Abstract
The sensory circumventricular organs (CVOs) are specialized collections of neurons and glia that lie in the midline of the third and fourth ventricles of the brain, lack a blood-brain barrier, and function as chemosensors, sampling both the cerebrospinal fluid and plasma. These structures, which include the organum vasculosum of the lamina terminalis (OVLT), subfornical organ (SFO), and area postrema (AP), are sensitive to changes in sodium concentration but the cellular mechanisms involved remain unknown. Epithelial sodium channel (ENaC)-expressing neurons of the CVOs may be involved in this process. Here we demonstrate with immunohistochemical and in situ hybridization methods that ENaC-expressing neurons are densely concentrated in the sensory CVOs. These neurons become c-Fos activated, a marker for neuronal activity, after various manipulations of peripheral levels of sodium including systemic injections with hypertonic saline, dietary sodium deprivation, and sodium repletion after prolonged sodium deprivation. The increases seen c-Fos activity in the CVOs were correlated with parallel increases in plasma sodium levels. Since ENaCs play a central role in sodium reabsorption in kidney and other epithelia, we present a hypothesis here suggesting that these channels may also serve a related function in the CVOs. ENaCs could be a significant factor in modulating CVO neuronal activity by controlling the magnitude of sodium permeability in neurons. Hence, some of the same circulating hormones controlling ENaC expression in kidney, such as angiotensin II and atrial natriuretic peptide, may coordinate ENaC expression in sensory CVO neurons and could potentially orchestrate sodium appetite, osmoregulation, and vasomotor sympathetic drive.
Keywords: area postrema, epithelial sodium channels, enac, organum vasculosum of the lamina terminalis, OVLT, subfornical organ
three blood-borne factors (angiotensin II, aldosterone, and sodium) act in the brain to affect sodium appetite (11). Peptide hormones, such as angiotensin II, bind to receptors in the sensory circumventricular organs (CVOs), which include the organum vasculosum of the lamina terminalis (OVLT), subfornical organ (SFO), and area postrema (AP). Aldosterone, the primary mineralocorticoid hormone, targets a subset of neurons in the nucleus tractus solitarius (NTS) that express the enzyme 11-β-hydroxysteroid dehydrogenase type 2 (HSD2), making them selectively responsive to this steroid (10). In addition, sodium depletion elicits c-Fos activation of the HSD2 neurons (10). The other brain sites that detect changes in plasma sodium levels are likely to reside in the CVOs (16). Plasma sodium levels are tightly maintained within a narrow range of ∼135–145 meq/l, and it is not clear how slight variations in sodium are sensed. Since sodium is the main determinant of plasma osmolality, it is conceivable that the CVOs function mainly as osmoreceptors (5) rather than sodium sensors. Regardless of the actual cellular mechanism(s) involved, CVO neurons detect changes in the chemical environment (solutes and peptides) in both the cerebrospinal fluid and plasma, and two of them, the OVLT and SFO, send efferent projections to nearby hypothalamic regions that are linked to downstream networks that regulate vasopressin release and the vasomotor sympathetic outflow (24). In contrast, the AP, via its connections to the HSD2 neurons (44) as well as to the other brain stem cell groups (48), are likely to modulate the forebrain sites that control the motor behaviors involved in sodium consumption (11, 46).
CVOs express epithelial sodium channels (ENaCs) (2). ENaCs (Scnn1) are nonvoltage-dependent, amiloride-sensitive sodium channels. These highly selective sodium channels conduct Na+ across the apical membrane of cells in salt-reabsorbing epithelia, such as in the distal nephron where they play a prominent role in regulating extracellular fluid volume, which is an important factor that determines blood pressure. In addition, the absorption of sodium via these channels determines the final concentration of urinary sodium. To date, the bulk of the research done on ENaCs has focused on the kidney (25), but ENaCs are present in other tissues, including in the sodium taste receptors of the tongue where they function as sodium detectors (6, 31). Besides central neurons (2, 50), ENaCs are expressed in astrocytes, endothelial cells, and the choroid plexus (2) raising the possibility that these channels participate in a range of different functions including the maintenance of critical Na+ levels in the extracellular space of the brain and spinal cord, regulation of [Na+] in the cerebrospinal fluid, and potentially detecting changes in plasma sodium levels. We were especially interested in the latter possibility. In the present study, the systemic sodium levels in rats were manipulated by various treatments, and after sufficient time for central nervous system (CNS) changes to occur, the patterns of c-Fos activity in ENaC-immunoreactive neurons of the sensory CVOs were analyzed.
c-Fos is a proto-oncogene that is a member of an immediate early gene family of transcription factors and is widely used as an indirect marker of neuronal activity (9, 23, 43). While most previous studies have used c-Fos immunohistochemistry as a marker for short-term neuronal changes (∼90–120 min), we found that this method is useful for detecting activated neurons after long-term periods of sodium deprivation (10). Thus in the present study we used this method to study the brain following both short- and long-term systemic sodium manipulations.
EXPERIMENTAL PROCEDURES
The experiments described here were approved by the Washington University School of Medicine (WUMS) Institutional Animal Care and Use Committee and followed National Institutes of Health guidelines. Sprague-Dawley adult male and female rats (Charles River Laboratories, Wilmington, MA) were provided access to tap water and standard rat chow (PicoLab rodent no. 20, 0.33% sodium; Lab-Diet, Richmond, IN) ad libitum. The rats were housed in a room with an automated lighting system: 12 h-12 h light-dark schedule (lights on at 5:30 AM; lights off at 5:30 PM) and with automatic climate control. The day before each experiment, the rats were housed in individual cages.
Normal material.
Between 7 and 10 AM, rats were anesthetized with freshly prepared 8.0% chloral hydrate (1 ml/100 g body wt ip; Sigma, St. Louis, MO) and perfused through the heart with 0.9% saline, followed by 4% paraformaldehyde in 0.1 M sodium phosphate buffer (pH = 7.4). The brains were removed and stored in fixative for 3–5 days. The brains were used for immunohistochemistry and in situ hybridization. All sections were cut in the transverse plane at 50 μm on a freezing microtome.
Immunohistochemistry (IHC).
Free floating sections were processed by a double indirect ABC immunofluorescence procedure that allowed for the covisualization of two different antigens. Triton-X 100 was omitted from all of the solutions to prevent the solubilization of ENaCs. All solutions were made in a 5% donkey serum in a 0.1 M sodium phosphate buffer (pH = 7.4) containing 0.01% sodium azide. All histochemical reactions were carried out on a rotary shaker at room temperature.
Frozen sections were incubated in rabbit anti c-Fos (1:8 K, EMD Millipore, Billerica, MA) or chicken anti c-Fos solution (1:1250; Abcam, Cambridge, MA) for 1–2 days, washed in 0.1 M potassium phosphate-buffered saline (KPBS, pH = 7.4), reacted with biotinylated donkey anti-rabbit or anti-chicken (1:250; Jackson ImmunoResearch, West Grove, PA) for 2.5 h, washed in KPBS, reacted with the ABC complex for 2 h (Vectastain Kit PK-4000, Vector Labs, Burlingame, CA), washed, and reacted with Cy3-streptavidin (1:250; Jackson) for 2 h, washed, and transferred to a rabbit anti-ENaC α-subunit antibody (1:1 K; StressMarq, Victoria, BC, Canada) or goat anti-ENaC α-subunit antibody solution (1:150, Everest Biotech, Oxfordshire, UK) for 1–2 days. The secondary ABC reaction for these ENaC antibodies followed the same protocol as just described, except biotinylated donkey anti-rabbit or donkey anti-goat antibodies, respectively, were used (1:250, Jackson). After 2.5 h incubation, followed by a KPBS wash, the sections were reacted with Cy2-streptavidin (1:250, Jackson). None of these dyes (Cy2 or Cy3) bled through when carried out as a single immunohistochemical procedure.
Antibodies.
A primary rabbit antibody against the ENaC α-subunit (Scnn1a) (SPC-403D, 1:1000; StressMarq Bioscience) was used. This antibody was produced against a synthetic peptide from the NH2-terminus of the ENaC α-subunit (amino acids 46–68, NP_113736; manufacturer antibody designation 3560-2). This antibody detects a single band at ∼85 kDa in membrane fractions from rat renal cortex in Western blots (18, 32). Immunostaining of brain stem sections was blocked when this antibody was preadsorbed with the peptide against which it was made. The ENaC α-subunit peptide had the following sequence: LGKGDKREEQGLGPEPSAPRQPTC-COOH and was purchased from Thermo Fisher Scientific (Rockford, IL). It was used at a concentration of 500 μg/ml in the blocking experiments. An alternative method sometimes used to demonstrate the specificity of an antibody depends on immunostaining tissue sections from knockout animals, but to date, no brain-specific ENaC knockout mice or rats are available. Finally, when the primary antibody was omitted from the immunostaining procedure, no staining resulted.
