Abstract
cFos activation in the anterior piriform cortex (aPC) occurs in early odor preference learning in rat pups (Roth and Sullivan 2005). Here we provide evidence that the pairing of odor as a conditioned stimulus and β-adrenergic activation in the aPC as an unconditioned stimulus generates early odor preference learning. β-Adrenergic blockade in the aPC prevents normal preference learning. Enhancement of aPC cAMP response element-binding protein (CREB) phosphorylation in trained hemispheres is consistent with a role for this cascade in early odor preference learning in the aPC. In vitro experiments suggested theta-burst-mediated long-term potentiation (LTP) at the lateral olfactory tract (LOT) to aPC synapse depends on N-methyl-d-aspartate (NMDA) receptors and can be significantly enhanced by β-adrenoceptor activation, which causes increased glutamate release from LOT synapses during LTP induction. NMDA receptors in aPC are also shown to be critical for the acquisition, but not expression, of odor preference learning, as would be predicted if they mediate initial β-adrenoceptor-promoted aPC plasticity. Ex vivo experiments 3 and 24 h after odor preference training reveal an enhanced LOT-aPC field excitatory postsynaptic potential (EPSP). At 3 h both presynaptic and postsynaptic potentiations support EPSP enhancement while at 24 h only postsynaptic potentiation is seen. LOT-LTP in aPC is excluded by odor preference training. Taken together with earlier work on the role of the olfactory bulb in early odor preference learning, these outcomes suggest early odor preference learning is normally supported by and requires multiple plastic changes at least at two levels of olfactory circuitry.
Keywords: olfactory, learning, memory, LTP, piriform cortex
rat pups born blind and deaf readily acquire a preference for an odor paired with unconditioned stimuli (US) that mimic maternal care (Sullivan and Hall 1988) or that activate the noradrenergic locus coeruleus system (Sullivan et al. 2000). A single 10-min pairing of a novel odor (to-be-conditioned stimulus or CS) and a US produces an odor preference memory lasting at least 24 h (Sullivan and Leon 1987; Sullivan and Wilson 2003). Such a preference assists the pup in maintaining proximity to the dam and should enhance survival. Human infants demonstrate similar early odor preference learning (Delaunay-El Allam et al. 2010).
The mechanisms underlying this form of learning have been extensively studied in the olfactory bulb. Blocking β-adrenoceptors in the bulb (Sullivan et al. 2000) or blocking N-methyl-d-aspartate (NMDA) receptors (NMDARs) in the bulb (Lethbridge et al. 2012) before training prevents learning but does not prevent expression of an already acquired preference. The β-adrenoceptor agonist isoproterenol infused directly into the bulbs before novel odor exposure produces learning (Lethbridge et al. 2012; Sullivan et al. 2000). These pharmacological results suggest plasticity of the odor representation in the bulb is both necessary and sufficient for early odor preference learning. The bulbar cellular changes associated with learning suggest that increased cAMP response element-binding protein (CREB) phosphorylation in mitral cells (McLean et al. 1999) and synaptic changes in mitral cell connections (Lethbridge et al. 2012), such as an increase in AMPA receptor (AMPAR) responses in the area of the odor representation (Yuan and Harley 2012), are among the modifications that support early odor preference learning.
Odor information from the olfactory bulb is transmitted over the lateral olfactory tract (LOT) to the anterior piriform cortex (aPC), among other sites (Isaacson 2010). The aPC has spatially diffused odor representations (Illig and Haberly 2003; Poo and Isaacson 2009; Stettler and Axel 2009), in contrast to those seen in the olfactory bulb (Takahashi et al. 2004; Wachowiak and Cohen 2001). The more diffused piriform representation is driven by LOT activity from olfactory bulb “hot spots.” Roth and Sullivan (2005) have shown increased cFos activation in both the olfactory bulb and the aPC, following odor preference training in rat pups, but a mechanistic study of the role of aPC in early odor preference learning has not been carried out.
In the present study we target the aPC using the technique of bilateral local infusions to assess its role in early odor preference learning. Follow-up experiments examine in vitro analogs of learning in an LOT-aPC slice preparation. In the last set of experiments, unilateral nostril occlusion during training is used to confine learning to one hemisphere (Kucharski and Hall 1987; Kucharski et al. 1986; Yuan and Harley 2012) and ex vivo studies of aPC slices examine the changes in LOT-evoked field excitatory postsynaptic potentials (fEPSPs) induced at 3 or at 24 h following training when odor preferences are readily demonstrable (Grimes et al. 2011).
MATERIALS AND METHODS
Animals and Ethics Statement
All experimental procedures were approved by the Institutional Animal Care Committee at Memorial University of Newfoundland with adherence to the guidelines set by the Canadian Council on Animal Care. Male and female Sprague-Dawley rat pups (Charles River) were used in this study. Animals were bred and pups were born onsite at the research facility. Litters were culled to 12 pups with equal numbers of males and females on postnatal day 1 (PD1; day of birth is designated PD0). Dams were maintained under a 12-h reverse light-dark cycle with ad libitum access to food and water.
Behavioral Studies
Behavioral experiments were carried out in a temperature controlled room at ∼28°C and followed the standard protocols previously established for early odor preference learning (McLean et al. 1999; Sullivan and Leon 1987) as described below. One-way ANOVAs and post hoc Fisher tests were used to determine statistical significance throughout the experiments.
Odor preference training and testing.
On PD7, pups were assigned to an odor plus stroking (O/S+) or an odor only (O/S−) condition. Pups were removed from the nest and placed on normal, unscented bedding for 10 min. After habituation, pups receiving conditioning training (O/S+) were placed on peppermint-scented bedding (0.3 ml of peppermint in 500 ml of bedding) and vigorously stroked with a paintbrush for 30 s, followed by a 30 s rest, for a total of 10 min. Pups in the nonlearning condition (O/S−) were placed in peppermint-scented bedding for 10 min following the habituation period. These pups were not stroked. Once odor conditioning was complete, pups were returned to the dam.
On PD8, pups were tested for odor preference memory using a two-choice odor preference procedure. The testing apparatus was a stainless steel chamber (30 × 20 × 18 cm) placed over two testing boxes. One box contained peppermint-scented bedding and the other contained normal, unscented bedding. Testing boxes were separated by a 2-cm neutral zone. For testing, pups were removed from the dam and placed in the neutral zone. Pups moved freely for 1 min, while the time spent over each testing box was measured, and were then removed from the test chamber for 1 min. This was repeated for a total of five trials. The average time spent over peppermint bedding was calculated for each pup. Direction preference was eliminated by alternating the orientation of the starting position of the pups in the neutral zone.
Cannula implantation surgery.
On PD6 pups were anesthetized via hypothermia and placed in a stereotaxic apparatus in a skull flat position. A horizontal incision was made just posterior to the eyes so that bregma was visible. Two small holes were drilled ∼2.5 mm anterior and 3.0–3.25 mm bilateral with respect to bregma. Two guide cannulae (Vita Needle, MA) with insect pins were lowered from the surface of the brain to a depth of 5.5–6.0 mm and were anchored to the skull with dental acrylic (Lang Dental). Two sutures were made on either side of the cannula, and pups were placed on warm bedding to recover before being placed back with the dam.
Intracerebral infusions.
Twenty-four hours following surgical cannula implantation, pups underwent odor training. Pups receiving infusions were removed from the dam, and insect pins were removed from the cannulae, which were cleared of any debris from the home cage. The pups were then placed on plastic hexagonal weighing dishes where they were infused with 1 μl of the desired solution at a rate of 0.25 μl/min. Infusions were followed by 6 min of rest to allow for diffusion of the solution into the brain. Pups were then returned to the dam for 10 min before training or testing. Following testing, pups received intracranial infusions of 4% methylene blue dye (Fisher Scientific) and were killed, and the brains were collected and sliced to confirm cannula placement. Pups with misplaced cannula were excluded from data analysis.
Pharmacological agents.
