Abstract
During spermatogenesis, the molecular mechanism that confers spermatid adhesion to the Sertoli cell at the apical ectoplasmic specialization (apical ES), a testis-specific F-actin-rich adherens junction, in the rat testis remains elusive. Herein, the activated form of focal adhesion kinase (FAK), p-FAK-Tyr397, a component of the apical ES that was expressed predominantly and stage specifically in stage VII-early stage VIII tubules, was found to be a crucial apical ES regulator. Using an FAK-Y397E phosphomimetic mutant cloned in a mammalian expression vector for its transfection vs. FAK and vector alone in adult rat testes in vivo, its overexpression was found to cause defects in spermiation. These defects in spermiation were manifested by entrapment of spermatids in the seminiferous epithelium in late stage VIII–X tubules and were mediated by a disruption on the spatiotemporal expression and/or mislocalization of actin regulatory protein actin-related protein 3, which induces branched actin polymerization, epidermal growth factor receptor pathway substrate 8 (an actin barbed end capping and bundling protein), and palladin (an actin cross-linking and bundling protein). This thus perturbed changes of F-actin organization at the apical ES to facilitate spermiation, which also led to a concomitant alteration in the distribution and upregulation of adhesion proteins nectin-2 and nectin-3 at the apical ES. As such, nectin-2 and -3 remained at the apical ES to anchor step 19 spermatids on to the epithelium, delaying spermiation. These findings illustrate a mechanistic pathway mediated by p-FAK-Tyr397 that regulates spermatid adhesion at the apical ES in vivo.
Keywords: spermatogenesis, focal adhesion kinase, focal adhesion kinase mutant, adherens junction, actin filament bundles, testis
in the rat testis, step 1 spermatids derived from secondary spermatocytes via meiosis undergo extensive morphological changes during spermiogenesis (8, 10, 20). Besides changes in cell shape via 19 steps in which round spermatids (step 1) transform into elongated spermatids (step 19), step 1 spermatids residing in the adluminal compartment but near the basal compartment and adjacent to the basement membrane must traverse back-and-forth the seminiferous epithelium during the epithelial cycle of spermatogenesis (8, 19, 22). As such, fully developed spermatids (i.e., spermatozoa) can be lined up at the luminal edge of the tubule lumen at late stage VIII of the epithelial cycle for spermiation (10, 20, 24). During spermiogenesis, a testis-specific adherens junction (AJ) appears at the Sertoli-spermatid (step 8) interface at stage VIII of the cycle known as apical ectoplasmic specialization (apical ES) (6, 27, 32). Once apical ES forms, it replaces desmosome and gap junction, becoming the only anchoring device that confers spermatid adhesion and polarity, and the apical ES persists through step 19 spermatids until its degeneration at spermiation (6, 16, 32, 36). The most obvious ultrastructural feature of the apical ES is the bundles of actin filaments that aligned perpendicular to the Sertoli cell plasma membrane that are sandwiched in between cisternae of the endoplasmic reticulum and the apposing plasma membranes of Sertoli-spermatid (6, 19, 27, 32). Thus, it is conceivable that the transport of developing spermatids across the epithelium during spermiogenesis requires extensive reorganization of these actin filament bundles, converting from their “bundled” to their “debundled” configuration intermittently.
Recent studies have shown that the organization of actin filament bundles is dynamically regulated via the stage-specific and spatiotemporal expression of epidermal growth factor receptor pathway substrate 8 (Eps8, an actin filament barbed-end capping and bundling protein) (13), palladin (an actin cross-linking and bundling protein) (25), and actin-related protein 3 (Arp3, which together with Arp2 forms the Arp2/3 complex that induces barbed end branched actin polymerization) (12). In short, these actin-binding/regulatory proteins are working synergistically to convert actin filaments at the apical ES from a bundled to a “debundled/branched” configuration and vice versa to facilitate spermatid transport across the epithelium during the epithelial cycle. However, the molecule(s) that coordinate and/or regulate these proteins remain unknown. Earlier studies have shown that focal adhesion kinase (FAK) is an integrated component of the apical and the basal ES. p-FAK-Tyr397 is exclusively and stage-specifically expressed at the apical ES at stage VII-early VIII of the cycle, but not at late stage VIII when spermiation takes, and virtually undetectable at the basal ES at these stages (2, 29). These findings thus suggest that p-FAK-Tyr397 may be crucial to maintain apical ES integrity from stage VII through early VIII of the cycle, and, if this hypothesis is correct, its overexpression in the testis in vivo should impede spermiation by delaying the release of sperms. We thus sought to investigate the effects on the apical ES function following overexpression of p-FAK-Tyr397 via the use of an FAK Y397E (Y, Tyr; E, Glu) phosphomimetic mutant in the rat testis, in particular changes on F-actin organization and if there were any alterations on the spatiotemporal expression of actin regulatory and adhesion proteins at the apical ES.
MATERIALS AND METHODS
Animals and antibodies.
Sprague-Dawley rats were obtained from Charles River Laboratories (Kingston, NY). Animals were kept and maintained at the Rockefeller University Comparative Bioscience Center (CBC) according to the applicable portions of the Animal Welfare Act and the guidelines in the Department of Health and Human Services publication Guide for the Care and Use of Laboratory Animals. Rats maintained in the CBC had free access to water and standard rat chow ad libitum at 21 ± 1°C with a light-dark cycle of 12:12 h. The use of rats for experiments reported herein was approved by the Rockefeller University Laboratory Animal Use and Care Committee with Protocol number 12–506. Antibodies used for the experiments reported herein were summarized in Table 1, including their working dilutions and applications.
Table 1.
Antibodies used for different experiments
| Working Solution |
|||||
|---|---|---|---|---|---|
| Antibody* | Host Species | Vendor | Catalog No. | IB | IF |
| Arp3 | Mouse | Sigma-Aldrich | A5979 | 1:3,000 | 1:200 |
| Eps8 | Mouse | BD Biosciences | 610143 | 1:5,000 | 1:100 |
| FAK | Rabbit | Millipore | 06-543 | 1:1,000 | |
| Nectin-2 | Goat | Santa Cruz Biotechnology | sc-14802 | 1:75 | |
| Nectin-3 | Goat | Santa Cruz Biotechnology | sc-14806 | 1:300 | 1:75 |
| Palladin | Rabbit | Protein Tech Group | 10853-1-AP | 1:1,000 | 1:100 |
| Goat IgG-HRP | Bovine | Santa Cruz Biotechnology | sc-2350 | 1:3,000 | |
| Rabbit IgG-HRP | Bovine | Santa Cruz Biotechnology | sc-2370 | 1:3,000 | |
| Mouse IgG-HRP | Bovine | Santa Cruz Biotechnology | sc-2371 | 1:3,000 | |
| Goat IgG-Alexa Fluor 555 | Donkey | Invitrogen | A21432 | 1:200 | |
| Rabbit IgG-Alexa Fluor 555 | Goat | Invitrogen | A21429 | 1:250 | |
| Mouse IgG-Alexa Fluor 555 | Goat | Invitrogen | A21424 | 1:250 | |
IB, immunoblot; IF, immunofluorescence analysis; Arp3, actin-related protein 3 that induces branched actin polymerization; Eps8, epidermal growth factor receptor pathway substrate 8; FAK, focal adhesion kinase; HRP, horseradish peroxidase.
Antibodies used herein cross-reacted with the corresponding proteins in rats as indicated by the manufacturer.
Construction of wild-type FAK and FAK phosphomimetic mutant in mammalian expression vector pCI-neo.
