Abstract
Results from a meta-analysis of aggregated data provoked a new analysis using individual data on the neuropsychological performance of occupationally exposed workers.
Data from eight studies examining 579 exposed and 433 reference participants were included, 28 performance variables analyzed. The performance scores were adjusted for well-known individual-level covariates; the influence of possible, but unknown study-level covariates was attenuated by means of a z-normalization. Associations between performance and exposure were estimated by ANOVAs and ANCOVAs, the latter representing multi-level models.
Four cognitive and motor performance variables each indicated significantly lower performances of exposed individuals when confounding was considered; slowed motor performances and deficits in attention and short-term memory were found. Performance on a single test was significantly related to the biomarker manganese in blood. The outcomes on susceptibility were weak.
The slowing of responses was the most distinct feature of performances of exposed workers. It remains unclear, whether this result is related to the employed tests or provides important information about early stages of the neurotoxic impairment. More specific cognitive tests need to be employed to answer this question. The lack of dose–response relationships was related to features of the biomarker: it does not reflect the Mn in brain responsible for changes in performances.
Keywords: Systematic review, Neurobehavioral toxicology, Neuropsychological test, Neurobiological model, Biomarker
1. Introduction
The serious neurological sequelae following massive exposure to manganese (Mn) have been described as early as 1837 (Couper, 1837). The efforts to decipher the neurotoxic effects and mechanisms of Mn, however, increased distinctly during the last decades. The number of publications ascertained in PubMed for the search-terms Mn AND neurotox* increased from 77 between 1981 and 1990 to 452 between 2001 and 2010. The research devoted to the topic is related to the fact that our contemporary life-style is accompanied by several sources of Mn exposure that add to the natural exposure; the use of MMT (methylcyclopentadienyl manganese tricarbonyl) as an anti-knock agent and the welding of Mn-containing steel may be mentioned. Mining of Mn ore and production of the Mn-containing materials are other sources that put workforce and members of the general public at risk of neurotoxic effects of a trace element that is essential at lower concentrations. In terms of environmental exposures some studies examined the neurobehavioral impact of Mn on adults, adolescents and children living in the vicinity of mining and manufacturing facilities (among others Lucchini et al., 2012; Mergler et al., 1999; Riojas-Rodriguez et al., 2010; Rodriguez-Agudelo et al., 2006).
In the 1980s epidemiological studies started investigating the neurobehavioral impact of Mn in the occupational field (Siegl and Bergert, 1982); this was followed by studies in many parts of the world; among them were South Africa (Myers et al., 2003), Norway (Bast-Pettersen et al., 2004), South Korea (Kim et al., 2007), and China (Cowan et al., 2009b). In 2009 we tried to quantify the existing evidence for neurotoxic effects by means of a metaanalysis of aggregated data (AD) (Meyer-Baron et al., 2009). Reduced motor and cognitive processing speed was substantiated when exposed and unexposed workers were compared. But, questions on exposure–effect relationships and the heterogeneity of study outcomes remained unresolved. Inconsistent relationships between performance scores and the biomarker Mn in blood (MnB) might have resulted from the use of aggregated data. Heterogeneity among neurobehavioral outcomes from different studies could not be addressed satisfactorily, because the influence of covariates could not be diminished.
For the above mentioned reasons we opted for a meta-analysis of individual participant data (IPD). This approach provides distinct advantages not only in terms of the consideration of confounders and the estimate of dose–response relationships, but also in terms of the investigation of individual-specific risk factors (Lambert et al., 2002; McElvenny et al., 2004; Stewart and Tierney, 2002); these are of special importance when susceptible subpopulations have to be protected from sequelae of neurotoxic substances.
It is an obstacle for an IPD analysis in neurobehavioral toxicology that behavior is determined by the social and cultural background; the behavior may therefore not be comparable among studies from diverse cultures. This may be one of the reasons for the observation by Curran and Hussong (2009) that the use of individual data in meta-analyses is relatively novel in behavioral science. We attempted to take account of the cultural differences and opted for an approach to attenuate their influence on outcomes by considering the performance level of reference samples. The methodology was described in a previous paper (Meyer-Baron et al., 2011).
Our current analysis sought to answer the following questions: (1) Can the neurobehavioral effects of Mn exposure be confirmed when confounding is considered? (2) Is there evidence for an exposure–effect relationship when performance scores are related to individual concentrations of the biomarker Mn in blood (MnB)? (3) Can individual-specific risk factors for Mn effects be identified?
2. Materials
The recruitment of the studies and the preparation of the data set will be briefly summarized; for details see Meyer-Baron et al. (2011).
Studies analogous to the AD meta-analysis on Mn (Meyer-Baron et al., 2009) were considered eligible for the present study, since the same criteria for the inclusion were employed: (1) occupational Mn exposure examined by an epidemiological study, (2) outcomes published, (3) exposed and control groups consisted of random samples, (4) standardized neuropsychological tests employed, (5) tests employed in different studies, (6) concentrations of MnB reported. The samples were random in the sense that the studies recruited samples from cohorts of active workers without (suspected) occupational diseases. Studies ascertained by February 2009 were considered.
When agreement about the supply of the data was obtained from the principal investigators, a contract about the confidential use of the anonymous data was signed. The raw data were renamed in a common way and checked for congruence and plausibility before a master data set was created.
Neuropsychological test variables were considered when at least two results from different studies were available. We included also those performance variables that were not reported in the original papers due to insignificant results.
3. Methods
Details on the methodology and reasons for each step of our analysis were explained previously (Meyer-Baron et al., 2011) and will only be briefly summarized.
Correlation analyses were conducted to identify substantially overlapping performance variables. Performance data were log transformed where skewness > 2.
Both individual- and study-level covariates were considered as potential confounders for performance scores. The test scores were adjusted for age, prior intellectual ability, consumption of alcohol, and smoking habits in order to take into account individual-level covariates. The most detailed measurement of covariates available from an individual study was used for the respective study. Additionally, the influence of feasible, but unknown study-level covariates like cultural background was attenuated by a z-normalization. Test scores were normalized by subtracting the mean of the reference group and dividing the difference by the SD of the same group.
Since not all performance tests were available from all studies, they were analyzed in the respective subsample, e.g. within the 4 studies that employed the Digit Span. The sign was reversed when tests provided latency measures or error scores so that higher values always represent better performances.
Fixed effect ANOVAs were used to estimate exposure-related differences between exposed and reference samples (GROUP); fixed effect ANCOVAs were used to estimate the influence of exposure-related differences across studies (STUDY). These multilevel analyses employed individual MnB data as the most refined individual exposure information and study as the exposure characterization at a higher, aggregated level. All effects were modeled as fixed effects; variances and regression parameters were allowed to differ across the individual studies.
Differences in the susceptibility to the impact of Mn were examined in exposed workers that differed in terms of the covariates age, alcohol consumption, or smoking habits. MnB data were stratified into 3 groups (tertiles) as were data on age. Alcohol and smoking were incorporated dichotomously due to limited data. The ANOVAs used test scores that had been adjusted only for the covariates not under scrutiny in the respective calculation. Criteria for the choice of performance tests were number of studies included, sample size and domain measured.
Computations were run using the statistical software SAS 9.2; PROC MIXED was used for computing the fixed effect ANOVAs and ANCOVAs. p-Values ≤ 0.05 were regarded as significant, p-values ≤ 0.10 were displayed and referred to as borderline significant.
