Abstract
Intermittent hypoxia (IH) is most often thought of for its role in morbidity associated with sleep-disordered breathing, including central nervous system pathology. However, recent evidence suggests that the nervous system fights back in an attempt to minimize pathology by increasing the expression of growth/trophic factors that confer neuroprotection and neuroplasticity. For example, even modest (“low dose”) IH elicits respiratory motor plasticity, increasing the strength of respiratory contractions and breathing. These low IH doses upregulate hypoxia-sensitive growth/trophic factors within respiratory motoneurons but do not elicit detectable pathologies such as hippocampal cell death, neuroinflammation, or systemic hypertension. Recent advances have been made toward understanding cellular mechanisms giving rise to IH-induced respiratory plasticity, and attempts have been made to harness the benefits of low-dose IH to treat respiratory insufficiency after cervical spinal injury. Our recent realization that IH also upregulates growth/trophic factors in nonrespiratory motoneurons and improves limb (or leg) function after incomplete chronic spinal injuries suggests that IH-induced plasticity is a general feature of motor systems. Collectively, available evidence suggests that low-dose IH may represent a safe and effective treatment to restore lost motor function in diverse clinical disorders that impair motor function.
Intermittent hypoxia (IH) has been the focus of considerable research in recent years, increasing awareness of its biological and clinical significance (2,470 “hits” for intermittent hypoxia; PubMed, June 2013). A major reason for this interest is that IH contributes to the pathology of serious medical conditions, such as sleep-disordered breathing (7, 66, 67). On the other hand, IH is sometimes used to enhance athletic performance (21, 59, 121, 127). Thus the effects of IH can be described as both “good” and “bad.” What factors distinguish whether IH elicits pathology vs. physiological enhancement? At least one major factor distinguishing IH and its impact in these disparate contexts relates to the hypoxic “dose”; IH protocols described in the literature vary considerably in the severity and duration of hypoxic episodes, the interepisode intervals, and the cumulative exposure time.
With obstructive sleep apnea (OSA), IH is characterized by brief (10 to >100 s) but frequent hypoxic episodes (5 to >100 per hour) for 8–12 h/day (8). This pattern of IH can continue for years in individuals with OSA and triggers multiple pathologies (see below). In contrast, protocols that enhance athletic performance consist of a single exposure per day that lasts for more than 1 h; these protocols are thought to improve aerobic performance by inducing hypoxia-sensitive genes that regulate oxygen delivery to the tissues. Thus the balance of pathology vs. functional benefits is closely related to details of the IH exposure, essentially an “IH dose” (FIGURE 1). Although many IH protocols are reported in the literature, the specific “dose” of IH exposure of most benefit is unknown and may depend on the pathophysiology and/or training regime involved.
FIGURE 1.
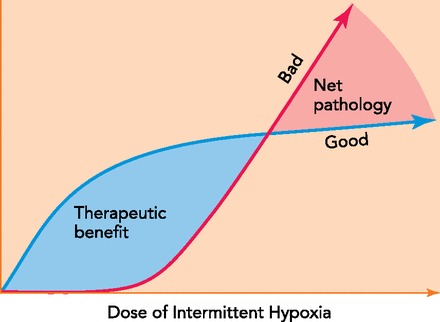
Varied exposures represent a dose of IH ranging from a few minutes per day to many hours per day over days to weeks
Although high-dose IH still elicits functional benefits (35), it shifts the balance from net benefit to unacceptable pathology. Conceptually, nearly all IH doses elicit beneficial (“good”) effects, including neuroprotection and the induction of respiratory and somatic motor plasticity. These low-dose IH exposures do not elicit detectable pathology (“bad”), such as hypertension (128), hippocampal apoptosis, or reactive gliosis (68). Although high-dose IH still elicits functional benefits (35, 63a), it shifts the balance from net benefit to unacceptable pathology. Finding an optimal IH dose is key to developing effective therapies for clinical disorders that impair motor function, such as spinal injury or ALS.
Two unexpected and only recently recognized benefits of IH are 1) improved respiratory and nonrespiratory somatic motor function, and 2) increased growth/trophic factor expression in the central nervous system (CNS). Even modest, low-dose IH protocols improve motor function and increase CNS expression of multiple growth/trophic factors, including brain-derived neurotrophic factor (BDNF), vascular endothelial growth factor (VEGF), and erythropoetin (EPO), albeit through distinct cellular mechanisms. BDNF is likely upregulated via chemoafferent activation, triggering serotonin release and initiating cell signaling cascades that increase BDNF synthesis; VEGF and EPO, in contrast, are HIF-regulated proteins. Each of these growth factors is expressed in motoneurons, and each confers neuroprotection and neuroplasticity. Here, we make the argument that at least some IH-induced motor plasticity results directly from an upregulation of these hypoxia-sensitive growth/trophic factors.
The focus of this brief review concerns the relatively unexplored, beneficial effects of IH in the CNS, with emphasis on recent discoveries concerning IH-induced motor plasticity. In specific, we will review 1) the hazards of high-dose IH; 2) the benefits of low-dose IH (i.e., neuroprotection and plasticity); 3) what is known concerning the roles of growth/trophic factors in mechanisms of IH-induced motor plasticity; and 4) the potential to harness low-dose IH as a therapeutic tool to treat motor deficits in devastating clinical disorders as diverse as spinal injury and ALS.
High-Dose IH
Although IH experienced by humans with OSA often occurs over years, most animal models involve exposure to chronic intermittent hypoxia (CIH) for more limited durations (4 days to 4 wk; Refs. 33, 41). For technical reasons relating to the speed at which oxygen can be exchanged in a chamber large enough to hold rodents, the frequency of hypoxic episodes during CIH is usually limited to ∼10–20/h, which most closely simulates the IH of only mild OSA. Most CIH protocols published in the literature also utilize relatively severe levels of hypoxemia within hypoxic episodes, reaching arterial saturations well below 85%. In contrast, OSA patients only occasionally reach similar desaturation levels.
Although OSA-induced pathology (7, 40, 66, 67) develops over years, at least some pathology can be reproduced in rats exposed to CIH for <1 mo, including systemic hypertension (32, 33, 66, 87, 94), impaired baroreflexes (45), and synaptic transmission in the nucleus of the solitary tract (58), metabolic syndrome (117), hippocampal cell death (41), and cognitive deficits (17, 44, 99).
CNS pathophysiology induced by CIH most likely arises from neuro-inflammation (41). Although the specific CNS cell types giving rise to these IH-induced toxic, neuro-inflammatory effects have not been conclusively demonstrated, microglia (i.e., the CNS resident immune cells) most likely play a dominant role (FIGURE 2; Refs. 42, 61). Microglia in the healthy CNS exhibit a surveillance phenotype that synthesizes and releases neuroprotective growth/trophic factors. On the other hand, “high-dose” IH (i.e., CIH) may activate microglia toward a toxic, pro-inflammatory phenotype that triggers pathology, including hippocampal apoptosis, impaired synaptic plasticity, and cognitive impairment (41). Acute CNS inflammation also undermines the capacity for spinal respiratory neuroplasticity (51, 52, 120).
FIGURE 2.
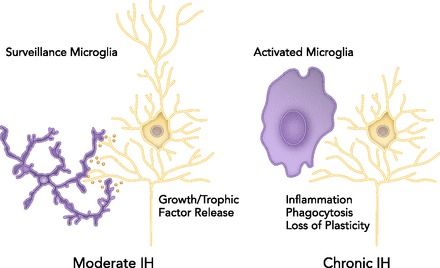
In the healthy CNS with no, or “low-dose” IH, microglia are in a “surveillance mode” that promotes neuron viability and function by releasing growth/trophic factors that confer neuro-protection and/or increase synaptic strength (i.e., plasticity)
In contrast, high doses of IH, such as chronic IH, may activate microglia to a toxic, pro-inflammatory phenotype that triggers neuronal apoptosis and undermines synaptic plasticity.
Despite ongoing CNS pathology, CIH also elicits unique forms of CNS neuroplasticity and metaplasticity (35, 63a, 85). Although the functional significance of CIH-induced plasticity to OSA remains unclear (70, 79), we now know that similar plasticity can be elicited by even modest (i.e., low dose) IH without apparent pathology (Refs. 68, 128; FIGURE 3).
