Abstract
The rising epidemic of diabetes is a pressing issue in clinical medicine worldwide from both healthcare and economic perspectives. This is fueled by overwhelming increases in the incidence and prevalence of obesity. Obesity and diabetes are characterized by both insulin resistance and endothelial dysfunction that lead to substantial increases in cardiovascular morbidity and mortality. Reciprocal relationships between insulin resistance and endothelial dysfunction tightly link metabolic diseases including obesity and diabetes with their cardiovascular complications. Therefore, therapeutic approaches that target either insulin resistance or endothelial dysfunction alone are likely to simultaneously improve both metabolic and cardiovascular pathophysiology and disease outcomes. Moreover, combination therapies with agents targeting distinct mechanisms are likely to have additive or synergistic benefits. Conventional therapies for diabetes and its cardiovascular complications that are both safe and effective are insufficient to meet rising demand. Large, robust, epidemiologic studies demonstrate beneficial metabolic and cardiovascular health effects for many functional foods containing various polyphenols. However, precise molecular mechanisms of action for food polyphenols are largely unknown. Moreover, translation of these insights into effective clinical therapies has not been fully realized. Nevertheless, some functional foods are likely sources for safe and effective therapies and preventative strategies for metabolic diseases and their cardiovascular complications. In this review, we emphasize recent progress in elucidating molecular, cellular, and physiological actions of polyphenols from green tea (EGCG), cocoa (ECG), and citrus fruits (hesperedin) that are related to improving metabolic and cardiovascular pathophysiology. We also discuss a rigorous comprehensive approach to studying functional foods that is essential for developing novel, effective, and safe medications derived from functional foods that will complement existing conventional drugs.
Keywords: insulin resistance, endothelial dysfunction, functional foods, polyphenols
obesity, prediabetes, metabolic syndrome, and diabetes are characterized by insulin resistance, endothelial dysfunction and resultant cardiovascular complications (38, 39, 45).1 Glucose intolerance and diabetes typically result from a combination of insulin resistance and impaired insulin secretion (17, 18, 66). Cardiovascular complications of metabolic diseases are related primarily to vascular endothelial dysfunction. Reciprocal relationships between insulin resistance and endothelial dysfunction help to link obesity and diabetes with their cardiovascular complications (39, 55, 57).
Conventional therapies for diabetes and its cardiovascular complications that are both safe and effective are insufficient to meet rising demand. Thus, developing alternative approaches for treatment as well as for prevention are imperative. One promising alternative therapeutic area comprises functional foods. That is, foods with health benefits beyond their nutritive value alone. A number of well-performed large epidemiologic studies demonstrate that consumption of functional foods containing bioactive polyphenols, including green tea, cocoa, and citrus fruits, are associated with dose-dependent improvement in metabolic and/or cardiovascular morbidity and mortality (7, 15, 28, 31, 32, 40, 46, 65, 90). In this review, we discuss the physiology of coordinated regulation of metabolic and cardiovascular actions of insulin, the pathophysiology of reciprocal relationships between insulin resistance and endothelial dysfunction, and a rigorous comprehensive approach to understanding molecular, cellular, and physiological mechanisms of actions for food polyphenols as potential therapeutic agents that simultaneously target improvement in metabolic and cardiovascular phenotypes. The scientific paradigm we outline here is a robust, efficient, and informative approach for understanding putative health benefits of functional foods. This approach is a useful means to discover and develop safe and effective novel therapies that complement existing conventional drugs for the treatment and prevention of diabetes and its cardiovascular complications.
Physiology of Insulin Action
Classical metabolic actions of insulin that help regulate glucose homeostasis include promotion of glucose uptake and utilization in skeletal muscle, suppression of hepatic glucose production, and inhibition of lipolysis in adipose tissue (18, 57). In recent years, it has become clear that a multitude of other biological actions of insulin in unconventional targets including vascular endothelium, brain, β-cell, kidney, gut, and other organs contribute importantly to both metabolic and cardiovascular homeostasis (55, 57, 58). Most, if not all, actions of insulin are initiated by the binding of insulin to specific cell surface insulin receptors, which are expressed on nearly every cell in the body (57). This initiates a complex signaling network that culminates in various biological actions of insulin in myriad tissues. For example, insulin signal transduction pathways promoting classical metabolic actions of insulin generally involve autophosphorylation of the insulin receptor tyrosine kinase that then phosphorylates insulin receptor substrates including IRS-1. Tyrosine phosphorylated IRS-1 binds and activates the lipid kinase phosphatidylinositol 3-kinase (PI3K) that then stimulates a serine kinase cascade (including PDK-1 and Akt) to promote translocation of the insulin-responsive glucose transporter GLUT4 from an intracellular compartment to the plasma membrane, where GLUT4 facilitates glucose entry into skeletal muscle and adipose tissue (61). In addition to PI3K-dependent insulin signaling, a MAPK-dependent branch of insulin signaling commences with tyrosine-phosphorylated IRS-1 or Shc binding to the SH2 domain of Grb-2, resulting in activation of the preassociated GTP exchange factor Sos (53, 61). This further activates the small GTP binding protein Ras, which then initiates a kinase phosphorylation cascade involving Raf, MAPK/extracellular signal-regulated kinase, and MAPK (61, 74). MAPK-dependent insulin signaling generally regulates effects of insulin to promote mitogenesis, growth, and differentiation.
Interestingly, in vascular endothelium, the PI3K branch of insulin signaling regulates activation of endothelial nitric oxide synthase (eNOS), leading to production of nitric oxide (NO), a potent vasodilator (50, 52, 95). In addition, PI3K-dependent insulin signaling in endothelium leading to phosphorylation of the transcription factor FOXO1 reduces synthesis and secretion of the vasoconstrictor peptide endothelin-1 (ET-1) (11, 57, 67, 73). At the same time, insulin signaling through MAPK-dependent pathways in endothelium promotes ET-1 synthesis and secretion (55, 57, 67). Thus, insulin regulates opposing vasoactive actions in vascular endothelium through relatively distinct major branches of insulin signaling pathways.
Pathophysiology of Insulin Resistance and Endothelial Dysfunction
Insulin resistance is typically defined as decreased sensitivity or responsiveness to the metabolic actions of insulin to promote glucose uptake and utilization (56). Insulin-resistant states are characterized by pathway-selective impairment in PI3K-dependent insulin signaling pathways with intact or augmented MAPK-dependent insulin signaling in both metabolic and vascular tissues. Compensatory fasting hyperinsulinemia attempts to maintain euglycemia with the unintended consequence of overdriving unaffected or augmented MAPK-dependent signaling and actions that are prohypertensive and inflammatory (57). In vitro models of insulin resistance in vascular endothelium are characterized by blunted PI3K-dependent eNOS expression and NO production. Simultaneously, signaling through MAPK pathways are substantially enhanced, leading to increased expression of endothelial adhesion molecules that are proatherogenic as well as increased mitogenic responsiveness of vascular smooth muscle cells (VSMC) in response to growth factors including insulin and VEGF that increase VSMC proliferation, another proatherogenic event (51).
