Abstract
Purpose.
To assess the impact on visual function of community glaucoma screening in an African American population using spectral-domain optical coherence tomography (SD-OCT).
Methods.
Using a Monte Carlo microsimulation model with a 10-year time horizon, we analyzed the efficacy of SD-OCT screening on visual field outcomes in a population of African Americans who are not otherwise seeking office-based care. Outcomes included classification of visual field severity, quality-adjusted life years, and direct health care costs.
Results.
Assuming a 60% follow-up rate, screening decreased the prevalence of undiagnosed glaucoma from 75% to 38%, and decreased the prevalence of severe visual field loss in patients with glaucoma from 29.1% to 23.9%. Conversely, screening increased the prevalence of mild visual field loss in patients with glaucoma from 9.2% to 18.7%. From initial screening through confirmatory eye examination, the screening program (“screen only”) cost $98 per screened individual, and $2561 per new diagnosis of glaucoma. When considering the costs of initial screening though the resultant treatment, the screening program (“screen and treat”) had an average annual cost of $79 and $2138, respectively, over a 10-year time period. The cost of one quality-adjusted life year (QALY) gained by screening, including management and treatment, in comparison with opportunistic case finding, ranged from $46,416 to $67,813.
Conclusions.
Our findings suggest that community SD-OCT screening in an African American population will minimize glaucoma-related visual morbidity. Ideally, strategies to maximize treatment efficacy through improved medication adherence and improved compliance with follow-up should be identified and implemented before instituting a screening program.
Keywords: comparative effectiveness, SD-OCT, screening, Markov model, glaucoma
Using a Monte Carlo microsimulation model with a 10-year time horizon, we evaluated the impact of spectral-domain optical coherence tomography screening among African American patients on visual field loss and evaluated the associated costs of screening and treatment.
Introduction
Despite being a treatable condition, glaucoma is the leading cause of irreversible blindness worldwide.1 When diagnosed early, glaucoma can usually be halted through medical interventions. However, since most early glaucoma patients are asymptomatic and currently available screening tests are not optimal, there is often a significant delay in diagnosis resulting in irreversible visual loss. Epidemiologic studies and surveys show only 50% of glaucoma patients in the United States and other developed nations receive treatment.2 Disease detection in minority communities, which are disproportionately affected by glaucoma, may be as low as 25%.3 In part due to delayed detection, African American and other minority communities suffer a tremendous burden from glaucoma-related visual loss, resulting in approximately 15 times the rate of blindness compared with Caucasians of comparable age.4 Given the severity of disease in African Americans, it may be beneficial to implement regular screening in African American communities, as recommended by the United States Preventive Services Task Force (2005).5
Until recently, there was no diagnostic test available with sufficiently high sensitivity and specificity that was both simple to administer and easy for the patient to perform. Spectral-domain optic coherence tomography (SD-OCT) imaging of the optic nerve fulfills these criteria, which in turn may make the device a strong candidate for community screening. To date, there is no published long-term clinical data on the effectiveness of glaucoma screening using SD-OCT. A randomized-controlled clinical trial comparing SD-OCT screening to no screening would be ideal, but such a trial would be both lengthy and costly. The purpose of this study was to estimate the potential outcomes of SD-OCT screening among African American patients in the United States using decision analysis to synthesize the current evidence and project outcomes over a 10-year time horizon. To do this, we created a mathematical Markov model that incorporated clinical trial data on glaucoma progression, effects of treatment, adherence to follow up after screening, and diagnostic performance of SD-OCT to estimate future visual field losses.
A Markov model is a computer-based algorithm that assigns each simulated patient to one of a finite number of discrete health states for a period of time called a cycle. At the end of each cycle, patients may remain in their health state or progress to a different health state based on probabilities that are specified for transitioning from one health state to another. All probabilities, health states, and outcomes were derived from peer-reviewed clinical trial data. In order to best reflect a real world scenario, we developed a Monte Carlo microsimulation model, which is a Markov model that allows for variability among the individuals. The primary outcome of our Markov model was to compare the degree of functional visual loss as measured by visual fields in screened and unscreened patients after the implementation of a community screening initiative in high-risk communities. Visual field loss rather than central visual acuity was selected as the study endpoint because patients with good central vicual acuity and glaucoma-related visual field loss may still suffer from a measurable decline in a broad range of daily tasks including reading and driving.6–9 The secondary intent of this study was to assess the cost of the screening implementation.
Methods
Decision Analytic Model
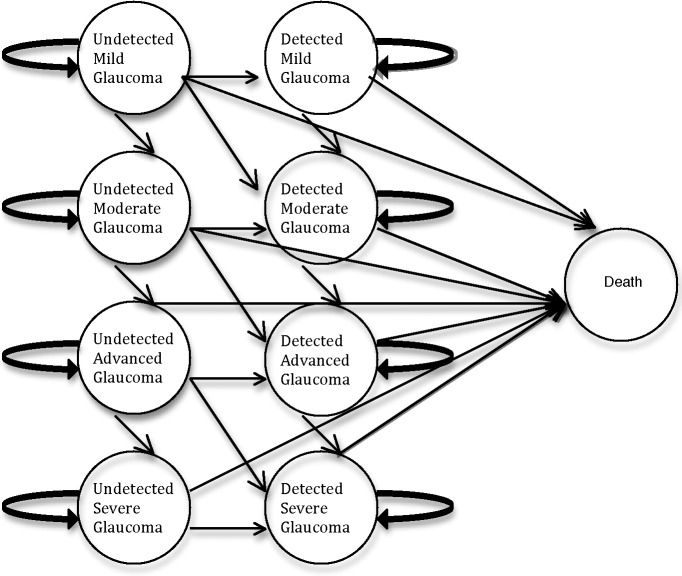
Our Markov model, diagrammed in Figure 1, assumes a one-time screening program for 50,000 African American patients age 50 years and older who are not seeking office-based care. The mean deviation (MD) of the visual field for each simulated glaucoma patient was randomly selected from a probability distribution function. Based on visual field MD and detection and treatment of glaucoma after the screening test, simulated patients were categorized into 1 of 10 health states: treated early, moderate, advanced, or severe glaucoma; untreated early, moderate, advanced, or severe glaucoma; glaucoma-free, and deceased. The visual field for each patient was then categorized and tracked for every year that a patient survived over the 10-year time horizon.10 In the model, detection of glaucoma during screening was based on the results of the worse eye. Given the relative paucity of data on bilateral visual field progression, the model was calibrated to the progression of the worse eye. All programming was performed with TreeAge Pro 2012. (TreeAge Software, Inc., Williamsport, MA, USA) Prevalence and incidence of disease, diagnostic characteristics of the OCT, baseline visual field characteristics, and risks of progression are detailed in Table 1.
