Abstract
Adult resting energy expenditure (REE) scales as height∼1.5, whereas body weight (BW) scales as height∼2. Mass-specific REE (i.e., REE/BW) is thus lower in tall subjects compared with their shorter counterparts, the mechanism of which is unknown. We evaluated the hypothesis that high-metabolic-rate brain mass scales to height with a power significantly less than that of BW, a theory that if valid would provide a potential mechanism for height-related REE effects. The hypothesis was tested by measuring brain mass on a large (n = 372) postmortem sample of Thai men. Since brain mass-body size relations may be influenced by age, the hypothesis was secondarily explored in Thai men age ≤45 yr (n = 299) and with brain magnetic resonance imaging (MRI) studies in Korean men (n = 30) age ≥20<30 yr. The scaling of large body compartments was examined in a third group of Asian men living in New York (NY, n = 28) with MRI and dual-energy X-ray absorptiometry. Brain mass scaled to height with a power (mean ± SEE; 0.46 ± 0.13) significantly smaller (P < 0.001) than that of BW scaled to height (2.36 ± 0.19) in the whole group of Thai men; brain mass/BW scaled negatively to height (−1.94 ± 0.20, P < 0.001). Similar results were observed in younger Thai men, and results for brain mass/BW vs. height were directionally the same (P = 0.09) in Korean men. Skeletal muscle and bone scaled to height with powers similar to that of BW (i.e., ∼2–3) in the NY Asian men. Models developed using REE estimates in Thai men suggest that brain accounts for most of the REE/BW height dependency. Tall and short men thus differ in relative brain mass, but the proportions of BW as large compartments appear independent of height, observations that provide a potential mechanistic basis for related differences in REE and that have implications for the study of adult energy requirements.
Keywords: body composition, nutritional requirements
energy requirements are largely determined by subject body weight (5b, 23). Since adult body weight increases as a function of height∼2 (33), taller subjects weigh more and have a greater energy requirement than their shorter counterparts (5b). The largest component of energy requirements in most adults is related to resting energy expenditure (REE), and REE scales as height∼1.5 (13). Accordingly, mass-specific resting energy requirements, defined as REE/body weight, decrease as height−0.5. Tall subjects thus appear to have a smaller magnitude mass-specific REE and from the energetic perspective can be considered relatively more “efficient” at rest. Greater stature and associated body weight are thus not accompanied by a proportionally larger resting energy need.
The mechanism leading to a smaller mass-specific REE with greater height is unknown, although one hypothesis is that relative to body weight tall and short subjects differ in their proportions of heat producing tissues (13,14). Total adipose tissue free mass (ATFM), skeletal muscle (SM) mass, and bone mass also scale approximately as height2, so ATFM and musculoskeletal mass appear to remain stable proportions of body weight independent of height (14). Adiposity, defined as %fat or adipose tissue, is largely independent of height (14).
In contrast to these other relatively large compartments, brain mass at ∼2% of body weight scales inconsistently to height with the limited observations variable between men and women (14). Moreover, some studies of adults fail to detect significant correlations whereas others observe significant but relatively weak associations between brain mass and stature (4, 8, 11, 14, 16, 17, 20, 27–31, 36, 38, 43, 44). We recently reported in a small adult sample that in men (n = 19) brain volume measured using magnetic resonance imaging (MRI) scaled to height with a power of less than 1 and that brain mass/body weight was negatively associated with stature (14). Since brain is a high-metabolic-rate organ (∼240 kcal·kg−1·day−1; Ref. 7), a relatively smaller brain mass may provide a partial mechanistic explanation for the lower REE/body weight observed in taller men.
The present study expands on these earlier observations by testing in a large sample of Thai men the hypothesis that brain mass scales to height with a power significantly less than that of body weight (i.e., ∼2). If valid, this hypothesis would provide a potential mechanism explaining the observation that mass-specific REE is smaller in magnitude in tall subjects compared with their shorter counterparts. To exclude the neurodegenerative effects of aging on our analyses, we also explored brain mass-body size relations in a subgroup of the Thai men age ≤45 yr and in a small group of young Korean men. A secondary study aim was to establish whether large body compartments such as ATFM, SM mass, and bone mass also scale in Asian men as height∼2.
These collective observations would, accordingly, not only provide insights into the genesis of human whole body REE but also create a foundation for establishing the fundamental relations between adult stature and body composition.
METHODS
Experimental Design and Rationale
The study focused on Thai men as a large adult autopsy cohort (n = 372) was available to critically test the hypothesis with carefully collected postmortem estimates of brain mass. The men ranged in age from 18 to 88 yr, and one concern expressed in earlier studies is the presence of age-related changes in brain mass (5a, 30, 31). We therefore also separately analyzed men in this cohort at or below the age of 45 yr (n = 299). Additionally, we specifically explored brain mass-body size relations in a small cohort of healthy young Korean men (n = 30) recruited within the restricted age range of ≥20<30 yr using MRI to estimate total brain volume. The scaling of large body compartments, ATFM, SM mass, and bone mass to height was evaluated in a third healthy cohort of 28 Asian men, largely Chinese, living in New York (NY), who completed whole body MRI and dual-energy X-ray absorptiometry (DXA) studies.
The study included only men of Asian extraction since our earlier investigation of brain mass-body size relations included an ethnically heterogeneous sample (14) and brain mass (16, 17, 31) and other body compartments (18) may vary across race groups. However, the country of origin differed between the men, including mainly Thailand, Korea, and China. The available sample of Thai women was considerably smaller than that of men, particularly when considering those age ≤45 yr, and we thus concentrated the present study on men.
Brain mass-body size relations.
The primary sample for brain mass evaluation included Thai men studied at the time of autopsy (3, 4). The forensic autopsies were performed at the Department of Pathology, Faculty of Medicine, Ramathibodi Hospital. All of the subjects selected were Thai adult (age ≥18 yr) men who died of a nonbrain injury with a survival time of less than 15 min. Forensic pathologists performed all autopsies within 24 h following death since the delay between death and autopsy can alter brain weight (28, 30). Body weight, length (i.e., height), and brain weight were evaluated and the collected data was used to establish the scaling of body weight and brain weight to height. The Ramathibodi Institutional Review Board approved the postmortem data collection.
