Abstract
Acetaldehyde is accumulated at high concentrations in the colonic lumen following ethanol administration. Previous studies demonstrated that acetaldehyde disrupts intestinal epithelial tight junctions and increases paracellular permeability. In the present study, we investigated the role of PP2A in the acetaldehyde-induced disruption of intestinal epithelial tight junctions. Caco-2 cell monolayers were exposed to 200–600 μM acetaldehyde for varying times, and the epithelial barrier function was evaluated by measuring transepithelial electrical resistance and inulin permeability. Acetaldehyde treatment resulted in a time-dependent increase in inulin permeability and redistribution of occludin and ZO-1 from the intercellular junctions. Treatment of cells with fostriecin (a PP2A-selective inhibitor) or knockdown of PP2A by siRNA blocked acetaldehyde-induced increase in inulin permeability and redistribution of occludin and ZO-1. The effects of fostriecin and acetaldehyde were confirmed in mouse intestine ex vivo. Acetaldehyde-induced tight junction disruption and barrier dysfunction were also attenuated by a PP2A-specific inhibitory peptide, TPDYFL. Coimmunoprecipitation studies showed that acetaldehyde increased the interaction of PP2A with occludin and induced dephosphorylation of occludin on threonine residues. Fostriecin and TPDYFL significantly reduced acetaldehyde-induced threonine dephosphorylation of occludin. Acetaldehyde failed to change the level of the methylated form of PP2A-C subunit. However, genistein (a tyrosine kinase inhibitor) blocked acetaldehyde-induced association of PP2A with occludin and threonine dephosphorylation of occludin. These results demonstrate that acetaldehyde-induced disruption of tight junctions is mediated by PP2A translocation to tight junctions and dephosphorylation of occludin on threonine residues.
Keywords: intestinal epithelium, dephosphorylation, barrier function
epithelial tight junctions serve to regulate the passage of ions and molecules between cells and to mark the division between apical and basolateral surfaces of cells in cellular differentiation (21). Thus the disruption of intestinal epithelial tight junctions has two undesirable consequences: unwanted substances such as endotoxins are allowed into the body, and depolarization of cells may be promoted (17, 31). Therefore, the maintenance of tight junctions is of critical importance to the functions of epithelia. Tight junctions are complex assemblies of transmembrane proteins (occludin, claudins, junctional adhesion molecules) (1), scaffolding proteins (zona occludens family, including ZO-1) (8), and signaling proteins such as protein kinases (2) and protein phosphatases (34). The interactions among these molecules keep the tight junctions intact and help to maintain epithelial polarization under normal conditions (39). Interactions of injurious factors with tight junctions may cause dissociation of the protein complex. Internalization of dissociated proteins may weaken tight junctions, resulting in increased transepithelial permeability.
Acetaldehyde, a metabolic product of ethanol, is highly toxic to cells owing to formation of adducts with DNA (5) and proteins (4, 22, 40), leading to epigenetic and posttranslational consequences in different cells (13, 43). Acetaldehyde is produced in the colonic lumen as a result of normal bacterial fermentation, which is elevated dramatically following alcohol consumption. A high level of acetaldehyde is accumulated in the colonic lumen (28). Acetaldehyde at concentration of 200–400 μM has been detected in plasma and saliva of alcoholics. Because of the volatility of acetaldehyde, its quantitation in biological fluids is considered underestimated. The highest level of acetaldehyde is developed in the colonic lumen as a result of oxidation of ethanol by microbial alcohol dehydrogenase; colonic microflora express very low levels of aldehyde dehydrogenase. Acetaldehyde concentration in rat colonic lumen following alcohol administration was recorded as high as 1 mM. A significant body of evidence shows that ethanol consumption induces intestinal epithelial barrier dysfunction and increased gut permeability, leading to endotoxemia in human subjects and experimental animals (27, 28, 31). In the intestinal epithelial cell monolayers in vitro, ethanol is less effective in disruption of tight junctions (10, 30). On the other hand, acetaldehyde disrupts tight junctions and increases paracellular permeability in Caco-2 cell monolayers (30). The precise mechanism involved in acetaldehyde-induced tight junction disruption is poorly understood.
Occludin, one of the transmembrane proteins of tight junctions, is phosphorylated on several evolutionarily conserved serine and threonine residues (32). Previous studies have shown that these residues are dephosphorylated and occludin is internalized into the cell as the tight junction weakens in the presence of hydrogen peroxide (35) or calcium-free medium (34). Protein phosphatase 2A (PP2A), a serine/threonine phosphatase, is a multimeric protein composed of catalytic C subunit, scaffolding A subunit and one of several regulatory B subunits (16). PP2A interacts with tight junctions and appears to play a role in the regulation of tight junction integrity, likely by modulating the phosphorylation of tight junction proteins on specific serine or threonine residues (33, 37). Few recent studies indicated that protein phosphatases are involved in ethanol-induced cellular dysfunction. Activation of PP1 is involved in ethanol-induced T cell suppression in mesenteric lymph nodes (19). PP2A activation mediates ethanol-induced dysfunction of hepatocytes (18) and cardiomyocytes (20).
The goal of our study was to investigate the role of PP2A in acetaldehyde-induced dephosphorylation of occludin and the disassembly of tight junctions. Caco-2 cell monolayers and mouse intestine were used as in vitro and ex vivo models of the intestinal epithelium. A selective pharmacological PP2A inhibitor, a PP2A translocation inhibitor peptide, and siRNA-mediated knockdown of PP2A were applied to determine the role of PP2A in acetaldehyde-induced tight junction disruption.
EXPERIMENTAL METHODS
Reagents.
Cell culture reagents were purchased from Invitrogen and Fisher Scientific. FITC-inulin, leupeptin, aprotinin, bestatin, pepstatin A, phenylmethylsulfonyl fluoride (PMSF), Triton X-100, malachite, green and protein-A Sepharose were purchased from Sigma-Aldrich (St. Louis, MO). Fostriecin and okadaic acid were purchased from Calbiochem EMD Chemicals (San Diego, CA). Protein phosphatase substrate phosphopeptide (KRpTIRR) was purchased from Millipore (Billerica, MA). Scrambled control RNA and siRNA specific to human PP2A-Cα (smart pool of 3 different siRNA) were purchased from Dharmacon (Lafayette, CO). Inhibitory peptide TPDYFL and control peptide (PALFTA) were custom synthesized by Genscript (Piscataway, NJ). All other chemicals were of analytical grade and purchased from Sigma-Aldrich or Fisher Thermo Scientific (Tustin, CA).
