Abstract
The colon mucus layers minimize the contact between the luminal flora and the epithelial cells, and defects in this barrier may lead to colonic inflammation. We now describe an ex vivo method for analysis of mucus properties in human colon and mouse small and large intestine. Intestinal explants were mounted in horizontal perfusion chambers. The mucus surface was visualized by adding charcoal particles on the apical side, and mucus thickness was measured using a micropipette. Mucus thickness, adhesion, and growth rate were recorded for 1 h. In mouse and human colon, the ability of the mucus to act as a barrier to beads the size of bacteria was also evaluated. Tissue viability was monitored by transepithelial potential difference. In mouse ileum, the mucus could be removed by gentle aspiration, whereas in colon ∼40 μm of the mucus remained attached to the epithelial surface. Both mouse and human colon had an inner mucus layer that was not penetrated by the fluorescent beads. Spontaneous mucus growth was observed in human (240 μm/h) and mouse (100 μm/h) colon but not in mouse ileum. In contrast, stimulation with carbachol induced a higher mucus secretion in ileum than colon (mouse ileum: Δ200 μm, mouse colon: Δ130 μm, human colon: Δ140 μm). In conclusion, while retaining key properties from the mucus system in vivo, this setup also allows for studies of the highly dynamic mucus system under well-controlled conditions.
Keywords: mucin, carbachol, colon, ileum, mucus growth
one of the major functions of the colon epithelium is to act as a protective barrier against the enormous amount of bacteria that resides in the lumen. To manage this task, the epithelial cells are sealed by tight junctions, secrete anions and fluid, and are covered by a dense adherent mucus layer that forms a physical barrier separating the luminal flora from the underlying epithelium (12, 14).
The thickness and adhesion properties of the intestinal mucus layers have been characterized in vivo in rodents showing a dominating loose layer in the small intestine and a two-layer system in the colon with an inner firmly adherent layer and an outer loosely adherent layer (2, 14). The importance of the colonic mucus as a part of the intestinal barrier became evident with the mouse lacking the Muc2 mucin that developed spontaneous colitis around the time of weaning (28, 29). Formation of a normal mucus layer involves a regulated secretory process where mucins are released, expanded, and hydrated to form the mucus gel. The rate of mucus release is under control of the enteric nervous system, and the exocytotic process is coordinated with anion and fluid secretion to allow for proper formation of the mucus (3, 20).
In human colon, regulation of mucus synthesis and secretion has been studied using [3H]glucosamine incorporation in cultured specimens (5, 6, 16). The organization of the mucus layer in situ has been studied using histological techniques in mouse, rat, and human colon (4, 14, 15, 19, 27). However, this approach does not permit studies of physiological regulation of mucus formation. The available information regarding thickness and properties of the mucus layer throughout the gastrointestinal tract is largely based on data from anesthetized rodents (2, 22, 26). This preparation necessitates major surgery and manipulation of the tissue that may affect the behavior of the system.
Here we describe a third approach, ex vivo measurements of mucus adhesion, properties, thickness, and growth, as a function of time in mouse and human colon and mouse small intestine. The principle of measuring mucus thickness is adapted from the in vivo technique where charcoal particles were added onto the mucosal surface and visualized by light microscopy (1, 2, 26). By using a perfusion chamber with separated luminal and serosal compartments, the tissue is easily accessible to pharmacological interventions, which makes it useful for experimental manipulation. With this setup, we were able to measure initial mucus thickness, spontaneous rate of mucus formation, and the functional properties of the mucus. In the present study, we evaluated mucus physiology in mouse colon and ileum and in endoscopic biopsies from human distal colon. The results confirmed formation of an outer loose layer and an inner firmly adherent layer in mouse colon and loose layer in mouse ileum. Both the mouse and human colon mucus formed a barrier between beads in the size of bacteria and the epithelial surface. Spontaneous mucus growth was lower in ileum than colon, whereas the reactivity to induced secretion was higher in ileum.
MATERIALS AND METHODS
Mouse distal ileum and distal colon.
Ethical approval for the animal experiments was granted by the Animal Ethics committee, University of Gothenburg. Mice (C57/Bl6 males, 10–12 wk old) were anesthetized with isoflurane and killed by cervical dislocation. The distal colon or distal ileum was dissected and flushed with ice-cold oxygenated (95% O2, 5% CO2) Krebs' solution with the following composition (in mM): 116 NaCl, 1.3 CaCl2, 3.6 KCl, 1.4 KH2PO4, 23 NaHCO3, and 1.2 MgSO4 (Merck), pH 7.4. The tissue was kept on ice for 30 min followed by opening along the mesenteric border and removal of the longitudinal muscle layer by blunt dissection. The specimen was divided into two pieces that were mounted in the horizontal perfusion chambers for thickness measurements or in the image chamber for studies of mucus penetrability. The dissection was performed in oxygenated ice-cold Krebs' solution.
Human subjects.
Study subjects were recruited among patients referred for colonoscopy at Sahlgrenska University Hospital, Gothenburg, Sweden. Biopsies from sigmoid colon were assessed from 28 control patients referred to colonoscopy for reasons such as unspecific bleeding, previous diverticulitis, and polyp surveillance in which the colonoscopy was macroscopically normal. The material consisted of 16 women (54 ± 4 yr) and 12 men (56 ± 3 yr). The study was performed after written informed consent was obtained from all of the subjects. Approval was granted by the Human Research Ethical Committee, University of Gothenburg.
Bowel preparation and colonoscopy.
In the evening preceding colonoscopy, the bowel was cleaned by ingestion of four liters of an osmotic laxative (Laxabon). When arriving at the endoscopy unit, the patient was given full information according to the ethical permit and signed the informed consent. The patient was then given premedication consisting of midazolam (1–2 mg) (Dormicum) and petidin (50 mg) (Petidin Meda) intravenously. Heart rate and peripheral oxygenation was monitored by pulse oximetry. If necessary, the patient received additional sedation during the procedure.