FIGURE 3.
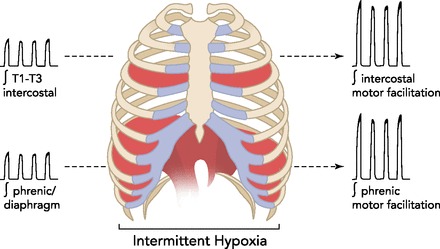
Low-dose IH elicits spinal respiratory motor plasticity
For example, a single presentation of acute intermittent hypoxia (AIH: 3–10 episodes, 5-min duration, 5-min intervals) elicits phrenic (and diaphragm), long-term facilitation (3, 118). Similar, but greater relative effects are observed in the inspiratory intercostal nerves/muscles (34a). These forms of respiratory motor plasticity can be harnessed to recover lost breathing capacity by exposing rats with cervical spinal injuries to daily AIH (7 days; Ref. 68). Similar motor plasticity is also observed in limb function (68, 119), demonstrating that IH elicits plasticity in diverse motor systems. Understanding mechanisms giving rise to respiratory motor plasticity may guide development of novel therapies to treat motor impairment in diverse clinical disorders, including spinal cord injury and ALS.
Low-Dose IH: The Good Without the Bad?
Early exploration of potential benefits from modest IH occurred in the former Soviet Union; IH was used to treat clinical disorders ranging from psychiatric depression to hypertension (for review, see Ref. 106). More recently, we have come to realize that modest IH enhances respiratory and nonrespiratory motor systems (27, 28, 68, 83, 84, 119, 120), including even single presentations of acute intermittent hypoxia (AIH; 3 to 15 total episodes; 5 min in duration with 5 min intervals) in adults (3, 118, 119) and neonates (80), as well as repetitive AIH (rAIH), consisting of repeated AIH presentations over days to weeks (27, 83, 120). Recently described rAIH protocols consist of daily AIH (dAIH: 10 episodes for 7 days; Refs. 68, 128) or repeated AIH (10 episodes) three days per wk for 4 to 10 wk (101). These protocols were developed with the idea that we can harness IH therapeutically by choosing a low dose where benefits outweigh pathology.
Our explorations of cellular/synaptic mechanisms giving rise to rAIH-induced motor plasticity reveal a common theme: the plasticity requires hypoxia-inducible growth/trophic factors also known to elicit CNS neuroprotection (27, 38, 85, 114). In this brief review, we will now focus on what is known concerning the roles of three hypoxia-sensitive growth/trophic factors in IH-induced motor plasticity: brain-derived neurotrophic factor (BDNF), vascular endothelial growth factor (VEGF), and erythropoietin (EPO).
BDNF
BDNF is a neurotrophin first known for its ability to confer neuroprotection in developing and adult animals (5, 54, 74, 75, 116, 122). Of particular note, BDNF is neuroprotective in spinal motoneurons (6, 13, 90), enhances diaphragm neuromuscular junctions (136, 73), and restores rhythmic phrenic activity below a cervical spinal hemisection (109). In other regions of the CNS, BDNF plays key roles in multiple forms of activity-dependent synaptic plasticity and learning and memory (12, 14, 64, 72, 102).
BDNF signals predominantly through its high-affinity receptor tyrosine kinase, tropomyosin-regulated kinase B (TrkB), as well as through a low-affinity receptor, p75 (2, 48, 96). Downstream TrkB signaling cascades include ERK/MAP kinases, PI3Kinase/Akt, Src, and PLCγ (2, 48, 96).
BDNF and Intermittent Hypoxia
BDNF expression is regulated by oxygen in whole brain (63), brain microvascular endothelial cells (123), hippocampus (37, 139), and the upper cervical spinal cord (4, 128). TrkB is regulated by hypoxia-inducible factor-1 (HIF-1), a key transcription factor regulating hypoxia-sensitive genes (76). Thus TrkB expression is upregulated in whole brain following hypoxia/ischemia (88).
The impact of prolonged IH on BDNF expression in the CNS is somewhat unclear since CIH (8 h/day; 14 days) has been reported to profoundly decrease hippocampal BDNF levels, an observation suggested to account for associated deficits in cognitive function and hippocampal synaptic plasticity (129). However, IH effects on hippocampal BDNF may be critically dependent on the duration of IH, since shorter exposures (4 h/day; 14 days) promote BDNF-dependent hippocampal neurogenesis and antidepressant effects (139).
Repetitive AIH (dAIH; 3 times/wk for 4 or 10 wk) increases BDNF expression in respiratory (68, 101) and nonrespiratory motoneurons (Satriotomo I, Dale EA, Mitchell GS, unpublished observations), demonstrating the potential to play key roles in motoneuron survival and plasticity. In association, rAIH upregulates TrkB and major downstream signaling molecules (ERK and Akt) in respiratory and nonrespiratory spinal motoneurons (Refs. 68, 101; Satriotomo I, Dale EA, Mitchell GS, unpublished observations).
BDNF and Respiratory Plasticity
AIH elicits long-lasting respiratory motor plasticity (FIGURE 3), including long-term facilitation of phrenic (pLTF; Refs. 3, 4a, 89) and diaphragm activity (118). Similar long-term facilitation is observed in inspiratory intercostal nerve (34a) and muscle activity (Navarette Opazo A, Mitchell GS, unpublished observations). Distinct cellular pathways give rise to phenotypically similar long-lasting phrenic motor facilitation (pMF), and several of these require BDNF synthesis and/or TrkB receptor activation (Ref. 27; FIGURE 4).
FIGURE 4.
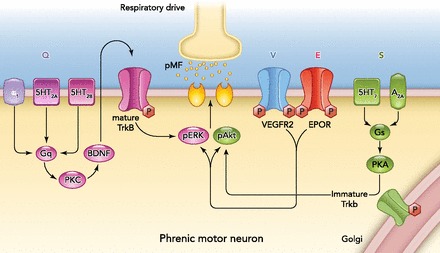
Working model of convergent pathways to long-lasting phrenic motor facilitation (pMF)
The “Q” pathway (left; purple) is elicited by intermittent activation of Gq-coupled metabotropic receptors (e.g., 5-HT2 or α1), followed by PKC activation, new BDNF synthesis, TrkB activation, and activation of ERK MAP kinases (pERK; Ref. 27). The mechanism whereby pERK elicits pMF remains unknown but may involve changes in respiratory motoneuron excitability and/or synaptic strength. The “S” pathway (right; green) is elicited by Gs-coupled metabotropic receptors (e.g., 5-HT7 and A2A), PKA activation, new synthesis of an immature TrkB isoform, and downstream signaling via Akt phosphorylation/activation (pAkt; Ref. 38). The mechanism whereby pAkt elicits pMF remains unknown but may involve changes in respiratory motoneuron excitability and/or synaptic strength. Although important details distinguish them, the BDNF/TrkB system plays a critical role in both the Q and S pathways to pMF. Other hypoxia-sensitive growth/trophic factors elicit pMF via ERK- and Akt-dependent mechanisms, including VEGF (V pathway) and EPO (E pathway). The biological significance of diverse cellular cascades to pMF remains unclear, but they may impart adaptability as an animal copes with diverse stimuli that differ in (for example) severity, pattern, or cumulative duration (89). Regardless, the existence of so many hypoxia-induced pathways gives many options as we attempt to devise repetitive AIH protocols for therapeutic benefit.
Following a single presentation of moderate AIH, pLTF requires serotonin-dependent BDNF synthesis followed by TrkB (4) and ERK MAP kinase activation (49a). Indeed, cervical spinal TrkB receptor activation is sufficient to elicit pMF without hypoxia (4). This cellular mechanism is referred to as the “Q pathway” to pMF, since multiple metabotropic Gq protein-coupled receptors elicit pMF by a similar BDNF synthesis-dependent mechanism; receptors known to elicit similar pMF include 5HT2A and 2B (69) as well as α1-adrenergic receptors (27).
A distinct cellular mechanism gives rise to pLTF following AIH, consisting of severe hypoxic episodes (89), or cervical spinal activation of adenosine 2A (38) or serotonin type 7 receptors (49). This alternate pathway to pMF is independent of new BDNF synthesis but requires new synthesis of an immature TrkB isoform that appears to autophosphorylate and signal from within the cell (38, 49). This cellular mechanism has been termed the “S pathway” to pMF since multiple Gs protein-coupled metabotropic receptors elicit pMF via this cellular cascade [5HT7 (49); A2A (38)]. Thus the BDNF/TrkB system plays critical roles in multiple forms of AIH-induced respiratory motor plasticity.