4. Results
4.1. Study and sample characteristics
One data set included in the AD meta-analysis was no longer retrievable due to changes in data processing (Chia et al., 1993). The principal investigators of altogether 8 studies provided their data. These studies represented 62% of the meta-analytical sample. Their original data are shown in Table 1. Individual data on airborne concentrations of Mn, however, were provided only by two studies (Bast-Pettersen et al., 2004; Lucchini et al., 1999).
Table 1.
Characteristics of the included studies.
| Reference |
N exposed/ reference |
Workplace of exposed subjects | MnBa [µg/L] exposed/referenceb | MnInhc [mg/m3] |
|---|---|---|---|---|
| 1. Bast-Pettersen et al. (2004) | 100/100 | Production of alloys, melting of ores, electric furnaces | 10.40 ± 3.23/9.09 ± 2.62 | 0.30 (GM)d |
| 2. Blond and Netterstrom (2007) and Blond et al. (2007) | 92/19 | Electro steel works, melting of scrap metal, manual and mechanical handling of Mn | 10.08 ± 2.61/NA | NA |
| 3. Lucchini et al. (1999) | 61/87 | Production of alloys, electric furnaces, crushing of ore, melting, casting | 9.68 ± 3.20/6 ± 1.69 | 0.05 (GM) |
| 4. Lucchini et al. (1997) | 35/37 | Production of alloys, electric furnaces, crushing of ore, melting | 10.02 ± 3.11/6.93 ± 1.46 | 0.19 (GM) |
| 5. Mergler et al. (1994) | 110/145 | Production of alloys, crushing and screening of raw materials and alloy products, casting, cooling of the product | 10.97 ± 4.51/7.47 ± 2.53 | 0.23 (GM) |
| 6. Sjögren et al. (1996) | 12/39 | Railway track company, welding, high alloy Mn steel | 8.58 ± 2.27/7.08 ± 2.63 | NA |
| 7. Wang et al. (2006) | 82/51 | Vehicle manufactures | 21.52 ± 23.24/13.30 ± 22.40 | 0.25 (AM)e |
| 8. Yuan et al. (2006) | 56/34 | Machine works | 48.50 ± 31.74/19.23 ± 12.32 | 0.14 (AM) |
Manganese in blood.
Means ± SD.
Inhalable manganese.
Geometric mean.
Arithmetic mean.
We excluded females due to their small proportion (4.4%) and the fact that 47 of 50 participated in the same study (Wang et al., 2006). Sixty-eight reference participants were excluded, because their MnB was higher than the median of the exposed participants of the same study. This was done to obtain two distinctly different samples for the group comparison.
The final sample comprised 579 exposed and 433 reference persons (57.1% of the eligible participants). As missing data occurred in independent, dependent, and confounding variables, the number of participants is sometimes smaller in the tables.
The included studies examined random samples of active, currently exposed workers; most of them had a cross-sectional design. Studies with a longitudinal design (Blond and Netterstrom, 2007; Blond et al., 2007) were treated like cross-sectional ones by using data from the first or single employment of the test.
Table 2 provides characteristics of the final sample of workers. The measures of confounders displayed are less sophisticated than those used for the adjustment of performance data. These comparable measures allowed imparting information on all or at least most of the studies. Measures provided only by a single study are not displayed. ‘‘Years of education’’ are displayed as a proxy for prior intellectual capability.
Table 2.
Characteristics of exposed and unexposed participants.
| Exposed | Reference participants | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | Range | n | Mean | SD | Range | p | |
| Age [years] | 579 | 42.5 | 9.6 | 19.2–68.0 | 431 | 42.4 | 8.7 | 22.0–64.0 | 0.87a |
| Education [years] | 361 | 9.6 | 2.6 | 1.0–18.0 | 350 | 10.2 | 2.8 | 4.0–18.0 | 0.01a |
| Exposure [years] | 489 | 17.2 | 8.5 | 0.50–44.0 | 433 | – | – | – | |
| MnBb [µg/L] | 558 | 15.2 | 17.3 | 0.7–160.5 | 386 | 7.5 | 4.4 | 2–41.4 | 0.00c |
| Alcohol | 573 | Yes: 443 no: 130 | 427 | Yes: 322 no: 105 | 0.48d | ||||
| Smoking | 578 | Yes: 297 no: 281 | 430 | Yes: 197 no: 233 | 0.06d | ||||
t Test.
Manganese in blood.
Wilcoxon test.
Chi2 test.
Exposed and unexposed participants differed significantly with respect to exposure variables. Education lasted significantly longer among the referents (0.6 years).
4.2. Test characteristics
Results from at least 2 studies were available for 28 performance variables obtained by standardized neuropsychological tests. The variables represented either a test (e.g. Digit Symbol), a subtest (e.g. Digit Span Forward, Digit Span Backward), or a test variable (e.g. mean time or SD in the simple reaction task (SRT)). Ten variables referred to cognitive and 18 to motor performance. Attention, memory and motor functioning were the neuropsychological domains examined. The neuropsychological function speed of information processing was measured by SRT SD, Trail Making A, and Digit Symbol; complex attention by Trail Making B; short-term and working memory by Digit Span Forward and Backward; recognition by Benton Visual Retention. Motor speed was measured by SRT, Tapping, Luria tests, and Catsys subtests on pronation/supination (Catsys: PC-based test system by Danish Product Development Ltd.); fine motor performance by Pursuit Aiming; dexterity by Santa Ana, static steadiness by tremor tests. In addition, we computed ratios for the different components of the Trail Making and Digit Span Test to assess the influence of additional test demands.
Due to inter-correlations of r ≥ 0.65 only preferred hand performances were analyzed where preferred and non-preferred hand performances were measured. The remaining tests were analyzed individually due to moderate inter-correlations (88% r < 0.40).
4.3. Neurobehavioral outcomes
Table 3 shows the outcomes of different approaches to analyze the relationship between exposure and performance.
Table 3.
Exposure–effect relationships estimated by ANOVAs (GROUP) and ANCOVAs (STUDY, MNB), the latter representing multi-level models. F-values are depicted. For abbreviations of studies see Table 1.
