Abstract
Men are at greater risk for renal injury and dysfunction after acute ischemia-reperfusion (I/R) than are women. Studies in animals suggest that the reason for the sex difference in renal injury and dysfunction after I/R is the protective effect of estrogens in females. However, a reduction in testosterone in men is thought to play an important role in mediating cardiovascular and renal disease, in general. In the present study, we tested the hypothesis that I/R of the kidney reduces serum testosterone, and that contributes to renal dysfunction and injury. Male rats that were subjected to renal ischemia of 40 min followed by reperfusion had a 90% reduction in serum testosterone by 3 h after reperfusion that remained at 24 h. Acute infusion of testosterone 3 h after reperfusion attenuated the increase in plasma creatinine and urinary kidney injury molecule-1 (KIM-1) at 24 h, prevented the reduction in outer medullary blood flow, and attenuated the increase in intrarenal TNF-α and the decrease in intrarenal VEGF at 48 h. Castration of males caused greater increases in plasma creatinine and KIM-1 at 24 h than in intact males with renal I/R, and treatment with anastrozole, an aromatase inhibitor, plus testosterone almost normalized plasma creatinine and KIM-1 in rats with renal I/R. These data show that renal I/R is associated with sustained reductions in testosterone, that testosterone repletion protects the kidney, whereas castration promotes renal dysfunction and injury, and that the testosterone-mediated protection is not conferred by conversion to estradiol.
Keywords: kidney injury molecule-1, sex, androgens, vascular endothelial growth factor
acute kidney injury (aki) is thought to affect ∼2–5% of all hospitalized individuals in the United States (31a), and the mortality rates are as high as 80% (5, 11, 31). Aging increases both the incidence and the mortality rates due to AKI in both men and women. Despite the importance of the problem, the mechanisms responsible for AKI have not been completely elucidated.
The incidence of acute renal failure in surgical patients is significantly higher in men than women (8, 16, 20). Animal studies have also reported sex differences in the adverse effects of ischemia reperfusion (I/R) of the kidney with males being more susceptible than females (18, 21). The mechanisms responsible for the sex differences in AKI in humans and the sex differences in animals are not clear.
Reductions in serum testosterone in men have been shown in numerous studies to occur with chronic disease, such as heart disease, obesity, hypertension, and renal disease (2, 13, 15, 24). The association of reduction in testosterone with increased cardiovascular disease has lead investigators to suggest that it is the reduction in testosterone that may contribute to chronic disease states rather than that the chronic disease is the cause of the reduction in circulating testosterone levels (15). There is also evidence that acute conditions may reduce androgen levels. For example, Pugh et al. (23) and Tripathi and Hegde (30) reported that serum androgen levels fell acutely following myocardial infarction (MI) in men. In fact, Militaru et al. (17) found that the level of testosterone on admission for MI had a positive correlation with the short-term mortality in men. There is also suppression of testosterone levels following acute traumatic brain injury (32, 33). In addition, in animal studies, intraperitoneal injection of LPS (a model of sepsis) caused a significant reduction in serum testosterone levels in male rats (26). Finally, and most importantly for the present studies, Robert et al. (27) found that renal I/R in rats resulted in reductions in testosterone levels at 1 and 5 days after reperfusion. However, whether the reduction in androgens is a consequence of I/R or plays a role in mediating injury associated with renal I/R is not clear.
The present studies were designed to evaluate the role played by testosterone in response to I/R of the kidney in male rats. The hypothesis tested was that I/R of the kidney in male rats results in reductions in serum testosterone, and infusion of testosterone 3 h after reperfusion attenuates renal injury.
METHODS
Animals.
Male Sprague-Dawley rats, aged 11–12 wk (350–370 g), were obtained from the vendor (Harlan Sprague Dawley, Indianapolis, IN) and allowed to equilibrate in a temperature-controlled environment with 12:12-h light-dark cycle for at least 1 wk. Rats were allowed ad libitum access to rat chow (Teklad 1% NaCl) and tap water. All procedures were approved by the Animal Care and Use Committee of the University of Mississippi Medical Center and followed the Guidelines for the Care and Use of Laboratory Animals, 2011 edition. Rats were placed into several groups depending on the experiment. In experiment 1, we examined the effect of I/R on serum testosterone and the effect of testosterone supplement on I/R injury: intact sham, intact sham + testosterone propionate, intact rats with renal I/R, intact rats with I/R + testosterone supplement (n = 5 or 6/group). In experiment 2, we investigated the effect of castration or supplemental testosterone on I/R injury: intact sham, intact with renal I/R, castrated rats with I/R, castrated rat with I/R and testosterone supplement (n = 5 or 6/group). In experiment 3, we explored the role of conversion to estradiol in mediating the attenuation of renal I/R injury with testosterone propionate: sham, rats with I/R injury, rats with I/R injury treated with testosterone, rats with I/R injury treated with anastrazole, aromatase inhibitor, and rats with I/R injury treated with anastrazole and testosterone (n = 5 or 6/group).
Renal I/R-AKI.