A goat antibody to the human ENaC α-subunit (EB08950: 1:150, Everest Biotech), which has a similar epitope as the rat, was also used. This was made against the 14-mer peptide EGNKLEEQDSSPPQ-COOH, which is a sequence found near the NH2 terminus of this protein. The specificity of this reagent was tested in double-labeling experiments. Sections from the rat medulla oblongata were double immunostained, first, with the rabbit antibody to the ENaC α-subunit and then, with the goat antibody to the human ENaC α-subunit. Because the motor neurons of the dorsal vagal and hypoglossal nuclei were robustly immunostained with both antibodies and the individual motor neurons spatially separated from each other, these two cell groups were excellent sites to compare the efficiency of the immunostaining of these two reagents. Even though the immunostaining obtained with the goat antiserum was weaker than the results obtained with the rabbit antiserum, both antibodies produced comparable results. The goat antibody detected 93% ± 1.4% of the dorsal vagal and hypoglossal neurons that were colabeled with the rabbit antibody. In a separate experiment, the 14-mer peptide that had been used to make the goat anti-ENaC antibody was mixed with the goat antibody (1:150 dilution) and used to immunostain sections. This peptide was used at a 667 μg/ml concentration. The addition of this peptide to the antibody solution blocked the immunostaining of the dorsal vagal and hypoglossal motor neurons.
A mouse monoclonal anti-NeuN antibody (NeuN; MAB377; 1:500; EMD Millipore) and a mouse monoclonal anti-tryptophan hydroxylase antibody (5-HT; synthetic enzyme for serotonin; MAB no. 5278; 1:4K; EMD Millipore) were also used in the present studies. These latter two antibodies have been documented as speciFic reagents in http://onlinelibrary.wiley.com/journal/10.1002/%28ISSN%291096-9861/homepage/jcn_antibody_database.htm.
In situ hybridization (ISH).
ENaC α-subunit probes were made using plasmids purchased from Open Biosystems (α: clone ID 7100777). cDNA fragments were generated by PCR using gene-specific primers coupled with T3 and T7 polymerase sequences, purchased from Integrated DNA Technologies (IDT, Coralville, IA; see Table 1).
Table 1.
Gene-specific primers
| Gene | Primer Sequence | Probe Length |
|---|---|---|
| α* | Forward: AATTAACCCTCACTAAAGGGGTCGCTTCAACCAGGCCCCC | 830 bp |
| Reverse: GTAATACGACTCACTATAGGCCACAGGCTCCACTGGCTGC | ||
| Forward: AATTAACCCTCACTAAAGGGGCTTGGTTGGCCCCGACTCC | 777 bp | |
| Reverse: GTAATACGACTCACTATAGGCTGCAGGGGGTGCGGGAAAG |
For epithelial sodium channel (ENaC) α-subunit, two probes were used in combination at 200 ng each per milliliter.
Digoxigenin (DIG) antisense RNA probes were made using PCR products as template and T7 RNA polymerase (Roche Diagnostics, Indianapolis, IN). Probes were made similarly using T3 polymerase (Promega, Madison, WI) and served as controls for determining nonspecific staining.
Frozen sections through the regions containing the CVOs were placed briefly in 0.01 M KPBS (pH = 7.4) and then transferred directly to prehybridization solution in tissue culture trays. Tissues were incubated for 1 h at room temperature and then 1 h at 37°C. The prehybridization buffer (pH =7.5) contained 0.6 M NaCl (Sigma, St. Louis, MO; Note: all other chemicals were purchased from Sigma except where noted), 0.1 M Tris·HCl, 0.01 M EDTA, 0.05% Na pyrophosphate, 5% dextran sulfate, 0.5 mg/ml yeast total RNA (Roche), 0.05 mg/ml yeast tRNA (Roche), 1× Denhardt's solution (Invitrogen, Carlsbad, CA), 50% formamide (Invitrogen), 0.05 mg/ml poly A, 10 μM of the four ribonucleotide triphosphates (Promega), 10 mM dithiothreitol, and 0.5 mg/ml herring sperm DNA. Probes were then added directly to wells at concentrations ranging from 400–600 ng/ml. Sections were incubated overnight at 55°C before being rinsed in 4x saline-sodium citrate (SSC) buffer/10 mM sodium thiosulfate, treated with RNaseA, rinsed in 2x SSC/10 mM sodium thiosulfate, and rinsed in 0.5x SSC at 37°C. Finally, sections were rinsed in 0.1x SSC for 1 h at 55°C.
After the rinses, sections were blocked in 10% normal horse serum (Invitrogen) and 0.1% Triton X-100 in Tris-buffered saline and then incubated in alkaline phosphatase labeled anti-DIG antibody (Roche) diluted 1:1000 in blocking solution overnight at 4°C. The next day, the sections were washed and colorized using nitro blue tetrazolium and 5-bromo-4-chloro-3-indolyl phosphate (Roche). The reaction was monitored with a dissecting microscope and stopped once the color reaction developed. Three separate 10-min washes were performed in 0.1 M Tris-1 mM EDTA, pH 8.5. Sections were then washed in KPBS, mounted on gelatin-coated glass slides, and cover slipped with 90% glycerol or UltraCruz mounting medium (SC-24941, Santa Cruz Biotechnology, Santa Cruz, CA), which contains 1.5 μg/ml of 4′,6-diamidino-2-phenylindole (DAPI) for DNA counterstaining. The cover glasses were sealed with finger nail polish; the slides were then stored in the refrigerator at 4°C.
Cell counts of the ENaC-expressing CVO neurons.
The data generated from the cell counts were intended as a way to describe the density of ENaC α-positive cells in the CVOs and compare them to nearby sites; they were not meant as a full catalogue of ENaC-expressing sites, which can be found at http://connectivity.brain-map.org/transgenic/experiment/81709690 in the Allen Brain Atlas.
A combination of direct and indirect fluorescence optics was used together to photoimage the CVOs and nearby brain regions. Since these preparations were cover slipped with a mounting medium that contained DAPI (4′,6-diamidino-2-phenylindole, which is a fluorescent dye that binds to the A-T rich regions of DNA), the nuclei were visible with fluorescence optics and the ENaC α-subunit positive cell bodies were visualized with brightfield optics.
Photomontages of sections from the AP, SFO, and OVLT were made. For the AP and surrounding region (n = 3 rats), the sections were sampled at 200-μm intervals starting with the rostral section at the bregma −14.04 mm level. The SFO (n = 4 rats) was sampled at 150-μm intervals, beginning at the bregma −1.08 mm level. The OVLT (n = 4 rats) were sampled at three levels: bregma −0.00 mm, −0.12 mm, and −1.08 mm levels, respectively. Bilateral cell counts were made from each region using the MetaMorph program (Molecular Devices, Sunnyvale, CA). Cell densities for each area were averaged, and the final value was expressed as a density measurement, namely, the number of ENaC-expressing cells per unit area. For control counts, we included the median preoptic region (MnPO) and supraoptic hypothalamic nucleus (SON) because both sites are known to be involved in fluid homeostasis. In the brain stem, three control sites were selected because of their proximity to the AP: these were the hypoglossal nucleus (XII), dorsal vagal nucleus (DMX), and NTS. All group data are presented as means ± SE. These mean values were evaluated by the one-way ANOVA and two-tailed Student's t-tests in GraphPad Prism software (San Diego, CA). A P value of ≤0.05 was chosen as signifying a level of statistical significance.
Digital images.
Brightfield images were taken on a Nikon microscope using a CCD camera with Nikon ACT-1 software (v2.62). Image cropping, resizing, and adjustments in brightness, contrast, sharpness, and color balance were performed using Adobe Photoshop CS3 (San Jose, CA).