| Test | Studies | Group | Study | Manganese in blood |
|---|---|---|---|---|
| All participants | Exposed participants | |||
| Simple Reaction Task SD | 1–6 | 7.801,734** | 2.155,386 (*) | 1.186,386 |
| Trail Making A | 1, 5 | 5.011,400* | 0.241,199 | 0.802,199 |
| Trail Making B | 1, 5 | 0.731,394 | 1.231,198 | 0.941,198 |
| Trails B/A | 0.071,394 | 1.721,198 | 1.962,198 | |
| Digit Symbol | 1, 7, 8 | 0.901,331 | 0.572,207 | 1.013,207 |
| Digit Symbol mean, SPES | 3, 6 | 0.121,185 | 0.451,69 | 0.152,69 |
| Digit Symbol log SD, SPES | 3, 6 | 1.581,185 | 0.951,69 | 0.372,69 |
| Digit Span Forward | 1, 5, 7, 8 | 6.911,564** | 0.593,309 | 1.554,309 |
| Digit Span Backward | 1, 5, 7, 8 | 0.071,564 | 2.343,309(*) | 1.594,309 |
| Digit Span Backward/Forward | 1, 5, 7, 8 | 0.481,564 | 1.753,309 | 0.804,309 |
| Digit Span Forward, SPESa | 3, 5, 6 | 5.061,417* | 1.132,171 | 0.943,171 |
| Benton Visual Retention | 5, 7, 8 | 0.901,395 | 2.662,213(*) | 0.453,213 |
| Simple Reaction Task time | 1–6 | 18.031,740*** | 5.705,389*** | 0.546,389 |
| Tapping dominant hand | 1, 5 | 2.251,398 | 0.411,199 | 0.182,199 |
| Tapping dominant hand, computer | 2, 3, 6 | 3.051,257(*) | 1.232,130 | 1.123,130 |
| Luria finger thumb dominant hand | 1, 3, 5, 6 | 22.981,585*** | 1.333,268 | 0.173,368 |
| Luria hand clench dominant hand | 3, 5, 6 | 26.491,418*** | 0.392,171 | 0.713,171 |
| Luria alternating | 3, 6 | 1.241,185 | 0.251,69 | 1.602,69 |
| Catsys pronation/supination, slow, mean | 2, 3 | 0.621,218 | 0.181,122 | 0.452,122 |
| Catsys log pronation/supination, slow, SD | 2, 3 | 0.151,213 | 0.131,119 | 0.382,119 |
| Catsys pronation–supination fast, mean | 2, 3 | 1.491,219 | 0.201,123 | 0.242,123 |
| Catsys log pronation–supination fast, SD | 2, 3 | 2.131,214 | 0.101,120 | 0.652,120 |
| Pursuit Aiming correct | 4, 7, 8 | 1.461,222 | 1.871,111 | 0.912,111 |
| Pursuit Aiming total | 7, 8 | 0.721,163 | 9.961,111** | 3.762,111* |
| Pursuit Aiming log errors | 7, 8 | 0.181,163 | 0.411,199 | 0.182,199 |
| Santa Ana dominant hand | 5, 7, 8 | 0.041,396 | 7.072,213** | 1.913,213 |
| Catsysb log Tremor intensity | 1–3 | 0.031,361 | 1.512,203 | 1.093,203 |
| Catsys Tremor center frequency | 1–3 | 0.011,361 | 1.762,203 | 0.833,203 |
| Catsys Tremor dispersion | 1–3 | 0.591,361 | 2.552,203(*) | 1.873,203 |
| Catsys Tremor harmonics | 1–3 | 4.941,361* | 1.782,203 | 2.143,203(*) |
Swedish Performance Evaluation System.
PC-based test system by Danish Product Development Ltd.
p ≤ 0.10.
p ≤ 0.05.
p ≤ 0.01.
p ≤ 0.001.
The first model (ANOVA, GROUP) analyzed group differences between exposed and reference samples. It took account of exposure in a dichotomous way (yes–no). Eight significant main effects indicated lower performance scores in the exposed compared to the non-exposed sample.
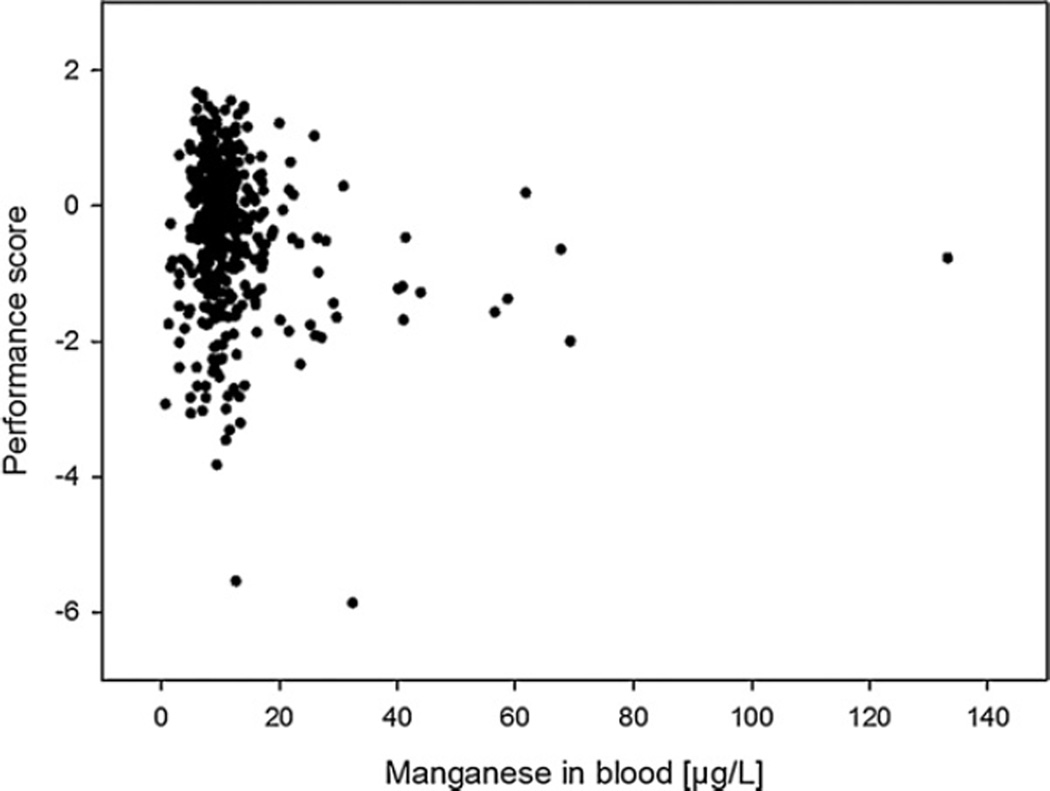
The second model (ANCOVA, STUDY, covariate MNB) evaluated the effect of exposure on performance in terms of studies and individuals. Three significant main effects of STUDY indicated differences among studies as meaningful for neurobehavioral test outcomes. Only one significant coefficient for MnB suggested a relationship between individual concentrations of the biomarker and performance. Fig. 1 depicts the relationship between MnB and motor performance measured by the SRT.
Fig. 1.
Individual performance data related to individual concentrations of the biomarker. Data of the Simple Reaction Task (SRT) are shown.
To analyze whether differences in concentrations of Mn among studies might have induced the effects of STUDY, we conducted an ANOVA estimating the main effect of STUDY on MnB in the exposed samples. A significant main effect (F8,67.2 = 16.01, p < 0.001) was obtained. Pair-wise comparisons showed that the majority of studies differed significantly from each other in terms of concentrations of MnB (Table 4).
Table 4.
Results of pair-wise comparisons of mean MnB concentrations of the studies. Information on significance was obtained by a fixed effect ANOVA. For abbreviations of studies see Table 1.
| Difference of means | |
|---|---|
| Significant | Insignificant |
| 1–2 | 1–3 |
| 1–6 | 1–4 |
| 1–7 | 1–5 |
| 1–8 | 3–4 |
| 3–2 | 3–6 |
| 3–5 | 4–5 |
| 3–7 | 4–6 |
| 3–8 | 6–2 |
| 4–2 | |
| 4–7 | |
| 4–8 | |
| 6–5 | |
| 6–7 | |
| 6–8 | |
| 7–2 | |
| 7–5 | |
| 7–8 | |
| 8–2 | |
| 8–5 | |
| 5–2 | |
To analyze whether kind of exposure might have induced performance differences among studies, we tried to compare the samples of welders and non-welders. However, as the group of non-welders comprised only studies from Europe and North America, the variance in concentrations of MnB was small and did not allow fitting regressions (data not shown).