Under isoflurane anesthesia, an abdominal incision was made, and the renal pedicles on both kidneys were bilaterally occluded with a microvascular clamp (bulldog) for 40 min. The temperature of the animal was maintained during the procedure within 35.5–37°C, as monitored by a rectal probe. At 40 min, both clamps were removed, and blood flow to the kidneys was reestablished. The surgical incision was sutured, and rats were allowed to recover with free access to food and water. Analgesia, using buprenorphine (Sigma Aldrich, St. Louis, MO; 0.01 mg/kg sc), was given following recovery from anesthesia. During the first 24 h following the procedure, animals were placed in metabolic cages for urine collection. Urine samples were stored at −80°C for kidney injury molecule-1 (KIM-1) and protein excretion. Blood samples from the jugular vein were drawn at 24 h after the procedure to evaluate serum creatinine.
Renal hemodynamics.
Two days after inducing AKI, some animals were anesthetized again with isoflurane, and placed laterally, exposing the left lumbar area. An incision was made on the left side, and the left kidney was isolated from the perirenal fat, and stabilized on a special plastic holder. A laser-Doppler probe (Transonic Systems, Ithaca, NY) attached to a BLF-21D laser-Doppler flow meter (Transonic Systems) was inserted into the kidney to a depth of 4–5 mm from the surface to measure the outer medullary blood flow (OMBF). The animal was allowed to stabilize for 30 min, after which recordings were collected for 20 min and analyzed with the Power Lab software (AD Instruments, Colorado Springs, CO). The final value of OMBF was expressed as Tissue Perfusion units (TPU).
After the measurements were done, the animal was euthanized by exsanguination under deep anesthesia, and both kidneys were removed, homogenized in RIPA buffer (Santa Cruz Biotechnology, Santa Cruz, CA) (1:4 tissue to volume ratio) containing proteinase inhibitor tablet (Roche Pharmaceuticals, Nutley, NJ), and stored at −80°C, for molecular determinations.
Testosterone infusion.
Following ischemia and 3 h after reperfusion, a continuous intravenous infusion of testosterone propionate (Sigma Aldrich, St. Louis, MO; 20 μg·kg−1·min−1 in 0.2% EtOH in 1.8 ml of 0.9% NaCl, given for 10 min) or vehicle was given via the femoral vein. This time point was chosen on the basis of our preliminary studies that immediately after reperfusion, there is a transient recovery in the outer medullary blood flow (OMBF), which after 3 h post reperfusion begins to drop again. We followed the animals for three additional hours and found that OMBF continued to be lower in I/R rats than in sham animals. Thus, we used the 3 h after reperfusion as a time point to infuse testosterone acutely in the hopes that we could attenuate the hypoperfusion, since testosterone has acute vasodilatory actions (22). In addition, mRNA expression of inflammatory markers (TNF-α, IL-6) is detected as early as 4–6 h after reperfusion in models of I/R of the kidney.
Serial testosterone levels in rats with I/R.
To determine the levels of serum testosterone that occur in rats subjected to sham surgery or I/R, rats were treated as described above except that blood (100 μl) was drawn from a femoral catheter at 3, 6, 12, and 24 h after reperfusion. For percent change from control, a separate group of rats that were not exposed to surgery was used as 100% (serum testosterone levels = 387.72 ± 93.27 ng/ml; n = 6). Serum testosterone was measured by Coat-A-Coat RIA, as we have previously described (25).
Anastrozole infusion.
To prevent conversion of testosterone to estradiol, male rats received the aromatase inhibitor, anastrazole (AstraZeneca, Wilmington, DE; 0.15 mg·kg−1·day−1, in water by gavage), or vehicle, starting 3 days before I/R and continued thereafter.
Indices of renal damage and function.
Loss of renal function was evaluated by quantifying the levels of plasma creatinine at 24 h after I/R, using a commercially available kit (Quantichrome creatinine assay kit; BioAssay Systems, Hayward, CA), from blood collected in EDTA via the jugular vein in isoflurane-anesthetized animals. Data are presented as micrograms creatinine per deciliter.
KIM-1.
KIM-1, a sensitive biomarker for proximal tubular injury, was measured in urine samples using a commercially available kit (R&D Systems, Minneapolis, MN; RKM100), according to the manufacturer's instructions. KIM-1 levels in urine were factored for the volume of urine collected over the first 24 h after reperfusion. Data are expressed as picograms per 24 h.
Urine and tissue protein.
Protein levels in kidney and urine levels were determined using the Bradford assay (Bio-Rad, Torrence, CA) and BSA as a standard. Urinary protein excretion is presented as milligrams of protein excreted per day. Tissue protein was measured as milligrams per milliliter homogenate.
TNF-α.
TNF-α levels in renal tissue were measured using a commercially available ELISA kit (R&D Systems, Minneapolis, MN; RTA00). Data are presented as picograms of TNF-α per microgram of protein.
VEGF.
VEGF was measured in kidney homogenates using a specific rat VEGF-ELISA kit (R&D Systems; RRV00). The data are presented as picograms of VEGF per microgram of protein.
Statistical analyses.
Data are shown as means ± SE. Data were analyzed by two-way ANOVA, and significant differences were designated at P < 0.05.
RESULTS
Experiment 1. Effect of renal I/R on serum testosterone and the effect of testosterone supplements on renal injury after I/R at 24 h after reperfusion.