Insulin stimulates MAPK-dependent ET-1 synthesis and secretion as well as PI3K-dependent NO production from vascular endothelium in healthy subjects to maintain normal net vascular tone. Pathophysiological imbalance between PI3K and MAPK signaling in response to insulin in the vasculature results in endothelial dysfunction that contributes substantially to insulin-resistant metabolic tissues in humans (10). Indeed, clinical physiology studies consistently show that diminished insulin-stimulated vasodilation is correlated with reduced insulin-stimulated vascular blood flow in subjects with obesity and/or type 2 diabetes (4, 12, 25, 41, 80, 82, 87). Furthermore, there is reduced NO activity (PI3K-dependent pathway) and augmented response of insulin to stimulate ET-1 production (MAPK-dependent pathway) in overweight, obese, and type 2 diabetes subjects (9, 43, 62, 77). Thus, an imbalance with overdriven MAPK-dependent signaling together with impaired PI3K-dependent signaling in the vascular endothelium leads to endothelial derangements that secondarily contribute to metabolic insulin resistance. These important pathophysiological relationships help link metabolic diseases with their cardiovascular complications (57). Since many of these pathophysiologies are present before the development of frank disease, it is possible that safe interventions including food polyphenols may also act as effective preventative measures.
Classic metabolic and cardiovascular diseases linked to the pathophysiology of insulin resistance include diabetes, metabolic syndrome, obesity, coronary heart disease, atherosclerosis, and hypertension. However, there are a host of other diseases associated with either or both insulin resistance and endothelial dysfunction. Some of these associations are at the level of epidemiological links, while others have putative pathophysiological mechanisms rooted in either insulin resistance or endothelial dysfunction. These other conditions include stroke, Alzheimer disease, nonalcoholic fatty liver disease, some forms of cancer, (e.g., breast and colon), and even aging (associated with increasing insulin resistance, although aging is not a disease per se). Thus, the concepts that we propose below apply to all of these conditions, although we focus on metabolic diseases and their cardiovascular complications.
Developing Therapeutics from Functional Foods
A rigorous, comprehensive approach to evaluating the safety and efficacy of functional foods and their bioactive components as useful therapeutic agents in humans is required but rarely implemented. Here, we outline a strategic paradigm that efficiently leads to identification and characterization of potential functional foods or nutritional supplements with compelling relevant therapeutic results, be they positive or negative (Fig. 1). Important characteristics that help identify promising foods or compounds include well-performed prospective longitudinal epidemiologic studies in large population cohorts with hard clinical endpoints of specific morbidity or mortality that strongly associate specific health benefits with consumption of particular foods or supplements. It is also helpful to have evidence of consistent dose-dependent effects in studies that are controlled for other sources of variance and specificity of effects on particular pathophysiological and clinical endpoints but for the exclusion of others (e.g., effects on cardiovascular death but not cancer death). Next, a single putative bioactive ingredient should be identified, purified, and characterized for further preclinical cellular and animal studies to elucidate mechanisms of action. Potential molecular and cellular mechanisms of action for the single active compound should be identified prior to commencement of observational or mechanistic animal studies of safety and efficacy. This is important because effects of the bioactive compound on relevant biomarkers that are consistent with a hypothesized mechanism of action help to bolster observational studies in vivo. In addition, it is important to characterize therapeutic dose ranges in vitro to set boundaries for effectiveness, test dose-response characteristics, and evaluate potential levels at which the compound may be toxic. These concentration characteristics of individual compounds will then be helpful in guiding the design of subsequent animal and human intervention trials.
Fig. 1.
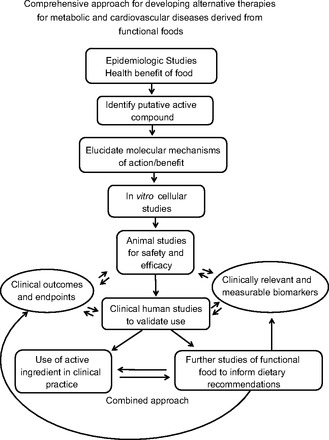
Comprehensive approach for developing alternative therapies for metabolic and cardiovascular diseases derived from functional foods.
After molecular and cell-based studies are completed, appropriate animal models should be used to test safety, efficacy, and dose-dependent effects. The dose-ranging studies performed in cell-based assays are useful for designing appropriate dosages to evaluate in vivo. Moreover, when dosing schemes for animal studies are being devised, the route of administration, bioavailability, tissue distribution, pharmacokinetics, pharmacodynamics, and identification and measurement of bioactive metabolites in blood and urine are all important parameters to consider and characterize, if possible. Doses that are effective in the in vitro setting are often somewhat higher than those required in the circulation or at the tissue level in in vivo settings, since physiological systems in the whole animal tend to be more sensitive and responsive than the somewhat artificial cell-based studies. These types of in vivo characterizations of therapeutic compounds are routine and expected for conventional drugs. However, for functional foods and nutritional supplements, these essential preclinical studies are often neglected or poorly executed. This contributes importantly to the lack of rigor in the overall characterization and therapeutic evaluation of compounds derived from functional foods and nutritional supplements.
Once compelling cellular and animal data are obtained, translation into rigorous patient-oriented clinical studies is appropriate and necessary. These should be placebo-controlled randomized, double-blind clinical studies with a single quantifiable, prospectively identified primary outcome that has the potential for clinical relevance and significance. Secondary clinical outcomes should be designed into the study to be informative with respect to putative mechanisms. Again, as with animal studies, it is essential to establish pharmacokinetic parameters, etc., and to have previously identified a putative mechanism of action and relevant biomarkers to evaluate during the clinical study to provide a proper context with which to evaluate the primary study outcome, whether it is positive or negative. Because these are studies of natural food compounds, important questions to consider include not only the ability of the compound to ameliorate pathophysiology in disease but also the potential to improve health in people at risk but without overt disease (e.g., would it be beneficial to lower the fasting glucose from 90 to 80?). Finally, one may consider the possibility of extending positive clinical studies with single compounds into prospective large longitudinal studies with the original functional food along with measurement of appropriate biomarkers, as this has the potential for tremendous worldwide impact, even in developing nations. Combined approaches could be considered as well, similar to the use of calcium supplementation in addition to consumption of calcium-rich foods to promote bone health. In the same way, food polyphenols, if proven safe for the general population, may also be considered in a preventative light. Compounds derived from functional foods available over the counter at sources including GNC are generally presumed to be safer than conventional drugs that require prescriptions. However, this may or may not be actually true. The oversight of functional foods and nutritional supplements is much more lax than that of ethical drugs. For example, EGCG (epigallocatechin gallate), which can be purchased easily at GNC has potential liver toxicity if taken at too high a dose (44, 49).