Confocal immunofluorescence images were obtained with an Olympus Fluoview FV500b laser-scanning microscope using either ×20 (NA 1.17) or ×40 (NA 1.35) oil objective lens in steps of 0.621 or 0.311 μm, respectively, through the tissue section. Image resolution was 1024 × 1024 pixels. One pixel in the X-Y plane was the minimum unit of resolution that covered an area roughly 0.6 μm ×0.6 μm. The z-frames were collapsed to a two-dimensional image. Photomontages were constructed, and adjustments in brightness and contrast were made using the Adobe Photoshop program. Manipulations of the confocal stacks, z-frame projections and pseudocoloration were performed using MetaMorph software (Molecular Devices). Cytoarchitectonic boundaries were added to the photoimages with the aide of the Adobe Illustrator program.
Hypertonic and isotonic saline injections.
On the day of the experiment, rats (350–400 g) were injected intraperitoneally with 2 ml of either hypertonic (2M) or isotonic (0.15M) NaCl at 8 to 9:30 AM, and then returned to their home cage for a 2-h period. Then the animals were anesthetized with 8% chloral hydrate (ip) and perfused through the heart with saline, followed by fixative (as above). Brains were removed and stored in fixative for 2–5 days and then were processed for immunohistochemistry.
Sodium deprivation and sodium repletion experiments.
Rats were housed in individual cages with wire screen floors to prevent these animals from being able to eat their waste products (a potential source of sodium). For 1 mo, they ate a low-sodium chow (0.01% Na, no. 85292, Harlan-Teklad; Madison, WI) and drank distilled water ad libitum. At the termination of the experiment, the rats were divided into three groups. Group 1 (n = 10) was anesthetized with 8.0% chloral hydrate and perfused as described above. Group 2 (n = 12) was presented with two water bottles: one filled with distilled water and the other with 2M NaCl solution. Group 3 (n = 4) was given a bottle containing 0.15M NaCl solution; preliminary studies showed that under these experimental conditions the rats did not drink distilled water and thus in this experiment a second bottle with only distilled water was not used. The amount of fluids that were consumed from both sources was recorded. After 2 h, the rats in groups 2 and 3 were anesthetized with chloral hydrate and perfused. The brains from these animals were removed and stored in fixative for 1 wk before a double immunohistochemical procedure for c-Fos and ENaC-α subunit.
Plasma sodium measurements.
A separate group of 24 rats was used for measurement of plasma sodium levels after various manipulations: intraperitoneal injections (2 ml) with 0.15 M or 2.0 M NaCl; sodium deprivation (4 wk); sodium repletion with 0.15 M NaCl after 4 wk of sodium deprivation; and sodium repletion with 2.M NaCl after 4 wk of sodium deprivation (see data in Fig. 5).
Fig. 5.
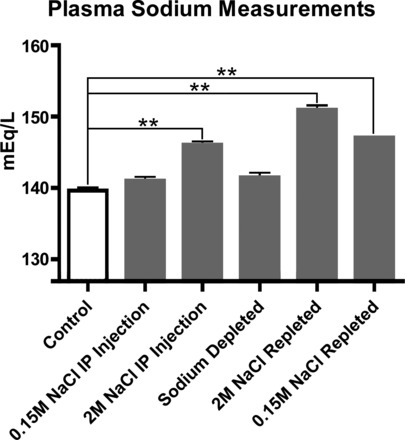
Plasma sodium concentrations after sodium injections, sodium deprivation, and sodium repletion. The plasma [Na+] values from normal rats (control) versus rats that received isotonic saline injections were not statistically different. Rats that received hypertonic saline injections or were sodium repleted had higher sodium plasma levels than control animals (P < 0.001). Sodium-deprived rats showed a small increase in plasma sodium levels compared with normal rats (P < 0.05), suggesting that some homeostatic mechanism withdraws sodium from sodium reservoirs, such as bone, to buffer extreme plasma sodium derangements.
To obtain the plasma samples, the rats were anesthetized with 8% chloral hydrate ip, and 3 to 4 ml of blood were drawn from the left ventricle of the heart in a 10-ml syringe containing heparin (50 Units in 0.5 ml, Abbott, Chicago, IL). Blood plasma was immediately separated by centrifugation (5,000 rpm, 5 min) and sent for determination of the plasma sodium concentration performed in the Core Laboratory for Clinical Studies in the Department of Medicine (WUMS, St. Louis, MO).
c-Fos immunohistochemistry.
A c-Fos antibody generated in rabbits against the peptide SGFNADYEASSSRC that corresponds to amino acids 4–17 of human c-Fos (1:8K; PC38, Calbiochem/EMD Millipore) or a polyclonal c-Fos antibody generated in chickens against the synthetic peptide corresponding to amino acids 168–380 of human c-Fos.(1:1250; ab14285, Abcam, Cambridge, MA) was used in the analysis of the CVOs. In a separate set of experiments, rats (n = 4) were injected intraperitoneally with 2 ml of 2 M NaCl, and after 2 h, the animals were anesthetized and perfused as above. Serial sections through the SFO were immunostained with rabbit anti c-Fos, followed by the chicken anti c-Fos antibody. Cells counts were made in a 1-in-2 series from the SFO sections, and we found that 89 ± 0.4% c-Fos activated identified with the rabbit anti c-Fos antibody were also immunostained with the chicken anti c-Fos antibody, indicating that the chicken antibody had a slightly lower avidity for c-Fos than the rabbit antibody.
All three CVOs were processed by a double immunofluorescence method to determine the colocalization of c-Fos and ENaC α-subunit immunoreactivity. For a general description of double-labeling procedures see recent publications from our laboratory (36, 37).
RESULTS
The anatomical nomenclature of the rat CVOs as described by McKinley and colleagues (35) is used in this report, with slight modifications for the terminology of the AP. All data are expressed as means ± SE.
Immunohistochemical (IHC) procedures.
ENaC α-subunit immunostaining was found throughout the AP, SFO, and OVLT. Figure 1 presents examples of ENaC α-expressing neurons. In Fig. 1A, ENaC+ neurons in the dorsal cap of the OVLT neurons have ENaC immunoreactivity in their cell bodies and NeuN immunostaining in their nuclei. Similar results were present in the lateral zone of the OVLT (data not shown). In Fig. 1B, the ventromedial core and outer shell of the SFO contain α-ENaC+ cell bodies and these neurons coexpress NeuN immunoreactivity in their nuclei. In Fig. 1C, ENaC α-subunit and tryptophan hydroxylase (synthetic enzyme for serotonin, 5-HT; hence, we will refer to these cells as “5-HT neurons”) was coexpressed in AP neurons; the 5-HT cells represent one neuronal phenotype in the AP that projects to the parabrachial region (37).
Fig. 1.
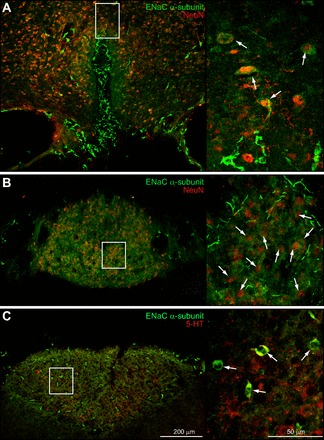
Epithelial sodium channel (ENaC) α-subunit immunoreactivity is present in the sensory circumventricular organs (CVOs). A: ENaC α-subunit immunostaining was localized in both the dorsal cap and lateral zone of the organum vasculosum of the lamina terminalis (OVLT). The higher-power photoimage (right) shows colocalization of ENaC α-subunit and NeuN immunoreactivity (arrows). B: ENaC α-immunoreactivity was distributed throughout the subfornical organ (SFO). The higher-power photoimage (right) provides an example of ENaC α-subunit and NeuN colocalization in the SFO ventromedial core (arrows). C: ENaC α-subunit immunostaining was colocalized in the tryptophan hydroxylase immunoreactive area postrema (AP) neurons (arrows), and these were concentrated in the ventrolateral part of this CVO. This enzyme is involved in the synthesis of serotonin (5-HT).
In situ hybridization (ISH) preparations of the sensory CVOs.