Serum testosterone was measured in male rats subjected to 40 min of renal ischemia, as described in methods, and plasma creatinine and serum testosterone levels were measured 24 h later. As shown in Fig. 1A, renal I/R resulted in increases in plasma creatinine of approximately three-fold, and increases in urinary KIM-1 excretion of more than five-fold (Fig. 1B), compared with sham rats. In rats in which testosterone propionate was given intravenously 3 h after renal reperfusion, plasma creatinine and urinary KIM-1 excretion were both reduced by 30% and 60%, respectively (Fig. 1, A and B), and urinary protein excretion was reduced by ∼54% (Fig. 1C).
Fig. 1.
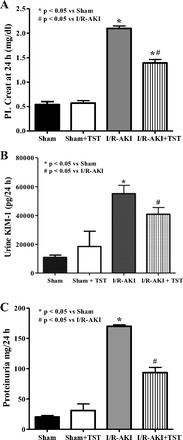
Testosterone supplementation attenuates plasma creatinine (A), KIM-1 (B), and proteinuria (C) 24 h after ischemia-reperfusion (I/R)-induced acute kidney injury (AKI) in male rats. Testosterone supplementation (TST) was given 3 h after reperfusion (or sham surgery) in males subjected to 40 min of ischemia. Data are expressed as milligrams creatinine per deciliter plasma (A), KIM-1 excretion per 24 h (B), and urinary protein excretion per 24 h (C). *P < 0.05 compared with sham. #P < 0.05 compared with I/R-AKI.
Serial measurement of testosterone.
To determine the effect of sham surgery and renal I/R with and without testosterone infusion, serial testosterone measurements were made in serum of rats beginning at 3 h after reperfusion. As shown in Fig. 2A, both sham rats and rats subjected to renal I/R had a 90% reduction in serum testosterone by 3 h after reperfusion (the time when testosterone was given in these studies), compared with untreated controls. Also, as shown in Fig. 2A, serum testosterone levels began to increase by 12 h in sham rats, but not in rats subjected to I/R. As shown in Fig. 2B, levels of serum testosterone at 6 h (3 h after testosterone infusion) were not different in sham + T than in I/R + T. By 12 h after reperfusion, testosterone levels had increased in sham and sham + T, but not in I/R rats.
Fig. 2.
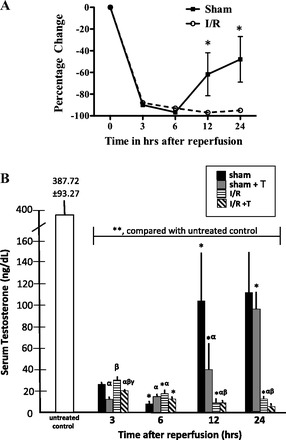
Serum testosterone levels measured serially every 3 h for 24 h following reperfusion after renal ischemia for 45 min in I/R or sham rats with or without testosterone supplementation. A: percentage change in serum testosterone levels at 3, 6, 12, and 24 h in sham (surgery only) and rats subjected to I/R (no testosterone supplements). Serum testosterone levels in sham and I/R rats are compared with age-matched untreated controls (serum testosterone = 387.72 ± 93.27 ng/dl). *P < 0.05 compared with I/R treated or untreated sham. B: numerical changes in serum testosterone levels in sham or I/R-treated rats with and without testosterone supplements given 3 h after reperfusion. Data are expressed as nanograms testosterone/deciliter serum. **P < 0.05 for all samples compared with untreated controls (387.72 ± 93.27 ng/dl); αP < 0.05 compared with sham; βP < 0.05 compared with sham+testosterone; γP < 0.05 compared with I/R; *P < 0.05 compared with previous time point for the same animal group.
Effect of renal I/R on renal injury markers at 48 h after reperfusion.
The OMBF that was measured 48 h after I/R was significantly reduced in rats with renal I/R and normalized in rats given testosterone propionate infusion (Fig. 3). To evaluate the effect of testosterone propionate infusion on inflammation 48 h after I/R, TNF-α was measured in kidney homogenates and was increased by three-fold but was reduced by 50% in rats that received testosterone after I/R (Fig. 4A). VEGF was also measured 48 h after I/R and was significantly reduced with I/R, but improved by testosterone infusion (Fig. 4B).
Fig. 3.
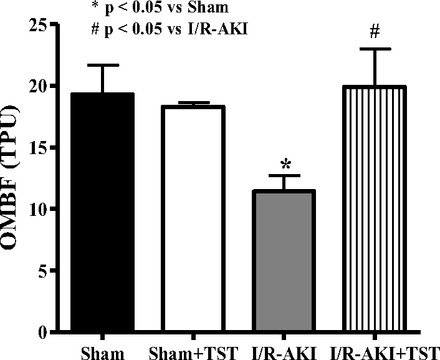
Testosterone supplementation improved outer medullary blood flow (OMBF) by 48 h in the same male rats subjected to I/R-induced AKI as in Figs. 1 and 2. Data are expressed as arbitrary tissue perfusion units (TPU) *P < 0.05 compared with sham. #P < 0.05 compared with I/R-AKI.
Fig. 4.
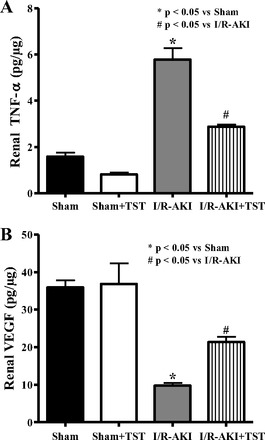
Testosterone supplementation reduces renal TNF-α (A) and VEGF (B) by 48 h in male rats subjected to I/R-induced AKI. Data are expressed as picogram of TNF-α/μg kidney tissue (A) and picogram of VEGF/μg kidney tissue (B). *P < 0.05 compared with sham. #P < 0.05 compared with I/R-AKI.