The full-length cDNA of wild-type rat FAK, including both start and stop codons (accession no. NM_013081.1), was amplified using total cDNAs from primary Sertoli cells isolated from 20-day-old rat testes (9, 18) by PCR using specific primers as earlier described (14). It was then cloned into the MluI/XbaI site of the pCI-neo vector (Promega). Single mutation of FAK-Tyr397 from Tyr (Y) to Glu (E) was performed via a three-step mutagenic PCR using the wild-type construct as the template as described (21, 31). In short, the desired mutation was first introduced by amplifying two overlapping sequences of FAK using two synthetic primer pairs (a mutagenic primer paired with a flanking primer and with the opposite orientation) as detailed elsewhere (14). The two PCR products were then merged by overlapping extension (31), from which the full-length cDNA containing the single mutation was subsequently amplified with primers to obtain the mutant as described (14). All sequences were verified by direct DNA sequencing at Genewiz to confirm the mutant cDNA construct as well as the full-length FAK cDNA. For transfection into Sertoli cells, plasmid DNA was prepared with the HiSpeed Plasmid Midi Kit (Qiagen). For the visualization of transfected cells to confirm transfection efficiency in vivo, testes were administered with pCI-neo/DsRed2 (red fluorescence).
Overexpression of FAK and FAK mutant in the adult rat testes in vivo.
FAK phosphomimetic mutant or FAK was cloned into the MluI/XbaI site of the Mammalian Expression Vector pCI-neo (Promega) as detailed elsewhere (14). Plasmid DNA, both the mutant FAK and empty vector, was obtained using a HiSpeed Plasmid Midi Kit (Qiagen). Plasmid DNA was then treated with the Mira-CLEAN Endotoxin Removal Kit (Mirus, Madison, WI) to remove any bacterial endotoxin contaminant. Rats were transfected with corresponding plasmid DNA via intratesticular injection using a 29-gauge needle as described (28, 30). On day 0, one testis of an adult rat (∼275–300 g body wt with a weight of ∼1.6 g, assuming a volume of ∼1.6 ml) received 15 μg pCI-neo empty vector DNA plasmid while the other testis received the same amount of FAK phosphomimetic mutant Y397E plasmid DNA or FAK plasmid DNA for transfection. Plasmid DNA (15 μg, for the mutant, FAK, or empty vector) suspended in 20 μl TE buffer (10 mM Tris, pH 8.0 at 22°C containing 1 mM EDTA) was combined with 140 μl TransIT-EE delivery solution (Mirus Bio) to a total volume of 160 μl and administered to each testis. Rats (n = 7 rats/time point in both treatment and control groups) received intratesticular injection of plasmid DNA daily for 3 days at days 0, 1, and 2 using the procedure earlier described (28) and modified in our laboratory for gene silencing using short hairpin RNA or small-interfering RNA for transfection in vivo (15, 25). In brief, plasmid DNA in TransIT-EE delivery solution in 160 μl was loaded on a 1-ml Insulin Syringe U-100 with a 29-gauge, 0.5-in. needle (Becton Dickinson). A new syringe was used for each testis for administration, including both treatment and control groups. The needle was first inserted in one end of a testis along the vertical axis, and the content was gently released as the needle was gradually removed from the testis. Thus, the 160-μl transfection medium and the plasmid DNA was evenly spread across the testis to optimize transfection. Three days after the last injection (i.e., on day 5), rats were killed by CO2 asphyxiation. This time point was selected based on pilot experiments since the phenotype observed on day 4 or day 7 was similar to that of day 5. Testes were immediately removed thereafter under aseptic conditions, snap-frozen in liquid nitrogen, and stored at −80°C to be used for lysate preparation, and to obtain frozen sections for histological analysis, immunohistochemistry, or dual-labeled immunofluorescence analysis. Some testes were also fixed in Bouin's fixative to obtain paraffin sections for histological analysis following hematoxylin and eosin staining.
Assessing the efficacy of FAK and FAK Y397E overexpression in the testis in vivo.
The efficacy of overexpression of pCI-neo/FAK and pCI-neo/FAK Y397E vs. pCI-neo empty vector alone in the testis in vivo was estimated by two approaches. First, a DsRed2 cDNA construct was cloned from pIRES2-DsRed2 (Clontech, Mountain View, CA) using the primer pair specific to DsRed2 (Table 2) and the pIRES2-DsRed2 plasmid DNA as a template, which was then ligated to the MluI/XbaI site of the mammalian expression vector pCI-neo. About 15 μg pCI-neo-DsRed2 plasmid DNA suspended in 160 μl TransIT-EE delivery solution were administered to the right testis of an adult rat with n = 3 rats on days 0, 1, and 2 (i.e., a total of 3 transfections) and rats were killed on day 5 (i.e., 3 days after the last transfection), with the left testis receiving pCI-neo vector alone. Second, lysates (∼800 μg protein) obtained from testes transfected with pCI-neo/FAK or pCI-neo/FAK Y397E vs. pCI-neo empty vector (served as a control) were subjected to immunoprecipitation (IP) using an anti-FAK antibody (Table 1) with normal rabbit IgG serving as a negative control. The resultant immunocomplexes were then examined by immunoblotting using an anti-FAK antibody to assess if there was an increase in the expression of FAK protein in the testis transfected with either FAK or FAK Y397E vector.
Table 2.
Primer pairs used for PCR to clone DsRed2 gene
| Gene Name | Primer Orientation | Primer Sequence | Nucleotide Position | Expected Size, bp |
|---|---|---|---|---|
| DsRed2 | Sense | 5′-ATGGCCTCCTCCGAGAACGTCAT-3′ | 1254–1276 | 678 |
| Antisense | 5′-CTACAGGAACAGGTGGTGGCGG-3′ | 1910–1931 |
Co-IP and immunoblotting.
Lysates were obtained from testes, prepared in IP lysis buffer [50 mM Tris, pH 7.4 at 22°C, containing 150 mM NaCl, 2 mM EGTA, 10% glycerol (vol/vol), and 1% Nonidet P-40 (vol/vol)], and supplemented with a protease inhibitor cocktail (Sigma-Aldrich) at 1:100 (protease inhibitor cocktail-buffer) dilution before its use. Protein concentrations were determined by using Bio-Rad DC protein assay kits (Bio-Rad). Co-IP was performed using freshly prepared rat testes lysate (800 μg protein/sample tube) as described (14). Briefly, 800 μg of lysate protein were precleared with normal IgG corresponding to the animal species (rabbit or goat IgG for FAK or nectin-3) of the primary antibody used in the subsequent step (3 μg IgG for each mg of lysate protein) for 2 h. Nonspecific IgG binding-protein complexes were pulled down by incubating with Protein A/G Plus Agarose (Santa Cruz) for 2 h to eliminate nonspecific interacting proteins. Following centrifugation, the clear supernatant (i.e., the precleared lysate) was subjected to IP using the specific primary antibody and incubated for 16 h overnight (4 μg IgG/mg of lysate protein) by mounting the samples in a Glas-Col (Terre Haute, IN) rotator at ~25 rpm. Protein immunocomplexes were then pulled down by Protein A/G Plus Agarose after a 6-h incubation on the Glas-Col rotator. Beads were washed with IP lysis buffer, and proteins were extracted in SDS-sample buffer and resolved by SDS-PAGE. Specific interacting proteins were identified by immunoblotting using antibodies listed in Table 1. Testis lysate protein (100 μg) without Co-IP served as positive control. Normal rabbit or goat IgG in substitution of the primary antibody was used for Co-IP and served as negative control. All steps were performed at room temperature. For immunoblotting, nitrocellulose membranes were blocked in 5% nonfat dry milk (wt/vol) and washed with TBS-0.1% Tween 20 buffer (15.2 mM Tris·HCl, 4.6 mM Tris, pH 7.4 at 22°C, containing 150 mM NaCl, 0.1% Tween 20). Primary and secondary antibodies were prepared using 0.1% BSA (wt/vol) in PBS-Tris (10 mM Tris, 10 mM sodium phosphate, pH 7.4 at 22°C, containing 0.15 M NaCl) and incubated with the blots overnight and 1 h, respectively. Proteins in blots were visualized using in-house enhanced chemiluminescence kits prepared in our laboratory as described (17). Images were acquired using a Fujifilm LAS-4000 Mini Luminescent Image Analyzer using Fujifilm MultiGauge software (version 3.1).
Dual-labeled immunofluorescence and histological analysis.