VEGF
VEGF was originally recognized for its roles in angiogenesis (24) and regulation of cell permeability (105), and is now known to play important roles in promoting neuronal survival and plasticity. Although several VEGF isoforms and receptor subtypes exist (98), VEGFA-165 and its primary receptor VEGFR-2 are most frequently studied. VEGFR-2 is a receptor tyrosine kinase that signals via ERK and Akt activation, similar to TrkB (34, 135). VEGF regulates hippocampal neurogenesis (31, 62) and long-term memory formation (19, 91). Of particular importance to this review, VEGF is expressed in spinal motoneurons (46), where it is neuroprotective and hypoxia-regulated (39, 115, 135).
VEGF and IH
VEGF expression is regulated by hypoxia-inducible factor 1 (HIF-1; Refs. 104, 124). Increased HIF-1α levels increase VEGF expression, and this upregulation is more robust with intermittent vs. sustained hypoxia (132, 133). With IH, robust HIF-1α stabilization occurs via novel NADPH oxidase, mTOR, and PKC-dependent mechanisms (132, 133). Repetitive AIH upregulates VEGF and VEGF-R2 in phrenic (101) and nonrespiratory spinal motoneurons, as well as the motor cortex (Satriotomo I, Dale EA, Mitchell GS, unpublished observations). These neurochemical data suggest the potential for VEGF to play important roles in motoneuron plasticity. One likely possibility is that VEGF enhances motor output over longer time domains relative to BDNF. If true, such effects may contribute to respiratory plasticity or meta-plasticity after CIH (63a) or chronic sustained hypoxia (93).
VEGF and the Neural Control of Breathing
The role of VEGF in ventilatory control has only recently been explored. The peripheral, carotid body chemoreceptors undergo profound structural plasticity after chronic sustained hypoxia, an effect attributed, at least in part, to VEGF (23). VEGF and VEGFR-2 are both expressed in phrenic motoneurons, where they elicit long-lasting ERK and Akt-dependent pMF (Ref. 29; FIGURE 4). Although rAIH upregulates VEGF and VEGFR-2 in phrenic motoneurons (101), there is no evidence that this effect enhances VEGF-induced pMF (25). Important questions remain concerning the (multiple) roles of VEGF in respiratory plasticity, particularly with prolonged IH.
EPO
EPO was originally described as a hematopoietic factor (9, 10, 56). However, EPO and its receptor (EPO-R) are also expressed in the mammalian CNS (11, 16, 30, 77, 78), where it is neuroprotective for hippocampal (110, 130) and spinal motoneurons (22, 53, 81, 86). EPO-induced neuroprotection is ERK- and Akt-dependent (57, 108, 138), similar to BDNF/TrkB and VEGF. EPO also regulates hippocampal synaptic plasticity (1, 126).
EPO and IH
EPO is regulated by HIF-1 (124, 125), and possibly HIF-2 (131). EPO exerts beneficial effects on exercise training (21, 59, 121, 127), and likely contributes to the efficacy of “live-high, train-low” theory where athletes live in higher altitudes to increase vascularization and red blood cell production but train (and compete) at lower altitudes where greater maximal oxygen consumption is possible. By increasing EPO at altitude, increased blood hemoglobin (and oxygen) concentrations increase aerobic scope and the ability to train with intensity at lower elevations. This theory does not address the question of whether there are other benefits to hypoxic training associated with the CNS. IH-induced EPO production exerts psychiatric antidepressant effects in rats and humans (36, 82), protects against ischemia-reperfusion injury (18), and improves learning and memory (1, 100, 107, 137). Collectively, the limited available evidence is consistent with a prominent role for EPO in various forms of plasticity, including respiratory and nonrespiratory motor plasticity. This is an area of research that warrants further investigation.
EPO and the Neural Control Of Breathing
EPO regulates breathing, acting via the peripheral chemoreceptors, brain stem respiratory neurons, and respiratory motoneurons. EPO overexpression in the CNS exaggerates ventilatory responses to severe hypoxia (111). After carotid denervation, these EPO overexpressing mice maintain breathing during severe hypoxia, whereas wild-type littermates experience life-threatening apneas. Downregulation of the soluble EPO receptor is necessary for ventilatory acclimatization to sustained hypoxia, indicating that EPO plays a role in this long-studied form of respiratory plasticity (113). EPO and EPO-R are found in multiple CNS regions of interest to respiratory control, including the pre-Bötzinger complex (111), the nucleus tractus solitarius (111), and phrenic motoneurons (26). Consequently, cervical spinal EPO injections elicit long-lasting pMF via ERK- and Akt-dependent mechanisms, similar to VEGF (Ref. 26; FIGURE 4). Thus EPO potentially plays important roles in multiple aspects of ventilatory control, including respiratory rhythm generation, chemo-afferent integration, and rAIH-induced respiratory motor plasticity (26, 112).
Significance
Biological Significance
Although there is clear evidence that prolonged IH elicits some pathology, the CNS appears to have considerable capacity to “fight back,” effectively minimizing functional impairment. Thus the brain adapts to sublethal stressors such as hypoxia, ischemia, and excitatory toxicity via preconditioning (“that which does not kill you, makes you stronger”). In particular, the ability to upregulate growth and trophic factors that confer neuroprotection following “low-dose” IH represents a form of preconditioning that would protect against future (or ongoing) hypoxic insults. The ability of these same growth factors to elicit motor plasticity is more difficult to explain, particularly given similar effects on both respiratory and nonrespiratory motor systems. Enhanced respiratory motor function after IH will preserve oxygenation in the event of future hypoxic events and may even prevent future hypoxic episodes by stabilizing breathing (e.g., preserving upper airway patency). The purpose of nonrespiratory somatic motor plasticity is less clear. An interesting possibility is that IH-induced motor plasticity is a phylogenetically ancient response to fluctuating oxygen levels experienced by aquatic vertebrates. For example, fish living in environments where seasonal ambient hypoxia is common frequently respond to low oxygen by swimming in search of water with higher oxygen levels (15, 55, 60, 92, 103). Indeed, many fish live in seasonally hypoxic water and have developed the capacity for air breathing to exploit the oxygen-rich environment above (i.e., facultative air breathing; for review, see Refs. 43, 97). In this case, swimming (locomotion) and breathing are linked, and are both associated with intermittent hypoxia experienced during the interbreath intervals. Facultative air breathing is quite common in fish and has developed in many genera (20, 43, 97). We speculate that the intermittent hypoxia increases growth/trophic factor expression, amplifying the capacity for linked motor behaviors: breathing and swimming. In terrestrial vertebrates, this same capacity may have been preserved, reflected as similar IH-induced motor plasticity in respiratory and nonrespiratory motor systems (68).
Our recent realization that multiple, distinct signaling pathways give rise to phenotypically similar phrenic motor facilitation (27) leaves us with a question: Why do so many (seemingly redundant) pathways to motor plasticity exist? These diverse pathways may 1) enable animals a range of responses in the face of stimuli that differ in severity and/or duration [e.g., severe vs. moderate acute intermittent hypoxia (89); acute vs. chronic intermittent or sustained hypoxia]; 2) enable alternative mechanisms of achieving motor facilitation when primary pathways are not functional [e.g., with inflammation or during acute spinal cord injury (52, 120)]; and/or 3) confer the capacity to express emergent properties, such as pattern sensitivity and/or metaplasticity (29a). Studies to understand these basic questions and to explore the potential translation to relevant therapeutics in cases of respiratory (and nonrespiratory) motor deficits are ongoing.
Clinical Significance
The impact of low-dose IH on respiratory and nonrespiratory somatic motor systems has considerable potential as a therapeutic approach to restore motor function in severe clinical disorders (83). This concept has been developed most extensively following chronic spinal cord injury (FIGURE 5). In particular, we now know that modest dAIH has remarkable capacity to restore lost breathing capacity and forelimb function in rats with chronic cervical spinal injuries (68). Of considerable importance, dAIH elicits phenotypic plasticity in spinal motoneurons (see below) and improved motor function without hypertension (128) or hippocampal pathology, such as reactive gliosis or neuronal cell death (68). This approach has been extended to humans with chronic, incomplete spinal injuries (>1 yr postinjury; ASIA C and D). In these individuals, even a single presentation of AIH (15 bouts) increases ankle strength for at least 4 h (119). In preliminary studies, dAIH appears to improve over-ground walking (47).