Examples of Paradigmatic Studies of Functional Foods
Below, we review studies of plant food polyphenols from green tea (EGCG), cocoa [epicatechin gallate (ECG)], and citrus fruits (hesperedin) that support putative cardiometabolic benefits of these compounds derived from functional foods. We organize our discussions according to the research paradigm set out above. The health benefits from food polyphenols are often attributed to their antioxidant properties. However, it is extremely important to emphasize at the outset that there is absolutely no rigorous scientific evidence to support an antioxidant mechanism for therapeutic benefits of food polyphenols. Indeed, the polyphenols we discuss below are actually mildly prooxidant in in vivo settings. Thus, the mechanisms of action for food polyphenols to promote metabolic and cardiovascular health are unrelated to scavenging oxidants. Moreover, many of the health benefits of food polyphenols are initiated by low level prooxidant signals from polyphenols in vivo (e.g., H2O2) that stimulate beneficial cellular signal transduction pathways leading to improved physiological homeostasis.
As described above, data on bioavailability, pharmacokinetics, and active metabolites in vivo for food polyphenols tend to be quite limited, especially in humans. Ingestion of two cups of green tea in humans results in peak plasma levels of EGCG of 0.2–0.3 μM. Drinking ten cups of green tea raises circulating levels of EGCG into the low micromolar range, where biological effects are observed in cell-based assays (93). Hesperidin, a food polyphenol derived from citrus, has an oral bioavailability of ∼5% and is hydrolyzed in vivo and conjugated into glucuronides and sulfoglucuronides (hesperitin, the active metabolite) (42). Consumption of 1.5 liters of orange juice results in circulating hesperitin concentrations in the low micromolar range sufficient to produce bioactive effects in cells in vitro (42). Similarly, cocoa binds to salivary proteins and reaches the small intestine largely intact, where roughly 20% of ECG (the major cocoa polyphenol) is absorbed (21). In general, bioavailability of food polyphenols tends to be relatively poor. However, when taken as single purified compounds at feasible dosages, circulating concentrations of active food-derived polyphenolic compounds and metabolites in the low micromolar range result in therapeutic concentrations where physiological bioactivity is plausible.
Green tea.
Large epidemiologic studies have shown that consumption of green tea is associated with decreased all-cause mortality and cardiovascular mortality, but not cancer mortality, in a dose-dependent fashion (40, 65). Similarly, epidemiologic studies show a decrease in type 2 diabetes among drinkers of green tea (29). Green tea contains the polyphenol EGCG. EGCG inhibits gluconeogenesis in hepatocytes and stimulates glucose uptake in rat skeletal muscle cells by using a PI3K-dependent mechanism that mimics metabolic actions of insulin (33, 86). Also, EGCG directly and acutely stimulates production of NO from primary endothelial cells by using a signaling pathway that involves low-level generation of H2O2 and possibly other reactive oxygen species that then goes on to stimulate a signaling cascade including sequential activation of the src family kinase fyn, PI3K, Akt, and eNOS (37). Interestingly, this pathway shares features in common with insulin signaling pathways regulating activation of eNOS and NO production in endothelial cells (39, 85). This increased production of NO tends to lower peripheral vascular resistance and increase blood flow through both conduit arteries as well as recruiting nutritive capillaries in skeletal muscle. Thus, the NO-mediated vasodilator actions of EGCG have primary benefits for cardiovascular homeostasis and secondary metabolic benefits mediated by increased delivery of substrates and hormones to metabolic targets (39). EGCG also decreases ET-1 expression, in part through regulation of FOXO1. This reduces vasoconstrictor tone and may also directly increase bioavailability of NO to improve endothelial function and oppose atherogenesis (73). Human aortic endothelial cells treated with EGCG have increased expression of heme oxygenase-1 mRNA, protein, and activity (69). Increased heme oxygenase-1 may mediate the anti-inflammatory actions of EGCG, accounting in part for its cardiovascular and metabolic benefits. Finally, suppression of hepatic gluconeogenesis in isolated hepatocytes through EGCG-activated AMPK helps to explain the beneficial metabolic actions of EGCG in glucose homeostasis (13).
In spontaneously hypertensive (SHR) rats (genetic model of human metabolic syndrome with hypertension, endothelial dysfunction, insulin resistance, and overweight), we demonstrated that 3 wk of EGCG administration reduces insulin resistance, improves endothelial dysfunction, opposes hypertension, protects against cardiac ischemia/reperfusion injury, and raises plasma adiponectin levels (68). Antidiabetic or lipid lowering properties of green tea or EGCG have been demonstrated in rodent models of type 2 diabetes as well as obesity (26, 76, 84, 94). In diabetic db/db mice, EGCG increases the size and number of pancreatic islets and improves β-cell morphology, leading to better glucose tolerance comparable to rosiglitazone (63). Moreover, in human subjects who smoke or those with coronary artery disease, consumption of green tea significantly reverses their endothelial dysfunction (59, 88). Meta-analyses show green tea consumption lowers both total and LDL-cholesterol levels and improves endothelial function as measured by flow-mediated dilation of the brachial artery (36, 71, 97). Green tea also lowers blood pressure, improves insulin sensitivity, and lowers cholesterol in obese, hypertensive patients (5, 81). In addition to direct and indirect metabolic and cardiovascular actions of EGCG discussed above, EGCG may also possess anti-inflammatory properties that contribute to its salutary effects on glucose and vascular homeostasis (35).
Citrus.
Citrus fruit consumption is associated in large epidemiologic and prospective studies with lower rates of coronary disease and ischemic stroke (15, 27, 31, 32, 90). Hesperidin, a predominant polyphenol found in citrus fruits, is one candidate that may mediate beneficial metabolic and vascular effects observed with citrus fruit consumption. Our laboratory has demonstrated that treatment of bovine aortic endothelial cells (BAEC) with hesperetin (a metabolite of hesperidin) acutely stimulates sequential activation of Src, PI3K, Akt, AMPK, and eNOS to produce NO (75). Similarly to EGCG, this pathway shares features in common with insulin signaling pathways regulating activation of eNOS and NO production in endothelial cells (39, 85, 96). Hesperidin may also have anti-inflammatory properties that contribute to its salutary effects on vascular homeostasis. We demonstrated that pretreatment of BAEC with hesperetin protects against TNFα-stimulated increases in expression of VCAM-1 and adhesion of monocytes to endothelial cells (75).
In animal studies, intake of hesperidin decreases infarct size in male Wistar rats after middle cerebral artery occlusion (72). In SHR rats, hesperidin causes a dose-dependent decrease in systolic blood pressure and improves endothelial dysfunction (91, 92). In diabetic rodents, hesperidin therapy has hypoglycemic and hypolipidemic effects and can lower circulating plasma free fatty acids, triglycerides, and total cholesterol along with lowering hepatic triglyceride levels and decreasing fatty acid oxidation (2, 3, 34). Interestingly, hesperidin augments the effects of exercise on cholesterol and insulin sensitivity (16).