ISH preparations showing mRNA localization of the ENaC α-subunit in the OVLT, SFO, and AP are presented in Fig. 2. The anti-sense probes hybridized with cells in all three CVOs. In the OVLT, dense collections of labeled cells were seen in the dorsal cap and lateral zone of this CVO (Fig. 2A); sense probes resulted in no cell labeling in the OVLT (Fig. 2A′). In the SFO, the anti-sense probes show the presence of closely packed, darkly stained cells in the ventromedial core and outer shell of this structure (Fig. 2B); the control tissues showed virtually no labeling throughout the body of the SFO. In the AP preparations, dense cell labeling was seen throughout this CVO (Fig. 2C). The control preparations of the AP showed no cell labeling in this CVO. Some slight residual staining was observed, however, along the outermost edge of the AP (Fig. 2C′). The subpostremal NTS region had virtually no α-ENaC+ cells, and the medial NTS had only relatively few α-ENaC+ cells. The motor neurons of the DMX and hypoglossal nucleus (XII) were strongly α-ENaC+, and in the control preparations the staining of the DMX and XII motor neurons was greatly reduced but not completely absent (Fig 2C′). Perinuclear labeling was present in the DMX and XII motor neurons, but this did not extend into cell bodies or proximal dendrites and may be related to the histochemical procedure. This does not obscure the principal findings for the AP neurons since the control preparations showed no ISH staining. Finally, it is important to note that ISH data corresponded precisely with the IHC findings.
Fig. 2.
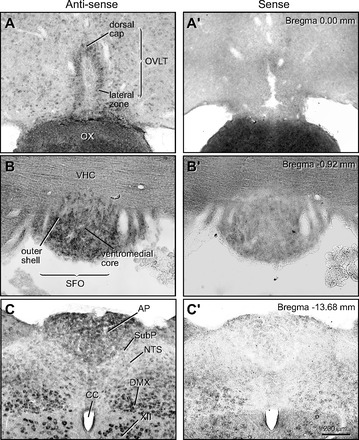
In situ hybridization (ISH) preparations showing mRNA ENaC α-subunit localization in the sensory CVOs. A: anti-sense ISH preparation showing ENaC α-subunit mRNA in the OVLT. Both the dorsal cap and lateral zone of the OVLT had labeled neurons. A′: no cellular staining was seen in the sense (control) ISH preparations. B: cell bodies in the ventromedial core and outer shell of the SFO labeled by anti-sense ISH preparation for the ENaC α-subunit mRNA. B′: sense (control) ISH preparations of SFO had almost no cell body labeling. C: anti-sense preparation of the AP showed a dense concentration of labeled cells, especially in its dorsal region. No signal was found in the subpostremal region (SubP) and only moderate labeling was seen in the medial part of the nucleus tractus solitarius (NTS). Dorsal motor nucleus of vagus nerve (DMX) and hypoglossal nerve (XII) showed strong cell body labeling. C′: the sense (control) ISH preparation of the dorsal medulla had virtually no signal in the AP. CC, central canal, OX, optic chiasm, VHC, ventral hippocampal commissure.
Cell counts of the ISH preparations.
The ENaC cell counts for the individual brain regions were as follows: SFO = 8.11 ± 1.1.7; OVLT 6.92 ± 0.27; AP = 6.33± 0.17; SON = 5.34± 0.28; NTS = 3.87± 0.12; MnPO = 3.52± 0.16; DMX = 1.96±0.14; and XII = 0.6±0.02 cells/μm2 × 10−4. Each of these cell counts was tested by a one-way ANOVA. Statistical differences among the three CVOs (SFO, OVLT, and AP) were not found. However, each of the CVOs was statistically different from the non-CVO regions (MnPO, NTS, DMX, XII) at a level of P < 0.01 or 0.001, except for the SON. The AP and OVLT were not statistically different from the SON, but the SFO was different by P < 0.01.
Figure 3 presents histograms that summarize these results. When the data from these cell counts were regrouped so a broader comparison could be made, namely, the cell density in the CVOs versus the non-CVOs (SON, MnPO, NTS, DMX, and XII), we found the ENaC cell population in the CVOs was roughly two times greater than the non-CVO areas. The cell density for the CVOs was 7.19 ± 0.46 versus non-CVO regions was 3.14±0.37 cells/μm2 × 10−4. These groups were tested by the two-tailed Student's t-test, and differed by P < 0.0001. Thus the CVOs have clearly more α-ENaC+ cells per unit area than the nearby brain regions. Since we did not make a complete analysis of the distribution of α-ENaC+ neurons throughout the brain, we are not making the claim that the CVOs are the most ENaC-rich region of the brain.
Fig. 3.
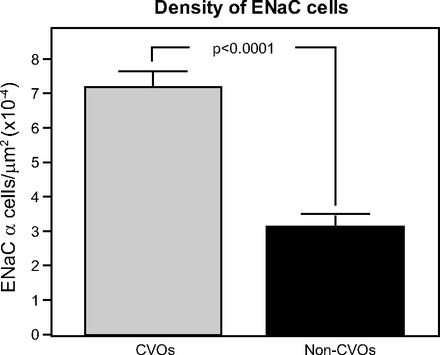
ENaC cell density in the CVOs was higher than nearby brain regions, which included the median preoptic nucleus (MnPO) and supraoptic hypothalamic nucleus (SON) (two sites involved in fluid and electrolyte regulation). The histogram compares the number of ENaC-positive cells found in CVOs with nearby brain regions. Bars represent means ± SE. Two-tailed Student's t-test shows statistical differences between the number of ENaC-expressing neurons in the CVOs and other brain regions.
c-Fos activation of the ENaC-expressing neurons of the CVOs following systemic sodium changes.
Figure 4 illustrates the five experimental conditions that were used to examine the c-Fos activation pattern in the ENaC-expressing CVO neurons following peripheral manipulations of sodium levels. Table 2 presents the results showing plasma sodium concentrations after these various manipulations.
Fig. 4.
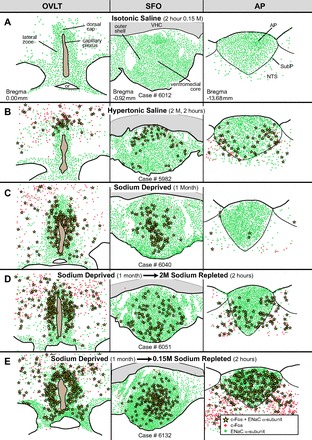
Line drawings made from photoimages of the sensory CVOs to show differences in the patterns of c-Fos activity after various peripheral sodium manipulations. ENaC α-subunit cells are indicated by green dots, c-Fos activated neurons are shown by red dots, and ENaC-expressing neurons that were c-Fos activated are illustrated by yellow stars. A: isotonic saline injections did not produce c-Fos activation of any of the CVOs. B: hypertonic saline injections induced c-Fos activity in the dorsal cap of the OVLT, outer shell of the SFO, as well as in the ventrolateral AP. C: dietary sodium deprivation for 1 mo induced c-Fos activity in the lateral zone of the OVLT and ventromedial core of the SFO and almost had no effect in the AP. D: sodium repletion, after 1 mo of sodium deprivation, with 2M NaCl resulted in c-Fos activation in the lateral zone of the OVLT, outer shell of the SFO and the ventrolateral zone of the AP. E: sodium repletion, after 1 mo of sodium deprivation, with 0.15M NaCl elicited widespread c-Fos activation in all three sensory CVOs.
Table 2.
Sodium and volume load after saline injections and repletion
| Weight, g | Sodium Injected, mg | Sodium Consumed, mg | Sodium/Weight, mg/g | Saline Injected, ml | Saline Consumed, ml | Water Consumed, ml | Liquid/Weight, ml/g | |
|---|---|---|---|---|---|---|---|---|
| Injection | ||||||||
| 0.15M NaCl | 321.5 ± 9.22 | 6.89 ± 0 | N/A | 0.022 ± 0.001 | 2.0 ± 0 | N/A | N/A | 0.006 ± 0.000 |
| 2M NaCl | 328.67 ± 5.34 | 91.96 ± 0 | N/A | 0.28 ± 0.004 | 2.0 ± 0 | N/A | N/A | 0.006 ± 0.000 |
| Repletion | ||||||||
| 0.15M NaCl | 175.5 ± 4.99 | N/A | 237.08 ± 26.69 | 1.35 ± 0.13 | N/A | 68.75 ± 7.74 | N/A | 0.39 ± 0.04 |
| 2M NaCl | 359.5 ± 18.75 | N/A | 214.57 ± 19.39 | 0.6 ± 0.05 | N/A | 4.67 ± 0.42 | 20.17 ± 3.96 | 0.07 ± 0.01 |
Values are means ± SE. N/A, not available. Sodium manipulations were used to elevate c-Fos activation patterns in epithelial sodium channel (ENaC)-expressing neurons of the sensory circumventricular organs (CVOs) The rats that received hypertonic saline injections were given 13 times more sodium per body weight than the animals receiving isotonic saline injections. Furthermore, sodium-repleted rats had sodium/body weight levels that greatly exceeded the levels seen after the hypertonic saline injections. This increase was by a factor of 5. The 0.15M NaCl repletion rats consumed 2.25 more times sodium per gram of body weight than the 2M-repleted rats. Sodium-repleted rats ingested more volume per body weight than was injected into the rats treated with hypertonic saline. The rats used in the 2M NaCl repletion and 0.15M NaCl repletion experiments drank 11 and 64 times, respectively, more volume per body weight than was injected.