Human colonic biopsies.
Biopsies from the sigmoid colon were obtained one at a time using a single-use large-capacity biopsy forcep (Olympus), and biopsies were instantly placed into oxygenated (95% O2, 5% CO2) ice-cold Krebs' solution with the same composition as described for the mouse specimens. The biopsies were incubated on ice for 30 min followed by mounting in the perfusion chamber for mucus thickness measurement or in the image chamber for studies of mucus penetrability. The biopsy was mounted by placing it on the backside of the apical chamber in a drop of oxygenated Krebs' solution. The biopsy was oriented with the mucosal side toward the apical chamber, held in place with a forceps, and spread out over the circular opening using a second forceps. Excessive Krebs' solution was removed by a paper tissue, and the apical chamber was turned around and mounted on top of the serosal chamber.
Chamber characteristics: Mucus thickness measurements.
The procedure described here was identical for the mouse small and large intestine and the human colonic biopsies. After being mounted, the apical chamber was filled with 150 μl oxygenized room temperature Krebs' solution containing d-mannitol (10 mM) (Sigma-Aldrich), sodium pyruvate (5.7 mM) (Sigma-Aldrich), and sodium-l-glutamate (5.1 mM) (Merck), pH 7.4. The basolateral chamber was perfused at a rate of 5 ml/min with room tempered Krebs' solution where d-mannitol was exchanged for d-glucose (Sigma-Aldrich) (10 mM), pH 7.4. The chamber was gradually heated to 37°C during a period of 10 min and kept at a constant temperature during the course of the experiment. Tissue viability was monitored using reference electrodes (Ref201; Radiometer) connected to the chamber via agar bridges (4% agar, 0.9% NaCl). Junction potential was compensated for by measuring background transepithelial potential difference (PD) in the assembled chamber before each experiment. Background PD was then subtracted from the tissue PD. The volume of the apical chamber was kept constant and unstirred during the whole experiment to avoid disturbing the mucus gel.
During the development of the method, three sets of experiments were performed for the human biopsies where the surface area and chamber volume were adjusted to improve the stability of the system. In the final setup, the surface diameter was 1.5 mm for the human colonic biopsies and 2.5 mm for the mouse small and large intestine and included PD measurements. The apical chamber volume was 150 μm, and the serosal volume was 165 μl.
Mucus thickness measurements.
To visualize the surface of the transparent mucus, a suspension of activated charcoal particles (Fluka) in Krebs' mannitol solution was added on the apical surface. The charcoal particles were allowed to sediment on to the mucus surface, and the thickness of the mucus was assessed by measuring the distance between the epithelial surface and the surface of the mucus (D) using a micropipette (OD: 1.2 mm, ID: 0.6 mm) pulled to a tip diameter of 5–10 μm. The micropipette was mounted in a micromanipulator (in house) connected to a digimatic indicator (Mitotoyo, Tokyo, Japan). The tissue was viewed through a stereomicroscope at ×50 magnification (Leica MZ125). The level of the epithelial surface was determined as the point where the tip of the micropipette and the epithelial surface was in the same focal plane. The micropipette was kept at a constant angle of 45°, and the vertical thickness of the mucus was obtained by multiplying the distance (D) with cos45 (26). To obtain an average mucus thickness over the epithelial surface, five points (epithelium and mucus) were measured, and the average thickness was used as a single value. In some experiments, the adhesive properties of the colonic mucus were assessed by measuring how much of the mucus could be removed by gentle aspiration. The total mucus thickness was measured as described above, followed by aspiration of the whole apical volume using a plastic Pasteur pipette (Cellprojects, PP-101, outer tip diameter 0.9 mm, inner tip diameter 0.7 mm, maximum volume 800 μl) during ∼3 s. The apical chamber was refilled with 150 μl KREB solution, new charcoal particles were added to the apical surface, and the remaining thickness was measured. Because of the structure of the ileal epithelium, the procedure for measuring mucus thickness is slightly different from that in the colon. In ileum, the initial mucus thickness is measured from the top of the villi to the mucus surface. The mucus is then aspirated as described above to visualize the surface epithelium in between the villi. To obtain the total mucus thickness as measured from the surface epithelium in between the villi to the mucus surface, the height of the villi is measured and added to the initial mucus thickness. Because of this, the ileal mucus has to be aspirated at the start of the experiment to allow for accurate measurements of the total mucus thickness.
To study the reactivity of the intestinal specimens, one set of experiments was performed where mucus secretion was induced by the cholinergic agonist carbachol (CCh). Baseline values were recorded for 30 min followed by serosal addition of CCh (1 mM in the serosal perfusate). The CCh-induced effect on mucus growth and PD was recorded for 30 min. To determine the magnitude of the response, the CCh-treated group was compared with an untreated control group.
Evaluation of mucus penetrability in colon.