Hyperlipidemic human subjects treated with hesperidin have lower circulating triglyceride levels (47, 48). In our recent clinical study of patients with metabolic syndrome, we demonstrated that hesperidin treatment (vs. placebo) substantially improved brachial artery flow-mediated dilation (FMD) compared with placebo treatment (7.78 vs. 10.26%, P = 0.02). In addition, hesperidin therapy reduced total cholesterol and apoB and increased HDL (75). These results are in keeping with cellular studies of hesperetin actions that mimic the ability of insulin to inhibit assembly and secretion of apoB-100-containing lipoproteins from hepatoma cells (6). Consistent with the anti-inflammatory effects of hesperetin in BAEC, we also observed significant decreases in circulating hsCRP, SAA protein, and sE-selectin in human subjects with metabolic syndrome treated with hesperidin (500 mg/day for 3 wk vs. placebo, P < 0.01) (75) as well as a trend toward improvement in insulin sensitivity as assessed by the surrogate index QUICKI (P = 0.06) (75). It is important to note that in our clinical intervention trial the positive prospectively designated primary outcomes and secondary positive physiological outcomes were fully consistent with the mechanism of action worked out in preclinical cellular and animal studies and supported by circulating biomarker data obtained during the clinical intervention trial.
Cocoa.
Cocoa is rich in monomeric (ECG and catechin) and oligomeric (procyanidin) flavanols (89). Epidemiologic evidence supports an inverse relationship between cocoa consumption and blood pressure, stroke, myocardial infarction, coronary heart disease, and cardiovascular and all-cause mortality (7, 8, 19, 20). In nondiabetic patients followed after acute myocardial infarction, chocolate consumption shows a strong dose-related inverse relationship to cardiac mortality (30).
Cocoa contains high levels of flavonoids, and in particular the polyphenol ECG, which enhances tyrosine phosphorylation in an insulin-like manner, activating the PI3K/Akt and AMPK pathways in the vascular endothelium to increase NO production (14, 21). In human coronary artery endothelial cells, epicatechin phosphorylates and activates eNOS through Akt and a complex with HSP90 (70). Incubating human umbilical vein endothelial cells with cocoa extract inhibits angiotensin-converting enzyme activity and enhances NO production (64).
Male mice treated with epicatechin have improved exercise performance and resistance to muscle fatigue. Mice treated with epicatechin alone (without exercise) have structural and metabolic changes in skeletal and cardiac muscle similar to those of mice undergoing exercise (60). Epicatechin alone and in combination with exercise has an additive effect to stimulate myocardial angiogenesis (70). Obese, diabetic db/db mice have prolonged lifespan, along with a decrease in markers of inflammation and LDL-cholesterol when treated with epicatechin (79). It is important to note that these animal studies are observational in nature and need to be followed up by more mechanistic studies in the future.
In human studies, cocoa ingestion acutely improves NO-dependent flow-mediated vasodilation and may contribute to amelioration of insulin resistance, hypertension, and metabolic syndrome (22). Grassi et al. showed that consumption of flavanol-rich dark chocolate for 2 wk decreased daytime and nighttime blood pressure, reduced insulin resistance, and improved NO-dependent vasorelaxation in a placebo-controlled (white chocolate) crossover study (24). Heart transplant recipients have improved coronary function and vasodilation, with decreased platelet reactivity 2 h after consumption of flavanol-rich dark chocolate (23). A recent Australian study showed the benefits of dark chocolate as a cost-effective measure in reducing cardiovascular events in subjects with metabolic syndrome (98). Our laboratory has shown that consumption of polyphenol-rich cocoa (vs. polyphenol-depleted cocoa placebo control) for 2 wk improves endothelial function in patients with hypertension without changing blood pressure, insulin sensitivity, or circulating inflammatory markers (54). A recent meta-analysis of flavonoids from cocoa included 24 studies (covering 1,106 participants) that studied the short-term effects of flavonoids. In this analysis, short-term cocoa consumption showed benefits in cardiovascular health, blood pressure, LDL-cholesterol levels, and insulin resistance. Since they were all short-term studies, no effect on BMI or worsening of diabetes was evident (78). Similar results were found in a smaller meta-analysis (83). Conclusions from these particular meta-analyses need to be considered with extreme caution, since most of the studies included in these analyses did not have appropriate blinding, randomization, or placebo controls.
PERSPECTIVES/CONCLUSIONS
Insulin signal transduction occurs through two major relatively distinct PI3K-dependent and MAPK-dependent pathways that have divergent effects on metabolic and vascular biology. In normal physiology, signaling through the PI3K pathway predominates, leading to increased insulin-dependent glucose uptake in muscle and fat with simultaneous improvements in vascular endothelial function. In the pathophysiological state of insulin resistance, PI3K signaling is selectively impaired, allowing signaling through the MAPK pathway to predominate. This leads to enhanced mitogenic activity and diminished NO-mediated vasodilation, contributing to endothelial dysfunction and metabolic insulin resistance.
Rigorous scientific investigation of functional foods and nutritional supplements for potential therapeutic health benefits represents an exciting new approach for deriving effective, safe treatments for diabetes and its cardiovascular complications that may complement existing conventional therapies. Use of dietary supplements is a popular practice that many people use to try to improve their health. However, it is essential to scientifically study, understand, and validate the benefits of functional foods and their derivatives by using the research paradigm outlined above. Without such research, there is no solid foundation for recommending functional foods for health benefits or disease prevention. The food polyphenols discussed above are often touted for their potent antioxidant activity. However, as explained above, the mechanisms of action to promote metabolic and cardiovascular health for food polyphenols is unrelated to scavenging oxidants and is most likely due to low-level prooxidant-induced signal transduction pathways that are beneficial for maintaining metabolic and cardiovascular homeostasis. Indeed, the American Diabetes Association recommends against routine supplementation with antioxidants, not only because of lack of evidence of efficacy but also because of concerns related to the long-term safety of these antioxidants (1). A rigorous approach to identify potentially beneficial foods from epidemiologic data and then undertaking systematic study in cells, animals, and finally humans is necessary to provide convincing evidence of safe therapeutic potential. Polyphenols derived from functional foods offer a novel and important approach for adjunctive treatment of the pathophysiology of metabolic disorders associated with insulin resistance and their cardiovascular complications.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: K.M.M. and M.J.Q. conception and design of research; K.M.M. and M.J.Q. analyzed data; K.M.M. and M.J.Q. interpreted results of experiments; K.M.M. prepared figures; K.M.M., S.C., F.G., and M.J.Q. drafted manuscript; K.M.M., F.G., and M.J.Q. edited and revised manuscript; K.M.M., F.G., and M.J.Q. approved final version of manuscript.
Footnotes
This article type is the topic of an Editorial by Peter D. Wagner (85a). The aim of this series is to provide practicing MDs and researchers an up-to-date physiological understanding of disease. Articles published in this series are intended to provide a thoughtful and lucid linkage of science to the patient. Go here for more information on this series and to browse the content (http://www.the-aps.org/mm/Publications/Journals/PIM).
