Abstract
In women, cardiac deaths attributable to tobacco exposure have reached the same high levels as men. Normally, sympathetic nerve activity (SNA) fluctuates according to the menstrual phase, but in habitual smokers, SNA levels remain constant. Our purpose is to extend these observations to other groups of women exposed to tobacco smoke and to explore potential mechanisms. We hypothesize that women exposed to secondhand smoke, but not former smokers, have nonfluctuating SNA compared with never smokers, and that impaired baroreflex suppression of SNA, and/or heightened central SNA responses, underlie this nonfluctuating SNA. We also hypothesize that female smokers have impaired nocturnal blood pressure dipping, normally mediated by modulation of SNA. In 49 females (19 never, 12 current, 9 former, 9 passive smokers), SNA was recorded (microneurography) during high- and low-hormone ovarian phases at rest, during pharmacological baroreflex testing, and during the cold pressor test (CPT). Twenty-four hour blood pressure (BP) monitoring was performed. Current and passive smokers, but not former smokers, had a nonfluctuating pattern of SNA, unlike never smokers in whom SNA varied with the menstrual phase. Baroreflex control of SNA was significantly blunted in current smokers, independent of menstrual phase. In passive smokers, SNA response to CPT was markedly increased. Nondipping was unexpectedly high in all groups. SNA does not vary during the menstrual cycle in active and passive smokers, unlike never and former smokers. Baroreflex control of SNA is blunted in current smokers, whereas SNA response to CPT is heightened in passive smokers. Smoking cessation is associated with return of the altered SNA pattern to normal.
Keywords: baroreflex control, tobacco, autonomic nervous system, cardiac risk in women
although risk of death from cigarette smoking has plateaued in men in the United States, the mortality risk continues to increase in women (50). In fact, risk, specifically cardiovascular risk, attributable to smoking in women is now the same as the high levels found in men (50). In both men and women, smoking cessation dramatically decreases this risk (50). Of further concern, the recent analysis of the Nurses' Health Study, which prospectively followed over 100,000 women without known coronary artery disease at baseline, revealed a strong dose-response relationship between cigarette smoking and sudden cardiac death (48). Consistent with prior reports (44), in this study even small amounts of daily cigarette consumption significantly increased sudden death risk in women. Once again, smoking cessation was associated with a significant decline in this sudden cardiac death risk (48). Although not examined in either of these recent studies, exposure to secondhand cigarette smoke (“passive smoking”) has also been identified as an important cardiovascular risk factor in women (6).
Although heart disease is the number-one killer in both men and women in the United States, the onset of heart disease in women compared with men is delayed by about a decade (7). Importantly, cigarette smoking in women obliterates this delay (7). Cigarette smoking is the most important modifiable risk factor for cardiac disease, and its risk is greatest in premenopausal women (37). In women ages 35–44, cigarette smoking confers a relative risk of myocardial infarction of 7.1, a much greater adverse effect than that found in similarly aged men who smoke (37). The mechanisms conferring the delay in cardiac disease in women, which is then eliminated by smoking, are unknown, but are presumably related to endogenous ovarian hormones. In premenopausal women, ovarian hormone levels undergo dramatic monthly fluctuations related to the menstrual cycle. Estrogen and progesterone are lowest during the early follicular (EF) phase, during menstrual flow, and peak during the midluteal (ML) phase, approximately 1 wk after ovulation. We (33, 40), and others (34), have found that in women who have never been exposed to tobacco smoke (“never smokers”), sympathetic nerve activity (SNA) also fluctuates during the menstrual cycle; SNA is lower during the EF phase, and higher during the ML phase. However, in current smokers, we found a different pattern (40). We found that in current smokers, SNA does not fluctuate during the menstrual cycle, despite the normal fluctuation of ovarian hormones (40). SNA is known to be a culprit in increasing cardiovascular risk in many patient populations, including patients with coronary artery disease, heart failure, and perioperative patients (4, 18, 19); altered SNA levels may also underlie the increased cardiovascular risk conferred by cigarette smoking.
Resting central sympathetic outflow directed to the heart and muscle is tonically suppressed by peripheral baroreceptors (reviewed in Ref. 5) and is also influenced by central nervous system factors as well (selected factors reviewed in Refs. 3, 11, 20). Acute tobacco exposure increases SNA in habitual smokers, by impairing baroreflex sensitivity (21, 30) as well as through central mechanisms (35). Importantly, the effect of chronic tobacco exposure on the baroreflex has not been studied. The goals of the current study are three. 1) One goal is to extend our knowledge of the pattern of SNA in premenopausal women exposed to tobacco smoke, including former smokers and passive smokers. We hypothesize that SNA levels will fluctuate during the menstrual cycle in former smokers, similar to the rising and falling SNA levels in never smokers, but not passive smokers, in whom SNA levels will be fixed. 2) We will investigate two potential mechanisms underlying the altered pattern of SNA in women exposed to tobacco smoke, including abnormal baroreflex control of SNA, and heightened central sympathetic responsiveness to a non-baroreflex-mediated stimulus [cold pressor test (CPT)]. We hypothesize that women exposed to tobacco smoke (current and passive smokers) will have altered, specifically attenuated, baroreflex control of SNA compared with never or former smokers. Third, since fluctuations in SNA underlie the nocturnal fall in blood pressure (dipping) (13, 25, 36), which is associated with lower cardiovascular risk (1, 14, 15, 26, 38), we will measure ambulatory blood pressures for 24 h in our subjects to determine if there is an increased incidence of nondipping in women exposed to tobacco smoke compared with those never exposed. We hypothesize that the incidence of nondipping will in fact be greater in those exposed to tobacco smoke (current and passive smokers).
MATERIALS AND METHODS
Subjects.
A total of 49 healthy premenopausal women, not taking oral contraceptive pills (OCPs), participated in these studies: 19 never smokers, 12 current smokers, 9 former smokers (quit > 1 yr), and 9 never smokers regularly exposed to secondhand smoke (exposure >1 h/wk by roommates n = 7; or by close social contact, n = 2). All were between the ages of 18 and 45 yr, with no chronic medical problems, did not use illicit drugs or drink ≥ 2 alcoholic drinks per day, and were on no medications including OCPs or nicotine gum/patches. No subjects were in exercise training programs. All had regular menstrual cycles of ∼4 wk duration. The experimental protocol was approved by the Institutional Review Board at the University of California, Los Angeles, and written informed consent was obtained from each volunteer.