Lidocaine-hydrochloride (4%, dissolved in phosphate buffered saline; Sigma-Aldrich), a reversible sodium channel blocker; muscimol (50 mM, dissolved in saline; Sigma-Aldrich), a GABAA agonist; propranolol hydrochloride (100 μM, dissolved in saline; Sigma-Aldrich), a β-adrenoceptor antagonist; various concentrations of the β-adrenoceptor agonist isoproterenol (5 μM, 50 μM, or 500 μM; dissolved in saline; Sigma-Aldrich); and d-(−)-2-amino-5-phosphonopentanoic acid (d-APV), an NMDAR antagonist (100 μM, dissolved in saline; Tocris), were infused into the aPC at various time points as described in results. Saline infusions were used in control animals.
Reversible nostril occlusion for ex vivo experiments.
Nose plugs were constructed using polyethylene 20 (PE 20) tubing, silk surgical thread, and human hair, as per procedures described previously (Cummings et al. 1997). To insert the plug, a small dab of a sterile local anesthetic, 2% Xylocaine (AstraZeneca), a lidocaine hydrochloride jelly, was applied to the left nostril. Pups were given one min to rest before the plug was gently inserted in the left nostril. After nostril plug insertion pups were placed on unscented bedding to habituate to the nose plug, followed by appropriate odor conditioning training.
pCREB Immunohistochemistry
pCREB immunohistochemistry was performed in two sets of experiments: first, following unilateral infusion of lidocaine into the aPC to test the silencing effect of lidocaine on the pyramidal cells (Fig. 1, C and D); second, following odor preference training to test the activation level of the pyramidal cells in the aPC (Fig. 2).
Fig. 1.
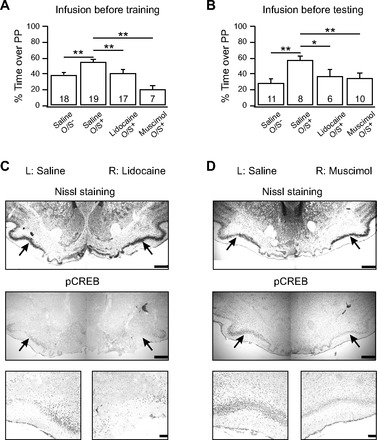
Piriform cortex silencing impairs early odor preference learning. A: lidocaine (4%) or muscimol (50 mM) infusions into the anterior piriform cortex (aPC) 10 min before odor + stroke (O/S+) training. B: lidocaine or muscimol infusion 10 min before odor preference testing. Bars represent percentage of time spent over peppermint-scented bedding during a 2-choice test. Error bars are means ± SE. *P < 0.05; **P < 0.01. C: Nissl staining and immunohistochemistry of phosphorylated cAMP response element-binding protein (pCREB) expression in the aPC 20–30 min following unilateral lidocaine injection. D: Nissal staining and pCREB staining in the aPC 20–30 min following unilateral muscimol infusion. Black arrows in low magnification indicate the pyramidal cell layer where less pCREB staining is evident following lidocaine (C) or muscimol (D) injection compared with saline injection. Higher magnification is shown of a portion of the pyramidal cell layer. Scale bars = 500 μm for low magnification and 100 μm for high magnification.
Fig. 2.
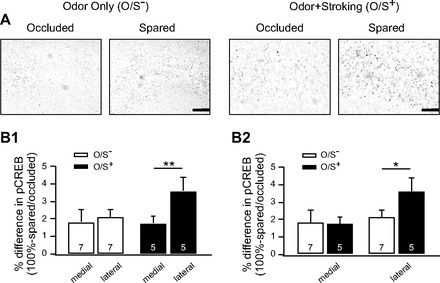
O/S+ training enhances pCREB in the lateral region of the aPC. A: examples of immunohistochemistry of pCREB expression in the aPC of pups who underwent O/S+ or odor only (O/S−) training. Arrows indicate the pyramidal cell layers. Scale bars = 100 μm. B1 and B2: analysis of the relative optic density of pCREB staining in the pyramidal cell layers in spared and occluded hemispheres 10 min following either O/S− or O/S+ training. B1 and B2 are taken from the same animals for comparisons on different aspects. Bars are percentage differences of pCREB expressions. Error bars are means ± SE. *P < 0.05; **P < 0.01.
Brain tissue collections.
TRANSIENT SILENCING USING LIDOCAINE.
On PD6 or 7, following hypothermia and stereotaxic surgeries, pups were infused with methylene blue dye (4%, Fisher Scientific) in one hemisphere and lidocaine (4%, dissolved in methylene blue solution, Fisher Scientific) in the other hemisphere. One microliter of dye solution was slowly administered into one hemisphere by gently pushing the syringe, while 1 μl of lidocaine solution was administered into the opposite hemisphere. After each application, the syringe was held in place for 30 s to ensure solution diffusion into the tissue. Pups were placed on warm bedding to recover for 20–30 min from the time of drug infusion. Animals were placed in a plastic container with pure peppermint oil soaked in a piece of tissue for 10 min. Pups were then anaesthetized with chloral hydrate intraperitoneally (400 mg/kg; Fisher Scientific) and perfused transcardially with saline (0.9%), followed by paraformaldehyde (4%, dissolved in 0.1 M PBS). Brains were collected and placed in paraformaldehyde overnight at 4°C. The following day, the tissue was transferred to a sucrose solution (20%) for an additional 24 h.
PYRAMIDAL CELL ACTIVATION FOLLOWING ODOR PREFERENCE LEARNING.
Pups with a single nostril occluded underwent either O/S+ or O/S− training. After training, the pups were returned to normal bedding for an additional 10 min before perfusion for brain collection as described above.
Immunohistochemistry.
For slicing, brains were mounted using Cryomatrix fixative (Thermo Scientific) and immediately placed in a cryostat at −20°C until fully frozen. Thirty-micrometer coronal sections were cut and two slices were collected every 200 μm and were mounted on chrome-gelatin coated slides in an alternating fashion, allowing for Nissl comparison staining. Following slicing, slides were kept at 4°C for 10 min before being brought to room temperature to dry. A pCREB antibody (1:100; Cell Signaling) was applied to the alternate slides. The antibody was dissolved in phosphate buffered saline with 0.2% Triton-X-100, 0.02% sodium azide, and 2% normal goat serum and left on sections overnight at 4°C in a humidified chamber. The following day, the slices were washed with PBS and a biotinylated anti-rabbit secondary antibody solution (Vectastain) was applied to the slices, followed by an avidin and biotinylated (A+B) enzyme amplification step. Finally, sections were stained using a diaminobenzidine tetrahydrochloride (DAB) reaction [50 mg DAB (Amresco); dissolved in 50 ml 0.1 M PBS, 50 ml dH2O, and 30 μl 30% H2O2], which was monitored for completion under an upright light microscope (Olympus). Slides were then dehydrated and coverslipped. Hemisphere sections on each slide were compared under the microscope to confirm there was less pCREB expression in the lidocaine-injected hemisphere.
Image analysis.
To obtain a quantitative comparison between occluded and spared hemispheres in O/S+ or O/S− conditions, image analysis was performed using a Bioquant system (R&M Biometrics). Images were obtained using a CCD camera and viewed with a Leitz microscope with a consistent light intensity. Slices were analyzed from rostral to caudal. An optical density (OD) reading of the background was taken near the midline of each slice to use for staining comparisons. A region of interest (ROI) was manually traced in both a lateral and a medial position in the pyramidal cell layer of the piriform cortex for both hemispheres. The relative OD (ROD) was calculated using the following formula: ROD = |(OD of ROI − OD of background)/OD of background|.
The medial and lateral ROD measurements were compared in the spared and occluded hemispheres of each animal and were also compared between experimental conditions (O/S+ vs. O/S−). To account for individual differences between animals, measurements were normalized (ratio of the spared hemisphere to the occluded hemisphere), and the percentage differences were calculated using the formula: %difference = 100% − (spared ROD/occluded ROD).
Values displayed indicate the percentage differences between medial and lateral regions of the spared and occluded hemispheres and between O/S+ and O/S− animals. Student's t-tests were used to determine statistical significance.