Analyses on susceptibility were conducted within the data of 5 tests (Tapping SPES, Luria finger thumb, SRT, SRT SD, Digit Span Forward). It was analyzed whether age, smoking habits or alcohol consumption increased the susceptibility to Mn effects. Age-related differences in the susceptibility were suggested by two significant interactions in the data of SRT and SRT SD (see Table 5). Regressions in the three groups showed a borderline significant effect of MnB on SRT only in the youngest group (β = 0.1545, p = 0.07 for the age group < 38 years). The trend was not seen in the age groups 38–47.1 or > 47.1 years. Insignificant regressions in each of the groups were obtained for the SRT SD scores. Smoking habits or alcohol consumption did not show any significant interactions with MnB (data not shown).
Table 5.
Main and interaction effects of age and manganese in blood (MnB) for two cognitive (lines 1 and 2) and three motor performance variables (lines 3–5). Groups were based on tertiles of the independent variables. Results obtained by ANOVAS. The z-normalized data used were adjusted for prior intellectual ability alcohol consumption, and smoking habits.
| Test | Age (3 groups) |
MnB (3 groups) |
Age × MnB |
|---|---|---|---|
| Simple Reaction Task SD | 0.102,402 | 2.912,402(*) | 3.214,402* |
| Digit Span Forward | 1.732,313 | 3.012,313(*) | 1.734,313 |
| Simple Reaction Task time | 2.372,407(*) | 1.272,407 | 3.454,407** |
| Tapping SPESa dominant hand | 0.822,136 | 1.112,136 | 0.704,136 |
| Luria finger thumb dominant hand | 7.092,272*** | 1.712,272 | 0.404,272 |
Swedish Performance Evaluation System.
p ≤ 0.10.
p ≤ 0.05.
p ≤ 0.01.
p ≤ 0.001.
5. Discussion
5.1. Neurobehavioral effects
The analysis of 8 epidemiological studies provided evidence for lower motor and cognitive performance scores in workers occupationally exposed to Mn when compared to reference workers. The speed of simple, repetitive and sequential movements was reduced, but only one of the tremor variables was affected; a more discoordinated tremor was found in exposed workers. In terms of cognitive functioning the speed of information processing, and short-term memory were lower. Deficits in short-term memory were confirmed by a paper–pencil and a computerized test. The deficits were found in workers exposed to mean concentrations of inhalable Mn ranging from 0.05 to 0.30 mg/m3.
The analysis replicated some of the outcomes of our AD meta-analysis (Meyer-Baron et al., 2009) and did this despite consideration of confounders and differences in the sample of included studies. Both analyses showed lower performances of exposed workers in processing speed and speed of movements, while manual dexterity and the recognition of memorized visual material were consistently unaffected. Compared to occupational studies that could not be included herein there is agreement about (1) reduced short-term memory (Chang et al., 2009; Myers et al., 2003), (2) maintained visual recognition (Myers et al., 2003), (3) more discoordinated tremor and reduced tapping performance (Chang et al., 2009), (4) maintained steadiness (Cowan et al., 2009b). Compared to environmental studies there is agreement about (1) reduced short-term memory (Mergler et al., 1999; Wasserman et al., 2011) and (2) slowed sequential movements (Mergler et al., 1999; Rodriguez-Agudelo et al., 2006).
The outcomes of the analysis are in accordance with established knowledge about the neurotoxic properties of Mn. The nuclei of the basal ganglia are target sites for the accumulation of Mn (Eriksson et al., 1992; Kim et al., 1999, 2007; Newland et al., 1989) and since the basal ganglia are part of different cortico-striatal loops (Alexander et al., 1986; Saint-Cyr, 2003), they are of importance for both cognitive and motor performances.
Especially the outcomes on the Digit Span Backward and Trails B appear interesting in terms of new data and hypotheses. Both tests, the first measuring the ability to concurrently maintain and manipulate information in working memory and the second addressing task-switching, may be considered as measuring executive functioning (McCabe et al., 2010; Sanchez-Cubillo et al., 2009). Neither these tests nor the composite measures, calculated to emphasize the executive part by considering the speed related component of the other sub-test, indicated deficiencies in executive functioning in exposed workers. This outcome cannot be straightforwardly explained, since basal ganglia circuits are closely related to executive functions like selection and adjustment of behavior (Chudasama and Robbins, 2006) or working memory (Hazy et al., 2007). The following ideas may be considered:
The data remained confounded. This general, methodological explanation cannot be ruled out. The measurement of covariates was not always adequate; for example the use of ‘years of education’ as an indicator of the prior intellectual capacity lacks refinement. In addition, practice effects may have affected sub-tests. Langenecker et al. (2007) pointed out that traditional neuropsychological measures such as Digit Span and Trail Making suffer from distinct practice effects. It is feasible that the accomplishment of Digit Span Forward and Trails A exerts some kind of practice effect on the second part of the tests.
A dose-related explanation suggests that the slowing of performances is the most important symptom that precedes other effects at an early stage of the neurotoxic impairment and/or at lower levels of exposure. Different performance alterations being related to different exposure conditions might be concluded from epidemiological data on pegboard tests that could not be analyzed herein. Cowan et al. (2009b) and Chang et al. (2009) found in active welders, Ellingsen et al. (2008) and Wastensson et al. (2012) in retired welders significantly lower performances in a pegboard test. Mergler et al. (1994), Bast-Pettersen et al. (2004), and Sjögren et al. (1996) did not find significant differences. The outcomes in the first group might be related to higher internal exposures due to the fine particles in the welding fume. In the second group alloy workers and shorter exposed welders were examined.
Our data seemed to support that significant differences were not obtained due to an increased inter-individual variation of test performances. Moderate mean differences between groups because of small differences in basal ganglia functioning might not reach significance because of increased variances in both groups. This was observed in the exposed and unexposed sample; the variance was about 3 times higher when Trails B was compared to Trails A. The coefficients of variation showed the same trend (CV = 0.43 and 0.37 for unexposed, CV = 0.42 and 0.35 for exposed participants). The increase in the cognitive demand of Trails B results from the requirement of additional and diverse abilities compared to Trails A. It was shown that the subtests do not only differ with respect to cognitive demands, but also with respect to motor control and visual selection (Arbuthnott and Frank, 2000). This means, the sources for variance of test scores are manifold in Trails B: different psychological functions, skills and strategies of the examined individual increase the inter-individual variation.
The uncertainties regarding the interpretation of the test outcomes challenge the employment of tests that help to further decipher the neurotoxic effects Mn exerts. Most obviously many studies placed emphasis on motor performance tests in the context of Mn related deficits. This approach should be reconsidered given current knowledge from neuroscience. Only one of the corticostriatal loops described by Alexander et al. (1986) is associated with motor performance; the others are associated with numerous cognitive functions (Alexander and Crutcher, 1990; Chudasama and Robbins, 2006). Additionally, in vivo measurements of the dopaminergic presynaptic nerve terminal dysfunction showed that in asymptomatic welders the uptake of 6-[18F]Fluoro-l-dopa was most affected in the caudate (Criswell et al., 2011). This nucleus is involved in the basal-ganglia circuits relevant for cognitive functions.
The neurobiological model by Humphries et al. (2006) further refines these loops by differentiating a selection and a control pathway that is related to different cortical structures. It allows thereby attributing observed performance alterations to distinct cortical structures and neurotransmitters. Due to the central role of the globus pallidus, subthalamic nucleus and striatum – structures that are compromised by excessive Mn deposition – this model seems of special interest for Mn related effects.