FIGURE 5.
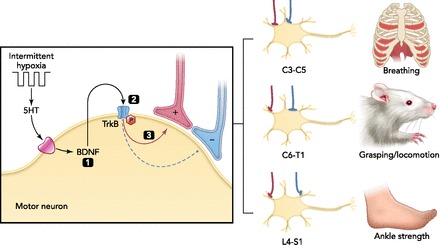
Hypothetical mechanism of motor plasticity following rAIH in rats and humans with chronic spinal injuries
Based on our increasing understanding of cellular mechanisms giving rise to AIH-induced phrenic motor facilitation (FIGURE 4) and daily AIH (7 days)-induced changes in key molecules within respiratory and nonrespiratory motor nuclei (68), we propose that a common mechanism underlies plasticity in both respiratory and nonrespiratory motoneurons. By enhancing synaptic inputs to motoneurons, rAIH amplifies whatever the relevant behavior is for that specific motor pool, including breathing (68), forelimb function during ladder walking (68), or ankle strength (119). In specific, we propose that rAIH elicits intermittent serotonin (5-HT) release within the respective motor nuclei, activating postsynaptic serotonin receptors and initiating new BDNF synthesis (4). BDNF-dependent activation of its high-affinity receptor tyrosine kinase TrkB subsequently strengthens spared synaptic pathways to motoneurons, improving motor function after spinal injury. Since the combination of SCI and rAIH increases TrkB phosphorylation and activation more than either stimulus alone (68), rAIH may be particularly effective after chronic spinal injury. Thus rAIH is safe, easy to administer, triggers spinal plasticity, and may be an effective therapeutic approach to enhance motor function in persons with chronic spinal injuries.
In rats with cervical spinal injuries, dAIH-induced recovery of lost respiratory and limb function is associated with an upregulation of BDNF and TrkB in the relevant cervical spinal motor nuclei, suggesting that the BDNF/TrkB plays a critical role in the mechanism of recovery (68). However, a direct, causal relationship between BDNF/TrkB upregulation and functional recovery was not explored in this study, nor were changes in VEGF or EPO. The specific roles of BDNF, VEGF, and EPO in rAIH-induced recovery of breathing and limb function after cervical spinal injury is a promising area for future research.
rAIH holds promise in the treatment of diverse clinical disorders that cause respiratory and nonrespiratory motor impairment. For example, AIH (at least temporarily) restores lost phrenic motor output at disease end-stage in a rodent model of ALS (89). Other disorders with associated motor deficits (e.g., stroke, cerebral palsy, PD, MS) may also benefit from rAIH, but these possibilities have not yet been explored. Collectively, currently available evidence strongly suggests that modest protocols of IH have considerable potential as a safe and effective means to restore lost motor function in patients with motor impairment from diverse clinical disorders.
Future Directions
Although we focused on the potential of IH to induce motor plasticity in clinical disorders that impair respiratory and nonrespiratory motor function, the same concepts raise interesting questions such as: Does rAIH improve neuro-motor function in normal individuals? Can rAIH be used to enhance athletic motor performance (i.e., coordination etc.)? We do know that AIH elicits respiratory motor plasticity (27, 85a) and upregulates BDNF expression near the phrenic motor nucleus in normal rats (4). Furthermore, rAIH upregulates BDNF and VEGF in respiratory (101, 128) and nonrespiratory motor nuclei in normal rats (101). Thus rAIH may enhance neuro-motor function and athletic performance in normal humans.
Along similar lines, very little information is available concerning the impact of developmental stage on IH-induced motor plasticity. We do know that chronic intermittent hypoxia in development attenuates adult AIH-induced pLTF (95) and that middle-aged males (not females) lose capacity for AIH-induced pLTF (134). Thus life stage is another crucial variable to take into consideration.
Finally, is it possible that upregulation of hypoxia-sensitive growth/trophic factors in regions of the brain associated with learning and memory could enhance cognitive function? These intriguing possibilities have not been adequately explored.
Acknowledgments
We thank J. J. Watters for critiquing parts of this manuscript and R. S. Dhillon for discussions concerning air-breathing in fish.
Footnotes
This study was supported by National Institutes of Health Grants R01 HL-080209 and R37 HL-69064; and P01 NS-057778 and T32 HL-07574 (E. A. Dale).
No conflicts of interest, financial or otherwise, are declared by the author(s).
Author contributions: E.A.D. and G.S.M. conception and design of research; E.A.D. performed experiments; E.A.D. and G.S.M. analyzed data; E.A.D. and G.S.M. interpreted results of experiments; E.A.D., F.B.M., and G.S.M. prepared figures; E.A.D., F.B.M., and G.S.M. drafted manuscript; E.A.D. and G.S.M. edited and revised manuscript; E.A.D., F.B.M., and G.S.M. approved final version of manuscript.
References
- 1.Adamcio B, Sargin D, Stradomska A, Medrihan L, Gertler C, Theis F, Zhang M, Mueller M, Hassouna I, Hannke K, Sperling S, Radyushkin K, El-Kordi A, Schulze L, Ronnenberg A, Wolf F, Brose N, Rhee JS, Zhang W, Ehrenreich H. Erythropoietin enhances hippocampal long-term potentiation and memory. BMC Biol 6: 37, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atwal JK, Massie B, Miller FD, Kaplan DR. The TrkN-Shc site signals neuronal surivival and local axon growth via MEK and PI3-Kinase. Neuron 27: 265–277, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Bach KB, Mitchell GS. Hypoxia-induced facilitation of respiratory activity is serotonin dependent. Respir Physiol 104: 251–260, 1996 [DOI] [PubMed] [Google Scholar]
- 4.Baker-Herman TL, Fuller DD, Bavis RW, Zabka AG, Golder FJ, Doperalski NJ, Johnson RA, Watters JJ, Mitchell GS. BDNF is necessary and sufficient for spinal respiratory plasticity following intermittent hypoxia. Nat Neurosci 7: 48–55, 2004 [DOI] [PubMed] [Google Scholar]
- 4a.Baker-Herman TL, Mitchell GS. Phrenic long-term facilitation requires spinal serotonin receptor activation and protein synthesis. J Neurosci 22: 6239–6246, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barde YA, Edgar D, Thoenen H. Purification of a new neurotrophic factor from mammalian brain. EMBO J 1: 549–553, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baumgartner BJ, Shine HD. Targeted transduction of CNS neurons with adenoviral vectors carrying neurotrophic factor genes confers neuroprotection that exceeds the transduced population. J Neurosci 17: 6504–6511, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berger S, Lavie L. Endothelial progenitor cells in cardiovascular disease and hypoxia-potential implications to obstructive sleep apnea. Transl Res 158: 1–13, 2011 [DOI] [PubMed] [Google Scholar]
- 8.Berry RB, Budhiraja R, Gottlieb DJ, Gozal D, Iber C, Kapur VK, Marcus CL, Mehra R, Parthasarathy S, Quan SF, Redline S, Strohl KP, Davidson Ward SL, Tangredi MM. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med 8: 597–619, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bert P. La Pression Barométrique: Recherches de Physiologie Expérimentale. Paris: Masson, 1878 [Google Scholar]
- 10.Bert P. Sur la richesse en hemoglobine du sang des animaux vivant sur les hauts lieux. CR Acad Sci Paris 94: 805–807, 1882 [Google Scholar]