Frozen sections (7 μm in thickness) of testes obtained in a cryostat at −21°C were fixed with 4% paraformaldehyde in PBS (10 mM sodium phosphate, pH 7.4 at 22°C containing 0.15 M NaCl) at room temperature for 10 min and permeabilized by using 0.1% Triton X-100 in PBS for 10 min. Sections were then blocked by 1% (wt/vol) BSA in PBS and incubated with specific primary antibodies (see Table 1) overnight, to be followed by an incubation of 1 h with Alexa-Fluor-conjugated secondary antibodies (Invitrogen). The specificity of the antibodies used for fluorescence microscopy as reported herein was either characterized earlier, such as anti-Arp3 (12), anti-Eps8 (13), and anti-palladin (25), or assessed by immunoblotting (e.g., nectin-2, nectin-3). Negative controls include the substitution of primary antibody with normal IgG of the same animal species or an omission of the secondary antibody. For F-actin staining, sections were incubated with FITC-conjugated phalloidin (1:75 in 1% BSA-PBS; Sigma-Aldrich) together with secondary antibody. Nuclei were visualized with Prolong Gold antifade reagent with 4′,6-diamidino-2-phenylindole (Invitrogen). For histological analysis, testes were fixed in Bouin's fixative, embedded in paraffin, sectioned with a microtome, and stained with hematoxylin-eosin. Images were acquired using an Olympus BX61 Fluorescence Microscope equipped with a DP71 digital camera, as described earlier (37, 38). Images in TIFF format were analyzed using Adobe PhotoShop in Creative Suite (version 3.0). All imaging data presented here were the results of a representative experiment with n = 7 rats from two independent experiments (i.e., 3 rats in one experiment and 4 rats in a second experiment), which yielded similar results. All testes within an experimental group, such as pCI-neo/FAK, pCI-neo/FAK Y397E vs. pCI-neo/control (Ctrl), were analyzed simultaneously in an experimental session to avoid interexperimental variations.
Image analysis to assess effects of overexpression of FAK Y397E phosphomimetic mutant in testes in vivo on target protein expression.
In experiments where we examined the effects of overexpression of FAK Y397E phosphomimetic mutant vs. pCI-neo empty (control) vector (or pCI-neo/FAK in selected experiments) on the expression of a selected target protein (e.g., Arp3, Eps8, palladin, nectin-2, and nectin-3) that impeded spermatid adhesion transport, frozen sections of testes were used for dual-labeled immunofluorescence analysis of the target protein (red fluorescence) vs. F-actin (stained by FITC-phalloidin) (green fluorescence). The intensity of fluorescence signals of the target protein (red fluorescence) at the apical ES surrounding the head of step 19 spermatids at the edge of the tubule lumen was quantified using ImageJ 1.45 (http://rsbweb.nih.gov/ij; U.S. National Institutes of Health, Bethesda, MD) in the pCI-neo/FAK Y397E (or pCI-neo/FAK in selected experiments) vs. the pCI-neo empty vector control groups. Initial pilot experiments have shown that these effects were limited to stage VII–VIII tubules. Thus, about 20 elongated spermatids from 4 different tubules at either stage VII or VIII were randomly selected and scored with n = 4 rat testes (i.e., a total of ∼300 spermatids in stage VII or VIII tubules). The fluorescence level of a target protein in stage VII tubules from pCI-neo/Ctrl rat testes was arbitrarily set at one, against which statistical comparison was performed between treatment and control groups in stage VII and VIII tubules, which thus yield information on stage-specific expression of the target protein, and these data can be for statistical analysis.
Assessing changes in the status of spermatogenesis following overexpression of FAK Y397E mutant in the testis in vivo.
To assess changes in the status of spermatogenesis after transfection of pCI-neo/FAK, pCI-neo/FAK Y397E mutant vs. empty pCI-neo vector in adult testes, testes were used for both fluorescence microscopy using frozen sections as well as histological analysis using paraffin sections with hematoxylin/eosin staining for analysis. Besides changes in the spatiotemporal and stage-specific expression of selected actin regulatory proteins (e.g., Arp3, Eps8, palladin) and adhesion proteins (e.g., nectin-2, nectin-3) at the apical ES that were noted in pilot experiments based on dual-labeled immunofluorescence analysis, defects in spermiation were also detected based on histological analysis. Defects in spermiation were assessed by examining ∼80 randomly selected late stage VIII and also stage IX–X tubules from cross sections of a rat testis, and a total of at least four rats was examined with a total of ∼300 stage VIII and IX–X tubules scored. A tubule was scored and annotated as having defects in spermiation when at least five elongating/elongated spermatids were retained in and/or entrapped within the seminiferous epithelium in a late VIII or stage IX–X tubule when spermiation had occurred. Data were expressed as percentage of defective tubules in testes from rats transfected with either FAK or FAK Y397E mutant vs. empty vector.
Statistical analysis.
All data presented herein were results of at least three independent experiments using different animals unless otherwise specified. Statistical significance was determined by one-way ANOVA followed by Dunnett's procedure using SigmaStat software (version 3.5).
RESULTS
Overexpression of p-FAK-Tyr397 using a phosphomimetic mutant FAK Y397E in vivo impedes spermiation in the rat testis.
Phosphomimetic FAK Y397E or FAK cloned into the pCI-neo mammalian expression vector as earlier described (14) vs. empty pCI-neo vector alone was used to transfect rat testes in vivo. Overexpression of FAK in the testis in both pCI-neo/FAK and pCI-neo/FAK Y397E groups vs. control (pCI-neo/Ctrl, vector alone) was confirmed by Co-IP using anti-FAK IgG (Fig. 1A), and these data were also summarized in a histogram (see Fig. 1A, inset), illustrating the efficacy of the in vivo overexpression. The efficacy using this approach was estimated to be 30.64 ± 0.53% (n = 360 tubules randomly selected from 3 rats, with 120 tubules from each rat testis scored by fluorescence microscopy) when rat testes were transfected with pCI-neo/DsRed2 and the number of tubules with red fluorescence was scored (Fig. 1B). When a total of ∼250 tubules at stage VIII–IX was randomly scored from testes of n = 5 rats (∼50 tubules at stage VIII–IX in each rat were scaored), ∼30% of stage VIII and 25% of stage IX–X tubules in testes overexpressed with the FAK Y397E mutant were found to display defects in spermiation (Table 3). Interestingly, while similar defects in spermiation were also noted in the FAK-overexpressed group, the percentages of tubules in the corresponding stage vs. the control group that were affected were ∼2.5- to 4-fold less compared with the FAK Y397E phosphomimetic mutant group (Table 3). The findings shown in Table 3 were summarized in Fig. 1, C and D; the defects of spermiation were clearly noted in late stage VIII and stage IX tubules in which step 19 spermatids were found to be “trapped” inside the seminiferous epithelium that failed to be transported to the adluminal compartment to be released at spermiation (see Fig. 1, C and D) in rats overexpressed with the FAK Y397E phosphomimetic mutant vs. FAK and control rats when cross sections of testes were examined by immunofluorescence microscopy (Fig. 1C) or histological analysis (Fig. 1D). These findings are also consistent with earlier reports that p-FAK-Tyr397 was crucial to maintain spermatid adhesion at stage VII-early VIII of the epithelial cycle (2, 29) when its expression at the apical ES was high at these stages but it was rapidly downregulated at late stage VIII at spermiation, illustrating its overexpression might impede spermatid adhesion and transport. Indeed, overexpression of the FAK Y397E phosphomimetic mutant that mimicked p-FAK-Tyr397 induced defects in spermiation in which elongated spermatids were entrapped in the epithelium as shown in Fig. 1, C and D, and Table 3. These findings also implicate that there was a defect in actin cytoskeleton dynamics in which actin filament bundles failed to undergo necessary reorganization to be converted to their debundled/branched configuration to facilitate spermatid transport and its release at spermiation. This thus prompted us to examine if there were any changes in the spatiotemporal expression of Arp3, Eps8, and palladin in stage VII and early stage VIII tubules in rats from the treatment vs. the control groups.
Fig. 1.