Electrophysiology
Tissue preparation and extracellular recording.
Animals were anesthetized with halothane inhalation and quickly decapitated. Brain tissue was extracted and placed in an ice-cold high glucose artificial cerebrospinal fluid (aCSF; in mM: 83 NaCl, 2.5 KCl, 0.5 CaCl2, 3.3 MgSO4, 1 NaH2PO4, 26.2 NaHCO3, 22 glucose, and 72 sucrose, equilibrated with 95% O2-5% CO2) for ∼10 min. Sagittal slices (400 μm) were cut using a vibrating blade (Vibratome 1000P; Leica Microsystems) and were incubated in an Isotemp 205 chamber (Fisher Scientific) at 34°C in the aforementioned solution for at least 60 min before use. Tissue slices were transferred to an RC-40 open bath recording chamber (Warner Instruments) which was continuously perfused with aCSF (in mML 119 NaCl, 2.5 KCl, 2.5 CaCl2, 1.3 MgSO4, 1 NaH2PO4, 26.2 NaHCO3, and 22 glucose, equilibrated with 95% O2-5% CO2) at 30–32°C and viewed with an upright microscope (Olympus BX51). Extracellular field potentials were recorded with glass pipettes filled with aCSF and placed in layer Ia of the aPC. A concentric bipolar stimulating electrode (FHC) was lowered into the LOT and delivered single test pulses, ranging from 10 to 80 μA.
Electrophysiological data were recorded with Multiclamp 700B (Molecular Devices), filtered at 2 kHz, and digitized at 10 kHz. Data acquisition and analysis were performed with pClamp10 and ClampFit 2.10 (Molecular Devices) and Igor Pro 6.10A (WaveMetrics). Student's t-tests were used to determine statistical significance.
In vitro electrophysiology.
Naïve PD7–11 animals were used for recording. A baseline of fEPSPs evoked by single pulse test stimulation was recorded at 0.05 Hz until the last 10 min were consistent and was then followed by theta-burst stimulation (TBS; 10 times 5 Hz trains, each train contains 5 pulses at 100 Hz). Protocols varied among one, four, or eight TBSs, with each TBS separated by 30 s. TBS was followed by the same single test pulse recording for 30–60 min. The stimulation intensity for recording and stimulation was determined as that at which 50% of the maximum response was evoked. The recordings were analyzed to determine the amount of long-term potentiation (LTP) at the LOT to aPC synapses. In subsets of experiments, the fEPSP input-output (I/O) relationship was measured as the ratio of the size of the presynaptic fiber volley (FV) to the slope of the fEPSP; the paired pulse ratio (PPR; 2nd EPSP amplitude/1st EPSP amplitude) was measured in two consecutive stimulations with an interval of 50 ms, using mid-range stimulation strength.
d-APV (50 μM; Tocris) and isoproterenol (20 μM; Sigma) were used in bath application in subsets of experiments. Drugs were washed into the recording chamber 5 min before the TBS and washed out following the TBS. The sizes of fEPSPs were measured, and PPRs during isoproterenol wash-in were compared before and immediately following the TBS.
Ex vivo electrophysiology.
Pups undergoing ex vivo electrophysiological analysis were subjected to odor conditioning with one nostril occluded as described earlier. Following a 5-min habituation period, pups underwent O/S+ or O/S− training. Upon completion, the plugs were removed and pups were returned to the dam. Pups were killed 3 or 24 h following training, and brain slices were prepared. The hemispheres of the brain were kept separately within the incubation chamber to achieve intra-animal control. I/O relationships and PPRs were measured as with in vitro experiments and compared between the spared and occluded slices. To reduce heterogeneities of the inputs in slices, two or three pairs of slices from the two hemispheres at the same cutting planes were used for recording and the results were averaged for each hemisphere. The stimulation and recording configurations were kept the same for each pair of slices. The slices from two hemispheres were recorded alternately. In another set of experiments, one and eight TBSs were used to induce LTPs at the LOT synapses in the nostril occluded and spared slices.
RESULTS
aPC is Critically Involved in Early Odor Preference Learning in Rats
Rainiki et al. (2009) showed that with strong shock and illness-related odor aversion learning, rat pups from 7–24 days of age increase 2-deoxy-d-glucose (2-DG) uptake in the posterior, but not the anterior, piriform cortex. At 7–8 days, however, mild shock pairings with odor induce odor preference and selective 2-DG uptake in both the olfactory bulb and aPC. To confirm the role of the aPC in early odor preference learning with a stroking US, two aPC silencing experiments were performed. The first employed lidocaine. Lidocaine is a transient sodium channel blocker, which silences neurons for up to 30–60 min (Martin 1991). On PD7, lidocaine was infused into the aPC 10 min before odor training or was infused on PD8 10 min before odor preference testing. To obtain a more specific silencing effect on neurons within the aPC, muscimol, a GABAa receptor agonist, was also used in the same protocols. Figure 1A shows that animals infused with lidocaine or muscimol before training spent less time over peppermint-scented bedding (lidocaine, 40.73 ± 4.23%, n = 17; muscimol, 20.34 ± 4.79%, n = 7) compared with saline-infused counterparts (saline O/S+, 54.81 ± 3.26%, n = 19). Saline-infused odor only (O/S−) animals (39.02 ± 2.70%, n = 18) were comparable to lidocaine pups. A one-way ANOVA reveals a significant difference among groups [F(3,57) = 10.77, P = 1.041 E−5]. Post hoc Fisher least significant difference testing demonstrates significant differences between saline O/S+ and saline O/S− groups (P = 0.001), saline O/S+ and lidocaine O/S+ animals (P = 0.005), and saline O/S+ and muscimol O/S+ groups (P = 1.074 E−6). Figure 1B shows that animals that underwent O/S+ training on PD7, followed by lidocaine or muscimol infusions before testing on PD8, demonstrated no preference for peppermint (lidocaine, 36.86 ± 7.92%, n = 6; muscimol, 34.92 ± 5.32, n = 10) compared with saline-infused counterparts (57.08 ± 4.69%, n = 7). Animals who received O/S− training and saline infusions also showed no preference for peppermint (28.98 ± 4.29%, n = 8). A one-way ANOVA shows significant between-groups differences [F(3,31) = 5.25, P = 0.005]. Post hoc testing demonstrates significant differences between saline O/S− and saline O/S+ animals (P = 5.675 E−4), saline O/S+ and lidocaine O/S+ animals (P = 0.024), and saline O/S+ and muscimol O/S+ animals (P = 0.006). These results show that the aPC is required for memory encoding and recall processes in odor preference learning: either the aPC directly participates in memory encoding or, as part of the olfactory circuitry, is necessary for odor perception during learning and memory.
To verify that pyramidal cells were silenced in response to lidocaine infusion, pCREB immunohistochemistry was performed following a unilateral lidocaine or muscimol infusion and peppermint odor exposure. Figure 1, C (lidocaine) and D (muscimol), shows pCREB expression in the aPC at low (middle) and high (bottom) magnifications. Hemispheres injected with lidocaine or muscimol show much less staining than saline-injected hemispheres (e.g., lidocaine ROD: 69.8 ± 12.3% of the saline-injected side, t = 6.50, P = 6.333 E−4, n = 7). Nissl staining (Fig. 1, top) confirms that pyramidal cell bodies are present and homogeneous in the aPC in both hemispheres. No spread to the olfactory bulb or the posterior PC was observed using both methylene blue infusion spread assessment and, more critically, pCREB immunohistochemistry reactivity in these two structures. This suggests that there was no silencing of adjacent areas. However, as we note elsewhere, feedforward and feedback electrophysiological interactions should be altered. There were also no long-lasting effects of lidocaine and muscimol at 24 h, the time of testing, judged by pCREB immunohistochemistry in aPC itself (n = 4, data not shown).