Figure 1.
Diagram of the Markov model used to evaluate the effect of screening on visual field status. Note that patients who were screened may be either undetected or detected based on their follow-up.
Table 1.
Probabilities of Health Outcomes
|
Model Input |
Base Case Estimate |
Sensitivity Analysis Range |
| Prevalence of perimetric glaucoma13 | ||
| 50–59 y | 4.1% | |
| 60–69 y | 6.7% | |
| 70–79 y | 14.8% | |
| ≥80 y | 23.2% | |
| 9-y incidence of perimetric glaucoma14 | ||
| 50–59 y | 3.6% | |
| 60–69 y | 6.6% | |
| ≥70 y | 7.9% | |
| Sensitivity of SD-OCT15 | 0.85 | 0.85–0.95 |
| Specificity of SD-OCT15 | 0.95 | 0.85–0.95 |
| Population baseline visual field, probability distribution function left skewed with right tail, measured in dB16 | Median −2.5 dB Range +2 to −17 | |
| Visual function loss/yr, probability distribution function left skewed with right tail, measured in dB18 | Median −0.5 dB Range 0 to −7 | |
| Hazard ratio of progression/age over 50 y19 | 1.01 | |
| Time horizon10 | 10 y | 2–10 |
| Reduction in visual field progression in treated patients | 0.5020–22 | 0.3–0.7 |
| Follow-up after screening28,29 | 0.60 | 0.40–0.80 |
Population
The simulated cohort represented African American individuals 50 years and older who had not been previously diagnosed with glaucoma and were willing to undergo a glaucoma screening test with SD-OCT. The model started with patients at age 50 years of age because it reflects the beginning of the rising incidence of glaucoma in African Americans. Since glaucoma is not believed to reduce life expectancy,11 the model was populated using average age distributions and age-specific mortality rates for African American individuals based on US census data.12 Glaucoma prevalence and incidence rates were derived from the Barbados Eye Study,13,14 as it is the only measure of incidence in an African-derived population. Within the model, patients were categorized as having either diagnosed or undiagnosed glaucoma at the time of glaucoma development in the model. Given the limited data on annual rates of disease detection in undiagnosed patients, patients with glaucoma at the start of the model who were not detected by screening were assumed not to transition from undiagnosed to diagnosed glaucoma state for the remainder of the model. However, incident cases had a 25% disease detection rate.3
Diagnostic Testing
The simulated diagnostic performance was derived from the work of Bengtsson and colleagues15 who evaluated the validity and accuracy of SD-OCT to detect disease in a population setting. In their study, Bengtsson and colleagues15 derived sensitivity and specific of the average retinal nerve fiber layer (RNFL) from the automated comparison with the normative database that is included in the machine's software. For their study, values that fell outside the lower fifth percentile were considered a positive screening test result. In our model, patients with unsuccessful or unreliable test results were assumed to remain undiagnosed.
Visual Field Loss
The magnitude of an individual's visual field damage at the time of screening was selected from a probability distribution function that was calculated to reproduce the population distribution of visual field defects.16 Since there is currently no universally recognized glaucoma staging system, the Glaucoma Staging System (GSS) scale developed by Mills et al.17 was selected as it is objective, reproducible, and clinically useful. Using the GSS scale, patients were categorized as early, mild, advanced, or severe based on their visual field's MD. Incident cases during the 10-year time horizon were assumed to enter the model with an early visual field defect.
The model was calibrated to match the rates of glaucoma progression as presented by Heijl and colleagues18 in “Natural history of open-angle glaucoma” from the Early Manifest Glaucoma Treatment Study (EMGT). To account for variability among rates of progression, an individual's rate of progression was selected from a probability distribution function (PDF) derived from Figure 1 of Heijl's study. The PDF was skewed to the right with approximately 75% of the patients progressing at rates of 0.0 to −1.5 dB/y. The median rate of visual function loss was −0.5 dB/y with a range from 0 to −7.0 dB/y. In order to account for the increasing risk of visual field progression with increasing age, a hazard ratio of 1.01 risk of progression per year of age was incorporated into the model.19
Glaucoma Detection and Treatment
A failed screening test was followed with one of two scenarios: patients could undergo a comprehensive glaucoma evaluation or fail to follow up and remain undetected. Our model assumed a follow-up rate after screening of 60%, which was derived from the Harkness Eye Institute's community screening program. Incident cases after screening were assumed to have a 25% disease detection rate by usual care.3 Diagnosed patients were assumed to receive treatment. Treated patients had a 50% reduction in visual field progression as derived from the Normal Tension Glaucoma Treatment Study,20 the Ocular Hypertension Treatment Study,21 and the Early Manifest Glaucoma Trial.22
Costs
The costs of glaucoma screening and diagnoses were derived from the 2013 national Medicare reimbursement rates current procedural terminology (CPT) codes (Table 2). The costs of screening, including personnel costs, device costs, and the cost for the SD-OCT image acquisition,23 are shown in Table 3. Due to the variability in pricing, space rental and advertising for screening were not considered in the total cost of screening. Components of the confirmatory examination were derived from the recommendations of the Preferred Practice Patterns. All costs are in 2013 dollars and reflect the societal perspective. Annual medical costs were based on disease severity and reflected real-world compliance rates and Medicare national average allowances.24 Future costs were converted to net present value using a discount rate of 3% annually.
Table 2.
Costs of Screening and Treatment
| Costs for confirmatory eye examination40 | |
| OCT screening test (CPT code 92133) | $51.03 |
| Comprehensive ophthalmologic examination (CPT code 92004) | $110.48 |
| Humphrey visual field (CPT code 92083) | $75.94 |
| Gonioscopy (CPT code 92020) | $23.76 |
| Total annual direct cost of glaucoma treatment by stage (converted to 2013 dollars)24 | |
| Early glaucoma (−0.01 to −6.00 dB) | $1835 |
| Moderate glaucoma (−6.01 to −12 dB) | $2188 |
| Advanced glaucoma (−12.01 to −20 dB) | $2374 |
| Severe glaucoma (>−20.01 dB) | $3511 |
Table 3.