Experimental protocol.
All volunteers were studied twice at approximately the same time of day, in a randomized order. Volunteers were studied on 1) days 1–4 after onset of menstrual flow (EF phase, “low hormone”), and 2) days 8–10 after detection (Midstream Ovulation test, Early-Pregnancy-Test.com) of the luteinizing hormone surge (ML phase, “high hormone”). Phase of menstrual cycle was confirmed by plasma estrogen and progesterone levels.
On the morning of the study, volunteers were permitted a light breakfast but abstained from caffeine and tobacco for at least 12 h before the study. A urine pregnancy test (Pregnancy Test strip, Early-Pregnancy-Test.com) was performed before each study. Volunteers were studied in a supine position in a quiet, semidark, temperature-controlled (21°C) Human Physiology Laboratory located in the UCLA General Clinical Research Center. An intravenous catheter was placed in the dominant arm for blood tests and drug infusion. A blood pressure (BP) cuff was placed on the upper arm, and electrocardiogram (ECG) patch electrodes were placed on the upper chest. The leg was positioned for microneurography, and a tungsten electrode was inserted into the peroneal nerve to obtain a satisfactory muscle (M) SNA recording. After a 10-min rest period, MSNA, HR, and BP were recorded for 10 min. The volunteer then performed the CPT by placing her hand in an ice water slurry for 2 min, while MSNA, HR, and BP were recorded. After a 20-min recovery period, arterial baroreflex activation (phenylephrine) and deactivation (nitroprusside) of SNA were performed. The laboratory study was then over. Prior to leaving the laboratory, the volunteer was fitted with a 24-h ambulatory blood pressure monitor (Ambulatory Blood Pressure Ultralite Monitor-90217, Spacelabs Healthcare, Issaquah, WA), which recorded systolic (SBP), diastolic (DBP), and mean arterial blood pressure (MAP) every 20 min while the volunteer was awake, and every 30 min during sleep.
Measurements.
MSNA was recorded using microneurography from the peroneal nerve as previously described (12, 33, 40, 52). Lead II of the ECG was recorded simultaneously with the neurogram using a multichannel digital data recorder (LabChart6 Pro, AD Instruments). MSNA was identified using previously described methods, and a satisfactory neurogram exhibited a signal-to-noise ratio >3:1. Sympathetic bursts were determined by visual inspection by a single investigator (H.R.M.) without knowledge of the volunteer's menstrual phase or smoking status. MSNA was expressed as burst frequency (bursts/min) and bursts per 100 heart beats (bursts/100 hb). Total activity per minute was determined by the sum of the heights of individual bursts per minute. Baroreflex activation and deactivation of MSNA was determined by recording BP every 2 min, and HR and MSNA continuously during three different 5-min infusions of phenylephrine and nitroprusside, respectively, in a closed-loop gain approach that includes multiple integrated responses (17, 22, 33). Phenylephrine was infused incrementally at doses of 0.3, 0.6, and 0.9 μg·kg−1·min−1, and nitroprusside was infused incrementally at doses of 0.4, 0.8, and 1.2 μg·kg−1·min−1, 5 minutes per infusion (17, 22, 33). BP, HR, and MSNA were averaged for each 5-min period. During 24-h ambulatory blood pressure recordings, the blood pressure monitor was preprogrammed with the volunteer's estimate of her sleep time and wake up time that day. SBP, DBP, and MAP were calculated by the device for the entire 24-h recording period, as well as for the wake and sleep periods. A “dipper” was defined as a day-night dip in SBP, DBP, or MAP ≥10% according to the American Heart Association Scientific Statement (43).
Statistical analysis.
Statistical analysis was performed by J.A.G. using SAS software (SAS 9.2, SAS, Cary, NC). Means were compared using a 4 × 2 repeated-measures analysis of variance (ANOVA) models for comparisons across the four groups and between EF low-hormone and ML high-hormone menstrual phases. The pairwise mean comparisons under this ANOVA model were assessed using the Fisher least significant difference (Fisher LSD) criterion.
Absolute change from baseline was used as outcomes for SNA, HR, and MAP. For the baroreflex sensitivity analysis, for each subject and menstrual phase, the six (2 drugs, 3 doses per drug) SNA changes (measured as bursts/min, bursts/100 hb, and total activity per minute) or HR changes (beats/min) from baseline values were regressed on the corresponding six MAP changes from baseline values to compute the SNA change or HR change per unit of MAP change rate (slope) for that subject and menstrual phase, a direct measure of the relationship between change in SNA or HR and change in MAP. The mean rates were then compared by group and menstrual status using the repeated-measures ANOVA as above. Estrogen and progesterone were compared on the log scale since their distribution followed the normal on this scale. Therefore geometric means are reported for estrogen and progesterone.
Results are reported as means ± SE. A P value ≤ 0.05 was considered statistically significant.
RESULTS
Descriptive characteristics.
Age (P = 0.38) and body mass index (P = 0.09) are displayed on Table 1 and did not differ among the groups. Current smokers smoked an average of 8.1 ± 1.6 cigarettes per day for a mean duration of 9.4 ± 2.0 yr. Former smokers had smoked an average of 10.9 ± 3.5 cigarettes per day for a mean duration of 3.2 ± 1.1 yr, and had stopped smoking an average of 3.9 ± 1.1 yr prior to the study. Passive smokers were exposed to cigarette smoke a mean of 7.9 ± 2.7 h/wk. Progesterone and estrogen were significantly higher during the ML phase compared with EF phase in each group (Table 1).
Table 1.
