatory interactions between muscle and the immune system during muscle regeneration. Am J Physiol Regul Integr Comp Physiol 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 533. Tomiya A, Aizawa T, Nagatomi R, Sensui H, Kokubun S. Myofibers express IL-6 after eccentric exercise. Am J Sports Med 32: 503–508, 2004 [DOI] [PubMed] [Google Scholar]
- 534. Torrente Y, Belicchi M, Marchesi C, Dantona G, Cogiamanian F, Pisati F, Gavina M, Giordano R, Tonlorenzi R, Fagiolari G, Lamperti C, Porretti L, Lopa R, Sampaolesi M, Vicentini L, Grimoldi N, Tiberio F, Songa V, Baratta P, Prelle A, Forzenigo L, Guglieri M, Pansarasa O, Rinaldi C, Mouly V, Butler-Browne GS, Comi GP, Biondetti P, Moggio M, Gaini SM, Stocchetti N, Priori A, D'Angelo MG, Turconi A, Bottinelli R, Cossu G, Rebulla P, Bresolin N. Autologous transplantation of muscle-derived CD133+ stem cells in Duchenne muscle patients. Cell Transplant 16: 563–577, 2007 [DOI] [PubMed] [Google Scholar]
- 535. Torrente Y, Belicchi M, Sampaolesi M, Pisati F, Meregalli M, D'Antona G, Tonlorenzi R, Porretti L, Gavina M, Mamchaoui K, Pellegrino MA, Furling D, Mouly V, Butler-Browne GS, Bottinelli R, Cossu G, Bresolin N. Human circulating AC133(+) stem cells restore dystrophin expression and ameliorate function in dystrophic skeletal muscle. J Clin Invest 114: 182–195, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 536. Torrente Y, Tremblay JP, Pisati F, Belicchi M, Rossi B, Sironi M, Fortunato F, El Fahime M, D'Angelo MG, Caron NJ, Constantin G, Paulin D, Scarlato G, Bresolin N. Intra-arterial injection of muscle-derived CD34(+)Sca-1(+) stem cells restores dystrophin in mdx mice. J Cell Biol 152: 335–348, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 537. Toti P, Villanova M, Vatti R, Schuerfeld K, Stumpo M, Barbagli L, Malandrini A, Costantini M. Nerve growth factor expression in human dystrophic muscles. Muscle Nerve 27: 370–373, 2003 [DOI] [PubMed] [Google Scholar]
- 538. Tsujinaka T, Ebisui C, Fujita J, Kishibuchi M, Morimoto T, Ogawa A, Katsume A, Ohsugi Y, Kominami E, Monden M. Muscle undergoes atrophy in association with increase of lysosomal cathepsin activity in interleukin-6 transgenic mouse. Biochem Biophys Res Commun 207: 168–174, 1995 [DOI] [PubMed] [Google Scholar]
- 539. Tureckova J, Wilson EM, Cappalonga JL, Rotwein P. Insulin-like growth factor-mediated muscle differentiation: collaboration between phosphatidylinositol 3-kinase-Akt-signaling pathways and myogenin. J Biol Chem 276: 39264–39270, 2001 [DOI] [PubMed] [Google Scholar]
- 540. Tzahor E. Heart and craniofacial muscle development: a new developmental theme of distinct myogenic fields. Dev Biol 327: 273–279, 2009 [DOI] [PubMed] [Google Scholar]
- 541. Uezumi A, Fukada S, Yamamoto N, Takeda S, Tsuchida K. Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nat Cell Biol 12: 143–152, 2010 [DOI] [PubMed] [Google Scholar]
- 542. Ustanina S, Carvajal J, Rigby P, Braun T. The myogenic factor Myf5 supports efficient skeletal muscle regeneration by enabling transient myoblast amplification. Stem Cells 25: 2006–2016, 2007 [DOI] [PubMed] [Google Scholar]
- 543. Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nature Cell Biol 9: 654–659, 2007 [DOI] [PubMed] [Google Scholar]
- 544. Van den Beld AW, de Jong FH, Grobbee DE, Pols HA, Lamberts SW. Measures of bioavailable serum testosterone and estradiol and their relationships with muscle strength, bone density, and body composition in elderly men. J Clin Endocrinol Metab 85: 3276–3282, 2000 [DOI] [PubMed] [Google Scholar]