Costs of Personnel and Equipment for Screening
|
Item |
Cost (Per Site) |
Total (×15 sites) |
| Administrative23 | $27,000 | $405,000 |
| Ophthalmic photographer23 | $40,500 | $697,500 |
| Optical coherence tomographer | $50,000 | $750,000 |
| Total | $1,762,000 | |
| Cost per person (50,000 people) | $35.24 |
Utilities
Quality-adjusted life years (QALYs) are a frequent measure of utility in decision analysis and cost effectiveness modeling. QALYs reflect a range from death to perfect health, anchored at zero to one, respectively, for each year of life. QALYs are used so that differing health outcomes can be evaluated using a single-health outcome indicator in comparative effectiveness analysis. In this study, QALYs were derived from the work of Rein and colleagues25 who quantified utility values associated with visual field MD. Our model was populated with a uniform background utility of 0.87, the average health utility for individuals 50 years of age and older.26
Sensitivity Analysis
Sensitivity analyses were performed to evaluate the impact of parameter uncertainties on the model's predictions. In a series of one-way sensitivity analyses, we varied the assumed values of the following variables: (1) age of screened cohort, (2) efficacy of treatment, and (3) follow-up after failed screening examination. Diagnostic performance of OCT was evaluated in a two-way sensitivity analyses.
Results
Model Validation
For validation of the prevalence and incidence in our model, we compared the predicted prevalence of glaucoma based upon our model to the known prevalence in the Barbados Eye Studies.13 The prevalence of glaucoma in 60-year olds in the Barbados Eye Study was 6.7%. Using a cohort of 50-year olds over a 10-year time period, our model simulated a prevalence of 6.2%. Likewise, the prevalence of glaucoma in 70- and 80-year olds in the Barbados Eye Study was 14.8% and 23.2%, respectively, and our model predicted a prevalence of 12.1% for 70-year olds and 23.7% for 80-year olds.
For validation of the magnitude of visual field loss, the model was used to predict the untreated arm of the St. Lucia Eye Study.27 The mean baseline MD in the St. Lucia Study was −6.0 and, over a 10-year period, patients progressed to a mean MD of −16.3 dB. Simulating the same baseline deviation, our model predicted a MD of −16.2 dB.
Base Case
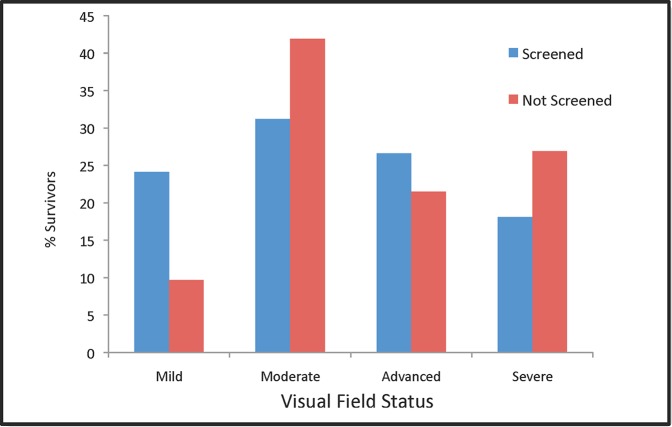
Results of the base case are displayed in Table 4. According to the model, implementation of the described glaucoma screening program decreases the prevalence of undiagnosed glaucoma from 75% to 38%, assuming a 60% follow-up rate after a failed screening test. The prevalence of severe visual field loss decreases from 29.1% to 23.9% in screened individuals with glaucoma (Table 4; Figs. 2, 3). Conversely, the prevalence of mild visual field loss increases from 9.2% to 18.7% in screened glaucoma patients. Table 5 displays visual field outcomes categorized by initial glaucoma severity at the time of screening.
Table 4.
Base Case Visual Field Outcomes Over 2, 5, 8, and 10 Years in Screened and Unscreened Individuals
|
Y |
Screened % of Survivors |
Not Screened % of Survivors |
Δ Screened vs. Not Screened |
Relative Risk Reduction |
||||||||||||
|
Sev |
Adv |
Mod |
Mild |
Sev |
Adv |
Mod |
Mild |
Sev |
Adv |
Mod |
Mild |
Sev |
Adv |
Mod |
Mild |
|
| 2 | 0.7 | 12.7 | 19.6 | 67 | 0.9 | 12.7 | 22.0 | 64.4 | 0.2 | 0.0 | 2.4 | −2.6 | 0.22 | 0.00 | 0.11 | −0.04 |
| 5 | 6.4 | 19.0 | 35.6 | 39.0 | 11.9 | 16.5 | 34.7 | 36.9 | 5.5 | −2.5 | −0.9 | −2.1 | 0.46 | −0.15 | −0.03 | −0.06 |
| 8 | 19.1 | 21.0 | 34.4 | 25.5 | 28.8 | 20.6 | 40.2 | 10.4 | 9.7 | −0.4 | 5.8 | −15.1 | 0.34 | −0.02 | 0.14 | −1.45 |
| 10 | 23.9 | 22.7 | 34.7 | 18.7 | 29.1 | 21.1 | 40.6 | 9.2 | 5.2 | −1.6 | 5.9 | −9.5 | 0.18 | −0.08 | 0.15 | −1.03 |
Sev, Severe; Adv, Advanced; Mod, Moderate.
Figure 2.
Percentage of patients in each visual field category after 10 years that were screened at 50-years old (blue) and in patients who were not screened (red).
Figure 3.
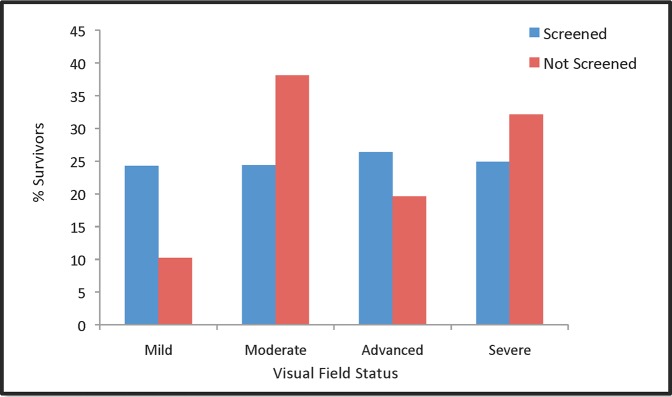
Percentage of patients in each visual field category after 10 years that were screened at 70-years old (blue) and in patients who were not screened (red).
Table 5.