- 11.Bernaudin M, Marti HH, Roussel S, Divoux D, Nouvelot A, MacKenzie ET, Petit E. A potential role for erythropoietin in focal permanent cerebral ischemia in mice. J Cereb Blood Flow Metab 19: 643–651, 1999 [DOI] [PubMed] [Google Scholar]
- 12.Bliss TVP, Collingridge G. A synaptic model of memory: long-term potentiation in the hippocampus. Nature 361: 31–39, 1993 [DOI] [PubMed] [Google Scholar]
- 13.Bradley DM, Beaman FD, Moore DB, Kidd K, Heaton MB. Neurotrophic factors BDNF and GDNF protect embryonic chick spinal cord motoneurons from ethanol neurotoxicity in vivo. Brain Res Dev Brain Res 112: 99–106, 1999 [DOI] [PubMed] [Google Scholar]
- 14.Bramham CR, Messaoudi E. BDNF function in adult synaptic plasticity: the synaptic consolidation hypothesis. Prog Neurobiol 76: 99–125, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Breitburg DL. Effects of hypoxia, and the balance between hypoxia and enrichment, on coastal fishes and fisheries. Estuaries 25: 767–781, 2002 [Google Scholar]
- 16.Brines ML, Ghezzi P, Keenan S, Agnello D, de Lanoerolle NC, Cerami C, Itri LM, Cerami A. Erythropoietin crosses the bloodbrain barrier to protect against experimental brain injury. Proc Natl Acad Sci USA 97: 10526–10531, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bucks RS, Olaithe M, Eastwood P. Neurocognitive function in obstructive sleep apnea: a meta-review. Respirology 18: 61–70, 2013 [DOI] [PubMed] [Google Scholar]
- 18.Cai Z, Manalo DJ, Wei G, Rodriguez ER, Fox-Talbot K, Lu H, Zweier JL, Semenza GL. Hearts from rodents exposed to intermittent hypoxia or erythropoietin are protected against ischemia-reperfusion injury. Circulation 108: 79–85, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Cao L, Jiao X, Zuzga DS, Liu Y, Fong DM, Young D, During MJ. VEGF links hippocampal activity with neurogenesis, learning and memory. Nat Genet 36: 827–835, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Chapman LJ, MacKenzie DJ. Behavioral responses and ecological consequences. In: ]?> Hypoxia, edited by Richards JG, Farrell AP, Brauner CJ. San Diego, CA: Academic, 2009, p. 25–77 [Google Scholar]
- 21.Chapman RF, Stray-Gundersen J, Levine BD. Individual variation in response to altitude training. J Appl Physiol 85: 1448–1456, 1998 [DOI] [PubMed] [Google Scholar]
- 22.Celik M, Gökmen N, Erbayraktar S, Akhisaroglu M, Konakc S, Ulukus C, Genc S, Genc K, Sagiroglu E, Cerami A, Brines M. Erythropoietin prevents motor neuron apoptosis and neurologic disability in experimental spinal cord ischemic injury. Proc Natl Acad Sci USA 99: 2258–2263, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen J, Dinger B, Jyung R, Stensaas L, Fidone S. Altered expression of vascular endothelial growth factor and FLK-1 receptor in chronically hypoxic rat carotid body. Adv Exp Med Biol 536: 583–591, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Connolly DT, Heuvelman DM, Nelson R, Olander JV, Eppley BL, Delfino JJ, Siegel NR, Leimgruber RM, Feder J. Tumor vascular permeability factor stimulates endothelial cell growth and angiogenesis. J Clin Invest 84: 1470–1478, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dale EA, Mitchell GS. Spinal vascular endothelial growth factor (VEGF) and erythropoietin (EPO) induced phrenic motor facilitation after repetitive acute intermittent hypoxia. Respir Physiol Neurobiol 185: 481–488, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dale EA, Satriotomo I, Mitchell GS. Cervical spinal erythropoietin induces phrenic motor facilitation via extracellular signal-regulated protein kinase and Akt signaling. J Neurosci 32: 5973–5983, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dale-Nagle EA, Hoffman MS, MacFarlane PM, Mitchell GS. Multiple pathways to long-lasting phrenic motor facilitation. Adv Exp Med Biol 669: 225–230, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dale-Nagle EA, Hoffman MS, MacFarlane PM, Satriotomo I, Lovett-Barr MR, Vinit S, Mitchell GS. Spinal plasticity following intermittent hypoxia: implications for spinal injury. Ann NY Acad Sci 1198: 252–259, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dale-Nagle EA, Satriotomo I, Mitchell GS. Spinal vascular endothelial growth factor induces phrenic motor facilitation via extracellular signal-regulated kinase and Akt signaling. J Neurosci 31: 7682–7690, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29a.Devinney MJ, Huxtable AG, Nichols NL, Mitchell GS. Hypoxia-induced phrenic long-term facilitation: emergent properties. Ann NY Acad Sci 1279: 143–153, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Digicaylioglu M, Bichet S, Marti HH, Wenger RH, Rivas LA, Bauer C, Gassmann M. Localization of specific erythropoietin binding sites in defined areas of the mouse brain. Proc Natl Acad Sci USA 92: 3717–3720, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fabel K, Fabel K, Tam B, Kaufer D, Baiker A, Simmons N, Kuo CJ, Palmer TD. VEGF is necessary for exercise-induced adult hippocampal neurogenesis. Eur J Neurosci 18: 2803–2812, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Fava C, Montagnana M, Favaloro EJ, Guidi GC, Lippi G. Obstructive sleep apnea syndrome and cardiovascular diseases. Semin Thromb Hemost 37: 280–297, 2011 [DOI] [PubMed] [Google Scholar]
- 33.Fletcher EX, Lesske J, Qian W, Miller CC, 3rd, Unger T. Repetitive, episodic hypoxia causes diurnal elevation of blood pressure in rats. Hypertension 19: 555–561, 1992 [DOI] [PubMed] [Google Scholar]
- 34.Franke TF, Kaplan DR, Cantley LC. PI3K: downstream AKTion blocks apoptosis. Cell 88: 435–437, 1997 [DOI] [PubMed] [Google Scholar]
- 34a.Fregosi RF, Mitchell GS. Long-term facilitation of inspiratory intercostal nerve activity following carotid sinus nerve stimulation in cats. J Physiol 477: 469–479, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fuller DD, Johnson SM, Olsen EB, Jr, Mitchell GS. Synaptic pathways to phrenic motoneurons are enhanced by chronic intermittent hypoxia after cervical spinal injury. J Neurosci 23: 2993–3000, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Girgenti MJ, Hunsberger J, Duman CH, Sathyanesan M, Terwillinger R, Newton SS. Erythropoietin induction by electroconvulsive seizure, gene regulation, and antidepressant-like behavioral effects. Biol Psychiatry 66: 267–274, 2009 [DOI] [PubMed] [Google Scholar]
- 37.Golan MH, Mane R, Molczadzki G, Zuckerman M, Kaplan-Louson V, Huleihel M, Perez-Polo JR. Impaired migration signaling in the hippocampus following prenatal hypoxia. Neuropharmacology 57: 511–522, 2009 [DOI] [PubMed] [Google Scholar]
- 38.Golder FJ, Ranganathan L, Satriotomo I, Hoffman M, Lovett-Barr MR, Watters JJ, Baker-Herman TL, Mitchell GS. Spinal adenosine A2a receptor activation elicits long-lasting phrenic motor facilitation. J Neurosci 28: 2033–2042, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gora-Kupilas K, Josko J. The neuroprotective function of vascular endothelial growth factor (VEGF). Folia Neuropathol 43: 31–39, 2005 [PubMed] [Google Scholar]
- 40.Gozal D, Hakim F, Kheirandish-Gozal L. Chemoreceptors, baroreceptors, and autonomic deregulation in children with obstructive sleep apnea. Respir Physiol Neurobiol 185: 177–185, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gozal D, Daniel JM, Dohanich GP. Behavioral and anatomical correlates for chronic episodic hypoxia during sleep in the rat. J Neurosci 21: 2442–2450, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Graeber MB. Changing face of microglia. Science 330: 783–788, 2010 [DOI] [PubMed] [Google Scholar]