The second subject cohort included healthy young Korean men in their twenties who were evaluated as part of a program aimed at developing national reference values for brain volume (19). Brain volume, converted to mass by use of the reported density of brain tissue (1.03 kg/l) (37), was evaluated by MRI with 1.5-mm contiguous slices as previously reported (20). The study was approved by the Korea University Medical Center, and all subjects signed an informed consent prior to participation. The collected data was used to evaluate the scaling of body weight and brain mass to height.
Large body compartment-body size relations.
Total body adipose tissue volume, SM volume, and ATFM were evaluated by whole-body MRI in Asian men age ≥18 yr at the NY Obesity Research Center. Compartment volumes were converted to mass using previously reported (7, 37) densities for adipose tissue (0.92 kg/l), SM (1.04 kg/l), and ATFM (1.04 kg/l). Bone mineral content (BMC) was measured by DXA on the same day and results were converted to bone mass as BMC/0.54 (12, 37). The collected data was used to evaluate the scaling of body weight, ATFM, SM mass, and bone mass to height. The study was approved by the Institutional Review Board of St. Luke's-Roosevelt Hospital, and all subjects signed an informed consent prior to participation. The NY Asian men were evaluated as part of a larger cross-sectional body composition study, and brain imaging studies were not included in the protocol (12, 14, 33).
Data from the three groups of Asian men was used to examine the study hypothesis and related topics by answering three questions: 1) How does body weight scale to height? 2) How do brain mass and brain mass/body weight scale to height? 3) How do adipose tissue, ATFM, SM, and bone scale to height? Question 1 was examined by evaluating the scaling of body weight to height in all three groups of men. Question 2 was examined by evaluating the allometric relationships of brain mass to height in the Thai and Korean men, and question 3 was evaluated in the corresponding analyses for adipose tissue mass, ATFM, SM mass, and bone mass in the NY Asian men.
Subjects
The Thai men included those succumbing to accidents, falls, electrocutions, suicides (e.g., overdoses), and homicides (e.g., gunshots) during the period February 2003–December 2007. Men were excluded who had any evidence of underlying disease, including infections, malignancies, or postmortem histological evidence of an acute or chronic illness. We excluded men succumbing from a fire-related death or from a treated chronic illness.
The Korean men were healthy young adults recruited through advertisements on the Korean University web page and local newspapers during the period August 2001–April 2004. Subjects were entered into the study following completion of a physical exam aimed at excluding any undetected neurological diseases.
The NY men were mainly Chinese with both parents of Asian extraction. All were healthy adults and had no evidence of acute or chronic disease based on a history, screening physical examination, and blood studies during the accrual period of June 1998–May 2003.
The Korean and NY Asian men were all well hydrated at the time of their physical examinations and imaging studies.
Measurements
Body weight and height.
Forensic pathologists supervised all of the Thai autopsies. Trained mortuary technicians measured body weight and length. Bodies were weighed naked with the same scale (Kern & Sohn, Balingen, Germany). Body length was measured from head to heel with a calibrated tape measure. All bodies were refrigerated at the same temperature (4°C) before weighing.
Body weight and height in the Korean and NY Asian men were measured via calibrated digital scales and stadiometers, respectively.
Brain mass.
Brains from the Thai men were removed within 10 min of autopsy initiation and prepared as recommended by The College of American Pathologists (32). The brain and spinal cord were first separated below the decussation of the pyramids and then promptly weighed with leptomeninges intact and unopened ventricles. The brains were then serially sectioned for gross examination followed by fixation for histological review.
Brain volume was evaluated in the Korean men using 1.5-mm-thick cross-sectional MRI images acquired via a 1.5-T Magnetom Vision system (Siemens, Erlangen, Germany) (20). Two trained observers independently segmented regions of interest, including whole brain, cerebellum, and lateral ventricles. Cerebrospinal fluid was separated from brain tissue and measured volumes were derived using V-work software (CyberMed, Seoul, Korea). The results from the two analysts were then averaged and converted to whole brain mass for presentation under results. The MRI measurements are not fully automated and therefore involve a human judgment component. Accordingly, we developed three measures of between-observer variation including the coefficient of variation (CV), the CVs' interquartile range (IQR) from multiple analysts, and the intraclass correlation coefficient (ICC). The respective CV, IQR, and ICC for brain mass evaluation are 2.9%, 1.6%, and 0.97.
Body composition.
Whole body MRI scans were completed in the NY Asian men with a 1.5-T General Electric scanner (6X Horizon, Milwaukee, WI), and images were then segmented by trained and quality-controlled analysts. Total body adipose tissue and SM volumes were calculated from the cross-sectional image data and converted to mass by using their previously reported tissue densities (7, 37). Adipose tissue-free mass was calculated as body weight minus adipose tissue mass. Respective CV, IQR, and ICC values for total body SM and adipose tissue mass are 2.1%, 1.8%, and 0.97 and 2.0%, 2.6%, 0.99.
Bone mineral content was measured for the whole body by use of a GE Lunar DPX system (Madison, WI, software version 3.6) (12). The CV for repeated measurements of bone mineral mass is 1.7%.
Statistical Methods
Baseline subject demographic characteristics are reported as means ± SD in tables and as mean powers ± standard error of the estimate (SEE) in the text and figures. The statistical analyses were carried out via SPSS (SPSS for Windows, 11.5, SPSS, Chicago, IL).
Allometric models.
The mathematical foundation for developing allometric models is extensively reviewed in earlier publications (2, 13, 14, 19, 26). Briefly, the classic general allometric model is expressed in a form of: Y = αXβ, where Y is outcome (e.g., brain mass), X is the predictor variable (e.g., height), β is the scaling exponent or power, and α is the proportionality constant. For instance, based on the work of Quetelet (34) and many others (14) since his seminal observations, β approximates 2 in these equations when body weight (Y) is scaled to height (X).
The scaling of brain mass/body weight can be examined by writing the general allometric model separately for brain mass and body weight, both scaled to height: brain mass = α1heightβ1 and body weight = α2heightβ2. Therefore, brain mass/weight = α1/α2heightβ1−β2. If brain mass and body weight scale differently to height (i.e., β1 ≠ β2), short and tall subjects will not have the same brain mass/body weight. In other words, the power of height when scaled to brain mass vs. body weight is not the same.