Antibodies.
Mouse monoclonal anti-PP2A Cα (free and methylated) and anti-E-cadherin were purchased from BD Transduction (Franklin Lakes, NJ). Mouse monoclonal anti-occludin (free and HRP-conjugated) and rabbit polyclonal anti-ZO-1, anti-phospho-threonine, and anti-phospho-serine antibodies were purchased from Invitrogen (Carlsbad, CA). Alexa Fluor 488-conjugated anti-mouse IgG antibody was purchased from Molecular Probes (Eugene, OR). Cy3-conjugated anti-rabbit IgG antibodies were from Sigma-Aldrich.
Cell culture and transfection.
Caco-2 cells purchased from the American Type Culture Collection were grown under standard culture conditions as described previously (30). Cells from passages 9–40 were grown on polycarbonate membranes in Transwell inserts for 7 days (6.5 mm), 11–14 days (12 mm), or 17–19 days (24 mm) and serum starved overnight prior to experimental treatments.
For siRNA transfection, Caco-2 cells (∼125,000 cells/well) were seeded in six-well cluster plates. After 24 h, cells were incubated in serum-free, antibiotic-free DMEM for an hour. Cells were transfected with siRNA or control RNA using 100 μl Optimem, 12 μl Plus reagent, and 7-μl oligofectamine with 150 μl DMEM and incubated for 6–8 h. Medium containing 10% serum was added, and cells were incubated at 37°C until the next day. Cell monolayers were trypsinized and seeded onto Transwell inserts. Transepithelial electrical resistance (TER) was monitored daily, and experiments were conducted on days 3 or 4, when TER stabilized at levels indicating a confluent monolayer.
Acetaldehyde and inhibitor treatments.
Acetaldehyde (200–400 μM) treatment was performed as previously described (3). Cells were preincubated in PBS containing 1.2 mM CaCl2 and 1 mM MgCl2, bovine serum albumin, and glucose for 1 h. Then cells were exposed to vapor-phase acetaldehyde to achieve 200–600 μM concentration in sealed culture plates (25). In some experiments cell monolayers were incubated with 50 nM fostriecin in DMEM in apical and basal wells for 16 h before acetaldehyde treatment. Genistein (100 μM) was administered 1 h prior to acetaldehyde treatment.
TPDYFL inhibitory peptide and corresponding control peptide were applied to cells by using the Chariot transfection reagent system (Active Motif, Carlsbad, CA) for permeabilization 2 h before acetaldehyde treatment. Permeabilization was optimized and confirmed by visualizing FITC-inulin internalization with and without Chariot transfection reagent by fluorescence microscopy.
Measurement of epithelial barrier function.
Barrier function was evaluated by measuring TER and inulin permeability.
TER.
TER was measured as described previously (30). A Millicell-ERS Electrical Resistance System (Millipore, Bedford, MA) was used. Basal TER of supporting semipermeable membrane of Transwells was subtracted from all values (80–100 Ω/cm2).
Unidirectional FITC-inulin flux.
Mature cell monolayers on Transwells were incubated in different experiments with 0.5 mg/ml FITC-inulin in the basal well. At the end of incubation with or without acetaldehyde, 100 μl each of medium from the apical and basal wells were sampled, and fluorescence was measured with a Flx-800 microplate fluorescence reader (BioTEK instruments, Winooski, VT). Flux into the apical well was calculated as the percentage of total basal well fluorescence per square centimeter surface area.
Immunoprecipitation.
Cells were washed with cold 20 mM Tris·HCl, pH 7.4, and proteins were extracted by using lysis buffer N (20 mM Tris, pH 7.4, containing 150 mM NaCl, 10 μg/ml leupeptin, 10 μg/ml pepstatin A, 10 μg/ml aprotinin, 10 μg/ml bestatin, and 0.1 mM PMSF). Protein extracts (400 μg) were incubated with 2 μg anti-occludin antibodies overnight at 4°C on a rocker. Immunocomplexes were precipitated with protein A/G-conjugated Sepharose beads. For PP2A phosphatase activity assays, beads were maintained on ice until the assay. For immunoblot analysis, beads were heated at 100°C for 10 min with Laemmli's sample buffer to release complexes.
Analysis of threonine phosphorylation.
Cells were lysed in lysis buffer D (0.3% SDS wt/vol, 10 mM Tris·HCl, pH 7.4, containing 10 μg/ml leupeptin, 10 μg/ml pepstatin A, 10 μg/ml aprotinin, 10 μg/ml bestatin, and 0.1 mM PMSF) preheated at 100°C. After repeated pipetting to homogenize samples, samples were heated at 100°C for 10 min. Protein extracts (400 μg) were incubated with anti-phospho-threonine antibodies overnight as described above. Protein complexes were immunoprecipitated with protein A Sepharose beads and extracted in Laemmli's sample buffer.
PP2A activity assay.
Anti-occludin immunocomplexes were resuspended in PPase buffer (50 mM HEPES, pH 7.2, 60 mM NaCl, 60 mM KCl, and protease inhibitors) to a volume of 20 μl. These samples were incubated at 30°C for 10 min with 5 μl (1 μg/μl) of phosphopeptide substrate. Free phosphate released during the activity was assayed by mixing with 100 μl malachite green in a 96-well microtiter plate and incubated at room temperature for 15 min. Absorbance was measured at 620 nm in a microplate reader (SpectraMax 190, Molecular Devices, Sunnydale, CA). The assay was performed in the presence or absence of 300 nM fostriecin and 100 nM okadaic acid. Units of PP2A activity represent picomoles of free phosphate generated under assay conditions.
Immunofluorescence microscopy.
Cell monolayers were fixed in 4% paraformaldehyde in TBST (20 mM Tris, pH 7.2, and 150 mM NaCl) for 15 min at room temperature. They were blocked in 4% milk in TBST and then incubated with primary antibodies for 90 min followed by incubation with secondary antibodies for 1 h. Fluorescence was visualized by using a Zeiss LSM 5 laser scanning confocal microscope, and images from 1-μm-thick x-y sections were collected. Images were stacked by using Image J software (National Institutes of Health, Bethesda, MD) and processed with Adobe Photoshop (Adobe Systems, San Jose, CA).
