Abstract
Pre-clinical and clinical studies have employed treatment with glucocorticoid receptor (GR) antagonists in an attempt to limit the deleterious behavioral and physiological effects of excess glucocorticoids. Here, we examined the effects of GR antagonists on neuroendocrine and behavioral stress responses, using two compounds: mifepristone, a GR antagonist that is also a progesterone receptor antagonist, and CORT 108297, a specific GR antagonist lacking anti-progestin activity. Given its well-documented impact on neuroendocrine and behavioral stress responses, imipramine (tricyclic antidepressant) served as a positive control. Male rats were treated for five days with mifepristone (10 mg/kg), CORT 108297 (30 mg/kg and 60 mg/kg), imipramine (10mg/kg) or vehicle and exposed to forced swim test (FST) or restraint stress. Relative to vehicle, imipramine potently suppressed adrenocorticotropin hormone (ACTH) responses to FST and restraint exposure. Imipramine also decreased immobility in the FST, consistent with antidepressant actions. Both doses of CORT 108297 potently suppressed peak corticosterone responses to FST and restraint stress. However, only the higher dose of CORT 108297 (60mg/kg) significantly decreased immobility in the FST. In contrast, mifepristone induced protracted secretion of corticosterone in response to both stressors, and modestly decreased immobility in the FST. Taken together, the data indicate distinct effects of each compound on neuroendocrine stress responses and also highlight dissociation between corticosterone responses and immobility in the FST. Within the context of the present study, our data suggest CORT 108297 may be an attractive alternative for mitigating neuroendocrine and behavioral states associated with excess glucocorticoid secretion.
Keywords: mifepristone, forced swim test, imipramine, antidepressants, corticosterone
Introduction
Depression is a serious neuropsychiatric disorder with a lifetime prevalence of 20%. A major factor in the onset or exacerbation of depression symptoms is hypothalamic pituitary adrenal (HPA) axis dysregulation (Juruena et al., 2004). Physiological or psychological stressors activate the HPA axis, culminating in the biosynthesis and release of glucocorticoids from the adrenal cortex. Glucocorticoids exert their biological effects by binding glucocorticoid receptors (mineralocorticoid receptors (MR) and glucocorticoid receptors (GR)). Notably, glucocorticoids provide a negative feedback signal at peripheral (pituitary) and central (hypothalamus, hippocampus, medial prefrontal cortex) targets to constrain excessive activation of this neuroendocrine system (Myers et al., 2012; Herman et al., 2012). This autoregulatory role of glucocorticoids is particularly important, as glucocorticoid hypersecretion is associated with somatic disease including obesity (Hryhorczuk et al., 2013) and psychopathology, most notably depression (Holsboer, 2000; Pariante, 2006).
A significant body of evidence suggests that depression is associated with impaired glucocorticoid signaling. For example, many depressed patients present with abnormal circadian cortisol secretion and/or deficits in GR mediated feedback (dexamethasone resistance) (Modell et al., 1997; Pariante, 2006). In this vein, successful antidepressant treatment normalizes cortisol concentrations in depressed individuals (Otoole et al., 1997; Piwowarska et al., 2012). Further, analyses of postmortem tissue from individuals with a history of depression indicate decreased GR mRNA expression in the frontal cortex and hippocampus (Modell et al., 1997; Webster et al., 1999; Webster 2002), suggesting that impaired GR signaling in central feedback areas may be an underlying factor in the pathophysiology of depression. Consistent with this finding, research in rodent models underscores the notion that GR signaling in central feedback areas is critical for appropriate mood and neuroendocrine responses. For example, increased depression-like and anxiety-like behaviors, as well as increased basal and stress-induced corticosterone secretion, are observed in genetic knockout mouse models targeting forebrain GR (medial prefrontal cortex, basolateral amygdala, and hippocampus) (FBGRKO) (Boyle et al., 2005; Boyle et al., 2006; Furay et al., 2008; Solomon et al., 2012). Notably, the tricyclic antidepressant, imipramine, reverses many of the abnormal neuroendocrine and behavioral stress responses observed in FBGRKO mice (Boyle et al., 2005). A putative mechanism by which antidepressants modulate aberrant glucocorticoid secretion is via GR regulation. For example, tricyclic antidepressants increase GR promoter activity, GR mRNA expression and GR binding in neuronal cell lines (Pepin et al., 1989; Pepin et al., 1991; Okugawa et al., 1999). Congruent with in vivo studies, antidepressant treatment increases hypothalamic and hippocampal GR mRNA expression in the brain (Peiffer et al., 1991). In aggregate, the preclinical and clinical findings suggest that dysfunction in central GR underlies depression symptomology and suggest that compounds targeting this receptor may be suitable treatment strategies for depression.
Given the reported deleterious effects of hypercortisolemia on mood regulation, GR antagonists are being advanced as potential antidepressants (Anacker et al., 2011). Antagonizing GR would provide a means of limiting the deleterious effects of chronically elevated glucocorticoids, and may be sufficient to up-regulate GR in central feedback areas such as the hippocampus and hypothalamus. Clinical studies indicate that brief treatment (4-8 days) with the GR antagonist, mifepristone, decreases symptoms of psychotic depression (Belanoff et al., 2001); although see (Carroll & Rubin, 2006). In addition, rodent studies demonstrate that mifepristone or selective GR antagonists like ORG34116, decrease depression-like behavior in a behavioral assay for antidepressant efficacy and behavioral despair (de Kloet et al.,1988; Bachmann et al., 2005; Wulsin et al., 2010). Further studies report that mifepristone prevents chronic stress-induced depression-like behavior, learning impairment and suppression of neurogenesis in rats (Aisa et al., 2007, Aisa et al., 2008; Oomen et al., 2007). Overall, these data specify a potentially promising role for GR antagonists, particularly mifepristone, as a treatment option to offset or prevent some of the emotional, cognitive and neuroplastic consequences of chronic stress exposure.
Our group has demonstrated that acute treatment with mifepristone modestly decreases immobility in the forced swim test (FST) and dampens HPA axis responses to FST exposure in rats (Wulsin et al., 2010). Furthermore, the data suggest that mifepristone attenuates neuroendocrine stress responses by increasing neuronal activation of stress inhibitory brain regions (medial prefrontal cortex and ventral subiculum of the hippocampus) and decreasing neuronal activation of stress excitatory brain regions (central amygdala). However, mifepristone is also a progesterone receptor (PR) antagonist, causing some uncertainty about mechanism of action.
In the present study, we examined the effects of a new, selective GR antagonist CORT 108297, on behavioral and neuroendocrine stress responses. Given the reported effects of glucocorticoid hypersecretion on physiology and behavior, we predicted that CORT 108297 would induce decreased neuroendocrine (ACTH and corticosterone) and decreased behavioral stress responses. Our data suggest that CORT 108297, at least when administered within the context of the present study, has antidepressant-like properties that mimic those of mifepristone. However, unlike mifepristone, CORT 108297 has a more potent effect at suppressing corticosterone responses during the peak phases of the stress response, but does not trigger rebound increases in cQorticosterone secretion at long post-stress latencies. Together, the data suggest that CORT 108297 may be a suitable alternative for treatment of neuroendocrine and behavioral conditions associated with excess glucocorticoid secretion.
Materials and Methods
Subjects
Male Sprague Dawley rats (Harlan Laboratories, Indianapolis, IN; 250-275 g) were housed two per cage in standard rat shoebox cages and acclimated to the housing conditions for 1 week prior to the initiation of the study. Rats were maintained in a temperature and humidity-controlled environment on a 12:12 LD (lights on 0600/ lights off at 1800) with food and water available ad libitum. All experimental manipulations occurred within the first 4 hours of lights on, and were conducted in accordance with the National Institutes of Health Guidelines for the Care and Use of Animals and approved by the University of Cincinnati Institutional Animal Care and Use Committee.