- 43.Graham JB. Air Breathing Fishes: Evolution, Diversity, and Adaptation. San Diego, CA: Academic, 1997 [Google Scholar]
- 44.Grogg-Damberger M, Ralls F. Cognitive dysfunction and obstructive sleep apnea: from cradle to tomb. Curr Opin Pulm Med 18: 580–587, 2012 [DOI] [PubMed] [Google Scholar]
- 45.Gu H, Lin M, Liu J, et al. Selective impairment of central mediation of baroreflex in anesthetized young adult Fischer 344 rats after chronic intermittent hypoxia. Am J Physiol Heart Circ Physiol 293: H2809–H2818, 2007 [DOI] [PubMed] [Google Scholar]
- 46.Hayashi T, Sakurai M, Abe K, Sadahiro M, Tabayashi K, Itoyama Y. Expression of angiogenic factors in rabbit spinal cord after transient ischaemia. Neuropathol Appl Neurobiol 25: 63–71, 1999 [DOI] [PubMed] [Google Scholar]
- 47.Hayes HB, Chavtal S, Ting LH, Rymer WZ, Mitchell GS, Trumbower RD. Effect of single-day acute intermittent hypoxia on overground walking speed and muscle coordination in persons with incomplete spinal cord injury. Soc Neurosci Abstract: 252.17/M16, 2012 [Google Scholar]
- 48.Hetman M, Kanning K, Cavanaugh JE, Xia Z. Neuroprotection by brain derived neurotrophic factor is mediated by extracellular signal-regulated kinase and phsophatidylinositol 3-kinase. J Biol Chem 274: 22569–22580, 1999 [DOI] [PubMed] [Google Scholar]
- 49.Hoffman MS, Mitchell GS. Spinal 5-HT7 receptor activation induces long-lasting phrenic motor facilitation. J Physiol 589: 1397–1407, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49a.Hoffman MS, Nichols NL, Macfarlane PM, Mitchell GS. Phrenic long-term facilitation after acute intermittent hypoxia requires spinal ERK activation but not TrkB synthesis. J Appl Physiol 113: 1184–1193, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hubold C, Lang UE, Gehring H, Schultes B, Schweiger U, Peters A, Hellweg R, Oltmanns KM. Increased serum brain-derived neurotrophic factor protein upon hypoxia in healthy young men. J Neural Transm 116: 1221–1225, 2009 [DOI] [PubMed] [Google Scholar]
- 51.Huxtable AG, Vinit S, Windelborn JA, Crader SM, Guenther CH, Watters JJ, Mitchell GS. Systemic inflammation impairs respiratory chemoreflexes and plasticity. Respir Physiol Neurobiol 178: 482–489, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huxtable AG, Smith SM, Vinit S, Watters JJ, Mitchell GS. Systemic LPS induces spinal inflammatory gene expression and impairs phrenic long-term facilitation following acute intermittent hypoxia. J Appl Physiol 114: 879–887, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Iwasaki Y, Ikeda K, Ichikawa Y, Igarashi O, Iwamoto K, Kinoshita M. Protective effect of interleukin-3 and erythropoietin on motor neuron death after neonatal axotomy. Neurol Res 24: 643–646, 2002 [DOI] [PubMed] [Google Scholar]
- 54.Jiang X, Zhu D, Okagaki P, Lipsky R, Wu X, Banaudha K, Mearow K, Strauss KI, Marini AM. N-methyl-d-aspartate and TrkB receptor activation in cerebellar granule cells: an in vitro model of preconditioning to stimulate intrinsic survival pathways in neurons. Ann NY Acad Sci 993: 134–145, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jones JRE. The reactions of fish to water of low oxygen concentration. J Exp Biol 29: 403–415, 1952 [Google Scholar]
- 56.Jourdanet D. Influence de la Pression de L'air sur la Vie de L'homme. Paris: Masson, 1875 [Google Scholar]
- 57.Kilic E, Kilic U, Soliz J, Bassetti CL, Gassmann M, Hermann DM. Brain derived erythropoietin protects from focal cerebral ischemia by dual activation of ERK-1/-2 and Akt pathways. FASEB J 19: 2026–2028, 2005 [DOI] [PubMed] [Google Scholar]
- 58.Kline DD, Ramirez-Navarro A, Kunze DL. Adaptive depression in synaptic transmission in the nucleus of the solitary tract after in vivo chronic intermittent hypoxia: evidence for homeostatic plasticity. J Neuroscience 27: 4663–4673, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Koistinen PO, Rusko H, Irjala Rajamaki AK, Penttinen K, Sarparanta VP, Karpakka J, Leppaluoto J. EPO, red cells, and serum tranferrin receptor in continuous and intermittent hypoxia. Med Sci Sports Exerc 32: 800–804, 2000 [DOI] [PubMed] [Google Scholar]
- 60.Kramer DL. Dissolved oxygen and fish behaviour. Environ Biol Fishes 18: 81–92, 1987 [Google Scholar]
- 61.Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci 19: 312–318, 1996 [DOI] [PubMed] [Google Scholar]
- 62.Lee E, Son H. Adult hippocampal neurogenesis and related neurotrophic factors. BMB Rep 42: 239–244, 2009 [DOI] [PubMed] [Google Scholar]
- 63.Lindvall O, Ernfors P, Bengzon J, Kokaia Z, Smith ML, Siesjoe BK, Persson H. Differential regulation of mRNAs for nerve growth factor, brain-derived neurotrophic factor, and neurotrophin 3 in the adult rat brain following cerebral ischemia and hypoglycemic coma. Proc Natl Acad Sci USA 89: 648–652, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63a.Ling L, Fuller DD, Bach KB, Kinkead R, Olson EB, Jr, Mitchell GS. Chronic intermittent hypoxia elicits serotonin-dependent plasticity in the central neural control of breathing. J Neurosci 21: 5381–5388, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lu B, Gottschalk W. Modulation of hippocampal synaptic transmission and plasticity by neurotrophins. Prog Brain Res 128: 231–241, 2000 [DOI] [PubMed] [Google Scholar]
- 65.Lu Y, Christian K, Lu B. BDNF: a key regulator for protein synthesis dependent LTP and long-term memory? Neurobiol Learn Mem 89: 312–323, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lurie A. Endothelial dysfunction in adults with obstructive sleep apnea. Adv Cardiol 46: 139–170, 2011 [DOI] [PubMed] [Google Scholar]
- 67.Lurie A. Inflammation, oxidative stress, and procoagulant and thrombotic activity in adults with obstructive sleep apnea. Adv Cardiol 46: 43–66, 2011 [DOI] [PubMed] [Google Scholar]
- 68.Lovett-Barr MR, Satriotomo I, Muir G, Wilkerson JER, Hoffman MS, Vinit S, Mitchell GS. Repetitive intermittent hypoxia induces respiratory and somatic motor recovery following chronic cervical spinal injury. J Neurosci 32: 3591–3600, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.MacFarlane PM, Mitchell GS. Episodic spinal serotonin receptor activation elicits long-lasting phrenic motor facilitation by an NADPH oxidase-dependent mechanism. J Physiol 587: 5469–5481, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mahamed S, Mitchell GS. Is there a link between intermittent hypoxia-induced respiratory plasticity and obstructive sleep apnoea? Exp Physiol 92: 27–37, 2007 [DOI] [PubMed] [Google Scholar]
- 71.Mahamed S, Mitchell GS. Respiratory long-term facilitation: too much or too little of a good thing? Adv Exp Med Biol 605: 224–227, 2008 [DOI] [PubMed] [Google Scholar]
- 72.Malenka RC, Nicoll RA. Long-term potentiation-a decade of progress? Science 285: 1870–1874, 1999 [DOI] [PubMed] [Google Scholar]
- 73.Mantilla CB, Zhan WZ, Sieck GC. Neurotrophins improve neuromuscular transmission in the adult rat diaphragm. Muscle Nerve 29: 381–386, 2004 [DOI] [PubMed] [Google Scholar]
- 74.Marini AM, Rabin SJ, Lipsky RHMocchetti. Activity dependent release of brain-derived neurotrophic factor underlies the neuroprotective effect of N-methyl-d-aspartate. J Biol Chem 273: 29394–29399, 1998 [DOI] [PubMed] [Google Scholar]