Both 2-DG (Raineki et al. 2009) and cFos (Roth and Sullivan 2005) increases in the aPC have been described accompanying odor preference training in 7- to 8-day-old rat pups. In the olfactory bulb, increased pCREB staining in the peppermint-encoding region is selectively associated with odor preference learning (McLean et al. 1999). In the present study, pyramidal neurons in the aPC were assessed for a role in odor preference memory encoding by measuring changes in pCREB levels in these cells shortly after odor training. To eliminate potential differences due to heterogeneity in background odors and immunohistochemical processing among different animals, unilateral nostril occlusions were employed. This allowed for intra-animal comparisons, where the occluded hemispheres were compared with the spared hemispheres. Pups underwent O/S− or O/S+ training and differences in pCREB expression in the aPC were observed (Fig. 2, A and B). RODs were measured in a lateral and a medial region in the aPC in both occluded and spared hemispheres. Data were normalized to account for individual differences and expressed as a percent difference [100% − (spared ROD/occluded ROD); Fig. 2B]. With the use of a paired sample t-test, O/S− animals showed no significant differences in normalized pCREB expression between medial (1.81 ± 0.70%) and lateral (2.10 ± 0.42%) regions of the aPC (n = 7, t = 0.36, P = 0.730), while O/S+ animals showed much higher staining in the lateral region (3.60 ± 0.76%) of the aPC than in the medial region (1.74 ± 0.43%, n = 5; t = 5.15, P = 0.007). Comparing medial and lateral regions between training conditions, a two sample t-test found no significant differences in the medial regions of the aPC between O/S− and O/S+ animals (t = 0.080, P = 0.939). However, there were significant differences in the lateral regions of the aPC between O/S− and O/S+ animals, with stronger staining in the O/S+ animals (t = 1.87, P = 0.046). These results suggest that O/S+ training activates pyramidal neurons more than O/S− training, specifically in the lateral regions of the aPC. This pattern of results also suggests that, as in olfactory bulb, pCREB-directed transcription may play a causal role in odor preference memory.
Odor Preference Learning is Dependent on NMDARs in the aPC
Since reversible lesions and CREB activation data were both consistent with a role for the aPC in early odor preference learning, the next series of experiments examined plasticity mechanisms in aPC that might support such learning. NMDA plasticity in the LOT input from the olfactory bulb to the piriform cortex is age dependent and most evident in young rat pups (Poo and Isaacson 2007). Such plasticity has been implicated in many associative learning models (Debanne 1996). NMDARs in the aPC were blocked using d-APV infusions 10 min before O/S+ training. When animals were tested the following day, it was found that blocking NMDARs in the aPC prevented early odor preference learning (Fig. 3A). Such animals spent significantly less time over peppermint-scented bedding compared with saline-infused counterparts (d-APV, 38.58 ± 4.27%, n = 15; saline O/S+, 54.81 ± 3.22%, n = 19). In addition, the saline-infused O/S− animals (39.02 ± 2.70%, n = 18) were comparable to d-APV-infused animals. A one-way ANOVA shows that there were significant differences among groups [F(2,51) = 7.85, P = 0.001]. Post hoc testing further shows that saline O/S− animals spent much less time over peppermint compared with saline O/S+ animals (P = 0.001) and d-APV infusion before O/S+ training significantly reduced the time that animals spent over peppermint bedding compared with saline O/S+ animals (P = 0.001). These results suggest that NMDAR activation in the aPC is required for early odor preference learning.
Fig. 3.
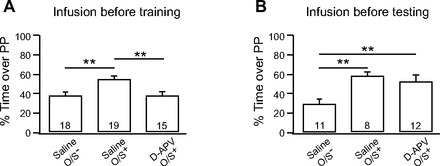
Odor preference learning is dependent on N-methyl-d-aspartate (NMDA) receptors in the piriform cortex. A: infusion of the NMDA receptor antagonist d-(−)-2-amino-5-phosphonopentanoic acid (d-APV; 100 μM) 10 min before O/S+ training. B: infusion of d-APV before odor preference testing. Bars are percentage of time spent over peppermint (PP)-scented bedding in a 2-choice test. Error bars are means ± SE. **P < 0.01.
To test whether d-APV infusion into the aPC alters odor perception instead of preventing learning, animals were trained with the O/S+ protocol and d-APV was infused before odor preference testing the next day. If blocking NMDARs in the aPC alters odor perception, it would be expected that d-APV infusion before testing would abolish the preference for peppermint in O/S+ animals. However, Fig. 3B showed that d-APV infusion before testing did not affect the preference for peppermint that was formed in O/S+ animals. When these animals were compared with learning (O/S+ with saline infusion) and nonlearning (O/S− with saline infusion) controls, a one-way ANOVA showed significant group effects [F(2,30) = 8.08, P = 0.002]. Pups infused with d-APV before testing showed similar results (51.80 ± 5.91%, n = 12) to those of saline-infused O/S+ animals (57.08 ± 4.70, n = 8, P = 0.496). Both groups spent significantly more time on the peppermint side than the control O/S− animals (28.98 ± 4.29%; P < 0.05).
Together, these results suggest that d-APV infusion did not affect odor perception, but rather the encoding of memory.
β-Adrenoceptor Activation in the aPC Can Mediate Early Odor Preference Learning
Previously it has been shown that direct pharmacological activation of β-adrenoceptors in the olfactory bulb is sufficient for early odor preference learning to occur (Lethbridge et al. 2012; Sullivan et al. 2000). However, it is not known to what extent natural release of norepinephrine during O/S+ learning engages the piriform cortex. We tested whether β-adrenoceptors in the aPC are involved in early odor preference learning. First, β-adrenoceptors were blocked by infusing propranolol directly into the aPC 10 min before O/S+ training. Propranolol-infused animals (39.59 ± 4.82%, n = 8) spent significantly less time on peppermint bedding than saline-infused O/S+ animals (54.81 ± 3.23%, n = 19) and were comparable to nonlearning saline-infused O/S− animals (39.02 ± 2.70%, n = 18; Fig. 4). Next, stroking was replaced with an intracranial infusion of varying concentrations of the β-adrenoceptor agonist isoproterenol during odor exposure. Animals infused with 5 μM isoproterenol (38.86 ± 4.29%, n = 6) or 50 μM isoproterenol (38.06 ± 8.68%, n = 6) did not learn to prefer peppermint; however, animals infused with 500 μM isoproterenol (58.41 ± 10.98%, n = 6) did show a preference for peppermint. The learning effect of 500 μM isoproterenol was blocked when coinfused with propranolol (27.38 ± 11.45%, n = 4). A one-way ANOVA shows significant differences among groups [F(6,60) = 3.66, P = 0.004]. Post hoc testing reveals significant differences between saline O/S+ and propranolol O/S+ animals (P = 0.023). In addition, there was a significant difference between saline O/S− animals and 500 μM isoproterenol animals (P = 0.010), and 500 μM isoproterenol and propranolol coinfusion groups (P = 0.004). These results suggest that early odor preference learning engages β-adrenoceptors in the aPC and that β-adrenoceptor activation in the aPC paired with a novel odor is sufficient to induce odor preference learning independent of the olfactory bulb β-adrenoceptor-induced learning effect.
Fig. 4.
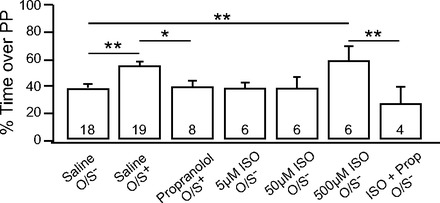
β-adrenoceptors in the piriform cortex are critical for learning. Infusion of the β-adrenoceptor antagonist propranolol (100 μM) before O/S+ training, or the β-adrenoceptor agonist isoproterenol (ISO; 5, 50, 500 μM) before O/S− training. Bars are percentage of time spent over peppermint-scented bedding in a 2-choice test. Error bars are means ± SE. *P < 0.05; **P < 0.01.