Impairments in basal ganglia circuitry have been most extensively studied in people suffering from degenerative diseases. For example a modified flanker task (Willemssen et al., 2009) that addresses response inhibition was shown to be able to differentiate PD patients from control participants (Beste et al., 2009a), reference participants from de novo PD patients (Willemssen et al., 2011), and PD patients (de novo or off-medication) from pre-symptomatic or symptomatic Huntington’s disease (HD) patients (Beste et al., 2009b). Also a selective modulation of response inhibition processes by the GRIN2B C2664T polymorphism could be shown by this test (Beste et al., 2010). The polymorphism increases the neurotransmission of the glutamatergic N-methyl-d-aspartate receptor 2B and is important in the HD neuropathology.
Differences in basal ganglia dysfunction can be shown more easily by this test due to its greater specificity. The test requires to focus the attention on the target stimuli and disregard information from distracting stimuli. Relevant and irrelevant stimuli consist of arrows that contain directional information where the stimulus-response mapping is obvious. Since the distracting flankers are provided at random, strategic behavior is not feasible. In these circumstances, the variance of the test performance reflects the capability to inhibit responses, a function that is closely related to basal ganglia dysfunction. It seems promising to employ specific tests like this flanker task, which allows inferences on the function affected.
5.2. Exposure–effect relationships
The influence of between-study variance that was unrelated to exposure was attenuated by the z-normalization; therefore the residual main effects of STUDY presumably reflected the influence of exposure characteristics of the studies on neurobehavioral performance. However, significant effects of STUDY occurred in 3 of 30 tests only. Also MnB, the most individual exposure measure, was significantly related to one of 30 test outcomes only. Study and MnB represent exposure in different ways; we will address doubts about the biomarker first.
The scatter plot of SRT scores depicts that MnB concentrations are inadequate for predicting the individual performance scores at hand (Fig. 1). Similar MnB values can obviously be associated with markedly different performance scores, even around 60 µg Mn/L where scores of exposed participants correspond to performances of reference participants (score = 0) or are distinctly lower (score = −2). This result is in line with weak and inconsistent relationships found in our AD meta-analysis analyzing a different sample of studies (Meyer-Baron et al., 2009). While performance scores might have been confounded there, the inclusion of covariates here did not result in the hypothesized significant relationship between biomarker and performance.
At first glance the outcome seems to contradict the results of 3 studies that confirmed exposure–effect relationships for MnB (Bowler et al., 2007; Chang et al., 2009; Lucchini et al., 1995). A hypothesis to reconcile these differences might be that the studies by Bowler et al. and Chang et al. examined highly exposed individuals. A recent study showed a somewhat closer relationship between airborne and MnB concentrations above concentrations of 0.1 mg/m3 (Pesch et al., 2012). A more adequate reflection of the external exposure by the biomarker might also result in a closer relationship between biomarker and effect. Regardless of higher exposures, a plethora of variables was analyzed in both studies (29 by Chang et al., 37 plus an unknown number by Bowler et al.), but only two variables in the first study and six in the latter were significantly related to. Chance and individual conditions have to be considered as feasible explanations for each of the studies.
Although there is a differentiation into biological markers of exposure and effect there is an acknowledgment of the continuum between these markers (Committee on Biological Markers of the National Research Council, 1987). A correlation of the marker to both exposure and effect should be given (Mercier and Robinson, 1993; Silbergeld and Davis, 1994) and a suitable biomarker should vary especially at low doses. The IPD neither confirm that MnB varies at lower concentrations nor that it is reasonably related to the neurobehavioral performance that reflects an early health impact of exposure.
The result is in accordance with outcomes from a Korean study (Kim et al., 2005) that examined relations between neurobehavioral performance and both MnB and the pallidal index (PI), an indicator of the Mn accumulated in the globus pallidus examined by magnetic resonance imaging. Motor speed, fine motor performance and short-term memory were significantly related to the PI, but not to MnB.
The most obvious underlying reason for the outcome may be seen in the discrepancy between half-times of Mn in blood and brain. It was shown in different animals and for different species of Mn that the half-time of Mn in brain is distinctly longer than in blood (about 2 h compared to 51–74 days after i.v. administration) (e.g. Takeda et al., 1995; Zheng et al., 2000). Half-time of brain Mn between 223 and 267 days was found in macaque monkeys after inhalation of trace amounts of54 MnCl2 for 30 min (Newland et al., 1987). Additionally, the intracellular binding and sequestration of Mn ions that prevent them from migration to the extracellular space have to be considered as reasons why MnB is an inadequate biomarker. Jiang et al. (2007) showed that the PI was clearly related to Mn in red blood cells, but not to MnB (r = 0.55, p = 0.02 vs. r = 0.09, p = 0.72). As in the Korean study, a significant correlation between PI and airborne concentrations was found.
However, even when the biomarker is inappropriate for explaining performance differences, it remains unclear why there is not a more pronounced effect of STUDY given the airborne concentrations shown in Table 1.
A feasible explanation is that the concentrations of inhalable Mn in Table 1 do not adequately reflect the real differences among the studies. The measurements of inhalable concentrations are hardly comparable: the fraction of measured Mn differed (total, inhalable, respirable dust), the sampling differed (personal vs. stationary, spot vs. series, current vs. average of past exposures), the statistical characterization of the sample differed (arithmetic vs. geometric means), and whether the concentrations are representative for the whole sample remains unclear. Insufficiently outlined measurement procedures further complicate comparisons. While it is hard to quantify measurement errors that confound the individual measurement, there is another more basic consideration: measurements of airborne concentrations, if they do not integrate measurements of a larger period, reflect current but not cumulative exposure which appears important for Mnrelated neurobehavioral effects (Lucchini et al., 1999; Newland, 1999; Newland and Weiss, 1992).
These obstacles might explain why it is difficult to prove exposure–effect relationships even at the level of STUDY. Apart from a stricter standardization of dust measurements the results call for research on a biomarker that reflects cumulative exposure since this is the most personal way of reflecting the individual exposure responsible for neurobehavioral changes. The Mn/Fe ratio in erythrocytes or plasma has been suggested as a sensitive and specific marker of exposure (Cowan et al., 2009a) and due to close interrelations between Mn and Fe (Fleming et al., 1998; Meltzer et al., 2010) this marker may represent a biomarker of effect in terms of effects that Mn exerts on Fe metabolism. However, the relationship between the Mn/Fe ratio and airborne concentrations has only been investigated in a small group of exposed workers (n = 20). There it was obviously related to current exposure and a single test performance (Cowan et al., 2009b). Further research is required to prove the ability of the Mn/Fe ratio to capture dose at the target organ at an individual level and to corroborate the relation between the biomarker and behavioral endpoints.
5.3. Person-specific risk factors
Our analyses on the susceptibility to Mn effects did not provide clear-cut results. No associations with use of tobacco or alcohol could be shown. There was a tendency that motor speed was decreased in the youngest tertile of workers, but the outcome was weak and not corroborated by other tests so that the meaning of this result is questionable.
5.4. Limitations of the analysis
The confounding of outcomes by study-level influences was attenuated as far as possible a posteriori. Given the few significant effects of STUDY, the z-normalization appears as an appropriate means to take account of multifaceted influences that may differ among the studies. It was an important advantage of the IPD analysis that individual-level covariates could be considered, but it remains unclear whether influences of individual-level covariates were sufficiently attenuated. The variance explained by these variables was small (Meyer-Baron et al., 2011) and there is evidence that not yet all the individual-level covariates adequately reflect the confounders. The correlation between years of education and intellectual capacity is for example unsatisfactory (Cervilla et al., 2000; Schmand et al., 1997) and the retrospective estimates of average quantities of alcohol may be biased (Del Boca and Darkes, 2003). Measures of verbal intelligence (Hedden and Gabrieli, 2004) and refined measures of alcohol consumption (Dawson, 2003) might decrease uncertainties.