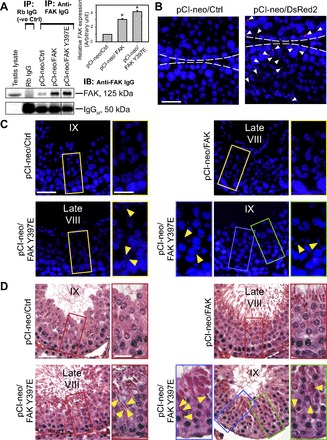
Overexpression of phosphorylated (p) focal adhesion kinase (FAK) p-FAK-Tyr397 via the use of FAK Y397E phosphomimetic mutant in rat testes in vivo impairs spermiation. Testes were transfected with plasmid DNA as described in materials and methods with n = 7 rats/time point in both treatment vs. control groups. A: the efficacy of overexpression was verified using coimmunoprecipitation (Co-IP) using an anti-FAK antibody (see Table 1) vs. normal rabbit (Rb) IgG (negative control) using 800 μg protein for each sample. Normal testis lysate (100 μg protein) without Co-IP served as a positive control. Histogram-summarized Co-IP results are shown in inset in A; each data point is a mean ± SD of n = 3, normalized against the corresponding IgG heavy chain (IgGH) level, of which the FAK protein level in pCI-neo/control (Ctrl) was arbitrary set at 1, against which statistical comparison was performed. *P < 0.05 vs. pCI-neo/Ctrl. B: in parallel experiments, rat testes (n = 3 rats) were transfected with either empty vector (control) vs. pCI-neo/DsRed2 vector to assess the efficacy of transfection. Positive transfection was confirmed by the presence of red fluorescence (DsRed2) in the epithelium (annotated by white arrowheads) by scoring 360 randomly selected tubules with 120 tubules from each rat testis of 3 rats in both groups (see B), with 30.64 ± 0.53% tubules found to have DsRed2 staining in tubules. “White” broken line annotates the relative location of the basement membrane in the tunica propria. Scale bar = 80 μm, which applies to both micrographs. C: frozen cross sections of testes from these rats were obtained in which cell nuclei were visualized by 4′,6-diamidino-2-phenylindole (DAPI). Elongated spermatids were entrapped deep inside the epithelium in late stage VIII and IX tubules from testes with overexpressed FAK Y397E phosphomimetic mutant (see “yellow” arrowheads), but not in testes overexpressed with empty vector (control) and also FAK. Boxed areas in micrographs were magnified and shown in adjacent images. Scale bar = 100 and 30 μm in magnified image, which apply to corresponding images in the panel. D: defects in spermiation were confirmed using paraffin sections of testes stained with hematoxylin-eosin. No obvious changes in spermatogenesis were detected in testes overexpressed with pCI-neo vector alone and also FAK. However, overexpression of FAK Y397E phosphomimetic mutant in testes led to defects in spermiation detected in considerably more tubules, ∼2.5-fold more than the pCI-neo/FAK group in which elongated spermatids remained entrapped in the epithelium in late stage VIII and IX tubules (see yellow arrowheads annotating entrapped spermatids in the epithelium). Boxed areas in micrographs were magnified and shown in adjacent images. Scale bar = 100 and 30 μm in magnified image, which apply to corresponding images in this panel.
Table 3.
Phenotypic changes in the seminiferous epithelium of rat testes following overexpression of FAK or FAK mutant Y397E vs. control rats
| Defects in Spermiation* |
||
|---|---|---|
| Treatment | Late stage VIII | Stage IX–X |
| pCI-neo/FAK vs. pCI-neo/Ctrl, % | 8.28±3.85 | 10.12±2.87 |
| pCI-neo/FAK Y397E vs. pCI-neo/Ctrl, % | 30.69±4.75 | 25.46±1.69 |
Each data point is a mean ± SD of 250 tubules with at least 50 randomly selected tubules from each testis scored from n = 5 rats.
Cross-sections of tubules that contained more than 5 elongated spermatids remained trapped in the seminiferous epithelium and failed to be released at stage VIII were scored as tubules with defects in spermiation, such as those shown in Fig. 1.
p-FAK-Tyr397 regulates apical ES via its effects on F-actin organization mediated by spatiotemporal expression of Arp3, Eps8, and palladin.
In normal rat testes, it was shown that the expression of p-FAK-Tyr397 was predominantly and exclusively expressed at the apical ES at the Sertoli-spermatid (step 19) interface at stage VII-early VIII tubules (29), being used to confer spermatid adhesion until late stage VIII when its expression was considerably reduced to facilitate the release of mature spermatids (i.e., spermatozoa) at spermiation (2, 4, 22, 29). If this postulate is correct, the prolonged existence of p-FAK-Tyr397 at the apical ES via an overexpression of its phosphomimetic mutant FAK Y397E should impede spermatid adhesion/transport and spermiation. In fact, data shown in Fig. 1 were shown to support this postulate since overexpression of FAK Y397E phosphomimetic mutant was effective in inducing defects in spermiation. We next performed studies to understand the molecular mechanism underlying these changes, and we speculated that p-FAK-Tyr397 mediates its effects via changes in the actin cytoskeleton dynamics at the apical ES. Arp3 (12), Eps8 (13), and palladin (25) were shown to display distinctively different patterns of spatiotemporal expression at the ES in stage VII–VIII tubules; we thus examined changes in their spatiotemporal expression at the apical ES at these stages in FAK Y397E phosphomimetic mutant vs. control rats overexpressed with pCI-neo empty vector alone. Below are these findings.
Arp3 and F-actin.
In control rat testes, Arp3 was robustly expressed at the apical ES in stage VII tubules but limited to the concave side of the spermatid head where extensively endocytic vesicle-mediated protein trafficking is known to take place, forming a giant endocytic vesicular ultrastructure formerly designated tubulobulbar complex (26, 39, 40), and colocalized with F-actin (filamentous form of actin, visualized by FITC-phalloidin) (Fig. 2A). This specific spatiotemporal expression of Apr3 is necessary to reorganize the F-actin network to a debundled/branched configuration to facilitate endocytic-mediated protein trafficking (1). Thus, “old” apical ES proteins can be recycled to the newly formed step 8 spermatids that arise at stage VIII to assemble “new” apical ES. However, Arp3 is absent in the blood-testis barrier (BTB) so that actin filament bundles can be maintained at the basal ES to confer BTB integrity. In stage VIII tubules, the expression of Arp3 at the apical ES was downregulated considerably to a level virtually nondetectable to prepare for the release of sperm at spermiation since it is no longer needed to confer actin dynamics at the site, but its expression was upregulated at the BTB to facilitate BTB restructuring (Fig. 2A). In testes with overexpression of FAK Y397E phosphomimetic mutant, Arp3 expression was mildly downregulated in stage VII tubules vs. controls; however, Arp3 persisted at the apical ES in stage VIII tubules vs. controls, rendering these tubules analogous to stage VII tubules (Fig. 2A). Furthermore, Arp3 was no longer restricted to the concave side of the spermatid head. Instead, Arp3 was localized further away from the apical ES site, no longer confined to the tip of the spermatid head, and not entirely colocalized with F-actin (Fig. 2A). Because of the absence of Arp3 at this site, F-actin persisted at the apical ES in stage VIII tubules vs. control testes, making these tubules more similar to stage VII tubules regarding the functional status of the F-actin network (Fig. 2A). These findings were also summarized in which the Arp3 signals at the apical ES shown in Fig. 2A were semiquantitatively analyzed by image analysis and shown in Fig. 2B. We next investigated the biomolecules that maintained F-actin at the apical ES in testes with overexpressed FAK Y397E phosphomimetic mutant.
Fig. 2.