Visual Field Outcomes at 2, 5, 8, and 10 Years Based on Baseline Visual Field
|
|
Screened, % of Survivors |
% Δ Screened vs. Not Screened |
Relative Risk Reduction |
|||||||||
|
Sev |
Adv |
Mod |
Mild |
Sev |
Adv |
Mod |
Mild |
Sev |
Adv |
Mod |
Mild |
|
| Mild VF loss at baseline | ||||||||||||
| 2 y | 0 | 0 | 15.1 | 84.9 | 0 | 0 | 6 | −6 | N/A | N/A | 0.28 | −0.08 |
| 5 y | 3.9 | 6.4 | 42.8 | 46.9 | 3.5 | 1.5 | 8.3 | −13.2 | 0.47 | 0.19 | 0.16 | −0.39 |
| 8 y | 11.7 | 11.3 | 50.2 | 26.8 | 8.1 | 0.5 | 4.3 | −12.9 | 0.41 | 0.04 | 0.08 | −0.93 |
| 10 y | 13.9 | 10.8 | 52.3 | 23 | 7.6 | −1.4 | 3.8 | −10 | 0.35 | −0.15 | 0.07 | −0.77 |
| Moderate VF loss at baseline | ||||||||||||
| 2 y | 0 | 0 | 100 | 0 | 0 | 0 | 0 | 0 | N/A | N/A | N/A | N/A |
| 5 y | 11.5 | 21.3 | 67.2 | 0 | 5.2 | 2 | −7.1 | 0 | 0.31 | 0.09 | −0.12 | N/A |
| 8 y | 22.6 | 29.6 | 47.8 | 0 | 6.9 | 7.4 | −14.3 | 0 | 0.23 | 0.2 | −0.43 | N/A |
| 10 y | 28.4 | 34.3 | 37.3 | 0 | 7.4 | 6.6 | −14 | 0 | 0.21 | 0.16 | −0.6 | N/A |
| Advanced VF loss at baseline | ||||||||||||
| 2 y | 4.5 | 95.5 | 0 | 0 | 4.2 | −4.2 | 0 | 0 | 0.48 | −0.05 | N/A | N/A |
| 5 y | 31.3 | 68.7 | 0 | 0 | 7.2 | −7.2 | 0 | 0 | 0.19 | −0.12 | N/A | N/A |
| 8 y | 45.6 | 54.4 | 0 | 0 | 11.8 | −11.8 | 0 | 0 | 0.21 | −0.28 | N/A | N/A |
| 10 y | 54.1 | 45.9 | 0 | 0 | 8.9 | −8.9 | 0 | 0 | 0.14 | −0.24 | N/A | N/A |
From initial screening through confirmatory eye examination, the screening program (“screen only”) incurred a one-time cost of $98 per screened individual, $377 to preserve 1-dB visual field, and $2561 per new diagnosis of glaucoma. When screening costs included the 10-year cost of management and treatment (with annual costs previously determined by Lee and colleagues24), the “screen and treat” costs rose considerably. Considering cost in terms of net present value over a 10-year time horizon, the cost per screened individual was $79, the cost to detect and preserve 1-dB visual field was $339, and the cost to detect and treat each new case of glaucoma was $2138. For purposes of cost-effectiveness analyses, the cost of one QALY gained by screening, including management and treatment, in comparison to opportunistic case finding ranged from $46,416 to $67,813.
Sensitivity Analysis
A sensitivity analysis was performed to evaluate the impact of parameter uncertainties on the outcomes of the model.
Since there is conflicting data on the ideal age to screen patients for glaucoma, we evaluated the impact of screening age on the model. (Table 6; Figs. 2, 3). While older patients have a higher prevalence of disease, younger patients have a greater average time from diagnosis to death and consequentially may benefit more from treatment. The sensitivity analyses demonstrates that after screening, the prevalence of severe visual field defects among glaucoma patients decreased from 26.9% to 18.1% (=Δ8.8%) in 50- to 59-year olds, and from 32.1% to 24.9% (=Δ7.2%) in 70- to 79-year olds, suggesting that younger patients have a slightly greater benefit from screening. The costs of “screen only” were relatively insensitive to patient age. However, the average annual costs per screened individual over a 10-year time horizon for “screen and treat” were $40, $71, and $119 for a 50-, 60-, and 70-year olds, respectively, due to the increasing prevalence of glaucoma and need for treatment with age. The cost of one QALY gained by screening in comparison to opportunistic case finding ranged from $46,532 to $58,611 in 50-year olds and from $37,522 to $49,654 in 70-year olds.
Table 6.
Visual Field Outcomes At 2, 5, 8, and 10 Years Based on Age at Screening
|
Y |
Screened, % of Survivors |
Not Screened, % of Survivors |
Δ Screened vs. Not Screened |
Relative Risk Reduction |
||||||||||||
|
Sev |
Adv |
Mod |
Mild |
Sev |
Adv |
Mod |
Mild |
Sev |
Adv |
Mod |
Mild |
Sev |
Adv |
Mod |
Mild |
|
| 50-y olds | ||||||||||||||||
| 2 | 0.2 | 13.7 | 20.1 | 66 | 0.6 | 13.4 | 25.3 | 60.7 | 0.4 | −0.3 | 5.2 | −5.3 | 0.67 | −0.02 | 0.21 | −0.09 |