Experiment 2. Effect of castration or supplemental testosterone on I/R injury.
In another study, rats were left intact or subjected to castration and subjected to renal I/R. I/R caused a 25% greater increase in plasma creatinine in castrated rats than in intact rats (Fig. 5A), and KIM-1 levels were twofold higher in castrated rats subjected to I/R than intact rats (Fig. 5B). When testosterone supplements were given intravenously to castrated rats 3 h after renal reperfusion, plasma creatinine was 25% lower than in intact rats with I/R (Fig. 5A), and urinary KIM-1 levels were 60% lower in castrated rats than in untreated intact rats. As we found previously, serum testosterone levels were significantly reduced by 24 h after I/R in control intact males, and while testosterone infusion improved levels compared with untreated castrated rats with I/R, the levels were still decreased compared with both intact sham and intact with I/R (Fig. 6).
Fig. 5.
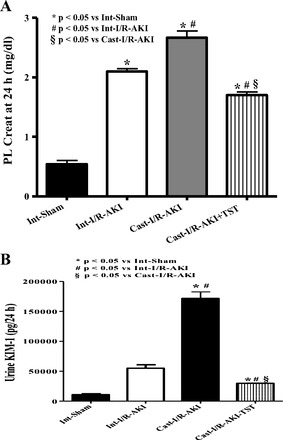
I/R-induced AKI in castration increased plasma creatinine (A) and urinary excretion of KIM-1 (B) compared with intact males with I/R, and testosterone supplements in castrated rats attenuated the increase in both. Int, intact males; cast, castrated males. Data are expressed as milligrams of creatinine per deciliter plasma (A) and KIM-1 excretion per 24 h (B). *P < 0.05 compared with intact sham. #P < 0.05 compared with intact with I/R-AKI. §P < 0.05 compared with untreated castrated rats with I/R-AKI.
Fig. 6.
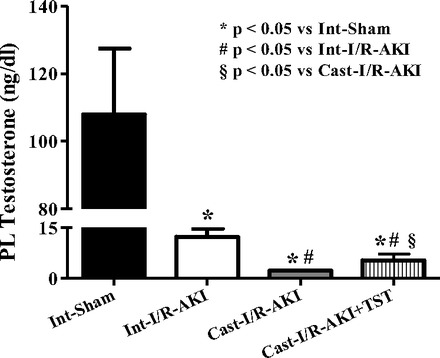
I/R-induced AKI significantly reduced plasma testosterone in intact males, and testosterone was further reduced in castrated rats, but it was slightly improved with testosterone supplements. Data are expressed as nanograms of testosterone/deciliter plasma. *P < 0.05 compared with intact sham. #P < 0.05 compared with intact with I/R-AKI. §P < 0.05 compared with untreated castrated rats with I/R-AKI.
Experiment 3. Role of conversion to estradiol in mediating the protection with testosterone propionate infusion against renal I/R injury.
Since female rats and women are protected from renal I/R injury and testosterone can be converted to estradiol by aromatase, we used an aromatase inhibitor, anastrazole, to make certain the protection afforded by testosterone after I/R was not merely mediated by immediate conversion to estradiol. Anastrazole alone for 3 days before I/R reduced plasma creatinine (Fig. 7A), KIM-1 excretion (Fig. 7B), and urinary protein excretion (Fig. 7C), measured 24 h after renal I/R but had no effect on serum testosterone 24 h after reperfusion (Fig. 8). Anastrazole plus testosterone infusion further reduced both plasma creatinine and urinary KIM-1 excretion (Fig. 7, A and B) but had no further protective effect on urinary protein excretion (Fig. 7C). Anastrazole alone reduced renal TNF-α levels after I/R to sham levels (Fig. 9), and testosterone plus anastrazole further reduced TNF-α to lower levels than sham.
Fig. 7.
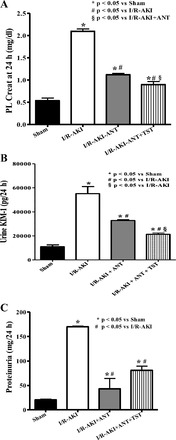
Chronic anastrozole (ANT), an aromatase inhibitor, attenuated plasma creatinine (A), KIM-1 excretion (B), and proteinuria (C) in intact male rats subjected to I/R-induced AKI. Testosterone supplementation with anastrazole further reduced plasma creatinine (A), KIM-1 excretion (B), but not proteinuria (C). Data are expressed as milligrams of creatinine per deciliter plasma (A), KIM-1 excretion per 24 h (B), and protein excretion per 24 h (C). *P < 0.05 compared with intact sham. #P < 0.05 compared with intact with I/R-AKI. §P < 0.05 compared with anastrozole-treated rats with I/R-AKI.
Fig. 8.