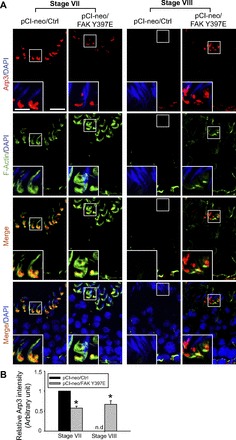
FAK Y397E phosphomimetic mutant-induced defects in spermiation in the rat testis are mediated by disrupting the stage-specific and spatiotemporal expression of actin-related protein 3 that induces branched actin polymerization (Arp3) and F-actin at the apical ES. A: in testes overexpressed with pCI-neo empty vector (pCI-neo/Ctrl), Arp3 (red) was high at the apical ES in stage VII tubules, but it was restricted to the concave side of the spermatid head, colocalized with F-actin (green), where the endocytic vesicle mediated protein trafficking known to occur to facilitate protein endocytosis, transcytosis, and recycling, so that “old” apical ES proteins can be used to assemble “new” apical ES for newly formed step 8 spermatids in stage VIII tubules. Thus, the upregulation of Arp3 at this site facilitated endocytic trafficking events. However, Arp3 expression was virtually nondetectable at the basal ectoplasmic specialization (ES) of the blood-testis barrier (BTB) when the BTB was intact. However, in stage VIII tubules, Arp3 expression was high at the basal ES of the BTB, colocalizing with F-actin, to facilitate BTB restructuring to allow the transport of preleptotene spermatocytes across the BTB. Arp3 expression at the apical ES was considerably diminished in stage VIII tubules to an almost nondetectable level, and F-actin was also barely visible at the apical ES, which thus facilitated spermiation. While overexpression of pCI-neo/FAK Y397E did not perturb the expression nor distribution of Arp3 and F-actin considerably at the apical ES in stage VII tubules, its overexpression upregulated Arp3 expression and retained a considerable amount of F-actin at the apical ES, rendering stage VIII tubules to mimic stage VII tubules regarding the expression and/or localization of Arp3 and F-actin at the apical ES. Cell nuclei visualized by DAPI. Scale bar = 60 and 15 μm in inset, which apply to corresponding micrographs and insets. B: image analysis of Arp3 (red) at the apical ES surrounding the head of step 19 spermatids (n = 300 spermatids from 4 rat testes) was performed (see materials and methods) in which the level of Arp3 in pCI-neo/Ctrl in stage VII tubules was arbitrarily set at 1. pCi-neo/FAK Y397E was compared with the control group in stage VII or VIII tubules. *P < 0.05; nd, nondetectable.
Eps8 and F-actin.
In control testes (pCI-neo/Ctrl), Eps8 was robustly expressed at the apical ES, colocalized with F-actin in stage VII tubules; however, its expression diminished considerably to a level virtually nondetectable in stage VIII tubules to facilitate spermiation (Fig. 3A). Following overexpression of the FAK Y397E phosphomimetic mutant, Eps8 expression persisted through stage VIII, colocalizing with F-actin, being used to maintain the actin filament bundles at the apical ES, and thereby conferring the apical ES integrity (Fig. 3A). Data shown in Fig. 3A were also expressed semiquantitatively by image analysis of Eps8 signals at the apical ES in both stage VII and VIII tubules in FAK Y397E phosphomimetic vs. control rat testes and shown in Fig. 3B. This thus explains the persistence of the F-actin network at the apical ES in stage VIII tubules following overexpression of the FAK Y397E phosphomimetic mutant vs. control testes (Fig. 3 vs. Fig. 2) since Eps8 was used to maintain the actin filament bundles, namely the F-actin network, at the apical ES.
Fig. 3.
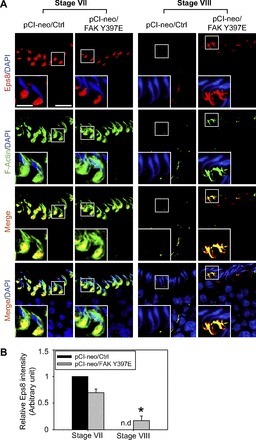
FAK Y397E phosphomimetic mutant-induced defects in spermiation in the rat testis is mediated by disrupting stage-specific and spatiotemporal expression of epidermal growth factor receptor pathway substrate 8 (Eps8) and F-actin at the apical ES. A: in control testes (pCI-neo/Ctrl), Eps8 (red) was highly expressed at the apical ES in stage VII tubules, colocalizing with F-actin (green), to maintain the integrity of actin filament bundles at the apical ES. However, Eps8 was considerably diminished to a level almost nondetectable (nd) at stage VIII, along with diminishing F-actin to facilitate spermiation. While overexpression of pCI-neo/FAK Y397E did not impede the expression and distribution of Eps8 in stage VII tubules, its overexpression retained the expression of Eps8 and also F-actin in stage VIII tubules, as well as their colocalization. This thus impeded spermiation, retaining elongated spermatids in the epithelium as shown in Fig. 1, C and D. Cell nuclei visualized by DAPI. Scale bar = 60 and 15 μm in inset, which apply to corresponding micrographs and insets. B: image analysis of Eps8 (red) at the apical ES surrounding the head of step 19 spermatids (n = 300 spermatids from 4 rat testes) in which the level of Eps8 in pCI-neo/Ctrl in stage VII tubules was arbitrarily set at 1. pCi-neo/FAK Y397E was compared with the control group in either stage VII or VIII tubules. *P < 0.05.
Palladin and F-actin.
To better understand the persistence of F-actin at the apical ES in stage VIII tubules following overexpression of FAK Y397E phosphomimetic mutant, we next examined changes in the spatiotemporal expression of palladin and F-actin (Fig. 4). In control testes expressed with empty pCI-neo vector, palladin was expressed in stage VII tubules that cover both the convex and the concave side of the elongated spermatids, colocalizing with F-actin; however, in stage VIII tubules, palladin restricted its expression mostly to the tip of the spermatid head when F-actin reorganized, converting to G-actin and diminising to a level virtually nondetectable at stage VIII (Fig. 4A), and these findings are consistent with a recent report (25). However, following overexpression of the FAK Y397E phosphomimetic mutant, while the distribution of palladin at the apical ES surrounding the spermatid head in stage VII tubules was similar to control testes, palladin at the spermatid head in stage VIII tubules was mostly localized to the tip of the head, robustly expressed at the convex side, associated with F-actin (Fig. 4A). These findings thus suggest that palladin was used to maintain the F-actin at the apical ES via changes in their distribution, not an upregulation in its expression, following overexpression of FAK Y397E phosphomimetic mutant, converting stage VIII tubules analogous to VII tubules regarding the organization of F-actin at the apical ES, even though the overall expression of palladin at the apical ES in VII–VIII tubules following overexpression of FAK Y397E in the rat testis remained relatively constant (Fig. 4B).
Fig. 4.
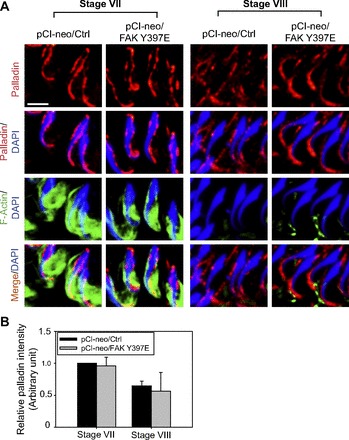
FAK Y397E phosphomimetic mutant-induced defects in spermiation in the rat testis are mediated by disrupting the stage-specific and spatiotemporal expression of palladin and F-actin at the apical ES. A: in control testes (pCI-neo/Ctrl), palladin (red) was highly expressed at the apical ES in stage VII tubules, colocalized with F-actin (green), that covered almost the entire head of elongated spermatids to maintain the integrity of the bundled actin filaments at the apical ES to confer adhesion. At stage VIII, palladin expression diminished and its localization shifted mostly to the tip (and on the convex side) of the elongated spermatid head, along with diminishing F-actin to facilitate spermiation. While overexpression of FAK Y397E phosphomimetic mutant did not appear to impede the expression of palladin and F-actin organization in stage VII tubules, its overexpression retained palladin at the tip (and on the convex side) of the elongated spermatid head, and also retained some F-actin, in stage VIII tubules as well as their partial colocalization. These changes thus retained actin filament bundles at the apical ES, which in turn impeded spermiation, retaining elongated spermatids in the seminiferous epithelium as shown in Fig. 1, C and D. Scale bar = 15 μm, which applies to other micrographs. B: this histogram summarizes results of image analysis of the fluorescence intensity of palladin (red) surrounding the head of step 19 spermatids in stage VII and VIII tubules such as those shown in A with n = 300 spermatids from 4 rat testes in which the level of palladin in pCI-neo/Ctrl in stage VII tubules was arbitrarily set at 1. pCi-neo/FAK Y397E was compared with the control group in either stage VII or VIII tubules. No statistically significant difference was found.