Preparation of detergent-insoluble fractions.
Cell monolayers were incubated for 15 min with lysis buffer-CS (Tris buffer containing 1.0% Triton X-100, 2 μg/ml leupeptin, 10 μg/ml aprotinin, 10 μg/ml bestatin, 10 μg/ml pepstatin-A, 1 mM vanadate, and 1 mM PMSF). Cell lysates were centrifuged at 15,600 g for 4 min at 4°C to sediment the high-density actin cytoskeleton (detergent-insoluble fraction). The “detergent-insoluble fraction” is likely to pull down lipid raft components along with F-actin filaments and therefore contains more than just the actin cytoskeleton. Supernatant was used as the detergent-soluble fraction. The pellet was suspended in 200 μl of lysis buffer-CS and sonicated to homogenize the actin cytoskeleton. Protein contents in different fractions were measured by the BCA method (Pierce Biotechnology, Rockford, IL). Triton-insoluble and Triton-soluble fractions were mixed with equal volume of Laemmli's sample buffer (2× concentrated) and heated at 100°C for 5 min.
Immunoblot analysis.
Proteins were separated by SDS-polyacrylamide gel (4–12% gradient) electrophoresis and transferred to nitrocellulose PVDF membranes. Membranes were blotted for different proteins by using specific antibodies in combination with HRP-conjugated anti-mouse IgG or HRP-conjugated anti-rabbit IgG antibodies. The blots were developed by the ECL chemiluminescence method (Amersham, Arlington Heights, IL). The bands were quantitated by densitometric analysis using Image J software (NIH).
PP2A methylation.
The level of methylated PP2A-Cα in cell extracts was evaluated by immunoblot analysis by using an antibody that specifically recognizes the methylated form of the PP2A C-subunit.
Acetaldehyde treatment of mouse intestine.
These studies were conducted using an IACUC-approved protocol. Adult male mice (C57BL/6; 12 wk old) were used for these studies. Segments of ileum were flushed and slit open longitudinally to prepare intestinal sheets. These tissues were incubated in DMEM at 37°C with or without 50 nM fostriecin for 15 min followed by incubation with acetaldehyde (400 μM) for 1 h. Similar to Caco-2 cell monolayers, acetaldehyde treatment was performed by exposure of intestinal tissues to vapor phase acetaldehyde in sealed cluster plates. Tissues were cryofixed in OCT and 10-μm cryosections were fixed and stained for occludin and ZO-1 by the immunofluorescence staining method as described above.
Statistical analyses.
Comparison between two groups was made by Student's t-tests for grouped data. Significance in all tests was set at 95% or greater confidence level.
RESULTS
Inhibition of PP2A activity attenuates acetaldehyde-induced barrier dysfunction and tight junction disruption.
Acetaldehyde is known to disrupt tight junctions and increase paracellular permeability in intestinal epithelium. However, the mechanism of acetaldehyde-induced tight junction disruption is poorly understood. The effects of endogenous PP2A activity on acetaldehyde-induced tight junction disruption were evaluated by using the PP2A-selective inhibitor fostriecin in Caco-2 cell monolayers. Acetaldehyde at 400 μM concentration increased unidirectional flux of inulin in a time-dependent manner, and pretreatment of cell monolayers with fostriecin significantly dampened this effect of acetaldehyde (Fig. 1A). Fostriecin, by itself, produced no significant effect on inulin flux. Incubation of cell monolayers with 200–600 μM acetaldehyde for 5 h did not significantly increase lactate dehydrogenase (LDH) activity in the incubation medium, indicating the absence of cell lysis by acetaldehyde under the present experimental conditions (Fig. 1B). On the other hand, incubation of cells with 0.05% Triton X-100 for 10 min increased LDH level in the medium (positive control).
Fig. 1.
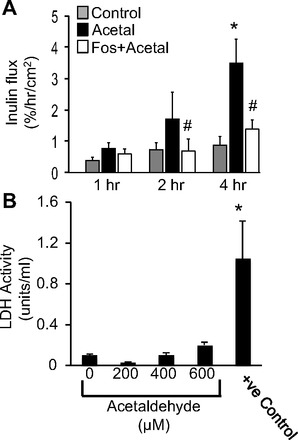
Inhibition of PP2A prevents acetaldehyde-induced barrier function in Caco-2 cell monolayers. A: Caco-2 cell monolayers were pretreated with fostriecin (Fos) 1 h prior to acetaldehyde administration. Unidirectional flux of FITC-inulin was measured during acetaldehyde (Acetal) treatment (400 μM for varying times). Values are means ± SE (n = 6). *Significantly different from values for control cell monolayers; #significantly (P < 0.05) different from corresponding values for acetaldehyde-treated cell monolayers in the absence of fostriecin. B: 5 h after incubation with varying concentrations of acetaldehyde, the apical medium was assessed for lactate dehydrogenase (LDH) activity. Cell monolayers treated with 0.1% Triton X-100 for 10 min were used as positive (+ve) control. Values are means ± SE (n = 4). *Significantly different from the value for control cell monolayer.
Immunofluorescence staining for tight junction proteins indicated that acetaldehyde exposure induced redistribution of occludin and ZO-1 from the intercellular junctions into intracellular compartments (Fig. 2A). Pretreatment with fostriecin attenuated acetaldehyde-induced redistribution of both occludin and ZO-1. Fostriecin in the absence of acetaldehyde appeared to enhance the junctional localization of occludin and ZO-1. Acetaldehyde exposure also resulted in redistribution of adherens junction proteins E-cadherin and β-catenin and this effect of acetaldehyde was blocked by fostriecin treatment (Fig. 2B).
Fig. 2.
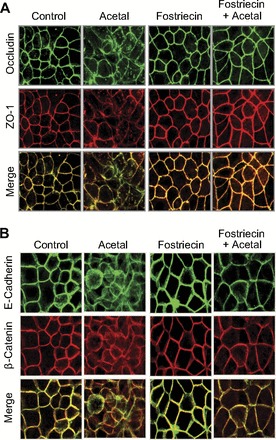
Inhibition of PP2A prevents acetaldehyde-induced disruption of tight junction and adherens junction in Caco-2 cell monolayers. Caco-2 cell monolayers incubated with 400 μM acetaldehyde for 5 h with or without fostriecin pretreatment were fixed and double stained for occludin and ZO-1 (A) or E-cadherin and β-catenin (B).
