Abstract
Motor learning often involves situations in which the somatosensory targets of movement are, at least initially, poorly defined, as for example, in learning to speak or learning the feel of a proper tennis serve. Under these conditions, motor skill acquisition presumably requires perceptual as well as motor learning. That is, it engages both the progressive shaping of sensory targets and associated changes in motor performance. In the present study, we test the idea that perceptual learning alters somatosensory function and in so doing produces changes to human motor performance and sensorimotor adaptation. Subjects in these experiments undergo perceptual training in which a robotic device passively moves the subject's arm on one of a set of fan-shaped trajectories. Subjects are required to indicate whether the robot moved the limb to the right or the left and feedback is provided. Over the course of training both the perceptual boundary and acuity are altered. The perceptual learning is observed to improve both the rate and extent of learning in a subsequent sensorimotor adaptation task and the benefits persist for at least 24 h. The improvement in the present studies varies systematically with changes in perceptual acuity and is obtained regardless of whether the perceptual boundary shift serves to systematically increase or decrease error on subsequent movements. The beneficial effects of perceptual training are found to be substantially dependent on reinforced decision-making in the sensory domain. Passive-movement training on its own is less able to alter subsequent learning in the motor system. Overall, this study suggests perceptual learning plays an integral role in motor learning.
Keywords: motor learning, perceptual learning, reaching movement, sensorimotor adaptation
motor learning is typically studied in the laboratory using sensorimotor adaptation tasks in which well-defined sensory targets are perturbed experimentally to study the characteristics of the subsequent adaptation. Procedures of this sort are used widely for studies of visuomotor adaptation (Krakauer et al. 1999), for force field learning (Shadmehr and Mussa-Ivaldi 1994), and for prism adaptation (Held and Hein 1958). However, much of initial skill learning involves situations in which the somatosensory targets of movement are poorly defined. Under these circumstances it is likely that perceptual experience and feedback, rather than the well-studied situations involving error-based learning, play a primary role in early learning by providing specificity to sensory targets and enabling subsequent sensorimotor adaptation.
There has been recent interest in the idea that factors other than sensory error contribute to human motor learning. Diedrichsen et al. (2010) showed that movement repetition, in the absence of load and the absence of error, alters the extent of subsequent force field adaptation. Huang et al. (2011) showed that there is a benefit to movement repetition, which is separate from that related to sensory error, in the context of visuomotor adaptation. Izawa and Shadmehr (2011) showed that reward and reinforcement on their own are capable of producing sensorimotor adaptation. Together these studies document the involvement in motor learning of mechanisms other than those typically associated with error-based adaptation. However, it is unclear whether the effects observed in these procedures that entail reinforcement and repetition result from benefits to sensory or motor function or the two in combination. In the present study, we have separated experimental manipulations of perceptual and motor function in time to assess the contribution to motor learning of somatosensory perceptual training. We find that perceptual learning even in the absence of active movement produces systematic changes to error-based learning.
There have been previous studies that have examined the effects of sensory training on subsequent somatosensory (Carey et al. 2002; Pleger et al. 2003) and motor performance (Carel et al. 2000; Lewis and Byblow 2004; Lotze et al. 2003). However, the possibility that perceptual learning contributes directly to motor learning has been little explored. In a study by Rosenkranz and Rothwell (2012) it was found that somatosensory discrimination training increased the excitability of primary motor cortex and improved measures of human motor learning. Wong et al. (2012) reported that passive movement of the arm along a desired trajectory increased the extent of motor learning. Vahdat et al. (2012) showed that perceptual learning results in changes to motor areas of the brain, suggesting that changes that occur in motor systems during motor skill acquisition may be partially attributable to perceptual learning.
In the present article, we ask if sensory training can result in perceptual change that is reflected in subsequent sensorimotor adaptation. We hypothesize that perceptual training helps to shape the sensory targets that guide motor learning. We will use the term sensory target or goal as a label to indicate a trajectory or vector of desired sensory values, a sensory plan that serves to regulate the generation of movements. We show that somatosensory feedback can shift the sensed position of the limb and improve perceptual acuity. We find that the sensory changes that result from this procedure can affect both the rate and the extent of motor learning, regardless of whether the perceptual training serves to increase or decrease movement related error. Our findings suggest that perceptual learning plays an integral role in motor learning and sensorimotor adaptation.
MATERIALS AND METHODS
Subjects and experimental conditions.
Seventy-two healthy right-handed subjects (29 men, 43 women, ages 18–45 yr) participated in our experiments. Subjects were excluded before testing if they had participated previously in studies of force field learning. All subjects were briefed on the experiment and signed a written consent form. The Institutional Review Board of McGill University approved all the experimental procedures.
The experiments involved a behavioral task in which subjects were seated in front of a two-degree of freedom robotic arm (InMotion2, Interactive Motion Technologies) and held the handle of the robot with their right hand. Seat height was adjusted for each subject to have 70° of shoulder abduction. An air sled supported the subject's arm, and seat straps were used to restrain the subject's trunk. A semi-silvered mirror placed just below eye level was used to project the target and hand position. The mirror blocked vision of the arm and the robot handle. Two 16-bit optical encoders at the robot's joints provided the position of the hand (Gurley Precision Instruments). Applied forces were measured using a force-torque sensor (ATI Industrial Automation) that was mounted below the robot handle.
Subjects in the main experiment were randomly assigned to one of four different conditions (Fig. 1). For the first three groups of subjects (n = 14 subjects in each group), the experiment was completed in a single session. The experiment begins with null field trials to establish a movement baseline. In these trials, the robot applies no forces to the subject's hand. These are followed by a sensory training procedure. Afterward, subjects repeat a second set of null field movements and then two sets of force field learning trials.
Fig. 1.
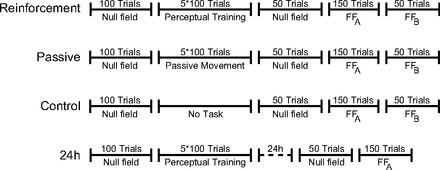
Schematic illustration showing the testing sequence in each of the experimental conditions. FFA and FFB, force field A and force field B.