The hypothesis was tested by examining the allometric relationships between body weight, brain mass, and height in the whole group of 372 Thai men. Allometric models were developed with coefficients α and β estimated by least squares multiple linear regression analysis based on log-transformed data in the form of logeY = logeα + βlogeX + ε, where ε is error term. Body weight or brain mass was set as the dependent variable and height and age as independent variables in the regression models (19, 26). Log α and β values along with R and SEE values for the series of developed regression models are presented under results. Statistical tests of the power of height when scaled to body weight vs. brain mass within the same group were performed by testing the significance of δ in the following model: logebrain mass − logebody weight = γ + δlogeheight + ε. The sequence of developing these models with related statistical tests were repeated for the Thai men age ≤45 yr and for the Korean men.
Allometric models were similarly developed describing the relations between adipose tissue mass, ATFM, SM mass, and bone mass with height in the NY Asian men. We also examined these relations for musculoskeletal mass, derived as the sum of SM and bone mass.
Scatterplots of allometric relations are presented in the figures and the data in the figures are fit with univariate regression models.
RESULTS
Group Characteristics
The baseline characteristics of the three groups of men are presented in Table 1. There were 372 total Thai men, 299 of whom were age ≤45 yr. The group as a whole had a mean age of ∼36 yr and a body mass index (BMI) of ∼22 kg/m2. Brain mass at the time of autopsy in the whole group of men was 1,334 ± 125 g (means ± SD) and was minimally larger (1,341 ± 121 g) in the younger group of men.
Table 1.
Characteristics of the Thai, Korean, and NY Asian men
| Thai Men |
Korean Men | NY Asian Men | |||
|---|---|---|---|---|---|
| Age <45 yr | All Subjects | ||||
| N | 299 | 372 | 30 | 28 | |
| Age, yr | 30.2±7.3 | 35.6±13.6 | 24.3±2.2 | 34.6±14.0 | |
| BW, kg | 62.5±11.1 | 62.1±10.9 | 71.4±10.1 | 67.3±8.5 | |
| Height, cm) | 167.6±6.4 | 167.1±6.5 | 175.3±3.7 | 172.3±5.9 | |
| BMI, kg/m2 | 22.2±3.4 | 22.2±3.3 | 23.2±2.9 | 22.7±2.5 | |
| Brain mass, g | 1,341±121 | 1,334±125 | 1,440±102 | NA | |
| AT mass, kg | NA | NA | NA | 13.7±4.4 | |
| SM mass, kg | NA | NA | NA | 27.7±3.8 | |
| Bone mass, kg | NA | NA | NA | 5.1±0.9 | |
| ATFM, kg | NA | NA | NA | 53.5±5.9 | |
Data are presented as group mean values ± SD; NA, data not available. AT, adipose tissue; ATFM, adipose tissue-free mass; BMI, body mass index; BW, body weight; NY, New York; SM, skeletal muscle.
There were 30 Korean men with a mean age of ∼24 yr with a BMI of ∼23 kg/m2. The group brain mass estimated by MRI was 1,440 ± 102 g.
There were 28 men in the NY Asian group with a mean age of ∼35 yr and a BMI of ∼23 kg/m2. Skeletal muscle was the largest of the evaluated tissue compartments (∼28 kg) and with added bone (∼5 kg) musculoskeletal mass was ∼33 kg or 49% of body weight. Skeletal muscle mass accounted for ∼60% of ATFM (∼54 kg). The total body adipose tissue mass was 13.7 kg or 20% of body weight. Adipose tissue mass was significantly correlated with age (r = 0.45, P < 0.01) whereas the correlations between body weight, ATFM, SM mass, and bone mass with age were all nonsignificant.
Allometric Analyses
Allometric analyses are presented in Table 2 for body weight and brain mass in the Thai and Korean men. The allometric analyses for body weight and body composition for the NY Asian men are presented in Table 3.
Table 2.
Height-related allometric regression models based on log-transformations for the Thai and Korean men
| All Subjects |
Age<45 yr |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ht | Age | Int | SEE | R (P) | Ht | Age | Int | SEE | R (P) | |||||||||
| BW, kg | ||||||||||||||||||
| Thai men | 2.36 | NS | −7.94 | 0.14 | 0.54 (<0.001) | 2.39 | NS | −8.12 | 0.14 | 0.54 (<0.001) | ||||||||
| Korean men | 3.03 | NS | −11.40 | 0.12 | 0.48 (<0.01) | |||||||||||||
| Brain mass, g | ||||||||||||||||||
| Thai men | 0.46 | −0.03 | 4.93 | 0.09 | 0.25 (<0.001) | 0.52 | NS | 4.57 | 0.09 | 0.21 (<0.001) | ||||||||
| Korean men | 1.25 | NS | 7.68 | 0.07 | 0.37 (<0.05) | |||||||||||||
| Brain/BW, g/kg | ||||||||||||||||||
| Thai men | −1.94 | −0.06 | 13.19 | 0.15 | 0.44 (<0.001) | −1.93 | −0.07 | 13.19 | 0.15 | 0.44 (<0.001) | ||||||||
| Korean men | −1.78 | NS | 12.20 | 0.15 | 0.25 (0.09) | |||||||||||||
NS, nonsignificant covariate. Ht, height; Int, intercept; SEE, standard error of the estimate.
Table 3.
Height-related allometric regression models based on log-transformations for the NY Asian men
| NY Asian Men |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ht, cm | Age | Int | SEE | R (P) | |||||
| BW, kg | 1.85 | NS | −5.33 | 0.11 | 0.49 (<0.01) | ||||
| AT, kg | NS | 0.34 | 1.47 | 0.29 | 0.40 (P=0.02) | ||||
| ATFM, kg | 2.16 | NS | −7.13 | 0.09 | 0.65 (<0.001) | ||||
| SM, kg | 2.24 | NS | −8.24 | 0.12 | 0.54 (<0.01) | ||||
| SM/Wt, kg/kg | NS | 0.10 | 0.59 | 0.07 | 0.45 (<0.01) | ||||
| Bone, kg | 2.75 | NS | 12.54 | 0.18 | 0.47 (<0.01) | ||||
| SM+B, kg | 2.29 | NS | 8.33 | 0.12 | 0.57 (<0.01) | ||||
| (SM+B)/Wt, kg/kg | NS | 0.10 | 0.40 | 0.07 | 0.45 (<0.01) | ||||
B, bone; SM, skeletal muscle mass; Wt, weight.
Body weight.