Induction of LTP at the LOT Synapse In Vitro Supports Learning Acquisition Mechanism
We next set out, using in vitro experiments, to characterize synaptic mechanisms occurring in the aPC that could support odor preference learning. We first characterized LTP at the LOT to aPC synapses. By stimulating LOT afferent inputs, and recording from the pyramidal cell dendritic layer Ia, LTP of pyramidal cell fEPSPs was induced by TBS protocols (Fig. 5). TBS protocols capture, to some extent, the natural sniffing-mediated input rhythms from olfactory bulb to aPC (Kepecs et al. 2006). Figure 5A shows LTP of fEPSPs following 1 TBS. A paired sample t-test comparing the normalized fEPSP slope (mV/ms) of the baseline to that 30 min post-TBS recording reveals that they are significantly different (post-LTP: 108.8 ± 3.8% of the baseline; n = 15, t = 2.31, P = 0.037). Furthermore, an eight TBS protocol induces a larger LTP (post-LTP: 116.5 ± 3.3%; n = 14, t = 4.93, P = 2.772 E−4; Fig. 5B). Therefore, consistent with previous reports (Franks and Isaacson 2005; Kanter and Haberly 1990), the LOT to aPC synapse is plastic at these ages and is capable of strengthening synaptic communication, a change that is proposed to underlie learning.
Fig. 5.
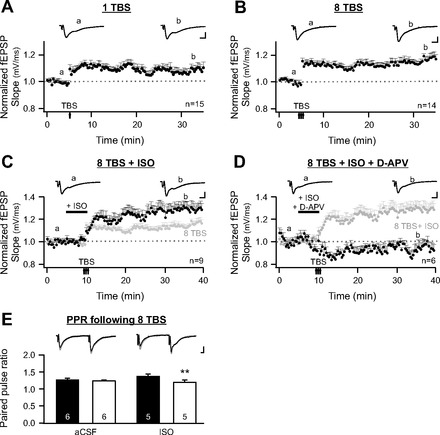
Long-term potentiation (LTP) at the lateral olfactory tract (LOT) to aPC synapse in vitro. A: time course of field excitatory postsynaptic potential (fEPSPs) recorded at LOT to aPC synapses with a 1 theta-burst stimulation (TBS) induction protocol. Typical fEPSP traces at time points a (baseline) and b (post-LTP) are illustrated. B: induction of fEPSP LTP by 8 TBSs. Note a larger LTP of field EPSPs was produced compared with that induced by 1 TBS. C: Induction of fEPSP LTP by 8 TBSs in the presence of ISO. Dark marks, 8 TBS + ISO; light marks, 8 TBS. D: induction of fEPSP LTP by 8 TBSs in the presence of both ISO and d-APV. Dark marks, 8 TBS + ISO + d-APV. Light marks, 8 TBS + ISO. E: paired pulse ratios (PPRs) of fEPSPs before and immediately following 8 TBSs in artificial cerebral spinal fluid (aCSF) or in the presence of isoproterenol. Example traces of fEPSPs recorded before TBS (black traces) are superimposed on the traces recorded immediately following TBS (grey traces). Scale bars = 0.5 mV/5 ms. Error bars are means ± SE. **P < 0.01.
We then investigated the influence of β-adrenoceptor activation on TBS-associated LOT LTP. Isoproterenol was bath applied to the slices, and eight TBSs were administered. Isoproterenol application increased the LTP magnitude compared with that induced by 8 TBSs alone (8 TBS + ISO: 129.9 ± 5.2%, n = 9; 8 TBS: 116.5 ± 3.3%, n = 14; t = 2.28, P = 0.033; Fig. 5C). Our behavioral results revealed that NMDARs were critical for early odor preference learning. LTP induced in the presence of isoproterenol was also blocked by d-APV (93.2 ± 5.1%, n = 6, t = 1.32, P = 0.245; Fig. 5D). What is the acute effect of isoproterenol on the LOT synapses that could lead to an enhanced LTP induction? To test if isoproterenol affects presynaptic release from LOT, we measured PPRs of two consecutive fEPSPs before and immediately following TBS induction. LOT synapses exhibited paired pulse facilitation (Fig. 5E). The PPRs in the aCSF perfused slices did not change following TBS (pre-TBS, 1.29 ± 0.033; post-TBS, 1.25 ± 0.024, n = 6, t = 1.97, P = 0.106). Conversely, for isoproterenol perfused slices, the PPRs were significantly lower post-TBS compared with pre-TBS (pre-TBS, 1.37 ± 0.078; post-TBS, 1.22 ± 0.055, n = 5, t = 6.32, P = 0.003; Fig. 5E), implying that there was an increase in presynaptic release following TBS in the presence of isoproterenol. While we cannot exclude a direct effect of isoproterenol on postsynaptic pyramidal cells during TBS induction, an increase in presynaptic release by isoproterenol could account for the larger LTP change. These results are consistent with the critical roles of NMDARs and β-adrenoceptors in vivo in early odor preference learning.
LOT to aPC LTP Is Expressed as Enhanced Postsynaptic Responses
We then explored the expression mechanisms of the LOT to aPC LTP. fEPSP I/O relationships and PPRs were measured to indicate post- and presynaptic changes correspondingly. The slope of the fEPSP was used for the I/O measurement and is largely composed of an AMPAR component (Franks and Isaacson 2005). We measured the fEPSP slope in the presence of NMDAR and AMPAR blockers. d-APV did not significantly change the fEPSP slope (95.1 ± 3.5% of the baseline, n = 5, t = 1.37, P = 0.244); however, the AMPAR antagonist 2,3-dioxo-6-nitro-1,2,3,4-tetrahydrobenzo[f]quinoxaline-7-sulfonamide disodium salt (NBQX; 20 μM) abolished the fEPSP in the presence of d-APV (n = 3, data not shown). Figure 6A shows a typical example of evoked fEPSPs in response to different stimulation intensities (top traces) and the changes following LTP (bottom traces). To characterize the postsynaptic changes relative to the LOT release that occur following LTP, the fEPSP I/O relationships were determined by comparing the presynaptic FV with the EPSP slope. A paired t-test reveals that the I/O was greater post-LTP compared with pre-LTP (pre-LTP: 1.26 ± 0.37; post-LTP: 1.57 ± 0.49, n = 15, t = 1.87, P = 0.041; Fig. 6A). To test whether there were presynaptic changes resulting from LTP, PPRs were measured pre- and 30 min post-LTP, following isoproterenol washout, either when TBS was applied during aCSF alone (pre-LTP, 1.20 ± 0.080; post-LTP, 1.23 ± 0.055, n = 6) or in the presence of isoproterenol (pre-LTP, 1.34 ± 0.063, post-LTP, 1.34 ± 0.047, n = 7; Fig. 6B). A paired sample t-test yielded no significant differences in the PPR before and after LTP, either in aCSF (t = 1.06, P = 0.336), or in the presence of ISO (t = 0.35, P = 0.738). Significant changes in the fEPSP I/O but not PPR suggest a postsynaptic expression of the LOT LTP.
Fig. 6.

Enhanced postsynaptic responses following in vitro LTP. A: input-output (I/O) relationships at LOT to aPC synapses before LTP and 30 min following LTP induction. Top: stimulation profiles of fEPSPs before LTP (pre-LTP) and 30 min following LTP (post-LTP). Middle: I/O curves of the slopes of the EPSPs and the sizes of the presynaptic fiber volley (FV), obtained pre-LTP (broken line with solid circles) and post-LTP (solid line with open circles). Bottom: difference in the I/O relationships before LTP vs. after LTP is summarized. B1: PPRs before LTP and 30 min following LTP, recorded in aCSF. B2: PPRs before LTP and 30 min following LTP. LTP was induced in the presence of ISO. Scale bars = 0.2 mV/5 ms. Error bars are means ± SE. *P < 0.05.