In all trials involving reaching movements (both null and force field conditions), subjects moved from a start position to an end position. The start position was ∼25 cm from the subject's chest along the body midline. Two circles, 1.5 cm in diameter, represented movement start and end points. The target position was 15 cm from the start in the sagittal plane. A smaller yellow circle (0.5 cm in diameter) provided feedback of hand position. Subjects were asked to move as straight as possible. Subjects were instructed to finish each movement in 700 ms following a visual cue. This duration was chosen because it is similar in magnitude to that of normal reaching movements of comparable amplitude. After completion of each trial, visual feedback of movement speed was provided. However, no trials were removed for movements faster or slower than the required duration. Visual feedback of the target and hand position was removed as soon as the subject left the start position. The target and cursor position reappeared at the end of movement. Subjects were instructed not to correct any end-point error when visual feedback was reintroduced. At the end of the trial, the robot moved the subject's hand straight back to the start position, without visual feedback.
The experiment started with 100 null field trials. Null field movements were followed by sensory training trials that were conducted in the absence of visual feedback. Subjects completed 5 blocks of 100 trials each, in which the robot moved the arm outward on one of a set of fan-shaped trajectories that deviated from the body midline by up to 8° to the right or left (Fig. 2A). Subjects in a somatosensory discrimination group were required to judge on each trial whether the robot had moved the arm to the right or left. In the last three blocks of perceptual training (300 trials), feedback on accuracy was given orally to provide reinforcement. A second group of subjects was tested in a passive movement condition in which the robot moved the arm through the same set of trajectories as those experienced by subjects in the discrimination group; however, no judgment was required and no feedback was given. A third set of subjects had no perceptual training at all and simply remained seated in the experimental setup for a period equivalent to that involved in perceptual training. These subjects served as a control group. In the first two blocks of trials in the somatosensory discrimination condition, feedback on judgment accuracy was withheld to provide a baseline measure of perceptual function before supervised training.
Fig. 2.
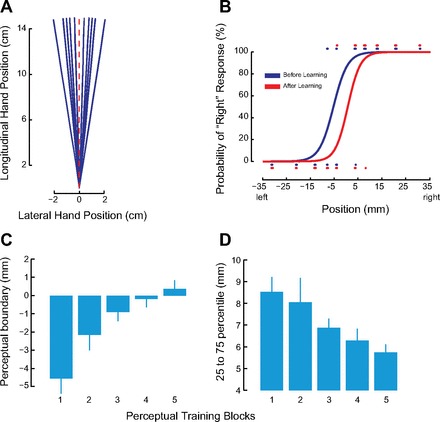
Somatosensory perceptual learning changes sensed limb position and perceptual acuity. A: the robot passively displaced the subject's arm along 1 of 10 trajectories. Top-down view shows the entire fan-shaped displacement pattern. B: systematic shift in the psychometric function of a representative subject as a result of perceptual reinforcement learning. Dots show tested limb positions and binary responses (blue represents start of training, red shows end of training). C: perceptual boundary changes over the course of training (mean over all subjects in the somatosensory discrimination group, ±SE). D: perceptual acuity increases with learning. As acuity increases, the distance between the 25th and 75th percentile of the psychometric function decreases (means ± SE).
In the perceptual training blocks, the robot was programmed to passively move the subjects' arm through 10 fan-shaped trajectories that were distributed equally to the right or left of the midline (Fig. 2A). All of the passive movements had the same velocity profile and were 15 cm in length. Visual feedback of target and handle position was eliminated as soon as the robot started the passive movement. We used lateral deviations of 8°, 5°, 4°, 3°, and 1.5° in both directions relative to the midline for sensory training. Each block of perceptual training involved 100 trials with the above angles tested 4, 10, 10, 14 and 12 times each, respectively.
Subjects were instructed not to resist the action of the robot to minimize active involvement of the motor system in the sensory training procedure. To assess this, we examined the forces that subjects applied to the robot handle during this procedure to estimate active motor force production during perceptual training. For subjects in the passive movement condition, to ensure that they were attending to the passive movements, on 10% of trials we briefly displayed the cursor position halfway through the passive movement and required subjects to report all such instances.
After sensory training, all subjects completed a second set of null field movements (50 trials). This was followed by 150 movements in a counterclockwise force field that pushed the subject's hand to the left in proportion to hand velocity. A final block of the experiment involved another 50 reaching movements in a clockwise field that pushed the hand to the right. The final block enabled us to assess the effect of perceptual training on anterograde interference, that is, on how the first force field learning task affected the learning of an opposite field.
The force field was applied according to Eq. 1:
| (1) |
where x and y are lateral and sagittal directions, fx and fy are the commanded force to the robot and vx and vy are hand velocities in Cartesian coordinates, and D defines the direction of force field. For the clockwise force field, D = 1, whereas in the counterclockwise condition, D = −1.
On five predefined trials (15, 85, 135, 139, and 143) during movements with a counterclockwise load, the robot was programmed to restrict subjects' movements to a straight-line connecting start and target points (“channel trials”). On these trials, the lateral deviation of the subject's hand was resisted by the robot (Scheidt et al. 2000). The stiffness and viscosity of the channel walls were set to 5,000 N/m and 50 N·s·m−1, respectively. These trials were used to record the lateral forces that subjects applied to the channel walls. These were compared with the ideal force that would be necessary to fully compensate for the robot-applied load, given the velocity of the hand (Eq. 1) and thus served as a measure of motor learning.
We also tested a fourth group of subjects (n = 10), for whom the experiment was divided into two sessions, which took place on 2 consecutive days (24-h group; see Fig. 1). The protocol for this 24-h group was similar to that of the somatosensory discrimination group except that we added a 24-h delay between the end of the perceptual training procedure and the subsequent null and force field trials. The 24-h group was not tested on the final clockwise force field at the end of the experiment. Subjects in this group did perceptual training with lateral deviations of 8°, 5°, 4°, 3°, and 1.5°. These were the same as those used in the other conditions.
In a control experiment, 20 new subjects were randomly assigned to one of two groups. The experimental procedures, with one exception, were identical to those of subjects in somatosensory discrimination and control groups of the main experiment (see Fig. 1). The difference was the direction of the force field. During force field learning trials, a clockwise rather than a counterclockwise field was used. During the trials, which followed, the direction of the force field was reversed. All other aspects of the experimental procedures were the same as those in the corresponding conditions in the main experimental sequence. The purpose of this control was to evaluate whether the effects of perceptual training were sensitive to the magnitude of kinematic error associated with direction of the force field.