Body weight was highly correlated with height for the Thai, Korean, and NY Asian men (Fig. 1). Body weight scaled similarly to height with powers of 2.36 ± 0.19 and 2.39 ± 0.22 (means ± SEE) in the whole group of Thai men (r = 0.54, P < 0.001) and in the Thai men age ≤45 yr (r = 0.54, P < 0.001) (Table 2). Body weight scaled with a higher power to height in the Korean men, but with a larger SEEs (3.03 ± 1.06). Body weight scaled to height with a power of 1.85 ± 0.64 in the NY Asian men (r = 0.49, P < 0.01). Age was not a significant covariate in any of these regression models (Table 2). Body weight in the three groups of men thus scaled to height with powers in the range of ∼2–3.
Fig. 1.
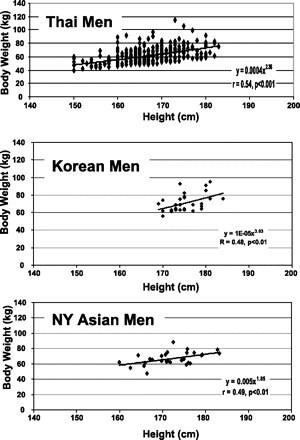
Body weight vs. height in the Thai (top), Korean (middle), and New York Asian (NY; bottom) men. The plotted data were fit with univariate power functions that are provided in each panel of the figure. The models are of the form Y = αXβ where Y is body weight, X is height, β is the scaling exponent or power, and α is the proportionality constant. All regression models in the figure are P < 0.01 and are presented in Table 2.
Brain mass.
Brain mass was significantly negatively correlated with age in the whole group of Thai men but not in those age ≤45 yr (Fig. 2) or the Korean men. Height was also significantly negatively correlated with age in the whole group of Thai men (r = 0.21, P < 0.001) but not in the Korean men.
Fig. 2.
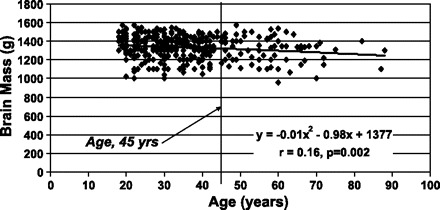
Brain mass vs. age in the Thai men. A vertical line separates the men above and below the age of 45 yr. The data were fit with a second order polynomial regression model.
Brain mass was significantly correlated with body weight (r = 0.31, P < 0.001), but body weight was not correlated with age (r = −0.07, P = not significant) in the whole group of Thai men with similar results for the men age ≤45 yr. The correlation between brain mass and body weight was nonsignificant in the Korean men.
The allometric analyses for the Thai and Korean men are presented in Table 2. Brain mass scaled to height with a power of 0.46 ± 0.13 (r = 0.24) (Fig. 3) and 0.52 ± 0.14 (r = 0.21) in the whole group of Thai men and men age ≤45 yr, respectively (both P < 0.001). The allometric regression model for the whole group of men included age as a significant predictor variable. Both powers, 0.46 and 0.51, were significantly less (P < 0.001) than those of the corresponding powers for body weight scaled to height (2.36 and 2.39).
Fig. 3.
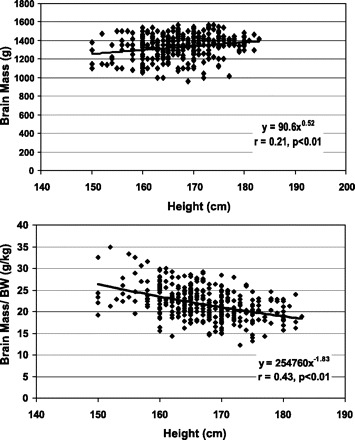
Brain mass (top) and brain mass/body weight (BW) (bottom) vs. height in the Thai men. The data are fit with univariate power functions that are shown in the figure. The plotted data were fit with univariate power functions that are provided in each panel of the figure. The models are of the form Y = αXβ where Y is brain mass or brain mass/BW, X is height, β is the scaling exponent or power, and α is the proportionality constant. Both regression models in the figure are P < 0.01 and are presented in Table 2.
Brain mass scaled to height with a power of 1.25 ± 0.59 (r = 0.37, P < 0.05) in the group of Korean men; age was not a significant predictor variable. This power of height (i.e., 1.25) is substantially less than the power of height when body weight is scaled to height (i.e., 3.03 ± 1.06), although this numerical difference did not reach statistical significance (P = 0.09). Brain mass in the two groups of men thus scaled to height with powers in the range of ∼0.5–1.2.
Brain mass, when expressed as a ratio to body weight in the Thai men, scaled negatively to height (Fig. 3) with a power of −1.94 ± 0.20 with age serving as a significant predictor variable (r = 0.44, P < 0.001). Results were nearly identical when the regression models were developed in the men age ≤45 yr (Table 3). The ratio of brain mass to body weight also scaled negatively to height (−1.78 ± 1.3) in the Korean men, but the correlation was nonsignificant (P = 0.18).
Body composition.
The allometric analyses of body composition for the NY Asian men are presented in Table 3. The associations between adipose tissue mass and % of body weight as adipose tissue with height were both nonsignificant (r = 0.07 and r = −0.16). All of the evaluated lean compartments, ATFM, SM mass, bone mass, and musculoskeletal mass scaled significantly to height with respective powers of 2.16 ± 0.50, 2.24 ± 0.70, 2.75 ± 1.00, and 2.29 ± 0.67 (Fig. 4). Age was a significant negative predictor in SM and bone mass regression models. Skeletal muscle mass and musculoskeletal mass expressed as a fraction of body weight were not significantly correlated with height. With the exception of adipose tissue mass, the evaluated large body compartments thus scaled to height with powers in range of ∼2–3.
Fig. 4.
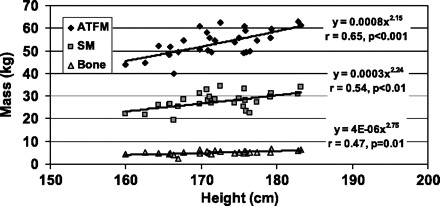
Adipose tissue free mass (ATFM), skeletal muscle (SM) mass, and bone mass vs. height in the NY Asian men. The data are fit with univariate power functions that are shown in the figure.
Energy expenditure.