| 5 | 6.4 | 15.3 | 38.6 | 39.7 | 11.4 | 14.8 | 36.6 | 37.2 | 5.0 | −0.5 | −2.0 | −2.5 | 0.44 | −0.03 | −0.05 | −0.07 |
| 8 | 18.9 | 15.8 | 40.9 | 24.4 | 27.5 | 16.0 | 47.2 | 9.3 | 8.6 | 0.2 | 6.3 | −15.1 | 0.31 | 0.01 | 0.13 | −1.62 |
| 10 | 18.1 | 26.6 | 31.2 | 24.1 | 26.9 | 21.5 | 41.9 | 9.7 | 8.8 | −5.1 | 10.7 | −14.4 | 0.33 | −0.24 | 0.26 | −1.48 |
| 60-y olds | ||||||||||||||||
| 2 | 0.4 | 12.8 | 23.4 | 63.4 | 0.6 | 12.9 | 24.9 | 61.6 | 0.2 | 0.1 | 1.5 | −1.8 | 0.33 | 0.01 | 0.06 | −0.03 |
| 5 | 8.3 | 16.6 | 35.2 | 39.9 | 12.6 | 15.7 | 35.2 | 36.5 | 4.3 | −0.9 | 0.0 | −3.4 | 0.34 | −0.06 | 0.00 | −0.09 |
| 8 | 18.4 | 22.5 | 37.6 | 21.5 | 29.5 | 25.6 | 37 | 7.9 | 11.1 | 3.1 | −0.6 | −13.6 | 0.38 | 0.12 | −0.02 | −1.72 |
| 10 | 20.1 | 25.8 | 29.8 | 24.3 | 27.9 | 22.9 | 39.8 | 9.4 | 7.8 | −2.9 | 10.0 | −14.9 | 0.28 | −0.13 | 0.25 | −1.59 |
| 70-y olds | ||||||||||||||||
| 2 | 0.8 | 11 | 22.2 | 66 | 1.2 | 10.7 | 26.1 | 62.1 | 0.4 | −0.3 | 3.9 | −4.0 | 0.33 | −0.03 | 0.15 | −0.06 |
| 5 | 7.7 | 17 | 40 | 35.3 | 10.7 | 17.1 | 37.0 | 35.1 | 3.0 | 0.1 | −3.0 | −0.1 | 0.28 | 0.01 | −0.08 | 0.00 |
| 8 | 21 | 19.4 | 33.7 | 25.9 | 31.1 | 19.0 | 39.1 | 10.8 | 10.1 | −0.4 | 5.4 | −15.1 | 0.32 | −0.02 | 0.14 | −1.40 |
| 10 | 24.9 | 26.4 | 24.4 | 24.3 | 32.1 | 19.6 | 38.1 | 10.2 | 7.2 | −6.8 | 13.7 | −14.1 | 0.22 | −0.35 | 0.36 | −1.38 |
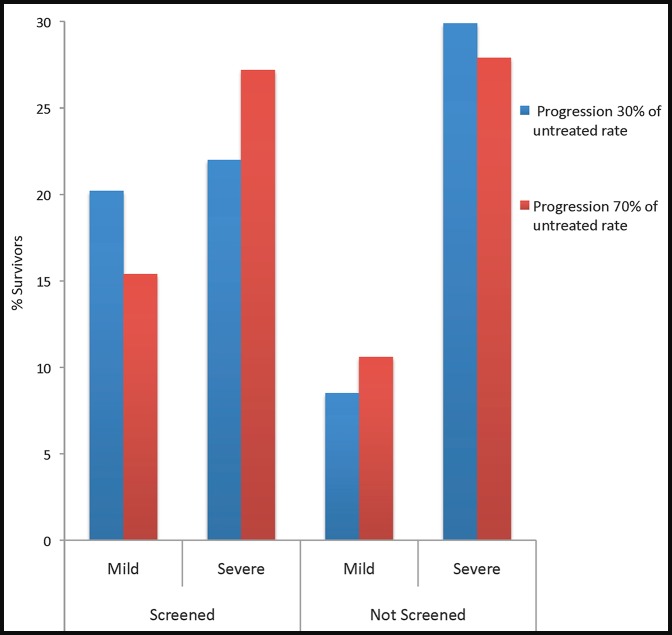
The sensitivity analysis considered a broad range of estimates for efficacy of treatment to account for uncertainty of this parameter (Table 7; Fig. 4). The base case assumed a 50% reduction in visual field progression in treated patients.20–22 When treatment decreased rates of progression to 70% of the untreated rate, the prevalence of severe visual field defects were minimally improved by screening when compared with no screening. When treatment decreased rates of progression to 30% of the untreated rate, prevalence of severe visual field defects in screened glaucoma patients relative to unscreened patients decreased from 29.9% to 22.0%. Specifically, improving the efficacy of treatment from 50% to 30% prevented an additional 1.9% of glaucoma patients from developing severe visual field loss, whereas decreasing the efficacy of treatment from 50% to 70% resulted in an additional 3.3% of glaucoma patients developing severe visual field loss. Efficacy of treatment had minimal impact on the cost of “screen only,” although improved medication efficacy lowered the cost of one QALY gained by screening and treatment to approximately $40,000.
Table 7.
Visual Field Outcomes at 2, 5, 8, 10 Years Based on Treatment Efficacy, Follow-up Rate, and Varied OCT Sensitivity and Specificity
|
|
Screened, % of Survivors |
Δ Not Screened vs. Screened |
Relative Risk Reduction |
|||||||||
|
Sev |
Adv |
Mod |
Mild |
Sev |
Adv |
Mod |
Mild |
Sev |
Adv |
Mod |
Mild |
|
| Rate of progression | ||||||||||||
| 30 | 22 | 17.3 | 40.5 | 20.2 | 7.9 | 1.1 | 2.7 | −11.7 | 0.26 | 0.06 | 0.06 | −1.38 |
| 50 | 23.9 | 22.7 | 34.7 | 18.7 | 5.2 | −1.6 | 5.9 | −9.5 | 0.18 | −0.08 | 0.15 | −1.03 |
| 70 | 27.2 | 19.2 | 38.2 | 15.4 | 0.7 | 1.1 | 3.0 | −4.8 | 0.03 | 0.05 | 0.07 | −0.45 |
| Follow-up rate | ||||||||||||
| 40 | 24.6 | 24.2 | 33.5 | 17.7 | 2.2 | −2.1 | 7.6 | −7.7 | 0.08 | −0.10 | 0.18 | −0.77 |
| 60 | 23.9 | 22.7 | 34.7 | 18.7 | 5.2 | −1.6 | 5.9 | −9.5 | 0.18 | −0.08 | 0.15 | −1.03 |
| 80 | 18.6 | 26.3 | 29.9 | 25.2 | 9.3 | −6.0 | 11.3 | −14.6 | 0.33 | −0.30 | 0.27 | −1.38 |
| Sensitivity/specificity | ||||||||||||
| 85/95 | 23.9 | 22.7 | 34.7 | 18.7 | 5.2 | −1.6 | 5.9 | −9.5 | 0.18 | −0.08 | 0.15 | −1.03 |
| 95/85 | 24.1 | 23.3 | 32 | 20.6 | 7.0 | −1.6 | 6.0 | −11.5 | 0.23 | −0.07 | 0.16 | −1.26 |
Figure 4.
Percentage of patients with visual field outcomes that were mild or severe at 10 years when treatment lowers progression to 30% of untreated rate (blue) and when treatment lowers progression to 70% of untreated rate (red) in screened and unscreened patients.