Data analysis.
Hand position and the force applied by the subject to the robot handle were both sampled at 400 Hz. The recorded signals were low-pass filtered at 40 Hz using a zero-phase lag second-order Butterworth filter. Position signals were numerically differentiated to produce velocities. The start and end of each trial were defined at 5% of peak tangential velocity. For analysis purposes, we calculated the perpendicular deviation of the hand at maximum velocity (PD) from a straight line connecting start and end positions. In this way, we obtained quantitative estimates of movement straightness that were used to assess learning.
For each experimental condition, we calculated the average PD on each trial in each force field condition. We assessed the change in PD over trials by fitting a single exponential function as a simple approximation to the data. In the counterclockwise condition, the equation takes the form P = a(1 − e−bn) + c. In this equation, P is the PD on trial n. This continuous domain equation can be well approximated in the discrete domain by P(n) = a[1 − 1(1 − b)n] + c, where b is the rate of learning. To obtain a robust estimate of the parameters, before fitting we smoothed the PD data using a nine-trial moving average window. To estimate the rate of learning in the clockwise force field condition, we used the following discrete domain equation: P(n) = a(1 − b)n + c.
For each experimental condition, we also calculated the average of PD in the first null field condition, the second null field condition (the final 50 trials in each case), and over the last 10 trials in the counterclockwise force field condition when performance had reached asymptotic levels. Two subjects (1 in somatosensory discrimination group and 1 in passive movement group) were removed from further analyses because their PD values in the null field or force field conditions fell outside of ±3 SD from the intersubject mean. We tested for differences in PD using repeated-measures ANOVA followed by Bonferroni-Holm-corrected comparisons.
We also quantified motor learning by measuring the lateral force in channel trials, normalized by the ideal force needed to fully compensate for the force field. We defined a force index (FI) as follows:
| (2) |
where fx(t) is the force applied by the subject in the lateral direction and vy(t) is the velocity in the direction of movement. The value 15 is the coefficient relating the applied force to hand velocity.
We further assessed learning by estimating the accuracy of the predictive control during force channel trials. To do so, we measured the time lag between normalized measures of the lateral force on the channel wall and the ideal force calculated from hand velocity that is needed to fully compensate the force field. The normalization scaled both measured and ideal force profiles by the peak ideal force in each channel trial to disentangle the effects of timing from force amplitude. Smaller time lags indicate better prediction of the expected force. The time lag between the two force profiles was estimated at the point at which the subject reached half of the maximum applied force on that trial. This point was used for this calculation rather than the peak force, because the force profile was found in some cases to be noisy around the peak. As an additional measure, we also calculated the time to reach 5% of the lateral applied force peak following movement start. This served as an estimate of the onset of the preparatory response.
Perceptual training.
The subject's perception of the boundary between left and right was estimated using the method of constant stimuli. Each block of perceptual training had 100 trials. We obtained an estimate of the perceptual boundary between right and left for each subject separately by fitting a logistic function to that subject's entire set of lateral deviations and associated binary (right/left) responses. The 50% point of the psychometric function was taken as the perceptual boundary. The distance between the 25th and 75th percentile was used as a measure of perceptual acuity. A smaller distance indicates a higher sensitivity in the discrimination task.
RESULTS
We studied the effects of perceptual learning on motor function by using a perceptual training task in which a robotic device passively moves the arm, which is hidden from view, outward along one of a set of fan-shaped paths (Fig. 2A). We tested separate groups of subjects using different versions of the somatosensory training protocol. Subjects in a somatosensory discrimination group were required to judge whether the robot displaced the hand to the right or the left of the midline, and feedback on response accuracy was provided. Subjects in a passive movement condition experienced passive limb displacements identical to those of the first group, but no decision was required and no feedback was given. These two tests let us determine the extent to which any improvements to motor learning following somatosensory training are due to the perceptual decision-making aspects of the somatosensory task as opposed to somatosensory exposure alone. A control group that did not participate in the somatosensory training protocol was also included.
We obtained quantitative measures of perceptual change for subjects in the somatosensory discrimination condition. Figure 2B shows psychometric functions before and after somatosensory discrimination training for a representative subject. As can be seen, before learning the perceptual boundary is located to the left of the midline. With training, the bias is removed. Figure 2, C and D, shows data for bias and acuity for subjects in the somatosensory discrimination group. For these subjects, we observed that with training, the perceptual boundary approached the actual boundary between left and right [t(13) = 3.37, P < 0.01, between the first and last blocks], and perceptual acuity increased [t(13) = 4.03, P < 0.001, between first and last]. To rule out the possibility of active motor outflow during perceptual training, we examined the forces that subjects applied to robot handle during this procedure. Measured forces were low throughout, averaging 0.52 N (±0.20) orthogonal to the displacement and 0.68 N (±0.23) in line with the displacement. The measured forces did not vary in any systematic fashion over the course of training or with the training direction.
The perceptual training trials were preceded and followed by movements in the absence of load (Fig. 3A). Movements in velocity-dependent force fields were also tested, after the second set of null field movements (after perceptual training). In all cases, the subject was required to move straight from the start to the end positions. In particular, we carried out two kinds of force field tests. A first set, designed to assess the rate of motor learning, used a force field that deflected the arm to the left in proportion to hand movement velocity. A second set, which followed immediately afterward, was designed to assess the resistance of the preceding motor learning to interference. In these tests the robot pushed the arm to the right, again in proportion to hand movement velocity. To rule out the possibility that factors other than perceptual learning might produce changes in movements and motor learning, subjects in a control group repeated similar tests of movement in null and force field conditions but in the absence of any kind of intervening somatosensory input.
Fig. 3.