Our results suggest that tall men have the same relative amounts of large body compartments (i.e., ATFM, skeletal muscle, and bone) compared with short men. By contrast, our results support the hypothesis that brain mass/body weight is significantly smaller in tall men. We modeled the effects these compartmental relations have on energy expenditure in the Thai men by estimating REE (kcal/day) according to the Harris-Benedict (HB) equation (REEHB) (10). We first evaluated the scaling of REEHB to height in the whole group of Thai men and in those age ≤45 yr (Table 4). Compared with body weight, REEHB scaled with a lower power to height (∼1.9 vs. ∼2.4) and REEHB/body weight scaled negatively to height with a power of ∼(−0.5) (P < 0.001 for both groups of Thai men).
Table 4.
REE-related allometric regression models based on log-transformations for the Thai men
| All Subjects |
Age<45 yr |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ht | Age | Int | SEE | R (P) | Ht | Age | Int | SEE | R (P) | |||||||||
| REEHB, kcal/day | 1.93 | −0.17 | −2.00 | 0.08 | 0.78 (<0.001) | 1.86 | −0.11 | −1.83 | 0.08 | 0.70 (<0.001) | ||||||||
| REEHB/BW, kcal·kg−1·day−1 | −0.47 | −0.19 | 6.25 | 0.06 | 0.73 (<0.001) | −0.56 | −0.15 | 6.59 | 0.06 | 0.55 (<0.001) | ||||||||
| NonBrain REE, kcal/day | 2.35 | −0.21 | −4.26 | 0.10 | 0.78 (<0.001) | 2.23 | −0.13 | −3.90 | 0.10 | 0.69 (<0.001) | ||||||||
| NonBrain REE/BW, kcal·kg−1·day−1 | NS | −0.23 | 4.00 | 0.06 | 0.82 (<0.001) | −0.19 | −0.17 | 4.53 | 0.05 | 0.62 (<0.001) | ||||||||
HB, Harris-Benedict equation; REE, resting energy expenditure.
We then assumed that REE represents the sum of two main contributions, brain with a mass-specific energy expenditure of 240 kcal·kg−1·day−1 and remaining tissue. This assumption then allowed us to divide REEHB into brain and nonbrain components (i.e., REEHB − [240 × brain mass in kg)]. Nonbrain REE scaled to height with powers very similar to the power observed when body weight is scaled to height (∼2.3 vs. ∼2.4). Nonbrain REE/weight was nonsignificantly related to height in the whole group of Thai men (Table 4) and significantly related to height with a small negative power of −0.19 ± 0.08 in the younger group of men.
DISCUSSION
Scaling of Body Composition to Height
Body mass scales across adults approximately as height2, an observation made almost two centuries ago by Quetelet (34) and again largely confirmed in the present study. The scaling of body mass to height reflects the weighted effects of each anatomical compartment, and our findings in Asian men suggest that ATFM, SM mass, and bone mass all scale to height with powers of ∼2. These large compartments, notably the structurally important musculoskeletal mass compartment, are the main determinants leading to the scaling of body mass as height2. As these lean tissue compartments scale across adult subjects with approximately the same power of height as body weight, short and tall subjects by inference have roughly the same relative amounts of ATFM, SM, and bone. By contrast, adipose tissue mass was highly variable between subjects and neither total adipose tissue mass nor percent of body weight as adipose tissue were significantly associated with height. The scaling across adult populations of body weight as height2 thus largely reflects the consistent scaling of lean tissues as height2. These observations in Asian men extend our earlier studies of both men and women of mixed ethnicity (14).
Body weight in the Thai men scaled as height∼2 whereas brain mass scaled as height∼0.5, the two powers differing significantly and strongly supporting our study hypothesis. Brain mass relative to body weight, accordingly, scaled negatively to height, expanding on our findings in a much smaller sample of ethnically mixed men (14). Excluding older men in the sample, above the age of 45 yr, did not measurably change these results for brain mass/body weight, and our findings were directionally similar but statistically nonsignificant for the much smaller sample of young Korean men.
Brain mass contributes minimally to total body mass (∼2%) (14, 37) and thus only has a small effect on the scaling of body weight to height. On the other hand, brain has a high mass-specific metabolic rate (∼240 kcal·kg−1·day−1) and accounts for ∼20% of energy expended at rest (7). The relatively smaller brain mass of tall subjects may thus account for the correspondingly lower REE/body weight also observed in tall subjects compared with their short counterparts (13). This hypothesis is supported by our modeling of energy expenditure derived using REE estimated with the HB equation (10). As with brain/body weight, REEHB/body weight also scaled negatively to height. Nonbrain REE, by contrast, approached a scaling relationship to height similar to that of body weight. Our estimates thus suggest that the previously reported lower REE/body weight in taller men (13) can largely be accounted for by their relatively smaller brain mass/body weight.
The only previous study we found in the literature related to the scaling of human brain mass to height was our own in a small sample of 19 ethnically mixed men (14). However, many studies over the past century have examined the relations between body size and brain mass or volume (e.g., 4, 8, 11, 14, 16, 17, 20, 27–31, 36, 38, 43, 44). Although there are limitations of most of these earlier studies (31), a consistent observation (Table 5) is the generally limited association between brain mass or volume and stature. Some studies report weak or modest associations between brain size and height whereas in others the correlations are nonsignificant. Relations between brain size and body weight are similarly variable between studies and reveal only modest associations.
Table 5.