The colonic explants were mounted in the same chamber as described above (surface diameter 1.5 mm). The apical chamber was filled with 1.5 ml Krebs' mannitol solution, and the serosal side was constantly perfused with Krebs' glucose solution containing Calcein Violet Blue tissue staining (1 μl/ml in the serosal perfusate; Invitrogen). The chamber was heated to 37°C during a period of 10 min and kept at a constant temperature during the whole experiment. The tissue was incubated for 20 min followed by removal of the majority of the apical solution, leaving ∼150 μl. A suspension of 1-μm-diameter far red fluorescent beads in Krebs' solution (5 μl) (FluoSpheres; Invitrogen) was then added to the apical surface, and the beads were left to sediment onto the mucus for 5 min. The apical chamber was then refilled with Krebs' mannitol solution, and the beads were allowed to sediment through the mucus for an additional 35 min. The distribution of the beads in the mucus was analyzed by taking confocal images in XY stacks (320 × 320 μm) using an LSM 700 Axio Examiner 2.1 confocal imaging system with a Plan-Apochromat ×20/1.0DIC water objective (Zeiss). The optical thickness of the section was 2.8 μm, and the sections were taken in 10-μm intervals. Images were processed using Volocity 5.5.1 software (Perkin-Elmer). Mucus penetrability was quantified by comparing the distance between the outer border of the beads and the epithelial surface with the distance between the most inner beads and the epithelial surface. The lowest point of beads was defined as the section where the bead intensity was <5% of the maximum bead intensity, which represents background fluorescence.
Immunostaining.
Specimens from mucus thickness measurements were fixed in Carnoy's fixative (60% dry MeOH, 30% chloroform, and 10% glacial acetic acid) followed by embedment in paraffin. Four-micrometer-thick sections were dewaxed and hydrated. Antigens were retrieved by microwave heating in 0.01 M citric acid buffer, pH 6, and the tissue was stained with either hematoxylin/eosin or with the anti-MUC2C3 antiserum (15). Fluorescein isothiocyanate-conjugated goat antirabbit immunoglobulin was used as secondary antibody. DNA was stained using the TO-PRO-3 iodide DNA staining (Invitrogen) according to the manufacturer's instructions. Pictures were obtained using a Nikon E1000 microscope with a ×20 objective or by an Axio Examiner Z1 LSM 700 confocal microscope using a plan apochromat ×20/1.3 DIC objective and the ZEN 2010 software.
The muc2-stained sections were used to determine which part of the intestinal tissue that responded to CCh treatment. The distribution of muc2-positive cells along the surface-crypt axis was compared between control and CCh-treated specimens. Because of technical reasons (chloroform-containing fixative), the tissue was removed from the perfusion chamber before fixation, which resulted in loss of most of the secreted mucus during the sample preparation. For this reason, we have not been able to correlate mucus thickness ex vivo with the amount of secreted mucus on the tissue sections.
Transmission electron microscopy.
Tissue specimens from the perfusion chamber were fixed in Karnovsky fixative (2% paraformaldehyde, 2.5% glutaraldehyde in 0.05 M sodium cacodylate buffer, pH 7.2) for 24 h followed by sequential staining using 1% OsO4 for 4 h, 1% tannic acid for 3 h, and 1% uranyl acetate overnight. Samples were dehydrated and embedded in epoxy resin (Agar 100). Ultrathin sections (50 nm) were cut using a Reichert Ultracut E microtome and collected on mesh copper support grids. The sections were contrasted using lead citrate and tannic acid and examined in a Zeiss 902 electron microscope.
Statistics.
Data are presented as means ± SD. A two-tailed Mann Whitney U-test was used when analyzing differences between the groups, and an F-test was used to test differences in variation between sample groups.
RESULTS
Chamber properties and methodological development: Defining surface area, chamber volume, and tissue thickness as important factors for a stable system.
Intestinal specimens from mouse and human colon as well as mouse ileum were studied as tissue explants. The tissue was mounted between two round openings in an Ussing-type horizontal chamber with an open apical chamber allowing the tissue to secrete mucus on its apical surface. Mucus is normally totally transparent, but the addition of charcoal made the upper surface visible through a dissection microscope, and it was possible to measure the mucus thickness with a glass capillary. The thickness of the mucus in colon was measured from the surface epithelium to the top of the mucus, whereas the thickness of the mucus in the small intestine was measured in two steps. The initial thickness was measured from the mucus surface to the villus tip. The mucus was then aspirated, and the mean villus height was estimated. These two values were then combined to give the total mucus thickness. A schematic drawing of the principle behind the measurements is shown in Fig. 1.
Fig. 1.
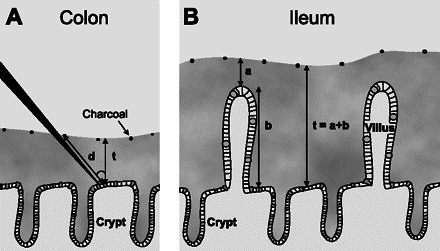
Schematic drawing of the principle of measuring mucus thickness ex vivo. A: colon. B: ileum. The distance between the epithelial surface and the surface of the mucus layer (d) is measured by a micropipette connected to a micromanipulator and a digimatic indicator. To obtain the vertical thickness of the mucus layer (t), the distance (d) is multiplied with the cosine of the angle of the micropipette, which in this case is 45° (t = d × cos45). In colon, which lacks villi, the epithelial surface is easily identified. In ileum, we used a two-step procedure to measure mucus thickness: first we measured the distance from the mucus surface to the tip of the villi and next we measured the height of the villi. Mucus thickness was then calculated as the sum of the two values.
The initial evaluation of the explant system was done on human colonic biopsies obtained during colonoscopy, since this was considered important for further use of the approach in studies of patients with inflammatory bowel disease. The first experiments were done with a chamber opening with a surface diameter of 1 mm. When using this small-sized epithelial surface, the survival of the tissue was poor, since shedding of the epithelium was observed both in the dissection microscope and after morphological evaluation. The surface diameter was then increased to 1.5 mm, which improved tissue viability as reflected by improved morphology. However, the intraindividual variation in mucus thickness after 1 h was still large, 700 ± 290 μm, n = 7, making comparisons between groups difficult.