Early Odor Preference Learning Induces LTP-like Changes at the LOT to aPC Synapse
Finally, we examined whether natural odor preference learning induces long-term synaptic changes at the LOT synapse in the aPC. Ex vivo electrophysiology was conducted following behavioral training. Such training involved unilateral nostril occlusion paired with O/S− or O/S+ training, followed by electrophysiological recordings at 3 or 24 h posttraining. At this age there is no contralateral communication between the two aPC areas (Kucharski and Hall 1987; Kucharski et al. 1986) so that learning can be confined unilaterally with single nostril occlusion (Yuan and Harley, 2012). We focused on recording from the sagittal slices containing the lateral portion of the aPC since the pCREB data demonstrated significant changes in that area following O/S+ training. We measured the fEPSP I/O relationship and PPRs from occluded and spared slices from the same animals. Because we compare fEPSP responses in different slices (spared and occluded), it is impossible to compare fEPSP slope with a single stimulation. Instead, the I/O relationship measuring the ratio of fEPSP slope to the presynaptic FV allows us to directly compare the relative number of functional AMPARs at LOT synapses from spared and occluded hemispheres of the same animals (Franks and Isaacson 2005). For example, a bigger I/O slope indicates a greater AMPAR response per LOT release site. A paired t-test showed that at 3 h posttraining, the O/S− condition (Fig. 7, A1 and A2) produced no changes in fEPSP I/O between occluded (1.11 ± 0.11) and spared (1.15 ± 0.14) hemispheres (n = 5, t = 0.423, P = 0.694; Fig. 7A1) and no difference in PPR between occluded (1.17 ± 0.08) and spared (1.21 ± 0.05) hemispheres (n = 5, t = 0.69, P = 0.527; Fig. 7A2). At 3 h posttraining, in the O/S+ condition (Fig. 7, B1 and B2), there was a steeper fEPSP I/O relationship in the spared (0.99 ± 0.12) hemisphere compared with the occluded hemisphere (0.70 ± 0.10; n = 9, t = 2.26, P = 0.027; Fig. 7B1), indicating a greater fEPSP for a given input. The PPR was also significantly lower in the spared (1.19 ± 0.04) hemisphere compared with the occluded hemisphere (1.36 ± 0.04; t = 3.67, P = 0.006; Fig. 7B2). Twenty-four hours post-O/S+ training (Fig. 7, C1 and C2), there was again a significant leftward shift in the fEPSP I/O relationship in the spared (0.85 ± 0.20) hemisphere compared with the occluded hemisphere (0.74 ± 0.19; n = 5, t = 4.07, P = 0.008; Fig. 7C1); however, there was no significant difference in the PPRs between the spared (1.18 ± 0.16) and occluded hemispheres (1.18 ± 0.17; n = 5, t = 0.04, P = 0.974; Fig. 7C2). These results suggest that postsynaptic LTP at the LOT synapse was sustained for up to 24 h following odor preference learning, and at a shorter period (3 h posttraining), there was also an increase in presynaptic release, most likely reflecting enhanced mitral cell output from the olfactory bulb (Lethbridge et al. 2012).
Fig. 7.
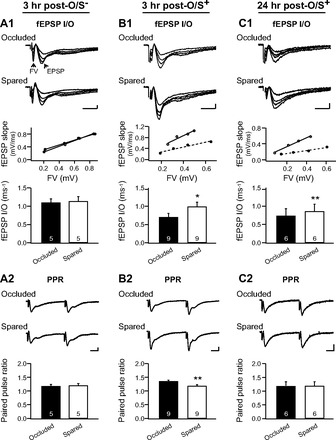
Learning induced LTP changes at the LOT to pyramidal cell synapse. A1 and A2: I/O relationships and PPRs at the LOT synapses in the piriform cortex 3 h postodor only (O/S−) training. Recordings from occluded and spared slices of the same animals were compared. A1, top: examples of fEPSP traces at various stimulation intensities in an occluded and a spared slice from the same animal. Middle: I/O relationship of the slopes of the EPSPs and the sizes of the presynaptic FV from these 2 slices (occluded: broken line with solid circles; spared: solid line with open circles). A1, bottom: average I/O from the 2 groups. A2, top: example traces of paired pulse recordings from an occluded and a spared slice from the same animal. A2, bottom: average PPRs of the 2 groups. B1 and B2: I/O and PPRs of fEPSPs in the piriform cortex 3 h post-O/S+ training. C1 and C2: I/O and PPRs of fEPSPs in the piriform cortex 24 h post-O/S+ training. Scale bars = 0.2 mV/5 ms. Error bars are means ± SE. *P < 0.05; **P < 0.01.
To further test if learning-induced LTP shares the same properties as those induced by TBS of the LOT, we performed LTP recording in spared vs. occluded slices 3–24 h posttraining. Figure 8 shows that in occluded slices, one TBS generated LTP of fEPSPs comparable to those generated in naïve slices in Fig. 5A (post-LTP: 109.9 ± 4.5% of the baseline; n = 7 slices from 4 animals, t = 2.21, P = 0.035), while eight TBSs further potentiated fEPSPs (post-LTP: 116.8 ± 7.4% of the baseline; t = 2.27, P = 0.032). In contrast, in spared slices, there was no potentiation of fEPSPs either by 1 TBS (post-LTP: 94.0 ± 10.4% of the baseline; n = 6 slices from 4 animals, t = 0.57, P = 0.705) or by a following set of 8 TBSs (post-LTP: 91.5 ± 10.1% of the baseline; t = 0.85, P = 0.436). These results suggest early odor preference learning results in LTP changes at the LOT synapses that are exclusive of further LTP induction at the same synapses.
Fig. 8.

Learning excludes LTP at the LOT synapses in vitro. Time course of fEPSPs recorded at LOT to aPC synapses with a 1 TBS induction protocol, followed by an 8 TBS protocol in spared (open circles) vs. occluded (solid circles) slices. Typical fEPSP traces at time points a (baseline), b (post-1 TBS), and c (post-8 TBS) are illustrated. Scale bars = 0.2 mV/5 ms.
DISCUSSION
As reviewed in the Introduction, pairing of a CS (odor) and a US (β-adrenoceptor activation) in the olfactory bulb is sufficient to produce early odor preference learning (Lethbridge et al. 2012; Sullivan et al. 2000). Blocking β-adrenoceptors in the olfactory bulb demonstrates that β-adrenergic activation is necessary for normal early olfactory learning (Sullivan et al. 2000). While this leads to the suggestion that plasticity in the rat pup olfactory bulb is both necessary and sufficient for early odor preference learning, there remains the possibility that other structures in the forebrain olfactory circuit play a role in supporting, or even in generating, this form of learning. The present experiments provide evidence that the aPC is also both necessary and sufficient for early odor preference learning. The evidence argues that early odor preference learning is normally supported by, and generated in, multiple forebrain memory circuits. We propose that normally, changes in the olfactory bulb drive changes in aPC. Thus earlier experiments that locally induced olfactory bulb circuitry enhancement to peppermint in turn entrained aPC, while blocking such enhancement prevented the recruitment of aPC changes. Here we show that bypassing the olfactory bulb change and inducing aPC change directly permits memory expression, but if aPC circuitry is prevented from altering then olfactory bulb change alone is not able to drive odor preference behavior.