It might be criticized that some of the reference participants were excluded on the basis of a biomarker that proved inappropriate for reflecting the dose of Mn in the brain of individuals. However, this was a decision made a priori, not a consequence of the findings. A sensitivity analysis including all participants showed the same results (data not shown).
Overall it has to be considered that the choice of the exposure measure is not under the control of those conducting the metaanalysis; they have to use what the study investigators report. MnB was the only exposure measure that reflected individual exposures and was available for all studies.
The analysis could not provide information on differences between welders and non-welders. It can be assumed that concentrations of Mn are higher in the brain of welders, among others because fine particles can reach the brain also via olfactory pathways (Brenneman et al., 2000; Thompson et al., 2011). Our data showed comparable effects in welders and non-welders: a reduced motor speed was found in tests where the Chinese welders, who were active for a longer time, were included (SRT) or not included (Tapping); executive functions were not impaired no matter whether these welders were included (Digit Span Backward) or not included (Trails B). However, it has to be considered that the sample for a thorough analysis of this question was too small. It seems worth mentioning that decreased performances were not limited to welding professions; the majority of included studies examined non-welders.
6. Conclusions
The outcomes confirmed and extended a previous meta-analysis of aggregated data. Lower cognitive and motor performances in Mn-exposed workers were found on the basis of a heterogeneous sample. As both individual-level and study-level covariates were considered, the confounding of the outcomes was markedly reduced. Nevertheless further standardization of procedures and refined measures of confounders should gain attention.
The analyses suggested that the biomarker MnB does not reflect the target dose of Mn. Since it was the only exposure measure available on an individual basis, evidence of a dose–response relationship could not be provided. A biomarker that is capable of reflecting long-term exposures also at the individual level is urgently required.
The performance deficits found in the analysis showed a reduced speed of performances, both in the cognitive and motor functioning domain. The main question is whether this outcome provides important diagnostic information on early stages of Mn neurotoxicity or might be related to the employed test procedures. A test demanding a multitude of psychological functions may not be appropriate to provide insights into basal ganglia-related functions that might be affected in individuals exposed to low levels of Mn. The consideration of findings from neurobiology and the use of more specific tests will help to answer this question.
Footnotes
Conflict of interest
None to be declared. The authors have sole responsibility for the writing and content of the paper.
References
- Alexander GE, Crutcher MD. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci. 1990;13(7):266–271. doi: 10.1016/0166-2236(90)90107-l. [DOI] [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Arbuthnott K, Frank J. Trail making test, part B as a measure of executive control: validation using a set-switching paradigm. J Clin Exp Neuropsychol. 2000;22(4):518–528. doi: 10.1076/1380-3395(200008)22:4;1-0;FT518. [DOI] [PubMed] [Google Scholar]
- Bast-Pettersen R, Ellingsen DG, Hetland SM, Thomassen Y. Neuropsychological function in manganese alloy plant workers. Int Arch Occup Environ Health. 2004;77(4):277–287. doi: 10.1007/s00420-003-0491-0. [DOI] [PubMed] [Google Scholar]
- Beste C, Baune BT, Domschke K, Falkenstein M, Konrad C. Dissociable influences of NR2B-receptor related neural transmission on functions of distinct associative basal ganglia circuits. Neuroimage. 2010;52(1):309–315. doi: 10.1016/j.neuroimage.2010.04.022. [DOI] [PubMed] [Google Scholar]
- Beste C, Dziobek I, Hielscher H, Willemssen R, Falkenstein M. Effects of stimulusresponse compatibility on inhibitory processes in Parkinson’s disease. Eur J Neurosci. 2009a;29(4):855–860. doi: 10.1111/j.1460-9568.2009.06621.x. [DOI] [PubMed] [Google Scholar]
- Beste C, Willemssen R, Saft C, Falkenstein M. Error processing in normal aging and in basal ganglia disorders. Neuroscience. 2009b;159(1):143–149. doi: 10.1016/j.neuroscience.2008.12.030. [DOI] [PubMed] [Google Scholar]
- Blond M, Netterstrom B. Neuromotor function in a cohort of Danish steel workers. Neurotoxicology. 2007;28(2):336–344. doi: 10.1016/j.neuro.2006.07.010. [DOI] [PubMed] [Google Scholar]
- Blond M, Netterstrom B, Laursen P. Cognitive function in a cohort of Danish steel workers. Neurotoxicology. 2007;28(2):328–335. doi: 10.1016/j.neuro.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Bowler RM, Roels HA, Nakagawa S, Drezgic M, Diamond E, Park R, et al. Dose–effect relationships between manganese exposure and neurological, neuropsychological and pulmonary function in confined space bridge welders. Occup Environ Med. 2007;64(3):167–177. doi: 10.1136/oem.2006.028761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenneman KA, Wong BA, Buccellato MA, Costa ER, Gross EA, Dorman DC. Direct olfactory transport of inhaled manganese ((54)MnCl(2)) to the rat brain: toxicokinetic investigations in a unilateral nasal occlusion model. Toxicol Appl Pharmacol. 2000;169(3):238–248. doi: 10.1006/taap.2000.9073. [DOI] [PubMed] [Google Scholar]
- Cervilla JA, Prince M, Joels S, Lovestone S, Mann A. Long-term predictors of cognitive outcome in a cohort of older people with hypertension. Br J Psychiatry. 2000;177:66–71. doi: 10.1192/bjp.177.1.66. [DOI] [PubMed] [Google Scholar]
- Chang Y, Kim Y, Woo ST, Song HJ, Kim SH, Lee H, et al. High signal intensity on magnetic resonance imaging is a better predictor of neurobehavioral performances than blood manganese in asymptomatic welders. Neurotoxicology. 2009;30(4):555–563. doi: 10.1016/j.neuro.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Chia SE, Foo SC, Gan SL, Jeyaratnam J, Tian CS. Neurobehavioral functions among workers exposed to manganese ore. Scand J Work Environ Health. 1993;19(4):264–270. doi: 10.5271/sjweh.1475. [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Robbins TW. Functions of frontostriatal systems in cognition: comparative neuropsychopharmacological studies in rats, monkeys and humans. Biol Psychol. 2006;73(1):19–38. doi: 10.1016/j.biopsycho.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Committee on Biological Markers of the National Research Council. Biological markers in environmental health research. Environ Health Perspect. 1987;74:3–9. doi: 10.1289/ehp.74-1474499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couper J. On the effects of black oxide of manganese when inhaled into the lungs. Br Ann Med Pharmacol. 1837;1:41–42. [Google Scholar]