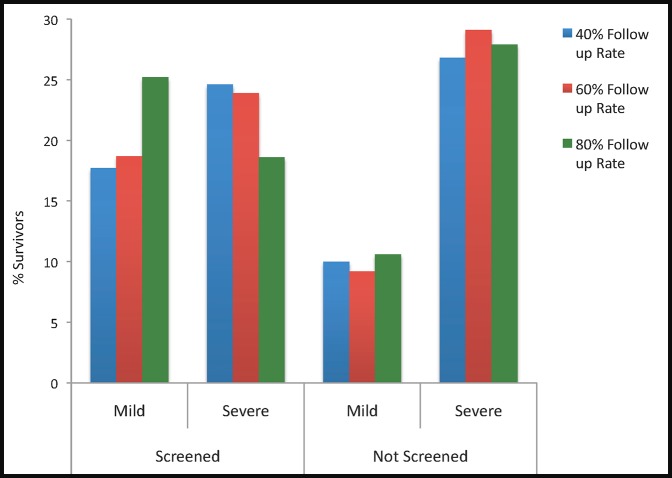
Studies on patient adherence with follow up after screening suggest that 40% to 65% of patients have a comprehensive ophthalmic examination after a failed glaucoma screening test.28,29 Varying the follow-up rate had a substantial impact on the outcomes of the model. (Table 7; Fig. 5) Among glaucoma patients, improving the follow-up rate from 60% to 80% prevented an additional 5.3% of glaucoma patients from developing severe visual field loss, whereas decreasing the follow-up rate to 40% resulted in an additional 0.7% of glaucoma patients developing severe visual field defects. “Screen only” costs were minimally affected by follow-up rate, but “screen and treat” annual costs ranged from $57 for 40% follow-up to $107 for an 80% follow-up over a 10-year time period.
Figure 5.
Percentage of patients with mild and severe visual field loss based on follow-up rate at 10 years of follow-up.
As there is limited data on the performance of SD-OCT in population screening, we reran the model while covarying the sensitivity and specificity of the average RNFL parameter. We assumed a correlation coefficient of −1, and covaried the two parameters between 85% and 95%. The diagnostic changes, however, had minimal impact on the visual impairment or cost generated in the base case (Table 7).
Discussion
The current gold standard for glaucoma screening is a comprehensive eye examination including an IOP measurement, dilated fundus examination, and visual field evaluation by an eye care specialist. However, a low percentage of individuals seek routine eye care in the office setting, leaving many individuals unscreened for glaucoma. An alternative is to supplement office-based screening with community-based screening for patients who might otherwise not be seen in a doctor's office. The intent of our Markov model was to assess the impact of community screening using SD-OCT on visual function, as measured by visual fields, due to glaucomatous damage. The secondary intent was to assess the cost of the screening implementation. Using patient level simulation data, we found that screening decreased the prevalence of undiagnosed glaucoma from 75% to 38%, assuming a 60% follow-up rate after failed screening test. In addition, for every 100 glaucoma patients screened with SD-OCT, there were five fewer patients with glaucoma-related severe visual field loss after 10 years than in their unscreened counterparts. Screening implementation would result in a one-time cost of $98 per screened individual. When screening costs included treatment, annual costs rose to $79 per patient screened over a 10-year time horizon, suggesting that the bulk of the incremental cost of screening is the result of disease management and treatment. The cost of one QALY gained by screening in comparison to opportunistic case finding ranged from $46,416 to $67,813. In comparison, the cost per QALY gained for the meningococcal polysaccharide vaccine is $138,000/QALY and the cost per QALY gained for installing rear seat belts ranges from $160,000 to $830,000/QALY gained.30
Our work is in keeping with recent studies that have found glaucoma screening to be cost effective. A recent microsimulation model by Ladapo and colleagues31 evaluated the implementation of frequency doubling technology as a community-screening tool for African American patients who are 50 to 59 years of age. The study found a 0.5% decrease in glaucoma-related blindness at a cost of $80 per screened individual when considering only the cost of the FDT and a confirmatory eye examination. In comparing the results of this study with ours, it is important to note that Ladapo's model used central visual acuity as the primary effectiveness endpoint. Increasing evidence suggests that even early visual field loss will have a negative impact on vision-related quality of life.32,33 As such, using central visual loss as a primary endpoint might fail to capture the true effectiveness of a glaucoma screening study. Another study evaluated the cost effectiveness of opportunistic glaucoma case detection during routine ophthalmologic visits in the United States. In this study, Rein and colleagues26 found a cost of $46,000 per QALY gained and concluded that office-based detection is a cost-effective way to reduce glaucomatous visual loss. Our study differs from Rein's model in two important ways. First, our study evaluated community- and not office-based detection. Factors such as financial limitations, difficulty with transportation, inadequate disease knowledge, and limited access to care may reduce the number of individuals seeking office-based screening. Second, although QALYs are a common measure of health-related utility, some experts argue that the concept of a QALY fails to accurately capture patients' actual perceptions and preferences.34 In glaucoma research, the inherent weaknesses of the QALY as a utility measure are compounded by limited data and varying assignment of QALY values when different methodological approaches are applied.35 Despite these limitations, to be comprehensive in our results we have taken into consideration the incremental cost per QALY gained. However, given the variability in glaucoma-specific QALYs, we chose to use visual function as measured by category of visual field loss as a more concrete and clinically meaningful effectiveness measure.
The results of this study are subject to the reliability of our parameters estimates. To ensure that the value of an individual parameter does not substantially affect our results, we ran a sensitivity analyses. These results suggest that visual function was markedly greater in screened patients than unscreened patients regardless of the variability of the parameter estimates. Poor follow-up rate and decreased treatment efficacy negatively impacted visual preservation.
There are at least six limitations of this study. First, we estimated the rate of visual field progression from the EMGTS, as it is the one longitudinal study that monitored high-tension glaucoma patients without treatment.22 However, given the significant visual disability and blindness in African-derived glaucoma patients, it has been hypothesized that African persons may have a faster rate of progression than their European-derived counterparts. However, we believe the use of the untreated EMGT arm was justified in this model as previous work has suggested an earlier onset of disease in African Americans, but no differences in rate of progression among racial groups. Furthermore, our visual field progression results are similar to the 10-year follow-up of the St. Lucia study, whose cohorts consisted primarily of African-derived persons.
Second, MD was used as the primary visual field classification in evaluating the functional damage of glaucoma. Confounding factors such as cataract might have an independent effect on MD. Since we did not consider cataract status for patients entering the model, the final MD for both the screened and unscreened cohorts in our model may underestimate the true final MD once the impact of cataract is considered. However, we anticipate this difference is slight as studies suggest that cataract extraction in patients with perimetric glaucoma only improves MD minimally.36
Third, several studies have demonstrated that treatment offers a 50% reduction in progression of glaucoma. Although this rate of reduction has been derived from several large-scale clinical trials of mixed race/ethnicity,20–22 it has not been evaluated in exclusively African-derived patients. It is possible that the higher incidence of visual morbidity in this population results from decreased response to treatment. Furthermore, it is known that patient adherence to treatment is suboptimal in the general patient population. A 50% reduction in progression may reflect a well-controlled and monitored clinical-trial population and not the population at large.