REFERENCES
- 1.Anonymous. Standards of medical care in diabetes—2012. Diabetes Care 35, Suppl 1: S11–S63, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Akiyama S, Katsumata S, Suzuki K, Ishimi Y, Wu J, Uehara M. Dietary hesperidin exerts hypoglycemic and hypolipidemic effects in streptozotocin-induced marginal type 1 diabetic rats. J Clin Biochem Nutr 46: 87–92, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Akiyama S, Katsumata S, Suzuki K, Nakaya Y, Ishimi Y, Uehara M. Hypoglycemic and hypolipidemic effects of hesperidin and cyclodextrin-clathrated hesperetin in Goto-Kakizaki rats with type 2 diabetes. Biosci Biotechnol Biochem 73: 2779–2782, 2009 [DOI] [PubMed] [Google Scholar]
- 4. Baron AD, Laakso M, Brechtel G, Edelman SV. Reduced capacity and affinity of skeletal muscle for insulin-mediated glucose uptake in noninsulin-dependent diabetic subjects. Effects of insulin therapy. J Clin Invest 87: 1186–1194, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bogdanski P, Suliburska J, Szulinska M, Stepien M, Pupek-Musialik D, Jablecka A. Green tea extract reduces blood pressure, inflammatory biomarkers, and oxidative stress and improves parameters associated with insulin resistance in obese, hypertensive patients. Nutr Res 32: 421–427, 2012 [DOI] [PubMed] [Google Scholar]
- 6. Borradaile NM, Carroll KK, Kurowska EM. Regulation of HepG2 cell apolipoprotein B metabolism by the citrus flavanones hesperetin and naringenin. Lipids 34: 591–598, 1999 [DOI] [PubMed] [Google Scholar]
- 7. Buijsse B, Feskens EJ, Kok FJ, Kromhout D. Cocoa intake, blood pressure, and cardiovascular mortality: the Zutphen Elderly Study. Arch Intern Med 166: 411–417, 2006 [DOI] [PubMed] [Google Scholar]
- 8. Buijsse B, Weikert C, Drogan D, Bergmann M, Boeing H. Chocolate consumption in relation to blood pressure and risk of cardiovascular disease in German adults. Eur Heart J 31: 1616–1623, 2010 [DOI] [PubMed] [Google Scholar]
- 9. Cardillo C, Campia U, Bryant MB, Panza JA. Increased activity of endogenous endothelin in patients with type II diabetes mellitus. Circulation 106: 1783–1787, 2002 [DOI] [PubMed] [Google Scholar]
- 10. Cardillo C, Nambi SS, Kilcoyne CM, Choucair WK, Katz A, Quon MJ, Panza JA. Insulin stimulates both endothelin and nitric oxide activity in the human forearm. Circulation 100: 820–825, 1999 [DOI] [PubMed] [Google Scholar]
- 11. Chen H, Lin AS, Li Y, Reiter CE, Ver MR, Quon MJ. Dehydroepiandrosterone stimulates phosphorylation of FoxO1 in vascular endothelial cells via phosphatidylinositol 3-kinase- and protein kinase A-dependent signaling pathways to regulate ET-1 synthesis and secretion. J Biol Chem 283: 29228–29238, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Clerk LH, Vincent MA, Jahn LA, Liu Z, Lindner JR, Barrett EJ. Obesity blunts insulin-mediated microvascular recruitment in human forearm muscle. Diabetes 55: 1436–1442, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Collins QF, Liu HY, Pi J, Liu Z, Quon MJ, Cao W. Epigallocatechin-3-gallate (EGCG), a green tea polyphenol, suppresses hepatic gluconeogenesis through 5′-AMP-activated protein kinase. J Biol Chem 282: 30143–30149, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cordero-Herrera I, Martin MA, Bravo L, Goya L, Ramos S. Cocoa flavonoids improve insulin signalling and modulate glucose production via AKT and AMPK in HepG2 cells. Mol Nutr Food Res 57: 974–985, 2013 [DOI] [PubMed] [Google Scholar]
- 15. Dauchet L, Ferrieres J, Arveiler D, Yarnell JW, Gey F, Ducimetiere P, Ruidavets JB, Haas B, Evans A, Bingham A, Amouyel P, Dallongeville J. Frequency of fruit and vegetable consumption and coronary heart disease in France and Northern Ireland: the PRIME study. Br J Nutr 92: 963–972, 2004 [DOI] [PubMed] [Google Scholar]
- 16. de Oliveira DM, Dourado GK, Cesar TB. Hesperidin associated with continuous and interval swimming improved biochemical and oxidative biomarkers in rats. J Int Soc Sports Nutr 10: 27, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. DeFronzo RA. Lilly lecture 1987. The triumvirate: beta-cell, muscle, liver. A collusion responsible for NIDDM. Diabetes 37: 667–687, 1988 [DOI] [PubMed] [Google Scholar]
- 18. DeFronzo RA. Pharmacologic therapy for type 2 diabetes mellitus. Ann Intern Med 131: 281–303, 1999 [DOI] [PubMed] [Google Scholar]
- 19. Djousse L, Hopkins PN, Arnett DK, Pankow JS, Borecki I, North KE, Curtis Ellison R. Chocolate consumption is inversely associated with calcified atherosclerotic plaque in the coronary arteries: the NHLBI Family Heart Study. Clin Nutr 30: 38–43, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Djousse L, Hopkins PN, North KE, Pankow JS, Arnett DK, Ellison RC. Chocolate consumption is inversely associated with prevalent coronary heart disease: the National Heart, Lung, and Blood Institute Family Heart Study. Clin Nutr 30: 182–187, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ellam S, Williamson G. Cocoa and human health. Annu Rev Nutr 33: 105–128, 2013 [DOI] [PubMed] [Google Scholar]
- 22. Fisher ND, Hughes M, Gerhard-Herman M, Hollenberg NK. Flavanol-rich cocoa induces nitric-oxide-dependent vasodilation in healthy humans. J Hypertens 21: 2281–2286, 2003 [DOI] [PubMed] [Google Scholar]
- 23. Flammer AJ, Hermann F, Sudano I, Spieker L, Hermann M, Cooper KA, Serafini M, Luscher TF, Ruschitzka F, Noll G, Corti R. Dark chocolate improves coronary vasomotion and reduces platelet reactivity. Circulation 116: 2376–2382, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Grassi D, Desideri G, Necozione S, Lippi C, Casale R, Properzi G, Blumberg JB, Ferri C. Blood pressure is reduced and insulin sensitivity increased in glucose-intolerant, hypertensive subjects after 15 days of consuming high-polyphenol dark chocolate. J Nutr 138: 1671–1676, 2008 [DOI] [PubMed] [Google Scholar]
- 25. Gudbjornsdottir S, Sjostrand M, Strindberg L, Lonnroth P. Decreased muscle capillary permeability surface area in type 2 diabetic subjects. J Clin Endocrinol Metab 90: 1078–1082, 2005 [DOI] [PubMed] [Google Scholar]