Changes in the spatiotemporal expression of nectin-2 and -3 in rat testes following overexpression of FAK Y397E phosphomimetic mutant.
To better understand the molecular mechanism(s) by which step 19 spermatids failed to undergo proper transport to complete spermiation due to defects of the apical ES in testes overexpressed with FAK Y397E phosphomimetic mutant, we examined the spatiotemporal expression of two apical ES adhesion proteins: nectin-2 and -3 at stage VII and early VIII tubules. It is noted that nectin-2 is known to be expressed by both Sertoli and spermatids, whereas nectin-3 is an exclusive late spermatid adhesion protein at the apical ES, and they can form nectin-2/nectin-2 or nectin-3/nectin-2 interactions at the spermatid/Sertoli cell interface (11, 23).
Nectin-2 and F-actin.
Nectin-2 was expressed at the apical ES in stage VII and early VIII tubules at the convex side of elongated spermatids (Fig. 5), consistent with earlier reports (11, 23). However, an increase in nectin-2 expression was detected in rat testes overexpressed with FAK Y397E phosphomimetic mutant at stage VII and VIII, suggesting nectin-2 was being used to confer adhesion at the apical ES (Fig. 5A). Rat testes with overexpressed FAK Y397E phosphomimetic mutant were found to have an upregulation of nectin-2 expression, and its staining was robust at the apical ES vs. control rats, mostly restricted to the convex side of the spermatid head (Fig. 5, A and B), partially colocalized with F-actin in stage VII–VIII tubules (Fig. 5B).
Fig. 5.
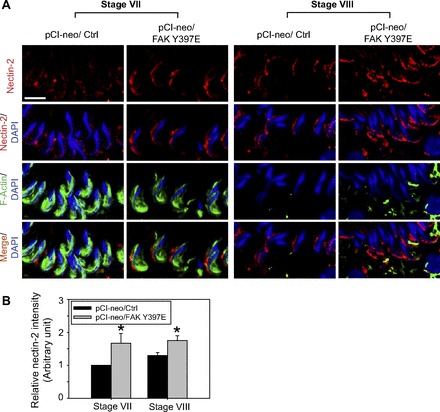
Overexpression of FAK Y397E phosphomimetic mutant in the rat testis in vivo upregulates the expression and alters the localization of nectin-2 at the apical ES in stage VII–VIII tubules. A: in control testes (pCI-neo/Ctrl), nectin-2 (a cell adhesion protein expressed by both Sertoli cell and spermatid at the apical ES) (red fluorescence) was expressed at the apical ES in stage VII tubules, partially colocalized with F-actin (green fluorescence), restricted almost exclusively to the convex side of the spermatid head, which become more predominant at the site in stage VIII tubules. Following overexpression of FAK Y397E phosphomimetic mutant, nectin-2 expression was upregulated in both stage VII and VIII tubules, largely restricted to the convex side of the spermatid head and partially colocalized with F-actin. Nuclei were visualized with DAPI (blue). Scale bar = 15 μm, which applies to other micrographs. B: this histogram summarizes results shown in A, illustrating the intensity of nectin-2 signals at the apical ES in the head of step 19 spermatids. Each bar is a mean ± SD of 300 randomly selected step 19 spermatids from 4 rat testes in which the level of nectin-2 in pCI-neo/Ctrl in stage VII tubules was arbitrarily set at 1. pCi-neo/FAK Y397E was compared with the control group in either stage VII or VIII tubules. *P < 0.05.
Nectin-3 and F-actin.
In normal rodent testes, nectin-3 was restricted to the apical ES, at the convex side of the spermatid head and robustly expressed in stage VII tubules; the expression of nectin-3 was considerably downregulated at stage VIII to a level virtually undetectable vs. stage VII, apparently being used to facilitate spermiation that occurred at late stage VIII (11, 23), and rat testes overexpressed with pCI-neo vector alone were consistent these findings (Fig. 6, A and B). In rat testes with overexpressed FAK, there was a mild upregulation of nectin-3 at the apical ES in stage VIII tubules vs. control testes transfected with empty vector even though no detectable change was noted in stage VII tubules (Fig. 6, A and B). While overexpression of FAK Y397E phosphomimetic mutant in the testis also did not alter the expression nor the pattern of distribution of nectin-3 at the apical ES at stage VII (Fig. 6A), it was found to induce overexpression of nectin-3 considerably, mostly localized to the convex side, nearing the tip of the spermatid head, partially colocalized with F-actin at stage VIII (Fig. 6, A and B). These changes in nectin-3 distribution pattern and expression rendered the stage VIII tubules more similar to stage VII tubules (Fig. 6A), and the persistent nectin-3 expression at the apical ES thereby conferred spermatid adhesion, perturbing spermatid transport, leading to defects in spermiation (Fig. 6B; also see Table 3). Because nectin-3 is an elongated spermatid-specific adhesion protein, we had used Co-IP to quantify if there was an increase in the steady-state level of nectin-3 in rat testes overexpressed with FAK and FAK Y397E vs. control (empty vector). Indeed, an increase in nectin-3 expression was detected in both groups vs. control (Fig. 6, C and D). It is noted that such an increase, by ∼1.5- to 2.5-fold for the FAK and FAK Y397E phosphomimetic mutant rat testes vs. control (Fig. 6D), was likely to be an underestimation since the efficacy of transfection using pCI-neo vector was estimated to be ∼30% (Fig. 1B).
Fig. 6.
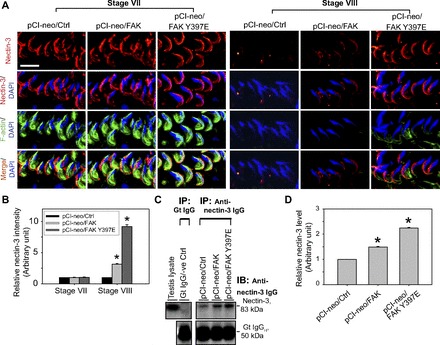
FAK Y397E mutant-induced defects in spermiation in the rat testis are mediated by retention of nectin-3 at the apical ES. A: changes in the stage-specific and spatiotemporal expression of nectin-3 (red) and F-actin (green) in the seminiferous epithelium following overexpression of pCI-neo/FAK Y397E and pCI-neo/FAK vs. control (pCI-neo empty vector) testes. In control rat testes, nectin-3, an adhesion integral membrane protein at the apical ES expressed exclusively by elongating/elongated spermatids, was prominently expressed at the apical ES in stage VII tubules, most predominantly at the convex side of the entire spermatid head and few staining at the concave side. However, the expression of this adhesion protein was considerably downregulated and virtually undetectable at the apical ES in stage VIII tubules, correlating with the onset of spermiation. However, in rat testes with overexpression of FAK and FAK Y397E phosphomimetic mutant, while changes in nectin-3 in stage VII tubules in both groups vs. the control empty vector group were negligible, a disruption on the stage-specific and spatiotemporal expression of nectin-3 in FAK-overexpressed rat testes was detected, and this effect was considerably more pronounced in the FAK Y397E phosphomimetic mutant rat testes. Nectin-3 remained highly expressed in late stage VIII tubules, colocalized partially with F-actin in pCI-neo/FAK Y397E testes. Nuclei were visualized with DAPI (blue). Scale bar = 15 μm, which applies to other micrographs. B: this histogram summarizes results shown in A illustrating the intensity of nectin-3 signals (red) at the apical ES in step 19 spermatids. Each bar is a mean ± SD of 300 randomly selected spermatids from 4 rat testes (i.e., ∼80 spermatids/rat testis) in which the level of nectin-3 in pCI-neo/Ctrl in stage VII tubules was arbitrarily set at 1. pCI-neo/FAK and pCI-neo/FAK Y397E were compared with the control group in either stage VII or VIII tubules. *P < 0.05. C: to further validate data shown in A and B and due to the limited protein loading capacity of SDS-polyacrylamide gel (∼200 μg protein/lane), nectin-3 restricted to step 18–19 spermatids in the rat testis was pulled down by Co-IP with ∼800 μg protein/sample using an anti-nectin-3 IgG (Table 1), and the immunocomplexes were used for immunoblotting (IB). Data were analyzed and shown in D. *P < 0.05.