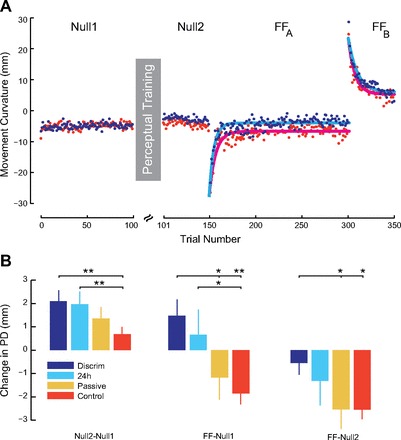
Reinforced perceptual learning increases the rate and extent of motor learning. A: experimental sequence and average lateral movement deviation in different phases of the experiment. For visualization purposes, only experimental subjects that underwent somatosensory discrimination training (blue) and control subjects that received no perceptual training, nor passive movement, of any kind at all (red) are shown. B: effect of perceptual training on movement and adaptation are shown as changes in movement deviation (means ± SE). Left, differences in deviation following sensory training relative to baseline. Middle, movement deviation relative to Null 1 baseline at the end of force field learning. Right, movement deviation at the end of force field learning relative to Null2. *P < 0.05; **P < 0.001.
We assessed the effects of perceptual training on movement and motor learning by measuring the curvature of the hand path (lateral deviation of the hand from a straight-line path at the point of maximum velocity) on a trial-by-trial basis. In all experimental conditions, movement curvature was low in the absence of load. The force field initially resulted in a substantial lateral deviation that was progressively reduced over the course of training. Figure 3A shows the effects of somatosensory training on movement. It can be seen that before training, deflections were similar for the training and control condition subjects (Null1). After training, there was less off-center deviation for discrimination group subjects (Null2). In force field learning, both the rate of learning and asymptotic performance were superior for subjects in somatosensory discrimination condition (blue). When the direction of the force field was switched from leftward to rightward, subjects in the somatosensory discrimination condition showed slower rates of unlearning of the previous force field.
We computed rates of decay of kinematic error, which serve as a measure of motor learning (see materials and methods). The estimated rate constant (mean ± 95% CI) in the counterclockwise force field was reliably greater for the discrimination condition (0.175 ± 0.019) and the passive training group (0.159 ± 0.004) than for the control condition subjects (0.136 ± 0.015). In the clockwise force field that followed, the rate constant was reliably less for the discrimination condition (0.097 ± 0.014) than for the control condition (0.128 ± 0.013). In interpreting these results, it should be noted that there were no differences between conditions at the start of force field training. In particular, we found no reliable differences between experimental conditions in lateral deviation of first movements in the force field [F(2,37) = 0.56, P > 0.5].
Motor learning was also assessed using measures of movement curvature (PD). Figure 3B shows tests conducted using changes in lateral deviation relative to baseline movements as a measure of performance. Figure 3B, left, shows that there were reliable changes in null field movements following somatosensory perceptual training [F(2,37) = 3.40, P < 0.05]. Figure 3B, middle, shows that there were also changes in asymptotic performance following motor learning, relative to initial baseline movements [F(2,37) = 5.54, P < 0.01]. Figure 3B, right, indicates differences in asymptotic performance following force field learning in relation to null field movements after perceptual training [F(2,37) = 3.46, P < 0.05]. In all cases, positive scores indicate improvements in performance, that is, reductions in curvature, relative to baseline. It can be seen in Fig. 3B, left, that somatosensory discrimination training resulted in reliable reductions in movement curvature under null field conditions compared with the control condition (P < 0.05, corrected for multiple comparisons). Figure 3B, middle, shows that there was less deviated asymptotic performance following motor learning for the somatosensory discrimination group than for either the control condition (P < 0.01) or the passive condition subjects (P ≃ 0.05). Figure 3B, right, shows that relative to the second null field, subjects in the discrimination training group performed better than those in either the control condition or the passive movement group (P < 0.05 in both cases). Moreover (also in Fig. 3B, right), it is shown that when the effects of the baseline shift are removed by subtracting out movement deviation in the second null field movements, subjects in the passive condition performed no better than control group subjects (P > 0.05).
We tested the persistence of changes to motor learning that result from somatosensory training by repeating both the null field and force field trials in a new group of subjects 24 h after somatosensory discrimination training. Figure 3B shows the results for these subjects (light blue). Tests conducted at a 24-h delay show that the effects of somatosensory training persist for at least 24 h following perceptual training. After somatosensory training, movements under null conditions were straighter and in subsequent force field learning reached less deviated asymptotic levels compared with control subjects (P < 0.05 in both cases).
Figure 4, A and B, shows measures of learning based on lateral force applied to the channel walls. The measured force profiles are normalized such that a maximum value of 1 indicates complete compensation for the applied load. Figure 4A shows that early in learning there were few differences in the level of force compensation between subjects in the somatosensory discrimination group and those in the passive movement and control groups. Late in learning (Fig. 4B) somatosensory discrimination group subjects applied forces closer to those needed to fully compensate the effect of the force field. Overall, one sees a gradient in the magnitude of force compensation and hence motor learning in which learning was greatest for subjects who underwent somatosensory discrimination training, least for control condition subjects, and intermediate for subjects exposed to passive movement alone. Figure 4C shows group-averaged data, based on a force index, the total applied force divided by total ideal force (see materials and methods). It can be seen that early in learning there were no differences in the force measure for the different experimental conditions [F(2,37) = 2.10, P > 0.1]. Late in learning there was a reliable difference between conditions [F(2,37) = 9.07, P < 0.001] in which the discrimination group performed significantly better than either control or passive condition subjects (P < 0.05, corrected for multiple comparison). Subjects tested following a 24-h delay showed retention of learning and applied forces that were reliably greater than those of subjects in the control group (P < 0.05). Thus, overall, it is shown that perceptual training has similar effects on both forces and kinematic measures of motor learning.
Fig. 4.
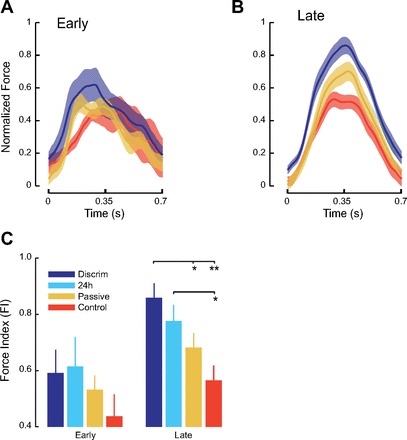
A: motor learning as reflected in lateral force production early in force field learning. Mean normalized force profiles (±SE) over the course of movement are shown. A value of 1 represents full compensation for the force field. B: lateral force production late in force field learning. Subjects in the reinforcement group show greatest learning; control subjects show least learning. Data for the 24-h condition are not shown but lie behind the passive group data. C: motor learning as evaluated by lateral forces applied to the channel walls, normalized by the ideal force, for movements early and late in learning (means ± SE). It can be seen that the force index increases from early to late in learning. *P < 0.05; **P < 0.001.