Drug Treatment
For Experiments 1 and 2, forty-eight rats were matched by bodyweight and were administered, CORT 108297 dissolved in dimethyl sulfoxide (DMSO) (30mg/kg s.c.(n=10) or 60 mg/kg s.c. (n=10) (Corcept Therapeutics, Menlo Park, CA), mifepristone dissolved in DMSO 10mg/kg s.c. (n=10) (Sigma-Aldrich), imipramine dissolved in saline 10mg/kg i.p. (n=10) (Sigma-Aldrich) or vehicle DMSO s.c. (n=4) or saline i.p. (n=4). Control groups consisted of both subcutaneous (s.c.) and intraperitoneal (i.p.) groups to control for the route of administration and both DMSO and saline to control for any potential differences between the compounds on neuroendocrine and behavioral stress responsiveness. As there were no statistical differences in neuroendocrine stress responses between the DMSO and saline treated animals, these groups were collapsed into one vehicle group. The doses and treatment regimen for mifepristone and imipramine were based on our previous study (Wulsin et al., 2010). The doses for CORT 108297 were chosen based on a range of doses that were previously published in (Belanoff et al., 2010; Asagami et al., 2011). The doses for mifepristone and imipramine were chosen based on our previously published findings of antidepressant-like effects in the forced swim test (Wulsin et al., 2010). The experimental animals were allowed approximately two weeks to recover between experiments 1 and 2. Animals remained in the same drug group for both experiments 1 and 2.
Experiment 1 Effects of GR Antagonists and Imipramine on Neuroendocrine and Behavioral Responses to Forced Swim Test
Rats were injected with CORT 108297 (30mg/kg or 60mg/kg), mifepristone (10mg/kg), imipramine (10mg/kg) or vehicle for 5 days. On the fifth day, animals were injected one hour prior to exposure to the FST. Blood samples were collected at 15 and 120 minutes after FST onset. Our laboratory (Wulsin et al., 2010) and others (Morley-Fletcher et al., 2004; Chaviaras et al., 2010) have consistently found an anti-depressant-like effect of imipramine in the forced swim test (FST); consequently, it was used as a positive control in this experiment.
Experiment 2 Effects of GR Antagonists and Imipramine on Neuroendocrine Responses to Restraint Stress
As in Experiment 2, rats were injected with CORT 108297 (30mg/kg s.c. or 60mg/kg s.c.), mifepristone (10mg/kg s.c.), imipramine (10mg/kg i.p.) or vehicle for 5 days. On the fifth day animals were injected one hour prior to exposure to the restraint stress challenge. Animals were gently placed in a well-ventilated Plexiglas restraint tube for 30 minutes. Blood samples were collected at 0 (pre-stress), 15, 30, 60 and 120 minutes after restraint stress onset. The 60 and 120 minute time points were free-bleed post-stress measures. As such, the animals had been removed from the restraint tubes and were placed back into the home cages.
Forced Swim Test
The FST was used according to methods in (Wulsin et al., 2010). Rats were exposed to the FST for 10 min during the light phase of the dark cycle (between 0800-1000h). The FST container was a Plexiglas cylinder 45 cm high and 20 cm in diameter filled with 31 cm of water (25±2°C). The session was videotaped. Two independent observers unaware of the treatment conditions completed the behavioral scoring. The following behaviors were scored: (i) climbing: -rapid movement of limbs in and out of the water with the body parallel to the cylinder, (ii) diving, (iii) swimming: -moving limbs in an active manner and making circular movements around the cylinder and (iv) immobility: not making any active movements or floating in the water without struggling. Each behavior was scored every 5s and the total counts of each behavior was summed for each animal and averaged within each treatment group.
Blood Collection
All blood samples (approximately 250μl) were collected into tubes containing 10 μl of 100mM EDTA and were immediately placed on ice. Samples were spun in a refrigerated centrifuge (4°C, 1500 × g, 15min) and stored at -20°C for subsequent measurement of plasma adrenocorticotropic hormone (ACTH) and corticosterone.
Radioimmunoassay
Plasma corticosterone concentrations were determined by using 125I radioimmunoassay (RIA) kits (MP Biomedicals, Inc., Orangeburg, NY). Plasma ACTH concentrations were determined by an RIA using a specific antiserum generously donated by Dr. William Engeland (University of Minnesota, Minneapolis, MN) at a dilution of 1:120,000, with 125I ACTH (Amersham Biosciences, Piscataway, NJ) as a labeled tracer (Jasper and Engeland, 1991). All samples were run in the same assay and the intra-assay coefficient of variation was less than 10% for both assays.
Statistical Analyses
Behavioral data were analyzed with one-way ANOVA or two-tailed t-test where applicable. Hormonal data were analyzed using a two way-repeated measures ANOVA with drug treatment as the between-subjects factor and time as the within or repeating factor. When necessary, data that violated Levene's homogeneity of variance were log transformed followed by the appropriate statistical analyses. The behavioral and hormonal data were analyzed such that each drug group was compared directly against the vehicle group. Fischer's LSD post hoc tests were used to compare individual group differences. For all data p ≤0.05 denotes statistical significance. In additional to significance testing, when appropriate, Cohen's d (t-tests) and eta squared (η2) (ANOVA) were calculated to express effect sizes. The effect size is a standardized value often used in conjunction with significance testing that represents the magnitude of the difference between or among groups. The effect size allows one to determine how much the independent variable (i.e., treatment) impacts the dependent variable (i.e., immobility or hormones). According to Cohen's guidelines (1988) Cohen's d of .2=small, .5=medium, and .8=large effect sizes, respectively. For η2 .01=small, .05=medium, and .14= large effect sizes, respectively.
Results
Experiment 1: Effects of GR Antagonists and Imipramine on Neuroendocrine and Behavioral Responses to Forced Swim Test
Effects of CORT 108297 on Neuroendocrine and Behavioral Stress Responses in the FST
As mentioned in the methods, all drug groups including CORT 108297, mifepristone and imipramine were compared relative to the vehicle group only. CORT 108297 (30mg/kg and 60 mg/kg) had no impact on circulating ACTH responses to FST exposure (p>0.05) (Figure 1A). However, there was a main effect of drug (F(2,25)=7.96, p<0.01, η2=.036), a main effect of time (F(1,50)= 213.43, p<0.01, η2=076) and a drug × time interaction for plasma corticosterone responses (F(2,50)=7.26, p <0.01, η2=.051). Relative to vehicle treatment, both doses of CORT 108297 decreased corticosterone stress responses at 15 minutes post FST onset (Figure 1B). The higher dose of CORT 108297 (60mg/kg) decreased immobility in the FST (p<0.05) (Figure 1C). Given that there was only a significant difference between CORT108297 (60mg/kg) and the vehicle group, we calculated Cohen's d for these two groups, with d=.95. This finding suggests a large effect size of CORT 108297 (60mg/kg) on immobility in the forced swim test.
Figure 1.