Injections of isotonic saline (Fig. 4A) did not produce c-Fos activation of any of the CVOs. This treatment did not affect plasma sodium levels (Fig. 5). In contrast, the hypertonic saline (Fig. 4B) induced c-Fos activity in the dorsal cap of the OVLT, outer shell of the SFO, as well as in the ventrolateral zone of the AP. Injections of hypertonic saline increased plasma sodium concentration by 4.6% (Fig. 5). After sodium deprivation (Fig. 4C), c-Fos activity was found predominantly in the lateral zone of the OVLT and ventromedial core of the SFO, and none was found in the AP. This treatment resulted in a 1.4% increase in plasma sodium concentration which was not significant (Fig. 5). In the sodium repletion experiments (Fig. 4, D and E), the OVLT showed a similar c-Fos expression pattern as seen in the sodium deprivation experiments wherein c-Fos activity was confined mainly to the lateral zone of the OVLT. In addition, in the repletion experiments, the patterns were the same, but the density of c-Fos activation varied as a result of the sodium concentration used. In the 2M NaCl repletion experiments (Fig. 4D), the outer shell of the SFO and ventrolateral zone of the AP were c-Fos activated, which was similar to the results found in the hypertonic saline experiments. These experiments resulted in an 8.2% increase in plasma sodium (Fig. 5). In the 0.15M NaCl repletion experiments (Fig. 4E), c-Fos activity in all three CVOs was widespread and extremely dense. Plasma sodium levels increased 5.3% (Fig. 5).
The differences between the two types of repletion experiments may relate to the taste pleasantness of the two NaCl solutions; the 2M solution would be repugnant while the 0.15 M would be highly pleasant given the length of time these animals were sodium deprived. As shown in Table 2, these two groups of rats consumed very large differences in the amount of sodium and fluid consumed. The 0.15M NaCl repletion group drank more than twice the amount of sodium per gram of body weight and over five times the volume of fluids per body weight than the 2M NaCl repletion group. These two differences may also account for considerably more c-Fos labeling in the CVOs of the 0.15M NaCl-repleted rats compared with the rats that consumed 2M NaCl.
Finally, there were topographical differences in the c-Fos expression in the AP in the repletion experiments, as well as in the hypertonic saline injection cases. The rostralmost AP sections were nearly devoid of c-Fos activity, whereas the caudalmost sections had large numbers of c-Fos expressing neurons. Colabeled ENaC and c-Fos neurons are shown in Fig. 6.
Fig. 6.
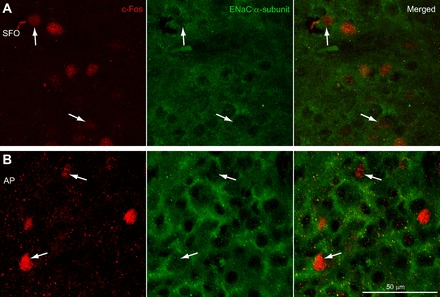
ENaC α-subunit colocalization in c-Fos activated neurons. A: ENaC-expressing SFO neurons were c-Fos activated following hypertonic saline injections. B: ENaC-expressing AP neurons were c-Fos activated following hypertonic saline injections.
DISCUSSION
Major findings.
This study demonstrates that: 1) the concentration of ENaC-expressing neurons in the sensory CVOs greatly exceeds that found in nearby brain sites by ∼2 times; 2) ENaC-expressing CVO neurons become c-Fos activated following intraperitoneal injections of hypertonic saline, while comparable injections of isotonic saline had no effect and these findings are in agreement with a previous study (22); 3) sodium deprivation induces c-Fos activation of ENaC-expressing neurons in the OVLT and SFO but has no noticeable effect in the AP; the latter result is different from what has been reported in sodium depletion experiment in which the diuretic drug furosemide was used to induce NaCl loss (35) perhaps because this drug blocks GABA-A receptors in the brain (29); 4) sodium repletion elicits c-Fos activation of ENaC-expressing neurons in all three sensory CVOs, especially in the AP; and 5) increases in c-Fos activity in the CVOs were correlated with increases plasma sodium concentration.
Because c-Fos activity was present in the OVLT and SFO 1 mo following sodium deprivation, the repletion experiments are complicated by this residual c-Fos labeling. When comparing the c-Fos patterns found in the sodium-repleted versus sodium-deprived animals, sodium repletion elicited additional c-Fos activity in the outer shell of the SFO and dorsal cap of the OVLT. Also, the density of c-Fos activity in the lateral zone of the OVLT appeared greater than in the sodium deprivation cases. However, the important underlying observation seems to be that neurons in the OVLT and SFO react to any significant change in sodium intake, either excessive intake or deficient intake. One interpretation of this observation is that these two regions are involved in balancing sodium intake or plasma levels. The fact that different regions of the OVLT and SFO are active during deprivation versus repletion seems to suggest that the heterogeneity of these regions may reflect either positive or negative control systems. In marked contrast to these two regions, the AP was inactive during sodium deprivation but active during any condition of elevated plasma sodium. This observation is consistent with previous studies that have shown that the AP is an important negative regulator of sodium appetite, which limits excessive sodium intake (for review see Ref. 11). This could mean that activity in the AP leads to diminished sodium appetite, whereas activity in parts of the OVLT or SFO that are uniquely active during sodium deprivation could conceivably be directed at increasing sodium appetite.
The actual mechanism (s) responsible for the c-Fos activation of the CVO neurons described here remains unknown. Manipulations of sodium levels may be one factor, but we cannot exclude the possibility that osmolality changes and/or the action of various hormones including angiotensin II may have been involved. Nevertheless, the key observation of the present study is the ENaC-expressing neurons of the CVOs are affected by these changes.
A number of different types of receptors have been identified in the SFO and AP using gene chips (19, 20), but this type of analysis is limited because it does not determine whether these putative receptors are localized in neurons, astrocytes, or endothelial cells. Thus single-cell analyses need to be performed to further advance our information regarding the CVOs. Moreover, the microarray studies do not distinguish whether or not there are unique regional distributions of receptors within the neuronal subpopulations within the AP, SFO, or OVLT, which potentially have unique functions. For example, are there variations with the ventromedial core versus the outer shell of the SFO, such has been shown by McKinley and coworkers (35).
Both OVLT and SFO were activated in high and low sodium states, but the pattern of c-Fos activation within each of these CVOs is distributed in a spatially complementary manner. In states of low sodium, the ventromedial core of the SFO was c-Fos activated, whereas in high-sodium states, the outer shell was activated. Moreover, the c-Fos activation patterns of the SFO between the two high-sodium states were different. When a normal rat was injected with hypertonic saline, the dorsal section of the outer shell of the SFO was heavily c-Fos positive (c-Fos+) with only a few c-Fos+ cells found in the more ventral sites.
When sodium-deprived rats were repleted with 2M or 0.15M sodium, analysis of the c-Fos pattern was complicated by the fact that residual c-Fos activated neurons from the sodium-deprived state were surely still present in the SFO and OVLT. In the sodium-deprived state, the ventromedial core of the SFO contained c-Fos+ cells, but c-Fos activity was also present in this site in the sodium-repleted state. There is no method that we aware of which would permit us to distinguish between “new” versus “old” c-Fos-activated neurons. Nonetheless, we favor the interpretation that both types of c-Fos+ neurons were present. Note, however, that more c-Fos+ neurons were present in outer shell of the SFO, especially in its ventral and ventrolateral parts, than present in the SFO during the sodium-deprived state. Since quantitative studies were not performed to verify these observations, future studies need to explore this issue. Finally, it is not clear whether the c-Fos activation seen in the outer shell of the SFO is directly related to sodium intake or is due to plasma osmolality changes due to the consumption of large volumes of saline.
The OVLT showed different “new” versus “old” c-Fos patterns of activation as well. After injection of hypertonic saline, only the dorsal cap was c-Fos+, whereas in both sodium-depleted and -repleted animals, the main site of c-Fos activation was the lateral zone of the OVLT. The sodium-repleted animals still showed c-Fos activation in the dorsal cap. Finally, the c-Fos expression pattern in OVLT and SFO for the sodium-depleted state appears similar to what has been reported following exogenous administration of angiotensin II (33, 34, 49) with the exception that the AP was not affected. In the sodium-deprived state, the SFO and OVLT neurons may have been c-Fos activated as the result of increased levels of circulating angiotensin II or possibly other hormones.