We assessed the acquisition of predictive control during learning by examining the time lag between the normalized lateral force exerted by subjects in channel trials and the normalized ideal force calculated from the hand velocity during movement (Fig. 5). For each subject, the mean prediction lag during the last three channel trials at the end of force field training was obtained. Figure 5A shows the mean normalized applied force in yellow and the mean normalized ideal force in blue for subjects in the perceptual discrimination condition. The distance between the vertical lines indicates the time lag at the point when subjects reached half of their maximum applied force. Figure 5, B and C, show similar curves for subjects in the passive movement and control conditions, respectively. Figure 5D shows that there were reliable differences in predictive control following somatosensory perceptual training [F(2,37) = 7.29, P < 0.005]. Subjects in the perceptual discrimination group were found to have significantly less prediction lag (mean lag = 27 ms) than subjects in the passive movement condition (mean lag = 46 ms; P < 0.05, corrected for multiple comparisons) and subjects in the control condition (mean lag = 67 ms; P < 0.01, corrected). Likewise, the onset of the preparatory response (the time to reach 5% of the maximum applied force) was earlier following somatosensory perceptual training [F(2,37) = 4. 96, P < 0.01]. The preparatory force response in the perceptual discrimination group started significantly earlier in time (mean onset = 6 ms following movement start) than in the passive movement condition (mean onset = 33 ms; P < 0.01, corrected for multiple comparisons) and in the control condition (mean onset = 26 ms; P < 0.05, corrected).
Fig. 5.
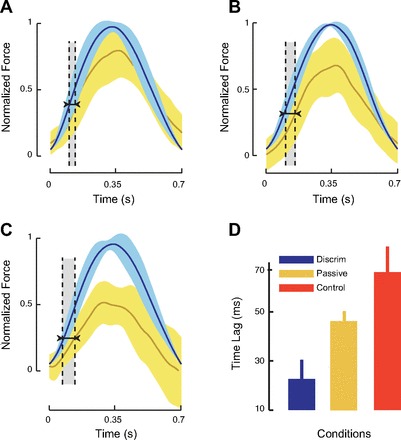
Reinforced perceptual learning facilitates the acquisition of predictive control during sensorimotor adaptation. The mean lateral force exerted by the subject in the channel trials is shown (yellow) in relation to the ideal force needed to fully compensate for the load (blue). A: reinforced perceptual training. B: passive movement. C: control. D: the time lag between actual and ideal force is least for the reinforcement group and greatest for the control condition subjects (means ± SE).
A control experiment was run to determine whether the changes to motor learning observed for subjects in the somatosensory discrimination condition resulted from changes to the magnitude of movement error, due to the perceptual manipulation. As it stands, the observed changes to motor learning may be present because the perceptual training manipulation moved the perceptual boundary to the right and thus increased the magnitude of error in the left-directed force field training trials. We reasoned that if the observed changes to measures of motor learning were due to the effect of the perceptual manipulation on movement error, then if we instead paired the same perceptual training procedure with a rightward force field, a decrease in the extent and rate of learning should be observed, because the target shift under these conditions serves to reduce the error due the force field. Alternatively, our effects might depend on factors other than movement error, for example, changes in perceptual acuity or other effects on motor function that derive from perceptual learning such as improvements in the capacity for precise force production. If this were the case, perceptual training might lead to improvements in performance regardless of the direction of the force field.
We found that following perceptual training there were changes to sensed limb position (perceptual boundary between left and right) [t(9) = 3.43, P < 0.01] and to measures of perceptual acuity [t(9) = 2.64, P < 0.05] that were the same as those in the main experimental manipulation. Estimates of the left/right boundary shifted to the body midline and perceptual acuity improved. Figure 2, C and D, shows the overall pattern, averaged over the present control experiment and the main experimental manipulation. Similar statistically reliable changes were observed in each individual case.
Figure 6A shows measures of movement curvature (PD) over the course of training for subjects tested in a rightward force field. The blue dots show movements for subjects in the perceptual discrimination condition; the red dots shows data for control subjects that were tested in a rightward force field, but without perceptual training. The effects are also similar to those observed in the main experimental manipulation. Specifically, we obtained a reliable statistical interaction indicating that changes in baseline movements and asymptotic values following force field learning differed for subjects in the perceptual discrimination and control condition trials [F(2,36) = 4.10, P < 0.05]. Whereas control condition subjects showed no changes in baseline curvature in the two tests of null field movement (P > 0.9), following perceptual training there was a reliable improvement in movement curvature under null field conditions (P < 0.02). Additionally, in the perceptual discrimination group, asymptotic measures of movement curvature following force field training were no different from those obtained in the second set of null field trials (P > 0.9). In contrast, estimates of asymptotic movement curvature in the control condition were reliably different from null field values (P < 0.01). This indicates incomplete compensation in control condition subjects.
Fig. 6.
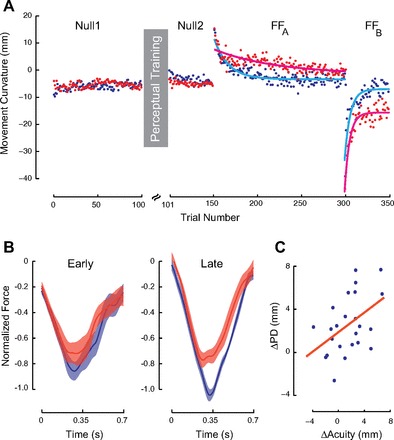
Somatosensory perceptual training improves the rate and extent of motor learning despite a perceptual boundary shift that serves to decrease error on subsequent movements. A: measures of movement curvature in association with perceptual training that is followed by a rightward force field and then a leftward field. Blue indicates subjects in the perceptual training condition; red shows control subjects. B: mean normalized force profiles over the course of training. Perceptual training results in increases in lateral force production relative to control subjects even when the associated perceptual shift serves to reduce kinematic error. C: increases in acuity with perceptual training are found to be systematically correlated with improvements in motor learning as measured by changes in movement curvature (ΔPD) between baseline and asymptotic performance in the force field. As a measure of acuity we used the distance in millimeters between the 25th and 75th percentile of the psychometric function. The values shown are the changes in distance from the early to the late phase of sensory training. Larger values correspond to greater acuity.