Baseline characteristics
| Never Smokers (n = 19) | Current Smokers (n = 12) | Former Smokers (n = 9) | Passive Smokers (n = 9) | |
|---|---|---|---|---|
| Age, yr | 27.9 ± 1.8 | 27.9 ± 2.1 | 30.9 ± 2.8 | 33.0 ± 2.8 |
| BMI, kg/m2 | 24.1 ± 0.8 | 21.6 ± 1.0 | 24.2 ± 1.3 | 25.3 ± 1.3 |
| Estrogen*, pg/ml | ||||
| EF | 77.4 ± 10.7 | 64.8 ± 10.4 | 67.3 ± 13.9 | 63.7 ± 8.0 |
| ML | 147.1 ± 18.5 | 172.6 ± 27.6 | 123.4 ± 23.7 | 234.1 ± 48.2 |
| Progesterone†, ng/ml | ||||
| EF | 0.5 ± 0.1 | 0.6 ± 0.1 | 0.8 ± 0.2 | 0.4 ± 0.1 |
| ML | 2.8 ± 0.8 | 4.4 ± 1.6 | 2.4 ± 1.1 | 7.2 ± 3.4 |
Values are means ± SE. BMI, body mass index. Early follicular (EF) phase vs. midluteal (ML) phase:
P < 0.01,
P < 0.05.
Resting sympathetic nerve activity and hemodynamics.
Resting MSNA was compared among the four groups at both EF and ML phases of the menstrual cycle to determine if MSNA levels waxed and waned in former and passive smokers similar to the pattern seen in never smokers (33, 40), or if the MSNA level remained relatively fixed, as previously seen in current smokers (40). We found a menstrual phase effect (P = 0.0001), no smoking effect (P = 0.70), and a menstrual phase × smoking interaction (P = 0.0080). As displayed in Fig. 1, MSNA was significantly lower during the EF (low-hormone) phase compared with the ML (high-hormone) phase in both the never-smoker and former smoker groups, but not in the current smoker or passive smoker groups. Results were the same when MSNA was analyzed as bursts/100 heart beats (Table 2). Resting HR (menstrual phase effect P = 0.10, smoking effect P = 0.21, menstrual phase × smoking interaction P = 0.91) and MAP (menstrual phase effect P = 0.76, smoking effect P = 0.25, menstrual phase × smoking interaction P = 0.71) were not different among the groups.
Fig. 1.
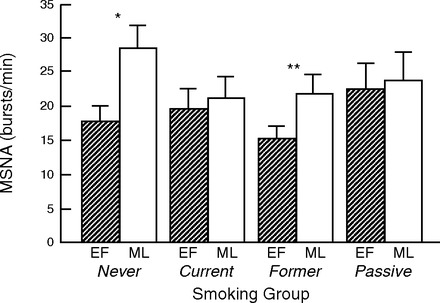
Comparison of resting sympathetic nerve activity (bursts per minute) in premenopausal women who are never, current, former, and passive smokers, according to menstrual phase. There was a menstrual phase effect (P = 0.0001), no smoking effect (P = 0.70), and a menstrual phase × smoking interaction (P = 0.0080). Muscle sympathetic nerve activity (MSNA) was significantly lower during the early follicular (EF; low-hormone) phase compared with the midluteal (ML; high-hormone) phase in both the never-smoker (*P < 0.00001) and former smoker (**P < 0.02) groups, but not in the current smoker (P = 0.48) or passive smoker (P = 0.65) groups.
Table 2.
Resting sympathetic nerve activity and hemodynamics
| Never Smokers (n = 19) | Current Smokers (n = 12) | Former Smokers (n = 9) | Passive Smokers (n = 9) | |
|---|---|---|---|---|
| MSNA, bursts/100 heart beats | ||||
| EF | 26.5 ± 3.2* | 30.7 ± 4.5 | 24.3 ± 3.6† | 33.4 ± 6.1 |
| ML | 41.6 ± 5.2 | 32.5 ± 4.8 | 34.5 ± 5.3 | 34.2 ± 6.2 |
| HR, beats/min | ||||
| EF | 67.0 ± 1.8 | 63.7 ± 2.6 | 63.0 ± 1.7 | 68.1 ± 3.7 |
| ML | 69.6 ± 1.9 | 64.8 ± 2.0 | 64.5 ± 1.2 | 69.6 ± 2.8 |
| MAP, mmHg | ||||
| EF | 72.0 ± 1.4 | 73.0 ± 1.8 | 74.7 ± 0.9 | 77.1 ± 2.3 |
| ML | 72.2 ± 2.1 | 71.1 ± 1.6 | 79.0 ± 2.3 | 76.3 ± 2.1 |
Values are means ± SE.
P < 0.00001,
P < 0.02.
Baroreflex control of MSNA and HR.
Since baroreflex input tonically inhibits central MSNA outflow, we tested the hypothesis that baroreflex sensitivity would vary according to the phase of the menstrual period in never smokers and former smokers in whom MSNA varies according to menstrual period, but that this pattern would be altered in the current and passive smokers in whom MSNA does not vary.
In fact, we did find a menstrual phase effect (P = 0.039), and smoking effect (P = 0.042) but not a menstrual phase × smoking interaction (P = 0.46). Baroreflex control of SNA tended to be greater (steeper slope) during the ML phase compared with the EF phase in all groups except the current smokers group, but this tendency did not reach statistical significance in any of the individual groups.
On the other hand, baroreflex control of SNA was significantly attenuated in the current smokers compared with never smokers during both phases of the menstrual period (Fig. 2A). Results were the same when MSNA was analyzed as bursts per minute and bursts per 100 heart beats (data not shown).
Fig. 2.
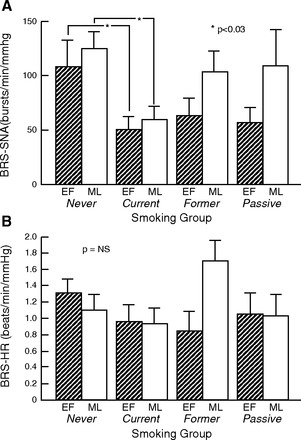
Comparison of baroreflex control of sympathetic nerve activity (SNA; A) and heart rate (HR; B) in premenopausal women who are never, current, former, and passive smokers, according to menstrual phase. A: baroreflex sensitivity (BRS) (total activity SNA·min−1·mmHg−1) was significantly blunted during both menstrual phases in current smokers compared with never smokers. BRS was not different between menstrual phases in any group. Results were the same when MSNA was measured as bursts/min or bursts/100 heart beats (data not shown). *P < 0.03, never smokers vs. current smokers. B: baroreflex control of HR was not different between menstrual phases in any group. TA, total activity.