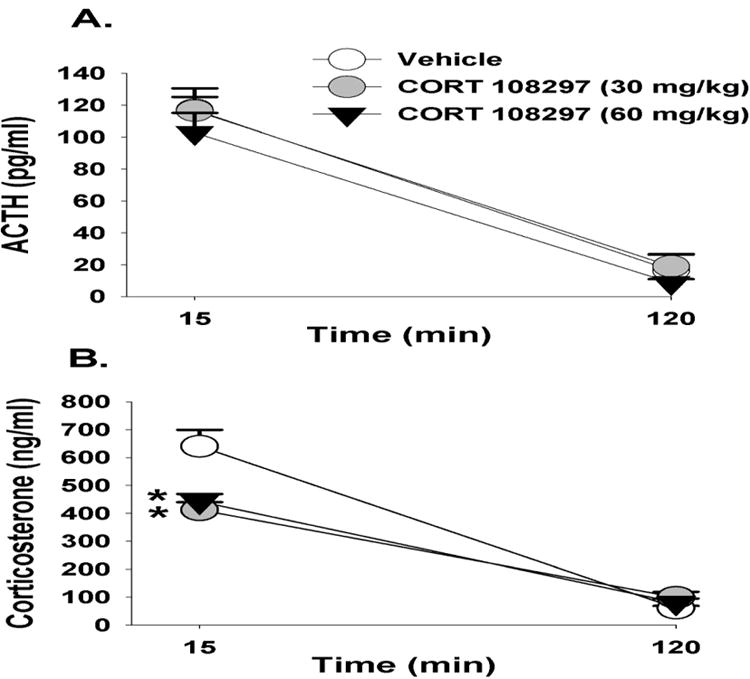
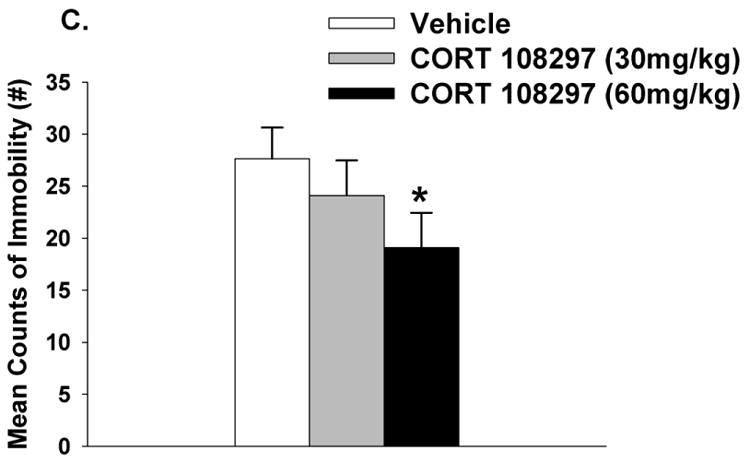
Plasma ACTH (A) and corticosterone (B) concentrations in animals treated with CORT 108297 or vehicle in response to a 10 minute FST exposure. Animals treated with CORT 108297 at 30mg/kg and 60mg/kg had significantly lower corticosterone concentrations at 15 minutes compared to vehicle treated animals. (C) Animals treated with CORT 108297 60mg/kg had significantly less immobility counts in the FST compared with vehicle treated animals. Data are represented as mean ± SEM., n=8-10 per group. * < 0.05 vs. vehicle treated groups.
Effects of Mifepristone on Neuroendocrine and Behavioral Stress Responses in the FST
Mifepristone did not impact circulating concentrations of ACTH in response to FST exposure (p>0.05) (Figure 2A). There was however a main effect of time (F(1,34)=48.72, p <0.01, η2=.51) on corticosterone secretion, and a drug × time interaction (F(1,34)=8.76, p <0.01, η2=.09). At the 120 minute time point, animals treated with mifepristone had higher corticosterone concentrations relative to the vehicle treated animals in response to FST (Figure 2B). Mifepristone did not modulate immobility in the FST, (p>0.05; Figure 2C).
Figure 2.
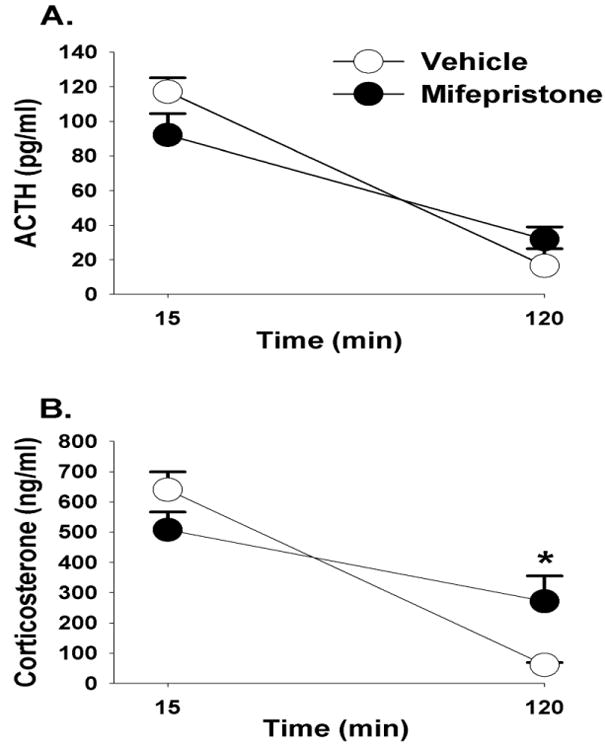
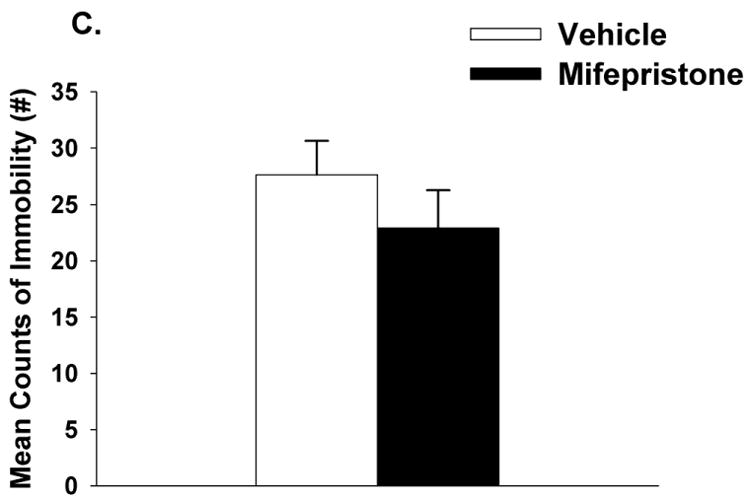
Plasma ACTH (A) and corticosterone (B) concentrations in animals treated with mifepristone or vehicle in response to a 10 minute FST exposure. Animals treated with mifepristone had significantly higher corticosterone concentrations at 120 minutes compared to vehicle treated animals. (C) There was no statistically significant difference in immobility counts between mifepristone and vehicle treated animals, although there was a trend p= 0.07. Data are represented as mean ± SEM., n=8-10 per group. * < 0.05 vs. vehicle treated groups.
Effects of Imipramine on Neuroendocrine and Behavioral Stress Responses in the FST
Overall, imipramine modulated circulating ACTH concentrations in response to FST exposure F(1,16)=31.13, p<0.01, η2=20). There was also a main effect of time (F(1,34)=95.08, p <0.01, η2=.49) and a drug × time interaction (F(1,34)=9.89, p<0.01, η2=.15). For example, at the 15 minute time point, rats treated with imipramine had lower circulating ACTH concentrations relative to vehicle treated animals (Figure 3A).
Figure 3.