- 545. Van Rooij E, Quiat D, Johnson BA, Sutherland LB, Qi X, Richardson JA, Kelm RJ, Jr, Olson EN. A family of microRNAs encoded by myosin genes governs myosin expression and muscle performance. Dev Cell 17: 662–673, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 546. Van Rooij E, Sutherland LB, Qi X, Richardson JA, Hill J, Olson EN. Control of stress-dependent cardiac growth and gene expression by a microRNA. Science 316: 575–579, 2007 [DOI] [PubMed] [Google Scholar]
- 547. Vandenburgh HH, Karlisch P, Shansky J, Feldstein R. Insulin and IGF-I induce pronounced hypertrophy of skeletal myofibers in tissue culture. Am J Physiol Cell Physiol 260: C475–C484, 1991 [DOI] [PubMed] [Google Scholar]
- 548. Velleman SG, Li X, Coy CS, McFarland DC. The effect of fibroblast growth factor 2 on the in vitro expression of syndecan-4 and glypican-1 in turkey satellite cells. Poult Sci 87: 1834–1840, 2008 [DOI] [PubMed] [Google Scholar]
- 549. Vertino AM, Taylor-Jones JM, Longo KA, Bearden ED, Lane TF, McGehee RE, Jr, MacDougald OA, Peterson CA. Wnt10b deficiency promotes coexpression of myogenic and adipogenic programs in myoblasts. Mol Biol Cell 16: 2039–2048, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 550. Viguie CA, Lu DX, Huang SK, Rengen H, Carlson BM. Quantitative study of the effects of long-term denervation on the extensor digitorum longus muscle of the rat. Anat Rec 248: 346–354, 1997 [DOI] [PubMed] [Google Scholar]
- 551. Villena J, Brandan E. Dermatan sulfate exerts an enhanced growth factor response on skeletal muscle satellite cell proliferation and migration. J Cell Physiol 198: 169–178, 2004 [DOI] [PubMed] [Google Scholar]
- 552. Volonte D, Liu Y, Galbiati F. The modulation of caveolin-1 expression controls satellite cell activation during muscle repair. FASEB J 19: 237–239, 2005 [DOI] [PubMed] [Google Scholar]
- 553. Wakelam MJ. The fusion of myoblasts. Biochem J 228: 1–12, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 554. Walker BE. An investigation of skeletal muscle regeneration with radioautography. Anat Rec 350, 1960 [Google Scholar]
- 555. Walsh K. Coordinate regulation of cell cycle and apoptosis during myogenesis. Prog Cell Cycle Res 3: 53–58, 1997 [DOI] [PubMed] [Google Scholar]
- 556. Warren GL, Hulderman T, Jensen N, McKinstry M, Mishra M, Luster MI, Simeonova PP. Physiological role of tumor necrosis factor alpha in traumatic muscle injury. FASEB J 16: 1630–1632, 2002 [DOI] [PubMed] [Google Scholar]
- 557. Watt DJ, Morgan JE, Clifford MA, Partridge TA. The movement of muscle precursor cells between adjacent regenerating muscles in the mouse. Anat Embryol 175: 527–536, 1987 [DOI] [PubMed] [Google Scholar]
- 558. Wehrman TS, von Degenfeld G, Krutzik PO, Nolan GP, Blau HM. Luminescent imaging of beta-galactosidase activity in living subjects using sequential reporter-enzyme luminescence. Nat Methods 3: 295–301, 2006 [DOI] [PubMed] [Google Scholar]
- 559. Weinberg RA. The retinoblastoma protein and cell cycle control. Cell 81: 323–330, 1995 [DOI] [PubMed] [Google Scholar]