Knockdown of PP2A-cα attenuates acetaldehyde-induced barrier dysfunction and tight junction disruption.
To further confirm the role of PP2A in acetaldehyde-induced tight junction disruption, we knocked down PP2A-Cα using a specific siRNA targeted to the catalytic subunit of PP2A. PP2A-Cα level was significantly reduced by siRNA transfection (Fig. 3A). Acetaldehyde increased inulin permeability in nonspecific RNA-transfected cell monolayers. But in PP2A-specific siRNA transfected cell monolayers, acetaldehyde-induced inulin permeability was significantly lower than that in NS-RNA-transfected cells (Fig. 3B). Knockdown of PP2A-Cα subunit also suppressed acetaldehyde-induced redistribution of occludin and ZO-1 from the intercellular junctions (Fig. 3C).
Fig. 3.
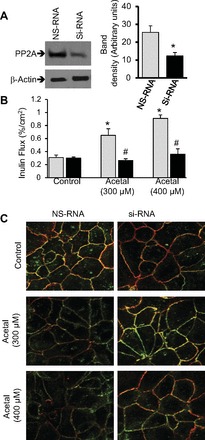
Knockdown of PP2A-Cα dampens acetaldehyde-induced barrier dysfunction and tight junction disruption. A: Caco-2 cells were transfected with siRNA specific for catalytic subunit of PP2A or nonspecific RNA (NS-RNA). Protein extracts were immunoblotted for PP2A-C and PP1. The graph shows the densitometric analysis of PP2A bands. Values are means ± SE (n = 4). *Significantly different (P < 0.05) from the value for NS-RNA. B: inulin flux was measured after 5 h of exposure to acetaldehyde (300 or 400 μM). Values are means ± SE (n = 4). *Significantly different from control values. #Significantly (P < 0.05) different from corresponding values for NS-RNA group. C: after incubation with varying amounts of acetaldehyde for 5 h the cell monolayers were fixed and stained for occludin (green) or ZO-1 (red).
Acetaldehyde induces dephosphorylation of occludin on threonine residues by a PP2A-dependent mechanism.
Previous studies indicated that tight junction disruption by calcium depletion or hydrogen peroxide treatment is associated with occludin dephosphorylation on threonine residues (9, 33, 37). The role of PP2A in acetaldehyde-induced tight junction disruption suggested that occludin dephosphorylation might be part of this effect. To determine the effect of acetaldehyde on occludin phosphorylation, we evaluated the levels of threonine-phosphorylated occludin in cells treated with acetaldehyde in the presence or absence of fostriecin. Acetaldehyde treatment rapidly reduced the level of threonine-phosphorylated occludin as early as 30 min of treatment (Fig. 4, A and B). Pretreatment of cell monolayers with fostriecin significantly attenuated acetaldehyde-induced occludin dephosphorylation (Fig. 4, A and B).
Fig. 4.
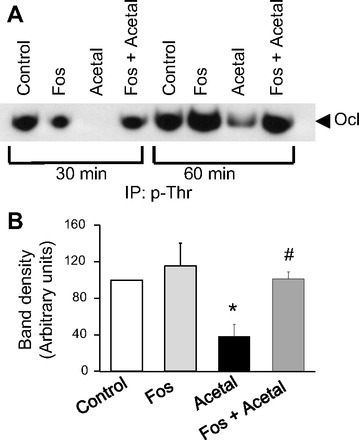
Acetaldehyde induces dephosphorylation of occludin on threonine residues by a PP2A-dependent mechanism. A: Caco-2 cell monolayers pretreated with or without fostriecin were exposed to 400 μM acetaldehyde for 30 or 60 min. Phospho-threonine (p-Thr) was immunoprecipitated (IP) from the denatured protein extracts and immunoblotted for occludin (Ocl). B: densitometric analysis of occludin bands from different experiments involving 60-min acetaldehyde treatment. Values are means ± SE (n = 3). *Significantly different from control value; #different from value for acetaldehyde group.
Acetaldehyde increases association of PP2A-cα with occludin.
To determine the effect of acetaldehyde on association of PP2A with the tight junction protein complex, we evaluated coimmunoprecipitation of PP2A with occludin in cell monolayers exposed to acetaldehyde for varying times. The fostriecin-sensitive protein threonine phosphatase activity in occludin immunocomplexes was rapidly increased by acetaldehyde treatment (Fig. 5A). Immunoblot analysis indicated that the association between occludin and PP2A also increased rapidly (Figs. 5B and 4C). This increase in interaction between occludin and PP2A was confirmed by precipitation of PP2A using microcystin-conjugated beads and immunoblot analysis for occludin (data not shown).
Fig. 5.
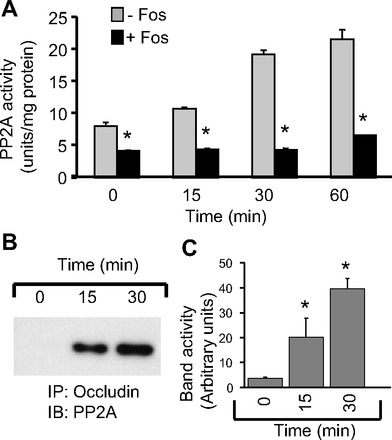
Acetaldehyde increases association of PP2A with occludin. A: immunoprecipitates of occludin from Caco-2 cell monolayers exposed to acetaldehyde (400 μM) for varying times were assayed for PP2A phosphatase activity in the absence (gray bars) or presence (black bars) of fostriecin. Values are means ± SE (n = 4). *Significantly different from corresponding values for activities in the absence of fostriecin. B and C: occludin immunoprecipitates prepared from cells incubated with acetaldehyde for varying times were immunoblotted (IB) for PP2A-C (B), and the PP2A band densities in different experiments were evaluated (C). Values are means ± SE (n = 3). *Significantly different from corresponding values for 0-min control.