Conversely, baroreflex control of HR did not differ according to menstrual phase (P = 0.35) or smoking group (P = 0.44), and there was no menstrual phase × smoking interaction (P = 0.11) (Fig. 2B).
Cold pressor effect on MSNA.
Since central factors also determine resting MSNA levels, we next explored the possibility that a strong, non-baroreflex-mediated stimulus such as the CPT would elicit differing MSNA responses according to menstrual phase in never and former smokers but not in current and passive smokers, suggestive of a non-baroreflex influence determining MSNA levels. We did not find a menstrual phase effect (P = 0.46), but there was a smoking effect (P = 0.036). There was no menstrual phase × smoking interaction (P = 0.44). MSNA (total activity/minute) responses to CPT were significantly greater, in fact ∼100% greater, in the passive smokers during both phases of the menstrual cycle compared with all other groups (Fig. 3A). Results were the same when MSNA was analyzed as bursts per minute and bursts per 100 heart beats (data not shown). MAP response (menstrual phase effect P = 0.52, smoking effect P = 0.29, menstrual phase × smoking interaction P = 0.45), and HR response (menstrual phase effect P = 0.95, smoking effect P = 0.71, menstrual phase × smoking interaction P = 0.76) during CPT were not different among the groups (Fig. 3, B and C).
Fig. 3.
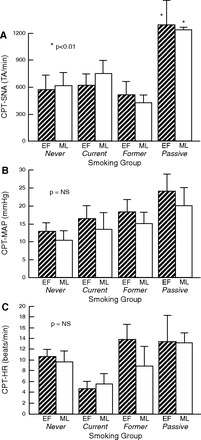
Comparison of increase in SNA (total activity/min, A), mean arterial pressure (MAP, B), and HR (C) during cold pressor testing (CPT). A: increase in SNA during CPT, a strong, non-baroreflex-mediated stimulus, was markedly augmented in passive smokers compared with all other groups. The increase in SNA was not different between the menstrual phases in any of the groups. Results were the same when MSNA was measured as bursts/min or bursts/100 heart beats (data not shown). *P < 0.01, passive smokers vs. never, current, or former smokers. B: increase in MAP was not statistically different between any group or menstrual phase. C: increase in HR was not statistically different between any group or menstrual phase.
Ambulatory 24 h BP recordings.
Since fluctuations in SNA underlie the nocturnal fall in blood pressure (dipping), which is associated with lower cardiovascular risk, we measured ambulatory BP for 24 h in all groups during each menstrual phase to determine if there was an increased incidence of nondipping in active and/or passive smokers compared with never and/or former smokers, and if this varied according to menstrual phase. Mean 24 h SBP, DBP, and MAP were not different among the groups, or within each group according to menstrual phase. Mean SBP, DBP, and MAP sleep or wake values were not different among the groups, or within each group according to menstrual phase (Table 3). Although the incidence of nondipping was surprisingly high (Fig. 4), there was no difference in nondipping between menstrual phases within any of the groups, or within menstrual phases among the groups. Data shown are MAP (Fig. 4) and are not different when analyzed by SBP or DBP.
Table 3.
24-h ambulatory blood pressure monitor
| Never Smokers (n = 19) |
Current Smokers (n = 12) |
Former Smokers (n = 9) |
Passive Smokers (n = 9) |
|||||
|---|---|---|---|---|---|---|---|---|
| EF | ML | EF | ML | EF | ML | EF | ML | |
| 24 h | ||||||||
| SBP | 106.2 (1.9) | 108.2 (2.0) | 107.2 (2.1) | 107.3 (2.0) | 106.7 (3.5) | 107.9 (1.8) | 109.3 (2.7) | 109.0 (2.2) |
| DBP | 67.4 (1.1) | 69.1 (1.4) | 66.8 (1.1) | 67.4 (1.6) | 66.2 (1.8) | 66.6 (1.4) | 66.2 (2.3) | 65.7 (2.0) |
| MAP | 80.4 (1.1) | 82.5 (1.4) | 80.7 (2.5) | 81.3 (1.7) | 79.9 (1.9) | 80.4 (1.0) | 81.4 (2.1) | 81.3 (1.8) |
| Day | ||||||||
| SBP | 111.3 (1.9) | 111.2 (2.0) | 110.3 (2.0) | 109.9 (2.0) | 111.9 (3.2) | 117 (1.4) | 112.7 (2.9) | 111.0 (2.3) |
| DBP | 72.3 (1.1) | 72.9 (1.4) | 71.0 (1.6) | 70.6 (1.9) | 69.8 (1.9) | 70.1 (1.2) | 69.3 (2.2) | 68.3 (2.0) |
| MAP | 85.0 (1.2) | 86.1 (1.4) | 83.6 (1.3) | 83.7 (1.8) | 83.3 (1.8) | 84.1 (1.0) | 84.3 (2.3) | 83.2 (1.8) |
| Night | ||||||||
| SBP | 97.7 (2.1) | 95.2 (5.4) | 102.1 (3.3) | 100.9 (2.6) | 99.3 (3.6) | 99.2 (3.3) | 102.0 (3.3) | 104.3 (3.6) |
| DBP | 58.9 (1.3) | 60.7 (1.9) | 62.2 (2.3) | 60.9 (1.6) | 60.7 (1.4) | 59.7 (2.6) | 60.9 (2.9) | 59.9 (2.7) |
| MAP | 72.6 (1.4) | 74.6 (1.8) | 76.2 (2.3) | 74.8 (1.8) | 74.1 (1.7) | 73.5 (2.5) | 76.0 (2.7) | 76.3 (3.1) |
Values are means (SE). SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure.
Fig. 4.
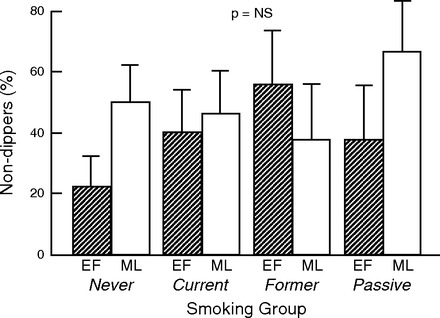
Ambulatory 24-h BP recordings: %nondippers according to smoking group. There was no difference in dipping within the groups between hormone phase, or among the 4 study groups.