- Cowan DM, Fan Q, Zou Y, Shi X, Chen J, Aschner M, et al. Manganese exposure among smelting workers: blood manganese – iron ratio as a novel tool for manganese exposure assessment. Biomarkers. 2009a;14(1):3–16. doi: 10.1080/13547500902730672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan DM, Zheng W, Zou Y, Shi X, Chen J, Rosenthal FS, et al. Manganese exposure among smelting workers: relationship between blood manganese–iron ratio and early onset neurobehavioral alterations. Neurotoxicology. 2009b;30(6):1214–1222. doi: 10.1016/j.neuro.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criswell SR, Perlmutter JS, Videen TO, Moerlein SM, Flores HP, Birke AM, et al. Reduced uptake of [(1)F]FDOPA PET in asymptomatic welders with occupational manganese exposure. Neurology. 2011;76(15):1296–1301. doi: 10.1212/WNL.0b013e3182152830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran PJ, Hussong AM. Integrative data analysis: the simultaneous analysis of multiple data sets. Psychol Methods. 2009;14(2):81–100. doi: 10.1037/a0015914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson DA. Methodological issues in measuring alcohol use. Alcohol Res Health. 2003;27(1):18–29. [PMC free article] [PubMed] [Google Scholar]
- Del Boca FK, Darkes J. The validity of self-reports of alcohol consumption: state of the science and challenges for research. Addiction. 2003;98(Suppl. 2):1–12. doi: 10.1046/j.1359-6357.2003.00586.x. [DOI] [PubMed] [Google Scholar]
- Ellingsen DG, Konstantinov R, Bast-Pettersen R, Merkurjeva L, Chashchin M, Thomassen Y, et al. A neurobehavioral study of current and former welders exposed to manganese. Neurotoxicology. 2008;29(1):48–59. doi: 10.1016/j.neuro.2007.08.014. [DOI] [PubMed] [Google Scholar]
- Eriksson H, Tedroff J, Thuomas KA, Aquilonius SM, Hartvig P, Fasth KJ, et al. Manganese induced brain lesions in Macaca fascicularis as revealed by positron emission tomography and magnetic resonance imaging. Arch Toxicol. 1992;66(6):403–407. doi: 10.1007/BF02035130. [DOI] [PubMed] [Google Scholar]
- Fleming MD, Romano MA, Su MA, Garrick LM, Garrick MD, Andrews NC. Nramp2 is mutated in the anemic Belgrade (b) rat: evidence of a role for Nramp2 in endosomal iron transport. Proc Natl Acad Sci U S A. 1998;95(3):1148–1153. doi: 10.1073/pnas.95.3.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazy TE, Frank MJ, O‘Reilly RC. Towards an executive without a homunculus: computational models of the prefrontal cortex/basal ganglia system. Philos Trans R Soc Lond Ser B Biol Sci. 2007;362(1485):1601–1613. doi: 10.1098/rstb.2007.2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedden T, Gabrieli JDE. Insights into the ageing mind: a view from cognitive neuroscience. Nat Rev Neurosci. 2004;5:85–97. doi: 10.1038/nrn1323. [DOI] [PubMed] [Google Scholar]
- Humphries MD, Stewart RD, Gurney KN. A physiologically plausible model of action selection and oscillatory activity in the basal ganglia. J Neurosci. 2006;26(50):12921–12942. doi: 10.1523/JNEUROSCI.3486-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Zheng W, Long L, Zhao W, Li X, Mo X, et al. Brain magnetic resonance imaging and manganese concentrations in red blood cells of smelting workers: search for biomarkers of manganese exposure. Neurotoxicology. 2007;28(1):126–135. doi: 10.1016/j.neuro.2006.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E, Kim Y, Cheong HK, Cho S, Shin YC, Sakong J, et al. Pallidal index on MRI as a target organ dose of manganese: structural equation model analysis. Neurotoxicology. 2005;26(3):351–359. doi: 10.1016/j.neuro.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Kim EA, Cheong HK, Choi DS, Sakong J, Ryoo JW, Park I, et al. Effect of occupational manganese exposure on the central nervous system of welders: 1H magnetic resonance spectroscopy and MRI findings. Neurotoxicology. 2007;28(2):276–283. doi: 10.1016/j.neuro.2006.05.013. [DOI] [PubMed] [Google Scholar]
- Kim Y, Kim KS, Yang JS, Park IJ, Kim E, Jin Y, et al. Increase in signal intensities on T1-weighted magnetic resonance images in asymptomatic manganese-exposed workers. Neurotoxicology. 1999;20(6):901–907. [PubMed] [Google Scholar]
- Lambert PC, Sutton AJ, Abrams KR, Jones DR. A comparison of summary patient-level covariates in meta-regression with individual patient data meta-analysis. J Clin Epidemiol. 2002;55(1):86–94. doi: 10.1016/s0895-4356(01)00414-0. [DOI] [PubMed] [Google Scholar]
- Langenecker SA, Zubieta JK, Young EA, Akil H, Nielson KA. A task to manipulate attentional load, set-shifting, and inhibitory control: convergent validity and test – retest reliability of the Parametric Go/No-Go Test. J Clin Exp Neuropsychol. 2007;29(8):842–853. doi: 10.1080/13803390601147611. [DOI] [PubMed] [Google Scholar]
- Lucchini R, Apostoli P, Perrone C, Placidi D, Albini E, Migliorati P, et al. Long term exposure to low levels of manganese oxides and neurofunctional changes in ferroalloy workers. Neurotoxicology. 1999;20(2–3):287–298. [PubMed] [Google Scholar]
- Lucchini R, Bergamaschi E, Smargiassi A, Festa D, Apostoli P. Motor function, olfactory threshold, and hematological indices in manganese-exposed ferroalloy workers. Environ Res. 1997;73(1–2):175–180. doi: 10.1006/enrs.1997.3702. [DOI] [PubMed] [Google Scholar]
- Lucchini R, Selis L, Folli D, Apostoli P, Mutti A, Vanoni O, et al. Neurobehavioral effects of manganese in workers from a ferroalloy plant after temporary cessation of exposure. Scand J Work Environ Health. 1995;21:143–149. doi: 10.5271/sjweh.1369. [DOI] [PubMed] [Google Scholar]
- Lucchini RG, Guazzetti S, Zoni S, Donna F, Peter S, Zacco A, et al. Tremor, olfactory and motor changes in Italian adolescents exposed to historical ferro-manganese emission. Neurotoxicology. 2012;33(4):687–696. doi: 10.1016/j.neuro.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe DP, Roediger HL, McDaniel MA, Balota DA, Hambrick DZ. The relationship between working memory capacity and executive functioning: evidence for a common executive attention construct. Neuropsychology. 2010;24(2):222–243. doi: 10.1037/a0017619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElvenny DM, Armstrong BG, Jarup L, Higgins JP. Meta-analysis in occupational epidemiology: a review of practice. Occup Med (Lond) 2004;54(5):336–344. doi: 10.1093/occmed/kqh049. [DOI] [PubMed] [Google Scholar]
- Meltzer HM, Brantsaeter AL, Borch-Iohnsen B, Ellingsen DG, Alexander J, Thomassen Y, et al. Low iron stores are related to higher blood concentrations of manganese, cobalt and cadmium in non-smoking, Norwegian women in the HUNT 2 study. Environ Res. 2010;110(5):497–504. doi: 10.1016/j.envres.2010.03.006. [DOI] [PubMed] [Google Scholar]
- Mercier MJ, Robinson AE. Use of biologic markers for toxic end-points in assessment of risks from exposure to chemicals. Int Arch Occup Environ Health. 1993;65(1 Suppl.):S7–S10. doi: 10.1007/BF00381300. [DOI] [PubMed] [Google Scholar]