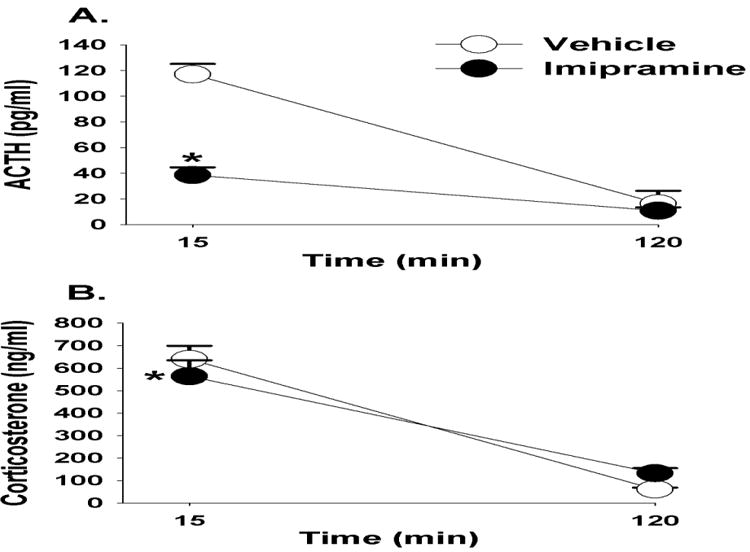
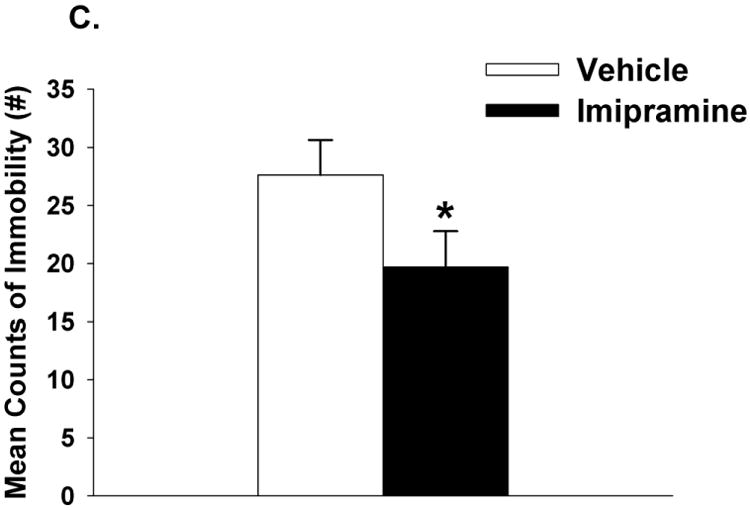
Plasma ACTH (A) and corticosterone (B) concentrations in animals treated with imipramine or vehicle in response to a 10 minute FST exposure. Animals treated with imipramine had significantly lower ACTH and corticosterone concentrations at 15 minutes compared to vehicle treated animals. (C) Imipramine treated animals had significantly lower immobility counts in the FST compared with vehicle treated animals. Data are represented as mean ± SEM., n=8-10 per group. * < 0.05 vs. vehicle treated groups.
There was a main effect of time (F(1,32)=195.19, p<0.01, η2=81) on plasma corticosterone responses to the FST, and a drug × time interaction (F(1,32)=9.89, p <0.01, η2=.04). Rats treated with imipramine had lower corticosterone concentrations at the 15 minute time point (Figure 3B). Imipramine also attenuated immobility counts in the FST (t(16)=1.9, p<0.05). Cohen's d=.93, suggesting a large effect size of imipramine on immobility in the FST (Figure 3C).
Experiment 2: Effects of GR Antagonists and Imipramine on Neuroendocrine Responses to Restraint Stress
Effects of CORT 108297 on Neuroendocrine Responses to Restraint Stress
Consistent with the FST data, neither dose of CORT 108297 affected circulating ACTH concentrations in response to restraint stress (p>0.05, Figures 4A & 4B). However, there was a main effect of drug (F(2,25)=5.54, p<0.05, η2=.05) and time (F(4,133)=70.67, p <0.01, η2=.58) on plasma corticosterone responses, and significant interaction of drug × treatment (F(8,133)=4.43, p<0.01, η2=.07). At the 15 and 30 minute time points, both doses of CORT 108297 significantly decreased plasma corticosterone responses relative to vehicle treatment. CORT 108297 (60mg/kg) also significantly decreased plasma corticosterone responses at the 15 minute time point relative to CORT 108297 (30mg/kg) (Figure 4C). In comparison to vehicle-treated animals only, CORT 108297 (60mg/kg) also decreased the integrated (area under the curve) corticosterone response to restraint stress (p<0.05; Figure 4D). Cohen's d = 1.4, suggesting a very strong effect of CORT 108297 (60mg/kg) on the integrated corticosterone response to restraint stress.
Figure 4.
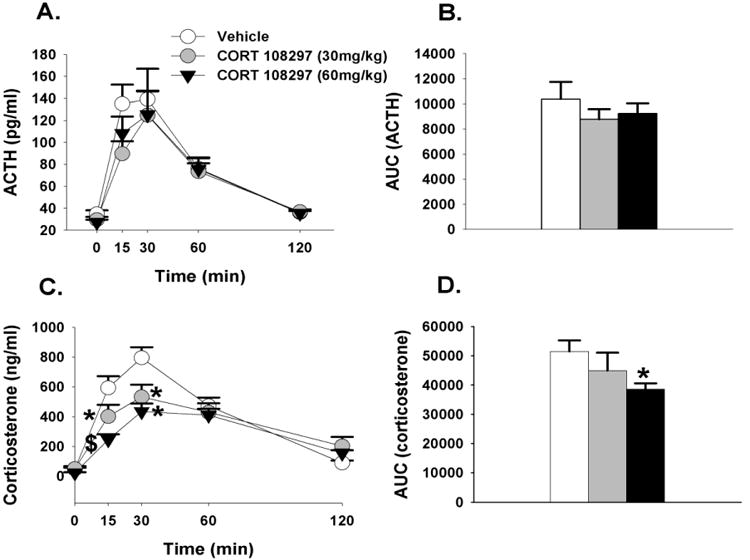
(A)Time course of ACTH in response to acute restraint challenge in animals treated with CORT 108297 or vehicle. (B) Integrated ACTH responses to restraint stress. (C) Time course of corticosterone responses to restraint stress. At the 15 and 30 minute time points animals treated with CORT 108297 30mg/kg and 60mg/kg have significantly lower corticosterone relative to vehicle treated animals. (D) Integrated corticosterone responses to restraint stress. Animals treated with CORT 108297 60mg/kg had significantly lower integrated corticosterone response relative to vehicle treated animals. Data are represented as mean ± SEM., n=8-10 per group. * 0.05 vs. vehicle treated groups, $ vs. all other groups.
Effects of Mifepristone on Neuroendocrine Responses to Restraint Stress
Mifepristone had no effect on circulating plasma ACTH responses to restraint stress (Figures 5A & 5B). There was however a main effect of time (F(4,88)=53.38 p<0.01, η2=.67) on plasma corticosterone secretion, and a drug × time interaction (F(4,88)=4.28, p<0.01, η2=.05). At the 15 minute time point, rats treated with mifepristone had lower plasma corticosterone concentrations relative to vehicle treated animals. However, at the 120 minute time point, rats treated with mifepristone had higher corticosterone concentrations, suggesting an impairment in GR mediated feedback. There was however no difference in the integrated corticosterone responses to restraint stress between mifepristone vs. vehicle treated animals (p>0.05; Figures 5C & 5D).
Figure 5.
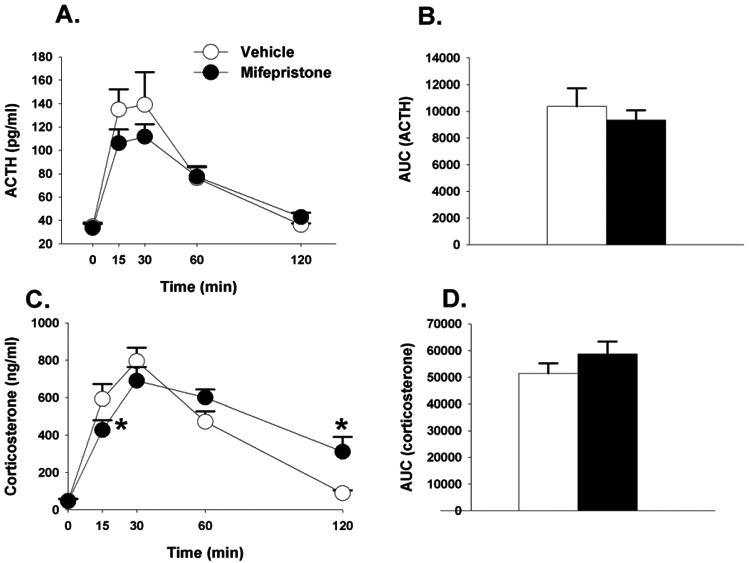
(A) Time course of ACTH in response to acute restraint challenge in animals treated with mifepristone or vehicle. (B) Integrated ACTH responses to restraint stress. (C) Time course of corticosterone responses to restraint stress. At the 15 minute time point animals treated with mifepristone had significantly lower corticosterone responses, but significantly higher corticosterone in response to restraint stress relative to vehicle treated animals. (D) Integrated corticosterone responses to restraint stress. Data are represented as mean ± SEM., n=8-10 per group. * < 0.05 vs. vehicle treated groups.