DISCUSSION
In the rat testis, germ cells remain attached to the Sertoli cell in the seminiferous epithelium at a Sertoli-to-germ cell ratio of ∼1:30–1:50 (33) during the epithelial cycle of spermatogenesis to obtain nourishment and structural supports since a significant number of these germ cells, in particular postmeiotic spermatids, are metabolically quiescent cells. These supports are mediated via gap junctions and perhaps intercellular bridges at the Sertoli-germ cell interface, including spermatogonia, spermatocytes, and step 1–7 spermatids (20, 32). Once step 8 spermatids appear at stage VIII of the epithelial cycle, a testis-specific AJ known as apical ES arises, which replaces desmosome and gap junction to become the only anchoring device, and it persists through step 19 spermatids, until it is degenerated to allow the release of sperm at spermiation (5, 6, 22). The apical ES is typified by the presence of an array of actin filament bundles that lie perpendicular to the Sertoli cell plasma membrane apposing the step 8–19 spermatid plasma membrane and are sandwiched in between the cisternae of endoplasmic reticulum and the Sertoli cell plasma membrane, surrounding almost the entire head of spermatids that confers both adhesion and polarity to spermatids (6, 32, 35). Because of these unique actin filament bundles that confer adhesive strength to the ES, the ES was shown to be one of the strongest anchoring junctions in mammalian epithelia, at least two times as strong as desmosomes when the force required to disrupt these junctions was quantified and compared (34). While the apical ES is an exceedingly strong adhesion junction, it is a highly dynamic ultrastructure since it must be rapidly reorganized to accommodate the transport of step 8–19 spermatids across the seminiferous epithelium during spermiogenesis. It is thus conceivable that these actin filament bundles at the apical ES require continuous but highly restrictive spatiotemporal breakdown and reorganization via cycles of “debundling” and “rebundling,” such that spermatids can be transported across the seminiferous epithelium while remaining attached to the Sertoli cell. Recent studies have shown that such extensive reorganization of the F-actin network at the apical ES is regulated via stage-specific and spatiotemporal expression of two major groups of actin regulatory proteins in the seminiferous epithelium: 1) actin bundling Eps8 (also an actin-barbed end-capping protein) (13) and palladin (also an actin cross-linking protein) (25); and 2) branched actin polymerization protein Arp3 (12), effectively converting actin filaments from their bundled to their “branched/debundled” configuration and vice versa.
While these recent findings have shed insightful information on the regulation of apical ES dynamics during spermatogenesis, such as the role of actin-bundling proteins Eps8 and palladin, and actin branched polymerization/debundling regulatory proteins Arp3 and drebrin E, the underlying molecular mechanism(s) and the involving biomolecule(s) that trigger these events remain unknown. Herein, we demonstrate that p-FAK-Tyr397 is one of the major players in these events. Unlike other epithelia in which FAK is restricted to the cell-extracellular matrix interface known as the focal adhesion complex (FAC) or focal contact that mediates integrin-based signaling to regulate cell movement (3, 7), p-FAK-Tyr397 was first demonstrated to be a component of the apical ES (2, 29) that displayed stage-specific and spatiotemporal expression in the rat testis (29). For instance, p-FAK-Tyr397 was upregulated at the apical ES and most predominant in stage VI–VII tubules; thereafter, its expression was reduced somewhat in early stage VIII tubules and became virtually undetectable by late stage VIII (29), suggesting that it might be used to confer spermatid adhesion until late in stage VIII before the release of sperm at spermiation. This concept has now been confirmed in the in vivo studies reported herein. p-FAK-Tyr397, via the overexpression of a FAK Y397E phosphomimetic mutant in the adult rat testis, was found to cause defects of spermiation in stage VIII tubules. This is possibly mediated by a retention of step 19 spermatid adhesion on the Sertoli cell in the epithelium, leading to a failure of spermatid transport in the epithelium. This concept is supported by the observations reported herein in which the expression of actin-bundling proteins Eps8 and palladin persisted at the apical ES in stage VIII tubules in testes with overexpressed FAK Y397E phosphomimetic mutant, and also Arp3 at the apical ES site where endocytic vesicle-mediated protein trafficking took place when these proteins should have been downregulated to a level virtually nondetectable in stage VIII in normal rat testes, such as in testes with overexpressed empty pCI-neo vector. In short, these stage VIII tubules, based on the morphological outlay of elongated spermatid heads vs. stage VII tubules, were functionally analogous to stage VII tubules regarding the functional status of their actin filament bundles, perturbing spermatid transport and adhesion that led to a defect in spermiation in which many spermatids failed to be transported to the luminal edge of the tubule lumen for spermiation; instead, many elongated spermatids remained entrapped in the epithelium in late stage VIII–X tubules. This postulate was further supported by the presence of the nectin-2-F-actin and nectin-3-F-actin complex at the apical ES. Such delay in the physiological downregulation of nectin-2 and -3 at the apical ES and the persistence of F-actin at the site are likely mediated by the continual upregulation of Eps8 and palladin at the ES, which confer the integrity of the actin filament bundles. In summary, overexpression of p-FAK-Tyr397 via the use of FAK Y397E phosphomimetic mutant appears to prolong the duration of stage VII, delaying its transition into stage VIII by upregulating nectin-2 and -3, as well as Eps8 and palladin. As such, elongated spermatids failed to undergo and to complete spermiation in FAK Y397E phosphomimetic-overexpressed testes vs. control testes. These findings thus suggest that p-FAK-Tyr397, which is highly expressed in stage VI–VII tubules, regulates spermatid adhesion by maintaining the stage-specific and spatiotemporal expression of Eps8, paladin, and Arp3 at the apical ES, so that actin-based cytoskeleton can be used as the attachment site by the cell adhesion proteins nectin-2 and -3.
GRANTS
This work was supported by National Institute of Child Health and Human Development Grants U54-HD-029990, Project 5, to C. Y. Cheng and R01-HD-056034 to C. Y. Cheng; National Science Foundation of China/Research Grants, Council of Hong Kong Joint Research Scheme (N_HKU 717/12), to W. M. Lee and Committee on Research and Conference Grants Seed Funding of the University of Hong Kong to W. M. Lee; and The Hong Kong General Research Fund HKBU261812 to C. K. Wong.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: H.-T.W., S.Y.T.L., K.-W.M., and C.Y.C. performed the experiments; H.-T.W., D.D.M., S.Y.T.L., K.-W.M., W.M.L., C.K.W., and C.Y.C. analyzed the data; H.-T.W., S.Y.T.L., and C.Y.C. prepared the figures; H.-T.W. and C.Y.C. drafted the manuscript; H.-T.W., D.D.M., S.Y.T.L., K.-W.M., W.M.L., C.K.W., and C.Y.C. approved the final version of the manuscript; C.Y.C. interpreted the results of the experiments; C.Y.C. contributed to the conception and design of the research; C.Y.C. edited and revised the manuscript.