Representative studies reporting correlations between adult brain mass or volume and body size
| Author (Reference No.) | Subjects | Comment |
|---|---|---|
| Pakkenberg and Voigt (28) | 765 men and 325 women; brain evaluated at autopsy. | In adults (724 men, 302 women), multiple regression with weight and height as brain mass predictors, only height significant. |
| Holloway (16) | 502 men and 165 women judged as “normal adults <age 65 yr” from the database reported in Ref. 28; brain evaluated at autopsy. | Brain mass correlation with height, after controlling for age, r = 0.29 for men (P = 0.001) and 0.04 for women (P = 0.32). |
| Passingham (30) | Portions of study reported in Ref. 28, men (734) and women (305); 198 men and 92 women age 18–45 yr; brain evaluated at autopsy. | Height and brain mass, r = 0.31 in all men (P < 0.001) and 0.20 in women (P < 0.001); age 18–45 yr, r = 0.20 in men (P < 0.004) and 0.12 in women (P < 0.12). |
| Skullerud (36) | 64 men and 17 women, ages 45–54 yr; 196 men and 190 women, ages 70–79 yr; brain evaluated at autopsy. | Brain mass correlated with height only in the older group. Reduced brain mass observed in subjects with low BMI. |
| Witelson (44) | Caucasian women (58) and men (42) with nonneurological cancers; brain evaluated at autopsy. | After adjustment for age, correlations between cerebral volume and height were nonsignificant; height accounted for 1–4% of brain size within each sex. |
| Peters et al. (31) | Healthy Caucasian men (69) and women (46), ages 19–41 yr; brain evaluated with MRI. | Brain mass correlations with height, r = 0.05 and 0.21, both nonsignificant. |
| Healthy Caucasian men (52) and women (30), ages 19–41 yr; brain evaluated with MRI. | Brain mass correlations with height, r = 0.004 (NS) and 0.33 (P = 0.04). | |
| Heymsfield et al. (14) | Healthy men (19) and women (57); brain evaluated with MRI. | Brain mass scaled to height in men (r = 0.46, P = 0.04) with a power of 0.83 and nonsignificantly (r = 0.003, P = NS) in the women. |
| Koh et al. (20) | Healthy Korean men (30) and women (30) in their twenties; brain evaluated with MRI. | Correlations of brain with body weight nonsignificant; correlation of brain with height significant in men (r = 0.37, P < 0.05) but not women. |
| Willerman (43) | 40 college students; brain evaluated with MRI. | After adjusting for sex, r = 0.09 and 0.10 for brain size versus height and weight, both nonsignificant. |
| Nopoulos (27) | Healthy men (42) and women (42); brain evaluated with MRI. | Significant correlation between cerebral tissue volume and height in women (r = 0.43, P = 0.003) but not men (r = 0.02, P = 0.867). |
| Spann and Dustmann (38) | Males (898) and women (430), ages 15 to 65 yr; without major illnesses or head trauma; brain evaluated at autopsy. | Brain mass, men>women at each height grouping; descriptive increase in brain mass (∼10%) with height (∼150–170/180 cm) for both men and women. |
| Ho et al. (17) | 1261 adults, 25–80 yr with those having brain pathology excluded; brain evaluated at autopsy. | Reported race, sex, and age differences in brain mass; sex and race-specific correlations between brain mass and height, r values 0.15–0.24, all significant with groups ranging in size from 218–414. |
| Garby et al. (8) | 1,598 healthy or apparently healthy subjects age >16 yr, 1,096 men and 512 women; brain evaluated at autopsy. | Reported sex and age differences in brain mass; among major body organs, smallest correlations between brain mass and combined effects of weight, height, and age. |
| Hartmann et al. (11) | 7,965 healthy adults free of brain disorders, 4,488 men and 3,477 women; brain evaluated at autopsy. | Reported sex, age, and BMI differences in brain mass; brain increases in mass, independent of sex, by ∼3.7 g/cm. |
The factors controlling brain mass must therefore differ to some extent from those controlling the noncentral nervous system components of body mass. Brain reaches near-peak mass by the age of 8–10 yr, long before the remainder of body mass completes growth processes (5a, 9). Other than aging, once maximal brain mass is achieved in early adulthood there is relatively little change in brain size across the life span, with the exception perhaps of neural tissue loss secondary to severe semistarvation (36). On the other hand, body mass and most associated compartments can vary widely during adulthood, as for example the growth of adipose tissue, SM, bone, liver, and heart that occurs with the development of obesity (12, 41). Hormones, such as growth hormone and IGF-1b, have minimal effects on postnatal brain mass but substantial effects on somatic tissues such as is observed in patients with acromegaly (6) or in transgenic rodent models overexpressing growth hormone (35).
Although brain mass remains relatively stable in mass across the adult life span, increasing evidence indicates that there are large between-individual differences in brain mass after controlling for body size that can be accounted for in part by heritable factors. Data from the Framingham cohort and offspring indicate genetic factors account for 55% of between-individual differences in brain white matter volume as measured by MRI (1). Moreover, five genes have been identified leading to the rare condition referred to as primary microcephaly that is associated with a head circumference >3 SD below normal and adult brain volumes in the range of 400–500 cm3 (5). The afflicted individuals are often mildly retarded but may have a body size at or near that of their unaffected siblings. The presently known genes causative for primary microcephaly do not appear to account for the large between-individual difference observed in brain volume among healthy adults (45), but clearly there are other as yet undiscovered regulators of brain growth that will help to unravel whether and how brain and body mass are related to each other among adults.
Importantly, these collective observations provide a working basis for understanding the very weak correlations between brain mass and height or body weight in the many previous studies that have examined these questions over the past century and those of the present report.
Relationship to Energy Requirements
Adult stature, in addition to genetic mechanisms (21), is extremely sensitive to prevailing nutritional and other environmental conditions (15). A premium must thus be placed on growing taller when conditions allow while at the same time minimizing the energetic “cost” imposed on adults. The greater body mass of tall subjects would necessitate larger energy requirements, but these costs are minimized by the scaling patterns observed in the present study.
As an example, body weight approximately doubles (50 kg→100 kg) when the height of a hypothetical man of age 20 yr and BMI 22 kg/m2 increases from 152 cm (5 ft) to 213 cm (7 ft). Our findings indicate that skeletal muscle and bone would also approximately double in mass, whereas Garby's formula (8), for example, predicts that brain mass would only increase by 0.26 kg from 1.43 to 1.69 kg. When height increases from 152 to 213 cm, Hartmann et al.'s analysis (11) predicts an increase in brain mass of 0.23 kg, and the data collected in Thai men (Table 2) similarly predicts only a small relative increase in brain weight of 0.21 kg. REE in our hypothetical man would be substantially higher had brain mass, with an energy cost of 240 kcal·kg−1·day−1, also doubled with greater height and associated weight from 1.43 to 2.86 kg (i.e., a Δ of 1.43 kg).
The lower mass-specific REE of tall subjects may also confer greater fasting endurance (22, 24), thus allowing longer survival when environmental conditions lead to absence of food supplies with complete cessation of dietary intake.
Study Limitations
Investigators have struggled to obtain high-quality data for more than a century when trying to study brain mass-physiological or cognitive relations. When testing the main study hypothesis in the present study we applied rigorous criteria for subject selection and assembled a large postmortem sample with adequate power, although ideally the study testing our primary hypothesis would be carried out in living subjects and include both men and women.