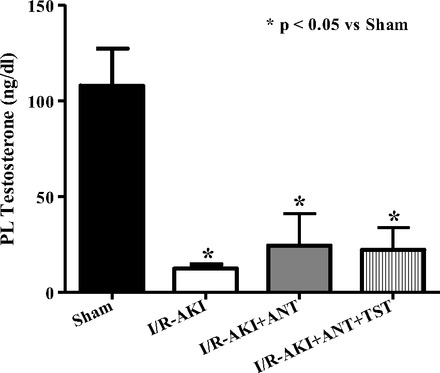
Effect of chronic anastrozole on plasma (PL) testosterone levels measured 24 h after reperfusion in control and testosterone-supplemented rats subjected to I/R-AKI. Data are expressed as nanograms of testosterone/deciliter plasma. *P < 0.05 compared with intact sham. #P < 0.05 compared with intact with I/R-AKI. §P < 0.05 compared with anastrozole-treated rats with I/R-AKI.
Fig. 9.
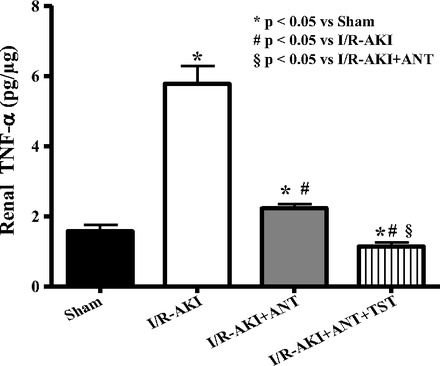
Chronic anastrazole reduced renal expression of TNF-α and the combination of anastrazole+testosterone further reduced renal TNF-α in male rats subjected to I/R-induced AKI. Data are expressed as picograms of TNF-α/μg kidney tissue. *P < 0.05 compared with intact sham. #P < 0.05 compared with intact with I/R-AKI. §P < 0.05 compared with anastrozole-treated rats with I/R-AKI.
DISCUSSION
The novel findings of these studies are that 1) testosterone levels measured serially are reduced with surgery in shams alone but begin to improve by 12 h after reperfusion; 2) testosterone levels are not different at 3 h postinfusion (6 h after reperfusion) in either sham or I/R treated with testosterone propionate; 2) castration exacerbates I/R injury; 3) intravenous testosterone supplementation 3 h after renal I/R in intact or castrated rats protects against renal injury and dysfunction; and 4) the protective effect of testosterone infusion on I/R injury is independent of conversion to estradiol. These data provide strong evidence that the sex difference in renal I/R injury in males is due, at least in part, to reductions in testosterone.
Chronic diseases such as obesity, hypertension, coronary heart disease, and chronic kidney disease (2, 13, 15, 24) are associated with reduced circulating testosterone levels. Although testosterone in the past has been thought to promote cardiovascular and renal disease, investigators are now suggesting that the reduction in testosterone may be a consequence of the disease process and that the reduced levels of testosterone are associated with worsening outcomes for several chronic diseases (15, 24). The mechanisms by which testosterone levels are decreased in chronic cardiovascular and renal diseases have not been elucidated.
With acute injury, the role played by testosterone is controversial. In humans with acute trauma admitted to the intensive care unit with acute respiratory distress syndrome, circulating testosterone levels were decreased within 48 h in men (10). Wagner et al. (33) reported that testosterone suppression is present in all men after traumatic brain injury. These investigators went on to find that with acute traumatic brain injury, testosterone levels remained reduced in plasma samples collected over the 7 days following injury (32). In animal studies, however, Park et al. (21) found that testosterone treatment in mice with renal I/R had no effect on plasma creatinine in males compared with untreated mice with I/R, but castration protected against I/R injury, and testosterone treatment of castrated mice with renal I/R increased plasma creatinine to levels found in untreated males with I/R. In contrast, Robert et al. (27) reported that I/R of the kidney in rats caused a reduction in circulating testosterone levels by day 1 after I/R but returned to normal levels by day 5. Albayrak et al. (1) reported that testosterone given intraperitoneally (100 mg/kg ip) protected against I/R of the intestine in rats (1). In mice in which aromatase gene is deleted (ArKO), Bell et al. (4) reported that myocardial injury was less following I/R in the heart with lower levels of lactate dehydrogenase, perhaps because of the increased testosterone levels in these animals. Borst et al. (6) also found that testosterone treatment (1 mg/day) protected against I/R injury in rat hearts. In contrast, Wang et al. (34) reported that dihydrotestosterone (DHT) treatment for 3 wk in rats reduced STAT3 activation and SOCS3 expression after I/R of the heart (34); increases in both SOC3 and STAT3 are thought to promote cardiac recovery after I/R, suggesting that DHT treatment promoted cardiac I/R injury.
The reason why there are differences in testosterone effects on I/R injury of various tissues is unclear. In our studies, we found a protective effect with acute testosterone infusion in our model of renal I/R in rats, whereas Park et al. (21) found that chronic testosterone treatment (500 μg·kg−1·day−1 for 14 days) in mice with renal I/R had no effect on plasma creatinine compared with untreated mice with I/R, but castration protected against AKI, and testosterone treatment of castrated mice with renal I/R increased plasma creatinine to levels found in untreated males with I/R (21). Neither flutamide (androgen receptor antagonist) nor cyproterone (which has both agonistic and antagonistic properties) had any effect on the plasma creatinine compared with untreated shams.