Evidence for aPC as a Critical Node in Early Olfactory Forebrain Learning Circuitry
Previous experiments using cFos activation to examine forebrain olfactory structures exhibiting immediate early gene activation following odor preference learning in rat pups of the same age as those used in the present experiments found only two structures with enhanced cFos activation, the olfactory bulb and the aPC (Roth and Sullivan 2005). Similar results were seen with 2-DG uptake (Raineki et al. 2009). Aversive odor training recruited the olfactory bulb, the posterior piriform cortex, but not the aPC, and the central amygdala (Raineki et al. 2009; Roth et al. 2006). In the present studies, with the use of pCREB activation as a marker of early transcriptional activation implicated in learning, the results confirm that the aPC is activated by peppermint odor. More compellingly, pairing a peppermint odor CS with stroking, a maternal mimic of US, produces increased pCREB activation in the aPC as it does in the olfactory bulb (McLean et al. 1999). This pCREB learning effect is selective to the lateral aspect of the aPC in the present study, but the possibility remains of more general involvement below the threshold of our optical density measurements. An earlier cFos developmental study of aPC activation by odor has shown that there is major spatial reorganization of odorant representation occurring at PD10 (Illig 2007) just at the end of the critical period for early odor preference learning. It is possible that the effects seen here are specific to the critical window period. LOT afferent LTP is also more readily elicited at this early age (Poo and Isaacson 2007). It is also the case that NMDA-dependent NE release is selectively elevated in cortex at this age (Brown 1993). In future studies, pharmacological manipulations could be used to alter the nature of odor learning (e.g., appetitive vs. aversive) or to modulate the critical period window (Moriceau and Sullivan 2004; Moriceau et al. 2006; Roth et al. 2006). The spatial correlates of pCREB activation with different forms of learning at the same age and with the same learning at different ages may be informative. Consistent with the gene activation evidence for a role of aPC in early odor preference learning, lidocaine or muscimol infusions, which prevented aPC participation either during training or during testing, also prevented the acquisition and expression of early odor preference learning. This result is consistent with a role for aPC in preference learning, but the effect could be indirect, for example, piriform cortex feedback to the olfactory bulb may modulate the bulbar representation (Boyd et al. 2012; Strowbridge 2009) to affect learning or relaying olfactory information per se through the aPC may be critical. Additional experiments here, however, provided evidence for a direct role of aPC mechanisms in the generation of early odor preferences.
Evidence for aPC Plasticity in the Generation of Early Odor Preference Learning
The NMDAR antagonist d-APV infused locally in the aPC before training prevented early odor preference learning in vivo. Again it was possible that this was an indirect effect mediated by a change in the modulation of sensory input either in the piriform cortex, or in the olfactory bulb. However, the failure of the NMDA antagonist to alter the expression of odor preference learning 24 h after normal training argues that sensory distortion is not an explanation for the ability of NMDA infusions to prevent learning.
In vitro experiments revealed a role for NMDARs in theta-burst LTP at the LOT to aPC synapse, as has been previously reported (Kanter and Haberly 1990). Of particular interest was the ability of β-adrenoceptor activation, via isoproterenol, to significantly strengthen theta-burst LTP at this synapse. During the application of isoproterenol, a reduction in the PPR suggested that one way in which isoproterenol enhanced LTP effects was through increasing the levels of glutamate released during the theta burst. Such an effect is consistent with the report that isoproterenol directly prevents rapid presynaptic habituation at the LOT to aPC synapse by modifying the phosphorylation of the metabotropic III receptor that is responsible for reduced transmitter release (Best and Wilson 2004). In vivo studies demonstrated that blocking β-adrenoceptors in the aPC, as in the olfactory bulb, prevented early odor preference learning. Finally, infusion of isoproterenol in aPC paired with peppermint produced learning. Taken together these data argue that β-adrenergic support of novel theta-burst odor input to the aPC leads to learning modifications at LOT to aPC synapses that can support the acquisition and expression of an olfactory preference.
Synaptic Changes Supporting Early Odor Preference Learning and Expression in the aPC
Ex vivo experiments carried out at 3 and at 24 h after training in slices from paired trained and untrained hemispheres revealed the LTP predicted at the LOT to aPC synapses based on the in vitro experiments. Paired pulse evidence suggested that at 3 h posttraining there was enhanced presynaptic release as well as an increase in postsynaptic responses. At 24 h, increased presynaptic release was no longer in evidence but enhanced postsynaptic responsiveness remained. These data argue that memory effects in the aPC may depend primarily on increased AMPA receptor currents or increased AMPA receptor insertion as seen at olfactory nerve to mitral cell synapses in the olfactory bulb (Cui et al. 2011; Lethbridge et al. 2012; Yuan and Harley 2012). Under natural learning conditions norepinephrine release in the aPC would enhance NMDAR currents and postsynaptic calcium events evoked by LOT glutamate release. Additionally, local LOT glutamate release could engage NMDA receptors on norepinephrine terminals to increase norepinephrine output in a positive feedback effect that would increase plasticity locally (Brown 1993; Wang et al. 1992). Local plasticity effects would support a synergistic recruitment of long-term synaptic potentiation and of the CREB cascade in pyramidal cells of the aPC. Another set of effects seen in the olfactory bulb, but not evaluated here, could include changes in the synchrony of neuronal firing (de Almeida et al. 2012; Doucette et al. 2011). Norepinephrine is known to suppress intracortical input relative to afferent LOT input in aPC and subsequently enhance the signal-to-noise ratio (Hasselmo et al. 1997).
Piriform Cortex and Olfactory Bulb Interactions in the Support of Odor Learning
Local infusions of the US isoproterenol in either the olfactory bulb (Lethbridge et al. 2012; Sullivan et al. 2000) or the aPC (Fig. 4), paired with odor can generate early odor preference learning and memory. Blockade of β-adrenoceptors at either site prevents natural odor preference acquisition. Consistent with a model in which noradrenergic input normally supplies the US at this developmental stage, both structures receive extensive innervation from the noradrenergic locus coeruleus nucleus (Fallon and Moore 1978; McLean et al. 1989; Shipley and Ennis 1996). LTP of olfactory nerve input to mitral cells (Lethbridge et al. 2012; Yuan and Harley 2012) and, here, of LOT input to aPC pyramidal cells accompanies early odor preference learning and may be the major mechanism underlying its expression. The two structures interact directly via feed forward input from mitral and tufted cell axons to aPC layers II and III, and via feedback input from pyramidal cells in layers II/III of aPC to the granule cells of the olfactory bulb (Boyd et al. 2012; Isaacson 2010; Strowbridge 2009), which have been shown to modulate rhythmic activity and synchronization in the olfactory bulb (Neville and Haberly 2003; Poo and Isaacson 2009). Recent data (Doucette et al. 2011) suggest the frequency of rhythmic activity in olfactory bulb odor representations (and possibly those of aPC) provides the signature for appetitive stimulus encoding in the olfactory bulb of adult rodents. If that occurs in the rat pup, plasticity effects at the first and second synapses and the interaction between these two structures may both increase the saliency of the peppermint odor signal and assign a positive valence to that signal. Since either structure can generate early olfactory preferences when odor and a noradrenergic stimulus are paired, it suggests either can initiate saliency and valence components. We suggest that preference learning stability would be optimized by recruiting both olfactory networks in the serial/parallel configuration that can be obtained in the real world [see also de Almeida et al. 2012 for a model of interactive effects between these two structures with cholinergic neuromodulation that may parallel effects seen with norepinephrine (Hasselmo et al. 1997)].
GRANTS
This work was supported by Canadian Institutes of Health Research Operating Grant MOP-102624 (to Q. Yuan).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: G.L.M., C.J.F., and Q.Y. performed experiments; G.L.M., C.J.F., and Q.Y. analyzed data; G.L.M., C.J.F., C.W.H., and Q.Y. interpreted results of experiments; G.L.M., C.J.F., and Q.Y. prepared figures; G.L.M., C.J.F., C.W.H., and Q.Y. drafted manuscript; G.L.M., C.J.F., C.W.H., and Q.Y. edited and revised manuscript; G.L.M., C.J.F., C.W.H., and Q.Y. approved final version of manuscript; C.W.H. and Q.Y. conception and design of research.
ACKNOWLEDGMENTS
We thank Hilary Fry, Andrea Darby-King, and Dr. Qinlong Hou for excellent technical assistance.