Although we attempted to narrow our focus to “Asian” men, there is clearly phenotypic variation among Asians from different regions and nations due to both inherited and environmental effects. Brain mass tended to be smaller in the Thai men compared with their Korean counterparts, although some of this variation may be accounted for by measurement method differences. Our estimates of brain mass in the Thai and Korean men are similar to those reported by using the same corresponding methods in Thai and Korean men reported by Narongchai and Narongchai (25) and Park et al. (29), respectively. Our brain mass/body weight and body composition analyses were conducted within each group and our conclusions should not be measurably influenced by variation across the three samples of Asian men. Nevertheless, our study should be considered only an initial step in explaining the interrelations between stature, body composition, and energy expenditure.
Another feature of our selected subject samples is that they did not include the full biological range of stature observed in healthy adults. Data on brain mass in very short and tall subjects who are otherwise considered healthy is very limited. Our brain and body composition evaluation protocols in Korean and NY Asian men also did not provide the opportunity to measure REE, and this is an important gap that needs to be filled in future studies.
Lastly, we did not evaluate the scaling of body composition to height beyond brain and several large compartments. It seems likely that with larger and more comprehensively evaluated subject groups that more “deviations” above and below the “height2” power rule will be detected.
Conclusions
Our findings establish in Asian men that brain mass and body weight scale differently to height. By contrast, musculoskeletal mass and body weight scale similarly to height. These collective findings reveal previously unforeseen relationships between body composition and stature: brain mass is relatively smaller in tall compared with short subjects whereas the proportion of body weight as musculoskeletal mass appears to be independent of stature. This stature-related organ-tissue “mix” likely in part explains the corresponding observation that mass-specific REE is inversely related to height. Our findings, including those based on predicted REE values, need to be confirmed and extended in larger ethnically diverse samples and in women.
Acknowledgments
The authors acknowledge the technical assistance of Chesley Heymsfield for help preparing the manuscript.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Atwood LD, Wolf PA, Heard-Costa NL, Massaro JM, Beiser A, D'Agostino RB, DeCarli C. Genetic variation in white matter hyperintensity volume in the Framingham Study. Stroke 35: 1609–1613, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Calder WA Jr. Size, Function, and Life History. Cambridge, MA: Harvard University Press, 1984
- 3.Chirachariyavej T, Limburanasombat S, Tiensuwan M. The relationship between bone and ash weight to body weight and body length of Thai corpses in Bangkok and central part of Thailand after cremation. J Med Assoc Thai 90: 1872–1878, 2007 [PubMed] [Google Scholar]
- 4.Chirachariyavej T, Ouyswat K, Sanggarnjanavanich S, Tiensuwan M, Peonim V, Sirikulchayanonta V. Normal internal organ weight of Thai adults correlated to body length and body weight. J Med Assoc Thai 89: 1702–1712, 2006 [PubMed] [Google Scholar]
- 5.Cox J, Jackson AP, Bond J, Woods CG. What primary microcephaly can tell us about brain growth. Trends Mol Med 12: 358–366, 2006 [DOI] [PubMed] [Google Scholar]
- 5a.Dekaban AS. Changes in brain weights during the span of human life: relation of brain weights to body heights and body weights. Ann Neurol 4: 345–356, 1978 [DOI] [PubMed] [Google Scholar]
- 5b.Food and Nutrition Board, Institute of Medicine of the National Academies.Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids Washington, D. C.: The National Academies Press, 2002. [http://www.nap.edu/books/0309085373/html/].
- 6.Freda PU, Shen W, Heymsfield SB, Reyes-Vidal CM, Geer EB, Bruce JN, Gallagher D. Lower visceral and subcutaneous but higher intermuscular adipose tissue depots in patients with growth hormone and insulin-like growth factor I excess due to acromegaly. J Clin Endocrinol Metab 93: 2334–2343, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gallagher D, Belmonte D, Deurenberg P, Wang Z, Krasnow N, Pi-Sunyer FX, Heymsfield SB. Organ-tissue mass measurement allows modeling of REE and metabolically active tissue mass. Am J Physiol Endocrinol Metab 275: E249–E258, 1998 [DOI] [PubMed] [Google Scholar]
- 8.Garby L, Lammert O, Kock KF, Thobo-Carlsen B. Weights of brain, heart, liver, kidneys, and spleen in healthy and apparently healthy adult Danish subjects. Am J Hum Biol 5: 291–296, 1993 [DOI] [PubMed] [Google Scholar]
- 9.Giedd JN. Structural magnetic resonance imaging of the adolescent brain. Ann NY Acad Sci 1021: 77–85, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Harris J, Benedict F. A Biometric Study of Basal Metabolism in Man. Washington, D. C.: Carnegie Institute of Washington, 1919
- 11.Hartmann P, Ramseier A, Gudat F, Mihatsch MJ, Polasek W. [Normal weight of the brain in adults in relation to age, sex, body height and weight.] Pathologe 15: 165–170, 1994 [DOI] [PubMed] [Google Scholar]
- 12.Hayes M, Chustek M, Wang Z, Gallagher D, Heshka S, Spungen A, Bauman W, Heymsfield SB. DXA: potential for creating a metabolic map of organ-tissue resting energy expenditure components. Obes Res 10: 969–977, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Heymsfield SB, Childers D, Beetsch J, Allison DB, Pietrobelli A. Body size and human energy requirements: reduced mass-specific resting energy expenditure in tall adults. J Appl Physiol 103: 1543–1550, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Heymsfield SB, Gallagher D, Mayer L, Beetsch J, Pietrobelli A. Scaling of human body composition to stature: new insights into body mass index. Am J Clin Nutr 86: 82–91, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hizli S, Abaci A, Büyükgebiz B, Büyükgebiz A. Nutritional stunting. Pediatr Endocrinol Rev 4: 186–195, 2007 [PubMed] [Google Scholar]