Effects of Imipramine on Neuroendocrine Responses to Restraint Stress
Imipramine modulated ACTH response to restraint stress. There was a main effect of drug (F(1,16)=5.23, p<0.05, η2=.05), a main effect of time (F(4,88)=28.61, p <0.01, η2=.16), and a drug × time interaction (F(4,88)=4.17, p <0.01, η2=.07). Imipramine decreased plasma ACTH concentrations at 15 and 30 minutes post restraint, as well as the integrated ACTH response (Figures 6A & B). For the integrated ACTH response to restraint stress, Cohen's d= 1.05. This finding indicates a very large effect size of imipramine on circulating stress-induced ACTH concentrations.
Figure 6.
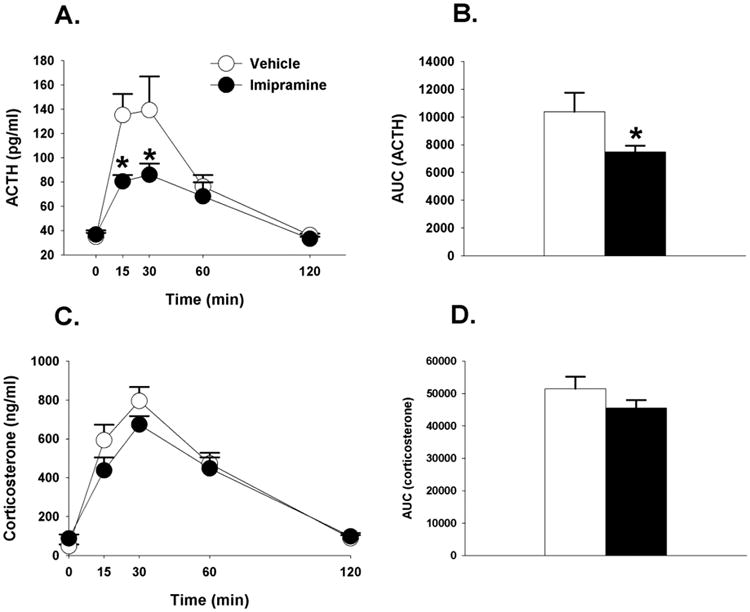
(A) Time course of ACTH in response to acute restraint challenge in animals treated with imipramine or vehicle. Animals treated with imipramine had significantly lower ACTH at 15 and 30 minute time points relative to vehicle treated animals. (B) Integrated ACTH response to restraint stress was significantly lower in imipramine treated animals. (C) Time course of corticosterone responses to restraint stress. (D) Integrated corticosterone responses to restraint stress. Data are represented as mean ± SEM., n=8-10 per group. * < 0.05 vs. vehicle treated groups.
Although not fully appreciated by the graph, (Figure 6C) there was a main effect of drug on corticosterone responses (F(1,16)=4.74, p<0.05, η2=.007), a significant main effect of time (F(4,88)=57.59, p<0.01, η2=07), but no significant drug × time interaction (p >0.05). There was no difference between imipramine and vehicle treated rats in the integrated corticosterone response to restraint stress (Figure 6D). Overall, within the context of the present study imipramine induced marginal effects on circulating corticosterone responses to stress.
Discussion
Here we present data demonstrating antidepressant-like actions of CORT 108297 (60mg/kg), in a behavioral assay of antidepressant efficacy. Both doses of CORT 108297 (30mg/kg and 60mg/kg) dampened corticosterone responses to FST and restraint stress during earlier time points (15 min and 30 min), but had no impact on later phases of the stress response. Mifepristone mildly attenuated stress responsiveness during earlier time points (i.e., 15 min), in response to restraint stress, but prolonged corticosterone responses at later time points (i.e., 120 min) to both restraint and FST exposure. Although both GR antagonists have similar antidepressant-like properties in the FST (albeit to varying degrees), our data indicate disparate effects of CORT 108297 and mifepristone on HPA-axis feedback responses. Overall, the data suggest that antagonizing GR with CORT 108297, at least under stress-evoking situations, protects the organism from heightened neuroendocrine and behavioral responsiveness to an acute, novel stressor.
Neuroendocrine Responses
CORT 108297 potently suppressed corticosterone responses to both FST and restraint stress only during the earlier activational phases of the stress response (i.e., 15 and 30 minutes). There were no differences in circulating corticosterone concentrations between animals treated with either dose of CORT 108297 or vehicle at later time points (60 and 120 minutes) during the inactivational phase. Because CORT 108297 did not induce a similar decrease in ACTH levels, these findings suggest decreased adrenal responsiveness to ACTH. This purported decreased adrenal responsiveness in animals treated with CORT 108297 likely translated into an overall net decrease in circulating glucocorticoid exposure, as the integrated response (area under the curve) to restraint stress was significantly lower in the CORT 108297 (60mg/kg) group compared with vehicle-treated animals. Future studies will be conducted to test this possibility.
On the other hand, animals treated with mifepristone have a strikingly different negative feedback profile to both psychogenic stressors. Consistent with our previous findings (Wulsin et al., 2010), during the initial phase of the stress response, mifepristone modestly attenuated peak ACTH and corticosterone responses to FST, yet prolonged the corticosterone responses to FST stress. This same HPA axis profile in mifepristone treated rats occurs during exposure to the restraint challenge, with an initial decrease in neuroendocrine stress responses at 15 and 30 minutes, but a protracted secretion of glucocorticoids later on during the 120 minute time point. These findings suggest a partial impairment in GR mediated negative feedback in the mifepristone treated animals, an effect discussed in Belanoff et al., 2001. One consequence of this partial impairment in GR mediated feedback in the mifepristone treated animals is a delayed recovery from the stress response. Although mifepristone appears to prolong corticosterone responses to stress, the initial stress dampening effects of the drug may be mediated in part by decreased central stress responsiveness. For example, we have previously found that mifepristone decreases neuronal activation in stress-excitatory areas like the central amygdala, while increasing neuronal activation in stress-inhibitory areas like the ventral subiculum of the hippocampus and the medial prefrontal cortex (Wulsin et al., 2010). These data also demonstrate that the modulatory role of both GR compounds on HPA axis activity is only evident during periods of central HPA axis stimulation, as there were no effects of CORT 108297 or mifepristone on basal circulating levels of ACTH and corticosterone. Previous reports indicate increased basal and stress-induced neuroendocrine responses in animals subjected to GR antagonism (van Haarst et al., 1996; Bachmann et al., 2003; Spiga et al., 2007) but these differences may be due to dosage, route of administration and duration of the treatment regimen.
In accord with previous reports in humans and rodents, imipramine diminished HPA axis responsiveness to stress. Animals treated with imipramine had an overall lower ACTH output over time in response to stress. Imipramine decreases HPA axis responsiveness (Michelson et al., 1997; Frost et al., 2003) and it is likely that the marked decrease in ACTH in our animals is due to less hypothalamic CRH release in response to psychogenic stress exposure (Butterweck et al., 2001; Basta-Kaim et al., 2005). Although imipramine markedly suppressed ACTH responsiveness, it only mildly decreased corticosterone levels. This finding is similar to a recent study by Pinter and colleagues (2011). Overall, the data presented here support a stress dampening effect of imipramine, likely at the level of the brain and pituitary to effectively curtail elevated CRH and ACTH secretion, respectively.
Mechanism of Action of CORT 108297 vs. Mifepristone
Given the remarkably different HPA axis profiles of CORT 108297 and mifepristone on the later phases of the stress response (i.e., 120 minutes), it appears that these compounds may interact with different GR mediated factors to differentially regulate HPA axis responsiveness. Consistent with this notion, a recent paper by Zalachoras and colleagues (2013) noted that CORT 108297 induces an interaction profile between GR and GR-mediated downstream factors that is distinct from the classical antagonist mifepristone and the full GR agonist dexamethasone.