As in the main experimental manipulation, subjects that received perceptual training showed greater amounts of learning and faster rates of adaptation than control subjects. The estimated rate constants (mean ± 95% CI) for the perceptual training and control conditions are 0.060 ± 0.011 and 0.014 ± 0.008, respectively. When the force field was reversed, the rate constant for the perceptual training condition was 0.125 ± 0.045 and that for the control was 0.166 ± 0.040. The latter rate constants were not reliably different (P > 0.10).
Figure 6B provides a comparison of data from channel trials for the subjects tested in this control experiment. It can be seen that force on the channel walls was initially similar for perceptual training and control subjects [t(18) = 0.44, P > 0.1], but at the end of force field learning perceptual training subjects showed reliably higher values, indicating more learning [t(17) = 2.603, P < 0.05]. Data for one subject that was more than 3 SD from the mean was removed from the second analysis.
We assessed the relationship between measures of perceptual and motor learning for the two force field directions. We observed no reliable relationship between either kinematic or force channel measures of learning and changes in perceptual bias (P > 0.1 for all tests). This was expected since measures of motor learning increase regardless of whether perceptual learning served to increase or decrease movement error due to the force field. In contrast, measures of perceptual acuity were correlated with measures of motor learning (r = 0.46, P < 0.02). In particular, the acuity change between baseline values and those obtained at the end of perceptual training were systematically related to changes in movement curvature (PD) between baseline and asymptotic performance in the force field.
We conducted a comparison of the effects of perceptual training on adaptation trials in a leftward vs. rightward force field. In addition to the effects reported above, there were also observed directional differences. However, they were unrelated to whether perceptual training serves to increase or decrease error in subsequent force field trials. Thus, although mean force applied to the channel walls at peak velocity (±SE) was greater for rightward than leftward loads (4.87 ± 0.26 vs. 4.08 ± 0.25 N, respectively), these same differences, in the same proportion, were present in the data from control subjects that did not undergo the perceptual manipulation (4.12 ± 0.30 vs. 3.075 ± 0.20 N, respectively). Thus there appear to be directional asymmetries in this task associated with left- vs. right-acting force fields. However, because they are observed in subjects in control conditions, they are unrelated to whether perceptual training serves to increase or decrease kinematic error.
DISCUSSION
The present findings show that perceptual training helps to define the somatosensory goals of movement and accordingly facilitates motor learning. Perceptual training is found to improve sensitivity to small deviations (reduced uncertainty in the somatosensory domain) and to aid in the development of a sensory plan, a desired sensory trajectory that guides subsequent movements. Changes following perceptual training are observed in the kinematic (hand's lateral deviation) and kinetic (force production level) characteristics of reaching movements during motor learning, and in the temporal profile of the compensatory response (force production lag).
The effects seen in this study do not appear to be due to changes in the magnitude of kinematic error that is produced by the perceptual training. The beneficial effects of perceptual training are observed regardless of whether the force field testing procedure serves to globally increase or decrease the magnitude of movement error. These benefits presumably stem from changes in somatosensory precision or acuity that result from perceptual training or possibly, as suggested by the increase in force measures with perceptual training, from a direct influence of perceptual learning on the motor system. The effects of perceptual training on the motor system are found to be substantially dependent on perceptual judgment and reinforcement. Sensory exposure on its own is less able to produce changes in motor learning. It is also seen that the effects of perceptual training are durable. The benefits for motor learning are evident in subjects who were tested for sensorimotor adaptation 24 h after completion of the perceptual training task.
Force field learning and visuomotor adaptation paradigms have been used extensively to study sensorimotor adaptation. There is ample evidence that these paradigms result in persistent change to both motor and somatosensory systems, but they provide a model of motor learning in the context of well-defined sensory targets and hence error-based learning. In situations outside of the laboratory, somatosensory goals early in learning are often poorly defined, and thus perceptual and motor learning must presumably occur in parallel.
In the present study we have designed a series of experiments in which it is possible to see the separate contributions of perceptual and motor components to sensorimotor adaptation. We have conducted perceptual training in the absence of active movement to dissociate perceptual from motor contributions to learning. That is, although the initial stages of motor learning presumably include both perceptual and motor refinements, here the perceptual refinements occur first in the context of passive movement perceptual training. Nevertheless, we find that perceptual training on its own is sufficient to modify movements and the learning that follows. Whether active movement under these conditions would enhance or suppress learning needs to be determined. However, in a study by Wong et al. (2012), subject-assisted proprioceptive training did not seem to have a beneficial effect on subsequent motor learning.
The current studies complement the findings of recent work on the effects on motor learning on sensory systems (Cressman and Henriques 2009; Haith et al. 2008; Mattar et al. 2013; Nasir and Ostry 2009; Ostry et al. 2010; Vahdat et al. 2011). In particular, it has been shown that sensorimotor adaptation results in changes to somatosensory perceptual function and to somatosensory areas of the brain that are correlated in magnitude with the extent of motor learning (Vahdat et al. 2011). These studies thus suggest that perceptual change is an integral part of motor learning.
The findings also complement those of a similarly designed neuroimaging study (Vahdat et al. 2012). In that experiment subjects underwent functional MRI scans of the resting brain before and after the same perceptual training protocol as used in the present study. Changes in functional connectivity were assessed after parceling out those effects that could be predicted on the basis of activity in sensory areas of the brain, and in particular, primary and second somatosensory cortex and ventral premotor cortex. It was found that even with these effects removed, there were still independent changes in functional connectivity in frontal motor areas and cerebellar cortex that were correlated with perceptual training measures. Thus changes to motor areas of the brain that occur in association with motor skill acquisition could be partially the result of perceptual learning.