To improve the stability of the system, the serosal chamber volume was increased from 17 to 165 μl, which significantly reduced both the intraindividual variation and the variability of total mucus thickness from 700 ± 290 to 450 ± 70 μm, P < 0.01. In addition, the spontaneous mucus growth rate and variability were reduced by 50% from 550 ± 290 to 240 ± 60 μm/h, P < 0.05. To verify the reproducibility of the method, two biopsies obtained in close proximity to each other from the same individual were studied in parallel for 30 min, and the thickness of the formed mucus layer was compared. The mucus thicknesses did not differ between the two samples [320 ± 60 vs. 310 ± 90 μm, P = not significant (NS)].
When using mouse colon and small intestine specimens, removal of the longitudinal muscle layer was essential for a stable preparation, since the epithelium was otherwise rapidly shed. The stability of the method was also tested in mouse colon and ileum by measuring two samples from the same animal in parallel. Similar to the observations in the human colon, the total mucus thickness in mouse colon did not differ between the two groups after 30 min incubation (130 ± 20 vs. 150 ± 50 μm, P = NS). Likewise, in mouse ileum, the initial thickness did not differ between the two groups (210 ± 9 vs. 210 ± 6 μm, P = NS). In this segment, the spontaneous growth during the following 30 min was not significantly different from zero.
Because the principle of the method is that the tissue is mounted in between two fluid-filled chambers, it is impossible to avoid tissue damage at the edge of the chamber. To reduce this effect, we increased the surface diameter to 1.5 mm in human colon biopsies and 2.5 mm in mouse intestine. The reason for using a smaller hole for the human experiments is that this is the largest diameter possible when using biopsies taken with forceps normally used in clinical practice. Unfortunately, the smaller hole did cause some artifacts, as discussed below. In some experiments using confocal microscopy (penetrability experiments), we used the smaller 1.5-mm hole also for mouse colon, since this size gave a flat epithelial surface that is more stable during the time it takes to record the confocal stacks.
The final setup, with the chamber mounted in a heating block with serosal perfusion tubes and the agar bridges, is shown in Fig. 2A. A top view of the epithelial surfaces of mouse distal colon, human distal colon, and mouse distal ileum viewed through the stereomicroscope before (top) and after (bottom) addition of charcoal to the apical surface is shown in Fig. 2B. A schematic drawing of the cross section of the perfusion chamber including the tissue, agar bridges, and the micropipette is shown in Fig. 2C.
Fig. 2.
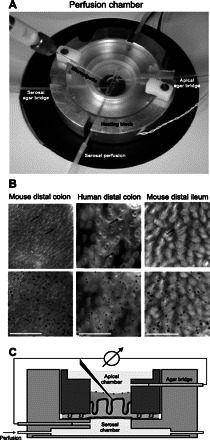
Perfusion chamber and top view of the intestinal specimens observed through the stereomicroscope. A: assembled chamber mounted in the heating block with serosal perfusion tubes, agar bridges, and micropipette. B: top view of mouse colon, human colon, and mouse ileum mounted in the perfusion chamber before (top) and after (bottom) charcoal was added to the apical surface. Scale bar 0.5 mm. Arrows mark the crypts in mouse and human colon and the villus tip in mouse ileum. C: schematic drawing of the tangential view of the perfusion chamber with the apical and basolateral chamber, intestinal specimen, agar bridges, and the micropipette.
Tissue viability.
To control that the tissue remained viable, transepithelial PD was measured in all experiments. In all three groups and during the entire experiment, the tissue generated a stable lumen negative PD (human colon: −1.8 ± 0.8 mV, mouse colon: −3.0 ± 1.6 mV, mouse ileum: −2.6 ± 0.9 mV). At the end of the experiment, the tissue was fixed for histological analysis in either Carnoy's fixative for light microscopy or in Karnowsky's fixative for transmission electron microscopy. Morphological evaluation showed a normal tissue structure with intact crypt and surface epithelium in the colon and intact villi and crypt structure in the ileum after 1 h incubation in the perfusion chamber (Fig. 3, A–C). Transmission electron microscopy confirmed an intact cell structure at the surface epithelium of the villus in the small intestine with preserved microvilli, cell-cell adhesion, and cell shape (Fig. 3D).
Fig. 3.
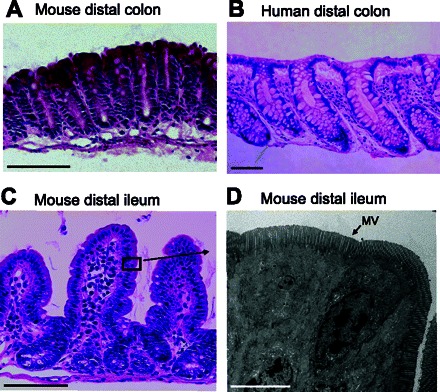
Histological evaluation of intestinal specimens after 1 h incubation in the perfusion chamber. A–C: hematoxylin/eosin staining of mouse colon, human colon, and mouse ileum. In mouse ileum, the black box represents the approximate location of the image in D. D: transmission electron micrograph of the epithelial cells in mouse ileum with intact microvilli (MV), cell-cell adhesion, and normal cell shape. A–C: scale bar 100 μm. D: scale bar 5 μm.
Adhesive properties of explant-generated mucus in human and mouse intestine.
In vivo studies of colon mucus have shown a two-layer system with an inner firmly adherent layer that is not possible to remove by gentle aspiration and an outer loose layer that can be easily aspirated (2, 15). In the same study, ileum was shown to generate mainly a loose mucus layer that could be easily aspirated. We now tested if similar properties also exist in ex vivo experiments. In the mouse colon, about 2/3 of the total mucus thickness could be removed directly after mounting by gentle aspiration, leaving a 40 ± 10-μm-thick firmly adherent mucus layer. Over a 1-h period, the colonic mucus thickness was restored to about the same thickness as the initial values. About 2/3 of this newly formed mucus could also be aspirated again, leaving a 50 ± 9-μm-thick adherent layer (Fig. 4A).