The AP showed almost no c-Fos activity in the sodium-deprived state. In contrast, the ventrolateral AP region was highly activated following hypertonic saline injections or after sodium repletion, suggesting that the latter group of AP neurons function as part of a negative feedback system. Presumably, these neurons are part of an ascending pathway that reaches the forebrain reward centers and causes a cessation of the motor activity associated with sodium consumption. One other point to be noted is that very large numbers of c-Fos+ neurons were present in the core and ventrolateral region of the AP in the repletion experiments. The total number c-Fos+ neurons found in the 0.15M experiments appeared to exceed that found than in the 2M repletion experiments. The reason for this difference is unknown but may be due to very large fluid volumes (∼40–70 ml) that were consumed in the former experiments.
Previous studies implicating the role of central ENaCs in control of blood pressure.
Brain ENaCs may be involved in the control of blood pressure since continuous intracerebroventricular infusion of benzamil, an amiloride analogue which selectively blocks these channels, prevents various forms of experimental hypertension. Benzamil prevented mineralocorticoid hypertension produced by chronic infusion of aldosterone (14) or deoxycorticosterone, a precursor in the aldosterone biosynthesis pathway (1). Central administration of benzamil also blocked the development of hypertension in Dahl salt-sensitive rats (15) and spontaneously hypertensive rats (39).
Benzamil was used at nanomolar or low micromolar concentrations in these experiments, which is within the range where it produces selective blockade of ENaCs (28). Central administration of other amiloride-related drugs, some of which had a greater affinity for the Na+/Ca2+ exchanger than ENaCs, also blocked DOCA-salt induced hypertension (26). Since it is unknown whether the latter drugs had blockade effects on ENaCs as well, some confusion remains because it is unknown whether the drug effect acts on brain ENaCs alone or in combination with the Na+/Ca2+ exchanger. Finally, the brain sites where the amiloride drugs act also remain unknown.
Hormonal changes occur after plasma sodium manipulations.
To relate our findings to previous studies, a useful starting point is to compare the present c-Fos data from the sodium repletion experiments with the c-Fos data from isotonic volume expansion studies (13, 40). As shown in Table 2, sodium-repleted animals consumed large amounts of fluids as they were in the process of reestablishing bodily sodium levels. One group of rats that drank both saline plus water consumed a total of ∼25 ml in a 2-h period (5 ml of 2 M NaCl and 20 ml of water); the other group drank ∼70 ml of 0.15M NaCl in 2 h. These intake volumes represent ∼7% and ∼40%, respectively, of the total body weight of these animals. Such large fluid ingestions almost certainly caused an expansion of blood volume that exceeded those used in previous studies (13, 40). Presumably, these volumes elicited similar hormonal responses as demonstrated by Godino and her colleagues, namely, large increases of atrial natriuretic peptide (ANP) and oxytocin, along with a decrease in vasopressin (13). All three of these neuropeptides target the brain, and one of them in particular, ANP, binds intensely to the SFO, OVLT, and AP (12, 30, 42). Considerably less is known regarding oxytocin and vasopressin binding sites in the CVOs. [3H]vasopressin binding occurs in the SFO and AP, whereas no [3H]oxytocin binding was found in any of the sensory CVOs (51). Furthermore, a potent nonpeptide vasopressin antagonist SR-49059 which binds preferentially to V1a receptors produced intense labeling in the SFO and AP but not in the OVLT (52). One possibility is that volume expansion elicits c-Fos activity in the CVO neurons due to parallel cellular mechanisms: one acting on ANP receptors and the other utilizing ENaCs. Previous studies in the kidney have shown that ENaCs are regulated by ANP (17, 53), and determining whether similar mechanisms are operational in the sensory CVO neurons will be an important area for future studies.
Under conditions of sodium restriction, angiotensin levels increase and this peptide has been shown to regulate ENaCs in the kidney (4). In the brain, it is possible that a similar type of dual cellular mechanism affects particular subsets of sensory CVO neurons where both ENaCs and angiotensin receptors are part of a common signaling system.
ENaCs as a component of the sodium signaling system in the sensory CVOs.
ENaCs belong to a family of channels that permit Na+ entry into cells. Unlike voltage-dependent sodium channels that are gated by voltage changes and carry the major inward current of action potentials in excitable cells, ENaCs lack a voltage sensor and allow inward sodium current to enter cells at all physiological potentials.
Conceivably, ENaCs could be key players in the CVOs that sense the initial phase of plasma Na+ changes and translate these chemical signals into neural activity affecting three separate but related centrally controlled functions: sodium appetite, sodium-induced changes affecting the cardiosympathetic outflow, and release of vasopressin. However, the direct sensing of extracellular sodium concentrations via a mechanism involving ENaCs would require them to discriminate sodium concentrations within a narrow range of plasma sodium ion variation. Such a requirement makes this an unlikely possibility. On the other hand, the activity and/or synthesis of ENaCs may respond to plasma sodium ion variation indirectly. Because of the absence of a blood-brain barrier in the sensory CVOs, CVO neurons are directly exposed to the plasma and may respond electrically to circulating hormones such as angiotensin II that bind to the receptors found in these sites. Whether individual neurons coexpress ENaCs and angiotensin II receptors or other receptors involved in sodium homeostasis is unknown and is an important issue that needs to be explored in future studies.
How would increased expression of ENaCs change membrane electrical excitability? The increased expression or activity of ENaCs would increase sodium leak current into the cell resulting in the cell's resting potential being moved closer to the action potential threshold producing an overall increase in neuronal excitability. Sodium leak currents have long been recognized as producing an increase in cell excitability (8, 47) and are believed to be a key factor in seizure disorders (41). As a result, many anti-epileptic compounds are preferential inhibitors of a portion of sodium leak current carried by voltage-dependent sodium channels. Under normal physiological conditions, sodium leak current carried by voltage-dependent sodium channels regulate cell excitability, as shown by their mediation of the pattern of bursting in neurons of the SFO (54). In addition to voltage-dependent sodium channels, it is becoming increasingly clear that ENaCs also carry a significant portion of persistent sodium leak current. ENaCs have been shown to be a significant determinant of membrane resting potential in both nonneuronal cells (7) and neuronal cells (50) where they regulate excitability in magnocellular cells of the rat supraoptic and paraventricular hypothalamic nuclei. Overall, increased ENaC activity in CVO neurons would depolarize the membrane potential and change their electrical firing pattern. Whether the net effect would be excitation or inhibition depends on the pool of other channel types present in the cell as well as the ENaC current. Depending on the magnitude of the ENaC sodium current, net excitability would result in neurons which were moved closer to their action potential threshold; in contrast, net inhibition would result in cells subjected to tonic depolarization where excitable channels are moved into a stable inactivated state. As mentioned, both voltage-dependent sodium channels and ENaCs produce persistent sodium currents and both may work hand-in-hand to control cell excitability. Each has its advantages and drawbacks. Persistent sodium currents from voltage-dependent sodium channels are voltage sensitive and thus open only in a particular voltage range, and vary in their amplitude. Furthermore, they may prove more difficult to regulate because the same channels produce both transient and persistent Na+ currents and downregulation of a single channel type will downregulate both persistent and transient Na currents. Alternatively, ENaCs are voltage independent (27) and can tune cell excitability over the entire voltage range. Overall, the flexibility of the system to modulate the electrical output of the CVOs is very great, and if common circulating hormones target ENaCs in a parallel way in kidney and CVOs, these hormones could well coordinate the brain-mediated functions such as sodium appetite as well as affect sodium uptake in the kidney.
Sensitivity of CVOs in detecting small fluctuations in osmolality.
SFO neurons detect osmotic changes as small as 1 mOsm, and these changes are translated into ∼0.1-Hz changes in firing frequency of these neurons (3). This suggests another indirect way that ENaCs could participate in responding to fluctuations in plasma sodium concentrations. Because of the very high sodium selectivity of these channels, cells with high levels of ENaC expression might be particularly sensitive to osmotic imbalances caused by sodium ion concentrations per se, thus enabling them to discriminate between osmotic changes caused by fluctuations in sodium versus other cations. Small changes in neuronal firing frequency, such as those noted by Anderson and coworkers (3), may be act in combination with specialized G protein-coupled receptors (GPCRs), such as those that have been implicated in sodium-water balance functions like vasopressin, angiotensin II, oxytocin, and/or ANP. Thus the parallel actions of ENaCs and GPCRs may ultimately determine the electrical activity of the CVO neurons.