Perceptual training in the present study is seen to affect motor learning and, afterward, the degree of anterograde interference, the ability of a previously learned motor task to reduce the amount of subsequent learning on an opposite motor task (Sing and Smith 2010). If perceptual training precedes a leftward force field, the interference on the subsequent rightward field is increased compared with the same control condition without perceptual learning (Fig. 3). However, the interference following perceptual training is reduced compared with the control condition if the order of force fields is reversed (Fig. 6). One possible explanation for these seemingly opposite effects of perceptual training on the subsequent anterograde interference is that the degree of interference depends on the amount of error experienced during the initial force field learning. Because of the direction of change in perceptual boundary, subjects in the perceptual training group sensed greater kinematic error during the initial leftward force field compared with the control condition, and hence they exhibited greater interference on the following rightward force field task. On the other hand, subjects in the perceptual training group who first experienced the rightward force field sensed less kinematic error compared with the corresponding control condition and therefore showed less interference on the following leftward force field task. This may suggest that two different mechanisms are responsible for initial acquisition vs. anterograde interference of a motor task; the former mainly depends on the precision of the sensory input, whereas the latter depends on the magnitude of the detected error.
It is observed in the present study that before perceptual training, the sensed boundary between the left and the right of the workspace lies to the left of the subject's body midline. The bias appears to be related to the hand used in the perceptual testing. Wilson et al. (2010) report the results of a systematic set of somatosensory perceptual tests using the left and the right hand. Their tests were similar to those used here, with the exception that in their tests, the judgments occurred in static rather than during passive movement of the limb. They observed that when the right hand is used for perceptual testing, it is perceived to the right of its actual position, as is the case in the present study. When perceptual testing involves the left hand, the opposite occurs: the hand is judged to be to the left of its actual position. This same directional bias is observed when subjects make active movement, without vision, to a target located in the body midline (Dizio and Lackner 1995). When subjects use their right hand, they end up to the left of the actual target. When they use their left, they end up to the right. These results are observed when subjects make unrestrained arm movements, and hence the effect is not related to the dynamics of an external manipulandum. The source of this proprioceptive bias is unknown, although factors related muscle spindle function and limb geometry have been suggested (Bergenheim et al. 2000; Herrmann and Flanders 1998; Jones et al. 2001).
The goal of the current study was to provide a training protocol that potentially maximizes the involvement of the perceptual network during training. Hence, we did not attempt to distinguish the effects of perceptual judgment and reinforcement learning during the perceptual training protocol. The first two blocks of perceptual training involved perceptual judgments without feedback, whereas blocks three to five involved both perceptual judgment and reinforced feedback, so any improvement that we observed can be attributed to either procedure or the two in combination.
Several investigators have examined the plasticity induced in cortical motor areas as a result of active movement training. The general finding has been that acquiring a new motor skill facilitates the induction of plasticity in motor cortex. For example, in a series of electrophysiological experiments on primates (Nudo et al. 1996; Plautz et al. 2000), Nudo and colleagues trained monkeys on a repetitive motor task that required the retrieval of food pellets from either a small- or large-diameter well. They found persistent changes in the movement representation in primary motor cortex with small-well training, in which a new motor skill emerged. This is in line with a recent study on spinal cord injured rats who trained on a combination of treadmill-based training and a robotic postural interface that promoted active involvement of their paralyzed hindlimbs (van den Brand et al. 2012). It was found that active engagement was necessary to induce cortical plasticity, which led to successful locomotor recovery. Automated step training failed to restore voluntary locomotion despite long periods of repeated training postinjury. These results support the idea that skill acquisition is important for the occurrence of cortical plasticity in the motor domain.
Similar results have been reported for plasticity in somatosensory cortex following sensory training. Recanzone et al. (1992) reported reorganization of the hand representation in primary somatosensory area 3b following a tactile frequency-discrimination task. In contrast, when monkeys received identical tactile stimulation of the hand but were attending to auditory stimuli, no significant reorganization was observed in somatosensory areas. In a recent study that is perhaps closest to the present report, Rosenkranz and Rothwell (2012) showed that sensory attention during a somatosensory frequency discrimination task results in changes to intracortical inhibition in primary motor cortex and augmented motor learning. The present results are consistent with these findings and show that skill acquisition in the somatosensory domain facilitates motor learning.
It is worth considering other recent work on the effects of sensory experience and repetition on motor learning. We show here that reinforced perceptual training can influence subsequent motor performance and learning. This is consistent with work by Huang et al. (2011), which shows that repeated movement in the context of visuomotor adaptation can enhance subsequent motor learning. However, one presently unresolved aspect of work on repetition and reward in motor learning is a discrepancy between the work of Diedrichsen et al. (2010) in which a directional movement bias was documented for repeated movements in a redundant dimension of the task, whereas in a similar study in which there was no redundancy in the task, repetition alone resulted in no bias in the movement direction (Huang et al. 2011). In the present study there was similarly no dimensional redundancy in the task, yet passive movement repetition biased subsequent force field learning. This is consistent with Diedrichsen et al.'s observation that sensory experience may have the capacity to influence the following movements. The source of these differences remains unclear, but the resolution of this issue will contribute to an understanding of the characteristics of sensory experience and perceptual learning that influence voluntary movement.
GRANTS
This research was supported by the National Institute of Child Health and Human Development (Grant R01-HD-075740) and by Le Fonds Quebecois de la Recherche sur la Nature et les Technologies (Québec).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.D., S.V., and D.J.O. conception and design of research; M.D. and S.V. performed experiments; M.D. and S.V. analyzed data; M.D., S.V., and D.J.O. interpreted results of experiments; M.D. and S.V. prepared figures; M.D., S.V., and D.J.O. drafted manuscript; M.D., S.V., and D.J.O. edited and revised manuscript; M.D., S.V., and D.J.O. approved final version of manuscript.