The reasons for the discrepancy between the findings of Park et al. (21) and our current data are also not clear. The animal model used was different, mice vs. rats. The length of time for androgen treatments was different between the studies, with Park et al. treating mice for 21 days with testosterone, while we gave testosterone acutely by intravenous infusion 3 h following reperfusion. In addition, the doses of testosterone were different, 500 μg·kg−1·day−1 chronically for 21 days (20) vs. a single acute dose of 20 μg·kg−1·min−1 × 10 min, in our present study. The level of testosterone produced by the supplements was not measured by Park et al. (21), but it was likely high prior to the renal I/R maneuver. The dose of testosterone may be important since Xu et al. (36) reported that circulating testosterone levels are also reduced in a rat model of streptozotocin diabetes and that supplemental high doses of androgens promote renal injury, but low doses, more physiological doses, protect against renal injury (36).
One surprising finding from the present studies is that sham surgery alone caused a precipitous reduction in serum testosterone levels. In our model of I/R, the serum testosterone levels remained low at 24 h after reperfusion, and although our results show improvement in measures of renal injury with testosterone infusion, we were not able to measure an increase in serum testosterone, even as soon as 3 h after infusion (6 h reperfusion). These data suggest that the low doses of testosterone that we are giving acutely are not enough to make a difference in serum levels by 3 h after infusion. The data also suggest that the mechanisms by which there is improvement in renal I/R injury may be mediated by acute or short-term mechanisms rather than genomic ones, although one could postulate that a 3-h reduction in serum androgens may upregulate androgen receptors that are located on proximal tubule cells and on endothelial cells in the kidney and could set up genomic changes and changes in intracellular signaling cascades. Future studies need to be done to determine whether, in fact, androgen receptors mediate the protective effects of testosterone infusion after renal I/R.
The mechanisms responsible for renal protection by testosterone treatment after I/R in our model are not clear. We measured TNF-α and found that the levels were attenuated in testosterone-treated I/R rats compared with untreated rats with I/R injury. Androgens have been shown previously to be anti-inflammatory (9, 19, 35). For example, DHT, via the androgen receptor, inhibits IL-1α and TNF-α-induced proinflammatory cytokine production in a rheumatoid cell line (35), and LPS treatment caused increased proinflammatory cytokines in a model of androgen ablation (19). Because TNF-α is produced by nonimmune cells in the kidney, such as medullary thick ascending limb, the reduction in TNF-α may not be contributory to the protection caused by testosterone, but may simply be an indication of less injury to the medulla in the presence of testosterone. Interestingly, Fijak et al. (9) reported that androgens have a direct effect on regulatory T-cell expansion and found a reduction in autoimmune orchiitis in the presence of testosterone supplements. It is tempting to propose that androgens may affect T cells and T-cell infiltration in the kidney after I/R. However, while Fijak et al. (9) found a reduction in macrophages and CD4+ T cells, they also found an upregulation of CD4+/CD25+/FOXP3+ T cells. Kinsey et al. (14) found that it was a reduction in this type of T cells that promoted renal injury in a mouse model of renal I/R (14). Needless to say, additional studies need to be performed to determine whether testosterone can modulate T cells to protect against I/R at this early time point.
We also measured VEGF in our animals subjected to renal I/R and found that it was increased with testosterone treatment compared with untreated rats, although not to control levels. Chade and Kelsen (7) reported that renal ischemia in a model of renal artery stenosis in the pig was associated with reductions in VEGF, and VEGF infusion protected against renal dysfunction and microvascular injury. It is possible that testosterone-mediated improvement of vascular function after renal I/R, by not permitting the significant reductions in VEGF, may have a beneficial effect on kidney function.
Although the presence of estradiol has been shown to be protective in renal I/R injury in both male and female rats (12, 28, 29), our data support the notion that the protective effect of testosterone on kidney function and injury after I/R was independent of conversion to estradiol, since aromatase inhibition in the presence of testosterone infusion protected better than testosterone alone, suggesting that higher levels of testosterone were achieved with aromatase inhibition. In addition, as reported by Bell et al. (4), aromatase-null mice were protected against I/R injury of the heart (4), we found that aromatase inhibition with anastrazole alone for 3 days prior to renal I/R protected against renal injury better than testosterone infusion alone, perhaps because of higher levels of endogenous testosterone produced prior to the I/R injury that may have also prevented the testosterone levels from falling so precipitously during I/R. In any case, anastrazole plus testosterone had an additive effect to further protect against renal injury.
Perspectives and Significance
In the present study, we found that renal I/R in male rats is associated with reductions in circulating levels of testosterone that are sustained for at least 24 h. Acute testosterone treatment protected against renal injury that was independent of conversion to estradiol. The mechanisms responsible for the protection with acute testosterone infusion are not clear from these studies, but attenuation of inflammation and attenuation of mediators of ischemia, such as VEGF, may be potential mechanisms by which testosterone could protect against renal I/R-mediated AKI. Individuals undergoing coronary artery bypass frequently develop AKI (27), and the present studies suggest that intravenous infusion of testosterone in men following coronary artery bypass could be a novel treatment to protect against renal I/R without adversely affecting the grafts. Future studies will be necessary to determine whether testosterone infusion could have translational applicability to clinical settings.