Fourth, since undetected patients are not receiving treatment, the model assumed that such patients had no societal cost. This assumption was made because the unit of analysis for the model was the worse eye and not the individual, and because there is insufficient data to describe indirect costs of visual loss. Particularly in more severe glaucoma, indirect costs are a tremendous driver of expense.37 As such, this model overestimates the incremental cost of screening over opportunistic case detection.
Fifth, because of limited data on the annual transition rates from undiagnosed to clinically diagnosed glaucoma, the model assumes that the study population would not otherwise seek office-based eye care and, therefore, would remain undiagnosed and untreated. Although this assumption may bias the model toward greater visual disability in the unscreened group, we felt this relative loss in effectiveness in the unscreened arm was partially offset by the assumption that untreated patients incurred no societal cost, as discussed above. Future studies should be aimed at assessing these transition probabilities.
Finally, there is a significant body of literature evaluating the diagnostic performance of SD-OCT in the office setting. However, there is limited SD-OCT data in the community setting and to date there is no published data with regard to SD-OCT community screening in an entirely African American population. Since the Bengtsson study15 was performed in an exclusively European-derived population, it was unclear whether the study's results were generalizable to our model. However, we felt its use was acceptable because prior studies have demonstrated that race does not appear to have an effect on the diagnostic performance of imaging.38 Furthermore, given the limited data, the model assumed that the SD-OCT technology, treatment efficacy, and life expectancy would remain unchanged over the 10-year time horizon of the study.
Conclusions
The results of this study highlight the importance of treatment efficacy and compliance with follow-up as important predictors of visual outcomes after screening. Poor follow-up for confirmatory eye examinations and decreased treatment efficacy resulting from lack of adherence are modifiable barriers to prevention of visual loss after screening. Ideally, strategies to improve compliance should be identified and implemented before instituting a screening program.
Our findings suggest that SD-OCT screening in an African American population will minimize glaucoma-related visual morbidity. Although there are moderate costs for the screening program, the United States has sizeable costs related to vision loss. Frick et al.39 estimated that total excess medical expenditures for blind and visually impaired patients in the United States was in excess of $5 billion annually (net present value approximately $5.62 billion in 2013) and even these sizeable estimates incorporate only direct costs. Particularly in more severe glaucoma, indirect costs are a tremendous driver of expense.39 Such costs can be decreased by earlier detection, potentially through an organized screening program such as the one described here.
Acknowledgments
Supported by grants from the National Cancer Institute, National Institutes of Health (KM1 CA 156709).
Disclosure: D.M. Blumberg, None; R. Vaswani, None; E. Nong, None; L. Al-Aswad, None; G.A. Cioffi, None
References
- 1. Broman AT, Quigley HA, West SK, et al. Estimating the rate of progressive visual field damage in those with open-angle glaucoma, from cross-sectional data. Invest Ophthalmol Vis Sci. 2008; 49: 66–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tielsch JM, Sommer A, Katz J, Royall RM, Quigley H, Javitt J. Racial variations in the prevalence of primary open-angle glaucoma. The Baltimore Eye Survey. JAMA. 1991; 266: 369–374 [PubMed] [Google Scholar]
- 3. Varma R, Ying-Lai M, Francis BA, et al. Prevalence of open-angle glaucoma and ocular hypertension in Latinos: the Los Angeles Latino Eye Study. Ophthalmology. 2004; 111: 1439–1448 [DOI] [PubMed] [Google Scholar]
- 4. Muñoz B, West SK, Rubin GS, et al. Causes of blindness and visual impairment in a population of older Americans: the Salisbury Eye Evaluation Study. Arch Ophthalmol. 2000; 118: 819–825 [DOI] [PubMed] [Google Scholar]
- 5. Fleming C, Whitlock E, Beil T, Smit B. Primary Care Screening for Ocular Hypertension and Primary Open-Angle Glaucoma. Agency for Healthcare Research and Quality (US): Rockville; 2005. Available at: Accessed February, 16 2013. [PubMed] [Google Scholar]
- 6. Mangione CM, Berry S, Spritzer K, et al. Identifying the content area for the 51-item National Eye Institute Visual Function Questionnaire: results from focus groups with visually impaired persons. Arch Ophthalmol. 1998; 116: 227–233 [DOI] [PubMed] [Google Scholar]
- 7. Ramulu PY, West SK, Munoz B, Jampel H, Friedman D. Driving cessation and driving limitation in glaucoma: the Salisbury Eye Evaluation Project. Ophthalmology. 2009; 116: 1846–1853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Janz NK, Musch DC, Gillespie BW, Wren PA, Niziol LM; for the Collaborative Initial Glaucoma Treatment Study (CIGTS) Investigators. Evaluating clinical change and visual function concerns in drivers and nondrivers with glaucoma. Invest Ophthalmol Vis Sci. 2009; 50: 1718–1725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McGwin G, Xie A, Mays A, et al. Visual field defects and the risk of motor vehicle collisions among patients with glaucoma. Invest Ophthalmol Vis Sci. 2005; 46: 4437–4441 [DOI] [PubMed] [Google Scholar]
- 10. Hernández RA, Burr JM, Vale LD. OAG. Screening Project Group. Economic evaluation of screening for open-angle glaucoma. Int J Technol Assess Health Care. 2008; 24: 203–211 [DOI] [PubMed] [Google Scholar]
- 11. Stein JD, Newman-Casey PA, Niziol LM, Gillespie GW, Lichter PR, Musch DC. Association between the use of glaucoma medications and mortality. Arch Ophthalmol. 2010; 128: 235–240 [DOI] [PubMed] [Google Scholar]
- 12. U.S. Census Bureau. 2010 Census of Population and Housing. Summary Population and Housing Characteristics, CPH-1-1. Washington, DC: United States U.S. Government Printing Office; 2013. [Google Scholar]