- 75.Marini AM, Jiang X, Wu X, Tian F, Zhu D, Okagaki P, Lipsky RH. Role of brain-derived neurotrophic factor and NF-kappaB in neuronal plasticity and survival: from genes to phenotype. Resto Neurol Neurosci 222: 121–130, 2004 [PubMed] [Google Scholar]
- 76.Martens LK, Kirschner KM, Warnecke C, Scholz H. Hypoxia-inducible factor-1 (HIF-1) is a transcriptional activator of the TrkB neurotrophin receptor gene. J Biol Chem 282: 14379–14388, 2007 [DOI] [PubMed] [Google Scholar]
- 77.Marti HH, Wenger RH, Rivas LA, Straumann U, Digicaylioglu M, Henn V, Yonekawa Y, Bauer C, Gassmann M. Erythropoietin gene expression in human, monkey and murine brain. Eur J Neurosci 8: 666–676, 1996 [DOI] [PubMed] [Google Scholar]
- 78.Masuda S, Okano M, Yamagishi K, Nagao M, Ueda M, Sasaki R. A novel site of erythropoietin production. Oxygen-dependent production in cultured rat astrocytes. J Biol Chem 269: 19488–19493, 1994 [PubMed] [Google Scholar]
- 79.Mateika JH, Narwani G. Intermittent hypoxia and respiratory plasticity in humans and other animals: does exposure to intermittent hypoxia promote or mitigate sleep apnoea? Exp Physiol 94: 279–296, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.McKay LC, Janczewski WA, Feldman JL. Episodic hypoxia evokes long-term facilitation of genioglossus muscle activity in neonatal rats. J Physiol 557: 13–18, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mennini T, De Paola M, Bigini P, Mastrotto C, Fumagalli E, Barbera S, Mengozzi M, Viviani B, Corsini E, Marinovich M, Torup L, Van Beek J, Leist M, Brines M, Cerami A, Ghezzi P. Nonhematopoietic erythropoietin derivatives prevent motoneuron degeneration in vitro and in vivo. Mol Med 7–8: 153–160, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Miskowiak KW, Vinberg M, Harmer CJ, Ehrenreich H, Kessing LV. Erythropoietin: a candidate treatment for mood symptoms and memory dysfunction in depression. Psychopharmacology (Berl) 219: 687–698, 2012 [DOI] [PubMed] [Google Scholar]
- 83.Mitchell GS. Respiratory plasticity following intermittent hypoxia: a guide for novel therapeutic approaches to ventilatory control disorders. In: Genetic Basis for Respiratory Control Disorders, edited by Gaultier C. New York: Springer, 2007 [Google Scholar]
- 84.Mitchell GS, Baker TL, Nanda SA, Fuller DD, Zabka AG, Hodgeman BA, Bavis RW, Mack KJ, Olsen EB., Jr Invited review: Intermittent hypoxia and respiratory plasticity. J Appl Physiol 90: 2466–2475, 2001 [DOI] [PubMed] [Google Scholar]
- 85.Mitchell GS, Johnson SM. Neuroplasticity in respiratory motor control. J Appl Physiol 94: 358–374, 2003 [DOI] [PubMed] [Google Scholar]
- 85a.Mitchell GS, Terada J. Should we standardize protocols and preparations used to study respiratory plasticity? Respir Physiol Neurobiol 177: 93–97, 2011 [DOI] [PubMed] [Google Scholar]
- 86.Nagańska E, Taraszewska A, Matyja E, Grieb P, Rafałowska J. Neuroprotective effect of erythropoietin in amyotrophic lateral sclerosis (ALS) model in vitro. Ultrastructural Study Folia Neuropathol 48: 35–44, 2010 [PubMed] [Google Scholar]
- 87.Nanduri J, Makarenko V, Reddy VD, Yuan G, Pawar A, Wang N, Khan SA, Zhang X, Kinsman B, Peng YJ, Kumar GK, Fox AP, Godley LA, Semenza GL, Prabhakar NR. Epigenetic regulation of hypoxic sensing disrupts cardiorespiratory homeostasis. Proc Natl Acad Sci USA 109: 2515–2520, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Narumiya S, Ohno M, Tanaka N, Yamano T, Shimada M. Enhanced expression of full-length TrkB receptors in young rat brain with hypoxic/ischemic injury. Brain Res 797: 278–286, 1998 [DOI] [PubMed] [Google Scholar]
- 89.Nichols NL, Gowing G, Satriotomo I, Nashold LJ, Dale EA, Suzuki M, Avalos P, Mulcrone PL, McHugh J, Svendsen CN, Mitchell GS. Intermittent hypoxia and stem cell implants preserve breathing capacity in a rodent model of ALS. Am J Respir Crit Care Med 187: 535–542, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Niu C, Yip HK. Neuroprotective signaling mechanisms of telomerase are regulated by brain-derived neurotrophic factor in rat spinal cord motor neurons. J Neuropathol Exp Neurol 70: 634–652, 2011 [DOI] [PubMed] [Google Scholar]
- 91.Pati S, Orsi SA, Moore AN, Dash PK. Intra-hippocampal administration of the VEGF receptor blocker PTK787/ZK222584 impairs long-term memory. Brain Res 1256: 85–91, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pihl L, Baden SP, Diaz RJ. Effects of periodic hypoxia on distribution of demersal fish and crustaceans. Mar Biol 108: 349–360, 1991 [Google Scholar]
- 93.Powell FL, Milsom WK, Mitchell GS. Time domains of the hypoxic ventilatory response. Respir Physiol 112: 123–134, 1998 [DOI] [PubMed] [Google Scholar]
- 94.Ramar K, Caples SM. Vascular changes, cardiovascular disease and obstructive sleep apnea. Future Cardiol 7: 241–249, 2011 [DOI] [PubMed] [Google Scholar]
- 95.Reeves SR, Mitchell GS, Gozal D.(2006). Early postnatal chronic intermittent hypoxia modifies hypoxic respiratory responses and long-term phrenic facilitation in adult rats. Am J Physiol Regul Integr Comp Physiol 290: R1664–R1671, 2006 [DOI] [PubMed] [Google Scholar]
- 96.Reichardt LF. Neurotrophin-regulated signaling pathways. Philos Trans R Soc Lond B Biol Sci 361: 1545–1564, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Reid SG, Sunin L, Milsom WK. The cardiorespiratory system in tropical fishes: structure, function, and control. Fish Physiol 21: 225–274, 2006 [Google Scholar]
- 98.Robinson CJ, Stringer SE. The splice variants of vascular endothelial growth factor (VEGF) and their receptors. J Cell Sci 114: 853–865, 2001 [DOI] [PubMed] [Google Scholar]
- 99.Row BW. Intermittent hypoxia and cognitive function: implications from chronic animal models. Adv Exp Med Biol 618: 51–67, 2007 [DOI] [PubMed] [Google Scholar]
- 100.Sargin D, El-Kordi A, Agarwal A, Muller M, Wojcik SM, Hassouna I, Sperking S, Nave KA, Ehrenreich H. Expression of constitutively active erythropoietin receptor in pyramidal neurons of cortex and hippocampus boosts higher cognitive functions in mice. BMC Biol 9: 27, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Satriotomo I, Dale EA, Dahlberg JM, Mitchell GS. Repetitive acute intermittent hypoxia increases expression of proteins associated with plasticity in the phrenic motor nucleus. Exp Neurol 237: 103–115, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Schinder AF, Poo M. The neurotrophin hypothesis for synaptic plasticity. Trends Neurosci 23: 639–645, 2000 [DOI] [PubMed] [Google Scholar]
- 103.Schurmann H, Claireaux G, Chartois H. Changes in vertical distribution of sea bass (Dicentrarchus labrax L) during a hypoxic episode. Hydrobiol 371/372: 207–213, 1998 [Google Scholar]
- 104.Semenza GL. Hypoxia-inducible factor 1 (HIF-1) pathway. Sci STKE 2007: cm8, 2007 [DOI] [PubMed] [Google Scholar]
- 105.Senger DR, Perruzzi CA, Feder J, Dvorak HF. A highly conserved vascular permeability factor secreted by a variety of human and rodent tumor cell lines. Cancer Res 46: 5629–5632, 1986 [PubMed] [Google Scholar]
- 106.Serebrovskaya TV. Intermittent hypoxia research in the former soviet union and the commonwealth of the independent states: history and review of the concept and selected applications. High Alt Med Biol 3: 205–221, 2002 [DOI] [PubMed] [Google Scholar]
- 107.Shao G, Zhang R, Wang ZL, Gao CY, Huo X, Lu GW.(2006–2007). Hypoxic preconditiong improves spatial cognitive ability in mic. Neurosignals 15: 314–321, 2006–2007 [DOI] [PubMed] [Google Scholar]