REFERENCES
- Bergenheim M, Ribot-Ciscar E, Roll JP. Proprioceptive population coding of two-dimensional limb movements in humans: I. Muscle spindle feedback during spatially oriented movements. Exp Brain Res 134: 301–310, 2000 [DOI] [PubMed] [Google Scholar]
- Carel C, Loubinoux I, Boulanouar K, Manelfe C, Rascol O, Celsis P, Chollet F. Neural substrate for the effects of passive training on sensorimotor cortical representation: a study with functional magnetic resonance imaging in healthy subjects. J Cereb Blood Flow Metab 20: 478–484, 2000 [DOI] [PubMed] [Google Scholar]
- Carey LM, Abbott DF, Puce A, Jackson GD, Syngeniotis A, Donnan GA. Reemergence of activation with poststroke somatosensory recovery: a serial fMRI case study. Neurology 59: 749–752, 2002 [DOI] [PubMed] [Google Scholar]
- Cressman EK, Henriques DY. Sensory recalibration of hand position following visuomotor adaptation. J Neurophysiol 102: 3505–3518, 2009 [DOI] [PubMed] [Google Scholar]
- Diedrichsen J, White O, Newman D, Lally N. Use-dependent and error-based learning of motor behaviors. J Neurosci 30: 5159–5166, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dizio P, Lackner JR. Motor adaptation to Coriolis force perturbations of reaching movements: endpoint but not trajectory adaptation transfers to the nonexposed arm. J Neurophysiol 74: 1787–1792, 1995 [DOI] [PubMed] [Google Scholar]
- Haith A, Jackson C, Miall R, Vijayakumar S. Unifying the sensory and motor components of sensorimotor adaptation. In: Advances in Neural Information Processing Systems 21, edited by Koller D, Bengio Y, Schuurmans D, Bottou L, Culotta A. Vancouver, BC: NIPS Foundation, 2008, p. 593–600 [Google Scholar]
- Held R, Hein AV. Adaptation of disarranged hand-eye coordination contingent upon re-afferent stimulation. Percept Mot Skills 8: 87–90, 1958 [Google Scholar]
- Herrmann U, Flanders M. Directional tuning of single motor units. J Neurosci 18: 8402–8416, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang VS, Haith A, Mazzoni P, Krakauer JW. Rethinking motor learning, and savings in adaptation paradigms: model-free memory for successful actions combines with internal models. Neuron 70: 787–801, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izawa J, Shadmehr R. Learning from sensory and reward prediction errors during motor adaptation. PLoS Comput Biol 7: e1002012, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KE, Wessberg J, Vallbo AB. Directional tuning of human forearm muscle afferents during voluntary wrist movements. J Physiol 536: 635–647, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakauer JW, Ghilardi MF, Ghez C. Independent learning of internal models for kinematic, and dynamic control of reaching. Nat Neurosci 2: 1026–1031, 1999 [DOI] [PubMed] [Google Scholar]
- Lewis GN, Byblow WD. The effects of repetitive proprioceptive stimulation on corticomotor representation in intact and hemiplegic individuals. Clin Neurophysiol 115: 765–773, 2004 [DOI] [PubMed] [Google Scholar]
- Lotze M, Braun C, Birbaumer N, Anders S, Cohen LG. Motor learning elicited by voluntary drive. Brain 126: 866–872, 2003 [DOI] [PubMed] [Google Scholar]
- Mattar AA, Darainy M, Ostry DJ. Motor learning and its sensory effects: time course of perceptual change and its presence with gradual introduction of load. J Neurophysiol 109: 782–791, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasir SM, Ostry DJ. Auditory plasticity and speech motor learning. Proc Natl Acad Sci USA 106: 20470–20475, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nudo RJ, Milliken GW, Jenkins WM, Merzenich MM. Use-dependent alterations of movement representations in primary motor cortex of adult squirrel monkeys. J Neurosci 16: 785–807, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostry DJ, Darainy M, Mattar AA, Wong J, Gribble PL. Somatosensory plasticity and motor learning. J Neurosci 30: 5384–5393, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plautz EJ, Milliken GW, Nudo RJ. Effects of repetitive motor training on movement representations in adult squirrel monkeys: role of use versus learning. Neurobiol Learn Mem 74: 27–55, 2000 [DOI] [PubMed] [Google Scholar]
- Pleger B, Foerster AF, Ragert P, Dinse HR, Schwenkreis P, Malin JP, Nicolas V, Tegenthoff M. Functional imaging of perceptual learning in human primary and secondary somatosensory cortex. Neuron 40: 643–653, 2003 [DOI] [PubMed] [Google Scholar]
- Recanzone GH, Merzenich MM, Jenkins WM, Grajski KA, Dinse HR. Topographic reorganization of the hand representation in cortical area 3b owl monkeys trained in a frequency-discrimination task. J Neurophysiol 67: 1031–1056, 1992 [DOI] [PubMed] [Google Scholar]
- Rosenkranz K, Rothwell JC. Modulation of proprioceptive integration in the motor cortex shapes human motor learning. J Neurosci 32: 9000–9006, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheidt RA, Reinkensmeyer DJ, Conditt MA, Rymer WZ, Mussa-Ivaldi FA. Persistence of motor adaptation during constrained, multi-joint, arm movements. J Neurophysiol 84: 853–862, 2000 [DOI] [PubMed] [Google Scholar]
- Shadmehr R, Mussa-Ivaldi FA. Adaptive representation of dynamics during learning of a motor task. J Neurosci 14: 3208–3224, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sing GC, Smith MA. Reduction in learning rates associated with anterograde interference results from interactions between different timescales in motor adaptation. PLoS Comput Biol 6: e1000893, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahdat S, Darainy M, Ostry DJ. Plasticity in motor system induced by somatosensory training. Program No. 275.09. 2012 Neuroscience Meeting Planner New Orleans, LA: Society for Neuroscience, 2012 [Google Scholar]
- Vahdat S, Darainy M, Milner TE, Ostry DJ. Functionally specific changes in resting-state sensorimotor networks after motor learning. J Neurosci 31: 16907–16915, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Brand R, Heutschi J, Barraud Q, DiGiovanna J, Bartholdi K, Huerlimann M, Friedli L, Vollenweider I, Moraud EM, Duis S, Dominici N, Micera S, Musienko P, Courtine G. Restoring voluntary control of locomotion after paralyzing spinal cord injury. Science 336: 1182–1185, 2012 [DOI] [PubMed] [Google Scholar]
- Wilson ET, Wong J, Gribble PL. Mapping proprioception across a 2D horizontal workspace. PLoS One 5: e11851, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong JD, Kistemaker DA, Chin A, Gribble PL. Can proprioceptive training improve motor learning? J Neurophysiol 108: 3313–3321, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]