Fig. 4.
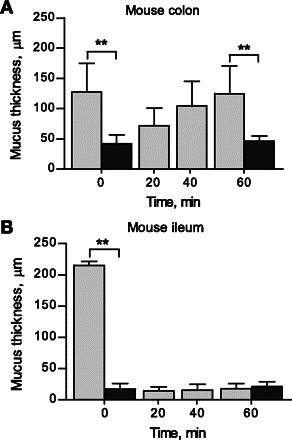
Growth of mucus layers in mouse colon and small intestine A: mouse colon (n = 6). B: mouse ileum (n = 5). The total mucus thickness was measured directly after mounting the explants (light gray bar), followed by aspiration of the loose mucus and measurement of the remaining attached mucus (dark gray bar). Spontaneous mucus growth was recorded for 1 h followed by repeated removal of the loose mucus and measurement of the remaining adherent mucus (dark gray bar). Data are presented as means ± SD. **P < 0.01.
In contrast to mouse colon, the mucus formed on the human colonic explants was not possible to remove by gentle aspiration, neither at the start of the experiment nor after 1 h incubation. A possible reason for this difference between mouse and human colon was that the latter experiments were performed using a smaller chamber opening (1.5 mm diameter in human colon vs. 2.5 mm diameter in mouse colon). When mouse colon was studied in the chamber with the 1.5-mm opening, less mucus could be aspirated.
In mouse ileum, >90% of the mucus could be removed by gentle aspiration, leaving only a thin mucus layer (20 ± 9 μm). In contrast to the colonic tissue, the ileal mucus layer was not restored after removal, i.e., mouse ileum did not have any measurable spontaneous mucus growth (Fig. 4B).
Mucus penetrability in mouse and human colon.
We have previously shown that the inner mucus layer of the mouse colon is impermeable to bacteria and beads in the size of bacteria (13, 15). To test if the mucus formed on human and mouse colonic explants have similar properties, the penetrability to fluorescent beads was studied. Mucus was allowed to form on top of the explants (1.5-mm-diameter opening) for 20 min before fluorescent beads with a diameter of 1 μm were added to the apical side and allowed to sediment through the mucus for 40 min followed by z-stack recordings obtained with a confocal microscope. In both mouse and human colon, the fluorescent beads penetrated ∼40% of the mucus, whereas the remaining 60% of the mucus was impenetrable to the beads (Fig. 5, A and B).
Fig. 5.
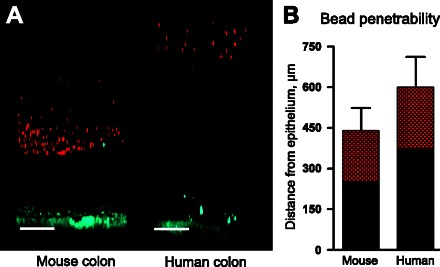
Mucus penetrability to 1 μm far red fluorescent beads in mouse and human colon. A: representative Z-stack projections from mouse and human colon with the tissue in blue and beads in red. Scale bar 100 μm. B: distribution of bead intensity in mouse (n = 6) and human (n = 8) colonic mucus. The red area represents the part of the mucus that is penetrated by the beads, whereas the black part represents the part of the mucus that is not penetrated by the beads. Data are presented as means ± SD.
Dynamics of spontaneous mucus growth in human and mouse intestine.
Next we compared the rate of mucus growth over time on the mouse and human explants. The spontaneous mucus growth was followed for 1 h. Both the growth rate (human: 240 ± 60 μm/h, mouse: 100 ± 60 μm/h) and the final mucus thickness (human: 480 ± 70 μm; mouse: 190 ± 40 μm) were higher in human than in mouse colon (Fig. 6, B and E).
Fig. 6.
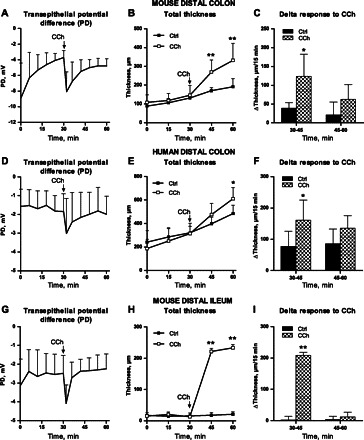
Spontaneous and carbachol (CCh)-induced mucus growth in mouse and human intestine. A, D, and G: transepithelial potential difference (PD) before and after CCh stimulation in mouse colon (n = 6), human colon (n = 7), and mouse ileum (n = 6). PD is given in mV with luminal polarity. B, E, and G: total mucus thickness in control and CCh-stimulated tissue from mouse colon, human colon, and mouse ileum. In mouse and human colon, the mucus was not aspirated before the experiment, whereas in mouse ileum the initial thickness represents the remaining mucus after aspiration. C, F, and I: Δincrease in mucus thickness in control (Ctrl) and CCh-treated tissue. Data are presented as means ± SD. *P < 0.05 and **P < 0.01.
CCh induces an increase in mucus thickness in both human and mouse intestine.
For this explant system to be truly useful, it should also respond to physiological stimuli and pharmacological interventions. Mucus and ion secretion was accordingly stimulated by serosal addition of the cholinergic agonist CCh (1 mM), which has been shown to induce both mucus and anion secretion in the small and large intestine of various species (11, 17, 18, 24, 25). The addition of CCh induced a rapid transient increase in PD in all three groups that was followed by mucus release observed as an increased mucus thickness (Fig. 6, A–B, D–E, and G–H). The CCh-induced mucus response peaked within 15 min of stimulation, and, after 30 min of stimulation, the effect declined toward control values (Fig. 6, C, F, and I). Because both mouse and human colon show high rates of spontaneous mucus growth, resulting in different baseline values, we also compared the magnitude of the CCh response after trying to adjust for differences in spontaneous mucus growth. This was done by subtracting the growth in the control groups from that of the CCh-treated groups. The results showed that the total CCh response was larger in mouse ileum than mouse colon (mouse ileum: Δ210 ± 20 μm, mouse colon: Δ130 ± 70 μm, P < 0.05), whereas it did not differ between mouse and human colon (mouse colon: Δ130 ± 70 μm, human colon 140 ± 80 μm, P = NS).