- 13. Leske MC, Connell AM, Schachat AP, Hyman L. The Barbados Eye Study. Prevalence of open angle glaucoma. Arch Ophthalmol. 1994; 112: 821–829 [DOI] [PubMed] [Google Scholar]
- 14. Leske MC, Wu SY, Honkanen R, et al. Nine-year incidence of open-angle glaucoma in the Barbados Eye Studies. Ophthalmology. 2007; 114: 1058–1064 [DOI] [PubMed] [Google Scholar]
- 15. Bengtsson B, Andersson S, Heijl A. Performance of time-domain and spectral-domain optical coherence tomography for glaucoma screening. Acta Ophthalmol. 2012; 90: 310–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Boland MV, Quigley HA. Evaluation of a combined index of optic nerve structure & function for glaucoma diagnosis. BMC Ophthalmol. 2011; 11: 6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mills RP, Budenz DL, Lee PP, et al. Categorizing the stage of glaucoma from pre-diagnosis to end-stage disease. Am J Ophthalmol. 2006; 141: 24–30 [DOI] [PubMed] [Google Scholar]
- 18. Heijl A, Bengtsson B, Hyman L, Leske MC; for the Early Manifest Glaucoma Trial Group. Natural history of open-angle glaucoma. Ophthalmology. 2009; 116: 2271–2276 [DOI] [PubMed] [Google Scholar]
- 19. Leske MC, Heijl A, Hussein M, et al. Factors for glaucoma progression and the effect of treatment: the early manifest glaucoma trial. Arch Ophthalmol. 2003; 121: 48–56 [DOI] [PubMed] [Google Scholar]
- 20. Collaborative Normal-Tension Glaucoma Study Group. Comparison of glaucomatous progression between untreated patients with normal-tension glaucoma and patients with therapeutically reduced intraocular pressures. Am J Ophthalmol. 1998; 126: 487–497 [DOI] [PubMed] [Google Scholar]
- 21. Kass MA, Heuer DK, Higginbotham EJ, et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002; 120: 701–713, discussion 829–830 [DOI] [PubMed] [Google Scholar]
- 22. Heijl A, Leske MC, Bengtsson B, Hyman L, Bengtsson B, Hussein M; for the Early Manifest Glaucoma Trial Group. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol. 2002; 120: 1268–1279 [DOI] [PubMed] [Google Scholar]
- 23. Ophthalmic Photographer Salary (United States). Available at: http://www.payscale.com/research/US/Job=Ophthalmic_Photographer/Hourly_Rate. Accessed November 15, 2013. [Google Scholar]
- 24. Lee PP, Walt JG, Doyle JJ, et al. A multicenter, retrospective pilot study of resource use and costs associated with severity of disease in glaucoma. Arch Ophthalmol. 2006; 124: 12–19 [DOI] [PubMed] [Google Scholar]
- 25. Rein DB, Wirth DE, Johnson C, Lee PP. Estimating quality-adjusted life year losses associated with visual field deficits using methodological approaches. Ophthalmic Epidemiology. 2007; 14: 258–264 [DOI] [PubMed] [Google Scholar]
- 26. Rein DB, Wittenborn JS, Lee PP, et al. The cost-effectiveness of routine office-based identification and subsequent medical treatment of primary open angle glaucoma in the United States. Ophthalmology. 2009; 116: 823–832 [DOI] [PubMed] [Google Scholar]
- 27. Wilson MR, Kosoko O, Cowan CL Jr, et al. Progression of visual field loss in untreated glaucoma patients and glaucoma suspects in St. Lucia, West Indies. Am J Ophthalmol. 2002; 134: 399–405 [DOI] [PubMed] [Google Scholar]
- 28. Quigley HA, Park CK, Tracey PA, Pollack IP. Community screening for eye disease by laypersons: the Hoffberger program. Am J Ophthalmol. 2002; 133: 386–392 [DOI] [PubMed] [Google Scholar]
- 29. Mansberger SL, Edmunds B, Johnson CA, Kent KJ, Cioffi GA. Community visual field screening: prevalence of follow-up and factors associated with follow-up of participants with abnormal frequency doubling perimetry technology results. Ophthalmic Epidemiol. 2007; 14: 134–140 [DOI] [PubMed] [Google Scholar]
- 30. Grosse SD, Teutsch SM, Haddix AC. Cost effectiveness research for United States public health policy. Annu Rev Public Health. 2007; 28: 365–391 [DOI] [PubMed] [Google Scholar]
- 31. Ladapo JA, Kymes SM, Ladapo J, Nwosu VC, Pasquale LR. Projected clinical outcomes of glaucoma screening in African American individuals. Arch Ophthalmol. 2012; 130: 365–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. McKean-Cowdin R, Varma R, Wu J, et al. for the Los Angeles Latino Eye Study Group. Severity of visual field loss and health- related quality of life. Am J Ophthalmol. 2007; 143: 1013–1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hyman LG, Komaroff E, Heijl A, Bengtsson B, Leske MC; for the Early Manifest Glaucoma Trial Group. Treatment and vision-related quality of life in the early manifest glaucoma trial. Ophthalmology. 2005; 112: 1505–1513 Medicare fee schedule of services. [DOI] [PubMed] [Google Scholar]
- 34. Coast J. Is economic evaluation in touch with society's health values? BMJ. 2004; 329: 1233–1236 Available at: http://www.bmj.com/rapid-response/2011/10/30/diminishing-returns-complex-calculations-qaly. Accessed April 2, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kymes SM. Is it time to move beyond the QALY in vision research? Ophthalmic Epidemiol. 2014; 21: 63–65 [DOI] [PubMed] [Google Scholar]
- 36. Smith SD, Katz J, Quigley HA. Effect of cataract extraction on the results of automated perimetry in glaucoma. Arch Ophthalmol. 1997; 115: 1515–1519 [DOI] [PubMed] [Google Scholar]
- 37. Thygesen J, Aagren M, Arnavielle S, et al. Late-stage, primary open-angle glaucoma in Europe: social and health care maintenance costs and quality of life of patients from 4 countries. Curr Med Res Opin. 2008; 24: 1763–1770 [DOI] [PubMed] [Google Scholar]
- 38. Girkin CA, Liebmann J, Fingeret M, Greenfield DS, Medeiros F. The effects of race, optic disc area, age, and disease severity on the diagnostic performance of spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2011; 52: 6148–6153 [DOI] [PubMed] [Google Scholar]
- 39. Frick KD, Gower EW, Kempen JH, Wolff JL. Economic impact of visual impairment and blindness in the United States. Arch Ophthalmol. 2007; 125: 544–550 [DOI] [PubMed] [Google Scholar]
- 40. Centers for Medicare and Medicaid Services. Available at: http://www.cms.gov/apps/physician-fee-schedule/. Accessed March 11, 2013. [PubMed] [Google Scholar]