Our findings regarding the CORT 108297 mediated effects on stress induced corticosterone release are in agreement with the aforementioned study conducted by Zalachoras and colleagues (2013). The authors report that CORT 108297 (20mg/kg) decreased the corticosterone response to restraint stress. However, unlike the present study, they report a significant decrease in stress induced ACTH concentrations with CORT 108297 vs. vehicle. Differences between the two studies in stress-induced ACTH release in response to CORT 108297 administration may be due to the dosage (20mg/kg vs. 30mg/kg and 60mg/kg), frequency of administration (once vs. twice daily) and duration of restraint stress exposure (15 minute vs. 30 minute). The authors also report decreased stress induced CRH in the PVN in animals treated with CORT 108297. Overall, the findings from the Zalachoras study indicate a GR agonist-like effect of CORT 108297 on suppression of neuroendocrine (ACTH and corticosterone) responses and PVN CRH mRNA expression. Of note, the agonist like properties of CORT 108297 is brain region specific. For example, CORT 108297 attenuates corticosterone-mediated reduction of hippocampal neurogenesis (suggestive of antagonistic properties) and does not increase central amygdalar CRH mRNA expression, as does the GR agonist dexamethasone.
There are potentially other factors that should be considered when determining the differences between the two GR compounds on stress-induced HPA axis function, including receptor occupancy, binding affinity, and selectivity for other steroid receptors. For example, it is important to consider the potency of both compounds to occupy GR in distinct brain areas. Mifepristone binds to GR with a greater affinity than does CORT 108297 (Clark 2008), and thus the difference in binding affinity in discrete HPA axis regulatory sites may play some role in the marked effects on HPA axis responses to stress (although this remains to be determined). In addition, whereas CORT 108297 is a non-steroidal GR antagonist with no reported activity at the progesterone receptor (PR), mifepristone is a steroidal GR and PR antagonist. Thus, one cannot rule out a potential additive effect of blocking both GR and PR on HPA axis responses. Given that progesterone is stress responsive and has stress-relieving properties in both males and females (Wirth, M., 2011; Purdy et al., 1991, Barbaccia,et al., 2001, Bitran et al.,1995, Paul & Purdy, 1992), it is tempting to speculate that the difference between these two compounds on HPA axis responses may be in part attributable to differential actions on PR.
Behavioral Responses
In the current study we used the modified version of the FST to assess depression-like behavior and neuroendocrine responses to an acute novel stressor. The data presented here demonstrate that selective GR antagonists decrease immobility in the FST, suggestive of anti-depressant actions. CORT 108297 decreased immobility only at the higher dose (60mg/kg), as there was no significant difference in immobility between animals treated with CORT 108297 (30mg/kg) and vehicle. Given that both doses of CORT 108297 decreased circulating corticosterone concentrations in the FST, the results from this study underscore dissociation between absolute corticosterone concentrations and immobility within this task. This is particularly worth noting as it is commonly assumed and heavily reported, that increased glucocorticoid concentrations induce depression-like behavior. At present, we do not fully understand why both doses of CORT 108297 exerted similar effects on neuroendocrine stress responses, while only the higher dose significantly decreased immobility in the FST relative to vehicle. Notably, there was a trend for decreased immobility in the CORT 108297 (30mg/kg) group relative to the vehicle treated group. These data suggest additional factors outside of simply corticosterone concentrations drive the behavioral differences observed in the FST. Clearly, future studies need to evaluate the effects of CORT 108297 on other depression-related behaviors with the use of different behavioral assays.
Previously, our laboratory, along with others, reported that mifepristone decreases immobility within the modified or traditional FST (de Kloet et al., 1988; Aisa et al., 2007; Aisa et al. 2008; Wulsin et al., 2010). However, this finding is not always reproduced (Beckley et al., 2011). In the present study, the effect of mifepristone on immobility did not achieve statistical significance, possibly due to inherent variability in the current study. For example, our previous study was conducted in singly housed males, whereas the present study used pair housed males. Given the reported role of housing conditions on stress responsiveness, we cannot rule out the possibility that this factor could have had a subtle effect on behavioral stress responses.
Mifepristone is currently being advanced as a treatment option for many neuropsychiatric conditions associated with HPA axis dysregulation including major depression, psychotic depression and bipolar disorder (Belanoff et al., 2001; Belanoff et al., 2002; Young et al., 2004; Howland, 2013). While we observed modest antidepressant-like effects of mifepristone in the FST, the extent to which these findings can be translated to the clinical findings is somewhat limited. For example, we examined the ability of mifepristone and CORT 108297 to mitigate neuroendocrine and behavioral responses to acute stressors such as restraint and FST in relatively “normal” animals. However, many of the aforementioned neuropsychiatric conditions are associated with remarkable and sustained alterations in HPA axis function. Nonetheless, the data presented here and in our previous findings (Wulsin, et al., 2010) echo the clinical findings suggesting that systemic treatment with mifepristone, at least under the context of the present study, exerts anti-depressant and perhaps stress buffering properties.
Importantly, our positive control (imipramine) was effective in decreasing immobility in the FST, a finding that validates the sensitivity of our FST procedure and is consistent with previous findings (Morley-Fletcher et al., 2004; Wulsin et al., 2010; Pinter et al., 2011).
Conclusions
It is not the intention of the present study to suggest that depression is simply due to dysfunction in GR. Indeed, recent findings by (Vincent et al., 2013) suggest that the story regarding disruption in central GR signaling and increased depression-like behavior is more complicated than previously appreciated. However, our data suggest that at least within the context of the present study, antagonizing GR with CORT 108297 may be an effective treatment option for altering depressed mood while limiting possible deleterious effects of excessive or prolonged glucocorticoid secretion. In light of the recent findings by Zalachoras and colleagues, (2013) reporting that CORT 108297 functions as a selective GR modulator, this compound may serve as an attractive alternative to classical GR antagonists which may dampen some of the beneficial effects of GR signaling in the peripheral and central nervous systems. These data may have important implications for the utility of this drug for other conditions that are associated with aberrant glucocorticoid activity including depression, obesity and Alzheimer's disease.
The extent to which the findings with CORT 108297 in the present study translate to rodent models of depression (i.e., chronic stress or genetic models) also remains to be determined. Consequently, we are determining the effectiveness of this compound to limit chronic stress-induced neuroendocrine, brain, and behavioral abnormalities. Overall, the data suggest that antagonizing GR with CORT 108297, at least under acute stress-evoking situations, dampens neuroendocrine and behavioral stress responses.
Highlights.