GRANTS
These studies were supported in part by the John Bower Foundation (to L. A. Juncos) and HL-69194, HL-66072 and HL-51971 (to J. F. Reckelhoff).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: A.S., K.C., and R.L. performed experiments; A.S., A.F.L.R., K.C., R.L., and L.A.J. analyzed data; A.S., A.F.L.R., K.C., J.F.R., and L.A.J. interpreted results of experiments; A.S. and A.F.L.R. prepared figures; A.S. and J.F.R. drafted manuscript; A.S., A.F.L.R., K.C., R.O.M., R.L., J.F.R., and L.A.J. approved final version of manuscript; A.F.L.R. and L.A.J. conception and design of research; A.F.L.R., R.O.M., R.L., J.F.R., and L.A.J. edited and revised manuscript.
REFERENCES
- 1. Albayrak Y, Halici Z, Odabasoglu F, Unal D, Keles ON, Malkoc I, Oral A, Yayla M, Aydin O, Unal B. The effects of testosterone on intestinal ischemia/reperfusion in rats. J Invest Surg 24: 283–291, 2011 [DOI] [PubMed] [Google Scholar]
- 2. Allan CA, McLachlan RI. Androgens and obesity. Curr Opin Endocrinol Diabetes Obes 17: 224–232, 2010 [DOI] [PubMed] [Google Scholar]
- 4. Bell JR, Mellor KM, Wollermann AC, Ip WT, Reichelt ME, Meachem SJ, Simpson ER, Delbridge LM. Aromatase deficiency confers paradoxical postischemic cardioprotection. Endocrinology 152: 4937–47, 2011 [DOI] [PubMed] [Google Scholar]
- 5. Benoit DD, Hoste EA, Depuydt PO, Offner FC, Lameire NH, Vandewoude KH, Dhondt AW, Noens LA, Decruyenaere JM. Outcome in critically ill medical patients treated with renal placement therapy for acute renal failure: comparison between patients with and those without haematological malignancies. Nephrol Dial Transplant 20: 552–558, 2005 [DOI] [PubMed] [Google Scholar]
- 6. Borst SE, Quindry JC, Yarrow JF, Conover CF, Powers SK. Testosterone administration induces protection against global myocardial ischemia. Horm Metab Res 42: 122–130, 2010 [DOI] [PubMed] [Google Scholar]
- 7. Chade AR, Kelsen S. Reversal of renal dysfunction by targeted administration of VEGF into the stenotic kidney: a novel potential therapeutic approach. Am J Physiol Renal Physiol 302: F1342–F1350, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chertow Lazarus JM GM, Paganini EP, Allgren RL, Lafayette RA, Sayegh MH. Predictors of mortality and the provision of dialysis in patients with acute tublar necrosis. The auriculin anaritide acute renal failure study group. J Am Soc Nephrol 9: 692–698, 1998 [DOI] [PubMed] [Google Scholar]
- 9. Fijak M, Schneider E, Klug J, Bhushan S, Hackstein H, Schuler G, Wygrecka M, Gromoll J, Meinhardt A. Testosterone replacement effectively inhibits the development of experimental autoimmune orchitis in rats: evidence for a direct role of testosterone on regulatory T cell expansion. J Immunol 186: 5162–5172, 2011 [DOI] [PubMed] [Google Scholar]
- 10. Heffernan DS, Dossett LA, Lightfoot MA, Fremont RD, Ware LB, Sawyer RG, May AK. Gender and acute respiratory distress syndrome in critically injured adults: a prospective study. J Trauma 71: 878–889, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hoste EA, Clermont G, Kersten A, Venkataraman R, Angus DC, De Bacquer D, Kellum JA. RIFLE criteria for acute kidney injury are associated with hospital mortality in critically ill patients: a cohort analysis. Crit Care 10: R73, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hutchens MP, Dunlap J, Hurn PD, Jarnberg PO. Renal ischemia: does sex matter? Anesth Analg 107: 239–249, 2008 [DOI] [PubMed] [Google Scholar]
- 13. Iglesias P, Carrero JJ, Díez JJ. Gonadal dysfunction in men with chronic kidney disease: clinical features, prognostic implications and therapeutic options. J Nephrol 25: 31–42, 2012 [DOI] [PubMed] [Google Scholar]
- 14. Kinsey GR, Sharma R, Huang L, Li L, Vergis AL, Ye H, Ju ST, Okusa MD. Regulatory T cells suppress innate immunity in kidney ischemia-reperfusion injury. J Am Soc Nephrol 20: 1744–1753, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Malkin CJ, Pugh PJ, Morris PD, Asif S, Jones TH, Channer KS. Low serum testosterone and increased mortality in men with coronary heart disease. Heart 96: 1821–1825, 2010 [DOI] [PubMed] [Google Scholar]
- 16. Mehta RL, Pascual MT, Gruta CG, Zhuang S, Chertow GM. Refining predictive models in critically ill patients with acute renal failure. J Am Soc Nephrol 13: 1350–1357, 2002 [DOI] [PubMed] [Google Scholar]
- 17. Militaru C, Donoiu I, Dracea O, Ionescu DD. Serum testosterone and short-term mortality in men with acute myocardial infarction. Cardiol J 17: 249–253, 2012 [PubMed] [Google Scholar]
- 18. Muller V, Losonczy G, Heemann U, Vannay A, Fekete A, Reusz G, Tulassay T, Szabó AJ. Sexual dimorphism in renal ischemia-reperfusion injury in rats: possible role of endothelin. Kidney Int 62: 1364–1371, 2002 [DOI] [PubMed] [Google Scholar]