DISCUSSION
The major findings of this study are the following. 1) In premenopausal women who are current or passive smokers, sympathetic outflow does not fluctuate over the menstrual cycle, unlike never smokers, in whom MSNA is higher during the ML phase and lower during the EF phase. 2) In former smokers who have quit smoking at least 1 yr, the pattern of sympathetic outflow fluctuates during the menstrual cycle, similar to never smokers. 3) In current smokers, baroreflex control of MSNA is attenuated during both phases of the ovarian cycle. 4) During a strong non-baroreflex stimulus, the cold pressor test, premenopausal women who are passive smokers have an exaggerated sympathetic response, approximately twice that of never smokers. 5) The incidence of the nocturnal fall in blood pressure (“dipping”), which is mediated by a circadian rhythm in SNA, was not different between women exposed and not exposed to tobacco smoke.
In premenopausal women not taking OCPs, we (33, 40), and others(34), have reported that resting MSNA follows a predictable pattern during the ovarian cycle characterized by higher levels during the ML phase, and lower levels during the EF phase; however, this pattern is disrupted in current smokers, in whom MSNA does not decline in the EF phase, resulting in a greater overall sympathetic burden (40). The present study confirms these findings and extends them to women who are former smokers or passive smokers. Consistent with reports that women exposed to secondhand smoke have increased cardiac risk—on par with that of current smokers(6)—MSNA in these passive smokers also followed the same pattern seen in current smokers. Similarly, consistent with the recent large population studies confirming that smoking cessation is associated with a marked reduction in cardiovascular mortality and sudden death risk (48, 50), MSNA in these former smokers followed the normal pattern found in never smokers. These findings support the notion that abnormal levels of SNA in premenopausal women exposed to cigarette smoke, via both active smoking and passive exposure, contribute to their increased cardiovascular risk, and that cardiovascular risk and the normal, fluctuating pattern of SNA over the menstrual cycle can be restored with smoking cessation.
Input from the peripheral baroreceptors tonically suppresses central sympathetic outflow, and acute fluctuations in this input help maintain blood pressure at a steady state. Attenuation of baroreflex sensitivity has been reported in conditions associated with chronically elevated MSNA, such as heart failure (5, 32). Although we observed a trend toward higher baroreflex sensitivity during the ML phase compared with the EF phase overall, baroreflex sensitivity was not significantly different during the menstrual cycle in any of the individual four groups, including never smokers, consistent with our prior study (33). However, the impact of chronic cigarette smoking on baroreflex sensitivity, independent of menstrual phase, was significant, and remarkable.
We found that baroreflex sensitivity was significantly attenuated in the current smokers during both menstrual phases, low-hormone EF and high-hormone ML; stated differently, current smokers have attenuated suppression (flatter slope) of MSNA compared with never smokers independent of hormonal influences. This finding does not directly explain why MSNA varies with the ovarian cycle in never smokers but not in current smokers, unless the blunted baroreflex attenuation of MSNA obscures any subtler variation in MSNA mediated by the ovarian hormones. It may, however, have important implications for understanding the poor prognosis and increased mortality risk in current smokers. Attenuated baroreflex sensitivity [most often baroreflex control of HR (16, 27, 28), but baroreflex control of MSNA as well (23)] has been identified as an independent risk factor for adverse cardiac events, including increased mortality, ventricular arrhythmias, and sudden arrhythmic death risk, in several populations, including post-myocardial infarction, chronic heart failure, and chronic hypertension populations (16, 23, 27, 28).
This novel finding of attenuated baroreflex suppression of MSNA in current smokers may be explained by abnormal transmission of arterial stretch, and/or abnormalities localized to the baroreceptor nerve endings themselves. In otherwise healthy teenagers and young adults without peripheral vascular disease, chronic active and passive tobacco exposure has been associated with increased vascular stiffness; vascular reactivity mediated by nitric oxide was found to be impaired (9, 10). Thus fluctuations in blood pressure transmitted to the baroreceptors through changes in stretch of the vascular wall are dampened. This impairment was reversible with elimination of tobacco exposure (9, 10). Thus decreased endothelium-dependent reactivity, a potentially reversible cause of increased vascular stiffness, may contribute to the attenuated baroreflex sensitivity in our study.
Another potential mechanism underlying the attenuated baroreflex sensitivity in current smokers may be a direct sequela of the particles and noxious compounds contained in cigarette smoke altering the baroreceptor afferent nerve endings themselves. In recordings from the carotid sinus nerves in animal models, reactive oxygen species (ROS) have been found to significantly attenuate baroreceptor activity in a dose-dependent and, importantly, reversible manner (29, 41). Increased smoking-induced ROS in current smokers may directly, yet reversibly, impair baroreflex sensitivity. Interestingly, intravenous administration of high doses of the antioxidant ascorbic acid acutely improves baroreflex sensitivity in hypertensive patients, and in patients with chronic heart failure, further supporting the notion that exposure to ROS may play an important role in attenuated baroreflex sensitivity in disease (8, 42). Antioxidants such as ascorbic acid improve endothelial function in habitual smokers, but the effect of antioxidants on baroreflex sensitivity in habitual smokers remains untested (24, 46).
Surprisingly, despite the nonfluctuating pattern of MSNA present in passive smokers, baroreflex sensitivity was not attenuated in passive smokers compared with never smokers. This is suggestive of a different, non-baroreflex-mediated mechanism of altered central sympathetic outflow induced by exposure to secondhand smoke. Consistent with this possibility, passive smokers had a remarkably different response to the CPT compared with never smokers; there was an exaggerated increase in SNA during CPT, approximately twice that of never smokers. The explanation for the exaggerated SNA response during CPT among passive smokers may be attributable to differences in the quantity or quality of smoke exposure. First, secondhand smoke exposure is likely shorter duration in our subjects: ∼8 h/wk of smoke exposure for passive smokers vs. ∼8 cigarettes/day in current smokers. It has been hypothesized that a lower exposure time to smoke in passive smokers compared with habitual smokers is insufficient to induce protective countermechanisms, such as induction of antioxidant enzyme activity (31, 39, 47). Smoke exposure in this setting may, paradoxically, be more lethal on a minute-to-minute basis compared with habitual smokers, thus explaining the nonlinear smoke exposure-response curve (44).