- 26. Hasegawa N, Yamda N, Mori M. Powdered green tea has antilipogenic effect on Zucker rats fed a high-fat diet. Phytother Res 17: 477–480, 2003 [DOI] [PubMed] [Google Scholar]
- 27. He FJ, Nowson CA, MacGregor GA. Fruit and vegetable consumption and stroke: meta-analysis of cohort studies. Lancet 367: 320–326, 2006 [DOI] [PubMed] [Google Scholar]
- 28. Hooper L, Kay C, Abdelhamid A, Kroon PA, Cohn JS, Rimm EB, Cassidy A. Effects of chocolate, cocoa, and flavan-3-ols on cardiovascular health: a systematic review and meta-analysis of randomized trials. Am J Clin Nutr 95: 740–751, 2012 [DOI] [PubMed] [Google Scholar]
- 29. Iso H, Date C, Wakai K, Fukui M, Tamakoshi A. The relationship between green tea and total caffeine intake and risk for self-reported type 2 diabetes among Japanese adults. Ann Intern Med 144: 554–562, 2006 [DOI] [PubMed] [Google Scholar]
- 30. Janszky I, Mukamal KJ, Ljung R, Ahnve S, Ahlbom A, Hallqvist J. Chocolate consumption and mortality following a first acute myocardial infarction: the Stockholm Heart Epidemiology Program. J Intern Med 266: 248–257, 2009 [DOI] [PubMed] [Google Scholar]
- 31. Joshipura KJ, Ascherio A, Manson JE, Stampfer MJ, Rimm EB, Speizer FE, Hennekens CH, Spiegelman D, Willett WC. Fruit and vegetable intake in relation to risk of ischemic stroke. JAMA 282: 1233–1239, 1999 [DOI] [PubMed] [Google Scholar]
- 32. Joshipura KJ, Hu FB, Manson JE, Stampfer MJ, Rimm EB, Speizer FE, Colditz G, Ascherio A, Rosner B, Spiegelman D, Willett WC. The effect of fruit and vegetable intake on risk for coronary heart disease. Ann Intern Med 134: 1106–1114, 2001 [DOI] [PubMed] [Google Scholar]
- 33. Jung KH, Choi HS, Kim DH, Han MY, Chang UJ, Yim SV, Song BC, Kim CH, Kang SA. Epigallocatechin gallate stimulates glucose uptake through the phosphatidylinositol 3-kinase-mediated pathway in L6 rat skeletal muscle cells. J Med Food 11: 429–434, 2008 [DOI] [PubMed] [Google Scholar]
- 34. Jung UJ, Lee MK, Park YB, Kang MA, Choi MS. Effect of citrus flavonoids on lipid metabolism and glucose-regulating enzyme mRNA levels in type-2 diabetic mice. Int J Biochem Cell Biol 38: 1134–1145, 2006 [DOI] [PubMed] [Google Scholar]
- 35. Kaszkin M, Beck KF, Eberhardt W, Pfeilschifter J. Unravelling green tea's mechanisms of action: more than meets the eye. Mol Pharmacol 65: 15–17, 2004 [DOI] [PubMed] [Google Scholar]
- 36. Kim A, Chiu A, Barone MK, Avino D, Wang F, Coleman CI, Phung OJ. Green tea catechins decrease total and low-density lipoprotein cholesterol: a systematic review and meta-analysis. J Am Diet Assoc 111: 1720–1729, 2011 [DOI] [PubMed] [Google Scholar]
- 37. Kim JA, Formoso G, Li Y, Potenza MA, Marasciulo FL, Montagnani M, Quon MJ. Epigallocatechin gallate, a green tea polyphenol, mediates NO-dependent vasodilation using signaling pathways in vascular endothelium requiring reactive oxygen species and Fyn. J Biol Chem 282: 13736–13745, 2007 [DOI] [PubMed] [Google Scholar]
- 38. Kim JA, Montagnani M, Chandrasekaran S, Quon MJ. Role of lipotoxicity in endothelial dysfunction. Heart Fail Clin 8: 589–607, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kim JA, Montagnani M, Koh KK, Quon MJ. Reciprocal relationships between insulin resistance and endothelial dysfunction: molecular and pathophysiological mechanisms. Circulation 113: 1888–1904, 2006 [DOI] [PubMed] [Google Scholar]
- 40. Kuriyama S, Shimazu T, Ohmori K, Kikuchi N, Nakaya N, Nishino Y, Tsubono Y, Tsuji I. Green tea consumption and mortality due to cardiovascular disease, cancer, and all causes in Japan: the Ohsaki study. JAMA 296: 1255–1265, 2006 [DOI] [PubMed] [Google Scholar]
- 41. Laakso M, Edelman SV, Brechtel G, Baron AD. Decreased effect of insulin to stimulate skeletal muscle blood flow in obese man. A novel mechanism for insulin resistance. J Clin Invest 85: 1844–1852, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Manach C, Morand C, Gil-Izquierdo A, Bouteloup-Demange C, Remesy C. Bioavailability in humans of the flavanones hesperidin and narirutin after the ingestion of two doses of orange juice. Eur J Clin Nutr 57: 235–242, 2003 [DOI] [PubMed] [Google Scholar]
- 43. Mather KJ, Mirzamohammadi B, Lteif A, Steinberg HO, Baron AD. Endothelin contributes to basal vascular tone and endothelial dysfunction in human obesity and type 2 diabetes. Diabetes 51: 3517–3523, 2002 [DOI] [PubMed] [Google Scholar]
- 44. Mazzanti G, Menniti-Ippolito F, Moro PA, Cassetti F, Raschetti R, Santuccio C, Mastrangelo S. Hepatotoxicity from green tea: a review of the literature and two unpublished cases. Eur J Clin Pharmacol 65: 331–341, 2009 [DOI] [PubMed] [Google Scholar]
- 45. Mercurio V, Carlomagno G, Fazio V, Fazio S. Insulin resistance: is it time for primary prevention? World J Cardiol 4: 1–7, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mink PJ, Scrafford CG, Barraj LM, Harnack L, Hong CP, Nettleton JA, Jacobs DR., Jr Flavonoid intake and cardiovascular disease mortality: a prospective study in postmenopausal women. Am J Clin Nutr 85: 895–909, 2007 [DOI] [PubMed] [Google Scholar]
- 47. Miwa Y, Mitsuzumi H, Sunayama T, Yamada M, Okada K, Kubota M, Chaen H, Mishima Y, Kibata M. Glucosyl hesperidin lowers serum triglyceride level in hypertriglyceridemic subjects through the improvement of very low-density lipoprotein metabolic abnormality. J Nutr Sci Vitaminol (Tokyo) 51: 460–470, 2005 [DOI] [PubMed] [Google Scholar]
- 48. Miwa Y, Yamada M, Sunayama T, Mitsuzumi H, Tsuzaki Y, Chaen H, Mishima Y, Kibata M. Effects of glucosyl hesperidin on serum lipids in hyperlipidemic subjects: preferential reduction in elevated serum triglyceride level. J Nutr Sci Vitaminol (Tokyo) 50: 211–218, 2004 [DOI] [PubMed] [Google Scholar]
- 49. Molinari M, Watt KD, Kruszyna T, Nelson R, Walsh M, Huang WY, Nashan B, Peltekian K. Acute liver failure induced by green tea extracts: case report and review of the literature. Liver Transpl 12: 1892–1895, 2006 [DOI] [PubMed] [Google Scholar]
- 50. Montagnani M, Chen H, Barr VA, Quon MJ. Insulin-stimulated activation of eNOS is independent of Ca2+ but requires phosphorylation by Akt at Ser(1179). J Biol Chem 276: 30392–30398, 2001 [DOI] [PubMed] [Google Scholar]