REFERENCES
- 1. Anitei M, Hoflack B. Bridging membrane and cytoskeleton dynamics in the secretory and endocytic pathways. Nat Cell Biol 14: 11–19, 2011 [DOI] [PubMed] [Google Scholar]
- 2. Beardsley A, Robertson DM, O'Donnell L. A complex containing α6β1-integrin and phosphorylated focal adhesion kinase between Sertoli cells and elongated spermatids during spermatid release from the seminiferous epithelium. J Endocrinol 190: 759–770, 2006 [DOI] [PubMed] [Google Scholar]
- 3. Boutros T, Chevet E, Metrakos P. Mitogen-activated protein (MAP) kinase/MAP kinase phosphatase regulation: roles in cell growth, death, and cancer. Pharmacol Rev 60: 261–310, 2008 [DOI] [PubMed] [Google Scholar]
- 4. Cheng CY, Mruk DD. The blood-testis barrier and its implication in male contraception. Pharmacol Rev 64: 16–64, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cheng CY, Mruk DD. An intracellular trafficking pathway in the seminiferous epithelium regulating spermatogenesis: a biochemical and molecular perspective. Crit Rev Biochem Mol Biol 44: 245–263, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cheng CY, Mruk DD. A local autocrine axis in the testes that regulates spermatogenesis. Nat Rev Endocrinol 6: 380–395, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cheng CY, Mruk DD. Regulation of blood-testis barrier dynamics by focal adhesion kinase (FAK). An unexpected turn of events. Cell Cycle 8: 3493–3499, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. de Kretser DM, Kerr JB. The cytology of the testis. In: The Physiology of Reproduction Vol 1, edited by Knobil E, Neill JB, Ewing LL, Greenwald GS, Markert CL, Pfaff DW. New York, NY: Raven, 1988, p. 837–932 [Google Scholar]
- 9. Grima J, Zhu LJ, Zong SD, Catterall JF, Bardin CW, Cheng CY. Rat testin is a newly identified component of the junctional complexes in various tissues whose mRNA is predominantly expressed in the testis and ovary. Biol Reprod 52: 340–355, 1995 [DOI] [PubMed] [Google Scholar]
- 10. Hess RA, de Franca LR. Spermatogenesis and cycle of the seminiferous epithelium. Adv Exp Med Biol 636: 1–15, 2008 [DOI] [PubMed] [Google Scholar]
- 11. Inagaki M, Irie K, Ishizaki H, Tanaka-Okamoto M, Miyoshi J, Takai Y. Role of cell adhesion molecule nectin-3 in spermatid development. Genes Cells 11: 1125–1132, 2006 [DOI] [PubMed] [Google Scholar]
- 12. Lie PPY, Chan AYN, Mruk DD, Lee WM, Cheng CY. Restricted Arp3 expression in the testis prevents blood-testis barrier disruption during junction restructuring at spermatogenesis. Proc Natl Acad Sci USA 107: 11411–11416, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lie PPY, Mruk DD, Lee WM, Cheng CY. Epidermal growth factor receptor pathway substrate 8 (Eps8) is a novel regulator of cell adhesion and the blood-testis barrier integrity in the seminiferous epithelium. FASEB J 23: 2555–2567, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lie PPY, Mruk DD, Mok KW, Su L, Lee WM, Cheng CY. Focal adhesion kinase-Tyr407 and -Tyr397 exhibit antagonistic effects on blood-testis barrier dynamics in the rat. Proc Natl Acad Sci USA 109: 12562–12567, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mok KW, Mruk DD, Silvestrini B, Cheng CY. rpS6 regulates blood-testis barrier dynamics by affecting F-actin organization and protein recruitment. Endocrinology 153: 5036–5048, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mruk DD, Cheng CY. Cell-cell interactions at the ectoplasmic specialization in the testis. Trends Endocrinol Metab 15: 439–447, 2004 [DOI] [PubMed] [Google Scholar]
- 17. Mruk DD, Cheng CY. Enhanced chemiluminescence (ECL) for routine immunoblotting. An inexpensive alternative to commercially available kits. Spermatogenesis 1: 121–122, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mruk DD, Cheng CY. An in vitro system to study Sertoli cell blood-testis barrier dynamics. Methods Mol Biol 763: 237–252, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mruk DD, Cheng CY. Sertoli-Sertoli and Sertoli-germ cell interactions and their significance in germ cell movement in the seminiferous epithelium during spermatogenesis. Endocr Rev 25: 747–806, 2004 [DOI] [PubMed] [Google Scholar]
- 20. Mruk DD, Silvestrini B, Cheng CY. Anchoring junctions as drug targets: role in contraceptive development. Pharmacol Rev 60: 146–180, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mruk DD, Wong CH, Silvestrini B, Cheng CY. A male contraceptive targeting germ cell adhesion. Nature Med 12: 1323–1328, 2006 [DOI] [PubMed] [Google Scholar]
- 22. O'Donnell L, Nicholls PK, O'Bryan MK, McLachlan RI, Stanton PG. Spermiation: the process of sperm release. Spermatogenesis 1: 14–35, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ozaki-Kuroda K, Nakanishi H, Ohta H, Tanaka H, Kurihara H, Mueller S, Irie K, Ikeda W, Sakai T, Wimmer E, Nishimune Y, Takai Y. Nectin couples cell-cell adhesion and the actin scaffold at heterotypic testicular junctions. Curr Biol 12: 1145–1150, 2002 [DOI] [PubMed] [Google Scholar]
- 24. Parvinen M. Regulation of the seminiferous epithelium. Endocr Rev 3: 404–417, 1982 [DOI] [PubMed] [Google Scholar]
- 25. Qian X, Mruk DD, Wong EWP, Lie PPY, Cheng CY. Palladin is a regulator of actin filament bundles at the ectoplasmic specialization in the rat testis. Endocrinology 154: 1907–1920, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Russell LD. Further observations on tubulobulbar complexes formed by late spermatids and Sertoli cells in the rat testis. Anat Rec 194: 213–232, 1979 [DOI] [PubMed] [Google Scholar]
- 27. Russell LD. Observations on rat Sertoli ectoplasmic (‘junctional’) specializations in their association with germ cells of the rat testis. Tissue Cell 9: 475–498, 1977 [DOI] [PubMed] [Google Scholar]
- 28. Russell LD, Saxena NK, Weber JE. Intratesticular injection as a method to assess the potential toxicity of various agents to study mechanisms of normal spermatogenesis. Gamete Res 17: 43–56, 1987 [DOI] [PubMed] [Google Scholar]
- 29. Siu MKY, Mruk DD, Lee WM, Cheng CY. Adhering junction dynamics in the testis are regulated by an interplay of β1-integrin and focal adhesion complex (FAC)-associated proteins. Endocrinology 144: 2141–2163, 2003 [DOI] [PubMed] [Google Scholar]
- 30. Su L, Mruk DD, Lie PPY, Silvestrini B, Cheng CY. A peptide derived from laminin-γ3 reversibly impairs spermatogenesis in rats. Nat Communs 3: 1185, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Vallejo AN, Pogulis RJ, Pease LR. PCR mutagenesis by overlap extension and gene SOE. CSH Protoc 2008: pdb.prot4861, 2008 [DOI] [PubMed] [Google Scholar]
- 32. Vogl AW, Vaid KS, Guttman JA. The Sertoli cell cytoskeleton. Adv Exp Med Biol 636: 186–211, 2008 [DOI] [PubMed] [Google Scholar]
- 33. Weber JE, Russell LD, Wong V, Peterson RN. Three dimensional reconstruction of a rat stage V Sertoli cell. II. Morphometry of Sertoli-Sertoli and Sertoli-germ cell relationships. Am J Anat 167: 163–179, 1983 [DOI] [PubMed] [Google Scholar]
- 34. Wolski KM, Perrault C, Tran-Son-Tay R, Cameron DF. Strength measurement of the Sertoli-spermatid junctional complex. J Androl 26: 354–359, 2005 [DOI] [PubMed] [Google Scholar]
- 35. Wong EWP, Cheng CY. Polarity proteins and cell-cell interactions in the testis. Int Rev Cell Mol Biol 278: 309–353, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wong EWP, Mruk DD, Cheng CY. Biology and regulation of ectoplasmic specialization, an atypical adherens junction type, in the testis. Biochem Biophys Acta 1778: 692–708, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wong EWP, Mruk DD, Lee WM, Cheng CY. Par3/Par6 polarity complex coordinates apical ectoplasmic specialization and blood-testis barrier restructuring during spermatogenesis. Proc Natl Acad Sci USA 105: 9657–9662, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wong EWP, Mruk DD, Lee WM, Cheng CY. Regulation of blood-testis barrier dynamics by TGF-β3 is a Cdc42-dependent protein trafficking event. Proc Natl Acad Sci USA 107: 11399–11404, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Young JS, Guttman JA, Vaid KS, Vogl AW. Cortactin (CTTN), N-WASP (WASL), clathrin (CLTC) are present at podosome-like tubulobulbar complexes in the rat testis. Biol Reprod 80: 153–161, 2009 [DOI] [PubMed] [Google Scholar]
- 40. Young JS, Vogl AW. Focal adhesion proteins zyxin and vinculin are co-distributed at tubulobulbar complexes. Spermatogenesis 2: 63–68, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]