Selective glucocorticoid receptor antagonist CORT 108297 decreases HPA axis responses and immobility in the forced swim test
Unlike mifepristone, CORT 108297 does not lead to increased circulating corticosterone concentrations during recovery from stress
Acknowledgments
The authors would like to thank Drs. Joseph Belanoff, Hazel Hunt and Robin Clark of Corcept Therapeutics both for generous donation of the drug and helpful comments on the manuscript. The authors received CORT 108297 from Corcept Therapeutics, but did not have any funding from the company to complete any of the experiments outlined in this study. In addition, the authors do not have any financial interest in the company. This work was supported by MH 049698 to JPH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aisa B, Tordera R, Lasheras B, Del Rio J, Ramirez MJ. Cognitive impairment associated to HPA axis hyperactivity after maternal separation in rats. Psychoneuroendocrinology. 2007;32:256–266. doi: 10.1016/j.psyneuen.2006.12.013. [DOI] [PubMed] [Google Scholar]
- Aisa B, Tordera R, Lasheras B, Del Rio J, Ramirez MJ. Effects of maternal separation on hypothalamic-pituitary-adrenal responses, cognition and vulnerability to stress in adult female rats. Neuroscience. 2008;154:1218–1226. doi: 10.1016/j.neuroscience.2008.05.011. [DOI] [PubMed] [Google Scholar]
- Anacker C, Zunszain PA, Carvalho LA, Pariante CM. The glucocorticoid receptor: pivot of depression and of antidepressant treatment? Psychoneuroendocrinology. 2011;36:415–425. doi: 10.1016/j.psyneuen.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anacker C, Zunszain PA, Cattaneo A, Carvalho LA, Garabedian MJ, Thuret S, Price J, Pariante CM. Antidepressants increase human hippocampal neurogenesis by activating the glucocorticoid receptor. Mol Psychiatry. 16:738–750. doi: 10.1038/mp.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asagami T, Belanoff JK, Azuma J, Blasey CM, Clark RD, Tsao PS. Glucocorticoid Receptor (GR-II) Antagonist Reduces Body Weight Gain in Mice. J Nutr Metab. 2011;235389 doi: 10.1155/2011/235389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann CG, Bilang-Bleuel A, De Carli S, Linthorst AC, Reul JM. The selective glucocorticoid receptor antagonist ORG 34116 decreases immobility time in the forced swim test and affects cAMP-responsive element-binding protein phosphorylation in rat brain. Neuroendocrinology. 2005;81:129–136. doi: 10.1159/000086413. [DOI] [PubMed] [Google Scholar]
- Bachmann CG, Linthorst AC, Holsboer F, Reul JM. Effect of chronic administration of selective glucocorticoid receptor antagonists on the rat hypothalamic-pituitary-adrenocortical axis. Neuropsychopharmacology. 2003;28:1056–1067. doi: 10.1038/sj.npp.1300158. [DOI] [PubMed] [Google Scholar]
- Barbaccia ML, Affricano D, Purdy RH, Maciocco E, Spiga F, Biggio G. Clozapine,but not haloperidol, increases brain concentrations of neuroactive steroids in the rat. Neuropsychopharmacology. 2001;4:489–97. doi: 10.1016/S0893-133X(01)00254-8. [DOI] [PubMed] [Google Scholar]
- Beckley EH, Scibelli AC, Finn DA. Progesterone receptor antagonist CDB-4124 increases depression-like behavior in mice without affecting locomotor ability. Psychoneuroendocrinology. 2011;6:824–33. doi: 10.1016/j.psyneuen.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belanoff JK, Flores BH, Kalezhan M, Sund B, Schatzberg AF. Rapid reversal of psychotic depression using mifepristone. J Clin Psychopharmacol. 2001;5:516–21. doi: 10.1097/00004714-200110000-00009. [DOI] [PubMed] [Google Scholar]
- Belanoff JK, Rothschild AJ, Cassidy F, DeBattista C, Baulieu EE, Schold C, Schatzberg AF. An open label trial of C-1073 (mifepristone) for psychotic major depression. Biol Psychiatry. 2002;52(5):386–92. doi: 10.1016/s0006-3223(02)01432-4. [DOI] [PubMed] [Google Scholar]
- Bitran D, Shiekh M, McLeod M. Anxiolytic effect of progesterone is mediated by the neurosteroid allopregnanolone at brain GABAA receptors. JNeuroendocrinol. 1995;3:171–7. doi: 10.1111/j.1365-2826.1995.tb00744.x. [DOI] [PubMed] [Google Scholar]
- Boyle MP, Brewer JA, Funatsu M, Wozniak DF, Tsien JZ, Izumi Y, Muglia LJ. Acquired deficit of forebrain glucocorticoid receptor produces depression-like changes in adrenal axis regulation and behavior. Proc Natl Acad Sci. 2005;2:473–8. doi: 10.1073/pnas.0406458102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle MP, Kolber BJ, Vogt SK, Wozniak DF, Muglia LJ. Forebrain glucocorticoid receptors modulate anxiety-associated locomotor activation and adrenal responsiveness. J Neurosci. 2006;7:1971–8. doi: 10.1523/JNEUROSCI.2173-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll BJ, Rubin RT. Is mifepristone useful in psychotic depression? Neuropsychopharmacology. 2006;12:2793–4. doi: 10.1038/sj.npp.1301170. [DOI] [PubMed] [Google Scholar]
- Chaviaras S, Mak P, Ralph D, Krishnan L, Broadbear JH. Assessing the antidepressant-like effects of carbetocin, an oxytocin agonist, using a modification of the forced swimming test. Psychopharmacology (Berl) 2010;1:35–43. doi: 10.1007/s00213-010-1815-x. [DOI] [PubMed] [Google Scholar]
- Clark RD. Glucocorticoid receptor antagonists. Curr Top Med Chem. 2008;8:813–838. doi: 10.2174/156802608784535011. [DOI] [PubMed] [Google Scholar]
- DeBattista C, Belanoff J. The use of mifepristone in the treatment of neuropsychiatric disorders. Trends Endocrinol Metab. 2006;17:117–121. doi: 10.1016/j.tem.2006.02.006. [DOI] [PubMed] [Google Scholar]
- De Kloet ER, De Kock S, Schild V, Veldhuis HD. Antiglucocorticoid RU 38486 attenuates retention of a behaviour and disinhibits the hypothalamic-pituitary adrenal axis at different brain sites. Neuroendocrinology. 1988;2:109–15. doi: 10.1159/000124900. [DOI] [PubMed] [Google Scholar]
- Furay AR, Bruestle AE, Herman JP. The role of the forebrain glucocorticoid receptor in acute and chronic stress. Endocrinology. 2008;11:5482–90. doi: 10.1210/en.2008-0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JP, McKlveen JM, Solomon MB, Carvalho-Netto E, Myers B. Neural regulation of the stress response: glucocorticoid feedback mechanisms. Braz J Med Biol Res. 2012;45:292–298. doi: 10.1590/S0100-879X2012007500041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holsboer F. The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology. 2000;23:477–501. doi: 10.1016/S0893-133X(00)00159-7. [DOI] [PubMed] [Google Scholar]
- Howland RH. Mifepristone as a therapeutic agent in psychiatry. J Psychosoc Nurs Ment Health Serv. 2013;51(6):11–4. doi: 10.3928/02793695-20130513-01. [DOI] [PubMed] [Google Scholar]
- Hryhorczuk C, Sharma S, Fulton SE. Metabolic disturbances connecting obesity and depression. Front Neurosci. 2013;7:177, 1–14. doi: 10.3389/fnins.2013.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasper MS, Engeland WC. Synchronous ultradian rhythms in adrenocortical secretion detected by microdialysis in awake rats. Am J Physiol. 1991;(5Pt 2):R1257–68. doi: 10.1152/ajpregu.1991.261.5.R1257. [DOI] [PubMed] [Google Scholar]
- Juruena MF, Cleare AJ, Pariante CM. The hypothalamic pituitary adrenal axis, glucocorticoid receptor function and relevance to depression. Rev Bras Psiquiatr. 2004;26:189–201. doi: 10.1590/s1516-44462004000300009. [DOI] [PubMed] [Google Scholar]
- Knable MB, Barci BM, Webster MJ, Meador-Woodruff J, Torrey EF Stanley Neuropathology Consortium. Molecular abnormalities of the hippocampus in severe psychiatric illness: postmortem findings from the Stanley Neuropathology Consortium. Mol Psychiatry. 2004;6:609–20. doi: 10.1038/sj.mp.4001471. [DOI] [PubMed] [Google Scholar]