Second, the nature and characteristics of unfiltered secondhand smoke (“sidestream smoke”) are different from those of smoke drawn through a filtered cigarette (“mainstream smoke”). Tobacco smoke is divided into two phases: the tar (particulate) phase, which is trapped by the filter, and the gas phase, which passes through the filter. The particulate matter (PM) in the tar phase includes airborne material of >0.01 μm, whereas PM in the gas phase is <0.01 μm. Exposure to PM < 2.5 μm (PM2.5) is associated with increased cardiovascular mortality (45). Secondhand smoke contains ∼85% sidestream (unfiltered) smoke and 15% exhaled mainstream smoke and thus has a higher concentration of unfiltered, toxic compounds and PM2.5 (2). In summary, since, in current and passive smokers, the smoke stimulus is different quantitatively (time exposed) and qualitatively (type of smoke/PM size), the interaction with the baroreceptors and the central nervous system may also differ.
Just as MSNA rises and falls over the course of a month in concert with rise and fall of ovarian hormones, MSNA is known to rise and fall within the course of 24 h, increasing during waking hours, and decreasing profoundly during deep stages of sleep (49). BP follows this 24-h rise and fall, and a >10% falling systolic, diastolic, and/or mean arterial pressure from wakefulness to sleep (“dipping”) is healthful; conversely, nondipping is associated with increased cardiovascular risk (1, 14, 15, 26). Recently, short-term exposure to air pollution, characterized by PM size < 10 μm, was found to be associated with blunted nocturnal dipping in a population of city-dwelling adults (51). We asked the question whether there is a difference in the incidence of dipping between smoking status and/or the phases of the menstrual cycle among the four study groups. The results were clear: there was no difference according to smoking status or menstrual cycle phase. Interestingly, however, there was an unexpectedly high incidence of nondipping in all groups. Nondipping has been identified as a cardiac risk factor in certain diseases, such as hypertension and chronic kidney disease, but its specificity in a normotensive, healthy population, including current and passive smokers, is unknown.
Limitations.
In this study of relatively young premenopausal women, we found that current tobacco exposure is associated with attenuated baroreflex control of MSNA. From these studies, we do not know whether this is an age- and/or sex-specific finding, or whether habitual tobacco exposure in older women and men is also associated with impaired baroreflex sensitivity. This is an important question, which may have mechanistic implications explaining why active tobacco exposure increases cardiac mortality and sudden cardiac death in older women and in men as well. Although the overall number of subjects enrolled in these studies was relatively large (∼50), the number per group was not. Thus although we found an overall statistically significant relationship between menstrual phase and baroreflex sensitivity, we did not find a statistically significant relationship within any of the groups. Perhaps with a larger number of subjects in each group, we would have found a statistically significant relationship.
Ascorbic acid has been shown to reverse, at least temporarily, endothelial dysfunction in habitual smokers. Since we hypothesize that a smoking-related increase in ROS may underlie the impaired baroreflex control in current smokers, it would have been of interest to administer ascorbic acid and reassess baroreflex sensitivity. Whereas this intervention was beyond the scope of the current study, it will be of interest to pursue this potentially therapeutic intervention in follow up studies.
In summary, in premenopausal women never smokers and former smokers, in whom cardiac risk is not increased, SNA directed to muscle follows a cyclical pattern, increasing during the ML high-hormone phase, and decreasing during the EF low-hormone phase. In contrast, in current and passive smokers, in whom cardiac and sudden death risk is increased, SNA remains at a relatively constant level throughout the menstrual cycle, despite the normal fluctuating ovarian hormone pattern. Importantly, in current smokers, baroreflex control of MSNA is significantly blunted independent of the ovarian phase. Interestingly, passive smokers have a markedly exaggerated response to the strong, non-baroreflex-mediated, cold pressor stimulus, suggestive of a central, neuroexcitatory effect of secondhand smoke. These findings support the notion that abnormal levels of SNA in premenopausal women exposed to cigarette smoke contribute to increased cardiovascular risk. Once the mechanisms underlying sustained SNA levels associated with smoking are confirmed, therapies can be directed accordingly, such as antioxidants (ascorbic acid) to restore baroreceptor sensitivity. Meanwhile, the finding that SNA patterns revert to the pattern seen in never smokers adds support to the notion that it is never too late for smoking cessation.
GRANTS
This work was support by the Tobacco-Related Disease Research Program Exploratory/Developmental Research Award 18XT-00115 (H. R. Middlekauff), and National Institutes of Health Grant MO1-RR00865 (UCLA General Clinical Research Center). J. Park is supported by National Heart, Lung, and Blood Institute Grant K23-HL-098744 and the Atlanta Research and Education Foundation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: H.R.M. and J.P. conception and design of research; H.R.M. performed experiments; H.R.M., H.A., and J.A.G. analyzed data; H.R.M. and J.P. interpreted results of experiments; H.R.M. prepared figures; H.R.M. drafted manuscript; H.R.M., J.P., H.A., and J.A.G. approved final version of manuscript; J.P., H.A., and J.A.G. edited and revised manuscript.