- 108.Shen J, Wu Y, Xu JY, Zhang J, Sinclair SH, Yanoff M, Xu G, Li W, Xu GT. ERK-and Akt-dependent neuroprotection by erythropoietin (EPO) against glyoxal-AGEs via modulation of Bcl-xL, Bax, and BAD. Invest Ophthalmol Vis Sci 51: 35–46, 2010 [DOI] [PubMed] [Google Scholar]
- 109.Sieck GC, Mantilla CB. Role of neurotrophins in recovery of phrenic motor function following spinal cord injury. Respir Physiol Neurobiol 169: 218–225, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sirén AL, Knerlich F, Poser W, Gleiter CH, Bruck W, Ehrenreich H. Erythropoietin and erythropoietin receptor in human ischemic/hypoxic brain. Acta Neuropathol (Berl) 101: 271–276, 2001 [DOI] [PubMed] [Google Scholar]
- 111.Soliz J, Joseph V, Soulage C, Becskei C, Vogel J, Pequignot JM, Ogunshola O, Gassmann M. Erythropoietin regulates hypoxic ventilation in mice by interacting with brainstem and carotid bodies. J Physiol 568: 559–571, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Soliz J, Soulage C, Hermann DM, Gassmann M. Acute and chronic exposure to hypoxia alters ventilatory pattern but not minute ventilation of mice overexpressing erythropoietin. Am J Physiol Regul Integr Comp Physiol 293: R1702–R1710, 2007 [DOI] [PubMed] [Google Scholar]
- 113.Soliz J, Gassmann M, Joseph V. Downregulation of soluble erythropoietin receptor in the mouse brain is required for the ventilatory acclimatization to hypoxia. J Physiol 583: 329–336, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Spedding M, Gressens P. Neurotrophins and cytokines in neuronal plasticity. Novartis Found Symp 289: 222–233; discussion 233–240, 2008 [DOI] [PubMed] [Google Scholar]
- 115.Storkebaum E, Lambrechts D, Carmeliet P. VEGF: once regarded as a specific angiogenic factor, now implicated in neuroprotection. Bioessays 26: 943–954, 2004 [DOI] [PubMed] [Google Scholar]
- 116.Tabakman R, Lecht S, Sephanova S, Arien-Zakay H, Lazarovici P. Interactions between the cells of the immune and nervouse system: neurotrophins as neuroprotection mediators in CNS injury. Prog Brain Res 146: 387–401, 2004 [DOI] [PubMed] [Google Scholar]
- 117.Tasali E, Ip MS. Obstructive sleep apnea and metabolic syndrome: alterations in glucose metabolism and inflammation. Proc Am Thoracic Soc 5: 207–217, 2008 [DOI] [PubMed] [Google Scholar]
- 118.Terada J, Mitchell GS. Diaphragm long-term facilitation following acute intermittent hypoxia during wakefulness and sleep. J Appl Physiol 110: 1299–310, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Trumbower RD, Jayaraman A, Mitchell GS, Rymer WZ. Exposure to acute intermittent hypoxia augments somatic motor function in humans with incomplete spinal cord injury. Neurorehabil Neural Repair 26: 163–172, 2012 [DOI] [PubMed] [Google Scholar]
- 120.Vinit S, Lovett-Barr MR, Mitchell GS. Intermittent hypoxia induces functional recovery following cervical spinal injury. Respir Physiol Neurobiol 169: 210–217, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Vogt M, Hoppeler H. Is hypoxia training good for muscles and exercise performance? Prog Cardiovasc Dis 52: 525–533, 2010 [DOI] [PubMed] [Google Scholar]
- 122.Walton M, Connor B, Lawlor P, Young D, Sirimanne E, Gluckman P, Cole G, Dragunow M. Neuronal death and survival in two models of hypoxic ischemic brain damage. Brain Res Brain Res Rev 29: 137–168, 1999 [DOI] [PubMed] [Google Scholar]
- 123.Wang H, Ward N, Boswell M, Katz DM. Secretion of brain-derived neurotrophic factor from brain microvascular endothelial cells. Eur J Neurosci 23: 1665–1670, 2006 [DOI] [PubMed] [Google Scholar]
- 124.Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic helix-loop-helix PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA 92: 5510–5514, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wang GL, Semenza GL. Purification and characterization of hypoxia inducible factor 1. J Biol Chem 270: 1230–1237, 1995 [DOI] [PubMed] [Google Scholar]
- 126.Weber A, Maier RF, Hoffmann U, Grips M, Hoppenz M, Aktas AG, Heinemann U, Obladen M, Schuchmann S. Erythropoietin improves synaptic transmission during and following ischemia in rat hippocampal slice cultures. Brain Res 958: 305–311, 2002 [DOI] [PubMed] [Google Scholar]
- 127.Wilber RL, Stray-Gundersen J, Levine BD. Effect of hypoxic “dose” on physiological responses and sea level performance. Med Sci Sports Exerc 39: 1590–1599, 2007 [DOI] [PubMed] [Google Scholar]
- 128.Wilkerson JER, Mitchell GS. Daily intermittent hypoxia augments spinal BDNF levels, ERK phosphorylation, and respiratory long-term facilitation. Exp Neurol 217: 116–123, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Xie H, Leung KL, Chen L, Chan YS, Ng PC, Fok TF, Wing YK, Li AM, Yung WH. Brain-derived neurotrophic factor rescues and prevents chronic intermittent hypoxia-induced impairment of hippocampal long-term synaptic plasticity. Neurobiol Dis 40: 155–162, 2010 [DOI] [PubMed] [Google Scholar]
- 130.Xiong Y, Mahmood A, Meng Y, Zhang Y, Qu C, Schallert T, Chopp M. Delayed administration of erythropoietin reducing hippocampal cell loss, enhancing angiogenesis and neurogenesis, and improving functional outcome following traumatic brain injury in rats: comparison of treatment with single and triple dose. J Neurosurg 113: 598–608, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yeo EJ, Cho YS, Kim MS, Park JW. Contribution of HIF-1α or HIF2α to erythropoietin expression: in vivo evidence based on chromatin immunoprecipitation. Ann Hematol 87: 11–17, 2008 [DOI] [PubMed] [Google Scholar]
- 132.Yuan G, Nanduri J, Khan S, Semenza GL, Prabhakar NR. Induction of HIF-1α expression by intermittent hypoxia: involvement of NADPH oxidase, Ca2+ signaling, prolyl hydroxylases and mTOR. J Cell Physiol 217: 674–685, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yuan G, Nanduri J, Bhasker CR, Semenza GL, Prabhakar NR. Ca2+/calmodulin kinase-dependent activation of hypoxia inducible factor 1 transcriptional activity in cells subjected to intermittent hypoxia. J Biol Chem 280: 4321–4328, 2005 [DOI] [PubMed] [Google Scholar]
- 134.Zabka AG, Behan M, Mitchell GS. Long term facilitation of respiratory motor output decreases with age in male rats. J Physiol 531: 509–514, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zachary I. Neuroprotective role of vascular endothelial growth factor: signaling mechanisms, biological function, and therapeutic potential. Neurosignals 14: 207–221, 2005 [DOI] [PubMed] [Google Scholar]
- 136.Zhan WZ, Mantilla CB, Sieck GC. Regulation of neuromuscular transmission by neurotrophins. Sheng Li Xue Bao 55: 617–624, 2003 [PubMed] [Google Scholar]
- 137.Zhang JX, Chen XQ, Du JZ, Chen QM, Zhu CY. Neonatal exposure to intermittent hypoxia enhances mice performance in water maze and 8-arm radial maze tasks. J Neurobiol 65: 72–84, 2005 [DOI] [PubMed] [Google Scholar]
- 138.Zhang F, Signore AP, Zhou Z, Wang S, Cao G, Chen J.(2006). Erythropoietin protects CA1 neurons against global cerebral ischemia in rat: potential signaling mechanisms. J Neurosci Res 83: 1241–1251, 2006 [DOI] [PubMed] [Google Scholar]
- 139.Zhu XH, Yan HC, Zhang J, Qu HD, Qiu XS, Chen L, Lis SJ, Cao X, Bean JC, Chen LH, Qin XH, Liu JH, Bai XC, Mei L, Gao TM. Intermittent hypoxia promotes hippocampal neurogenesis and produces antidepressant-like effects in adult rats. J Neurosci 30: 12653–12663, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]