- 51. Montagnani M, Golovchenko I, Kim I, Koh GY, Goalstone ML, Mundhekar AN, Johansen M, Kucik DF, Quon MJ, Draznin B. Inhibition of phosphatidylinositol 3-kinase enhances mitogenic actions of insulin in endothelial cells. J Biol Chem 277: 1794–1799, 2002 [DOI] [PubMed] [Google Scholar]
- 52. Montagnani M, Ravichandran LV, Chen H, Esposito DL, Quon MJ. Insulin receptor substrate-1 and phosphoinositide-dependent kinase-1 are required for insulin-stimulated production of nitric oxide in endothelial cells. Mol Endocrinol 16: 1931–1942, 2002 [DOI] [PubMed] [Google Scholar]
- 53. Motley ED, Kabir SM, Gardner CD, Eguchi K, Frank GD, Kuroki T, Ohba M, Yamakawa T, Eguchi S. Lysophosphatidylcholine inhibits insulin-induced Akt activation through protein kinase C-alpha in vascular smooth muscle cells. Hypertension 39: 508–512, 2002 [DOI] [PubMed] [Google Scholar]
- 54. Muniyappa R, Hall G, Kolodziej TL, Karne RJ, Crandon SK, Quon MJ. Cocoa consumption for 2 wk enhances insulin-mediated vasodilatation without improving blood pressure or insulin resistance in essential hypertension. Am J Clin Nutr 88: 1685–1696, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Muniyappa R, Iantorno M, Quon MJ. An integrated view of insulin resistance and endothelial dysfunction. Endocrinol Metab Clin North Am 37: 685–711, ix–x, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Muniyappa R, Lee S, Chen H, Quon MJ. Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usage. Am J Physiol Endocrinol Metab 294: E15–E26, 2008 [DOI] [PubMed] [Google Scholar]
- 57. Muniyappa R, Montagnani M, Koh KK, Quon MJ. Cardiovascular actions of insulin. Endocr Rev 28: 463–491, 2007 [DOI] [PubMed] [Google Scholar]
- 58. Muniyappa R, Quon MJ. Insulin action and insulin resistance in vascular endothelium. Curr Opin Clin Nutr Metab Care 10: 523–530, 2007 [DOI] [PubMed] [Google Scholar]
- 59. Nagaya N, Yamamoto H, Uematsu M, Itoh T, Nakagawa K, Miyazawa T, Kangawa K, Miyatake K. Green tea reverses endothelial dysfunction in healthy smokers. Heart 90: 1485–1486, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Nogueira L, Ramirez-Sanchez I, Perkins GA, Murphy A, Taub PR, Ceballos G, Villarreal FJ, Hogan MC, Malek MH. (−)-Epicatechin enhances fatigue resistance and oxidative capacity in mouse muscle. J Physiol 589: 4615–4631, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Nystrom FH, Quon MJ. Insulin signalling: metabolic pathways and mechanisms for specificity. Cell Signal 11: 563–574, 1999 [DOI] [PubMed] [Google Scholar]
- 62. Okon EB, Chung AW, Rauniyar P, Padilla E, Tejerina T, McManus BM, Luo H, van Breemen C. Compromised arterial function in human type 2 diabetic patients. Diabetes 54: 2415–2423, 2005 [DOI] [PubMed] [Google Scholar]
- 63. Ortsater H, Grankvist N, Wolfram S, Kuehn N, Sjoholm A. Diet supplementation with green tea extract epigallocatechin gallate prevents progression to glucose intolerance in db/db mice. Nutr Metab (Lond) 9: 11, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Persson IA, Persson K, Hagg S, Andersson RG. Effects of cocoa extract and dark chocolate on angiotensin-converting enzyme and nitric oxide in human endothelial cells and healthy volunteers—a nutrigenomics perspective. J Cardiovasc Pharmacol 57: 44–50, 2011 [DOI] [PubMed] [Google Scholar]
- 65. Peters U, Poole C, Arab L. Does tea affect cardiovascular disease? A meta-analysis. Am J Epidemiol 154: 495–503, 2001 [DOI] [PubMed] [Google Scholar]
- 66. Polonsky KS. Lilly Lecture 1994. The beta-cell in diabetes: from molecular genetics to clinical research. Diabetes 44: 705–717, 1995 [DOI] [PubMed] [Google Scholar]
- 67. Potenza MA, Marasciulo FL, Chieppa DM, Brigiani GS, Formoso G, Quon MJ, Montagnani M. Insulin resistance in spontaneously hypertensive rats is associated with endothelial dysfunction characterized by imbalance between NO and ET-1 production. Am J Physiol Heart Circ Physiol 289: H813–H822, 2005 [DOI] [PubMed] [Google Scholar]
- 68. Potenza MA, Marasciulo FL, Tarquinio M, Tiravanti E, Colantuono G, Federici A, Kim JA, Quon MJ, Montagnani M. EGCG, a green tea polyphenol, improves endothelial function and insulin sensitivity, reduces blood pressure, and protects against myocardial I/R injury in SHR. Am J Physiol Endocrinol Metab 292: E1378–E1387, 2007 [DOI] [PubMed] [Google Scholar]
- 69. Pullikotil P, Chen H, Muniyappa R, Greenberg CC, Yang S, Reiter CE, Lee JW, Chung JH, Quon MJ. Epigallocatechin gallate induces expression of heme oxygenase-1 in endothelial cells via p38 MAPK and Nrf-2 that suppresses proinflammatory actions of TNF-alpha. J Nutr Biochem 23: 1134–1145, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ramirez-Sanchez I, Aguilar H, Ceballos G, Villarreal F. (−)-Epicatechin-induced calcium independent eNOS activation: roles of HSP90 and AKT. Mol Cell Biochem 370: 141–150, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ras RT, Zock PL, Draijer R. Tea consumption enhances endothelial-dependent vasodilation; a meta-analysis. PLoS One 6: e16974, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Raza SS, Khan MM, Ahmad A, Ashafaq M, Khuwaja G, Tabassum R, Javed H, Siddiqui MS, Safhi MM, Islam F. Hesperidin ameliorates functional and histological outcome and reduces neuroinflammation in experimental stroke. Brain Res 1420: 93–105, 2011 [DOI] [PubMed] [Google Scholar]
- 73. Reiter CE, Kim JA, Quon MJ. Green tea polyphenol epigallocatechin gallate reduces endothelin-1 expression and secretion in vascular endothelial cells: roles for AMP-activated protein kinase, Akt, and FOXO1. Endocrinology 151: 103–114, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Reusch JE, Bhuripanyo P, Carel K, Leitner JW, Hsieh P, DePaolo D, Draznin B. Differential requirement for p21ras activation in the metabolic signaling by insulin. J Biol Chem 270: 2036–2040, 1995 [DOI] [PubMed] [Google Scholar]