REFERENCES
- 1.Agarwal R, Andersen MJ. Prognostic importance of ambulatory blood pressure recordings in patients with chronic kidney disease. Kidney Int 69: 1175–1180, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Ambrose JA, Barua RS. The pathophysiology of cigarette smoking and cardiovascular disease: an update. J Am Coll Cardiol 43: 1731–1737, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Arnold AC, Sakima A, Kasper SO, Vinsant S, Garcia-Espinosa MA, Diz DI. The brain renin-angiotensin system and cardiovascular responses to stress: insights from transgenic rats with low brain angiotensinogen. J Appl Physiol 113: 1929–1936, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Auerbach AD, Goldman L. beta-Blockers and reduction of cardiac events in noncardiac surgery: scientific review. JAMA 287: 1435–1444, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Baily RG, Sinoway LI. Insight into human baroreceptor function using multiple indices of neural activity. Heart Fail 6: 33–41, 1990 [PubMed] [Google Scholar]
- 6.Barnoya J, Glantz SA. Cardiovascular effects of secondhand smoke: nearly as large as smoking. Circulation 111: 2684–2698, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Bello N, Mosca L. Epidemiology of coronary heart disease in women. Progr Cardiovasc Dis 46: 287–295, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Bruno RM, Daghini E, Ghiadoni L, Sudano I, Rugani I, Varanini M, Passino C, Emdin M, Taddei S. Effect of acute administration of vitamin C on muscle sympathetic activity, cardiac sympathovagal balance, and baroreflex sensitivity in hypertensive patients. Am J Clin Nutr 96: 302–308, 2012 [DOI] [PubMed] [Google Scholar]
- 9.Celermajer DS, Adams MR, Clarkson P, Robinson J, McCredie R, Donald A, Deanfield JE. Passive smoking and impaired endothelium-dependent arterial dilatation in healthy young adults. N Engl J Med 334: 150–154, 1996 [DOI] [PubMed] [Google Scholar]
- 10.Celermajer DS, Sorensen KE, Georgakopoulos D, Bull C, Thomas O, Robinson J, Deanfield JE. Cigarette smoking is associated with dose-related and potentially reversible impairment of endothelium-dependent dilation in healthy young adults. Circulation 88: 2149–2155, 1993 [DOI] [PubMed] [Google Scholar]
- 11.Chan SH, Chan JY. Brain stem oxidative stress and its associated signaling in the regulation of sympathetic vasomotor tone. J Appl Physiol 113: 1921–1928, 2012 [DOI] [PubMed] [Google Scholar]
- 12.Delius W, Hagbarth KE, Hongell A, Wallin BG. General characteristics of sympathetic activity in human muscle nerves. Acta Physiol Scand 84: 65–81, 1972 [DOI] [PubMed] [Google Scholar]
- 13.Ebata H, Hojo Y, Ikeda U, Ishida H, Natsume T, Shimada K. Differential effects of an alpha 1-blocker (doxazosin) on diurnal blood pressure variation in dipper and non-dipper type hypertension. Hypertens Res 18: 125–130, 1995 [DOI] [PubMed] [Google Scholar]
- 14.Fagard RH. Dipping pattern of nocturnal blood pressure in patients with hypertension. Exp Rev Cardiovasc Ther 7: 599–605, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Fagard RH, Thijs L, Staessen JA, Clement DL, De Buyzere ML, De Bacquer DA. Night-day blood pressure ratio and dipping pattern as predictors of death and cardiovascular events in hypertension. J Hum Hypertens 23: 645–653, 2009 [DOI] [PubMed] [Google Scholar]
- 16.Farrell TG, Odemuyiwa O, Bashir Y, Cripps TR, Malik M, Ward DE, Camm AJ. Prognostic value of baroreflex sensitivity testing after acute myocardial infarction. Br Heart J 67: 129–137, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferguson DW, Berg WJ, Roach PJ, Oren RM, Mark AL. Effects of heart failure on baroreflex control of sympathetic neural activity. Am J Cardiol 69: 523–531, 1992 [DOI] [PubMed] [Google Scholar]
- 18.Fonarow GC. Comprehensive adrenergic blockade post myocardial infarction left ventricular dysfunction. Cardiol Clin 26: 79–89, vii, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Francis GS, Cohn JN, Johnson G, Rector TS, Goldman S, Simon A. Plasma norepinephrine, plasma renin activity, and congestive heart failure. Relations to survival and the effects of therapy in V-HeFT. II The V-HeFT VA Cooperative Studies Group. Circulation 87: VI40–VI48, 1993 [PubMed] [Google Scholar]
- 20.Gabor A, Leenen FH. Central neuromodulatory pathways regulating sympathetic activity in hypertension. J Appl Physiol 113: 1294–1303, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grassi G, Seravalle G, Calhoun DA, Bolla GB, Giannattasio C, Marabini M, Del Bo A, Mancia G. Mechanisms responsible for sympathetic activation by cigarette smoking in humans. Circulation 90: 248–253, 1994 [DOI] [PubMed] [Google Scholar]
- 22.Grassi G, Seravalle G, Cattaneo BM, Lanfranchi A, Vailati S, Giannattasio C, Del Bo A, Sala C, Bolla GB, Pozzi M. Sympathetic activation and loss of reflex sympathetic control in mild congestive heart failure. Circulation 92: 3206–3211, 1995 [DOI] [PubMed] [Google Scholar]
- 23.Grassi G, Seravalle G, Dell'Oro R, Facchini A, Ilardo V, Mancia G. Sympathetic and baroreflex function in hypertensive or heart failure patients with ventricular arrhythmias. J Hypertens 22: 1747–1753, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Heitzer T, Just H, Munzel T. Antioxidant vitamin C improves endothelial dysfunction in chronic smokers. Circulation 94: 6–9, 1996 [DOI] [PubMed] [Google Scholar]
- 25.Hojo Y, Noma S, Ohki T, Nakajima H, Satoh Y. Autonomic nervous system activity in essential hypertension: a comparison between dippers and non-dippers. J Hum Hypertens 11: 665–671, 1997 [DOI] [PubMed] [Google Scholar]
- 26.Kim BK, Lim YH, Lee HT, Lee JU, Kim KS, Kim SG, Kim JH, Lim HK, Shin J. Non-dipper pattern is a determinant of the inappropriateness of left ventricular mass in essential hypertensive patients. Korean Circ J 41: 191–197, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koutelou M, Katsikis A, Flevari P, Theodorakis G, Livanis E, Georgiadis M, Voudris V, Kremastinos D. Predictive value of cardiac autonomic indexes and MIBG washout in ICD recipients with mild to moderate heart failure. Ann Nucl Med 23: 677–684, 2009 [DOI] [PubMed] [Google Scholar]