- 560. Wen Y, Bi P, Liu W, Asakura A, Keller C, Kuang S. Constitutive notch activation upregulates pax7 and promotes the self-renewal of skeletal muscle satellite cells. Mol Cell Biol 32: 2300–2311, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 561. Weyman CM, Wolfman A. Mitogen-activated protein kinase kinase (MEK) activity is required for inhibition of skeletal muscle differentiation by insulin-like growth factor 1 or fibroblast growth factor 2. Endocrinology 139: 1794–1800, 1998 [DOI] [PubMed] [Google Scholar]
- 562. Whalen RG, Harris JB, Butler-Browne GS, Sesodia S. Expression of myosin isoforms during notexin-induced regeneration of rat soleus muscles. Dev Biol 141: 24–40, 1990 [DOI] [PubMed] [Google Scholar]
- 563. Wheeler EF, Bothwell M. Spatiotemporal patterns of expression of NGF and the low-affinity NGF receptor in rat embryos suggest functional roles in tissue morphogenesis and myogenesis. J Neurosci 12: 930–945, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 564. White JD, Scaffidi A, Davies M, McGeachie J, Rudnicki MA, Grounds MD. Myotube formation is delayed but not prevented in MyoD-deficient skeletal muscle: studies in regenerating whole muscle grafts of adult mice. J Histochem Cytochem 48: 1531–1544, 2000 [DOI] [PubMed] [Google Scholar]
- 565. Williams AH, Liu N, van Rooij E, Olson EN. MicroRNA control of muscle development and disease. Curr Opin Cell Biol 21: 461–469, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 566. Wilson A, Trumpp A. Bone-marrow haematopoietic-stem-cell niches. Nat Rev Immunol 6: 93–106, 2006 [DOI] [PubMed] [Google Scholar]
- 567. Wokke JH, Van den Oord CJ, Leppink GJ, Jennekens FG. Perisynaptic satellite cells in human external intercostal muscle: a quantitative and qualitative study. Anat Rec 223: 174–180, 1989 [DOI] [PubMed] [Google Scholar]
- 568. Woodley DT, Rao CN, Hassell JR, Liotta LA, Martin GR, Kleinman HK. Interactions of basement membrane components. Biochim Biophys Acta 761: 278–283, 1983 [DOI] [PubMed] [Google Scholar]
- 569. Wozniak AC, Anderson JE. Nitric oxide-dependence of satellite stem cell activation and quiescence on normal skeletal muscle fibers. Dev Dyn 236: 240–250, 2007 [DOI] [PubMed] [Google Scholar]
- 570. Wu Z, Luby-Phelps K, Bugde A, Molyneux LA, Denard B, Li WH, Suel GM, Garbers DL. Capacity for stochastic self-renewal and differentiation in mammalian spermatogonial stem cells. J Cell Biol 187: 513–524, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 571. Wu Z, Woodring PJ, Bhakta KS, Tamura K, Wen F, Feramisco JR, Karin M, Wang JY, Puri PL. p38 and extracellular signal-regulated kinases regulate the myogenic program at multiple steps. Mol Cell Biol 20: 3951–3964, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 572. Yablonka-Reuveni Z, Rivera AJ. Temporal expression of regulatory and structural muscle proteins during myogenesis of satellite cells on isolated adult rat fibers. Dev Biol 164: 588–603, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 573. Yablonka-Reuveni Z, Rudnicki MA, Rivera AJ, Primig M, Anderson JE, Natanson P. The transition from proliferation to differentiation is delayed in satellite cells from mice lacking MyoD. Dev Biol 210: 440–455, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 574. Yaffe D. Cellular aspects of muscle differentiation in vitro. Curr Top Dev Biol 4: 37–77, 1969 [DOI] [PubMed] [Google Scholar]
- 575. Yamada M, Sankoda Y, Tatsumi R, Mizunoya W, Ikeuchi Y, Sunagawa K, Allen RE. Matrix metalloproteinase-2 mediates stretch-induced activation of skeletal muscle satellite cells in a nitric oxide-dependent manner. Int J Biochem Cell Biol 40: 2183–2191, 2008 [DOI] [PubMed] [Google Scholar]