- 75. Rizza S, Muniyappa R, Iantorno M, Kim JA, Chen H, Pullikotil P, Senese N, Tesauro M, Lauro D, Cardillo C, Quon MJ. Citrus polyphenol hesperidin stimulates production of nitric oxide in endothelial cells while improving endothelial function and reducing inflammatory markers in patients with metabolic syndrome. J Clin Endocrinol Metab 96: E782–E792, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Sabu MC, Smitha K, Kuttan R. Anti-diabetic activity of green tea polyphenols and their role in reducing oxidative stress in experimental diabetes. J Ethnopharmacol 83: 109–116, 2002 [DOI] [PubMed] [Google Scholar]
- 77. Shemyakin A, Bohm F, Wagner H, Efendic S, Bavenholm P, Pernow J. Enhanced endothelium-dependent vasodilatation by dual endothelin receptor blockade in individuals with insulin resistance. J Cardiovasc Pharmacol 47: 385–390, 2006 [DOI] [PubMed] [Google Scholar]
- 78. Shrime MG, Bauer SR, McDonald AC, Chowdhury NH, Coltart CE, Ding EL. Flavonoid-rich cocoa consumption affects multiple cardiovascular risk factors in a meta-analysis of short-term studies. J Nutr 141: 1982–1988, 2011 [DOI] [PubMed] [Google Scholar]
- 79. Si H, Fu Z, Babu PV, Zhen W, Leroith T, Meaney MP, Voelker KA, Jia Z, Grange RW, Liu D. Dietary epicatechin promotes survival of obese diabetic mice and Drosophila melanogaster. J Nutr 141: 1095–1100, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Steinberg HO, Baron AD. Vascular function, insulin resistance and fatty acids. Diabetologia 45: 623–634, 2002 [DOI] [PubMed] [Google Scholar]
- 81. Suliburska J, Bogdanski P, Szulinska M, Stepien M, Pupek-Musialik D, Jablecka A. Effects of green tea supplementation on elements, total antioxidants, lipids, and glucose values in the serum of obese patients. Biol Trace Elem Res 149: 315–322, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Tack CJ, Ong MK, Lutterman JA, Smits P. Insulin-induced vasodilatation and endothelial function in obesity/insulin resistance. Effects of troglitazone. Diabetologia 41: 569–576, 1998 [DOI] [PubMed] [Google Scholar]
- 83. Tokede OA, Gaziano JM, Djousse L. Effects of cocoa products/dark chocolate on serum lipids: a meta-analysis. Eur J Clin Nutr 65: 879–886, 2011 [DOI] [PubMed] [Google Scholar]
- 84. Tsuneki H, Ishizuka M, Terasawa M, Wu JB, Sasaoka T, Kimura I. Effect of green tea on blood glucose levels and serum proteomic patterns in diabetic (db/db) mice and on glucose metabolism in healthy humans. BMC Pharmacol 4: 18, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Vincent MA, Montagnani M, Quon MJ. Molecular and physiologic actions of insulin related to production of nitric oxide in vascular endothelium. Curr Diab Rep 3: 279–288, 2003 [DOI] [PubMed] [Google Scholar]
- 85a. Wagner PD. Physiology in Medicine—Déjá vu all over again. J Appl Physiol; 10.1152/japplphysiol.00028.2013 [DOI] [PubMed] [Google Scholar]
- 86. Waltner-Law ME, Wang XL, Law BK, Hall RK, Nawano M, Granner DK. Epigallocatechin gallate, a constituent of green tea, represses hepatic glucose production. J Biol Chem 277: 34933–34940, 2002 [DOI] [PubMed] [Google Scholar]
- 87. Westerbacka J, Vehkavaara S, Bergholm R, Wilkinson I, Cockcroft J, Yki-Jarvinen H. Marked resistance of the ability of insulin to decrease arterial stiffness characterizes human obesity. Diabetes 48: 821–827, 1999 [DOI] [PubMed] [Google Scholar]
- 88. Widlansky ME, Hamburg NM, Anter E, Holbrook M, Kahn DF, Elliott JG, Keaney JF, Jr, Vita JA. Acute EGCG supplementation reverses endothelial dysfunction in patients with coronary artery disease. J Am Coll Nutr 26: 95–102, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Wollgast J, Pallaroni L, Agazzi ME, Anklam E. Analysis of procyanidins in chocolate by reversed-phase high-performance liquid chromatography with electrospray ionisation mass spectrometric and tandem mass spectrometric detection. J Chromatogr A 926: 211–220, 2001 [DOI] [PubMed] [Google Scholar]
- 90. Yamada T, Hayasaka S, Shibata Y, Ojima T, Saegusa T, Gotoh T, Ishikawa S, Nakamura Y, Kayaba K. Frequency of citrus fruit intake is associated with the incidence of cardiovascular disease: the Jichi Medical School cohort study. J Epidemiol 21: 169–175, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Yamamoto M, Suzuki A, Hase T. Short-term effects of glucosyl hesperidin and hesperetin on blood pressure and vascular endothelial function in spontaneously hypertensive rats. J Nutr Sci Vitaminol (Tokyo) 54: 95–98, 2008 [DOI] [PubMed] [Google Scholar]
- 92. Yamamoto M, Suzuki A, Jokura H, Yamamoto N, Hase T. Glucosyl hesperidin prevents endothelial dysfunction and oxidative stress in spontaneously hypertensive rats. Nutrition 24: 470–476, 2008 [DOI] [PubMed] [Google Scholar]
- 93. Yang CS, Wang X, Lu G, Picinich SC. Cancer prevention by tea: animal studies, molecular mechanisms and human relevance. Nat Rev Cancer 9: 429–439, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Yang M, Wang C, Chen H. Green, oolong and black tea extracts modulate lipid metabolism in hyperlipidemia rats fed high-sucrose diet. J Nutr Biochem 12: 14–20, 2001 [DOI] [PubMed] [Google Scholar]
- 95. Zeng G, Nystrom FH, Ravichandran LV, Cong LN, Kirby M, Mostowski H, Quon MJ. Roles for insulin receptor, PI3-kinase, and Akt in insulin-signaling pathways related to production of nitric oxide in human vascular endothelial cells. Circulation 101: 1539–1545, 2000 [DOI] [PubMed] [Google Scholar]
- 96. Zeng G, Quon MJ. Insulin-stimulated production of nitric oxide is inhibited by wortmannin. Direct measurement in vascular endothelial cells. J Clin Invest 98: 894–898, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Zheng XX, Xu YL, Li SH, Liu XX, Hui R, Huang XH. Green tea intake lowers fasting serum total and LDL cholesterol in adults: a meta-analysis of 14 randomized controlled trials. Am J Clin Nutr 94: 601–610, 2011 [DOI] [PubMed] [Google Scholar]
- 98. Zomer E, Owen A, Magliano DJ, Liew D, Reid CM. The effectiveness and cost effectiveness of dark chocolate consumption as prevention therapy in people at high risk of cardiovascular disease: best case scenario analysis using a Markov model. BMJ 344: e3657, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]