- 28.La Rovere MT, Bigger JT, Jr, Marcus FI, Mortara A, Schwartz PJ. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction ATRAMI (Autonomic Tone and Reflexes After Myocardial Infarction) Investigators. Lancet 351: 478–484, 1998 [DOI] [PubMed] [Google Scholar]
- 29.Li Z, Mao HZ, Abboud FM, Chapleau MW. Oxygen-derived free radicals contribute to baroreceptor dysfunction in atherosclerotic rabbits. Circ Res 79: 802–811, 1996 [DOI] [PubMed] [Google Scholar]
- 30.Mancia G, Groppelli A, Di Rienzo M, Castiglioni P, Parati G. Smoking impairs baroreflex sensitivity in humans. Am J Physiol Heart Circ Physiol 273: H1555–H1560, 1997 [DOI] [PubMed] [Google Scholar]
- 31.McCusker K, Hoidal J. Selective increase of antioxidant enzyme activity in the alveolar macrophages from cigarette smokers and smoke-exposed hamsters. Am Rev Respir Dis 141: 678–682, 1990 [DOI] [PubMed] [Google Scholar]
- 32.Middlekauff HR, Mark AL. The treatment of heart failure: the role of neurohumoral activation. Intern Med 37: 112–122, 1998 [DOI] [PubMed] [Google Scholar]
- 33.Middlekauff HR, Park J, Gornbein JA. Lack of effect of ovarian cycle and oral contraceptives on baroreceptor and nonbaroreceptor control of sympathetic nerve activity in healthy women. Am J Physiol Heart Circ Physiol 302: H2560–H2566, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Minson CT, Halliwill JR, Young TM, Joyner MJ. Influence of the menstrual cycle on sympathetic activity, baroreflex sensitivity, and vascular transduction in young women. Circulation 101: 862–868, 2000 [DOI] [PubMed] [Google Scholar]
- 35.Narkiewicz K, van de Borne PJ, Hausberg M, Cooley RL, Winniford MD, Davison DE, Somers VK. Cigarette smoking increases sympathetic outflow in humans. Circulation 98: 528–534, 1998 [DOI] [PubMed] [Google Scholar]
- 36.Narkiewicz K, Winnicki M, Schroeder K, Phillips BG, Kato M, Cwalina E, Somers VK. Relationship between muscle sympathetic nerve activity and diurnal blood pressure profile. Hypertension 39: 168–172, 2002 [DOI] [PubMed] [Google Scholar]
- 37.Njolstad I, Arnesen E, Lund-Larsen PG. Smoking, serum lipids, blood pressure, and sex differences in myocardial infarction. A 12-year follow-up of the Finnmark Study. Circulation 93: 450–456, 1996 [DOI] [PubMed] [Google Scholar]
- 38.Ohkubo T, Hozawa A, Yamaguchi J, Kikuya M, Ohmori K, Michimata M, Matsubara M, Hashimoto J, Hoshi H, Araki T, Tsuji I, Satoh H, Hisamichi S, Imai Y. Prognostic significance of the nocturnal decline in blood pressure in individuals with and without high 24-h blood pressure: the Ohasama study. J Hypertens 20: 2183–2189, 2002 [DOI] [PubMed] [Google Scholar]
- 39.Otsuka R, Watanabe H, Hirata K, Tokai K, Muro T, Yoshiyama M, Takeuchi K, Yoshikawa J. Acute effects of passive smoking on the coronary circulation in healthy young adults. JAMA 286: 436–441, 2001 [DOI] [PubMed] [Google Scholar]
- 40.Park J, Middlekauff HR. Altered pattern of sympathetic activity with the ovarian cycle in female smokers. Am J Physiol Heart Circ Physiol 297: H564–H568, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peng YJ, Nanduri J, Zhang X, Wang N, Raghuraman G, Seagard J, Kumar GK, Prabhakar NR. Endothelin-1 mediates attenuated carotid baroreceptor activity by intermittent hypoxia. J Appl Physiol 112: 187–196, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Piccirillo G, Nocco M, Moise A, Lionetti M, Naso C, di Carlo S, Marigliano V. Influence of vitamin C on baroreflex sensitivity in chronic heart failure. Hypertension 41: 1240–1245, 2003 [DOI] [PubMed] [Google Scholar]
- 43.Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves J, Hill MN, Jones DW, Kurtz T, Sheps SG, Roccella EJ. Recommendations for blood pressure measurement in humans and experimental animals. 1. Blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation 111: 697–716, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Pope CA, 3rd, Burnett RT, Krewski D, Jerrett M, Shi Y, Calle EE, Thun MJ. Cardiovascular mortality and exposure to airborne fine particulate matter and cigarette smoke: shape of the exposure-response relationship. Circulation 120: 941–948, 2009 [DOI] [PubMed] [Google Scholar]
- 45.Pope CA, 3rd, Burnett RT, Thun MJ, Calle EE, Krewski D, Ito K, Thurston GD. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA 287: 1132–1141, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Raitakari OT, Adams MR, McCredie RJ, Griffiths KA, Stocker R, Celermajer DS. Oral vitamin C and endothelial function in smokers: short-term improvement, but no sustained beneficial effect. J Am Coll Cardiol 35: 1616–1621, 2000 [DOI] [PubMed] [Google Scholar]
- 47.Raupach T, Schafer K, Konstantinides S, Andreas S. Secondhand smoke as an acute threat for the cardiovascular system: a change in paradigm. Eur Heart J 27: 386–392, 2006 [DOI] [PubMed] [Google Scholar]
- 48.Sandhu RK, Jimenez MC, Chiuve SE, Fitzgerald KC, Kenfield SA, Tedrow UB, Albert CM. Smoking, smoking cessation, and risk of sudden cardiac death in women. Circ Arrhythm Electrophysiol 5: 1091–1097, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Somers VK, Dyken ME, Mark AL, Abboud FM. Sympathetic-nerve activity during sleep in normal subjects. N Engl J Med 328: 303–307, 1993 [DOI] [PubMed] [Google Scholar]
- 50.Thun MJ, Carter BD, Feskanich D, Freedman ND, Prentice R, Lopez AD, Hartge P, Gapstur SM. 50-year trends in smoking-related mortality in the United States. N Engl J Med 368: 351–364, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tsai DH, Riediker M, Wuerzner G, Maillard M, Marques-Vidal P, Paccaud F, Vollenweider P, Burnier M, Bochud M. Short-term increase in particulate matter blunts nocturnal blood pressure dipping and daytime urinary sodium excretion. Hypertension 60: 1061–1069, 2012 [DOI] [PubMed] [Google Scholar]
- 52.Wallin BG, Fagius J. Peripheral sympathetic neural activity in conscious humans. Annu Rev Physiol 50: 565–576, 1988 [DOI] [PubMed] [Google Scholar]