- Kitchener P, Di Blasi F, Borrelli E, Piazza PV. Differences between brain structures in nuclear translocation and DNA binding of the glucocorticoid receptor during stress and the circadian cycle. Eur J Neurosci. 2004;7:1837–46. doi: 10.1111/j.1460-9568.2004.03267.x. [DOI] [PubMed] [Google Scholar]
- Michelson D, Galliven E, Hill L, Demitrack M, Chrousos G, Gold P. Chronic imipramine is associated with diminished hypothalamic-pituitary-adrenal axis responsivity in healthy humans. J Clin Endocrinol Metab. 1997;82:2601–2606. doi: 10.1210/jcem.82.8.4172. [DOI] [PubMed] [Google Scholar]
- Modell S, Yassouridis A, Huber J, Holsboer F. Corticosteroid receptor function is decreased in depressed patients. Neuroendocrinology. 1997;3:216–22. doi: 10.1159/000127275. [DOI] [PubMed] [Google Scholar]
- Morley-Fletcher S, Darnaudery M, Mocaer E, Froger N, Lanfumey L, Laviola G, Casolini P, Zuena AR, Marzano L, Hamon M, Maccari S. Chronic treatment with imipramine reverses immobility behaviour, hippocampal corticosteroid receptors and cortical 5-HT(1A) receptor mRNA in prenatally stressed rats. Neuropharmacology. 2004;47:841–847. doi: 10.1016/j.neuropharm.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Muller M, Holsboer F, Keck ME. Genetic modification of corticosteroid receptor signalling: novel insights into pathophysiology and treatment strategies of human affective disorders. Neuropeptides. 2002;36:117–131. doi: 10.1054/npep.2002.0896. [DOI] [PubMed] [Google Scholar]
- Myers B, McKlveen JM, Herman JP. Neural Regulation of the Stress Response: The Many Faces of Feedback. Cell Mol Neurobiol. 2012 doi: 10.1007/s10571-012-9801-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okugawa G, Omori K, Suzukawa J, Fujiseki Y, Kinoshita T, Inagaki C. Long-term treatment with antidepressants increases glucocorticoid receptor binding and gene expression in cultured rat hippocampal neurones. J Neuroendocrinol. 1999;11:887–95. doi: 10.1046/j.1365-2826.1999.00405.x. [DOI] [PubMed] [Google Scholar]
- Pariante CM. The glucocorticoid receptor: part of the solution or part of the problem? J Psychopharmacol. 2006;20:79–84. doi: 10.1177/1359786806066063. [DOI] [PubMed] [Google Scholar]
- Paul SM, Purdy RH. Neuroactive steroids. FASEB J. 1992;6:2311–22. Review. [PubMed] [Google Scholar]
- Peeters BW, Ruigt GS, Craighead M, Kitchener P. Differential effects of the new glucocorticoid receptor antagonist ORG 34517 and RU486 (mifepristone) on glucocorticoid receptor nuclear translocation in the AtT20 cell line. Ann N Y Acad Sci. 2008;1148:536–541. doi: 10.1196/annals.1410.072. [DOI] [PubMed] [Google Scholar]
- Peiffer A, Veilleux S, Barden N. Antidepressant and other centrally acting drugs regulate glucocorticoid receptor messenger RNA levels in rat brain. Psychoneuroendocrinology. 1991;6:505–15. doi: 10.1016/0306-4530(91)90034-q. [DOI] [PubMed] [Google Scholar]
- Pepin MC, Beaulieu S, Barden N. Antidepressants regulate glucocorticoid receptor messenger RNA concentrations in primary neuronal cultures. Brain Res Mol Brain Res. 1989;1:77–83. doi: 10.1016/0169-328x(89)90031-4. [DOI] [PubMed] [Google Scholar]
- Pepin MC, Govindan MV, Barden N. Increased glucocorticoid receptor gene promoter activity after antidepressant treatment. Mol Pharmacol. 1992;6:1016–22. [PubMed] [Google Scholar]
- Pintér O, Domokos Á, Mergl Z, Mikics É, Zelena D. Do stress hormones connect environmental effects with behavior in the forced swim test? Endocr J. 2011;5:395–407. doi: 10.1507/endocrj.k10e-375. [DOI] [PubMed] [Google Scholar]
- Piwowarska J, Chimiak A, Matsumoto H, Dziklińska A, Radziwoń-Zaleska M, Szelenberger W, Pachecka J. Serum cortisol concentration in patients with major depression after treatment with fluoxetine. Psychiatry Res. 2012;198(3):407–11. doi: 10.1016/j.psychres.2012.01.029. [DOI] [PubMed] [Google Scholar]
- Purdy RH, Morrow AL, Moore PH, Jr, Paul SM. Stress-induced elevations of gamma-aminobutyric acid type A receptor-active steroids in the rat brain. ProcNatl Acad Sci. 1991;10:4553–7. doi: 10.1073/pnas.88.10.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiga F, Harrison LR, Wood SA, Atkinson HC, MacSweeney CP, Thomson F, Craighead M, Grassie M, Lightman SL. Effect of the glucocorticoid receptor antagonist Org 34850 on basal and stress-induced corticosterone secretion. J Neuroendocrinol. 2007;19:891–900. doi: 10.1111/j.1365-2826.2007.01605.x. [DOI] [PubMed] [Google Scholar]
- Spiga F, Knight DM, Droste SK, Conway-Campbell B, Kershaw Y, MacSweeney CP, Thomson FJ, Craighead M, Peeters BW, Lightman SL. Differential effect of glucocorticoid receptor antagonists on glucocorticoid receptor nuclear translocation and DNA binding. J Psychopharmacol. 2011;25:211–221. doi: 10.1177/0269881109348175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon MB, Furay AR, Jones K, Packard AE, Packard BA, Wulsin AC, Herman JP. Deletion of forebrain glucocorticoid receptors impairs neuroendocrine stress responses and induces depression-like behavior in males but not females. Neuroscience. 2012;203:135–43. doi: 10.1016/j.neuroscience.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Haarst AD, Oitzl MS, Workel JO, de Kloet ER. Chronic brain glucocorticoid receptor blockade enhances the rise in circadian and stress-induced pituitary-adrenal activity. Endocrinology. 1996;137:4935–4943. doi: 10.1210/endo.137.11.8895366. [DOI] [PubMed] [Google Scholar]
- Veldhuis HD, De Korte CC, De Kloet ER. Glucocorticoids facilitate the retention of acquired immobility during forced swimming. Eur J Pharmacol. 1985;115:211–217. doi: 10.1016/0014-2999(85)90693-4. [DOI] [PubMed] [Google Scholar]
- Vincent MY, Hussain RJ, Zampi ME, Sheeran K, Solomon MB, Herman JP, Khan A, Jacobson L. Sensitivity of depression-like behavior to glucocorticoids and antidepressants is independent of forebrain glucocorticoid receptors. Brain Res. 2013;1525:1–15. doi: 10.1016/j.brainres.2013.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster MJ, Knable MB, O'Grady J, Orthmann J, Weickert CS. Regional specificity of brain glucocorticoid receptor mRNA alterations in subjects with schizophrenia and mood disorders. Mol Psychiatry. 2002;2002:9, 985–994. doi: 10.1038/sj.mp.4001139. [DOI] [PubMed] [Google Scholar]
- Wirth MM. Beyond the HPA Axis: Progesterone-Derived Neuroactive Steroids in Human Stress and Emotion. Front Endocrinol (Lausanne) 2011;2:19. doi: 10.3389/fendo.2011.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulsin AC, Herman JP, Solomon MB. Mifepristone decreases depression-like behavior and modulates neuroendocrine and central hypothalamic-pituitary-adrenocortical axis responsiveness to stress. Psychoneuroendocrinology. 2010;35:1100–1112. doi: 10.1016/j.psyneuen.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young AH, Gallagher P, Watson S, Del-Estal D, Owen BM, Ferrier IN. Improvements in neurocognitive function and mood following adjunctive treatment with mifepristone (RU-486) in bipolar disorder. Neuropsychopharmacology. 2004;29(8):1538–45. doi: 10.1038/sj.npp.1300471. [DOI] [PubMed] [Google Scholar]
- Zalachoras I, Houtman R, Atucha E, Devos R, Tijssen AM, Hu P, Lockey PM, Datson NA, Belanoff JK, Lucassen PJ, Joëls M, de Kloet ER, Roozendaal B, Hunt H, Meijer OC. Differential targeting of brain stress circuits with a selective glucocorticoid receptor modulator. Proc Natl Acad Sci. 2013 doi: 10.1073/pnas.1219411110. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]