- 576. Yamada M, Tatsumi R, Kikuiri T, Okamoto S, Nonoshita S, Mizunoya W, Ikeuchi Y, Shimokawa H, Sunagawa K, Allen RE. Matrix metalloproteinases are involved in mechanical stretch-induced activation of skeletal muscle satellite cells. Muscle Nerve 34: 313–319, 2006 [DOI] [PubMed] [Google Scholar]
- 577. Yan D, Dong Xda E, Chen X, Wang L, Lu C, Wang J, Qu J, Tu L. MicroRNA-1/206 targets c-Met and inhibits rhabdomyosarcoma development. J Biol Chem 284: 29596–29604, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 578. Yang SY, Goldspink G. Different roles of the IGF-I Ec peptide (MGF) and mature IGF-I in myoblast proliferation and differentiation. FEBS Lett 522: 156–160, 2002 [DOI] [PubMed] [Google Scholar]
- 579. Yeow K, Phillips B, Dani C, Cabane C, Amri EZ, Derijard B. Inhibition of myogenesis enables adipogenic trans-differentiation in the C2C12 myogenic cell line. FEBS Lett 506: 157–162, 2001 [DOI] [PubMed] [Google Scholar]
- 580. Yildiz O. Vascular smooth muscle and endothelial functions in aging. Ann NY Acad Sci 1100: 353–360, 2007 [DOI] [PubMed] [Google Scholar]
- 581. Yoshimoto M, Chang H, Shiota M, Kobayashi H, Umeda K, Kawakami A, Heike T, Nakahata T. Two different roles of purified CD45+c-Kit+Sca-1+Lin− cells after transplantation in muscles. Stem Cells 23: 610–618, 2005 [DOI] [PubMed] [Google Scholar]
- 582. Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA. Induced pluripotent stem cell lines derived from human somatic cells. Science 318: 1917–1920, 2007 [DOI] [PubMed] [Google Scholar]
- 583. Yu Y, Ge N, Xie M, Sun W, Burlingame S, Pass AK, Nuchtern JG, Zhang D, Fu S, Schneider MD, Fan J, Yang J. Phosphorylation of Thr-178 and Thr-184 in the TAK1 T-loop is required for interleukin (IL)-1-mediated optimal NFkappaB and AP-1 activation as well as IL-6 gene expression. J Biol Chem 283: 24497–24505, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 584. Zammit PS, Golding JP, Nagata Y, Hudon V, Partridge TA, Beauchamp JR. Muscle satellite cells adopt divergent fates: a mechanism for self-renewal? J Cell Biol 166: 347–357, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 585. Zammit PS, Heslop L, Hudon V, Rosenblatt JD, Tajbakhsh S, Buckingham ME, Beauchamp JR, Partridge TA. Kinetics of myoblast proliferation show that resident satellite cells are competent to fully regenerate skeletal muscle fibers. Exp Cell Res 281: 39–49, 2002 [DOI] [PubMed] [Google Scholar]
- 586. Zarnegar R, Michalopoulos GK. The many faces of hepatocyte growth factor: from hepatopoiesis to hematopoiesis. J Cell Biol 129: 1177–1180, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 587. Zhang JM, Wei Q, Zhao X, Paterson BM. Coupling of the cell cycle and myogenesis through the cyclin D1-dependent interaction of MyoD with cdk4. EMBO J 18: 926–933, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 588. Zhang P, Wong C, Liu D, Finegold M, Harper JW, Elledge SJ. p21(CIP1) and p57(KIP2) control muscle differentiation at the myogenin step. Genes Dev 13: 213–224, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 589. Zhao M, New L, Kravchenko VV, Kato Y, Gram H, di Padova F, Olson EN, Ulevitch RJ, Han J. Regulation of the MEF2 family of transcription factors by p38. Mol Cell Biol 19: 21–30, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 590. Zhong W, Chia W. Neurogenesis and asymmetric cell division. Curr Opin Neurobiol 18: 4–11, 2008 [DOI] [PubMed] [Google Scholar]