- 16.Holloway RL. Within-species brain-body weight variability: a reexamination of the Danish data and other primate species. Am J Phys Anthropol 53: 109–121, 1980 [DOI] [PubMed] [Google Scholar]
- 17.Ho KC, Roessmann U, Straumfjord JV, Monroe G. Analysis of brain weight. II. Adult brain weight in relation to body height, weight, and surface area. Arch Pathol Lab Med 104: 640–645, 1980 [PubMed] [Google Scholar]
- 18.Jones A Jr, Shen W, St-Onge MP, Gallagher D, Heshka S, Wang Z, Heymsfield SB. Body-composition differences between African American and white women: relation to resting energy requirements. Am J Clin Nutr 79: 780–786, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Kaitaniemi P. Testing the allometric scaling laws. J Theor Biol 228: 149–153, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Koh I, Lee MS, Lee NJ, Park KW, Kim KH, Kim H, Rhyu IJ. Body size effect on brain volume in Korean youth. Neuroreport 16: 2029–2032, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Lettre G, Jackson AU, Gieger C, Schumacher FR, Berndt SI, Sanna S, Eyheramendy S, Voight BF, Butler JL, Guiducci C, Illig T, Hackett R, Heid IM, Jacobs KB, Lyssenko V, Uda M; Diabetes Genetics Initiative; FUSION; KORA; Prostate, Lung Colorectal, and Ovarian Cancer Screening Trial; Nurses' Health Study; SardiNIA, Boehnke M, Chanock SJ, Groop LC, Hu FB, Isomaa B, Kraft P, Peltonen L, Salomaa V, Schlessinger D, Hunter DJ, Hayes RB, Abecasis GR, Wichmann HE, Mohlke KL, Hirschhorn JN. Identification of ten loci associated with height highlights new biological pathways in human growth. Nat Genet 40: 584–591, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lindstedt SL, Boyce MS. Seasonality, fasting endurance, and body size in mammals. Am Nat 125: 873–878, 1985 [Google Scholar]
- 23.Lusk G. The Science of Nutrition (4th ed.). Philadelphia, PA: Saunders, 1928
- 24.Millar JS, Hickling GJ. Fasting endurance and the evolution of mammalian body size. Funct Ecol 4: 5–12, 1990 [Google Scholar]
- 25.Narongchai P, Narongchai S. Study of the normal internal organ weights in Thai population. J Med Assoc Thai 91: 747–753, 2008 [PubMed] [Google Scholar]
- 26.Nevill AM, Holder RL. Scaling, normalizing, and per ratio standards: an allometric modeling approach. J Appl Physiol 79: 1027–1031, 1995 [DOI] [PubMed] [Google Scholar]
- 27.Nopoulos P, Flaum M, O'Leary D, Andreasen NC. Sexual dimorphism in the human brain: evaluation of tissue volume, tissue composition and surface anatomy using magnetic resonance imaging. Psychiatry Res 98: 1–13, 2000 [DOI] [PubMed] [Google Scholar]
- 28.Pakkenberg H, Voigt J. Brain weight of the Danes. Acta Anat (Basel) 56: 297–307, 1964 [Google Scholar]
- 29.Park S, Lee JK, Kim JI, Lee YJ, Lim YK, Kim CS, Lee C. In vivo organ mass of Korean adults obtained from whole-body magnetic resonance data. Radiat Prot Dosimetry 118: 275–279, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Passingham RE. Brain size and intelligence in man. Brain Behav Evol 16: 253–270, 1979 [DOI] [PubMed] [Google Scholar]
- 31.Peters M, Jäncke L, Staiger JF, Schlaug G, Huang Y, Steinmetz H. Unsolved problems in comparing brain sizes in Homo sapiens. Brain Cogn 37: 254–285, 1998 [DOI] [PubMed] [Google Scholar]
- 32.Powers JM. Practice guidelines for autopsy pathology. Autopsy procedures for brain, spinal cord, and neuromuscular system Autopsy Committee of the College of American Pathologists. Arch Pathol Lab Med 119: 777–783, 1995 [PubMed] [Google Scholar]
- 33.Pierson RN Jr, Wang J, Heymsfield SB, Russell-Aulet M, Mazariegos M, Tierney M, Smith R, Thornton JC, Kehayias J, Weber DA. Measuring body fat: calibrating the rulers. Intermethod comparisons in 389 normal Caucasian subjects. Am J Physiol Endocrinol Metab 261: E103–E108, 1991 [DOI] [PubMed] [Google Scholar]
- 34.Quetelet LAJ. A Treatise on Man and the Development of his Faculties Edinburgh: Chambers, 1842. In: Comparative Statistics in the 19th Century. Farnborough, UK: Gregg International, 1973
- 35.Shea BT, Hammer RE, Brinster RL. Growth allometry of the organs in giant transgenic mice. Endocrinology 121: 1924–1930, 1987 [DOI] [PubMed] [Google Scholar]
- 36.Skullerud K. Variations in the size of the human brain. Influence of age, sex, body length, body mass index, alcoholism, Alzheimer changes, and cerebral atherosclerosis. Acta Neurol Scand Suppl 102: 1–94, 1985 [PubMed] [Google Scholar]
- 37.Snyder WS, Cook MJ, Nasset ES, Karhausen LR, Howells GP, Tipton. Report of the Task Group on Reference Man. Oxford, UK: Pergamon, 1975
- 38.Spann W, Dustmann HO. [Weight of the human brain and its dependence on age, body length, cause of death and occupation.] Dtsch Z Gesamte Gerichtl Med 56: 299–317, 1965 [PubMed] [Google Scholar]
- 41.Webster JD, Hesp R, Garrow JS. The composition of excess weight in obese women estimated by body density, total body water and total body potassium. Hum Nutr Clin Nutr 38: 299–306, 1984 [PubMed] [Google Scholar]
- 42.Wheeler PE. The thermoregulatory advantages of large body size for hominids foraging in savannah environments. J Hum Evol 23: 351–362, 1992 [Google Scholar]
- 43.Willerman L, Schultz R, Rutledge JN, Bigler ED. In vivo brain size and intelligence. Intelligence 15: 223–238, 1991 [Google Scholar]
- 44.Witelson SF, Beresh H, Kigar DL. Intelligence and brain size in 100 postmortem brains: sex, lateralization and age factors. Brain 129: 386–398, 2006 [DOI] [PubMed] [Google Scholar]
- 45.Woods RP, Freimer NB, De Young JA, Fears SC, Sicotte NL, Service SK, Valentino DJ, Toga AW, Mazziotta JC. Normal variants of Microcephalin and ASPM do not account for brain size variability. Hum Mol Genet 15: 2025–2029, 2006 [DOI] [PubMed] [Google Scholar]