PP2A translocation inhibitor prevents acetaldehyde-induced tight junction disruption.
PP2A activity is known to be regulated by posttranslational modifications of the catalytic subunit by carboxymethylation of L309 at the COOH-terminal end (6). The COOH-terminal sequence TPDYFL is conserved from yeast to humans (15) and was found to inhibit PP2A translocation in cardiac myocytes (7). Therefore, we evaluated the effect of this peptide on acetaldehyde-induced occludin phosphorylation and tight junction disruption. TPDYFL significantly blocked acetaldehyde-induced increase in inulin permeability (Fig. 6A); the control peptide with scrambled sequence did not affect inulin permeability in untreated or acetaldehyde-treated cell monolayers (data not shown). Acetaldehyde-induced redistribution of occludin and ZO-1 from the intercellular junctions was attenuated by TPDYFL (Fig. 6B), but not by scrambled peptide. TPDYL also blocked acetaldehyde-induced threonine dephosphorylation of occludin (Fig. 6C).
Fig. 6.
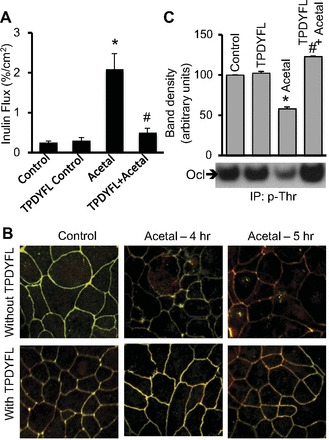
TPDYFL attenuates acetaldehyde-induced tight junction disruption and occludin dephosphorylation. A: Caco-2 cell monolayers were pretreated with PP2A translocation inhibitor peptide (500 ng/ml) or control peptide prior to acetaldehyde (400 μM) (Acetal) exposure for 5 h. Inulin permeability was measured. Values are means ± SE (n = 4). *Significantly different from control value; #different from value for acetaldehyde group. B: cell monolayers incubated with acetaldehyde for 4 or 5 h in the absence or presence of TPDYFL were fixed and stained for occludin (green) and ZO-1 (red) by immunofluorescence staining method. Fluorescence images were collected via a confocal microscope. C: phospho-threonine was immunoprecipitated from denatured protein extracts of Caco-2 cells exposed to 400 μM acetaldehyde with or without TPDYFL and immunoblotted for occludin. Occludin band densities from different experiments were quantitated by densitometric analysis. Values are means ± SE (n = 3). *Significantly different from control value; #different from value for acetaldehyde group.
Tyrosine kinase activity mediates acetaldehyde-induced threonine dephosphorylation of occludin.
PP2A multimeric protein complex assembly and subcellular translocation are known to be regulated by carboxymethylation of L309 or phosphorylation of Y307 (6, 11, 26). To determine the role of carboxymethylation of PP2A, we evaluated the effect of acetaldehyde on the level of methylated PP2A. Results showed a lack of significant change in methylated PP2A in acetaldehyde-treated cells compared with control cells (Fig. 7A), suggesting that change in methylation is not a part of the mechanism in acetaldehyde-induced tight junction disruption. Previous study demonstrated that tyrosine kinase activity plays a role in acetaldehyde-induced tight junction regulation (3). To determine the cross talk between tyrosine kinase activity and PP2A activity we evaluated the effect of genistein on interaction between occludin and PP2A C-subunit and threonine phosphorylation of occludin. Genistein significantly attenuated acetaldehyde-induced increase in coimmunoprecipitation of PP2A with occludin (Fig. 7B) and threonine dephosphorylation of occludin (Fig. 7, C and D). Acetaldehyde or genistein did not alter the level of total occludin in these cells (Fig. 7C). Acetaldehyde treatment also caused threonine dephosphorylation of claudin-5, but this effect of acetaldehyde was unaffected by genistein (Fig. 7C).
Fig. 7.
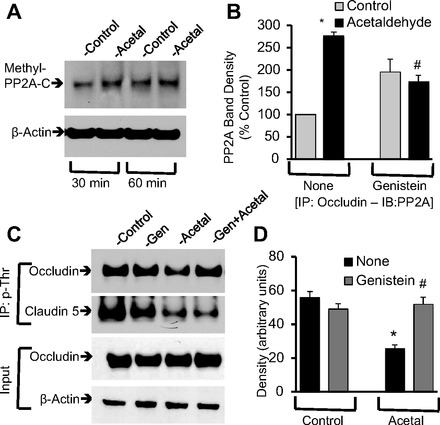
Tyrosine kinase activity mediates acetaldehyde-induced occludin dephosphorylation. A: protein extracts from Caco-2 cell monolayers treated with acetaldehyde (400 μM) for 30 or 60 min were immunoblotted for methyl-PP2A-C. B: cell monolayers were pretreated with genistein for 1 h prior to incubation with acetaldehyde for 30 min. Occludin from native cell extracts was immunoprecipitated, and the immunocomplexes were immunoblotted for PP2A and occludin. PP2A band densities were quantitated and normalized to corresponding occludin band densities. Values are calculated percent of control value for each experiment. Values are means ± SE (n = 3). *Significantly different from control value; #different from value for acetaldehyde without genistein. C and D: phospho-threonine was immunoprecipitated from denatured protein extracts of Caco-2 cells exposed to 400 μM acetaldehyde with or without genistein (Gen) pretreatment and immunoblotted for occludin or claudin-5. Occludin band densities from different experiments were quantitated and presented as arbitrary units. Values are means ± SE (n = 3). *Significantly different from control value; #different from value for acetaldehyde without genistein.
Inhibition of PP2A activity suppresses acetaldehyde-induced tight junction disruption in mouse intestine.
To establish the physiological significance of PP2A role in acetaldehyde-induced disruption of intestinal epithelial tight junctions, we determined the effect of acetaldehyde on tight junction integrity in mouse ileum ex vivo and the effect of fostriecin on acetaldehyde-mediated tight junction disruption. Results show that occludin and ZO-1 were colocalized at the intercellular junctions of the epithelial monolayer in tissues incubated ex vivo without acetaldehyde, but exposure to acetaldehyde resulted in loss of junctional distribution of both occludin and ZO-1 (Fig. 8). Pretreatment of tissue with fostriecin blocked acetaldehyde-induced redistribution of occludin and ZO-1 from the epithelial junctions. Fostriecin alone showed no considerable influence on the distribution of these tight junction proteins.