CCh induces goblet cell exocytosis in the colonic and small intestinal crypts.
To determine which part of the intestinal epithelium that responded to CCh treatment, sections from control and CCh-stimulated specimens were stained against the muc2 mucin, and the distribution pattern of muc2-positive cells was compared. In mouse colon, CCh treatment was associated with less muc2-positive cells in the lower part of the crypt, whereas filled goblet cells were still seen at the top of the crypt (Fig. 7, A and B). In human colon, the secretory process occurred in the direction top of the crypt toward the bottom of the crypt (Fig. 7, C and D). Sections from CCh-treated mouse ileum contained very few muc2-positive cells, and secreted material could be seen in the crypt lumen, thus the whole small intestinal crypt appeared to responds to treatment (Fig. 7, E and F).
Fig. 7.
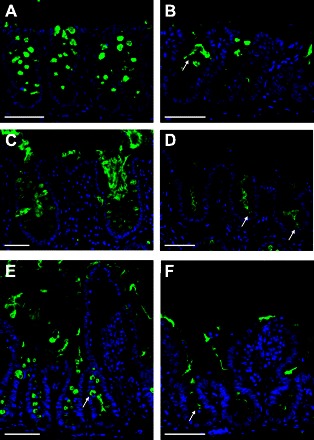
Histological analysis of muc2-containing goblet cells in control and CCh-treated specimens. A: mouse distal colon, control tissue. Muc2-positive cells are present along the whole crypt length. B: mouse distal colon, CCh treated. Muc2-secreting cells (arrow) are seen in the upper part of the crypt but not in the lower part of the crypt. C: human distal colon, control tissue. Muc2-positive cells are present along the whole crypt, and secreted material is seen in the crypt lumen. D: human distal colon, CCh treated. The upper parts of the crypts do not contain any filled goblet cells, whereas a few cells toward the lower part of the crypt show signs of ongoing secretion (arrows). E: mouse distal ileum, control tissue. Muc2-positive cells are present along the whole crypt length (arrow). F: mouse distal ileum, CCh treated. Residues of secreted muc2 are seen in the crypt lumen (arrow). Green, muc2; blue, DNA. Scale bar 50 μm.
DICUSSION
In the present study, we have used an Ussing chamber-like setup to study the dynamics of mucus formation in human colonic biopsies and mouse small and large intestine. The explants are mounted in an upright position, allowing the formed mucus to remain on the tissue explant at the same time as the mucus can be visualized. By combining this approach with measurements of mucus penetrability to fluorescent beads in size of bacteria, one can obtain data on mucus thickness, spontaneous and stimulated rates of mucus formation, and its biophysical properties. Data of this type are not available in the literature, in particular, not for humans.
Many aspects of previously reported in vivo behavior of the mucus system were reproduced in our setup (2). A direct comparison between mouse colon and ileum showed that the colonic mucus consisted of a loose and a firmly attached layer, whereas the mouse ileum formed mainly a loose layer. This is similar to that described in vivo for the rat (2). In both the small and large intestine, the mucus is built around the muc2 mucin. How the same protein can form a mucus gel with different adhesive properties in the two segments is not fully understood. However, both the secretory capacity of the epithelium and the presence of bacteria are known to affect mucus properties. In the small intestine, loss of cystic fibrosis transmembrane conductance regulator-mediated anion secretion results in mucus accumulation, whereas, in colon, the adhesive property of the mucus is induced by bacteria (9, 15). In the present study, the mucus formed in both mouse and human colonic explants also separated beads in the size of bacteria from the epithelial surface, suggesting that also the human colonic mucus has the ability to form a physical barrier separating the luminal bacteria from the epithelial surface.
The main advantage of the setup described here is the opportunity to follow changes in mucus properties over time. To allow for physiological interpretations, the viability of the tissue is of course crucial. This was evaluated in two ways, by light and electron microscopy and by monitoring transepithelial PD. Such measurements have been extensively used in studies of intestinal ion transport. The characteristic lumen negative PD requires active transport via the basolateral Na+-K+-ATPase; thus, a stable negative PD is only present in viable tissue exhibiting a normal ATP production (7). Low energy production and thus less viable tissue will be readily recognized as a decline in baseline PD. Both basal PD and the magnitude and time frame of the CCh responses are in accordance with previously published data from Ussing chamber experiments, which show that the viability of the preparation holds the same standard as well established ex vivo methods used for studies of epithelial transport (10, 17, 21, 30). We therefore conclude that the tissue is not only morphologically intact but also remains functionally viable in terms of both basal transport and reactivity to secretagogues.
This is the first study that measures mucus growth as a function of time in live human colonic tissue. Previous studies of mucus properties in human colon have focused on preserving the mucus on fixed tissue to estimate its thickness at a particular time point (19, 27). However, our data clearly show that mucus formation is a dynamic system that needs to be studied as a function of time. At least the loose mucus layer is expected to be severely affected by mechanical forces, including laxatives that are regularly used prior to the colonoscopy. It is therefore encouraging that the two studies that have measured mucus thickness in freshly resected tissue and snap-frozen biopsies report values similar to our starting mucus thickness of 200 μm (8, 23).