REFERENCES
- Best AR, Wilson DA. Coordinate synaptic mechanisms contributing to olfactory cortical adaptation. J Neurosci 24: 652–660, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd AM, Sturgill JF, Poo C, Isaacson JS. Cortical feedback control of olfactory bulb circuits. Neuron 76: 1161–1174, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown LM. Developmental alterations in N-methyl-d-aspartate stimulated [3H]norepinephrine release in rat brain cortex and hippocampus. Neurosci Lett 154: 43–46, 1993 [DOI] [PubMed] [Google Scholar]
- Cui W, Darby-King A, Grimes MT, Howland JG, Wang YT, McLean JH, Harley CW. Odor preference learning and memory modify GluA1 phosphorylation and GluA1 distribution in the neonate rat olfactory bulb: testing the AMPA receptor hypothesis in an appetitive learning model. Learn Mem 18: 283–291, 2011 [DOI] [PubMed] [Google Scholar]
- Cummings DM, Henning HE, Brunjes PC. Olfactory bulb recovery after early sensory deprivation. J Neurosci 17: 7433–7440, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Almeida LB, Idiart MA, Linster C. A model of cholinergic modulation in olfactory bulb and piriform cortex. J Neurophysiol 109: 1360–1377, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debanne D. Associative synaptic plasticity in hippocampus and visual cortex: cellular mechanisms and functional implications. Rev Neurosci 7: 29–46, 1996 [DOI] [PubMed] [Google Scholar]
- Delaunay-El Allam M, Soussignan R, Patris B, Marlier L, Schaal B. Long-lasting memory for an odor acquired at the mother's breast. Dev Sci 13: 849–863, 2010 [DOI] [PubMed] [Google Scholar]
- Doucette W, Gire DH, Whitesell J, Carmean V, Lucero MT, Restrepo D. Associative cortex features in the first olfactory brain relay station. Neuron 69: 1176–1187, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallon JH, Moore RY. Catecholamine innervation of the basal forebrain. III. Olfactory bulb, anterior olfactory nuclei, olfactory tubercle and piriform cortex. J Comp Neurol 180: 533–544, 1978 [DOI] [PubMed] [Google Scholar]
- Franks KM, Isaacson JS. Synapse-specific downregulation of NMDA receptors by early experience: a critical period for plasticity of sensory input to olfactory cortex. Neuron 47: 101–114, 2005 [DOI] [PubMed] [Google Scholar]
- Grimes MT, Smith M, Li X, Darby-King A, Harley CW, McLean JH. Mammalian intermediate-term memory: new findings in neonate rat. Neurobiol Learn Mem 95: 385–391, 2011 [DOI] [PubMed] [Google Scholar]
- Hasselmo ME, Linster C, Patil M, Ma D, Cekic M. Noradrenergic suppression of synaptic transmission may influence cortical signal-to-noise ratio. J Neurophysiol 77: 3326–3339, 1997 [DOI] [PubMed] [Google Scholar]
- Illig KR. Developmental changes in odor-evoked activity in rat piriform cortex. Neuroscience 145: 370–376, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illig KR, Haberly LB. Odor-evoked activity is spatially distributed in piriform cortex. J Comp Neurol 457: 361–373, 2003 [DOI] [PubMed] [Google Scholar]
- Isaacson JS. Odor representations in mammalian cortical circuits. Curr Opin Neurobiol 20: 328–331, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanter ED, Haberly LB. NMDA-dependent induction of long-term potentiation in afferent and association fiber systems of piriform cortex in vitro. Brain Res 525: 175–179, 1990 [DOI] [PubMed] [Google Scholar]
- Kepecs A, Uchida N, Mainen ZF. The sniff as a unit of olfactory processing. Chem Senses 31: 167–179, 2006 [DOI] [PubMed] [Google Scholar]
- Kucharski D, Hall WG. New routes to early memories. Science 238: 786–788, 1987 [DOI] [PubMed] [Google Scholar]
- Kucharski D, Johanson IB, Hall WG. Unilateral olfactory conditioning in 6-day-old rat pups. Behav Neural Biol 46: 472–490, 1986 [DOI] [PubMed] [Google Scholar]
- Lethbridge R, Hou Q, Harley CW, Yuan Q. Olfactory bulb glomerular NMDA receptors mediate olfactory nerve potentiation and odor preference learning in the neonate rat. PLoS One 7: e35024, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JH. Autoradiographic estimation of the extent of reversible inactivation produced by microinjection of lidocaine and muscimol in the rat. Neurosci Lett 127: 160–164, 1991 [DOI] [PubMed] [Google Scholar]
- McLean JH, Harley CW, Darby-King A, Yuan Q. pCREB in the neonate rat olfactory bulb is selectively and transiently increased by odor preference-conditioned training. Learn Mem 6: 608–618, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean JH, Shipley MT, Nickell WT, Aston-Jones G, Reyher CK. Chemoanatomical organization of the noradrenergic input from locus coeruleus to the olfactory bulb of the adult rat. J Comp Neurol 285: 339–349, 1989 [DOI] [PubMed] [Google Scholar]
- Moriceau S, Sullivan RM. Corticosterone influences on mammalian neonatal sensitive-period learning. Behav Neurosci 118: 274–281, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S, Wilson DA, Levine S, Sullivan RM. Dual circuitry for odor-shock conditioning during infancy: corticosterone switches between fear and attraction via amygdala. J Neurosci 26: 6737–6748, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neville KR, Haberly LB. Beta and gamma oscillations in the olfactory system of the urethane-anesthetized rat. J Neurophysiol 90: 3921–3930, 2003 [DOI] [PubMed] [Google Scholar]
- Poo C, Isaacson JS. An early critical period for long-term plasticity and structural modification of sensory synapses in olfactory cortex. J Neurosci 27: 7553–7558, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poo C, Isaacson JS. Odor representations in olfactory cortex: “sparse” coding, global inhibition, and oscillations. Neuron 62: 850–861, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raineki C, Shionoya K, Sander K, Sullivan RM. Ontogeny of odor-LiCl vs. odor-shock learning: similar behaviors but divergent ages of functional amygdala emergence. Learn Mem 16: 114–121, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth TL, Moriceau S, Sullivan RM. Opioid modulation of Fos protein expression and olfactory circuitry plays a pivotal role in what neonates remember. Learn Mem 13: 590–598, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth TL, Sullivan RM. Memory of early maltreatment: neonatal behavioral and neural correlates of maternal maltreatment within the context of classical conditioning. Biol Psychiatry 57: 823–831, 2005 [DOI] [PubMed] [Google Scholar]
- Shipley MT, Ennis M. Functional organization of olfactory system. J Neurobiol 30: 123–176, 1996 [DOI] [PubMed] [Google Scholar]
- Stettler DD, Axel R. Representations of odor in the piriform cortex. Neuron 63: 854–864, 2009 [DOI] [PubMed] [Google Scholar]
- Strowbridge BW. Role of cortical feedback in regulating inhibitory microcircuits. Ann NY Acad Sci 1170: 270–274, 2009 [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Hall WG. Reinforcers in infancy: classical conditioning using stroking or intra-oral infusions of milk as UCS. Dev Psychobiol 21: 215–223, 1988 [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Leon M. One-trial olfactory learning enhances olfactory bulb responses to an appetitive conditioned odor in 7-day-old rats. Brain Res 432: 307–311, 1987 [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Stackenwalt G, Nasr F, Lemon C, Wilson DA. Association of an odor with activation of olfactory bulb noradrenergic beta-receptors or locus coeruleus stimulation is sufficient to produce learned approach responses to that odor in neonatal rats. Behav Neurosci 114: 957–962, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan RM, Wilson DA. Molecular biology of early olfactory memory. Learn Mem 10: 1–4, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi YK, Kurosaki M, Hirono S, Mori K. Topographic representation of odorant molecular features in the rat olfactory bulb. J Neurophysiol 92: 2413–2427, 2004 [DOI] [PubMed] [Google Scholar]
- Wachowiak M, Cohen LB. Representation of odorants by receptor neuron input to the mouse olfactory bulb. Neuron 32: 723–735, 2001 [DOI] [PubMed] [Google Scholar]
- Wang JK, Andrews H, Thukral V. Presynaptic glutamate receptors regulate noradrenaline release from isolated nerve terminals. J Neurochem 58: 204–211, 1992 [DOI] [PubMed] [Google Scholar]
- Yuan Q, Harley CW. What a nostril knows: olfactory nerve-evoked AMPA responses increase while NMDA responses decrease at 24-h post-training for lateralized odor preference memory in neonate rat. Learn Mem 19: 50–53, 2012 [DOI] [PubMed] [Google Scholar]