Fig. 8.
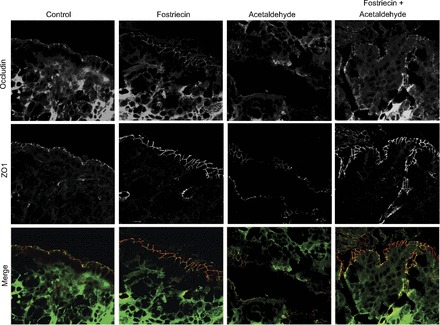
Inhibition of PP2A prevents acetaldehyde-induced tight junction disruption in mouse ileum ex vivo. Opened segments of mouse ileum were incubated with or without fostriecin for 20 min prior to exposure to 400 μM acetaldehyde for 1 h. Cryosections of these tissues were fixed and double-stained for occludin (green) and ZO-1 (red). Images were collected by using a laser scanning confocal microscope. This experiment was repeated twice with similar results.
DISCUSSION
Acetaldehyde, the most toxic product of ethanol metabolism, is involved in tissue injury in different organ systems (23). Although acetaldehyde is well known to disrupt intestinal epithelial tight junctions and increase paracellular permeability (3, 29, 30), the mechanism involved in this process of tight junction disruption is poorly understood. Evidence indicates that intracellular signaling molecules such as protein kinases and protein phosphatases regulate epithelial tight junctions (12). Previous studies demonstrated that PP2A plays a crucial role in epithelial tight junction regulation (25, 33, 35). Results of our present study indicate that PP2A translocation to tight junctions and PP2A-dependent dephosphorylation of occludin are involved in the mechanism of acetaldehyde-induced tight junction disruption.
Fostriecin is a selective inhibitor of PP2A activity (42). Our present study shows that pretreatment of Caco-2 cell monolayers with fostriecin significantly prevents acetaldehyde-induced barrier dysfunction without altering cell viability. Attenuation of acetaldehyde-induced redistribution of occludin and ZO-1 by fostriecin indicated that PP2A activity is involved in acetaldehyde-induced disruption of tight junctions, which explains the increase in inulin permeability. The role of PP2A in acetaldehyde-induced tight junction regulation was further confirmed by knockdown of PP2A using PP2A-Cα-specific siRNA. Knockdown of PP2A dampened the effects of acetaldehyde on inulin permeability and attenuated redistribution of occludin and ZO-1 from the intercellular junctions. Previous studies showed that overexpression of PP2A enhanced tight junction integrity in MDCK cells (24). Disruption of tight junctions by calcium depletion involved increased association of PP2A with the tight junction protein complex, and calcium-mediated reassembly of tight junctions was associated with dissociation of PP2A from tight junctions in Caco-2 cell monolayers. Inhibition of PP2A activity or knockdown of PP2A-Cα resulted in acceleration of calcium-induced tight junction assembly (33). Furthermore, PP2A activity was found to be involved in hydrogen peroxide-mediated disruption of tight junctions in Caco-2 cells monolayers. Our present study demonstrates that PP2A is involved in acetaldehyde-induced disruption of tight junctions.
The present study indicates that PP2A inhibition by fostriecin also attenuates acetaldehyde-induced redistribution of E-cadherin and β-catenin, suggesting that acetaldehyde-induced disruption of the adherens junction is also mediated by PP2A activity. The adherens junction is not a physical barrier to macromolecular transport across the epithelium. However, it is known to indirectly regulate the integrity of tight junctions and therefore influence the barrier function. Previous studies indicated that redistribution of E-cadherin and β-catenin plays a crucial role in acetaldehyde-induced barrier dysfunction in Caco-2 cell monolayers (38).
A significant body of evidence indicates that tight junctions are regulated by protein phosphorylation. Occludin is phosphorylated on serine and threonine residues, and this phosphorylation is regulated by protein kinases such as c-Src, c-Yes, PKCη, PKCζ, and CK2 (14, 41) and protein phosphatases such as PTP1B, PP1, and PP2A (36–38). Our data show that acetaldehyde induces a rapid dephosphorylation of occludin and threonine residues. The occludin dephosphorylation was effectively blocked by fostriecin, indicating that PP2A played a role in this effect of acetaldehyde. Coimmunoprecipitation studies demonstrated that acetaldehyde increased the association of PP2A with occludin, indicating an enhanced interaction of PP2A with the tight junction protein complex. Previous studies showed that PP2A directly binds to the COOH-terminal domain of occludin (33, 37). Therefore, it is likely that acetaldehyde enhances the direct interaction between occludin and PP2A.
Further studies were conducted to seek insight into the mechanism of acetaldehyde-induced PP2A translocation and occludin phosphorylation on threonine residues. Subcellular translocation of PP2A is known to be regulated by posttranslational modification at the COOH-terminal sequence TPDYFL (6, 11, 26). Previous studies demonstrated that a synthetic peptide with this sequence attenuated PP2A translocation in cardiac myocytes (7). Our present study demonstrates that TPDYFL prevented acetaldehyde-induced tight junction disruption and barrier dysfunction, indicating that PP2A translocation is an essential step in the mechanism of acetaldehyde-induced tight junction disruption.
Prevention of acetaldehyde-induced tight junction disruption by TPDYFL suggested that the acetaldehyde-induced PP2A translocation may be mediated by tyrosine phosphorylation or carboxy-methylation. Phosphorylation of Y307 in the TPDYFL sequence of PP2A influences the interaction between the B-subunit and the AC-dimer of PP2A (26). Similarly, methylation of L309 is required for the interaction of PP2A C-subunit with the B-subunit (6). Our study shows that methylated PP2A is present in the untreated Caco-2 cells, but acetaldehyde failed to alter the level of methyl-PP2A, indicating that methylation is not involved in the mechanism of acetaldehyde-induced PP2A translocation, occludin dephosphorylation, or tight junction disruption.