Our data on mucus thickness in mouse also agree closely with those obtained in vivo, both with regard to the total thickness and thickness of the inner firmly adherent layer (15, 22). The finding of an almost completely loose mucus layer in the mouse ileum is also in agreement with previous findings in anesthetized rats; however, both the total thickness and the spontaneous mucus growth were numerically lower in our preparation (2). In the study by Atuma et al. (2), the authors mention that the ileal mucus turned opaque over time, which made accurate measurements difficult. In our study, the mucus remained clear during the whole experiment, and opaque mucus was only seen at the edges of the chamber where the tissue was squeezed in between the two chamber halves. Normal mucus is transparent, and it is possible that formation of this opaque mucus is due to leakage of extracellular fluid during the preparation. Thereby, both the thickness and spontaneous growth might be overestimated in this segment when exposed to invasive surgery.
In mouse colon, 2/3 of the mucus could be removed by gentle aspiration, leaving the inner firmly adherent mucus layer. In contrast, the mucus formed on the human explants could not be easily removed by the same method. The reason for this discrepancy is unclear; however, one source of error might be the different size of the human and mouse chamber openings (1.5 vs. 2.5 mm). This possibility is supported by the finding that it was also more difficult to remove murine mucus using the 1.5-mm opening. Although the human mucus was not possible to remove by gentle aspiration, the penetrability to beads was similar in mouse and human colon, showing that the human mucus undergoes changes in its properties that make the outer part of the mucus more permeable than the mucus close to the epithelial surface. Based on this, we consider it likely that also the human colon has an outer loose mucus layer in vivo.
The mouse ileum did not have any measurable spontaneous mucus growth but was instead more reactive to CCh-induced mucus secretion than mouse colon. In the small intestine, the main defense mechanisms against bacterial invasion are fluid secretion, secretion of antibacterial peptides, and motor activity to flush the bacteria distally, i.e., a highly dynamic system that possibly also includes a rapid mucus secretion when necessary (12).
One of the major research interests regarding the colonic mucus layer is its role in inflammatory bowel disease. Mucus properties in patients with ulcerative colitis and Crohn's disease have been studied with histological techniques, and the results point in the direction of a thinner mucus layer (27). However, virtually nothing is known about the dynamics of mucus formation in these patients and how these features are related to pathology. Our method will enable testing the hypothesis that mucus dysfunction is a key element in the pathophysiology of inflammatory bowel disease, in particular ulcerative colitis. In addition, this method will be a useful tool in studies of regulation of spontaneous and induced mucus secretion, the effect of ions and fluid secretion on mucus secretion and properties, as well as the effects of endogenous and exogenous proteases and mucus-associated proteins on mucus properties.
GRANTS
This work was supported by the Swedish Research Council (nos. 7461, 8288, 21027, and 342-2004-4434), The Swedish Cancer Foundation, The Knut and Alice Wallenberg Foundation (KAW2007.0118), IngaBritt and Arne Lundberg Foundation, Sahlgren's University Hospital, EU-FP7 IBDase (no. 200931), Wilhelm and Martina Lundgren's Foundation, Torsten and Ragnar Söderbergs Foundations, The Swedish Foundation for Strategic Research-The Mucosal Immunobiology and Vaccine Center, and the Mucus-Bacteria-Colitis Center of the Innate Immunity Program (2010–2014).
DISCLOSURES
The authors have no conflicts of interest to disclose.
ACKNOWLEDGMENTS
We thank the electron microscopy unit and the centre for cellular imaging at the Sahlgrenska academy for technical assistance.
Author contributions: Conceived and designed the experiments: J. K. Gustafsson, M. E. V. Johansson, G. C. Hansson, H. Sjövall. Performed the experiments: J. K. Gustafsson, A. Ermund, M. E. V. Johansson, A. Schütte. Analyzed the data: J. K. Gustafsson, G. C. Hansson, H. Sjövall. Wrote the paper: J. K. Gustafsson, G. C. Hansson, H. Sjövall.
REFERENCES
- 1. Allen A, Pearson JP. The gastrointestinal adherent mucous gel barrier. Methods Mol Biol 125: 57–64, 2000. [DOI] [PubMed] [Google Scholar]
- 2. Atuma C, Strugala V, Allen A, Holm L. The adherent gastrointestinal mucus gel layer: thickness and physical state in vivo. Am J Physiol Gastrointest Liver Physiol 280: G922–G929, 2001. [DOI] [PubMed] [Google Scholar]
- 3. Bertrand CA, Laboisse CL, Hopfer U. Purinergic and cholinergic agonists induce exocytosis from the same granule pool in HT29-Cl.16E monolayers. Am J Physiol Cell Physiol 276: C907–C914, 1999. [DOI] [PubMed] [Google Scholar]
- 4. Cope GF, Heatley RV, Kelleher J, Axon AT. In vitro mucus glycoprotein production by colonic tissue from patients with ulcerative colitis. Gut 29: 229–234, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Finnie IA, Campbell BJ, Taylor BA, Milton JD, Sadek SK, Yu LG, Rhodes JM. Stimulation of colonic mucin synthesis by corticosteroids and nicotine. Clin Sci (Lond) 91: 359–364, 1996. [DOI] [PubMed] [Google Scholar]