- 19. Ongaro L, Castrogiovanni D, Giovambattista A, Gaillard RC, Spinedi E. Enhanced proinflammatory cytokine response to bacterial lipopolysaccharide in the adult male rat after either neonatal or prepubertal ablation of biological testosterone activity. Neuroimmunomodulation 18: 254–260, 2011 [DOI] [PubMed] [Google Scholar]
- 20. Paganini EP, Halstenberg WK, Goormastic M. Risk modeling in acute renal failure requiring dialysis: the introduction of a new model. Clin Nephrol 46: 206–211, 1996 [PubMed] [Google Scholar]
- 21. Park KM, Kim JI, Ahn Y, Bonventre AJ, Bonventre JV. Testosterone is responsible for enhanced susceptibility of males to ischemic renal injury. J Biol Chem 279: 52282–52292, 2004 [DOI] [PubMed] [Google Scholar]
- 22. Perusquía M, Espinoza J, Montaño LM, Stallone JN. Regional differences in the vasorelaxing effects of testosterone and its 5-reduced metabolites in the canine vasculature. Vascul Pharmacol 56: 176–182, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pugh PJ, Channer KS, Parry H, Downes T, Jones TH. Bio-available testosterone levels fall acutely following myocardial infarction in men: association with fibinolytic factors. Endocr Res 28: 161–173, 2002 [DOI] [PubMed] [Google Scholar]
- 24. Rao SR, Kini S, Tamler R. Sex hormones and bariatric surgery in men. Gend Med 8: 300–311, 2011 [DOI] [PubMed] [Google Scholar]
- 25. Reckelhoff JF, Zhang H, Granger JP. Testosterone exacerbates hypertension and reduces pressure-natriuresis in male SHR. Hypertension 31: 435–439, 1998 [DOI] [PubMed] [Google Scholar]
- 26. Reddy MM, Mahipal SVK, Subhashini J, Reddy MC, Reddanna P. Bacterial lipopolysaccharide-induced oxidative stress in the impairment of steroidogenesis and spermatogenesis in rats. Reprod Toxicol 22: 493–500, 2006 [DOI] [PubMed] [Google Scholar]
- 27. Robert R, Ghazali DA, Favreau F, Mauco G, Hauet T, Goujon JM. Gender difference and sex hormone production in rodent renal ischemia reperfusion injury and repair. J Inflamm (Lond) 8: 14, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Satake A, Takaoka M, Nishikawa M, Yuba M, Shibata Y, Okumura K, Kitano K, Tsutsui H, Fuji K, Kobuchi S, Ohkita M, Matsumura Y. Protective effect of 17β-estradiol on ischemic acute renal failure through the PI3K/Akt/eNOS pathway. Kidney Int 73: 308–317, 2008 [DOI] [PubMed] [Google Scholar]
- 29. Takaoka M, Yuba M, Fujii T, Ohkita M, Matsumura Y. Oestrogen protects against ischaemic acute renal failure in rats by suppressing renal endothelin-1 overproduction. Clin Sci (Lond) 103 Suppl 48: 434S–437S, 2002 [DOI] [PubMed] [Google Scholar]
- 30. Tripathi Y, Hegde BM. Serum estradiol and testosterone levels following acute myocardial infarction in men. Indian J Physiol Pharmacol 42: 291–294, 1998 [PubMed] [Google Scholar]
- 31. Uchino S, Bellomo R, Goldsmith D, Bates S, Ronco C. An assessment of the RIFLE criteria for acute renal failure in hospitalized patients. Crit Care Med 34: 1913–1917, 2006 [DOI] [PubMed] [Google Scholar]
- 31a. U.S. Renal Data System. USRDS 2012 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2012, p. 121–131 [Google Scholar]
- 32. Wagner AK, McCullough EH, Niyonkuru C, Ozawa H, Loucks TL, Dobos JA, Brett CA, Santarsieri M, Dixon CE, Berga SL, Fabio AJ. Acute serum hormone levels: characterization and prognosis after severe traumatic brain injury. Neurotrauma 28: 871–888, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wagner J, Dusick JR, McArthur DL, Cohan P, Wang C, Swerdloff R, Boscardin WJ, Kelly DF. Acute gonadotroph and somatotroph hormonal suppression after traumatic brain injury. J Neurotrauma 27: 1007–1019, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang M, Wang Y, Abarbanell A, Tan J, Weil B, Herrmann J, Meldrum DR. Both endogenous and exogenous testosterone decrease myocardial STAT3 activation and SOCS3 expression after acute ischemia and reperfusion. Surgery 146: 138–144, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Xu J, Itoh Y, Hayashi H, Takii T, Miyazawa K, Onozaki K. Dihydrotesterone inhibits interleukin-1α or tumor necrosis factor-α-induced proinflammatory cytokine production via androgen receptor-dependent inhibition of nuclear factor-κB activation in rheumatoid fibroblast-like synovial cell line. Biol Pharm Bull 34: 1724–1730, 2011 [DOI] [PubMed] [Google Scholar]
- 36. Xu Q, Prabhu A, Xu S, Manigrasso MB, Maric C. Dose-dependent effects of dihydrotestosterone in the streptozotocin-induced diabetic rat kidney. Am J Physiol Renal Physiol 297: F307–F315, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]