Another mechanism by which PP2A translocation may be regulated is phosphorylation of Y307 (26). Our previous study demonstrated that tyrosine kinase is involved in acetaldehyde-induced tight junction disruption (3). In the present study, the prevention of acetaldehyde-induced increase in association of PP2A with occludin and occludin dephosphorylation on threonine indicate that tyrosine kinase activity does play a role in acetaldehyde-induced PP2A translocation. According to the previous study, phosphorylation of PP2A on Y307 negatively regulates its interaction with the B-subunit (26). Y307E mutation of PP2A C-subunit leads to loss of its interaction with the B-subunit and alters its substrate specificity. Therefore, we speculate that tyrosine kinase activity and tyrosine phosphorylation of PP2A may increase its affinity for occludin, causing dephosphorylation of occludin on threonine residues. Our study also shows that acetaldehyde induces dephosphorylation of claudin-5 on threonine residues. However, genistein failed to prevent this dephosphorylation, indicating that the mechanism of claudin-5 dephosphorylation is distinct from that of occludin dephosphorylation.
Finally, to establish the physiological significance of PP2A-mediated regulation of tight junction integrity in acetaldehyde-treated intestinal epithelium, we determined the effect of acetaldehyde and fostriecin on tight junction integrity in mouse intestine. Acetaldehyde induced redistribution of occludin and ZO-1 from the intercellular junctions of epithelial monolayers in mouse ileum incubated in vitro with acetaldehyde, indicating that acetaldehyde disrupts tight junctions in mouse intestinal epithelium. A complete block of acetaldehyde-induced tight junction disruption by fostriecin demonstrated that acetaldehyde-induced tight junction disruption in mouse intestinal epithelium is also mediated by PP2A activity.
This study demonstrates that PP2A translocation plays a crucial role in acetaldehyde-induced disruption of tight junctions and increase in paracellular permeability in the intestinal epithelium. Acetaldehyde-induced PP2A translocation to tight junctions is likely mediated by protein tyrosine phosphorylation. PP2A-mediated occludin dephosphorylation on threonine residues is an integral part of the mechanism involved in acetaldehyde-induced tight junction disruption.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
M.D. and R.K.R. conception and design of research; M.D. and K.C. performed experiments; M.D., K.C., and G.S. analyzed data; M.D., G.S., and R.K.R. interpreted results of experiments; M.D. prepared figures; M.D. drafted manuscript; M.D., K.C., G.S., and R.K.R. edited and revised manuscript; M.D., K.C., G.S., and R.K.R. approved final version of manuscript.
REFERENCES
- 1.Aijaz S, Balda MS, Matter K. Tight junctions: molecular architecture and function. Int Rev Cytol 248: 261–298, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Andreeva AY, Piontek J, Blasig IE, Utepbergenov DI. Assembly of tight junction is regulated by the antagonism of conventional and novel protein kinase C isoforms. Int J Biochem Cell Biol 38: 222–233, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Atkinson KJ, Rao RK. Role of protein tyrosine phosphorylation in acetaldehyde-induced disruption of epithelial tight junctions. Am J Physiol Gastrointest Liver Physiol 280: G1280–G1288, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Biewald J, Nilius R, Langner J. Occurrence of acetaldehyde protein adducts formed in various organs of chronically ethanol fed rats: an immunohistochemical study. Int J Mol Med 2: 389–396, 1998 [DOI] [PubMed] [Google Scholar]
- 5.Brooks PJ, Theruvathu JA. DNA adducts from acetaldehyde: implications for alcohol-related carcinogenesis. Alcohol 35: 187–193, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Bryant JC, Westphal RS, Wadzinski BE. Methylated C-terminal leucine residue of PP2A catalytic subunit is important for binding of regulatory Bα subunit. Biochem J 339: 241–246, 1999 [PMC free article] [PubMed] [Google Scholar]
- 7.Deshmukh PA, Blunt BC, Hofmann PA. Acute modulation of PP2a and troponin I phosphorylation in ventricular myocytes: studies with a novel PP2a peptide inhibitor. Am J Physiol Heart Circ Physiol 292: H792–H799, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Fanning AS, Jameson BJ, Jesaitis LA, Anderson JM. The tight junction protein ZO-1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton. J Biol Chem 273: 29745–29753, 1998 [DOI] [PubMed] [Google Scholar]
- 9.Farshori P, Kachar B. Redistribution and phosphorylation of occludin during opening and resealing of tight junctions in cultured epithelial cells. J Membr Biol 170: 147–156, 1999 [DOI] [PubMed] [Google Scholar]
- 10.Fisher SJ, Swaan PW, Eddington ND. The ethanol metabolite acetaldehyde increases paracellular drug permeability in vitro and oral bioavailability in vivo. J Pharmacol Exp Ther 332: 326–333, 2010 [DOI] [PubMed] [Google Scholar]
- 11.Gentry MS, Li Y, Wei H, Syed FF, Patel SH, Hallberg RL, Pallas DC. A novel assay for protein phosphatase 2A (PP2A) complexes in vivo reveals differential effects of covalent modifications on different Saccharomyces cerevisiae PP2A heterotrimers. Eukaryot Cell 4: 1029–1040, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gonzalez-Mariscal L, Tapia R, Chamorro D. Crosstalk of tight junction components with signaling pathways. Biochim Biophys Acta 1778: 729–756, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Hao Q, Maret W. Aldehydes release zinc from proteins. A pathway from oxidative stress/lipid peroxidation to cellular functions of zinc. FEBS J 273: 4300–4310, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Jain S, Suzuki T, Seth A, Samak G, Rao R. Protein kinase phosphorylates occludin and promotes assembly of epithelial tight junctions. Biochem J 437: 289–299, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Janssens V, Goris J. Protein phosphatase 2A: a highly regulated family of serine/threonine phosphatases implicated in cell growth and signalling. Biochem J 353: 417–439, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Janssens V, Goris J. Protein phosphatase 2A: a highly regulated family of serine/threonine phosphatases implicated in cell growth and signalling. Biochem J 353: 417–439, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laurent-Puig P, Blons H, Cugnenc PH. Sequence of molecular genetic events in colorectal tumorigenesis. Eur J Cancer Prev 8, Suppl 1: S39–S47, 1999 [PubMed] [Google Scholar]