Other types of sodium channels of importance for detection of plasma sodium levels.
In the SFO and OVLT, astrocytes have been described that express Nax channels (Scn7a) and function as sodium sensors (55). The cells open their Nax channels in response to increased extracellular Na+, which permits Na+ to enter these cells (21). This resulted in increased anaerobic glucose metabolism in these cells, causing an elevation in intracellular lactate (45). As the lactate builds up, it eventually becomes dispersed throughout the CVO and exerts a paracrine effect by exciting local GABA neurons (45). The Nax channel is probably not directly involved in the stimulation of sodium appetite because it is activated by large increases in extracellular sodium that exceed 150 mM such as occurs during periods of prolonged water deprivation or after ingestion of hypertonic saline (11). Nax channels are also present in the neurons of the OVLT, SFO, and AP (38), so more work will be needed to resolve their role in the regulation of sodium appetite and blood pressure.
Perspectives and Significance
Intraperitoneal injections of hypertonic saline elicited c-Fos activation of ENaC+ neurons in all three CVOs, while isotonic saline injections were without an effect. Sodium deprivation resulted in c-Fos activation of ENaC-expressing neurons in the OVLT and SFO but not in the AP. However, sodium repletion following 1 mo of sodium deprivation produced c-Fos activation in ENaC+ neurons in AP, as well as the OVLT, and SFO. These data raise the possibility that ENaCs expressed in CVO neurons may underlie a common mechanism for coordinating a concert of many aspects essential to sodium ion metabolism. Such aspects may include osmoregulation, sodium appetite, and central regulation of vasomotor sympathetic drive. Since all of these factors must be tightly coordinated with sodium uptake in kidney, it is plausible that circulating hormonal factors, such as angiotensin II, ANP, and related hormones as well as solutes (Na+) that modulate ENaC expression in kidney may also affect the ENaC-expressing neurons of the CVOs.
GRANTS
This study was supported by National Institutes of Health Grants RO1-HL-25449 (to A. D. Loewy), RO1-HL-089742 (to P. A. Gray), and R01-NS0661871-01 (to L. B. Salkoff), and NS057105, Neuroscience Blueprint Core Grant, as well as the Bakewell Imaging Center Fund.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: R.L.M. and A.D.L. conception and design of research; R.L.M. and M.H.W. performed experiments; R.L.M. and A.D.L. analyzed data; R.L.M., P.A.G., and A.D.L. interpreted results of experiments; R.L.M. and A.D.L. prepared figures; R.L.M., M.H.W., P.A.G., L.B.S., and A.D.L. drafted manuscript; R.L.M., M.H.W., P.A.G., L.B.S., and A.D.L. edited and revised manuscript; R.L.M., M.H.W., P.A.G., L.B.S., and A.D.L. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Xay Van Nguyen for expert help with the surgery and histology, Marcy Hartstein for excellent work creating the computer graphics, and Dennis Oakley of the Bakewell Neuroimaging Laboratory for assistance with the confocal microscopy.
REFERENCES
- 1.Abrams JM, Engeland WC, Osborn JW. Effect of intracerebroventricular benzamil on cardiovascular and central autonomic responses to DOCA-salt treatment. Am J Physiol Regul Integr Comp Physiol 299: R1500–R1510, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amin MS, Wang HW, Reza E, Whitman SC, Tuana BS, Leenen FH. Distribution of epithelial sodium channels and mineralocorticoid receptors in cardiovascular regulatory centers in rat brain. Am J Physiol Regul Integr Comp Physiol 289: R1787–R1797, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Anderson JW, Washburn DL, Ferguson AV. Intrinsic osmosensitivity of subfornical organ neurons. Neuroscience 100: 539–547, 2000 [DOI] [PubMed] [Google Scholar]
- 4.Beutler KT, Masilamani S, Turban S, Nielsen J, Brooks HL, Ageloff S, Fenton RA, Packer RK, Knepper MA. Long-term regulation of ENaC expression in kidney by angiotensin II. Hypertension 41: 1143–1150, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Bourque CW. Central mechanisms of osmosensation and systemic osmoregulation. Nature Rev Neurosci 9: 519–531, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Chandrashekar J, Kuhn C, Oka Y, Yarmolinsky DA, Hummler E, Ryba NJ, Zuker CS. The cells and peripheral representation of sodium taste in mice. Nature 464: 297–301, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chavez JC, de la Vega-Beltran JL, Escoffier J, Visconti PE, Trevino CL, Darszon A, Salkoff L, Santi CM. Ion permeabilities in mouse sperm reveal an external trigger for SLO3-dependent hyperpolarization. PLos One 8: e60578, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crill WE. Persistent sodium current in mammalian central neurons. Annu Rev Physiol 58: 349–362, 1996 [DOI] [PubMed] [Google Scholar]
- 9.Dragunow M, Faull R. The use of c-fos as a metabolic marker in neuronal pathway tracing. J Neurosci Methods 29: 261–265, 1989 [DOI] [PubMed] [Google Scholar]
- 10.Geerling JC, Engeland WC, Kawata M, Loewy AD. Aldosterone target neurons in the nucleus tractus solitarius drive sodium appetite. J Neurosci 26: 411–417, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geerling JC, Loewy AD. Central regulation of sodium appetite. Exp Physiol 93: 177–209, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Gibson TR, Wildey GM, Manaker S, Glembotski CC. Autoradiographic localization and characterization of atrial natriuretic peptide binding sites in the rat central nervous system and adrenal gland. J Neurosci 6: 2004–2011, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Godino A, Giusti-Paiva A, Antunes-Rodrigues J, Vivas L. Neurochemical brain groups activated after an isotonic blood volume expansion in rats. Neuroscience 133: 493–505, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Gomez-Sanchez EP, Gomez-Sanchez CE. Effect of central amiloride infusion on mineralocorticoid hypertension. Am J Physiol Endocrinol Metab 267: E754–E758, 1994 [DOI] [PubMed] [Google Scholar]
- 15.Gomez-Sanchez EP, Gomez-Sanchez CE. Effect of central infusion of benzamil on Dahl S rat hypertension. Am J Physiol Heart Circ Physiol 269: H1044–H1047, 1995 [DOI] [PubMed] [Google Scholar]
- 16.Gourine AV, Kasparov S. Astrocytes as brain interoceptors. Exp Physiol 96: 411–416, 2011 [DOI] [PubMed] [Google Scholar]
- 17.Guo LJ, Alli AA, Eaton DC, Bao HF. ENaC is regulated by natriuretic peptide receptor-dependent cGMP signaling. Am J Physiol Renal Physiol 304: F930–F937, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hager H, Kwon TH, Vinnikova AK, Masilamani S, Brooks HL, Frokiaer J, Knepper MA, Nielsen S. Immunocytochemical and immunoelectron microscopic localization of α-, β-, and γ-ENaC in rat kidney. Am J Physiol Renal Physiol 280: F1093–F1106, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Hindmarch C, Fry M, Yao ST, Smith PM, Murphy D, Ferguson AV. Microarray analysis of the transcriptome of the subfornical organ in the rat: regulation by fluid and food deprivation. Am J Physiol Regul Integr Comp Physiol 295: R1914–R1920, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Hindmarch CC, Fry M, Smith PM, Yao ST, Hazell GG, Lolait SJ, Paton JF, Ferguson AV, Murphy D. The transcriptome of the medullary area postrema: the thirsty rat, the hungry rat and the hypertensive rat. Exp Physiol 96: 495–504, 2011 [DOI] [PubMed] [Google Scholar]