- 6. Finnie IA, Dwarakanath AD, Taylor BA, Rhodes JM. Colonic mucin synthesis is increased by sodium butyrate. Gut 36: 93–99, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Frizzell RA, Koch MJ, Schultz SG. Ion transport by rabbit colon. I. Active and passive components. J Membr Biol 27: 297–316, 1976. [DOI] [PubMed] [Google Scholar]
- 8. Fyderek K, Strus M, Kowalska-Duplaga K, Gosiewski T, Wedrychowicz A, Jedynak-Wasowicz U, Sladek M, Pieczarkowski S, Adamski P, Kochan P, Heczko PB. Mucosal bacterial microflora and mucus layer thickness in adolescents with inflammatory bowel disease. World J Gastroenterol 15: 5287–5294, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Grubb BR, Gabriel SE. Intestinal physiology and pathology in gene-targeted mouse models of cystic fibrosis. Am J Physiol Gastrointest Liver Physiol 273: G258–G266, 1997. [DOI] [PubMed] [Google Scholar]
- 10. Gyomorey K, Garami E, Galley K, Rommens JM, Bear CE. Non-CFTR chloride channels likely contribute to secretion in the murine small intestine. Pflugers Arch 443, Suppl 1: S103–S106, 2001. [DOI] [PubMed] [Google Scholar]
- 11. Halm DR, Halm ST. Secretagogue response of goblet cells and columnar cells in human colonic crypts. Am J Physiol Cell Physiol 278: C212–C233, 2000. [DOI] [PubMed] [Google Scholar]
- 12. Hecht G. Innate mechanisms of epithelial host defense: spotlight on intestine. Am J Physiol Cell Physiol 277: C351–C358, 1999. [DOI] [PubMed] [Google Scholar]
- 13. Johansson ME, Gustafsson JK, Sjoberg KE, Petersson J, Holm L, Sjovall H, Hansson GC. Bacteria penetrate the inner mucus layer before inflammation in the dextran sulfate colitis model. PLoS One 5: e12238, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Johansson ME, Larsson JM, Hansson GC. The two mucus layers of colon are organized by the MUC2 mucin, whereas the outer layer is a legislator of host-microbial interactions. Proc Natl Acad Sci USA 108, Suppl 1: 4659–4665, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Johansson ME, Phillipson M, Petersson J, Velcich A, Holm L, Hansson GC. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc Natl Acad Sci USA 105: 15064–15069, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. MacDermott RP, Donaldson RM, Jr, Trier JS. Glycoprotein synthesis and secretion by mucosal biopsies of rabbit colon and human rectum. J Clin Invest 54: 545–554, 1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mall M, Wissner A, Seydewitz HH, Kuehr J, Brandis M, Greger R, Kunzelmann K. Defective cholinergic Cl− secretion and detection of K+ secretion in rectal biopsies from cystic fibrosis patients. Am J Physiol Gastrointest Liver Physiol 278: G617–G624, 2000. [DOI] [PubMed] [Google Scholar]
- 18. Matos JE, Sausbier M, Beranek G, Sausbier U, Ruth P, Leipziger J. Role of cholinergic-activated KCa1.1. Acta Physiol (Oxf) 189: 251–258, 2007. [DOI] [PubMed] [Google Scholar]
- 19. Matsuo K, Ota H, Akamatsu T, Sugiyama A, Katsuyama T. Histochemistry of the surface mucous gel layer of the human colon. Gut 40: 782–789, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Neutra MR, O'Malley LJ, Specian RD. Regulation of intestinal goblet cell secretion. II. A survey of potential secretagogues. Am J Physiol Gastrointest Liver Physiol 242: G380–G387, 1982. [DOI] [PubMed] [Google Scholar]
- 21. Osbak PS, Bindslev N, Poulsen SS, Kaltoft N, Tilotta MC, Hansen MB. Colonic epithelial ion transport is not affected in patients with diverticulosis (Abstract). BMC Gastroenterol 7: 37, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Petersson J, Schreiber O, Hansson GC, Gendler SJ, Velcich A, Lundberg JO, Roos S, Holm L, Phillipson M. Importance and regulation of the colonic mucus barrier in a mouse model of colitis. Am J Physiol Gastrointest Liver Physiol 300: G327–G333, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pullan RD, Thomas GA, Rhodes M, Newcombe RG, Williams GT, Allen A, Rhodes J. Thickness of adherent mucus gel on colonic mucosa in humans and its relevance to colitis. Gut 35: 353–359, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Roumagnac I, Laboisse C. A mucus-secreting human colonic epithelial cell line responsive to cholinergic stimulation. Biol Cell 61: 65–68, 1987. [DOI] [PubMed] [Google Scholar]
- 25. Specian RD, Neutra MR. Mechanism of rapid mucus secretion in goblet cells stimulated by acetylcholine. J Cell Biol 85: 626–640, 1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Strugala V, Allen A, Dettmar PW, Pearson JP. Colonic mucin: methods of measuring mucus thickness. Proc Nutr Soc 62: 237–243, 2003. [DOI] [PubMed] [Google Scholar]
- 27. Strugala V, Dettmar PW, Pearson JP. Thickness and continuity of the adherent colonic mucus barrier in active and quiescent ulcerative colitis and Crohn's disease. Int J Clin Pract 62: 762–769, 2008. [DOI] [PubMed] [Google Scholar]
- 28. Van der Sluis M, De Koning BA, De Bruijn AC, Velcich A, Meijerink JP, Van Goudoever JB, Buller HA, Dekker J, Van Seuningen I, Renes IB, Einerhand AW. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology 131: 117–129, 2006. [DOI] [PubMed] [Google Scholar]
- 29. Velcich A, Yang W, Heyer J, Fragale A, Nicholas C, Viani S, Kucherlapati R, Lipkin M, Yang K, Augenlicht L. Colorectal cancer in mice genetically deficient in the mucin Muc2. Science 295: 1726–1729, 2002. [DOI] [PubMed] [Google Scholar]
- 30. Wallon C, Braaf Y, Wolving M, Olaison G, Soderholm JD. Endoscopic biopsies in Ussing chambers evaluated for studies of macromolecular permeability in the human colon. Scand J Gastroenterol 40: 586–595, 2005. [DOI] [PubMed] [Google Scholar]