- 18.Li X, Schwacha MG, Chaudry IH, Choudhry MA. A role of PP1/PP2A in mesenteric lymph node T cell suppression in a two-hit rodent model of alcohol intoxication and injury. J Leukoc Biol 79: 453–462, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Liangpunsakul S, Sozio MS, Shin E, Zhao Z, Xu Y, Ross RA, Zeng Y, Crabb DW. Inhibitory effect of ethanol on AMPK phosphorylation is mediated in part through elevated ceramide levels. Am J Physiol Gastrointest Liver Physiol 298: G1004–G1012, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma H, Yu L, Byra EA, Hu N, Kitagawa K, Nakayama KI, Kawamoto T, Ren J. Aldehyde dehydrogenase 2 knockout accentuates ethanol-induced cardiac depression: role of protein phosphatases. J Mol Cell Cardiol 49: 322–329, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matter K, Aijaz S, Tsapara A, Balda MS. Mammalian tight junctions in the regulation of epithelial differentiation and proliferation. Curr Opin Cell Biol 17: 453–458, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Mauch TJ, Donohue TM, Jr, Zetterman RK, Sorrell MF, Tuma DJ. Covalent binding of acetaldehyde selectively inhibits the catalytic activity of lysine-dependent enzymes. Hepatology 6: 263–269, 1986 [DOI] [PubMed] [Google Scholar]
- 23.Niemela O. Distribution of ethanol-induced protein adducts in vivo: relationship to tissue injury. Free Radic Biol Med 31: 1533–1538, 2001 [DOI] [PubMed] [Google Scholar]
- 24.Nunbhakdi-Craig V, Craig L, Machleidt T, Sontag E. Simian virus 40 small tumor antigen induces deregulation of the actin cytoskeleton and tight junctions in kidney epithelial cells. J Virol 77: 2807–2818, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nunbhakdi-Craig V, Machleidt T, Ogris E, Bellotto D, White CL, 3rd, Sontag E. Protein phosphatase 2A associates with and regulates atypical PKC and the epithelial tight junction complex. J Cell Biol 158: 967–978, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ogris E, Gibson DM, Pallas DC. Protein phosphatase 2A subunit assembly: the catalytic subunit carboxy terminus is important for binding cellular B subunit but not polyomavirus middle tumor antigen. Oncogene 15: 911–917, 1997 [DOI] [PubMed] [Google Scholar]
- 27.Purohit V, Bode JC, Bode C, Brenner DA, Choudhry MA, Hamilton F, Kang YJ, Keshavarzian A, Rao R, Sartor RB, Swanson C, Turner JR. Alcohol, intestinal bacterial growth, intestinal permeability to endotoxin, and medical consequences: summary of a symposium. Alcohol 42: 349–361, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rao R. Endotoxemia and gut barrier dysfunction in alcoholic liver disease. Hepatology 50: 638–644, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rao RK. Acetaldehyde-induced barrier disruption and paracellular permeability in Caco-2 cell monolayer. Methods Mol Biol 447: 171–183, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Rao RK. Acetaldehyde-induced increase in paracellular permeability in Caco-2 cell monolayer. Alcohol Clin Exp Res 22: 1724–1730, 1998 [PubMed] [Google Scholar]
- 31.Rao RK, Seth A, Sheth P. Recent Advances in Alcoholic Liver Disease I. Role of intestinal permeability and endotoxemia in alcoholic liver disease. Am J Physiol Gastrointest Liver Physiol 286: G881–G884, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Sakakibara A, Furuse M, Saitou M, Ando-Akatsuka Y, Tsukita S. Possible involvement of phosphorylation of occludin in tight junction formation. J Cell Biol 137: 1393–1401, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seth A, Sheth P, Elias BC, Rao R. Protein phosphatases 2A and 1 interact with occludin and negatively regulate the assembly of tight junctions in the CACO-2 cell monolayer. J Biol Chem 282: 11487–11498, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Seth A, Sheth P, Elias BC, Rao R. Protein phosphatases 2A and 1 interact with occludin and negatively regulate the assembly of tight junctions in the CACO-2 cell monolayer. J Biol Chem 282: 11487–11498, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Seth A, Yan F, Polk DB, Rao RK. Probiotics ameliorate the hydrogen peroxide-induced epithelial barrier disruption by a PKC- and MAP kinase-dependent mechanism. Am J Physiol Gastrointest Liver Physiol 294: G1060–G1069, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sheth P, Delos Santos N, Seth A, LaRusso NF, Rao RK. Lipopolysaccharide disrupts tight junctions in cholangiocyte monolayers by a c-Src-, TLR4-, and LBP-dependent mechanism. Am J Physiol Gastrointest Liver Physiol 293: G308–G318, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Sheth P, Samak G, Shull JA, Seth A, Rao R. Protein phosphatase 2A plays a role in hydrogen peroxide-induced disruption of tight junctions in Caco-2 cell monolayers. Biochem J 421: 59–70, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sheth P, Seth A, Atkinson KJ, Gheyi T, Kale G, Giorgianni F, Desiderio DM, Li C, Naren A, Rao R. Acetaldehyde dissociates the PTP1B-E-cadherin-beta-catenin complex in Caco-2 cell monolayers by a phosphorylation-dependent mechanism. Biochem J 402: 291–300, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shin K, Fogg VC, Margolis B. Tight junctions and cell polarity. Annu Rev Cell Dev Biol 22: 207–235, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Sorrell MF, Tuma DJ. The functional implications of acetaldehyde binding to cell constituents. Ann NY Acad Sci 492: 50–62, 1987 [DOI] [PubMed] [Google Scholar]
- 41.Suzuki T, Elias BC, Seth A, Shen L, Turner JR, Giorgianni F, Desiderio D, Guntaka R, Rao R. PKC eta regulates occludin phosphorylation and epithelial tight junction integrity. Proc Natl Acad Sci USA 106: 61–66, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Walsh AH, Cheng A, Honkanen RE. Fostriecin, an antitumor antibiotic with inhibitory activity against serine/threonine protein phosphatases types 1 (PP1) and 2A (PP2A), is highly selective for PP2A. FEBS Lett 416: 230–234, 1997 [DOI] [PubMed] [Google Scholar]
- 43.Wyatt TA, Kharbanda KK, Tuma DJ, Sisson JH, Spurzem JR. Malondialdehyde-acetaldehyde adducts decrease bronchial epithelial wound repair. Alcohol 36: 31–40, 2005 [DOI] [PubMed] [Google Scholar]