- Mergler D, Baldwin M, Belanger S, Larribe F, Beuter A, Bowler R, et al. Manganese neurotoxicity, a continuum of dysfunction: results from a community based study. Neurotoxicology. 1999;20(2–3):327–342. [PubMed] [Google Scholar]
- Mergler D, Huel G, Bowler R, Iregren A, Belanger S, Baldwin M, et al. Nervous system dysfunction among workers with long-term exposure to manganese. Environ Res. 1994;64(2):151–180. doi: 10.1006/enrs.1994.1013. [DOI] [PubMed] [Google Scholar]
- Meyer-Baron M, Knapp G, Schaper M, van Thriel C. Performance alterations associated with occupational exposure to manganese – a meta-analysis. Neurotoxicology. 2009;30(4):487–496. doi: 10.1016/j.neuro.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Meyer-Baron M, Schaper M, Knapp G, Lucchini R, Albini E, Bast-Pettersen R, et al. Statistical means to enhance the comparability of data within a pooled analysis of individual data in neurobehavioral toxicology. Toxicol Lett. 2011;206(2):144–151. doi: 10.1016/j.toxlet.2011.07.003. [DOI] [PubMed] [Google Scholar]
- Myers JE, Thompson ML, Ramushu S, Young T, Jeebhay MF, London L, et al. The nervous system effects of occupational exposure on workers in a South African manganese smelter. Neurotoxicology. 2003;24(6):885–894. doi: 10.1016/S0161-813X(03)00081-0. [DOI] [PubMed] [Google Scholar]
- Newland MC. Animal models of manganese’s neurotoxicity. Neurotoxicology. 1999;20(2–3):415–432. [PubMed] [Google Scholar]
- Newland MC, Ceckler TL, Kordower JH, Weiss B. Visualizing manganese in the primate basal ganglia with magnetic resonance imaging. Exp Neurol. 1989;106(3):251–258. doi: 10.1016/0014-4886(89)90157-x. [DOI] [PubMed] [Google Scholar]
- Newland MC, Cox C, Hamada R, Oberdorster G, Weiss B. The clearance of manganese chloride in the primate. Fundam Appl Toxicol. 1987;9(2):314–328. doi: 10.1016/0272-0590(87)90054-6. [DOI] [PubMed] [Google Scholar]
- Newland MC, Weiss B. Persistent effects of manganese on effortful responding and their relationship to manganese accumulation in the primate globus pallidus. Toxicol Appl Pharmacol. 1992;113(1):87–97. doi: 10.1016/0041-008x(92)90012-h. [DOI] [PubMed] [Google Scholar]
- Pesch B, Weiss T, Kendzia B, Henry J, Lehnert M, Lotz A, et al. Levels and predictors of airborne and internal exposure to manganese and iron among welders. J Expo Sci Environ Epidemiol. 2012;22(3):291–298. doi: 10.1038/jes.2012.9. [DOI] [PubMed] [Google Scholar]
- Riojas-Rodriguez H, Solis-Vivanco R, Schilmann A, Montes S, Rodriguez S, Rios C, et al. Intellectual function in Mexican children living in a mining area and environmentally exposed to manganese. Environ Health Perspect. 2010;118(10):1465–1470. doi: 10.1289/ehp.0901229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Agudelo Y, Riojas-Rodriguez H, Rios C, Rosas I, Sabido Pedraza E, Miranda J, et al. Motor alterations associated with exposure to manganese in the environment in Mexico. Sci Total Environ. 2006;368(2–3):542–556. doi: 10.1016/j.scitotenv.2006.03.025. [DOI] [PubMed] [Google Scholar]
- Saint-Cyr JA. Frontal – striatal circuit functions: context, sequence, and consequence. J Int Neuropsychol Soc. 2003;9(1):103–127. doi: 10.1017/s1355617703910125. [DOI] [PubMed] [Google Scholar]
- Sanchez-Cubillo I, Perianez JA, Adrover-Roig D, Rodriguez-Sanchez JM, Rios-Lago M, Tirapu J, et al. Construct validity of the Trail Making Test: role of task-switching, working memory, inhibition/interference control, and visuomotor abilities. J Int Neuropsychol Soc. 2009;15(3):438–450. doi: 10.1017/S1355617709090626. [DOI] [PubMed] [Google Scholar]
- Schmand B, Smit JH, Geerlings MI, Lindeboom J. The effects of intelligence and education on the development of dementia. A test of the brain reserve hypothesis. Psychol Med. 1997;27(6):1337–1344. doi: 10.1017/s0033291797005461. [DOI] [PubMed] [Google Scholar]
- Siegl P, Bergert KD. A method of early diagnostic monitoring in manganese exposure. Z Gesamte Hyg. 1982;28(8):524–526. [PubMed] [Google Scholar]
- Silbergeld EK, Davis DL. Role of biomarkers in identifying and understanding environmentally induced disease. Clin Chem. 1994;40(7 Pt. 2):1363–1367. [PubMed] [Google Scholar]
- Sjögren B, Iregren A, Frech W, Hagman M, Johansson L, Tesarz M, et al. Effects on the nervous system among welders exposed to aluminium and manganese. Occup Environ Med. 1996;53:32–40. doi: 10.1136/oem.53.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart LA, Tierney JF. To IPD or not to IPD? Advantages and disadvantages of systematic reviews using individual patient data Eval Health Prof. 2002;25(1):76–97. doi: 10.1177/0163278702025001006. [DOI] [PubMed] [Google Scholar]
- Takeda A, Sawashita J, Okada S. Biological half-lives of zinc and manganese in rat brain. Brain Res. 1995;695(1):53–58. doi: 10.1016/0006-8993(95)00916-e. [DOI] [PubMed] [Google Scholar]
- Thompson KJ, Molina RM, Donaghey T, Savaliya S, Schwob JE, Brain JD. Manganese uptake and distribution in the brain after methyl bromide-induced lesions in the olfactory epithelia. Toxicol Sci. 2011;120(1):163–172. doi: 10.1093/toxsci/kfq387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Yang Y, Wang X, Xu S. The effect of occupational exposure to metals on the nervous system function in welders. J Occup Health. 2006;48(2):100–106. doi: 10.1539/joh.48.100. [DOI] [PubMed] [Google Scholar]
- Wasserman GA, Liu X, Parvez F, Factor-Litvak P, Ahsan H, Levy D, et al. Arsenic and manganese exposure and children’s intellectual function. Neurotoxicology. 2011;32(4):450–457. doi: 10.1016/j.neuro.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wastensson G, Sallsten G, Bast-Pettersen R, Barregard L. Neuromotor function in ship welders after cessation of manganese exposure. Int Arch Occup Environ Health. 2012;85(6):703–713. doi: 10.1007/s00420-011-0716-6. [DOI] [PubMed] [Google Scholar]
- Willemssen R, Falkenstein M, Schwarz M, Muller T, Beste C. Effects of aging, Parkinson’s disease, and dopaminergic medication on response selection and control. Neurobiol Aging. 2011;32(2):327–335. doi: 10.1016/j.neurobiolaging.2009.02.002. [DOI] [PubMed] [Google Scholar]
- Willemssen R, Muller T, Schwarz M, Falkenstein M, Beste C. Response monitoring in de novo patients with Parkinson’s disease. PloS one. 2009;4(3):e4898. doi: 10.1371/journal.pone.0004898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan H, He S, He M, Niu Q, Wang L, Wang S. A comprehensive study on neurobehavior, neurotransmitters and lymphocyte subsets alteration of Chinese manganese welding workers. Life Sci. 2006;78(12):1324–1328. doi: 10.1016/j.lfs.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Zheng W, Kim H, Zhao Q. Comparative toxicokinetics of manganese chloride and methylcyclopentadienyl manganese tricarbonyl (MMT) in Sprague-Dawley rats. Toxicol Sci. 2000;54(2):295–301. doi: 10.1093/toxsci/54.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]