- 21.Hiyama TY, Watanabe E, Ono K, Inenaga K, Tamkun MM, Yoshida S, Noda M. Na(x) channel involved in CNS sodium-level sensing. Nat Neurosci 5: 511–512, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Ho JM, Zierath DK, Savos AV, Femiano DJ, Bassett JE, McKinley MJ, Fitts DA. Differential effects of intravenous hyperosmotic solutes on drinking latency and c-Fos expression in the circumventricular organs and hypothalamus of the rat. Am J Physiol Regul Integr Comp Physiol 292: R1690–R1698, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Hoffman GE, Smith MS, Verbalis JG. c-Fos and related immediate early gene products as markers of activity in neuroendocrine systems . Front Neuroendocrinol 14: 173–213, 1993. [DOI] [PubMed] [Google Scholar]
- 24.Johnson AK, Loewy AD. Circumventricular organs, and their role in visceral functions. In: Central Regulation of Autonomic Functions, edited by Loewy AD, Spyer KM. New York: Oxford University Press, 1990, p. 247–267 [Google Scholar]
- 25.Kashlan OB, Kleyman TR. Epithelial Na(+) channel regulation by cytoplasmic and extracellular factors. Exp Cell Res 318: 1011–1019, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keep RF, Si X, Shakui P, Ennis SR, Betz AL. Effect of amiloride analogs on DOCA-salt-induced hypertension in rats. Am J Physiol Heart Circ Physiol 276: H2215–H2220, 1999 [DOI] [PubMed] [Google Scholar]
- 27.Kellenberger S, Schild L. Epithelial sodium channel/degenerin family of ion channels: a variety of functions for a shared structure. Physiol Rev 82: 735–767, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Kleyman TR, Cragoe EJ., Jr Amiloride and its analogs as tools in the study of ion transport. J Membr Biol 105: 1–21, 1988 [DOI] [PubMed] [Google Scholar]
- 29.Korpi ER, Kuner T, Seeburg PH, Luddens H. Selective antagonist for the cerebellar granule cell-specific gamma-aminobutyric acid type A receptor. Mol Pharmacol 47: 283–289, 1995 [PubMed] [Google Scholar]
- 30.Kurihara M, Saavedra JM, Shigematsu K. Localization and characterization of atrial natriuretic peptide binding sites in discrete areas of rat brain and pituitary gland by quantitative autoradiography. Brain Res 408: 31–39, 1987 [DOI] [PubMed] [Google Scholar]
- 31.Lin W, Finger TE, Rossier BC, Kinnamon SC. Epithelial Na+ channel subunits in rat taste cells: localization and regulation by aldosterone. J Comp Neurol 405: 406–420, 1999 [DOI] [PubMed] [Google Scholar]
- 32.Masilamani S, Kim GH, Mitchell C, Wade JB, Knepper MA. Aldosterone-mediated regulation of ENaC alpha, beta, and gamma subunit proteins in rat kidney. J Clin Invest 104: R19–R23, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McKinley MJ, Allen AM, Burns P, Colvill LM, Oldfield BJ. Interaction of circulating hormones with the brain: the roles of the subfornical organ and the organum vasculosum of the lamina terminalis. Clin Exp Pharmacol Physiol 25: S61–R67, 1998 [DOI] [PubMed] [Google Scholar]
- 34.McKinley MJ, Badoer E, Oldfield BJ. Intravenous angiotensin II induces Fos-immunoreactivity in circumventricular organs of the lamina terminalis. Brain Res 594: 295–300, 1992 [DOI] [PubMed] [Google Scholar]
- 35.McKinley MJ, McAllen RM, Davern P, Giles ME, Penschow J, Sunn N, Uschakov A, Oldfield BJ. The sensory circumventricular organs of the mammalian brain. Adv Anat Embryol Cell Biol 172: 1–122, 2003 [DOI] [PubMed] [Google Scholar]
- 36.Miller RL, Knuepfer MM, Wang MH, Denny GO, Gray PA, Loewy AD. Fos-activation of FoxP2 and Lmx1b neurons in the parabrachial nucleus evoked by hypotension and hypertension in conscious rats. Neuroscience 218: 110–125, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller RL, Stein MK, Loewy AD. Serotonergic inputs to FoxP2 neurons of the pre-locus coeruleus and parabrachial nuclei that project to the ventral tegmental area. Neuroscience 193: 229–240, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nehme B, Henry M, Mouginot D, Drolet G. The expression pattern of the Na(+) sensor, Na(X) in the hydromineral homeostatic network: a comparative study between the rat and mouse. Front Neuroanat 6: 26, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nishimura M, Ohtsuka K, Nanbu A, Takahashi H, Yoshimura M. Benzamil blockade of brain Na+ channels averts Na(+)-induced hypertension in rats. Am J Physiol Regul Integr Comp Physiol 274: R635–R644, 1998 [DOI] [PubMed] [Google Scholar]
- 40.Randolph RR, Li Q, Curtis KS, Sullivan MJ, Cunningham JT. Fos expression following isotonic volume expansion of the unanesthetized male rat. Am J Physiol Regul Integr Comp Physiol 274: R1345–R1352, 1998 [DOI] [PubMed] [Google Scholar]
- 41.Rogawski MA, Loscher W. The neurobiology of antiepileptic drugs. Nat Rev Neurosci 5: 553–564, 2004 [DOI] [PubMed] [Google Scholar]
- 42.Saavedra JM. Regulation of atrial natriuretic peptide receptors in the rat brain. Cell Mol Neurobiol 7: 151–173, 1987 [DOI] [PubMed] [Google Scholar]
- 43.Sagar SM, Sharp FR, Curran T. Expression of c-fos protein in brain: metabolic mapping at the cellular level. Science 240: 1328–1331, 1988 [DOI] [PubMed] [Google Scholar]
- 44.Sequeira SM, Geerling JC, Loewy AD. Local inputs to aldosterone-sensitive neurons of the nucleus tractus solitarius. Neuroscience 141: 1995–2005, 2006 [DOI] [PubMed] [Google Scholar]
- 45.Shimizu H, Watanabe E, Hiyama TY, Nagakura A, Fujikawa A, Okado H, Yanagawa Y, Obata K, Noda M. Glial Nax channels control lactate signaling to neurons for brain [Na+] sensing. Neuron 54: 59–72, 2007 [DOI] [PubMed] [Google Scholar]
- 46.Shin JW, Geerling JC, Stein MK, Miller RL, Loewy AD. FoxP2 brainstem neurons project to sodium appetite regulatory sites. J Chem Neuroanat 42: 1–23, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stafstrom CE, Schwindt PC, Crill WE. Repetitive firing in layer V neurons from cat neocortex in vitro. J Neurophysiol 52: 264–277, 1984 [DOI] [PubMed] [Google Scholar]
- 48.Stein MK, Loewy AD. Area postrema projects to FoxP2 neurons of the pre-locus coeruleus and parabrachial nuclei: brainstem sites implicated in sodium appetite regulation. Brain Res 1359: 116–127, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sunn N, McKinley MJ, Oldfield BJ. Circulating angiotensin II activates neurones in circumventricular organs of the lamina terminalis that project to the bed nucleus of the stria terminalis. J Neuroendocrinol 15: 725–731, 2003 [DOI] [PubMed] [Google Scholar]
- 50.Teruyama R, Sakuraba M, Wilson LL, Wandrey NE, Armstrong WE. Epithelial Na+ sodium channels in magnocellular cells of the rat supraoptic and paraventricular nuclei. Am J Physiol Endocrinol Metab 302: E273–E285, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tribollet E. Vasopressin, and oxytocin receptors in the rat brain. In: Handbook of Chemical Neuroanatomy, edited by Bjorklund A, Hokfelt T. Amsterdam: Elsevier, 1992, p. 289–320 [Google Scholar]
- 52.Tribollet E, Raufaste D, Maffrand J, Serradeil-Le Gal C. Binding of the non-peptide vasopressin V1a receptor antagonist SR-49059 in the rat brain: an in vitro and in vivo autoradiographic study. Neuroendocrinology 69: 113–120, 1999 [DOI] [PubMed] [Google Scholar]
- 53.Wang W, Li C, Nejsum LN, Li H, Kim SW, Kwon TH, Jonassen TE, Knepper MA, Thomsen K, Frokiaer J, Nielsen S. Biphasic effects of ANP infusion in conscious, euvolumic rats: roles of AQP2 and ENaC trafficking. Am J Physiol Renal Physiol 290: F530–F541, 2006 [DOI] [PubMed] [Google Scholar]
- 54.Washburn DL, Anderson JW, Ferguson AV. A subthreshold persistent sodium current mediates bursting in rat subfornical organ neurones. J Physiol 529: 359–371, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Watanabe E, Hiyama TY, Shimizu H, Kodama R, Hayashi N, Miyata S, Yanagawa Y, Obata K, Noda M. Sodium-level-sensitive sodium channel Na(x) is expressed in glial laminate processes in the sensory circumventricular organs. Am J Physiol Regul Integr Comp Physiol 290: R568–R576, 2006 [DOI] [PubMed] [Google Scholar]


